Abstract
Introduction:
Evidence on incision lengths for ports and cardiopulmonary bypass (CPB) cannulation in robotic cardiac surgery is limited. This study aimed to assess these metrics and influencing factors.
Methods:
204 patients underwent robotic mitral valve repair (MVR) (54.9%), totally endoscopic coronary artery bypass grafting (TECAB) (30.9%), and minimally invasive direct coronary artery bypass grafting (MIDCAB) (14.2%). Total incision length (TIL) was measured intraoperatively and defined as the sum of thoracic incisions, portholes, and incisions for cannulation. In both univariate and multivariate analyses, TIL was calculated based on demographic and intraoperative variables. Additionally, TIL was linked with postoperative outcomes.
Results:
The median length of thoracic access incisions and ports was 11.5 (5.0–51.0) cm, while for cannulation access, it was 5.0 (3.0–13.0) cm. The median total incision length was 16.5 (10.0–62.0) cm. Thirteen pre- and intraoperative variables were associated with TIL on univariate analysis. Multivariate analysis revealed that BMI (p = 0.003), procedure type (p < 0.001), conversion to sternotomy (p < 0.001), technical challenges (p = 0.034) and total procedure time (p < 0.001) were associated with extended incision length. Multivariate testing additionally showed an association of TIL with blood transfusion (p = 0.004) and hospital stay (p < 0.001).
Conclusions:
Incision length in robotic cardiac surgery is primarily linked to obesity, procedure type, surgical technical problems, conversion to sternotomy, and procedure time. Longer incisions are associated with an increased number of blood transfusions and longer hospital stay
Introduction
Robotic technology enables cardiac surgery to be performed through small incisions and port holes rather than through a large sternotomy. Robotic cardiac surgery is mainly implicated in less invasive and totally endoscopic mitral valve surgery and coronary bypass grafting. Unless completely endoscopic, such as totally endoscopic coronary artery bypass grafting (TECAB) or totally endoscopic mitral valve repair, these procedures are carried out through a small working incision known as a minithoracotomy on the left or right chest to access the heart.
Existing literature describes the length of the minithoracotomy and robotic ports relatively non-quantitatively, often only referring to them in brief as “small”, or “short”. In those that do mention incision lengths, they are frequently described with a large range and are inconsistent across publications (1–12). In addition to these thoracic ports, the femoral artery and vein are also exposed in the groin through a small incision and the cannulae for cardiopulmonary bypass are inserted. Information on the access size for heart lung machine cannulation is also very limited (4).
Due to discrepancies and a lack of precisely measured evidence on this subject, this study aimed to quantify the lengths of individual incisions made on the patient's body, outline their locations, and identify factors influencing total incision length in robotically assisted coronary artery bypass and mitral valve surgery.
Materials and methods
From July 2021 to April 2024, 218 patients underwent robotic cardiac surgery at UPMC Presbyterian or UPMC Passavant hospital. Of these, 112 (54.9%) were robotic mitral valve repair (MVR), 63 (30.9%) were robotic totally endoscopic coronary artery bypass grafting (TECAB), and 29 (14.2%) were robotically assisted minimally invasive direct coronary artery bypass grafting (MIDCAB). Of these 204 patients, the median age was 63 (24–85) years, 150 (73.5%) were male, and the median STS risk of mortality was 0.5 (0.1–4.5) %. The remaining 14 procedures (6.4%) were rare interventions, which were excluded from our analysis. Of the 204 patients included, nine (4.4%) underwent conversions to sternotomy due to bleeding issues (n = 5, 55.6%), complex mitral valve pathology requiring replacement (n = 1, 11.1%), the inability to tolerate single lung ventilation (n = 1, 11.1%), mitral valve re-repair due to access through minithoracotomy being restricted (n = 1, 11.1%), and placement of a vein graft to PCI target in hybrid coronary intervention for intraoperative ischemia (n = 1, 11.1%).
Surgical technique
All procedures were carried out using the da Vinci Xi surgical robot [Intuitive, Sunnyvale, CA].
For robotic mitral valve repair, a minithoracotomy was placed in the 4th intercostal space on the right side of the chest. Three robotic ports were placed for the left and right arms of the robotic instrument and the left atrial retractor. Patients were cannulated in the right groin. An additional small incision was placed in the axillary fold for insertion of the left atrial suction tube and the Chitwood clamp. Patients were placed on cardiopulmonary bypass and cardioplegia was induced through a cardioplegia cannula in the aortic root after transthoracic aorta clamping. The left atrium was opened, and the mitral valve repair was performed with assistance by a patient side surgeon.
In robotic MIDCAB, instrument ports were placed in the 3rd, 5th, and 7th intercostal spaces on the patient's left lateral chest and docked to the surgical robot. CO2 was insufflated at pressures of 8 mmHg. The internal mammary artery was harvested in skeletonized technique and the pericardium was opened robotically. The target vessel was identified, and the location of the mini thoracotomy defined videoscopically. All patients were cannulated prophylactically in the groin or axillary depending on the grade of atherosclerosis on the aortoiliac level. After undocking the robot, a left sided mini thoracotomy was carried out and the graft to coronary artery anastomosis was performed on the beating heart using the Octopus Nuvo [Medtronic, Minneapolis, MN] for mechanical stabilization of the target vessel.
In robotic TECAB, the internal mammary harvesting was carried out as described for robotic MIDCAB. Additional ports were placed parasternally in the 4th intercostal interspace and subcostally for insertion of assisting instruments. Patients were cannulated in the groin using the Edwards Intraclude System [Edwards, Irvine, CA]. In procedures performed on the arrested heart, an endoaortic occlusion balloon was positioned into the aortic root under transesophageal echocardiography guidance. After initiation of cardiopulmonary bypass, the heart was arrested using the aortic occlusion balloon. In beating heart TECAB, the target vessel was stabilized using robotic long tip forceps brought in through a subcostal port and was snared with silastic tape. In both versions, the anastomosis was carried out in complete endoscopic fashion using robotic instrumentation.
In all three operations, patients were cannulated in the ipsilateral groin to the thoracic incisions. If more than mild aortoiliac atherosclerosis was present, we chose the axillary artery for arterial heart-lung machine access. All robotic MIDCAB patients were cannulated prophylactically, and a supportive pump run was initiated immediately in cases of technical difficulties or hemodynamic instability.
The incision lengths were measured intraoperatively at completion of the case with a sterile ruler. A scheme with the incision sites is shown in Figure 1. Total incision length (TIL) was defined as the sum of all thoracic ports and mini-incisions, the incisions for cannulation, and the length of the sternotomy if conversion to sternotomy became necessary.
Figure 1
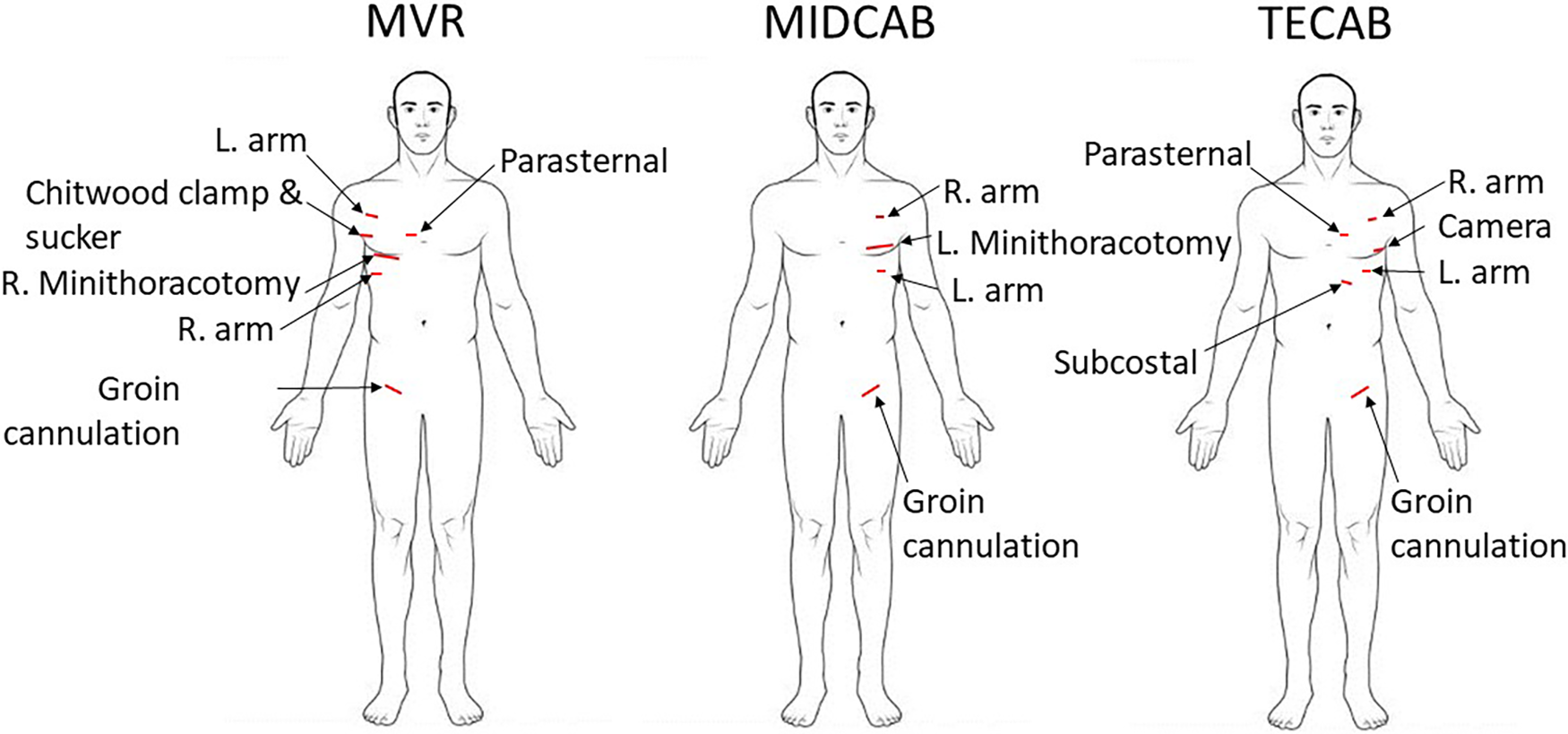
Schematic depicting access incisions for robotic mitral valve repair (MVR), robotic minimally invasive direct coronary artery bypass (MIDCAB), and robotic totally endoscopic coronary artery bypass (TECAB).
Ethical statement
This study was approved by the University of Pittsburgh Institutional Review Board (IRB #21080141) and patients gave informed consent.
Statistical analysis
Statistical analysis was conducted using SPSS Statistics, version 28.0.1.1. Total incision length was correlated with preoperative, intraoperative, and postoperative variables. Categorical variables are shown as absolute values and percentages and continuous variables are shown as median, minimum, and maximum. Correlations were calculated using the Spearman's rho correlation coefficient. Differences between groups were calculated using Mann–Whitney U-test and Kruskall-Wallis test. A multivariate analysis for predictors of total incision length was conducted using multiple linear regression. Additionally, the association between total incision length and outcome variables was analyzed using multivariate analysis of variance (MANOVA) after converting the variable “total incision length” into quartiles. A p-value < 0.05 was considered statistically significant.
Results
In this series of robotic cardiac surgical procedures, there was no hospital mortality, and no postoperative permanent stroke occurred. There were two revisions for bleeding, both done in a less invasive fashion, and one robotically in a TECAB patient. 121 out of 204 patients (59.3%) were extubated in the operating room. The median hospital stay was 4 (2–25) days.
Incision length details
The various incision lengths for the three types of robotic operations are shown in Table 1. The median length of the port incisions ranged from 1 cm to 2 cm, with the overall range extending from 1 cm to 4 cm. The minithoracotomy in robotic MVR was shorter than the minithoracotomy in robotic MIDCAB [7.0 (5.0–15.0) cm vs. 9.0 (5.0–12.0 cm), p < 0.001]. The shortest thoracic incision length (sum of all chest incisions) was seen for robotic TECAB [7.5 (5.0–51.0) cm] as compared to MIDCAB [12.5 (7.0–17.0) cm] and MVR [12.0 (9.5–35.5) cm, p < 0.001]. The shortest total incision length was also seen in robotic TECAB with 12.5 [10.0–62.0] cm, (p = 0.022), which was 4.5 and 6.0 cm shorter than both MVR [17 (12.5–40.0) cm] and MIDCAB [18.5 (11.0–30.0) cm], respectively.
Table 1
| n | MVR | MIDCAB | TECAB | p-value | Total |
|---|---|---|---|---|---|
| 112 | 29 | 63 | 204 | ||
| Left instrument port (cm) | 1.0 [1.0–2.5] | 1.0 [1.0–2.5] | 1.0 [1.0–2.0] | 0.488 | 1.0 [1.0–2.5] |
| Right instrument port (cm) | 1.0 [1.0–3.0] | 1.5 [1.0–2.5] | 1.5 [1.0–2.5] | <0 . 001 | 1.0 [1.0–3.0] |
| Left atrial retractor port (cm) | 1.0 [1.0–3.0] | n/a | n/a | ||
| Chitwood clamp/Left atrial suction tube incision (cm) | 2.0 [1.0–3.0] | n/a | n/a | ||
| Octopus Nuvo incision (cm) | n/a | 1.0 [1.0–1.5] | n/a | ||
| Minithoracotomy (cm) | 7.0 [5.0–15.0] | 9.0 [5.0–12.0] | n/a | <0 . 001 | 7.0 [5.0–15.0] |
| Camera port (cm) | n/a | n/a | 1.0 [1.0–3.0] | ||
| Parasternal assistance port (cm) | n/a | n/a | 1.0 [1.0–2.5] | ||
| Subcostal port (cm) | n/a | n/a | 2.0 [1.0–4.0] | ||
| Thoracic incision length (cm) | 12.0 [9.5–35.5] | 12.5 [7.0–19.0] | 7.5 [5.0–51.0] | <0 . 001 | 11.5 [5.0–51.0] |
| Cannulation incision length (cm) | 5.0 [3.0–11.0] | 5.0 [4.0–13.0] | 5.0 [4.0–12.0] | <0 . 001 | 5.0 [3.0–13.0] |
| Sternotomy in converted patients (cm) | 17.0 [17.0–22.0] | n/a | 21.0 [15.0–22.0] | <0 . 001 | 21.0 [15.0–22.0] |
| Total incision length (cm) | 17.0 [12.5–40.0] | 18.5 [11.0–30.0] | 12.5 (10.0–62.0) | <0 . 001 | 16.5 [10.0–62.0] |
Incision lengths by procedure type.
Values are measured in centimeters (cm) and represented with median and range.
Statistically significant results (p < 0.05) are highlighted in bold.
Preoperative factors influencing incision length
Multiple body habitus measurements positively correlated with increased TIL, including weight (R = 0.179, p = 0.011), body surface area (R = 0.179, p = 0.011), and body mass index (R = 0.251, p < 0.001, Figure 2). Additionally, smoking status corresponded to longer total incision length (p = 0.039), and forced expiratory volume in one second (FEV1) (R = −0.196, p = 0.008, Figure 3) and diffusing capacity of the lungs for carbon monoxide (DLCO) (R = −0.173, p = 0.037) were inversely correlated with TIL. Table 2 lists the twenty-two preoperative variables investigated.
Figure 2
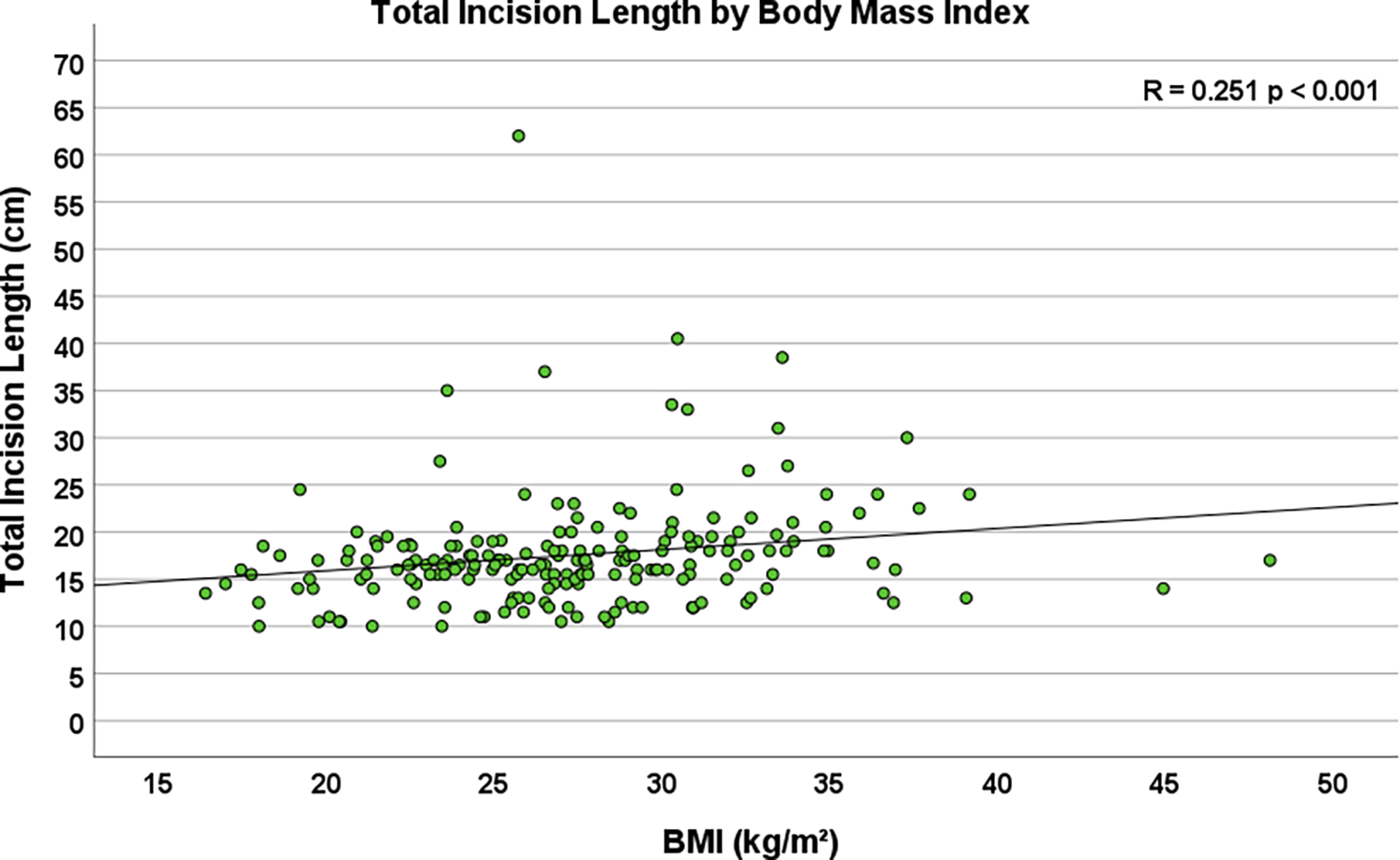
Scatterplot depicting the correlation between body mass Index (BMI) and total incision length (n = 204, R = 0.251, p < 0.001).
Figure 3
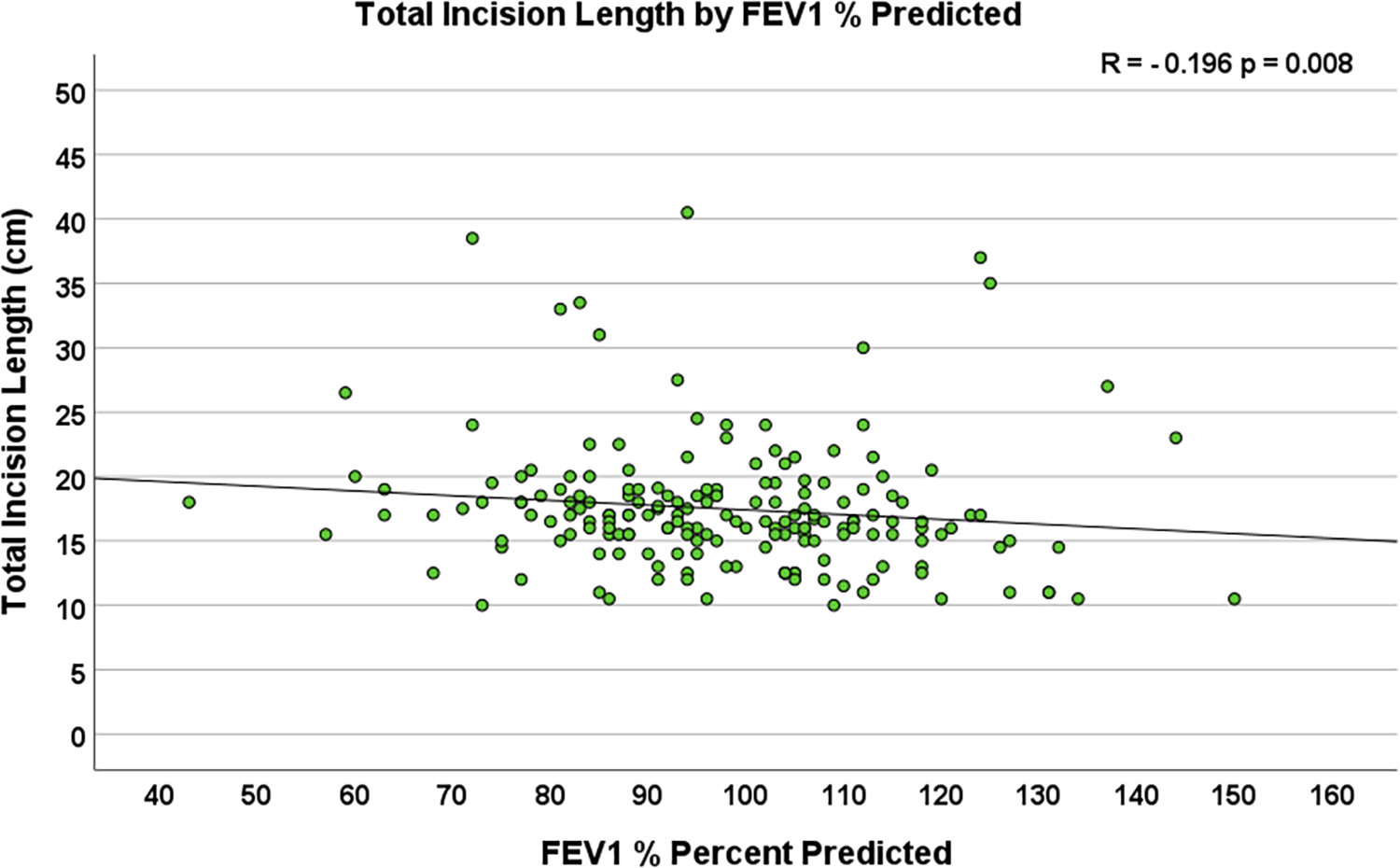
Scatterplot depicting the correlation between preoperative forced expiratory volume in One second (FEV1) % predicted and total incision length (n = 204, R = −0.196, p = 0.008).
Table 2
| Variable | Total incision length (cm) | |||||
|---|---|---|---|---|---|---|
| n = 204 | Percent (%) | Median | Range | R | p-value | |
| Demographics | ||||||
| Age (years) | −0.013 | 0.850 | ||||
| Sex | ||||||
| Male | 150 | 73.5 | 16.5 | 10.0–62.0 | 0.327 | |
| Female | 54 | 26.5 | 17.0 | 10.0–38.5 | ||
| Height (cm) | −0.076 | 0.283 | ||||
| Weight (kg) | 0.179 | 0 . 011 | ||||
| BMI (kg/m2) | 0.251 | <0 . 001 | ||||
| BSA (m2) | 0.179 | 0 . 011 | ||||
| LVEF (%) | 0.054 | 0.451 | ||||
| Risk factors | ||||||
| Smoking | ||||||
| No | 120 | 58.8 | 16.0 | 10.5–62.0 | 0 . 039 | |
| Former | 60 | 29.4 | 17.5 | 10.0–40.5 | ||
| Current | 24 | 11.8 | 16.5 | 10.0–30.0 | ||
| Diabetes mellitus | ||||||
| No | 167 | 81.9 | 16.5 | 10.0–62.0 | 0.096 | |
| Yes | 37 | 18.1 | 17.5 | 10.0–38.5 | ||
| Last A1C level | 0.023 | 0.746 | ||||
| Hypertension | ||||||
| No | 53 | 26.0 | 17.0 | 10.5–35.0 | 0.606 | |
| Yes | 151 | 74.0 | 16.5 | 10.0–62.0 | ||
| Comorbidities | ||||||
| COPD | ||||||
| No | 156 | 76.5 | 16.5 | 10.0–62.0 | 0.138 | |
| Yes | 48 | 23.5 | 17.0 | 12.0–38.5 | ||
| FEV1% predicted | −0.196 | 0 . 008 | ||||
| DLCO % predicted | −0.173 | 0 . 037 | ||||
| Creatinine | −0.084 | 0.241 | ||||
| CVD | ||||||
| No | 184 | 90.2 | 16.5 | 10.0–62.0 | 0.616 | |
| Yes | 20 | 9.8 | 17.0 | 10.0–20.0 | ||
| PVD | ||||||
| No | 199 | 97.5 | 16.5 | 10.0–62.0 | 0.426 | |
| Yes | 5 | 2.5 | 18.0 | 15.5–20.0 | ||
| Liver disease | ||||||
| No | 199 | 97.5 | 16.5 | 10.0–62.0 | 0.826 | |
| Yes | 5 | 2.5 | 14.0 | 12.0–33.5 | ||
| Preoperative labs | ||||||
| WBC | −0.028 | 0.700 | ||||
| Hemoglobin | −0.002 | 0.981 | ||||
| Hematocrit | 0.023 | 0.750 | ||||
| Platelet count | 0.007 | 0.926 | ||||
| Total albumin | −0.067 | 0.349 | ||||
Total incision length by demographics and preoperative variables.
Values are measured in centimeters (cm) and represented with median and range.
Statistically significant results (p < 0.05) are highlighted in bold.
Intraoperative factors influencing incision length
Incisions were longer in patients with complex operations [17.0 (11.0–40.0) cm] defined as procedures technically more extensive than placement of a left internal mammary artery (LIMA) to the left anterior descending artery (LAD) or correction of a simple P2 pathology. Patients who underwent the less complex procedure had a TIL of 16.5 [10.0–62.0] cm, (p = 0.012). In patients where no surgical technical problem occurred, the total incision length median was 16.0 (10.0–24) cm. In those patients where technical challenges did occur, the incision length median was 17.0 (10.0–62.0) cm. The total incision length for patients converted to sternotomy was 35.0 (27.5–62.0) cm as compared to 16.5 (10.0–30.0) cm those who did not require conversion (p < 0.001).
Operative times, which included cardiopulmonary bypass time (R = 0.224, p = 0.001), myocardial ischemic time (R = 0.208, p = 0.003), and total procedure time (R = 0.274, p < 0.001, Figure 4), were highly significantly associated with total incision length. Extubation status in the operating room did not have a significant association with TIL. Table 3 lists the seven intraoperative and eleven postoperative variables investigated.
Figure 4
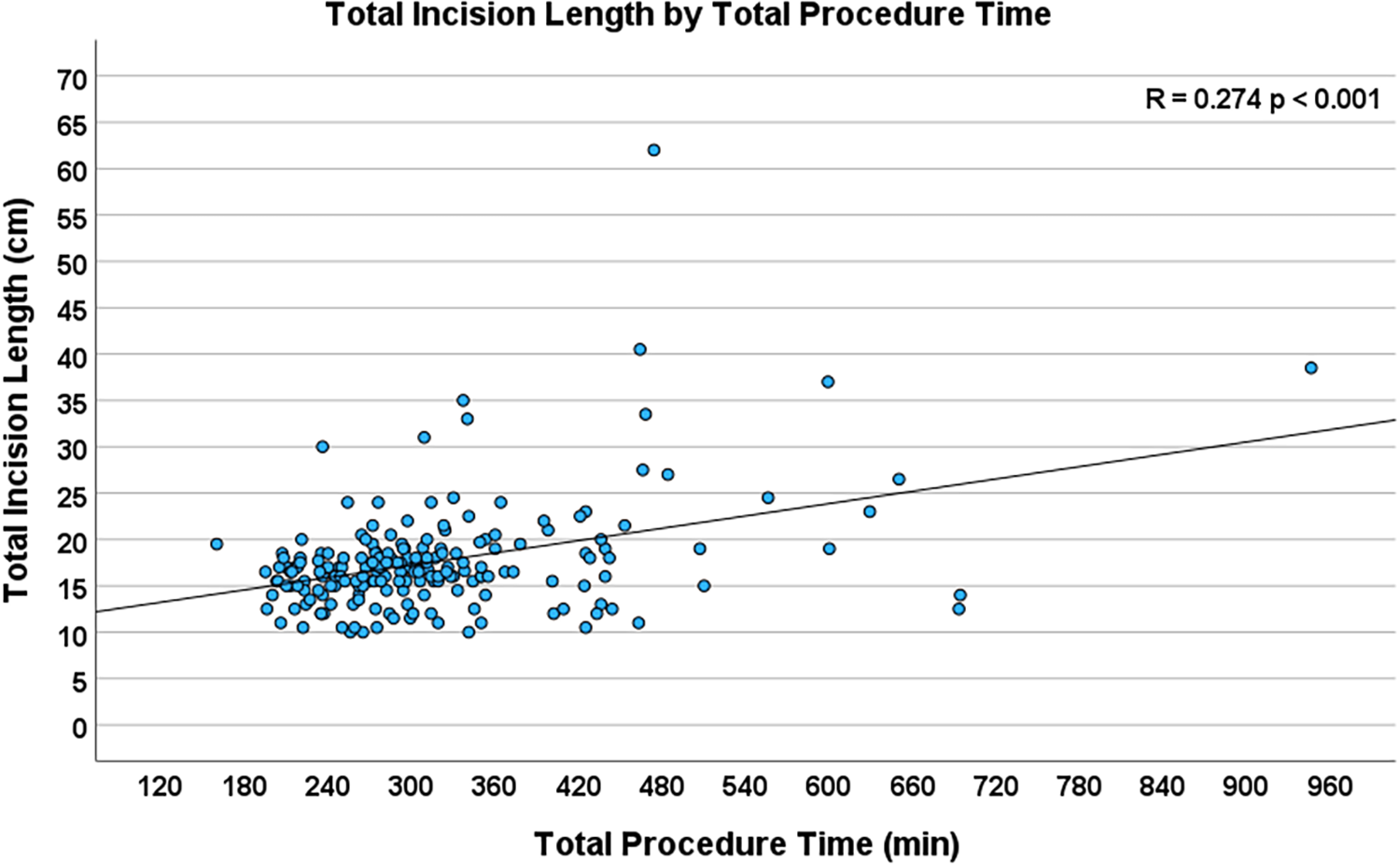
Scatterplot depicting the correlation between total procedure time and total incision length (n = 204, R = 0.274, p < 0.001).
Table 3
| Variable | Total incision length (cm) | |||||
|---|---|---|---|---|---|---|
| n = 204 | Percent (%) | Median | Range | R | p-value | |
| Intraoperative variables | ||||||
| Conversion to sternotomy | ||||||
| No | 195 | 95.6 | 16.5 | 10.0–30.0 | <0.001 | |
| Yes | 9 | 4.4 | 35.0 | 27.5–62.0 | ||
| Complex procedure | ||||||
| No | 133 | 65.2 | 16.5 | 10.0–62.0 | 0.012 | |
| Yes | 71 | 34.8 | 17.0 | 11.0–40.0 | ||
| Any technical challenge | ||||||
| No | 84 | 41.2 | 16.0 | 10.0–24.0 | 0.044 | |
| Yes | 120 | 58.8 | 17.0 | 10.0–62.0 | ||
| Cannulation problem | ||||||
| No | 164 | 80.4 | 16.5 | 10.0–40.5 | 0.784 | |
| Yes | 40 | 19.6 | 16.5 | 10.0–62.0 | ||
| CPB time | 0.224 | 0.001 | ||||
| Myocardial ischemic time | 0.208 | 0.003 | ||||
| Total procedure time | 0.274 | <0.001 | ||||
| Postoperative variables | ||||||
| Ventilation time | 0.197 | 0.005 | ||||
| Total number of RBC units transfused | 0.198 | 0.005 | ||||
| Total days in ICU | 0.124 | 0.083 | ||||
| Total days in hospital | 0.229 | 0.001 | ||||
| No | 202 | 16.5 | 10.0–62.0 | 0.555 | ||
| Yes | 2 | 18.0 | 16.5–19.0 | |||
| Postop permenant neurologic deficit | ||||||
| No | n/a | |||||
| Yes | ||||||
| Postop new atrial fibrilation | ||||||
| No | 153 | 75.0 | 16.5 | 10.0–62.0 | 0.596 | |
| Yes | 51 | 25.0 | 17.0 | 10.5–33.5 | ||
| Postop pnuemonia | ||||||
| No | n/a | |||||
| Yes | ||||||
| Postop renal failure | ||||||
| No | n/a | |||||
| Yes | ||||||
| Postop dialysis | ||||||
| No | n/a | |||||
| Yes | ||||||
| Postop deep thoracic wound infection | ||||||
| No | n/a | |||||
| Yes | ||||||
Total incision length by intra-and postoperative variables.
Values are measured in centimeters (cm) and represented with median and range.
Statistically significant results (p < 0.05) are highlighted in bold.
Table 4 shows the univariate association of pre- and intraoperative factors with TIL and the correlation of TIL with postoperative outcomes for each of the three operations studied. BMI was highly significantly associated with TIL in both robotic mitral valve repair and robotic TECAB and there was a corresponding trend in robotic MIDCAB. Increased tissue thickness in obese patients requires larger access to reach the ribcage and mitral valve. Figure 5 shows representative CT scans that demonstrate the anatomical differences between an obese and a non-obese patient, highlighting variations in soft tissue thickness, distance to the mitral valve, and their corresponding incision lengths. Conversion to sternotomy also showed a relation with increased incision length in robotic MVR and TECAB. Of note no conversion happened in robotic MIDCAB. Total procedure time was significantly associated with TIL in all three procedure variations.
Table 4
| n = 204 | Robotic MVR | Robotic MIDCAB | Robotic TECAB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 112 | 29 | 63 | ||||||||||
| Median | Range | R | p-value | Median | Range | R | p-value | Median | Range | R | p-value | |
| Preop and intraop factors and TIL | ||||||||||||
| Weight | 0.274 | 0 . 003 | 0.367 | 0.054 | 0.272 | 0 . 035 | ||||||
| BMI | 0.392 | <0 . 001 | 0.368 | 0.054 | 0.342 | 0 . 008 | ||||||
| BSA | 0.274 | 0 . 003 | 0.367 | 0.054 | 0.272 | 0 . 035 | ||||||
| Smoking | 0 . 004 | 0.266 | 0.787 | |||||||||
| No | 17.0 | 13.5–26.5 | 16.0 | 13.0–24.0 | 13.0 | 10.5–62.0 | ||||||
| Current | 16.0 | 12.5–17.0 | 19.0 | 11.0–30.0 | 12.5 | 10.0–24.0 | ||||||
| Former | 18.5 | 15.0–40.5 | 18.5 | 16.0–27.0 | 12.5 | 10.0–33.0 | ||||||
| FEV1% predicted | −0.84 | 0.392 | −0.054 | 0.589 | −0.252 | 0.074 | ||||||
| DLCO % predicted | −0.105 | 0.343 | −0.054 | 0.807 | −0.228 | 0.467 | ||||||
| Conversion to sternotomy | <0 . 001 | n/a | 0 . 015 | |||||||||
| No | 17.0 | 12.5–26.5 | 12.5 | 10.0–24.0 | ||||||||
| Yes | 37.0 | 33.5–40.5 | 33.0 | 27.5–62.0 | ||||||||
| Complex procedure | 0.106 | 0.643 | 0.158 | |||||||||
| No | 17.0 | 12.5–35.0 | 18.0 | 11.0–30.0 | 12.5 | 10.0–62.0 | ||||||
| Yes | 17.0 | 13.5–40.5 | 19.0 | 16.0–20.0 | 14.0 | 11.0–37.0 | ||||||
| Any technical challenge | 0 . 006 | 0.118 | 0.282 | |||||||||
| No | 16.5 | 12.5–24.0 | 17.5 | 11.0–21.0 | 12.5 | 10.0–20.0 | ||||||
| Yes | 17.6 | 14.5–40.5 | 19.0 | 13.0–30.0 | 13.5 | 10.0–62.0 | ||||||
| CPB time | 0.298 | 0 . 001 | 0.427 | 0 . 024 | 0.137 | 0.297 | ||||||
| Myocardial Ischemic time | 0.271 | 0 . 004 | 0.303 | 0.132 | 0.101 | 0.450 | ||||||
| Total procedure time | 0.471 | <0 . 001 | 0.548 | 0 . 003 | 0.283 | 0 . 033 | ||||||
| Postop outcomes and TIL | ||||||||||||
| Ventilation time | 0.320 | <0 . 001 | 0.208 | 0.287 | 0.289 | 0 . 028 | ||||||
| Number of blood Transfusions | 0.188 | 0 . 048 | 0.209 | 0.285 | 0.287 | 0 . 026 | ||||||
| Hospital stay length | 0.270 | 0 . 004 | 0.040 | 0.839 | 0.380 | 0 . 003 | ||||||
Univariate analysis of pre- and intraoperative factors and postoperative outcomes with total incision length (TIL) for each of the three operations studied: robotic mitral valve repair (MVR), robotic minimally invasive direct coronary artery bypass grafting (MIDCAB), and totally endoscopic coronary artery bypass grafting (TECAB).
Values are measured in centimeters (cm) and represented with median and range.
Statistically significant results (p < 0.05) are highlighted in bold.
Figure 5
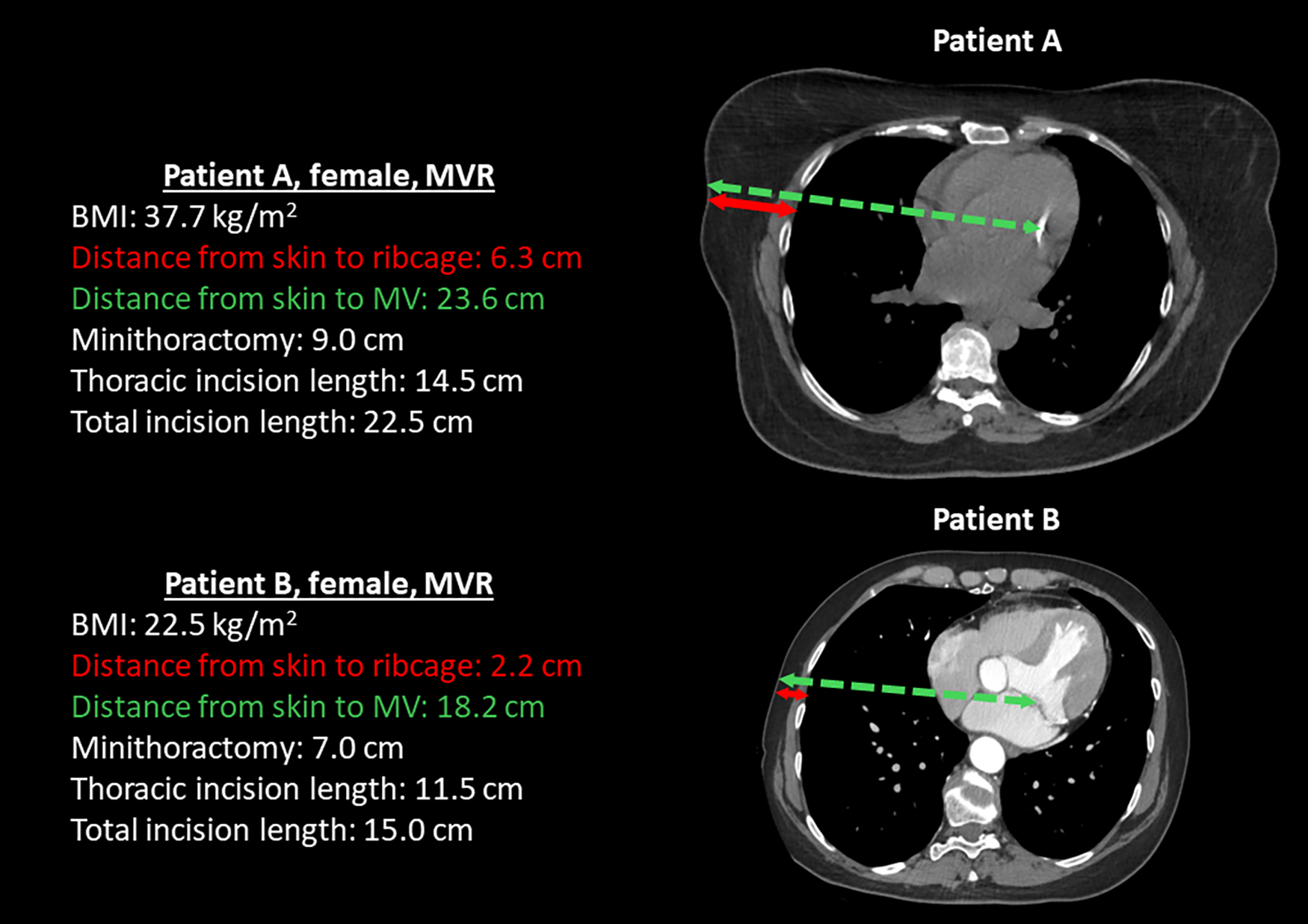
Representative CT scans of two female patients who underwent mitral valve repair (MVR), illustrating anatomical differences between an obese patient (patient A) and a non-obese patient (patient B). The scans highlight variations in soft tissue thickness, the distance to the mitral valve, and the corresponding incision lengths required for surgical access.
Incision length and postoperative outcomes
Increased total incision length was significantly associated with increased postoperative hospital length of stay (R = 0.229, p = 0.001, Figure 6). Additionally, longer incision lengths correlated with increased ventilation time (R = 0.197, p = 0.005), and an increased number of units of blood transfusions (R = 0.227, p = 0.019). The total incision length was 17.0 (10.5–40.5) cm in the patients who had reached full activity at the 4 weeks postoperative visit and 16.5 (10.0–62.0) cm in those who had not reached full activity level at the same time point (p = 0.153).
Figure 6
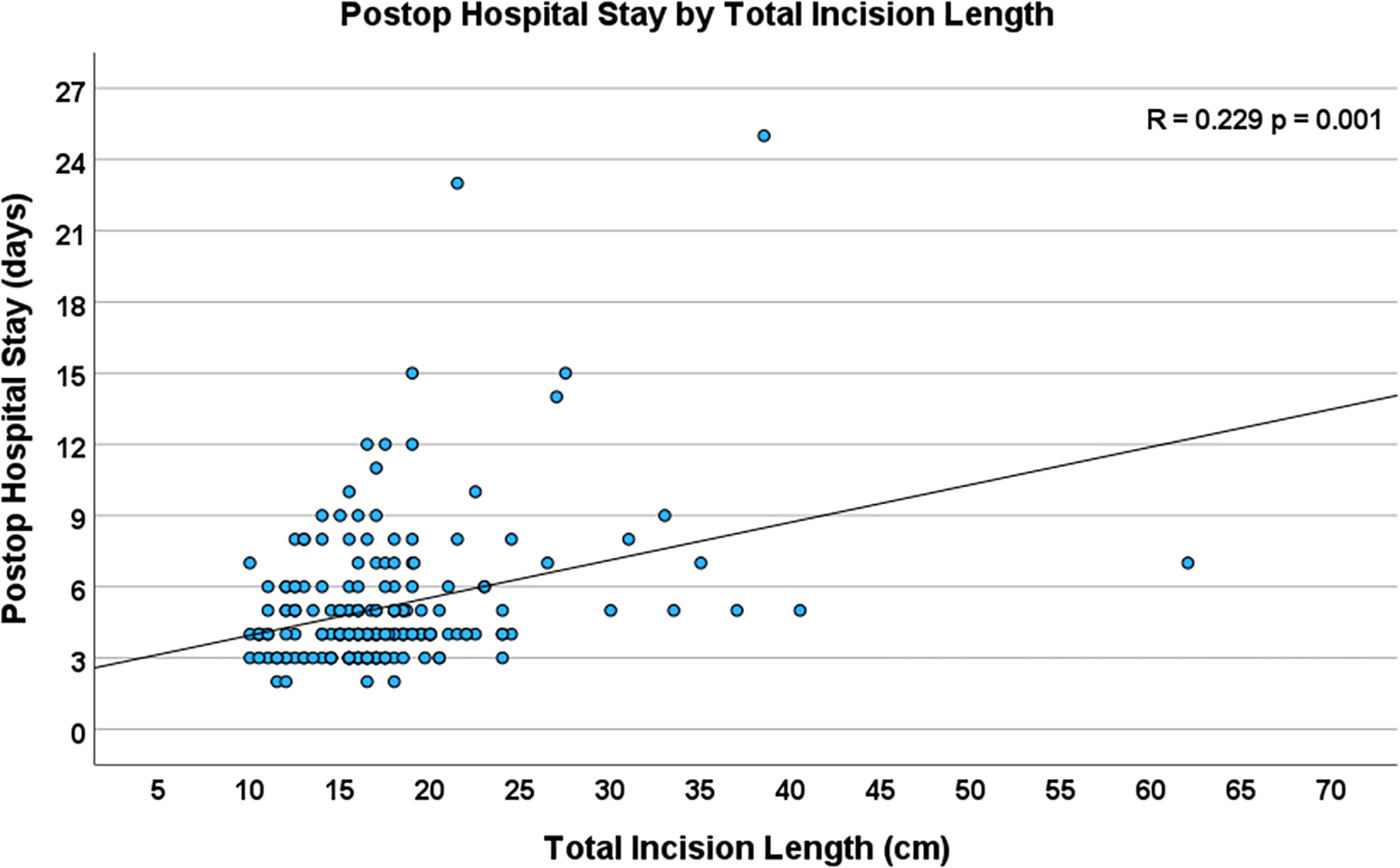
Scatterplot depicting the correlation between total incision length and total postoperative hospital stay (n = 204, R = 0.229, p = 0.001).
Table 5 presents both the univariate and multivariate predictors of TIL, along with the univariate and multivariate associations between TIL and postoperative outcome variables. It also includes a sub analysis of patients who did not undergo conversion to sternotomy.
Table 5
| Variable | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| p-value | p-value | |
| Predictors of total incision length (TIL) | ||
| Demographics | ||
| Weight (kg) | 0.011 | 0.433 |
| BMI (kg/m2) | <0 . 001 | 0.003 |
| BSA (m2) | 0.011 | 0.376 |
| Risk factors | ||
| Smoking | 0.039 | 0.466 |
| FEV1% predicted | 0.008 | 0.517 |
| DLCO % predicted | 0.037 | 0.898 |
| Intraoperative variables | ||
| Procedure type | <0 . 001 | <0 . 001 |
| Complex procedure | 0.012 | 0.648 |
| Any technical challenge | 0.044 | 0.034 |
| Conversion to sternotomy | <0 . 001 | <0 . 001 |
| CPB time | 0.001 | 0.856 |
| Myocardial ischemic time | 0.003 | 0.099 |
| Total procedure time | <0 . 001 | <0 . 001 |
| TIL association with postoperative outcome | ||
| Postoperative variables | ||
| Ventilation time | 0.005 | 0.089 |
| Number of blood transfusions | 0.019 | 0.004 |
| Hospital stay | 0.001 | <0.001 |
Univariate and multivariate analysis of predictors of total incision length (TIL) as well as the univariate and multivariate associations between TIL and postoperative outcome variables.
Multivariate predictors remained statistically significant even when patients who underwent conversion to sternotomy were excluded.
Statistically significant results (p < 0.05) are highlighted in bold.
Discussion
Preoperative variables
Before conducting this retrospective study, we hypothesized that anatomical factors, such as body habitus, would strongly influence the length of these incisions. Our work suggests correlations between BMI and total incision length in robotic cardiac surgery. This finding was observed by Brunaud et al., where they identified a positive correlation between incision length and body mass index in standard thyroidectomy and parathyroidectomy procedures (13). While our literature search did not reveal corresponding studies in cardiac surgery publications, we found multiple articles describing how BMI influenced postoperative outcomes, including increased mortality, morbidity, and cost (14, 15). Notably, one smaller study found that robotic mitral valve surgery was no less safe to perform in patients with a BMI over 30 compared to those with a BMI under 30 (16). While there seems to be some conflicting evidence, the literature generally leans towards a higher BMI negatively influencing outcomes, with our study suggesting longer incisions and increased tissue trauma in this patient population. We also found that total incision length correlated positively with weight and body surface area. Additionally, on univariate analysis, we noted a longer TIL in patients with impaired lung function, namely lower FEV1 (% of predicted) and DLCO. We could not identify any papers in the literature elaborating on this finding.
Intraoperative variables
The final lengths of the port incisions were found to range between 1 and 3 cm, despite the inserted metal robotic ports being 0.8 cm in diameter. Possible explanations for this include the surgeon initially making a slightly longer incision, the movement of the metal port extending the incision during procedures requiring greater arm mobility, and enlarging the port incision due to bleeding from the port. The literature does not specifically expand on this question, but the reported estimates for port sizes are in a similar range of our measurements (9–12).
It was interesting for us to observe that the right instrument port in MIDCAB and TECAB was significantly longer than in robotic MVR. A potential explanation is that this port travels through the pectoralis major muscle, which is well vascularized and tends to bleed, and occasionally port enlargements are necessary. In addition, the excursions of the right instrument in robotic coronary bypass surgery are more extensive than in robotic mitral surgery in which the workspace is relatively confined.
Additionally, the MIDCAB minithoracotomy was, on average, 2 cm longer than the corresponding minithoracotomy for robotic MVR. We believe that this occurs because in robotic MVR, the surgeon operates within a very narrow, cone-shaped operative field that has little variability between patients, whereas in robotic MIDCAB, the anatomical location of a patient's target vessels can be considerably variable.
It was anticipated to find variations in incision length among the three operations studied. TECAB, performed entirely endoscopically through ports, showed the shortest total incision length. In contrast, both MIDCAB and robotic MVR, which involve a minithoracotomy, resulted in similar, longer incisions.
Early and late publications on robotic mitral valve surgery do not present precise lengths of incisions. For example, the primary right minithoracotomy in MVR has been reported from 3 to 8 cm in length (1–5). A 3–6 cm incision is similarly characterized for the left minithoracotomy in MIDCAB (6, 7). Cannulation incisions are also infrequently discussed. In one of the first published series of mitral valve repair, Nifong states a 5–6 cm minithoracotomy in robotic MVR, and reports a 2 cm groin incision for cannulation (4).
Incisions in robotic mitral valve repair are likely slightly longer than those in classic videoscopic non-robotic mitral valve repair because, in the latter, the surgeon uses only one port for the camera in addition to the minithoracotomy. In robotic procedures, three additional ports are placed: one for the right instrument, one for the left instrument, and one for the left atrial retractor. We believe that the added level of precision in robotic approaches justifies these extra port incisions. In our literature search for this study, we did not find precise measurements for the robotic port incisions in robotic TECAB. Balkhy et al. describes placement of “standard” ports in the 2nd, 4th, and 6th intercostal spaces, which on the robotic system he used corresponds to three 8 mm incisions. In addition, he inserted a 12 mm subcostal port and a 15 mm port in the 2nd intercostal space for insertion the Flex A automated anastomotic device (10). Yang et al. in a series of robotic coronary bypass surgery reports three 0.8–1 cm incisions for the camera and two instrument ports (12). Overall, for robotic ports, which TECAB uses exclusively, publications describe the diameters as ranging from 0.5 to 2 cm (8–12).
One of our key findings was that as operation times increased, the total incision length also increased. Lee et al. investigated this relationship in laparoscopic vs. single-incision cholecystectomy and, like us, found that longer incisions were associated with significantly longer operating times (17). Similarly, the research by Brunaud and colleagues on thyroid and parathyroid resection considered the relationship of operation time and incision length and found a significant correlation with the duration of the procedure (13). On the other hand, Chung et al. studied traditional vs. mini-incision total hip replacement and found some similar outcomes for the shorter incision operation, such as a shorter length of hospital stay, but they did not state that longer incisions were associated with significantly longer operating times (18).
Brunaud's group attributed increases in incision lengths to the surgical complexity and case-specific technical difficulties (13). Similarly, we observed a slight increase in total incision length (TIL) in patients who underwent complex robotic coronary bypass surgery or mitral valve repair. Our study also indicated that longer operating times are more likely to occur when technical challenges are encountered. These challenges may require additional incisions to be made, or previously made incisions might need to be lengthened to achieve adequate vision and space for addressing the issues. These problems varied in complexity, with the most common consisting of port placement and cannulation problems, bleeding issues, unforeseen anatomical variations, intraoperative revisions of primary reconstructions e.g., for residual mitral valve regurgitation, and problems related to robotic technology. Technical challenges in surgery, even when minor, were meticulously documented in our operative reports and were present in 41% of operations. Technical challenges appeared to lead to an increase in TIL of 1 cm, which seems acceptable.
In 4.4% of patients in our series, technical challenges required the conversion of the robotic surgical approach to a traditional, midline sternotomy for patient safety and the assurance of a successful coronary revascularization or mitral valve repair. There were no conversions in our MIDCAB cases. Our findings were consistent with early studies, such as one by Glower et al., which compared incision lengths in sternotomy and port access mitral repair and found them to measure 26 cm and 8 cm, respectively (19). In comparison, the sternotomies in our conversions ranged from 15 cm to 22 cm. When placing our overall incision length of 16.5 cm in robotic cardiac surgery against the average sternotomy of 21 cm, it can be noted that robotic coronary and mitral surgery reduced the incision length by approximately 22% in our series. Although this reduction might seem less significant than anticipated, it is important to consider that the smaller incisions distribute the surgical trauma more evenly and leave the breastbone completely intact. This also does not account for the additional large incision necessary to harvest the saphenous vein commonly used in open coronary artery bypass grafting operations.
Are the predictors for TIL individually present in robotic MVR, MIDCAB, and TECAB?
The primary goal of our study was to examine robotic cardiac surgery as a whole, encompassing various procedures. A sub-analysis revealed that the univariate predictors, conversion to sternotomy and procedure time, were associated with TIL in all procedure subtypes analyzed. Multivariate testing confirmed their association with TIL, and in our opinion, both factors are plausible.
Incision length and perioperative outcomes
In a paper published in 2011, Bonatti et al. demonstrated a strong correlation between procedure time in robotic TECAB and the number of blood transfusions (20). This agrees with our finding that the number of transfusions was related to incision length and surgical trauma.
As can be estimated from our graphs, the hospital stay was approximately 5 days if the incision length was roughly between 10 cm and 15 cm. If the incision length was between 35 and 40 cm, the hospital stay was around 7 days. Multiple earlier studies comparing smaller incisions in cardiac surgery with sternotomy have demonstrated that reduced incision length resulted in shorter hospital stay (1, 19–23). Other papers, however, could not show such an effect (24, 25).
Multivariate analysis of factors
Among the preoperative factors associated with increased incision length, only obesity remained significant on multiple regression analysis. This finding suggests that patients with a higher BMI may require closer attention and preparation for potential increased surgical trauma. Procedure type was consistently a strong predictor of total incision length in multivariate testing, highlighting the need to develop fully endoscopic procedures, which resulted in the smallest TILs. Additionally, increased surgical trauma due to technical difficulties and the need for full chest opening via sternotomy were strongly associated with increased incision length in multivariate analysis. Continued refinement of procedural techniques and training methods is likely necessary to address this challenge.
Total procedure time was also associated with longer incisions in multivariate testing, suggesting that more extended surgical work was needed for these patients. The analysis revealed a correlation between longer TIL and an increased number of blood transfusions and longer hospital stays, indicating that minimizing incisional trauma could reduce postoperative morbidity in robotic cardiac surgery.
Even when patients who were converted to sternotomy were excluded, the majority of the factors we analyzed remained statistically significant. This confirms that conversion cases did not significantly skew the data, ensuring consistency in patients who completed a robotic operative course via ports or mini-incisions.
Sequelae of increased surgical incision length in experimental models
The decreased clinical trauma we observed is supported by laboratory studies that found increased surgical trauma correlated with elevated levels of inflammatory markers and activation of immune cells (26). Ioannidis et al. focused on incision length in a mouse model. They found that serum inflammatory markers, including CINC1/IL-8, TNF-α, and NO, significantly increased in mice who received incisions vs. the control group who did not have an incision. Additionally, the serum inflammatory markers increased proportionally for mice who received 10 cm incisions compared to the group who received a 1 cm incision, ultimately showing that there is a greater systemic inflammatory response that correlates with incision length (27).
Future directions
We hope that future papers report precise measurements of incision length and explore it as a variable of significance, given its importance for patient cosmesis and recovery. Furthermore, it would be desirable to further reduce the incision length in robotically assisted, minimally invasive cardiac surgery. One strategy would be to perform more totally endoscopic procedures such as the totally endoscopic approach for mitral valve repair which has been developed by Dr. Sloane Guy and colleagues. They used five 8 mm ports instead of a right minithoracotomy for robotic mitral valve repair (21). As our program was recently created, we have not yet begun performing this version of mitral valve repair but plan to do so in the future. Another strategy is to use percutaneous heart-lung machine cannulation and closure devices to eliminate the need for groin incisions. This method has also been carried out successfully in the clinical setting (22, 23). Reducing the size of robotic ports will also depend on the efforts of the manufacturers of robotic devices. There are, however, challenges in reducing the port and instrument diameters as the function and strengths of the robotic instruments may be impaired. It will be up to the surgeon and their team to consciously reduce the size of the incisions to the smallest possible length while respecting all aspects of patient safety and procedure efficacy. As any robotic program matures, a reduction in technical difficulties and conversions to sternotomy can be expected. Although there is an obvious difference in incision length between approaches, it may be reasonable to advocate for incision length to become a mandatory metric in studies comparing minimally invasive and conventional cardiac surgery. Based on our findings, greater awareness of incision length—both within surgical teams and the broader cardiac surgery community—would be beneficial. One potential direction for future work is to compare the preoperatively marked incision length with the final intraoperative length achieved. Incorporating these metrics into robotic cardiac surgery databases would help standardize reporting and support more meaningful comparisons across techniques.
Limitations
Our study is a small, single-center, single-surgeon, retrospective analysis without a control group. Low risk and anatomically well-suited patients were primarily selected for the robotic approach. Although the primary surgeon was experienced in robotic cardiac surgery, a new robotic cardiac surgery program was installed and the associated team learning effects were present.
Conclusions
We conclude that the primary incisions for robotic cardiac surgery are relatively small, but additional access for robotic ports and cannulation lead to a considerable total incision length. However, total incision length is still markedly shorter than a standard sternotomy, the surgical trauma is dispersed, and the sternum remains intact. Total incision length is strongly dependent on procedure type, with the totally endoscopic approach exhibiting the shortest overall measurement. Incisions are generally longer in obese patients. Technical difficulties, conversions to sternotomy, and total procedure time are strongly correlated with total incision length. According to our data, longer incisions are associated with increased need for blood transfusions and longer hospital stay. These results remain consistent even when patients who were converted to sternotomy are excluded from analysis.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Pittsburgh Institutional Review Board (IRB #21080141). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TR: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. AJ: Data curation, Writing – review & editing. MW: Data curation, Writing – review & editing. KP: Data curation, Writing – review & editing. SA: Data curation, Writing – review & editing. KD: Data curation, Writing – review & editing. NH: Data curation, Writing – review & editing. RD: Data curation, Writing – review & editing. SW: Writing – review & editing. DK: Writing – review & editing. IS: Writing – review & editing. JB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was approved by the University of Pittsburgh Institutional Review Board (IRB #21080141) and patients gave informed consent.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TIL, total incision length; TECAB, totally endoscopic coronary artery bypass grafting; MIDCAB, minimally invasive direct coronary artery bypass grafting; MVR, mitral valve repair; CPB, cardiopulmonary bypass; BMI, body mass index; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity of the lungs for carbon monoxide; LIMA, left internal mammary artery; LAD, left anterior descending artery; MANOVA, multivariate analysis of variance.
References
1.
Folliguet T . Mitral valve repair robotic versus sternotomy. Eur J Cardiothorac Surg. (2006) 29(3):362–6. 10.1016/j.ejcts.2005.12.004
2.
Gillinov AM Mihaljevic T Javadikasgari H Suri RM Mick SL Navia JL et al Early results of robotically assisted mitral valve surgery: analysis of the first 1,000 cases. J Thorac Cardiovasc Surg. (2018) 155(1):82–91.e2. 10.1016/j.jtcvs.2017.07.037
3.
Musumeci F Mariscalco G Ranocchi F Tosi D Persichetti P . Transareolar robotic-assisted access to the mitral valve. Innovations. (2015) 10(6):438–40. 10.1097/imi.0000000000000213
4.
Nifong LW Chitwood WR Pappas PS Smith CR Argenziano M Starnes VA et al Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg. (2005) 129(6):1395–404. 10.1016/j.jtcvs.2004.07.050
5.
Reichenspurner H Boehm DH Welz A Schmitz C Wildhirt S Schulze C et al Minimally invasive coronary artery bypass grafting: port-access approach versus off-pump techniques. Ann Thorac Surg. (1998) 66(3):1036–40. 10.1016/S0003-4975(98)00706-1
6.
Marin-Cuartas M Sá MP Torregrossa G Davierwala PM . Minimally invasive coronary artery surgery: robotic and nonrobotic minimally invasive direct coronary artery bypass techniques. JTCVS Techniques. (2021) 10:170–7. 10.1016/j.xjtc.2021.10.008
7.
Gofus J Cerny S Shahin Y Sorm Z Vobornik M Smolak P et al Robot-assisted vs. conventional MIDCAB: a propensity-matched analysis. Front Cardiovasc Med. (2022) 9:943076. 10.3389/fcvm.2022.943076
8.
Bonatti J Schachner T Bonaros N Oehlinger A Ruetzler E Friedrich G et al How to improve performance of robotic totally endoscopic coronary artery bypass grafting. Am J Surg. (2008) 195(5):711–6. 10.1016/j.amjsurg.2007.11.010
9.
Mandal K Alwair H Nifong WL Chitwood WR . Robotically assisted minimally invasive mitral valve surgery. J Thorac Dis. (2013) 5(Suppl 6):S694–703. 10.3978/j.issn.2072-1439.2013.11.01
10.
Balkhy HH Wann LS Krienbring D Arnsdorf SE . Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg. (2011) 92(3):821–7. 10.1016/j.athoracsur.2011.04.103
11.
Folliguet TA Dibie A Philippe F Larrazet F Slama MS Laborde F . Robotically-assisted coronary artery bypass grafting. Cardiol Res Pract. (2010) 2010:175450. 10.4061/2010/175450
12.
Yang M Wu Y Wang G Xiao C Zhang H Gao C . Robotic total arterial off-pump coronary artery bypass grafting: seven-year single-center experience and long-term follow-up of graft patency. Ann Thorac Surg. (2015) 100(4):1367–73. 10.1016/j.athoracsur.2015.04.054
13.
Brunaud L Zarnegar R Wada N Ituarte P Clark OH Duh QY . Incision length for standard thyroidectomy and parathyroidectomy: when is it minimally invasive?Arch Surg. (2003) 138(10):1140–3. 10.1001/archsurg.138.10.1140
14.
Wu W Ding R Chen J Yuan Y Song Y Yan M et al Effect of body mass index on clinical outcomes after robotic cardiac surgery: is there an obesity paradox? BMC Cardiovasc Disord. (2023) 23(1):271. 10.1186/s12872-023-03277-w
15.
Ghanta RK LaPar DJ Zhang Q Devarkonda V Isbell JM Yarboro LT et al Obesity increases risk-adjusted morbidity, mortality, and cost following cardiac surgery. J Am Heart Assoc. (2017) 6(3):e003831. 10.1161/JAHA.116.003831
16.
Senay S Cacur O Bastopcu M Gullu AU Kocyigit M Alhan C . Robotic mitral valve operations can be safely performed in obese patients. J Card Surg. (2021) 36(9):3126–30. 10.1111/jocs.15758
17.
Lee PC Lo C Lai PS Chang JJ Huang SJ Lin MT et al Randomized clinical trial of single-incision laparoscopic cholecystectomy versus minilaparoscopic cholecystectomy. Br J Surg. (2010) 97(7):1007–12. 10.1002/bjs.7087
18.
Chung W Liu D Foo L . Mini-Incision total hip replacement—surgical technique and early results. J Orthop Surg (Hong Kong). (2004) 12(1):19–24. 10.1177/230949900401200105
19.
Glower DD Landolfo KP Clements F Debruijn NP Stafford-Smith M Smith PK et al Mitral valve operation via port access versus median sternotomy1. Eur J Cardiothorac Surg. (1998) 14(Supplement_1):S143–7. 10.1016/S1010-7940(98)00123-7
20.
Bonatti J Schachner T Wiedemann D Weidinger F Kolbitsch C Knotzer H et al Factors influencing blood transfusion requirements in robotic totally endoscopic coronary artery bypass grafting on the arrested heart. Eur J Cardiothorac Surg. (2011) 39(2):262–7. 10.1016/j.ejcts.2010.05.035
21.
Yost CC Rosen JL Wu M Komlo CM Goldhammer JE Guy TS . How I perform totally endoscopic robotic mitral valve repair. Ann Cardiothorac Surg. (2022) 11(6):629–31. 10.21037/acs-2022-rmvs-16
22.
Bonatti J Kiaii B Alhan C Cerny S Torregrossa G Bisleri G et al The role of robotic technology in minimally invasive surgery for mitral valve disease. Expert Rev Med Devices. (2021) 18(10):955–70. 10.1080/17434440.2021.1960506
23.
Amabile A Morrison A LaLonde M Agarwal R Mori M Hameed I et al Two hundred robotic mitral valve repair procedures for degenerative mitral regurgitation: the Yale experience. Ann Cardiothorac Surg. (2022) 11(5):52532. 10.21037/acs-2022-rmvs-73
24.
Halkos ME Vassiliades TA Myung RJ Kilgo P Thourani VH Cooper WA et al Sternotomy versus nonsternotomy LIMA-LAD grafting for single-vessel disease. Ann Thorac Surg. (2012) 94(5):1469–77. 10.1016/j.athoracsur.2012.05.049
25.
Paparella D Fattouch K Moscarelli M Santarpino G Nasso G Guida P et al Current trends in mitral valve surgery: a multicenter national comparison between full-sternotomy and minimally-invasive approach. Int J Cardiol. (2020) 306:147–51. 10.1016/j.ijcard.2019.11.137
26.
Aosasa S Ono S Mochizuki H Tsujimoto H Osada S Takayama E et al Activation of monocytes and endothelial cells Depends on the severity of surgical stress. World J Surg. (2000) 24(1):10–6. 10.1007/s002689910003
27.
Ioannidis A Arvanitidis K Filidou E Valatas V Stavrou G Michalopoulos A et al The length of surgical skin incision in postoperative inflammatory reaction. JSLS. (2018) 22(4):e2018.00045. 10.4293/JSLS.2018.00045
Summary
Keywords
cardiac surgery & percutaneous cardiovascular interventions, minimally invasive & robotic surgery, incision length, coronary artery surgery, clinical outcomes, valve surgery, TECAB, MIDCAB
Citation
Rubino TE Jr, Jackson A, Winter M, Punu K, Ashraf SF, Dufendach K, Hess N, Deitz R, Waterford SD, Kaczorowski D, Sultan I and Bonatti J (2025) Small cuts, big questions: the impact of incision length in minimally invasive robotic cardiac surgery. Front. Cardiovasc. Med. 12:1575779. doi: 10.3389/fcvm.2025.1575779
Received
12 February 2025
Accepted
10 June 2025
Published
26 June 2025
Volume
12 - 2025
Edited by
Gabor Erdoes, University Hospital of Bern, Switzerland
Reviewed by
Gianluigi Bisleri, University of Toronto, Canada
Alfred Kocher, Medical University of Vienna, Austria
Updates
Copyright
© 2025 Rubino, Jackson, Winter, Punu, Ashraf, Dufendach, Hess, Deitz, Waterford, Kaczorowski, Sultan and Bonatti.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Johannes Bonatti bonattijo@upmc.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.