- Division of Cardiology, Department of Internal Medicine, Kosin University College of Medicine, Busan, Republic of Korea
Iron deficiency (ID) is common in patients with cardiovascular disease. Up to 60% of patients with coronary artery disease and even higher percentages of patients with heart failure (HF) or pulmonary hypertension have ID. However, the evidence for an association between ID including anemia and arrhythmias, particularly atrial fibrillation (AF) is less clear. The prevalence of ID increases with the severity of cardiac and renal dysfunction and would be more common in women. Increased blood loss due to antithrombotic therapy or gastrointestinal or renal disease and insufficient dietary iron intake, reduced iron absorption secondary to low-grade inflammation associated with congestion or reduced gastric acidity may cause ID. Both anemia and ID are associated with poor clinical outcomes, each may confer risk factors independently. There is growing evidence that ID is an important therapeutic target in patients with HF with reduced ejection fraction (HFrEF), even in the absence of anemia. Intravenous ferric carboxymaltose improved symptoms, ID-related quality of life, and exercise capacity and reduced hospitalizations for worsening HF in patients with HFrEF even mildly reduced EF (<50%). ID is easy to treat and effective in patients with HFrEF. These patients should be investigated for possible ID. Malnutrition has also been linked to cardiovascular disease. Both selenium and iron deficiencies have been associated with worse clinical outcomes in patients with HF. And selenium deficiency was associated with new-onset AF in nonsmoking participants. Interventional studies investigating the effects of optimizing the micronutrient status in at-risk populations are needed to assess causality, especially in those with ID. These recommendations may be extended to those populations based on evidence from future clinical trials.
1 Introduction
Iron is required by all organ systems for a variety of metabolic processes, such as erythropoiesis, mitochondrial function, oxygen transport, cardiac and skeletal muscle metabolism, immune and nervous systems, inflammatory response, and lipid metabolism (1).
Iron deficiency (ID) is common (2), and recent trials have shown it to be an important treatment target in patients with heart failure (HF) with reduced ejection fraction (HFrEF) (3, 4). However, ID appears to be common in a wide range of cardiovascular (CV) diseases. This review aimed to summarize the available data from epidemiological, and clinical studies on ID of arrhythmias and cardiovascular diseases. We followed the PRISM A guidelines and registration information (5).
2 Definition of iron deficiency
Anemia is a hematological disorder caused by a deficiency of red blood cells, most commonly defined as a decrease in hemoglobin concentration (6). Estimates of anemia prevalence vary by gender and age. Globally, the prevalence of anemia is estimated to be 10%–30% in non-pregnant women, 11%–12% in men, and 20%–24% in the elderly (7, 8). Among the various causes, anemia due to ID is the most common. Likewise, the prevalence of ID varies by race, gender, and age. In developing countries, the prevalence of ID can be as high as 41%–63% of women and 13% of men, whereas ID anemia can occur in 20%–39% of women and 4% of men (9). However, because comprehensive data are limited, these figures may underestimate the true burden of ID, especially non-anemic ID. There are numerous definitions and guidelines for diagnosing ID (10). Iron stores can be obtained from serum iron, ferritin, and transferrin levels (11). However, due to the nature of ferritin as an acute phase reactant, elevated serum ferritin levels also occur in other chronic inflammatory diseases, regardless of iron status. In chronic inflammatory conditions, serum ferritin production increases 2.5-fold (12). In these cases, additional evidence of low transferrin saturation under 20% and assessment of inflammatory markers (e.g., erythrocyte sedimentation rate and C-reactive protein) are needed to better assess ID (12, 13). In particular, a ferritin level of less than 100 ug/L or a combination of a ferritin level between 100 and 299 ug/L and a transferrin saturation of less than 20% are now guidelines recommended criteria for the diagnosis of ID in patients with heart failure. As explained below.
3 What mechanisms of chronic inflammation make iron deficiency?
Iron supplementation is thought to be beneficial in part because HF, similar to chronic kidney disease, cancer, and other inflammatory diseases, is associated with increased systemic inflammation and subsequent abnormal homeostasis of systemic iron (14, 15). Our understanding of the potential mechanisms of this phenomenon, termed functional ID, has evolved from several observations in chronic inflammatory diseases. Pro-inflammatory cytokines, such as interleukin-6, mediate the production and release of hepcidin (16). Hepcidin is a peptide that regulates the storage and release of iron by mediating the activity of iron proteins and iron transport proteins (4). In chronic inflammatory diseases, overexpression of hepcidin contributes to ID primarily by reducing iron protein levels and trapping iron in duodenal enterocytes and macrophages. Additionally, interferon-γ, lipopolysaccharide, and tumor necrosis factor-α upregulate the expression of divalent metal transporter 1, thereby increasing iron uptake in macrophages (17). Thus, abnormally high uptake and retention of iron occurs within the storage cells of the reticuloendothelial system, which limits iron availability for erythroid progenitor cells and erythropoiesis. Finally, inflammatory cytokines such as interleukin-1 and tumor necrosis factor-α also contribute to final anemia by suppressing erythropoietin expression and increasing erythrocyte phagocytosis (18). Therefore, chronic inflammatory diseases such as HF reduce the amount of iron available for erythropoiesis, resulting in a multifaceted pathophysiological process that precedes the development of anemia. This reduction in iron availability supports the benefit of intravenous iron supplementation in patients with heart failure (19). Notably, oral iron supplementation has not been shown to be beneficial, unlike intravenous replacement. Oral administration of iron is generally inadequate to prevent sequestration of stored iron in enterocytes and macrophages due to poor compliance and limited gastrointestinal absorption through mucosal edema (20).
4 What evidences of heart failure are related with iron deficiency and anemia?
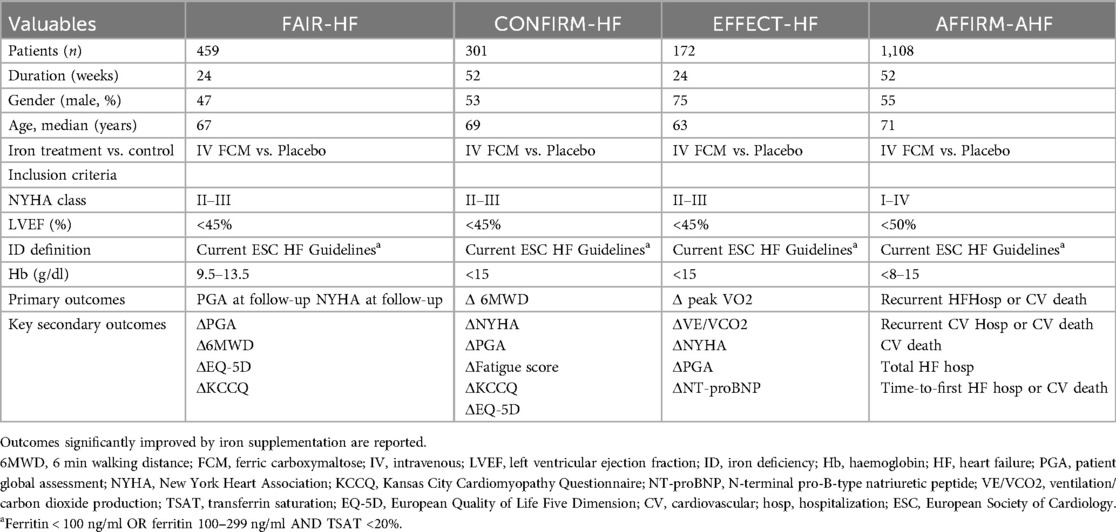
Iron supplementation is an established treatment for heart failure patients with comorbidities (Figure 1). Up to half of patients with HFrEF have evidence of ID (21), and the prevalence may be even higher in HF patients with preserved ejection fraction up to 60% (22). Furthermore, this high prevalence of ID occurs even in the absence of anemia (23). Although treatment of anemia and ID with subcutaneous erythropoietin and oral iron supplementation has not been shown to be beneficial, trials of intravenous iron supplementation have shown a consistent benefit. In FAIR-HF trial, which recruited HF patients with ID, showed that intravenous ferric carboxymaltose had a significant benefit compared to placebo for improvements in patient global assessment, the 6-min walk test, and quality of life (4). Importantly, these benefits were similar, regardless of whether the patients were anemic or not. The previous study also demonstrated that ferric carboxymaltose resulted in improvements in the 6-min walk test, New York Heart Association class, patient global assessment, and health-related quality of life compared to placebo (CONFIRM-HF trial) (24). And, the intravenous iron supplementation treatment group maintained and decreased maximal oxygen consumption compared to the control group at 24-week follow-up (EFFECT-HF trial) (23). Moreover, intravenous iron supplementation reduces recurrent hospitalization after discharge for acute HF (AFFIRM-AHF trial) (25). These are summarized about previous randomized controlled trials on iron deficiency in heart failure in Table 1.
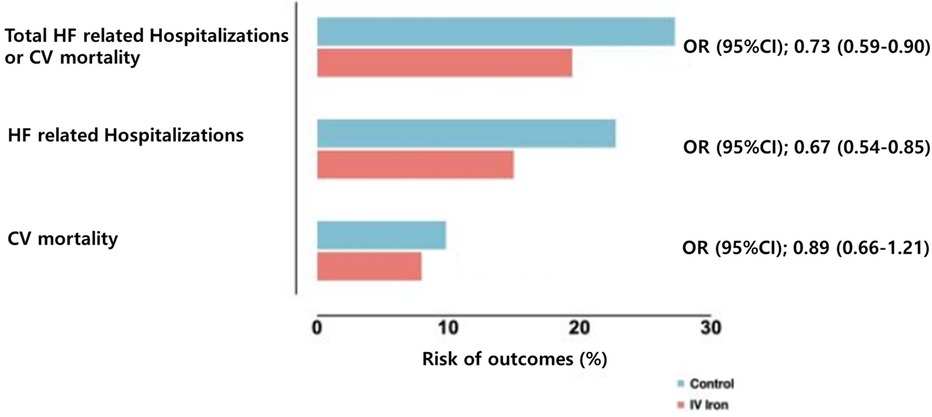
Figure 1. Risk of cardiovascular outcomes with intravenous iron vs. placebo. Data from Graham et al. (45) pooling data from multiple randomized controlled trials on intravenous iron supplementation in HF with iron deficiency. HF, heart failure; CV, cardiovascular; OR, odds ratio; CI, confidence interval.
5 What is the potential role of anemia and iron deficiency for arrhythmia including atrial fibrillation?
The role of anemia and ID in patients with heart failure is well known. However, relatively little research has been done on the impact this condition may have on patients with atrial fibrillation (AF). However, the high prevalence of AF once again underlines the susceptibility of this population to arrhythmias. This occurs despite the fact that AF and HF commonly coexist, share major risk factors, have similar pathophysiological mechanisms, and predispose to each other. Both conditions have similar consequences, including reduced cardiac output, oxygen uptake, and maximal work capacity (26). Moreover, emerging evidence suggests that inflammation plays an important role in the pathogenesis of AF. For example, studies have shown that patients with AF have increased lymphocyte cell activity, increased myocyte death, increased inflammatory markers, and an increased neutrophil/lymphocyte ratio compared to control patients without AF (27). Signaling pathways present in inflammatory diseases can also induce atrial remodeling and limit atrial conduction through the mediation of matrix metalloproteinase (MMP) 2 and MMP-9 (28). Finally, anemia and ID can lead to severe myocardial hypertrophy and ventricular dilatation, which may predispose patients to HF and AF (29). Therefore, the interrelationship between AF, HF, and inflammation raises the strong possibility that the association between anemia and ID in AF may be similar to that observed in HF.
To date, there is limited research assessing the prevalence of anemia in patients with AF (30–32). In subgroup analysis of AFCAS registry, 30% of patients with atrial fibrillation with measurable hemoglobin who underwent coronary stenting were anemic (33). This prevalence was similar to the prevalence in another Danish registry study that reported a prevalence of 34% in a population of all AF patients (34). In contrast, the prevalence was 12% in another study, suggesting some variation among different AF populations (35). A recent meta-analysis of available data from 28 studies found that the weighted proportion of AF patients with anemia was 16% (36).
Previous study (Danish registry) also found that anemic patients had a significantly increased risk of major bleeding events, stroke, thromboembolic events, and all-cause mortality compared with non-anemic patients with AF (34). Two analyzes of individuals from controlled trials of various oral anticoagulants also found an association with anemia and increased risk of major bleeding and all-cause death (35, 37). In previous meta-analysis, anemia was associated with a 78% increased risk of all-cause mortality, and every 1 g/dl decrease in hemoglobin was associated with a 24% increased risk (36). Furthermore, anemia was also associated with a 15% increased risk of stroke or systemic thromboembolism and a 78% increased risk of major bleeding. Several recent cohort studies have reported an association between anemia and risk of hospitalization for heart failure (30, 38). And, at least one study suggested that anemia may be associated with clinical recurrences of AF (39). In this study, patients with anemia had a higher incidence of post-ablation AF than patients without anemia. This association requires replication but is likely due to an adverse effect of anemia on atrial remodeling.
Nonetheless, these studies on anemia in patients with AF raise the potential relevance of anemia in subsequent complications. However, studies assessing the effects of anemia on other AF symptoms such as functional capacity and exercise tolerance are limited. Recent study reported the quality of life using a validated questionnaire among AF patients with/without anemia. Although quality of life at baseline did not appear to be different, patients with anemia did not improve their quality of life as significantly as patients without anemia at follow-up (30).
These associations were biologically plausible. The impact of anemia on mortality may be mediated by the established association between anemia and other adverse events such as stroke, HF, and hemorrhage (40, 41). Conversely, anemia may be an indicator of general frailty, the latter potentially causing confounding. However, given the magnitude of the reported association, further investigation would be needed into the prognostic impact of anemia in AF and whether treatment of this comorbidity is beneficial. Potential mechanisms linking atrial fibrillation, heart failure, iron deficiency, and anemia are shown Figure 2.
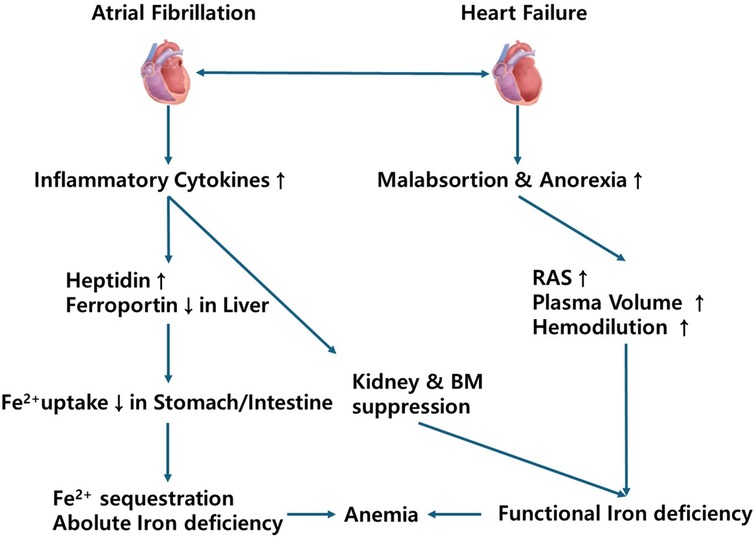
Figure 2. Potential mechanisms linking atrial fibrillation, heart failure, iron deficiency, and anemia. RAS, renin angiotensin system; BM, bone marrow.
6 What is clinical link between iron deficiency and atrial fibrillation?
There is a paucity of data regarding ID in patients with AF. Previous retrospective study has been published that analyzed data on ID in patients with AF (42). In this study, using a diagnostic threshold of ferritin level less than 100 ug/L, or a combination of a ferritin level between 100 and 299 ug/L and transferrin saturation under 20%, 47.6% of individuals with AF had ID. And, the prevalence of ID appeared to be higher in patients with permanent AF than in those with paroxysmal or persistent AF (43). A recent large-scale analysis of a national sample of inpatients found that 2.5% of patients hospitalized with atrial fibrillation (AF) were diagnosed with ID anemia (31). In cross-sectional analyses, ID anemia was associated with a longer length of stay and worse inpatient outcomes (e.g., myocardial infarction, renal injury, and vasopressor/ventilation requirement) excluding mortality. Despite its significant size, the prevalence reported in this study is likely underestimated due to the use of hospital coding data. As is the case with heart failure and other cardiovascular diseases, additional studies in diverse populations are needed to better characterize the prevalence of ID and ID anemia and to evaluate the potential relevance of these conditions to atrial fibrillation symptoms and complications.
7 Clinical significance and future treatment directions of the relationship between iron deficiency and atrial fibrillation
There is evidence that patients with AF may have significant rates of anemia and intellectual disability. Moreover, anemia appears to be associated with poor clinical outcomes in patients with AF. However, data on ID in AF are limited, preliminary findings suggest that the prevalence of ID may be non-significant or may also be correlated with severity and clinical outcome of AF. Although there is clearly a paucity of reports in this area, and these limited data should be interpreted with caution at this point in time. These initial findings suggest a strong possibility that anemia and ID may represent therapeutic targets for patients with AF. Moreover, this potential is supported by the close interrelationship between AF and HF, for which the role and benefits of iron supplementation have been established.
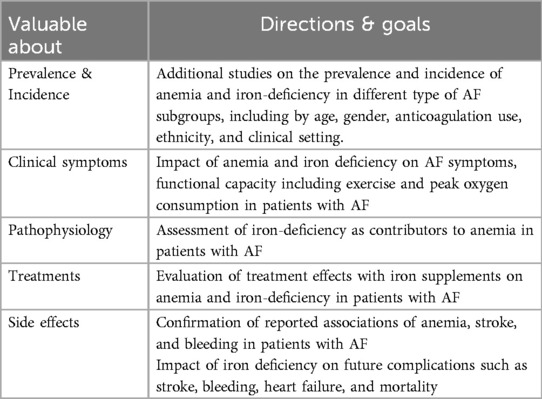
The RESAFE-HF trial is designed to further clinically evaluate the effect treating ID with intravenous ferric carboxymaltose (FCM) has on the arrhythmic burden of patients with HFrEF and Cardiac implantable electronic devices (CIEDs), while confirming via real-world data the benefits as for heart contractility, functional status, quality of life, HF hospitalizations, and survival (44). Several design decisions render RESAFE-HF uniquely suited to explore the effect of IV FCM on the arrhythmic burden of patients with HFrEF and ID. Importantly, only patients with implanted CIEDs were included in the study, which is expected to aid in the investigation of the effect of IV FCM in the arrhythmic burden of HFrEF patients in the aspect of that most CIEDs today hold a record of all atrial and ventricular tachycardia that fulfil sensor criteria. Thus, a reliable estimation of patients' arrhythmic burden can be derived by interrogating these devices. Eventhough, there is little data to support for those aforementioned theories. Therefore, future observational studies should provide additional estimates of the prevalence of anemia and ID in patients with AF, clarify the association of anemia and ID with clinical outcomes, and characterize the impact of anemia and ID on AF symptoms and functional capacity. Importantly, these studies were conducted in patients with and without heart failure, which provides insight into the potentially confounding effects of this condition. If this line of investigation proves promising, clinical trials to modify ID may be worthwhile. If successful, this may lead to routine screening and treatment of anemia and ID in patients with AF, as evaluated in the context of HF. Considering the increasing burden of AF worldwide, anemia and ID may be novel treatment strategies to evaluate in future studies. These are summarized about future directions of research and treatments for iron deficiency and arrhythmia in Table 2.
8 Conclusion
Both anemia and ID are highly prevalent in individuals with AF. Moreover, anemia and ID may be associated with worsening symptoms and outcomes in patients with AF. Future studies will be required to confirm the prevalence of anemia and ID across different populations with AF, better characterize the associations with outcomes, and ultimately determine whether correction of anemia and ID is a novel management strategy for patients with AF.
Author contributions
SI: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Special thanks to the editorial board members of MDPI and Biomedicines.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1573095/full#supplementary-material
References
1. Savarese G, von Haehling S, Butler J, Cleland JGF, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Eur Heart J. (2023) 44:14–27. doi: 10.1093/eurheartj/ehac569
2. Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. (2003) 24:S99–103. doi: 10.1177/15648265030244S206
3. Hsu HS, Li CI, Liu CS, Lin CC, Huang KC, Li TC, et al. Iron deficiency is associated with increased risk for cardiovascular disease and all-cause mortality in the elderly living in long-term care facilities. Nutrition. (2013) 29:737–43. doi: 10.1016/j.nut.2012.10.015
4. Kaur A, Kumar R. Iron deficiency and its relationship with chronic heart failure- A review. Cardiovasc Hematol Agents Med Chem. (2024) 23:161–70. doi: 10.2174/0118715257313681240913112017
5. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
6. Leung AKC, Lam JM, Wong AHC, Hon KL, Li X. Iron deficiency Anemia: an updated review. Curr Pediatr Rev. (2024) 20:339–56. doi: 10.2174/1573396320666230727102042
7. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. (2009) 12:444–54. doi: 10.1017/S1368980008002401
8. Goodnough LT, Schrier SL. Evaluation and management of anemia in the elderly. Am J Hematol. (2014) 89:88–96. doi: 10.1002/ajh.23598
9. Hanna-Rivero N, Tu SJ, Elliott AD, Pitman BM, Gallagher C, Lau DH, et al. Anemia and iron deficiency in patients with atrial fibrillation. BMC Cardiovasc Disord. (2022) 22:204. doi: 10.1186/s12872-022-02633-6
10. Sezik HA, Can H, Kurnaz MA, Tuna M, Ay Z. Use of iron supplements in children aged 1–2 years with iron deficiency anemia: a cross-sectional study. Pak J Med Sci. (2015) 31:1227–32. doi: 10.12669/pjms.315.7334
11. Ahmed F, Coyne T, Dobson A, McClintock C. Iron status among Australian adults: findings of a population based study in Queensland, Australia. Asia Pac J Clin Nutr. (2008) 17:40–7.18364325
12. Carmichael ED, Apple CG, Kannan KB, Gardener A, Anton S, Efron PA, et al. Chronic critical illness in patients with sepsis is associated with persistent Anemia, inflammation, and impaired functional outcomes. Am Surg. (2023) 89:2563–71. doi: 10.1177/00031348221104252
13. Rocha BML, Cunha GJL, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol. (2018) 71:782–93. doi: 10.1016/j.jacc.2017.12.027
14. Fu S, Huang J, Feng Z, Wang H, Xu H, Wu M, et al. Inflammatory indexes and anemia in chronic kidney disease: correlation and survival analysis of the national health and nutrition examination survey 2005–2018. Renal Fail. (2024) 46:2399314. doi: 10.1080/0886022X.2024.2399314
15. Hashmi MF, Aeddula NR, Shaikh H, Rout P. Anemia of chronic kidney disease. In StatPearls. Treasure Island (FL): StatPearls (2025).
16. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. Hepcidin-The culprit explaining disturbed iron homeostasis in chronic renal disease?: IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113:1271–1276, 2004. J Am Soc Nephrol. (2005) 16:287–90. doi: 10.1681/01.asn.0000926688.20809.aa
17. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. (2005) 352:1011–23. doi: 10.1056/NEJMra041809
18. Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. (1998) 18:555–9. doi: 10.1089/jir.1998.18.555
19. Lofruthe N, Gallitz I, Traeger L, Baumer N, Schulze I, Kuhlmann T, et al. Intravenous iron carboxymaltose as a potential therapeutic in Anemia of inflammation. PLoS One. (2016) 11:e0158599. doi: 10.1371/journal.pone.0158599
20. McDonagh T, Macdougall IC. Iron therapy for the treatment of iron deficiency in chronic heart failure: intravenous or oral? Eur J Heart Fail. (2015) 17:248–62. doi: 10.1002/ejhf.236
21. Foust R, Clarkson S, Nordberg M, Joly J, Griffin R, May J. Iron deficiency among hospitalized patients with congestive heart failure. J Healthcare Quality. (2024) 46:220–7. doi: 10.1097/JHQ.0000000000000432
22. Ternushchak TM, Tovt-Korshynska MI, Feysa SV. Iron deficiency and heart failure with preserved ejection fraction. Wiad Lek. (2024) 77:1996–2001. doi: 10.36740/WLek/195167
23. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Bohm M, Doletsky A, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. (2017) 136:1374–83. doi: 10.1161/CIRCULATIONAHA.117.027497
24. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. (2019) 7:36–46. doi: 10.1016/j.jchf.2018.07.015
25. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. (2020) 396:1895–904. doi: 10.1016/S0140-6736(20)32339-4
26. Tan MC, Vignarajah A, Yeo YH, Tamirisa K, Russo AM, Lee JZ, et al. Atrial fibrillation ablation in heart failure: difference in 3-year outcomes between reduced and preserved ejection fraction. Heart Rhythm. (2025). doi: 10.1016/j.hrthm.2024.12.045
27. Ihara K, Sasano T. Role of inflammation in the pathogenesis of atrial fibrillation. Front Physiol. (2022) 13:862164. doi: 10.3389/fphys.2022.862164
28. Packer M. Characterization, pathogenesis, and clinical implications of inflammation-related atrial myopathy as an important cause of atrial fibrillation. J Am Heart Assoc. (2020) 9:e015343. doi: 10.1161/JAHA.119.015343
29. Schwartz AJ, Converso-Baran K, Michele DE, Shah YM. A genetic mouse model of severe iron deficiency anemia reveals tissue-specific transcriptional stress responses and cardiac remodeling. J Biol Chem. (2019) 294:14991–5002. doi: 10.1074/jbc.RA119.009578
30. Hashimoto K, Kimura T, Ikemura N, Katsumata Y, Fujisawa T, Miyama H, et al. Burden of mild (<13 g/dl) Anemia in patients with atrial fibrillation (A report from a multicenter registry with patient-reported outcomes). Am J Cardiol. (2021) 57:48–55. doi: 10.1016/j.amjcard.2021.06.045
31. Minhas AMK, Sagheer S, Shekhar R, Sheikh AB, Nazir S, Ullah W, et al. Trends and inpatient outcomes of primary atrial fibrillation hospitalizations with underlying iron deficiency Anemia: an analysis of the national inpatient sample database from 2004 to 2018. Curr Probl Cardiol. (2022) 47:101001. doi: 10.1016/j.cpcardiol.2021.101001
32. Zhang Z, Jiang C, He L, Bai Y, Wu J, Hu R, et al. Associations of anemia with death and major bleeding in patients with atrial fibrillation: a report from the Chinese atrial fibrillation registry study. Clin Cardiol. (2022) 45:91–100. doi: 10.1002/clc.23764
33. Puurunen M, Kiviniemi T, Nammas W, Schlitt A, Rubboli A, Nyman K, et al. Impact of anaemia on clinical outcome in patients with atrial fibrillation undergoing percutaneous coronary intervention: insights from the AFCAS registry. BMJ Open. (2014) 4:e004700. doi: 10.1136/bmjopen-2013-004700
34. Bonde AN, Blanche P, Staerk L, Gerds TA, Gundlund A, Gislason G, et al. Oral anticoagulation among atrial fibrillation patients with anaemia: an observational cohort study. Eur Heart J. (2019) 40:3782–90. doi: 10.1093/eurheartj/ehz155
35. Westenbrink BD, Alings M, Granger CB, Alexander JH, Lopes RD, Hylek EM, et al. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: insights from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J. (2017) 185:140–9. doi: 10.1016/j.ahj.2016.12.008
36. Tu SJ, Hanna-Rivero N, Elliott AD, Clarke N, Huang S, Pitman BM, et al. Associations of anemia with stroke, bleeding, and mortality in atrial fibrillation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. (2021) 32:686–94. doi: 10.1111/jce.14898
37. Westenbrink BD, Alings M, Connolly SJ, Eikelboom J, Ezekowitz MD, Oldgren J, et al. Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: insights from the RE-LY trial. J Thromb Haemostasis. (2015) 13:699–707. doi: 10.1111/jth.12874
38. Krittayaphong R, Pumprueg S, Thongsri T, Wiwatworapan W, Choochunklin T, Kaewkumdee P, et al. Impact of anemia on clinical outcomes of patients with atrial fibrillation: the COOL-AF registry. Clin Cardiol. (2021) 44:415–23. doi: 10.1002/clc.23559
39. Kim M, Hong M, Kim JY, Kim IS, Yu HT, Kim TH, et al. Clinical relationship between anemia and atrial fibrillation recurrence after catheter ablation without genetic background. Int J Cardiol Heart Vasc. (2020) 27:100507. doi: 10.1016/j.ijcha.2020.100507
40. An Y, Ogawa H, Yamashita Y, Ishii M, Iguchi M, Masunaga N, et al. Causes of death in Japanese patients with atrial fibrillation: the fushimi atrial fibrillation registry. Eur Heart J Qual Care Clin Outcomes. (2019) 5:35–42. doi: 10.1093/ehjqcco/qcy033
41. Anker SD, Voors A, Okonko D, Clark AL, James MK, von Haehling S, et al. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: data from the OPTIMAAL trial. Eur Heart J. (2009) 30:1331–9. doi: 10.1093/eurheartj/ehp116
42. Keskin M, Ural D, Altay S, Argan O, Borklu EB, Kozan O. Iron deficiency and hematinic deficiencies in atrial fibrillation: a new insight into comorbidities. Turk Kardiyol Dern Ars. (2018) 46:103–10. doi: 10.5543/tkda.2018.51001
43. Imai R, Higuchi T, Morimoto M, Koyamada R, Okada S. Iron deficiency Anemia due to the long-term use of a proton pump inhibitor. Intern Med. (2018) 57:899–901. doi: 10.2169/internalmedicine.9554-17
44. Bakogiannis C, Mouselimis D, Tsarouchas A, Papadopoulos CE, Theofillogiannakos EK, Lechat E, et al. Iron therapy and severe arrhythmias in HFrEF: rationale, study design, and baseline results of the RESAFE-HF trial. ESC Heart Fail. (2023) 10:1184–92. doi: 10.1002/ehf2.14276
Keywords: iron deficiency, atrial fibrillation, cardiovascular disease, anemia, arrhythima
Citation: Im SI (2025) Advances in iron deficiency and iron-related arrhythmias and cardiovascular diseases. Front. Cardiovasc. Med. 12:1573095. doi: 10.3389/fcvm.2025.1573095
Received: 8 February 2025; Accepted: 30 June 2025;
Published: 11 July 2025.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Ryoko Kitada, Osaka Metropolitan University, JapanShingo Ota, Wakayama Medical University, Japan
Copyright: © 2025 Im. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Il Im, c3VuZ2lsczg5MzJAbmF2ZXIuY29t
 Sung Il Im
Sung Il Im