Abstract
Background and aims:
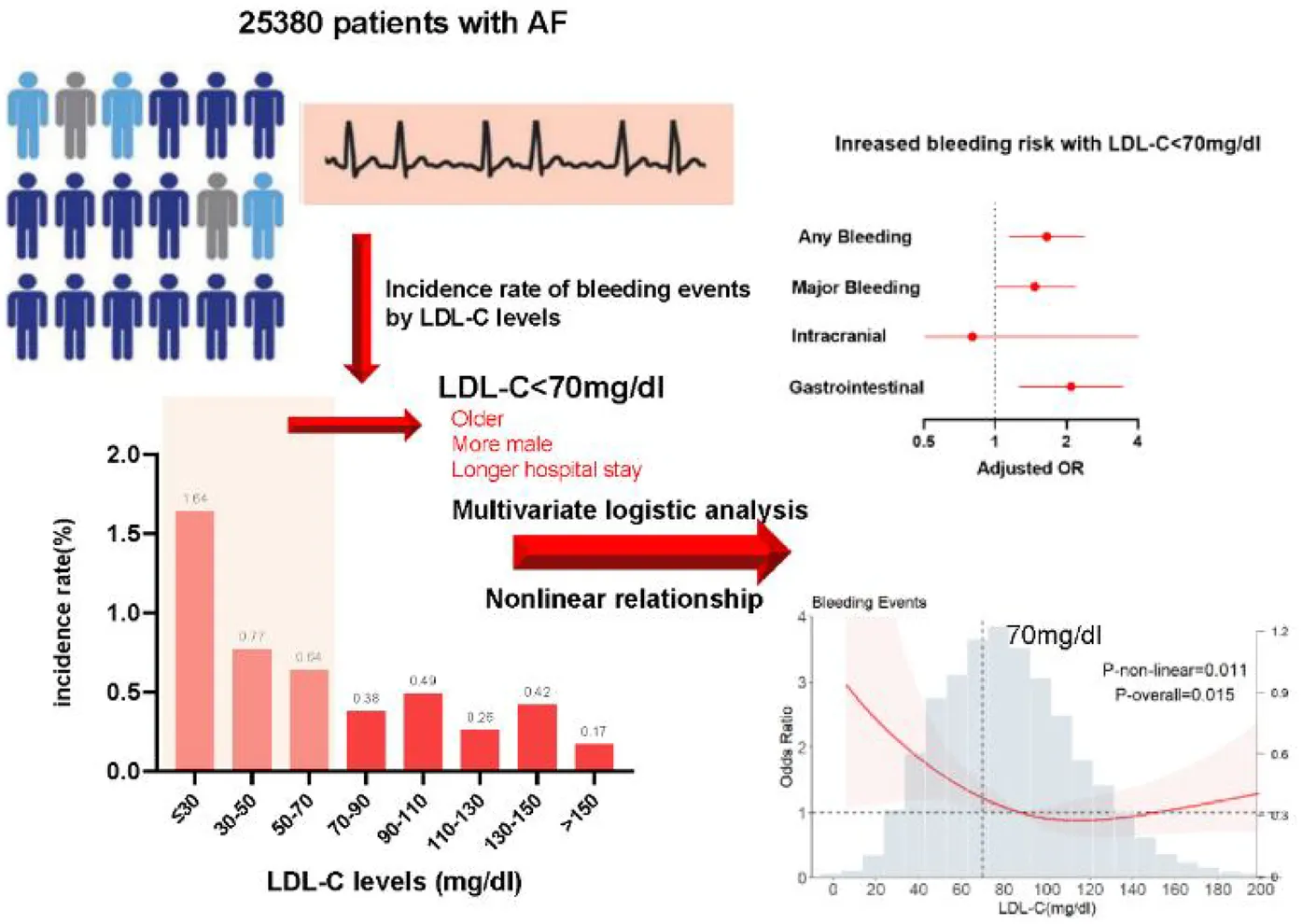
Emerging evidence indicates a relationship between low-density lipoprotein cholesterol (LDL-C) levels and bleeding. However, data regarding the relationship between LDL-C levels and bleeding events in patients with atrial fibrillation (AF) remain unfilled. This study is aimed to examine the relationship between LDL-C levels and the risk of in-hospital bleeding in patients with AF.
Methods and results:
In this multi-centered observational study, 25,380 patients with AF were enrolled; 14,071 (55.4%) and 11 309 (44.6%) were men and women, respectively, and the mean age was 69.51 ± 11.88 years. After adjusting for covariates, with LDL-C ≥ 70 mg/dl as the reference, LDL-C < 70 mg/dl was associated with a higher risk of any bleeding event [adjusted odds ratio [aOR]: 1.63, 95% confidence interval [CI]: 1.12–2.35; P = 0.009], major bleeding events (aOR: 1.48, 95% CI: 0.99–2.20; P = 0.05), and gastrointestinal bleeding events (aOR: 2.11, 95% CI: 1.27–3.50; P = 0.004) in the multivariate logistic regression model. The restricted cubic spline model showed an L-shaped relationship for bleeding events, with a higher risk at lower LDL-C levels. The nonlinear relationship between LDL-C levels and the risk of bleeding persisted among the subgroups.
Conclusions:
This nationwide and multi-centered AF registry study found an L-shaped relationship between LDL-C levels at admission and in-hospital bleeding events, with a greater risk at lower LDL-C levels. Further studies are needed to establish LDL-C as a factor for risk stratification and management of bleeding events in patients with AF.
Clinical Trial Registration:
[http://www.clinicaltrials.gov], identifier [NCT02309398].
Introduction
Atrial fibrillation (AF) is a common type of arrhythmia in the older adult population. Globally, 60 million (95% uncertainty interval: 32.5–42.6 million) individuals had AF or atrial flutter in 2022 (1), and 12 million were from China (2). AF is related to increased cardiovascular morbidity and mortality, and thus, it has a high global health and economic burden. Identifying patients with AF who are at the highest risk for bleeding events after anticoagulants is crucial for making informed clinical decisions to minimize bleeding complications. As such, biomarkers for predicting the bleeding risk have become important.
Low-density lipoprotein cholesterol (LDL-C) is a metabolic protein that regulates vascular disease through various mechanisms. It is well recognized as a risk factor for the development of atherosclerotic cardiovascular disease (ASCVD) events. High LDL-C levels consistently predict the risk of future ASCVD events in various populations worldwide (3). The current American Heart Association (AHA) guideline recommends that if a 10-year ASCVD risk ≥20% is estimated, a maximally tolerated statin dose should be used to lower LDL-C levels by >50% when the LDL-C is >70 mg/dl (4). A Korean study also found a unique relationship between LDL-C levels and mortality when lipid-lowering drugs were not used. This relationship followed a U-shaped pattern, indicating that higher and lower LDL-C levels were associated with an increased risk (5). Although emerging research has suggested that low levels of LDL-C are associated with bleeding (6–10), data regarding the relationship between low levels of LDL-C and bleeding events in patients with AF are limited.
Thus, this study aimed to investigate the relationship between LDL-C levels and the occurrence of in-hospital bleeding events in patients enrolled in the Improving Care for Cardiovascular Disease in China-Atrial Fibrillation (CCC-AF) project (11), which offered a substantial sample size to explore these relationships. We hypothesized that, similar to the results observed in randomized clinical trials, the LDL-C level is a biomarker for in-hospital bleeding events in individuals with AF.
Methods
Data collection and patients
The CCC-AF was launched in February 2015 as a cooperative project between the Chinese Society of Cardiology and AHA across 30 provinces in China. The methods and baseline data from the CCC-AF project, a national registry to improve AF management, have been previously reported (11). Patients with AF were included based on electrocardiograph (ECG) results recorded using a 12-lead ECG, 24 h Holter, or other rhythm monitors. The data elements and definitions of each variable were in accordance with the American College of Cardiology/AHA recommendations for AF clinical data standards (12, 13). The current study included patients with baseline LDL-C assessments from 236 hospitals that participated in the CCC-AF project between February 2015 and December 2019. The CCC-AF project was approved by the institutional review board of Beijing Anzhen Hospital, with a waiver for informed consent (number 2014018).
Definitions
The data collected included patient demographics, medical history, and laboratory test results at admission. The CHA2DS2-VASc score was calculated according to age, congestive heart failure, hypertension, diabetes, stroke or transient ischemic attacks, vascular disease, and female sex (14). The HAS-BLED score was calculated according to uncontrolled hypertension, abnormal renal and/or hepatic function, stroke, bleeding history or predisposition, age >65 years, drugs, or excessive alcohol use (15). LDL-C values were obtained within 24 h of the first medical contact. Patients with LDL-C levels ≥70 mg/dl estimated to have a 7.5% higher risk for ASCVD within 10 years are recommended to start using moderate-intensity statin or maximally tolerated statin with 20% higher risk therapy in the current AHA guidelines (4). Thus, we estimated the relationship between LDL-C levels <70 mg/dl and study outcomes with LDL-C levels ≥70 mg/dl as the reference.
Outcomes
An in-hospital bleeding event was defined as any occurrence of bleeding, including intracranial and gastrointestinal bleeding. Major bleeding events included fatal bleeding events, hemoglobin count decreasing to >20 g/L, total amount of transfusion >2U, or bleeding in vital organs or locations. Ischemic events included coronary heart disease, acute coronary syndrome, ischemic stroke, transient ischemic attack (TIA), pulmonary embolism, and peripheral arterial disease. All-cause deaths included in-hospital deaths regardless of the cause. The clinical outcome was defined as any occurrence of bleeding events, ischemic events, or all-cause death.
Statistical analyses
Continuous variables were expressed as means and standard deviations or interquartile ranges. They were compared using the t-test if the data conformed to a normal distribution or the Wilcoxon rank-sum test if they did not. Categorical variables were compared using the appropriate chi-square test or Fisher's exact test. We conducted univariate and multivariate logistic analyses in four models to investigate the independent determinants of clinical patient status according to LDL-C levels as a continuous variable or as a categorical variable: (i) crude logistic regression models; (ii)multivariable-adjusted logistic regression models adjusted for age and sex;(iii) multivariable-adjusted logistic regression models adjusted for sex, age, tertiary hospital, hospital stay, smoking history, drinking history, medical history including Diabetes mellitus, hypertension, stroke or TIA, heart failure, chronic obstructive pulmonary disease (COPD), chronic liver disease, bleeding history, chronic kidney disease, anemia, cancer, left ventricular ejection fraction (LVEF) ≤40%, prior antiarrhythmic drugs, prior catheter ablation, prior surgical ablation, prior statins, prior antiplatelet, prior anticoagulant; (iv) multivariable-adjusted logistic regression models adjusted for variates in model 3 with 1,000 bootstrapping replications performed for internal validation. LDL-C levels were further stratified according to a stepwise increase of 20 mg/dl from <30 mg/dl to 150 mg/dl. Univariate logistic regression models were used to examine the incidence and relationship of bleeding risk with clinical outcomes.
The nonlinear relationship was evaluated using restricted cubic splines in logistic regression models, and the models were adjusted for covariates included in the multivariable logistic model unless otherwise specified. To test whether the nonlinear relationship between LDL-C level and bleeding outcomes was consistent across the established subgroups. The overall nonlinear P-value and interactive P values were calculated and presented. All statistical analyses were performed using SPSS (version 27) and R (version 4.3.2). A two-sided P-value of <0.05 was considered significant.
Patient and public involvement statement
The public and patients have not been engaged in the research question proposal, design, recruitment, and implementation of the study. The results will be dispersed to study participants by public reporting.
Results
Patient characteristics
In total, 25,380 patients were included. Among them, 14,071 (55.4%) and 11,309 (44.6%) patients were men and women, respectively, and the mean age was 69.51 ± 11.88 years. Bleeding events occurred more often in patients with LDL-C < 70 mg/dl, 54/7,449 (0.7%), than in patients with LDL-C < 70 mg/dl, 68/17,931(0.4%). The patient characteristics are shown in Table 1. Overall, 122 bleeding events, 93 all-cause deaths, and 959 ischemic events occurred within a median hospital stay of 8.77 days. The incidence rates of in-hospital bleeding, ischemic events, all-cause death, and clinical events according to eight LDL-C categories are presented in Figure 1.
Table 1
| Characteristics | LDL ≥ 70 mg/dl | LDL < 70 mg/dl | Total population | P-value |
|---|---|---|---|---|
| Age, years, mean ± SD | 68.63 (11.98) | 71.62 (11.38) | 69.5 (11.88) | <0.001 |
| Age, years, n (%) | <0.001 | |||
| ≤64 | 6,181 (34.5) | 1,787 (24.0) | 7,968 (31.4) | |
| 65–74 | 5,629 (31.4) | 2,303 (30.9) | 7,932 (31.3) | |
| ≥75 | 6,121 (34.1) | 3,359 (45.1) | 9,480 (37.4) | |
| Age groups | 0.005 | |||
| 2015 and before | 2 (0.0) | 2 (0.0) | 4 (0.0) | |
| 2016 | 23 (0.1) | 4 (0.1) | 27 (0.1) | |
| 2017 | 3,628 (20.3) | 1,451 (19.5) | 5,079 (20.0) | |
| 2018 | 7,148 (39.9) | 2,854 (38.4) | 10,002 (39.5) | |
| 2019 and after | 7,114 (39.7) | 3,120 (42.0) | 10,234 (40.4) | |
| Length of stay (SD) | 8.60 (5.41) | 9.18 (5.77) | 8.77 (5.52) | <0.001 |
| Male, n (%) | 9,780 (54.5) | 4,291 (57.6) | 14,071 (55.4) | <0.001 |
| Han nationality, n (%) | 17,400 (97.0) | 7,245 (97.3) | 24,645 (97.1) | 0.335 |
| Tertiary hospitals, n (%) | 12,593 (70.2) | 5,221 (70.1) | 17,814 (70.2) | 0.824 |
| Medical insurance, n (%) | 0.710 | |||
| High cover | 11,264 (62.8) | 4,671 (62.7) | 15,935 (62.8) | |
| Moderate cover | 3,741 (20.9) | 1,584 (21.3) | 5,325 (21.0) | |
| Low cover | 2,926 (16.3) | 1,194 (16.0) | 4,120 (16.2) | |
| Sinus rhythm, n (%) | 3,949 (22.0) | 1,325 (17.8) | 5,274 (20.8) | <0.001 |
| Medical history, n (%) | ||||
| Drinking | 2,369 (13.2) | 973 (13.1) | 3,342 (13.2) | 0.748 |
| Smoking | 3,870 (21.6) | 1,585 (21.3) | 5,455 (21.5) | 0.590 |
| Hypertension | 9,778 (54.5) | 4,246 (57.0) | 14,024 (55.3) | <0.001 |
| Diabetes mellitus | 2,893 (16.1) | 1,506 (20.2) | 4,399 (17.3) | <0.001 |
| CAD and PCI | 4,778 (26.6) | 2,513 (33.7) | 7,291 (28.7) | <0.001 |
| Stroke or TIA | 2,268 (12.6) | 1,251 (16.8) | 3,519 (13.9) | <0.001 |
| PAD | 280 (1.6) | 140 (1.9) | 420 (1.7) | 0.071 |
| Heart failure | 1,709 (9.5) | 970 (13.0) | 2,679 (10.6) | <0.001 |
| Major bleed | 261 (1.5) | 146 (2.0) | 407 (1.6) | 0.004 |
| COPD | 797 (4.4) | 477 (6.4) | 1,274 (5.0) | <0.001 |
| Prior MI | 653 (3.6) | 429 (5.8) | 1,082 (4.3) | <0.001 |
| Chronic liver disease | 399 (2.2) | 213 (2.9) | 612 (2.4) | 0.003 |
| Chronic kidney disease | 320 (1.8) | 228 (3.1) | 548 (2.2) | <0.001 |
| Heart rate, bpm (SD) | 90.05 (26.62) | 89.15 (26.59) | 89.78 (26.6) | 0.488 |
| BMI, kg/m2 (SD) | 24.53 (3.83) | 24.14 (3.92) | 24.41 (3.85) | 0.039 |
| Blood pressure, median (SD) | ||||
| SBP, mmHg | 80.81 (14.08) | 78.55 (14.03) | 131.4 (21.27) | 0.751 |
| DBP, mmHg | 132.08 (21.31) | 129.79 (21.11) | 80.14 (14.10) | 0.196 |
| eGFR, ml/min/1.73 m2 (SD) | 93.61 (53.93) | 89.31 (52.96) | 92.34 (53.67) | 0.011 |
| eGFR < 60 ml/min/1.73 m2 | 3,060 (17.1) | 1,686 (22.6) | 4,746 (18.7) | <0.001 |
| Moderate and severe renal insufficiency | 3,122 (17.4) | 1,726 (23.2) | 4,848 (19.1) | <0.001 |
| Left atrial diameter (mm) (SD) | 40.99 (7.62) | 42.50 (8.21) | 41.41 (7.82) | <0.001 |
| LVEF (%) (SD) | 57.63 (11.14) | 56.91 (11.40) | 57.42 (11.22) | <0.001 |
| LVEF ≤ 40%, n (%) | 1,465 (8.2) | 629 (8.4) | 2,094 (8.3) | 0.470 |
| Total cholesterol, mmol/L (SD) | 4.51 (1.82) | 3.17 (2.17) | 3.98 (2.35) | <0.001 |
| LDL-C, mg/dl (SD) | 105.84 (28.71) | 52.82 (12.40) | 90.28 (34.79) | <0.001 |
| CHA2DS2-VASc score, median (SD) | 2.99 (1.74) | 3.38 (1.72) | 3.01 (1.89) | 0.101 |
| HASBLED risk score 0–1 | 12,440 (69.4) | 5,609 (75.3) | 18,049 (71.1) | <0.001 |
| HASBLED risk score ≥2 | 5,491 (30.6) | 1,840 (24.7) | 7,331 (28.9) | <0.001 |
| AF type, n (%) | <0.001 | |||
| First diagnosed | 5,602 (31.2) | 2,162 (29.0) | 7,764 (30.6) | |
| Paroxysmal | 6,571 (36.6) | 2,424 (32.5) | 8,995 (35.4) | |
| Persistent | 3,503 (19.5) | 1,680 (22.6) | 5,183 (20.4) | |
| Long-standing | 2,255 (12.6) | 1,183 (15.9) | 3,438 (13.5) | |
| Prior treatment, n (%) | ||||
| Prior concomitant with antiplatelet | 1,172 (6.5) | 733 (9.8) | 1,905 (7.5) | <0.001 |
| Prior treatment with anticoagulant | 3,538 (19.7) | 1,791 (24.0) | 5,329 (21.0) | <0.001 |
| Prior Antiarrhythmic drugs | 1,715 (9.6) | 565 (7.6) | 2,280 (9.0) | <0.001 |
| Prior Catheter ablation | 701 (3.9) | 240 (3.2) | 941 (3.7) | 0.008 |
| Prior cardioversion | 113 (0.6) | 28 (0.4) | 141 (0.6) | 0.013 |
| Prior surgical ablation | 53 (0.3) | 20 (0.3) | 73 (0.3) | 0.714 |
Clinical characteristics of patients stratified by the level of low-density lipoprotein cholesterol.
AF, atrial fibrillation; CAD, coronary artery disease; PCI, percutaneous coronary intervention; TIA, transient ischemic attack; PAD, peripheral artery disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LDL-C, low-density lipoprotein cholesterol.
Figure 1
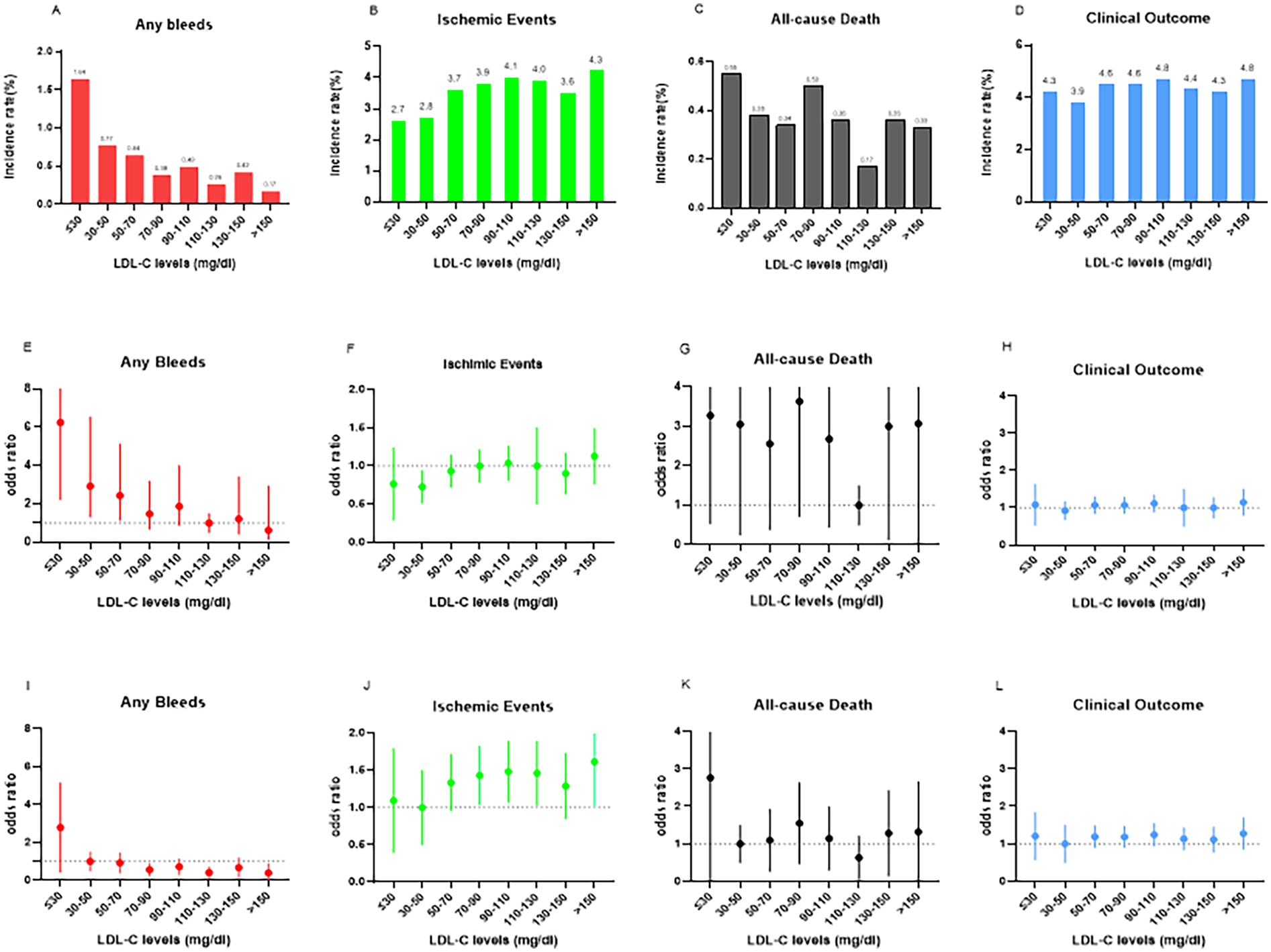
In-hospital bleeding events, ischemic events, all-cause death, and clinical outcomes according to the eight LDL-C level categories (A–D) In-hospital bleeding events, ischemic events, all-cause death and clinical outcomes according to the eight LDL-C categories. The numbers in parentheses indicate incidence in each LDL-C category. (E–H) Odds ratios (OR) and 95% confidence intervals (CIs) in the eight LDL-C level categories. The reference is set at LDL-C of 110-130 mg/dl in any bleeding event, ischemic events, clinical outcome, and all-cause death. (I–L) ORs and 95% CIs in the eight LDL-C level categories with LDL-C levels of 30-50 mg/dl as reference. LDL-C, low-density lipoprotein cholesterol.
Characteristics of patients stratified by LDL-C level
The mean LDL-C was 90.28 mg/dl in the total population and 52.82 mg/dl and 105.84 mg/dl in the low (<70 mg/dl) and high (≥70 mg/dl) LDL-C groups, respectively. The incidence of bleeding events was 44.6% in the low LDL-C group and 55.4% in the high LDL-C group. The low LDL-C group tended to be older and have a medical history of diabetes mellitus, history of bleeding, liver or kidney disease, higher blood pressure, stroke or TIA, history of major bleeding, and longer hospital stay (all P < 0.001), compared with the high LDL-C group. With respect to prior treatment, the low LDL-C group was more likely to have anticoagulant and antiplatelet and less likely to have received antiarrhythmic drugs or to have undergone cardioversion or catheter ablation, compared with the high LDL-C group (all P < 0.05). There were no significant between-group differences in ethnicity, tertiary hospitals, medical insurance, history of alcohol use and smoking, blood pressure, LVEF, or prior surgical ablation (all P > 0.05).
Relationship between LDL-C levels and in-hospital bleeding events
The independent predictors of patient outcomes are shown in Table 2. With LDL-C (≥70 mg/dl) as the reference, LDL-C < 70 mg/dl was associated with a higher risk of any bleeding event [adjusted odds ratio [aOR]: 1.63, 95% confidence interval [CI]: 1.12–2.35; P = 0.009], major bleeding events (aOR: 1.48, 95% CI: 0.99–2.20; P = 0.05), and gastrointestinal bleeding events (aOR: 2.11, 95% CI: 1.27–3.50; P = 0.004) in the multivariate logistic regression model (Model 3 in Table 2). These findings persisted in bootstrapping validation (Model 4 in Table 2). No association was observed between LDL-C < 70 mg/dl and intracranial bleeds. Notably, statistical significance was found for ischemic events in patients with LDL-C < 70 mg/dl in all models in Table 2, but not mortality. For the association between anticoagulant history and LDL-C levels, anticoagulants, but not antiplatelets, were associated with an increased probability of bleeding in the low LDL-C group. Additional analyses showed that, other than the original risk factors in the HAS-BLED score, anemia and smoking also increased the risk of bleeding in this group (Table 3). However, a history of cancer and age >65 years did not increase the risk.
Table 2
| Event | As a continuous variable | P-value | As a categorical variable <70 mg/dl with ≥70 mg/dlas reference | P-value |
|---|---|---|---|---|
| Model 1 | ||||
| Any bleeding | 0.99 (0.98,0.99) | 0.003 | 1.91 (1.32, 2.74) | <0.01 |
| Major | 0.98 (0.98,0.99) | <0.01 | 1.72 (1.17, 2.53) | 0.005 |
| Intracranial | 0.98 (0.97,1.01) | 082 | 0.80 (0.16, 3.97) | 0.780 |
| Gastrointestinal | 0.98 (0.97,0.99) | <0.01 | 2,56 (1.57, 4.19) | <.01 |
| All-cause deaths | 1.00 (0.99,1.00) | 0.274 | 0.98 (0.62,1.54) | 0.946 |
| Ischemic events | 1.00 (1.00,1.00) | 0.039 | 0.84 (0.72,0.97) | 0.023 |
| Clinical outcome | 1.00 (0.99,1.00) | 0.324 | 0.94 (0.82,1.08) | 0.438 |
| Model 2 | ||||
| Any bleeding | 0.99 (0.98,0.99) | 0.02 | 1.03 (1.01,1.05) | <0.01 |
| Major | 0.99 (0.98,0.99) | 0.002 | 1.55 (1.05,2.28) | 0.02 |
| Intracranial | 1.00 (0.98,1.02) | 0.93 | 0.65 (0.13,3.28) | 0.60 |
| Gastrointestinal | 0.98 (0.97,0.99) | <0.01 | 1.06 (1.03,1.08) | <0.01 |
| All-cause deaths | 1.00 (0.99,1.00) | 0.91 | 0.80 (0.51,1.25) | 0.333 |
| Ischemic events | 1.00 (1.00,1.00) | 0.001 | 0.77 (0.66,0.89) | <0.01 |
| Clinical outcome | 1.00 (1.00,1.00) | 0.015 | 0.86 (0.75,0.99) | 0.037 |
| Model 3 | ||||
| Any bleeding | 0.99 (0.98,1.00) | 0.08 | 1.63 (1.12,2.35) | <0.01 |
| Major | 0.99 (0.98,0.99) | 0.005 | 1.48 (0.99,2.20) | 0.05 |
| Intracranial | 0.99 (0.97,1.01) | 0.775 | 0.80 (0.15,4.11) | 0.780 |
| Gastrointestinal | 0.98 (0.97,0.99) | 0.001 | 2.11 (1.27,3.50) | 0.004 |
| All-cause deaths | 1.00 (0.99,1.00) | 0.848 | 0.78 (0.49,1.25) | 0.31 |
| Ischemic events | 1.00 (1.00,1.00) | 0.001 | 0.72 (0.62,0.84) | <0.01 |
| Clinical outcome | 1.00 (1.00,1.00) | 0.003 | 0.81 (0.70,0.93) | 0.003 |
| Model 4 | ||||
| Any bleeding | 0.99 (0.98,1.00) | 0.07 | 1.63 (1.13,2.36) | 0.009 |
| Major | 0.99 (0.98,0.99) | 0.005 | 1.49 (1.00,2.21) | 0.046 |
| Intracranial | 0.99 (0.97,1.01) | 0.768 | 0.81 (0.15,4.17) | 0.801 |
| Gastrointestinal | 0.98 (0.97,0.99) | 0.001 | 2.12 (1.28,3.52) | 0.003 |
| All-cause deaths | 1.00 (0.99,1.00) | 0.80 | 0.77 (0.48,1.23) | 0.28 |
| Ischemic events | 1.00 (1.00,1.00) | 0.001 | 0.73 (0.63,0.85) | <0.01 |
| Clinical outcome | 1.00(1.00,1.00) | 0.006 | 0.81(0.71,0.94) | 0.005 |
Relationship of low-density lipoprotein cholesterol levels with in-hospital bleeding.
Model 1: crude model.
Model 2: adjusted for age and sex.
Model 3: adjusted for sex, age, tertiary hospital, hospital stay, smoking history, drinking history, medical history including Diabetes mellitus, hypertension, stroke or TIA, heart failure, COPD, chronic liver disease, bleeding history, chronic kidney disease, anemia, cancer, LVEF ≤ 40%, prior antiarrhythmic drugs, prior catheter ablation, prior surgical ablation, prior statins, prior antiplatelet, prior anticoagulant.
Model 4: adjusted for covariates in model 3 with 1,000 bootstrapping replications performed.
AF, atrial fibrillation; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; LDL-C, low-density lipoprotein cholesterol.
Table 3
| Variables | OR(95%CI) | P-value | P-interaction |
|---|---|---|---|
| Age >65 | 1.36 (0.61–3.03) | 0.18 | 0.05 |
| Smoke | 2.31 (1.16–4.58) | 0.01 | 0.83 |
| Drinking | 0.86 (0.31–2.41) | 0.78 | 0.40 |
| Cancer | 0.38 (0.11–1.24) | 0.10 | 0.20 |
| Anemia | 0.15 (0.06–0.39) | <0.01 | <0.01 |
| Cr >200 | 4.95 (2.31–10.59) | <0.01 | 0.97 |
| Anticoagulant history | 0.53 (0.30–0.93) | 0.02 | 0.57 |
| Antiplatelets | 0.73 (0.32–1.62) | 0.44 | 0.82 |
| Bleeding history | 15.48 (7.90–30.07) | <0.01 | 0.15 |
| DM | 1.01 (0.51–1.96) | 0.96 | 0.29 |
| Hypertension | 0.75 (0.44–1.28) | 0.29 | 0.29 |
| Tertiary hospitals | 1.63 (0.98–2.71) | 0.05 | 0.47 |
Univariable analysis of factors associated with in-hospital bleeding in AF patients with LDL-C < 70 mg/dl.
AF, atrial fibrillation; DM, diabetes mellitus.
The incidence rates of in-hospital bleeding and ischemic events significantly differed by LDL-C level category (both P < 0.05). After logistic regression, the level of LDL-C (70 mg/dl) and lower increased the risk of bleeding events with the lowest risk of 110–130 mg/dl as a reference, and the level of LDL-C and higher increased the risk of ischemic events with the lowest risk of 30–50 mg/dl as a reference (Figure 1). For further study, when comparing each endpoint among the LDL-C level categories (lower than the endpoint with higher as a reference), the results showed a constant increasing trend of risk for bleeding at LDL-C levels <70 mg/dl. This effect stopped being significant when the LDL-C levels reached ≥70 mg/dl (P > 0.05). Meanwhile, an opposite trend was found for ischemic events, with a decreasing trend of risk at LDL-C levels <70 mg/dl, and this stopped being significant when the LDL-C levels reached ≥70 mg/dl (Figure 2).
Figure 2
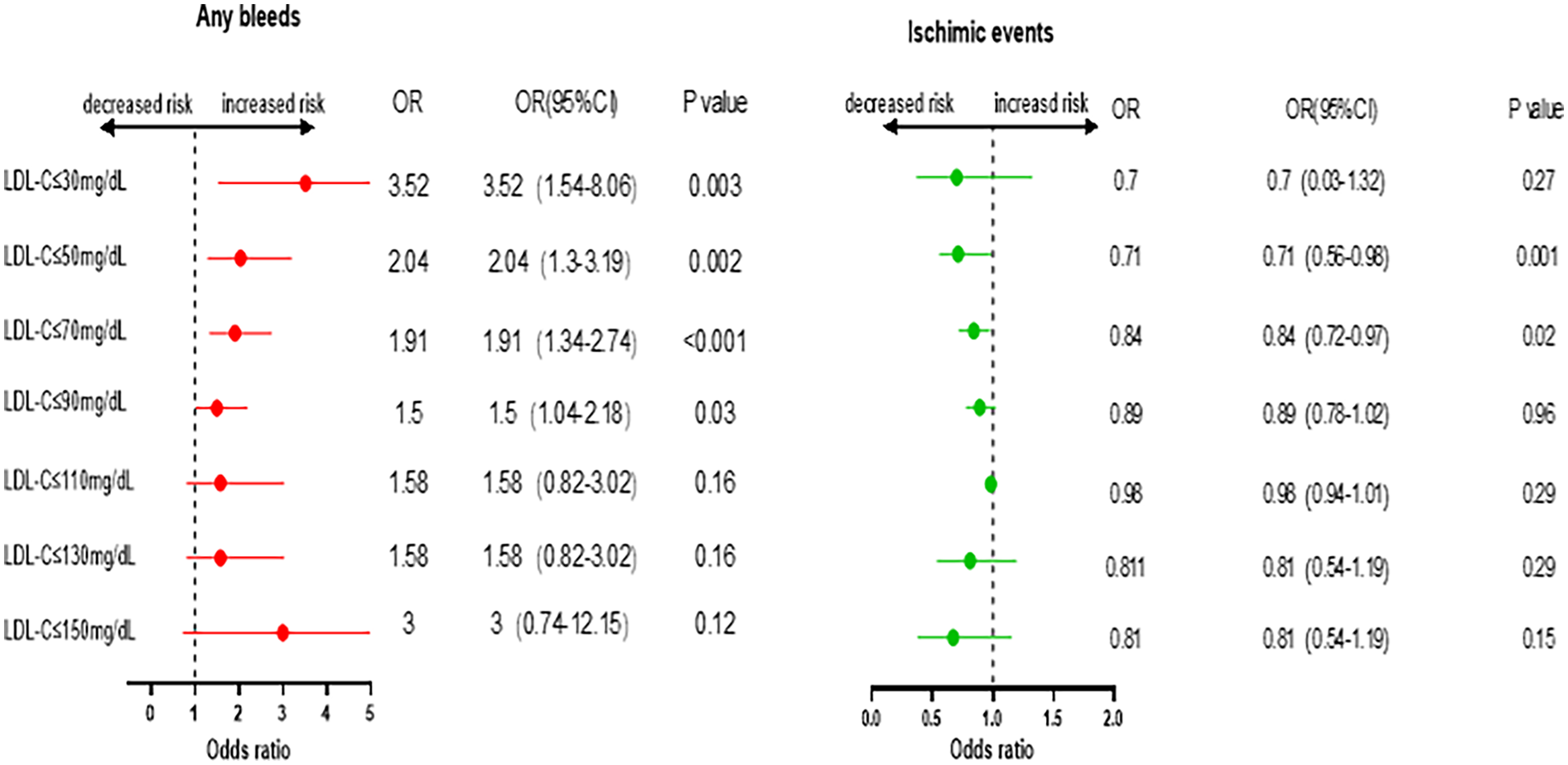
Odds ratio and 95% confidence intervals for Any bleeds and ischemic events. Comparing lower than the endpoint with higher as reference.
Nonlinear relationship between LDL-C levels and study outcomes
The reference point was set at the lowest risk of bleeding in each plot (LDL-C level of 110 mg/dl). LDL-C levels were found to have a nonlinear, L-shaped relationship with in-hospital bleeding, with a higher risk at lower LDL-C levels (P for nonlinearity = 0.011, P for overall = 0.015). Further, there was a nonlinear relationship between LDL-C levels and ischemic events, with lower risk at lower LDL-C levels (P for nonlinearity = 0.007, P for overall < 0.001). However, LDL-C levels did not demonstrate a nonlinear relationship with all-cause death and clinical outcomes (P for nonlinearity = 0.856 and =0.176, respectively) (Figure 3). The nonlinear relationship between LDL-C levels and the risk of bleeding outcomes remained in the subgroups stratified by sex, age, anticoagulant use, antiplatelet use, statin use, anemia (Figure 4) and Diabetes mellitus, hypertension and eGFR < 60 (Supplementary Figure S1). We also conducted subgroup analyses stratified by HAS-BLED scores. The results showed no significant interaction between LDL-C levels and HAS-BLED categories (P-interaction = 0.158), while the nonlinear relationship remained significant (P-overall = 0.002; P-nonlinear = 0.004) (Supplementary Figure S1).
Figure 3
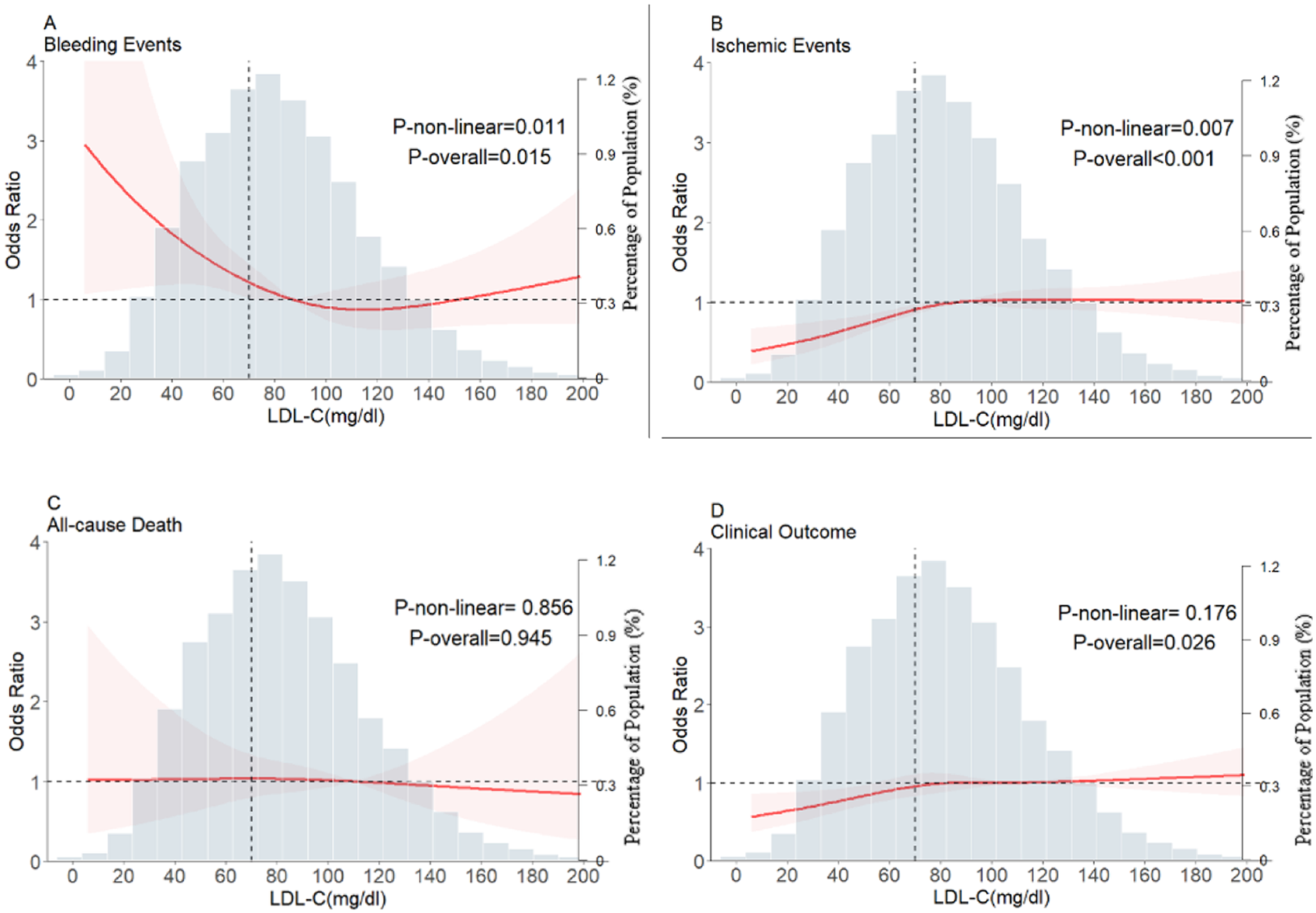
Restricted cubic spline plots for major bleeding events, ischemic events, all-cause death, and clinical outcome according to LDL-C levels after covariate adjustment. (A) Bleeding events, (B) ischemic events, (C) all-cause death, (D) clinical outcomes. The background histograms (light grey color) represent the percentage of the density distribution of LDL-C in the study population (right y-axis). The heavy red lines represent the estimated adjusted odds ratios, with the pink-shaded ribbons denoting the 95% confidence intervals. The vertical dotted lines indicate the threshold value for LDL-C, at 70 mg/dl. The horizontal dotted lines represent the OR of 1.0. The reference point for the lowest risk is 110 mg/dl (Knot = 4). OR, Odds ratio; LDL-C, low-density lipoprotein cholesterol.
Figure 4
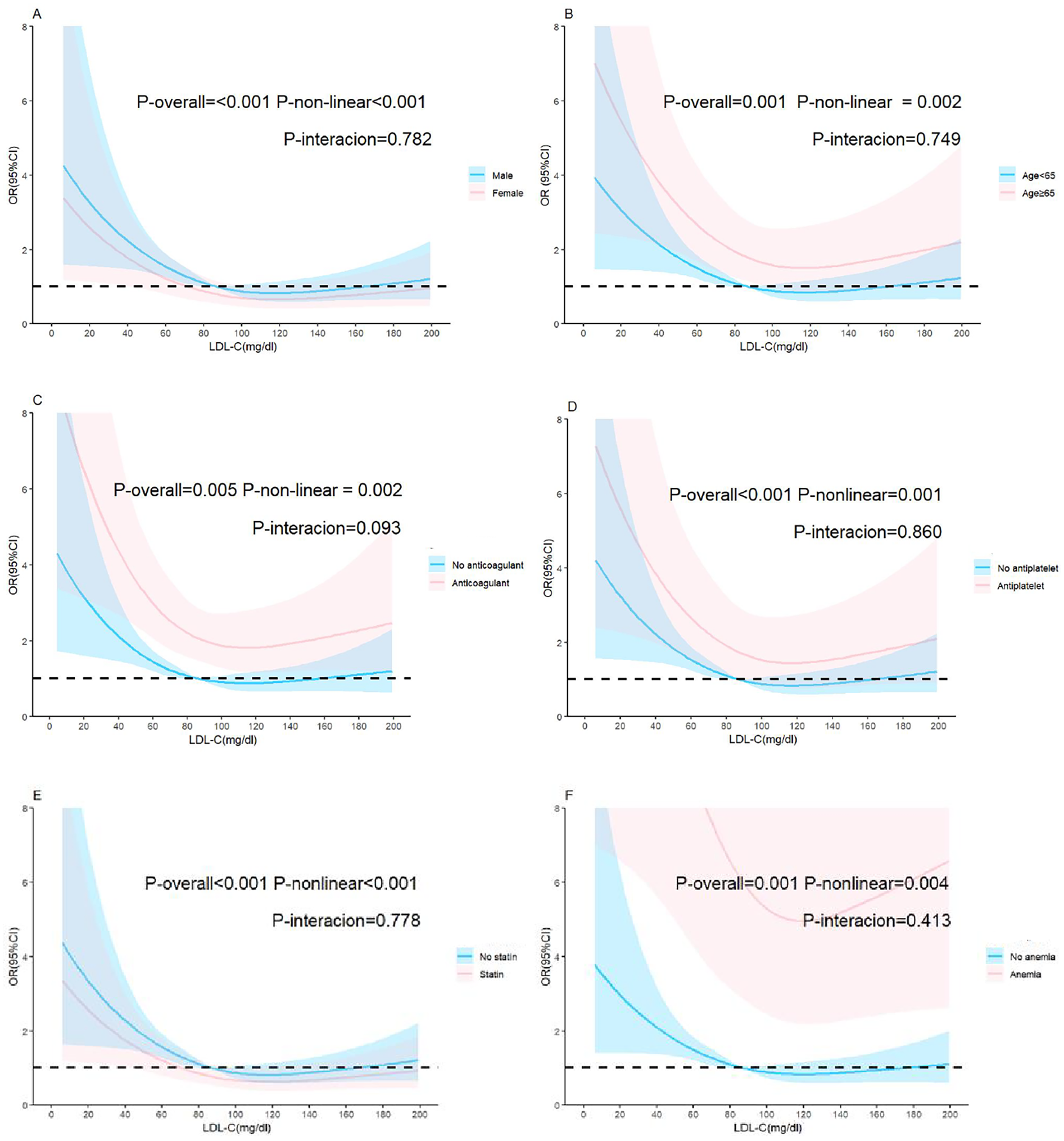
Adjusted relationship of LDL-C and bleeding in the restricted cubic spline subgroups. (A) Sex, (B) age, (C) anticoagulant intake, (D) antiplatelet intake, (E) statin intake, (F) anemia. The reference value (OR = 1) is set as the median value for each subgroup. The solid lines represent the adjusted ORs of LDL-C levels across the entire range, and the shaded areas represent the corresponding 95% confidence intervals. OR, Odds ratio; LDL-C, low-density lipoprotein cholesterol.
Discussion
The principal findings of this large-scale national AF registry-based dataset were as follows. First, 0.45% of the patients with AF had bleeding, and this rate was markedly lower than that in Western cohorts. Second, after adjusting for covariates, with LDL-C levels ≥70 mg/dl as the reference, LDL-C levels <70 mg/dl were associated with a higher risk of bleeding and a lower risk of ischemic events. Low LDL-C levels were strongly associated with the risk of gastrointestinal bleeding but not with that of intracranial bleeding. Third, anticoagulants, anemia, and smoking, in addition to the original risk factors in the HAS-BLED score, were associated with increased probabilities of bleeding in patients with lower LDL-C levels but not in those with higher LDL-C levels. Fourth, the restricted cubic spline analyses found an L-shaped relationship between LDL-C levels and in-hospital bleeding. The nonlinear relationship between LDL-C levels and the risk of bleeding outcomes persisted among the subgroups. These findings have important implications for the current management of AF.
Low LDL-C levels are associated with clinical outcomes, and some studies have attempted to determine what levels are too low (16). Recent evidence indicates that low LDL-C levels are associated with poor bleeding outcomes (8–10, 17). With the prevalence of statin use in patients with AF and cardiovascular disease (CVD), marked lowering of LDL-C levels has become realistic. In contrast to previous findings, our research suggests that low LDL-C levels are related to bleeding events in patients with AF. Although the mechanisms by which low LDL-C can promote bleeding are still unclear, some possible explanations exist. First, low LDL-C levels are strongly related to more microbleeds and erythrocyte fragility due to low cholesterol in the erythrocyte membrane.Disrupting intracellular cholesterol hemostasis might result in defect of terminal erythroid differentiation in vivo (18, 19). Second, LDL-C, a vital part of the platelet cell membrane, plays an essential role in platelet activation, tissue factor expression, and impaired coagulation function (20, 21).Third, low LDL-C levels are metabolically associated with Proprotein convertase subtilisin/kexin type 9 (PCSK9) concentrations (21–23). PCSK9 boosts platelet activation by binding with platelet CD36. Consequently, when LDL-C levels are low, and plasma PCSK9 levels decrease, the PCSK9/CD36-mediated downstream signaling pathways of cyclooxygenase-1/thromboxane A2 are inhibited. This phenomenon leads to increased platelet inhibition and impaired blood clotting mechanisms (23).
We found that among all kinds of bleeding events (intracranial, gastrointestinal, and others), gastrointestinal bleeding events were more directly related to low LDL-C levels than were intracranial bleeding events. These findings differ from those of some studies (9, 24), and this can be explained as follows: First, only a small number of intracranial hemorrhages were recorded in our trial. Second, the patients recruited for the CCC-AF project were mainly from the cardiology department, whereas patients with ischemic or hemorrhagic stroke as their first diagnosis without knowledge of AF in the neurology department were not included.
We found that in patients with low LDL-C levels (<70 mg/dl), anemia, in addition to the risk factors in the HAS-BLED score, also increased the risk of bleeding. The P-value for interaction between LDL-C < 70 mg/dl and anemia was <0.01. Low hemoglobin levels were found in some research to be a predictor for all-cause death and composite events such as increased hospitalization, bleeding, and thromboembolic events, as reported in previous studies (25, 26). In our sensitivity analysis adjusted for anemia, the strength of the relationship between low LDL-C and major bleeding events was unchanged. Notably, patients with LDL-C levels <70 mg/dl and a history of cancer did not have an increased risk of bleeding. However, some previous studies concluded that cancer complicates the clinical history of patients with AF and increases the risks of all-cause mortality, major bleeding, and intracranial hemorrhage in these patients. The risk of bleeding exceeds that of thromboembolism (27, 28).
The restricted cubic spline model showed a nonlinear and L-shaped relationship between LDL-C levels and bleeding events. Consistent with the theory that low LDL-C levels are an indirect marker of certain body degeneration traits, the relationship between low LDL-C levels and the risk of bleeding was more evident in the anemia and age >65 years subgroups. The frailty index has been studied and recommended as a tool to predict unplanned hospitalizations and other adverse outcomes, such as all-cause mortality and bleeding, but not stroke (29). Hemoglobin levels are also an independent predictor of all-cause death and composite events, such as thromboembolism and major hemorrhage (25, 26). To exclude interaction effect between covariates and LDL-C, we did subgroups including anticoagulant use, antiplatelet use, statin use, and anemia. The interaction P-value showed none. Of note, subgroup analyses stratified by HAS-BLED score demonstrated that a score ≥2 was associated with a higher bleeding risk, further validating HAS-BLED as a reliable tool for bleeding risk assessment. Additionally, the interaction P-value suggested that LDL-C may be an independent risk factor for bleeding.
We conducted a sensitivity analysis across the full range of LDL-C levels to identify the LDL-C levels associated with a higher risk of bleeding events. By comparing each endpoint of the LDL-C groups (lower than the endpoint with higher as a reference), we found an increasing trend for bleeding with a steady increase in risk at LDL-C levels below 70 mg/dl. This effect stopped being significant when LDL-C levels reached ≥70 mg/dl. In contrast, an opposite trend was found for ischemic events, with a consistent decrease in risk at LDL-C levels <70 mg/dl, and this stopped being significant when the levels reached ≥70 mg/dl. These results show that there is no safe zone for bleeding and ischemic events. Maintaining LDL-C levels >70 mg/dl may be best to prevent bleeding. However, once patients are assessed to have a high risk of ASCVD, LDL-C levels should still be strictly monitored to be ≤70 mg/dl because the incidence of bleeding is lower than that of ASCVD.
With the wider application of PCSK9 inhibitors, there will be a high prevalence of patients with LDL-C levels ≤70 mg/dl. It is essential to provide physicians with guidelines to identify patients requiring careful monitoring of their LDL-C levels and the appropriate range during clinical follow-up.
Strengths and limitations
The major strengths of this study are its large sample size and coverage, which provided sufficient power to explain the relationship between LDL-C levels and in-hospital bleeding events and enabled the results to be representative of the national population. Nevertheless, we acknowledge some limitations. First, this study has inherent limitations associated with an observational design, including the inability to establish a causal association between low LDL-C levels and bleeding risk in AF. Despite our efforts to adjust for many important confounders, there is potential for residential and unmeasured confounding. Second, although many studies found that low LDL-C levels were related to bleeding, more evidence of a clear biological mechanism by which low LDL-C can promote bleeding is warranted. Third, this study only included in-hospital events and lacked long-term follow-up, therefore, the bleeding events and mortality rate were low. Thus, our study may be underpowered to fully evaluate the association between low LDL-C levels and bleeding events.
Conclusion
Our results demonstrate an L-shaped relationship between LDL-C levels and bleeding events, with lower LDL-C levels associated with a higher risk of bleeding events. LDL-C levels may need to be considered in the risk stratification and management of patients with AF.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: raw data are available upon reasonable request with the corresponding author. Requests to access these datasets should be directed to De-yong Long: dragon2008@vip.sina.com.
Ethics statement
The studies involving humans were approved by the CCC-AF project was approved by the institutional review board of Beijing Anzhen Hospital, with a waiver for informed consent (number 2014018). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Written informed consent was waived by the institutional review board of Beijing Anzhen Hospital because the study involved minimal risk to participants, the data were collected anonymously, and the research could not practicably be conducted without the waiver.
Author contributions
X-yT: Writing – original draft, Formal analysis, Methodology. JL: Conceptualization, Writing – review & editing. Z-qS: Formal analysis, Methodology, Writing – review & editing. QH: Formal analysis, Methodology, Writing – review & editing. YZ: Formal analysis, Methodology, Writing – review & editing. C-XJ: Formal analysis, Methodology, Writing – review & editing. R-bT: Formal analysis, Supervision, Writing – review & editing. C-hS: Formal analysis, Methodology, Writing – review & editing. MN: Methodology, Writing – review & editing. C-qJ: Formal analysis, Methodology, Writing – review & editing. LF: Formal analysis, Methodology, Writing – review & editing. WW: Formal analysis, Methodology, Writing – review & editing. XZ: Formal analysis, Methodology, Writing – review & editing. C-yL: Formal analysis, Methodology, Writing – review & editing. S-nL: Formal analysis, Methodology, Writing – review & editing. X-yG: Formal analysis, Methodology, Writing – review & editing. TL: Data curation, Formal analysis, Methodology, Writing – review & editing. M-mL: Formal analysis, Methodology, Writing – review & editing. NY: Data curation, Formal analysis, Methodology, Writing – review & editing. Y-cH: Data curation, Formal analysis, Methodology, Writing – review & editing. JuL: Data curation, Formal analysis, Methodology, Writing – review & editing. JiL: Data curation, Formal analysis, Methodology, Writing – review & editing. JX: Supervision, Writing – review & editing. D-yL: Supervision, Writing – review & editing, Formal analysis, Methodology. J-zD: Supervision, Writing – review & editing. DZ: Supervision, Writing – review & editing. C-sM: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The CCC-AF Project is a collaborative project of the AHA and the Chinese Society of Cardiology. The AHA received funding from Pfizer through an independent grant for learning and change from AstraZeneca as a quality improvement initiative. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgments
We would like to thank all CCC-AF project investigators and their patients for contributing to these data (Supplementary Table S1). The study protocol is listed on http://www.clinicaltrials.gov (NCT02309398, Supplementary Table 1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1574796/full#supplementary-material
References
1.
Elliott AD Middeldorp ME Van Gelder IC Albert CM Sanders P . Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. (2023) 20:404–17. 10.1038/s41569-022-00820-8
2.
Ma C Wu S Liu S Han Y . Chinese guidelines for the diagnosis and management of atrial fibrillation. Pacing Clin Electrophysiol. (2024) 47:714–70. 10.1111/pace.14920
3.
Cannon CP . Low-Density lipoprotein cholesterol. J Am Coll Cardiol. (2020) 75:2119–21. 10.1016/j.jacc.2020.03.033
4.
Grundy SM Stone NJ Bailey AL Beam C Birtcher KK Blumenthal RS et al 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 139:e1082–143. 10.1161/CIR.0000000000000625
5.
Sung K-C Huh JH Ryu S Lee J-Y Scorletti E Byrne CD et al Low levels of low-density lipoprotein cholesterol and mortality outcomes in non-statin users. JCM. (2019) 8:1571. 10.3390/jcm8101571
6.
Feng X Tang Q Cheng C Xu S . Low serum lipid levels, use of statin and cerebral microbleeds: a systematic review and meta-analysis. J Clin Neurosci. (2021) 94:216–25. 10.1016/j.jocn.2021.10.032
7.
Yang Q Sun D Pei C Zeng Y Wang Z Li Z et al LDL cholesterol levels and in-hospital bleeding in patients on high-intensity antithrombotic therapy: findings from the CCC-ACS project. Eur Heart J. (2021) 42:3175–86. 10.1093/eurheartj/ehab418
8.
Hrabovský V Mendlová A Zadák Z Bláha V Hyšpler R Tichá A et al Cholesterol metabolism in acute upper gastrointestinal bleeding, preliminary observations. Wien Klin Wochenschr. (2012) 124:815–21. 10.1007/s00508-012-0292-0
9.
Cheng Y Qiao L Jiang Z Dong X Feng H Gui Q et al Significant reduction in the LDL cholesterol increases the risk of intracerebral hemorrhage: a systematic review and meta-analysis of 33 randomized controlled trials. Am J Transl Res. (2020) 12:463–77.
10.
Ma Y Li Z Chen L Li X . Blood lipid levels, statin therapy and the risk of intracerebral hemorrhage. Lipids Health Dis. (2016) 15:43. 10.1186/s12944-016-0213-8
11.
Hao Y Liu J Smith SC Huo Y Fonarow GC Ge J et al Rationale and design of the improving care for cardiovascular disease in China (CCC) project: a national registry to improve management of atrial fibrillation. BMJ Open. (2018) 8:e020968. 10.1136/bmjopen-2017-020968
12.
Buxton AE Calkins H Callans DJ DiMarco JP Fisher JD Greene HL et al ACC/AHA/HRS 2006 key data elements and definitions for electrophysiological studies and procedures: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (ACC/AHA/HRS writing committee to develop data standards on electrophysiology). J Am Coll Cardiol. (2006) 48:2360–96. 10.1016/j.jacc.2006.09.020
13.
McNamara RL Brass LM Drozda JP Go AS Halperin JL Kerr CR et al ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing commitee to develop data standards on atrial fibrillation). J Am Coll Cardiol. (2004) 44:475–95. 10.1016/j.jacc.2004.06.041
14.
Barnes GD Gu X Haymart B Kline-Rogers E Almany S Kozlowski J et al The predictive ability of the CHADS2 and CHA2DS2-VASc scores for bleeding risk in atrial fibrillation: the MAQI(2) experience. Thromb Res. (2014) 134:294–9. 10.1016/j.thromres.2014.05.034
15.
Pisters R Lane DA Nieuwlaat R de Vos CB Crijns HJGM Lip GYH . A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the euro heart survey. Chest. (2010) 138:1093–100. 10.1378/chest.10-0134
16.
Plummer S . How low is too low? A retrospective analysis of very low LDL-C levels in veterans. Fed Pract. (2022) 39:e0334. 10.12788/fp.0334
17.
Vinci P Di Girolamo FG Panizon E Tosoni LM Cerrato C Pellicori F et al Lipoprotein(a) as a risk factor for cardiovascular diseases: pathophysiology and treatment perspectives. Int J Environ Res Public Health. (2023) 20:6721. 10.3390/ijerph20186721
18.
Lu Z Huang L Li Y Xu Y Zhang R Zhou Q et al Fine-tuning of cholesterol homeostasis controls erythroid differentiation. Adv Sci (Weinh). (2022) 9:e2102669. 10.1002/advs.202102669
19.
Fessler MB Rose K Zhang Y Jaramillo R Zeldin DC . Relationship between serum cholesterol and indices of erythrocytes and platelets in the US population. J Lipid Res. (2013) 54:3177–88. 10.1194/jlr.P037614
20.
Blaha M Kostal M Lanska M Blaha V Foralova I Filip S et al The decrease of mean platelet volume after extracorporeal LDL-cholesterol elimination. Atheroscler Suppl. (2013) 14:77–81. 10.1016/j.atherosclerosissup.2012.10.019
21.
Nagy B Jr Jin J Ashby B Reilly MP Kunapuli SP . Contribution of the P2Y12 receptor-mediated pathway to platelet hyperreactivity in hypercholesterolemia. J Thromb Haemostasis. (2011) 9:810–9. 10.1111/j.1538-7836.2011.04217.x
22.
Tavori H Giunzioni I Linton MF Fazio S . Loss of plasma proprotein convertase subtilisin/kexin 9 (PCSK9) after lipoprotein apheresis. Circ Res. (2013) 113:1290–5. 10.1161/CIRCRESAHA.113.302655
23.
Qi Z Hu L Zhang J Yang W Liu X Jia D et al PCSK9 (proprotein convertase subtilisin/kexin 9) enhances platelet activation, thrombosis, and myocardial infarct expansion by binding to platelet CD36. Circulation. (2021) 143:45–61. 10.1161/CIRCULATIONAHA.120.046290
24.
Zhao Y Zhou Y Zhou H Gong X Luo Z Li J et al Low-density lipoprotein cholesterol, statin therapy, and cerebral microbleeds: the CIRCLE study. Neuroimage Clin. (2023) 39:103502. 10.1016/j.nicl.2023.103502
25.
Kodani E Inoue H Atarashi H Okumura K Yamashita T Origasa H , J-RHYTHM Registry Investigators, et al. Impact of hemoglobin concentration and platelet count on outcomes of patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM registry. Int J Cardiol. (2020) 302:81–7. 10.1016/j.ijcard.2019.11.127
26.
Dong M Xu C Zhou J Yuan Z investigators CCC-AF . Influence of hemoglobin concentration on the in-hospital outcomes in newly diagnosed heart failure patients with atrial fibrillation: finding from CCC-AF (improving care for cardiovascular disease in China-atrial fibrillation) project. Medicine (Baltimore). (2022) 101:e28978. 10.1097/MD.0000000000028978
27.
Pastori D Menichelli D Di Rocco A Farcomeni A Sciacqua A Pignatelli P et al Bleeding and thrombotic events in atrial fibrillation patients with cancer: a systematic review and meta-analysis. Intern Emerg Med. (2023) 18:655–65. 10.1007/s11739-022-03156-w
28.
Pastori D Marang A Bisson A Menichelli D Herbert J Lip GYH et al Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: a nationwide cohort study. Cancer. (2021) 127:2122–9. 10.1002/cncr.33470
29.
Perera V Bajorek BV Matthews S Hilmer SN . The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. (2008) 38:156–62. 10.1093/ageing/afn293
Summary
Keywords
atrial fibrillation, low-density lipoprotein cholesterol, bleeding risk, nonlinear relationship, CCC-AF project
Citation
Tong X-y, Lin J, Sun Z-q, He Q, Zhan Y, Jiang C-X, Tang R-b, Sang C-h, Ning M, Jia C-q, Feng L, Wang W, Zhao X, Li C-y, Li S-n, Guo X-y, Liu T, Li M-m, Yang N, Hao Y-c, Liu J, Liu J, Xie J, Long D-y, Dong J-z, Zhao D and Ma C-s (2025) Low-density lipoprotein cholesterol levels and in-hospital bleeding in patients with atrial fibrillation: findings from CCC-AF project. Front. Cardiovasc. Med. 12:1574796. doi: 10.3389/fcvm.2025.1574796
Received
11 February 2025
Accepted
14 April 2025
Published
12 June 2025
Volume
12 - 2025
Edited by
Jiong-Wei Wang, National University of Singapore, Singapore
Reviewed by
Akhmetzhan Galimzhanov, Semey State Medical University, Kazakhstan
Jie Yin, The First Affiliated Hospital of Shandong First Medical University, Shandong Provincial Qianfoshan Hospital, China
Updates
Copyright
© 2025 Tong, Lin, Sun, He, Zhan, Jiang, Tang, Sang, Ning, Jia, Feng, Wang, Zhao, Li, Li, Guo, Liu, Li, Yang, Hao, Liu, Liu, Xie, Long, Dong, Zhao and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: De-yong Long dragon2008@vip.sina.com Jin Xie 153162220@qq.com
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.