Abstract
Background:
Acute necrotizing encephalopathy (ANE) is a rare condition characterized by multiple symmetrical brain lesions mainly involving the thalamus. Acute fulminant myocarditis is a diffuse inflammatory disease of the myocardium characterized by acute onset, rapid progression, and a high risk of death. Its pathogenesis involves excessive activation of the innate immune system and the formation of an inflammatory storm. Both conditions are thought to be caused by viral infections. We present a case of ANE with fulminant myocarditis. Reporting this case is important due to the rarity and the critical interplay of these two severe conditions occurring simultaneously.
Case presentation:
A 16-year-old student presented with a 3-day history of high fever, cough, and expectoration, followed by multiple episodes of convulsive seizures. Despite high doses of vasoactive medications, the patient exhibited low blood pressure and elevated lactate levels. Portable echocardiography revealed diffuse decreased left ventricular motion with severe left ventricular dysfunction (ejection fraction < 30% by visual estimation). The patient was diagnosed with acute fulminant myocarditis. The patient remained comatose with a Glasgow coma scale (GCS) score of 3 (E1VeM1). Brain CT and MRI revealed bilateral striatal, thalamic, and brainstem lesions, typical of ANE. Consequently, a diagnosis of ANE accompanied by fulminant myocarditis was considered. The treatment regimen included high doses of glucocorticoids, immunoglobulins, tocilizumab, and V-A ECMO (Veno-arterial extracorporeal membrane oxygenation) life support. The patient showed significant recovery of cardiac function and was discharged after approximately 24 days of rehabilitation.
Conclusion:
This case report highlights the coexistence of ANE and fulminant myocarditis. The underlying mechanisms remain unclear. Early recognition of these two conditions is crucial for prognosis, though challenging. This report underscores the need for heightened awareness and prompt, comprehensive treatment strategies to improve outcomes in such complex cases.
Background
Acute necrotizing encephalopathy (ANE) is a rare, acute, life-threatening condition characterized by symmetrical brain lesions affecting the thalami, putamina, cerebral and cerebellar white matter, and brain stem tegmentum following various viral illnesses (1, 2). Fulminant myocarditis (FM) is a rapid progressing, acute diffuse inflammatory disease of the myocardium with a high risk of death (3). Infection is the most common cause of the diseases, and viruses are the predominant pathogens (2, 4). Here, we report a case of ANE accompanied by fulminant myocarditis. To the best of our knowledge, this is the rare reported case of coexisting FM and ANE.
Case presentation
A 16-year-old student presented to the emergency department of a local hospital with a 3-day history of high-grade fever, cough, and expectoration, followed by multiple episodes of convulsive seizures. The patient had no underlying disease, significant family history, or current medications. Clinical examination revealed a comatose patient with a Glasgow Coma Scale (GCS) score of 6/15. She was intubated due to the low GCS and transferred to the intensive care unit. Simultaneously, the patient experienced a rapid decrease in blood pressure and an increase in lactate levels, and blood pressure as low as 80/50 mmHg and lactate peaking at 10.8 mmol/L. Despite high doses of vasoactive medications, she maintained low blood pressure and elevated lactate levels. The doses of norepinephrine and dopamine administered were 1.5 ug/kg/min and 10 ug/kg/min, respectively. Consequently, her physician contacted us for extracorporeal membrane oxygenation (ECMO). The patient was then transported to our hospital on venoarterial (V-A) ECMO.
Portable echocardiography revealed diffuse decreased left ventricular motion with severe left ventricular dysfunction [ejection fraction (EF) <30% by visual estimation]. Laboratory findings showed marked elevated cardiac troponin I(TnI) (3.814 ng/ml, reference range 0–0.3 ng/ml), serum creatine phosphokinase isoenzyme (CK-MB) (26.54 ng/ml, reference range 0–6.36 ng/ml), N-terminal pro-brain type natriuretic peptide (>35,000 pg/ml, reference range 0–300 pg/ml), and D-dimer (>20 ug/ml, reference range 0–0.5 ug/ml). Mycoplasma pneumoniae IgM(MP-IgM) antibody was also positive. Additionally, the patient had acute liver failure, acute kidney injury, and coagulation dysfunction. Laboratory test results are presented in Table 1. And Time line was shown in Table 2.
Table 1
| Laboratory test | Laboratory test results | |||
|---|---|---|---|---|
| Before admission | Reference range | Day 1 post-admission | Reference range | |
| TnI(ng/ml) | 3.814 | 0–0.3 | 4.7 | 0.01–0.023 |
| NT-proBNP(pg/ml) | >35,000 | 0–300 | 20,000 | 300–450 |
| Leukocytes (10*109/L) | 10.82 | 4–10 | 6.53 | 3.5–9.5 |
| Hemoglobin (g/L) | 130 | 110–170 | 95 | 130–175 |
| Platelets(10*109/L) | 75 | 100–300 | 37 | 125–350 |
| CRP(mg/L) | ND | - | 25.9 | 0–4 |
| PCT(ng/ml) | 9.76 | 0–0.5 | 6.39 | 0–0.046 |
| Il-6(pg/ml) | 131 | 0–1.5 | 21.9 | 0–7 |
| ALT(U/L) | 2,234 | 0–50 | 3,219 | 9–50 |
| AST(U/L) | 5,779 | 0–38 | 1,532 | 15–40 |
| Albumin(g/L) | 31.8 | 35.3–52.9 | 25.3 | 40–55 |
| BUN(mmol/L) | 10.9 | 1.7–8.3 | 8.99 | 3.1–8.0 |
| Cr(mmol/L) | 129 | 35–80 | 104.4 | 57–97 |
| NA + (mmol/L) | 148 | 135–144 | 144.8 | 137–147 |
| LDH(U/L) | 5,851 | 0–65 | 3,315 | 120–250 |
| Glucose(mmol/L) | 6 | 3.9–5.8 | 8.1 | 3.9–6.1 |
| PT(s) | 32 | 9–14 | 40.3 | 9.4–12.5 |
| Fibrinogen(g/L) | 0.346 | 0–5 | 0.4 | 2.39–4.98 |
| D-dimer(ug/ml) | 193 | 0–0.5 | 253 | 0–0.5 |
| Lactate(mmol/L) | 9.58 | 0.5–1.6 | 10.8 | 0.5–1.6 |
The laboratory test results.
TnI, troponin I; NT-pro-BNP, N-terminal pro-brain natriuretic peptide; CRP, C-reactive protein; PCT, procalcitonin; ND, not performed; BUN, blood urea nitrogen; Cr, creatinine; LDH, lactate dehydrogenase; PT, prothrombin time.
Table 2
| Time line | Treatment | Condition |
|---|---|---|
| D1 | NO | High fever, cough, and expectoration. |
| D2 | Mechanical ventilation and Vasoactive drug support | Multiple episodes of convulsive seizures, GCS E1VeM1. |
| ICU in the local hospital. | ||
| D2 | V-A ECMO. | Then the patient went into shock, and bood pressure as low as 80/50 mmHg and lactate peaking at 10.8 mmol/L. |
| Cardiac function deterioration | ||
| D3 | ECMO | The patient was then transported to our hospital on V-A ECMO. |
| Ventilatory | ||
| Azithromycin Methylprednisolone | ||
| Immunoglobulin | ||
| D6 | Stop using sedatives and analgesics | Remained in a coma(E1VeM1) |
| D9 | ECMO was removed | Remained in a coma(E1VeM1) |
| D12 | Tocilizumab | CT revealed lesions involving the bilateral striatum, thalamus, and brainstem, typical for ANE. |
| D13 | Ehabilitation | Responding to painful stimuli |
| D16 | Ehabilitation | Transported to the ehabilitation department |
| D40 | Ehabilitation | Discharge |
| Walk and speak clearly |
Time line.
On admission, vital signs were as follows: temperature: 36.9°C, heart rate: 114 beats per minute (bpm), respiratory rate: 13 cycles per minute (cpm), blood pressure: 84/73 mmHg, oxygen saturation (SpO2): 98%. The GCS was graded 3 (E1VeM1). The pupils size was 2 mm, equal but unreactive to light.
The treatment regimen included ECMO support, ventilatory support, azithromycin anti-infection therapy, methylprednisolone(500 mg/day of methylprednisolone for 3 days), and immunoglobulin(2 g/kg total dose of IVIG over 5 days). To address elevated intracranial pressure (ICP), the patient received intravenous mannitol (20%, 125 ml every 6 h) with close monitoring of neurological status and electrolytes. The patient had repeated convulsions during the course of the disease, so we immediately gave antiepileptic drug (AED) which including intravenous sodium valproate (1 mg/kg/hour continuous intravenous pumping) for 5 days after admission. Following treatment, cardiac function recovered up to 38% of EF on the sixth day of ECMO therapy, and weaning from ECMO became possible 6 days after treatment initiation.
On the third day of admission, sedation and analgesia were discontinued, but the patient remained in a coma with a GCS score of 3(E1VeM1). Lumbar puncture revealed an opening pressure of 130 mmH2O. Cerebrospinal fluid (CSF) analysis revealed an elevated erythrocyte count of 20 × 106/L with a normal leukocyte count. CFS protein levels were elevated. CSF glucose, and chloride levels were normal, and the culture and cytology of the CSF were negative. Laboratory test results are presented in Table 3.
Table 3
| Results of relevant serum and CSF studies | ||||
|---|---|---|---|---|
| Infectious | Immunologic | Results | Reference range | |
| HBV | Negative | Antinuclear antibody | Negative | |
| HIV | Negative | C3 Complement(g/L) | 0.203 | 0.7–104 |
| Syphilis | Negative | C4 Complement(g/L) | 0.09 | 0.1–0.04 |
| EBV IgG/DNA | Negative | IgG(g/L) | 8.11 | 8.6–17.4 |
| CMV DNA | Negative | IgM(g/L) | 0.88 | 0.5–2.8 |
| SRSA-COV-2 PCR | Negative | IgA(g/L) | 1.65 | 1.0–4.2 |
| Respiratory Viral PCRa | Negative | |||
| Respiratory Viral IgMb | Negative | |||
| Mycoplasma Pneumonia IgG/M | positive/weakly Positive | |||
| Genetics | RANBP2 | Negative | ||
| CSF | Basic | Infectious | ||
| Glucose (mmol/L) | 4.31(Reference range: 2.5–4.5) | Bacterial and fungal culture | Negative | |
| Protein (g/L) | 0.81(Reference range: 0.15–0.45) | Next Generation Sequencing | Negative | |
| RBC (10*109/L) | 25(Reference range: 0) | Ink stain | Negative | |
| WBC (10*109/L) | 2(Reference range: 0–8) | Acid-fast stain | Negative | |
Relevant serum and CSF studies in our patient.
HBV, hepatitis B virus; HIV, human immunodeficiency virus; EBV, Epstein–Barr virus; IgG, immunoglobulin G; CMV, cytomegalovirus; IgM, immunoglobulin M; SARS-COV-2, severe acute respiratory syndrome coronavirus disease 2019; IgA, immunoglobulin A; PCR, polymerase chain reaction; IgE, immunoglobulin E; DNA, deoxyribonucleic acid; WNL, within normal limits; RANBP2, RAN-binding protein 2.
Adenovirus, influenza A&B, respiratory syncytial virus (RSV), and Mycoplasma pneumoniae.
Echovirus, Coxsackievirus, adenovirus, respiratory syncytial virus (RSV), parainfluenza virus-1,2,3, and Chlamydia pneumoniae.
Brain Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) revealed lesions involving the bilateral striatum, thalamus, and brainstem, typical for ANE (Figures 1A,B,I–L). A diagnosis of ANE accompanied by fulminant myocarditis was considered. Therefore, tocilizumab was included in the treatment regimen. Tocilizumab was used in hospital day 9 (8 mg/hg of tocilizumab for 1day). On the tenth day of admission, the patient began to regain consciousness. She was transferred to a general ward after 13 days. Echocardiography showed a significant improvement in left ventricular function (EF = 61%). The patient was discharged after approximately 24 days of rehabilitation. At the time of discharge, the patient could walk and speak clearly. She was discharged with a prescription for cardioprotective agents, including metoprolol, sacubitril/valsartan, spironolactone, furosemide, and dapagliflozin. One month after discharge, the patient was able to walk on her own, her speech was cleared, there were no remaining symptoms, and her heart function was normal. A repeat CT revealed that the lesions in the head had reduced (Figures 1G,H).
Figure 1
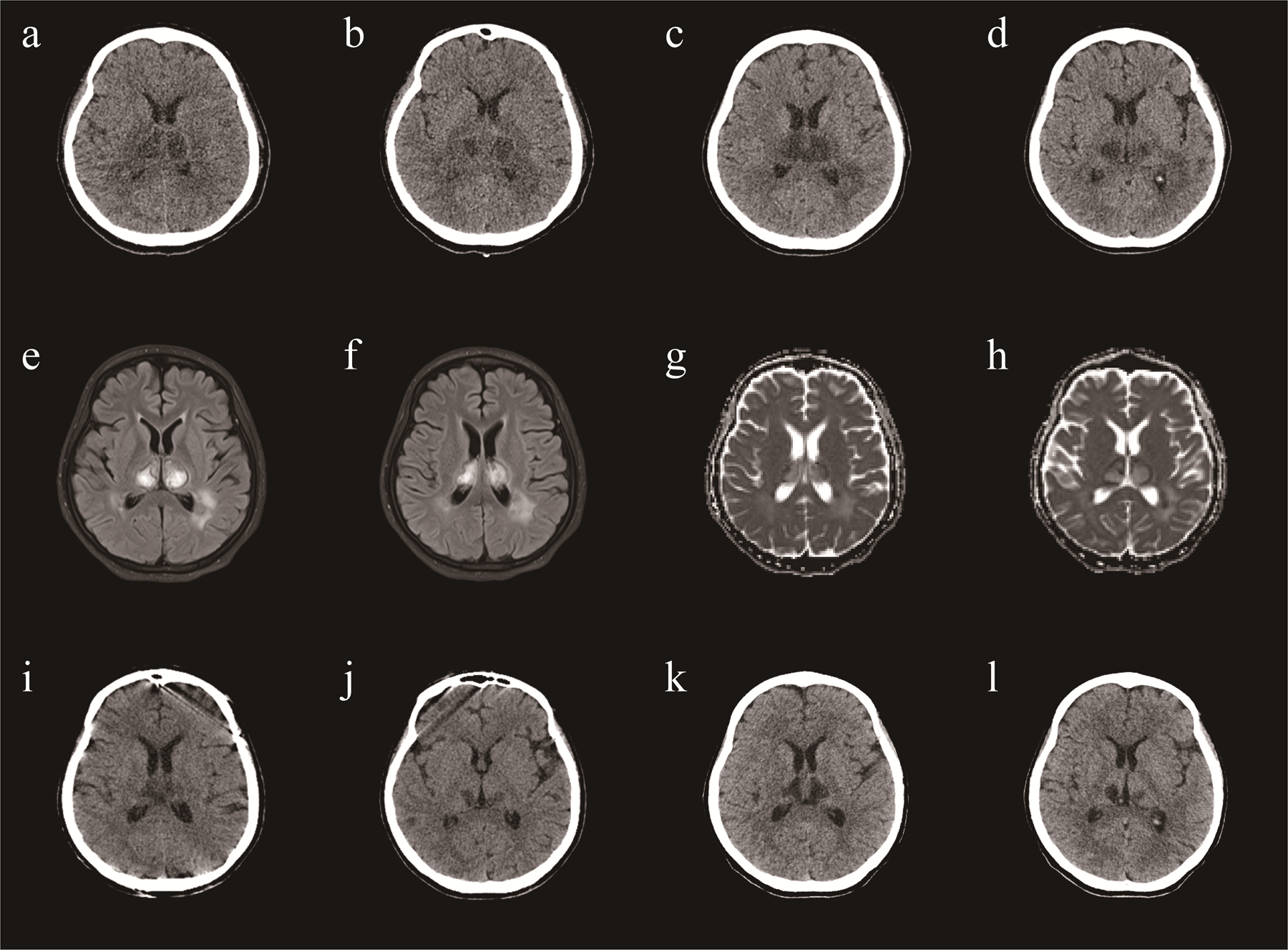
A brain CT (a,b) demonstrating symmetric lesions involving thalami, striatum and brain stem, consistent with acute necrotizing encephalopathy in day 12. A repeat CT revealed that the lesions in the head had reduced (c,d) in day 20. A repeat CT revealed that the lesions in the head had reduced (e,f) in day 38. One month after discharge, a repeat CT revealed that the lesions in the head had reduced (g,h). MRI (i,j) demonstrating symmetric lesions involving thalami, striatum and brain stem, consistent with acute necrotizing encephalopathy in day 22.
Discussion
ANE typically arises secondary to neurological complications of influenza or other viral infections (1, 5, 6). The disease progresses rapidly and often results in severe disability or death. Characteristic neuro-radiologic findings include multifocal and symmetric lesions, mainly affecting the bilateral thalami, cerebral periventricular white matter, brainstem tegmentum, and cerebellum (1, 7). Treatment of ANE is mainly supportive, with early high-dose intravenous steroids (methylprednisolone) leading to better outcomes. Tocilizumab may also be beneficial in improving morbidity outcomes (5, 8–10). Additionally, plasma exchange may be associated with increased survival in patients with ANE (10, 11).
FM is mainly caused by a variety of viral infections and is characterized by the rapid onset of cardiogenic shock (3, 12–14). In this case, the patient exhibited features such as sudden onset, rapid emergence of severe hemodynamic disorders, severe myocardial injury, and diffuse decreased ventricular wall movement, aligning with the diagnostic criteria of fulminant myocarditis (3). Active comprehensive treatment should be initiated as soon as possible for fulminant myocarditis, including anti-infection, antiviral therapy, glucocorticoids, gamma globulin, life support measures, and general treatment to nourish the myocardium and reduce cardiac load, etc (3, 4, 15, 16).
This is the rare case of ANE and FM coexisting in the same patient. ANE is most often seen following viral infection, and common viruses include influenza virus, SARS-CoV-2 and so on (2, 8, 10, 17). About FM, viral infection is also considered a major cause of myocarditis, including Coxsackie B virus, adenovirus and so on (4). The pathogenetic testing was positive for MP antibodies in this case, whereas all other pathogenetic tests yielded negative results. According to previous literatures, we found that MP can also cause ANE or FM (18, 19). MP-IgM can be detected within 1 week after clinical onset, and peaked at 3 weeks (20, 21). MP-IgG antibody can be detected about 2 weeks after infection, peaked at 5 weeks and maintained for a long time (20, 21). The median duration of persistence of MP DNA in the body is 7 weeks after infection, and the period cannot be shortened even if adequate antibiotic treatment is given (22). However, PCR-positive patients at presentation are far less than single IgM-positive patients, and PCR-negative patients, especially lately presented, are common over 20%–50% of study subjects (23). In this patient, the MP-IgM in the local hospital was positive, while the IgM and IgG antibodies were positive in our hospital, although the PCR was negative. Therefore, MP was considered to be the cause of the case.
It is believed that the cytokine storm caused by excessive innate immune activation caused by various causes and rapid triggering of immune cells to release a large number of inflammatory factors, also known as “inflammatory storm”, is an important reason for FM (3, 12). The “inflammatory storm” formed by a large number of cytokines and inflammatory mediators, including soluble growth stimulating expression gene 2 protein (sST2),interleukin-1 (IL-1), IL-6 and tumor necrosis factor (TNF)-α among others, may be the main cause of severe myocardial injury and pump failure (4). The most common hypothesis for the pathogenesis of ANE is also a “cytokine storm” involving interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), IL-10, IL-15 among others (5). IL-6 palys a import role in the pathogenesis of ANE and higher IL-6 levels correlate with more severe disease. Therefore, the causes of the two diseases may be similar and the treatment of them include glucocorticoids, immunoglobulin and so on. IL-6 inhibition is a natural choice when considering a more targeted approach to treating ANE and FM. At present, some studies suggest that tocilizumab can be used in the treatment of FM (24, 25). The proportion of patients who was ANE found to have a good outcome after receiving tocilizumab was much higher than those not receiving tocilizumab (17). IL-6 receptor blockade exerts cardiac beneficial effects by antiviral and immunomodulatory actions after induction of an acute murine CVB3 virus myocarditis (26). For this patient, we administered high doses of glucocorticoids, immunoglobulins, tocilizumab, and V-A-ECMO life support. Laboratory tests showed positive mycoplasma antibodies, and azithromycin was administered to treat the infection.
In conclusion, we report a rare case of ANE associated with FM. Physicians should be aware of this uncommon but life-threatening complication when patients with fulminant myocarditis develop consciousness disturbances.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of First Hospital of Lanzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JM: Writing – original draft. CP: Writing – review & editing. NB: Data curation, Resources, Writing – review & editing. SZ: Formal analysis, Writing – review & editing. PM: Visualization, Writing – review & editing. YW: Writing – review & editing. LA: Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Editage (https://www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANE, acute necrotizing encephalopathy; CK-MB, creatine phosphokinase isoenzyme; CSF, cerebrospinal fluid; CT, computed tomography; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; FM, fulminant myocarditis; GCS, glasgow coma scale; IL, interleukin; MRI, magnetic resonance imaging; SpO2, peripheral capillary oxygen saturation; TNF-α, tumor necrosis factor-alpha; V-A ECMO, veno-arterial extracorporeal membrane oxygenation
References
1.
Mizuguchi M Abe J Mikkaichi K Noma S Yoshida K Yamanaka T et al Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. (1995) 58(5):555–61. 10.1136/jnnp.58.5.555
2.
Weitkamp JH Spring MD Brogan T Moses H Bloch KC Wright PF . Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J. (2004) 23(3):259–63. 10.1097/01.inf.0000115631.99896.41
3.
Section of Precision Medical of Chinese Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology, Working Group on Adult Myocarditis. Chinese expert consensus statement on clinical diagnosis and treatment of fulminant myocarditis in adults[J]. Chin J Cardiol. (2017) 45(9):742–52. 10.3760/cma.j.issn.0253-3758.2017.09.004
4.
Jiang J Shu H Wang DW Hui R Li C Ran X et al Chinese society of cardiology guidelines on the diagnosis and treatment of adult fulminant myocarditis. Sci China Life Sci. (2024) 67:913–39. 10.1007/s11427-023-2421-0
5.
Wu X Wu W Pan W Wu L Liu K Zhang HL . Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediat Inflamm. (2015) 2015(1):792578. 10.1155/2015/792578
6.
Gowda VK Srinivasan VM . Recurrent familial acute necrotizing encephalopathy of childhood (ANEC). Indian J Pediatr. (2023) 90:730–730. 10.1007/s12098-023-04598-6
7.
Li H Sun C Chi S Wang Y Wu L Qin X . Use of MRI in the diagnosis and prognosis of acute necrotizing encephalopathy in a Chinese teenager. Medicine (Baltimore). (2019) 98:e17797. 10.1097/MD.0000000000017797
8.
Alsolami A Shiley K . Successful treatment of influenza-associated acute necrotizing encephalitis in an adult using high-dose oseltamivir and methylprednisolone: case report and literature review. Open Forum Infect Dis. (2017) 4:ofx145. 10.1093/ofid/ofx145
9.
Bashiri FA Al Johani S Hamad MH Kentab AY Alwadei AH Hundallah K et al Acute necrotizing encephalopathy of childhood: a multicenter experience in Saudi Arabia. Front Pediatr. (2020) 8:526. 10.3389/fped.2020.00526
10.
Fang Y Gao Q Jin W Li J Yuan H Lin Z et al Clinical characteristics and prognostic analysis of acute necrotizing encephalopathy of childhood: a retrospective study at a single center in China over 3 years. Front Neurol. (2023) 14:1308044. 10.3389/fneur.2023.1308044
11.
Li K Zhang T Liu G Jin P Wang Y Wang L et al Plasma exchange therapy for acute necrotizing encephalopathy of childhood. Pediatr Investig. (2021) 5:99–105. 10.1002/ped4.12280
12.
Hang W Chen C Seubert JM Wang DW . Fulminant myocarditis: a comprehensive review from etiology to treatments and outcomes. Sig Transduct Target Ther. (2020) 5(1):287. 10.1038/s41392-020-00360-y
13.
Pollack A Kontorovich AR Fuster V Dec GW . Viral myocarditis—diagnosis, treatment options and current controversies. Nat Rev Cardiol. (2015) 12:670–80. 10.1038/nrcardio.2015.108
14.
Caforio AL Pankuweit S Arbustini E Basso C Gimeno-Blanes J Felix SB et al Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:2636–48. 10.1093/eurheartj/eht210
15.
Maisch B Ruppert V Pankuweit S . Management of fulminant myocarditis: a diagnosis in search of its etiology but with therapeutic options. Curr Heart Fail Rep. (2014) 11:166–77. 10.1007/s11897-014-0196-6
16.
Liao X Li B Wen J Fan Z . Clinical application of extracorporeal membrane oxygenation in the treatment of fulminant myocarditis. Rev Cardiovasc Med. (2024) 25:114. 10.31083/j.rcm2504114
17.
Fischell SZ Fischell J Kliot T Tumulty J Thompson SJ Raees MQ . Case report: acute necrotizing encephalopathy: a report of a favorable outcome and systematic meta-analysis of outcomes with different immunosuppressive therapies. Front Neurol. (2023) 14:1239746. 10.3389/fneur.2023.1239746
18.
Zhu C Hu B Li X Han W Liang Y Ma X . A case report of mycoplasma pneumoniae-induced fulminant myocarditis in a 15-year-old male leading to cardiogenic shock and electrical storm. Front Cardiovasc Med. (2024) 11:1347885. 10.3389/fcvm.2024.1347885
19.
Neilson DE Adams MD Orr CMD Schelling DK Eiben RM Kerr DS et al Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Human Genet. (2009) 84:44–51. 10.1016/j.ajhg.2008.12.009
20.
Gao L Sun Y . Laboratory diagnosis and treatment of mycoplasma pneumoniae infection in children: a review. Ann Med. (2024) 56:2386636. 10.1080/07853890.2024.2386636
21.
Meyer Sauteur PM Pánisová E Bachmann LM Ambroggio L Berger C . Evaluation of IgM lateral flow assay as a screening tool for mycoplasma pneumoniae infection in childhood pneumonia. J Clin Microbiol. (2020) 58:10–1128. 10.1128/jcm.01498-20
22.
Nilsson AC Bjorkman P Persson K . Polymerase chain reaction is superior to serology for the diagnosis of acute mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. (2008) 8:1–8. 10.1186/1471-2180-8-93
23.
Jeon HE Kang HM Yang EA Han HY Han SB Rhim JW et al Early confirmation of mycoplasma pneumoniae infection by two short-term serologic IgM examination. Diagnostics. (2021) 11:353. 10.3390/diagnostics11020353
24.
Coyle J Igbinomwanhia E Sanchez-Nadales A Danciu S Chu C Shah N . A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. Case Rep. (2020) 2:1331–6.
25.
Savage E Wazir T Drake M Cuthbert R Wright G . Fulminant myocarditis and macrophage activation syndrome secondary to adult-onset still’s disease successfully treated with tocilizumab. Rheumatology. (2014) 53(7):1352–3. 10.1093/rheumatology/keu019
26.
Savvatis K Müller I Fröhlich M Pappritz K Zietsch C Hamdani N et al Interleukin-6 receptor inhibition modulates the immune reaction and restores titin phosphorylation in experimental myocarditis. Basic Res Cardiol. (2014) 109:1–4. 10.1007/s00395-014-0449-2
Summary
Keywords
acute necrotizing encephalopathy, acute fulminant myocarditis, shock, IL-6, tocilizumab (IL-6 inhibitor)
Citation
Ma J, Pan C, Bai N, Zhang S, Mi P, Wa Y and Lu A (2025) A rare case report: acute necrotizing encephalopathy and acute fulminant myocarditis. Front. Cardiovasc. Med. 12:1574397. doi: 10.3389/fcvm.2025.1574397
Received
10 February 2025
Accepted
12 May 2025
Published
27 May 2025
Volume
12 - 2025
Edited by
Guo-wei Tu, Fudan University, China
Reviewed by
Ujjawal Roy, Bangur Institute of Neurosciences, India
Yu-Ming Chang, National Cheng Kung University Hospital, Taiwan
Updates
Copyright
© 2025 Ma, Pan, Bai, Zhang, Mi, Wa and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Andong Lu lad12230904@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.