Abstract
Atrial fibrillation (AF) is a complex arrhythmia driven by intricate pathophysiological mechanisms, with atrial fibrosis and inflammation emerging as central players in its initiation and perpetuation. Key pathways, including the renin-angiotensin-aldosterone system (RAAS), TGF-β/Smad signaling, and pro-inflammatory cytokine cascades (e.g., TNF-α/NF-κB, IL-6/STAT3), contribute to fibrotic remodeling and electrophysiological dysfunction. These pathways promote extracellular matrix deposition, fibroblast activation, and heterogeneous conduction, creating a substrate for AF maintenance. Contemporary therapeutic approaches predominantly target rhythm control via catheter ablation techniques and pharmacological interventions with antiarrhythmic agents. Nevertheless, the efficacy of anti-inflammatory approaches, such as corticosteroids and colchicine, remains uncertain due to limited robust clinical evidence, highlighting the need for further investigation. Advanced fibrosis quantification modalities, particularly late gadolinium-enhanced magnetic resonance imaging and electroanatomic mapping, have emerged as valuable tools for optimizing ablation strategies. Furthermore, emerging evidence highlights significant sex-based disparities in atrial fibrosis distribution and electrophysiological substrate characteristics, suggesting the potential for gender-specific therapeutic approaches. This comprehensive review systematically examines the pathophysiological roles of atrial fibrosis and inflammation in AF progression, with particular emphasis on their intricate bidirectional relationship. Through detailed elucidation of these mechanistic interactions, we aim to facilitate the development of novel therapeutic interventions to enhance clinical management of AF.
1 Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, characterized by irregular atrial electrical activity and ineffective atrial contractions, leading to decreased cardiac function. More than 37.5 million people worldwide suffer from AF. In the past 20 years, the global incidence and prevalence of AF have both increased by more than 30%, and it is expected to continue to increase in the next 30 years (1). In China, there are approximately 7.9 million patients with AF, with a weighted prevalence of 1.8% (2). AF can significantly increase the risk of death, stroke, heart failure (HF), cognitive dysfunction, and dementia, seriously affecting the quality of life of patients (3–5), and causing a huge burden on the health and economy of patients. Catheter ablation and antiarrhythmic drugs (e.g., amiodarone and flecainide), have emerged as cornerstone therapeutic modalities for atrial fibrillation (AF) management (6).
As a complex heterogeneous arrhythmia, the occurrence, persistence, and occurrence of complications of AF involve multiple factors. The main pathogenesis of AF includes the presence of atrial ectopic electrical activity and reentry, involving atrial electrophysiological and structural remodeling (7, 8). Atrial fibrosis is a characteristic change in atrial structural remodeling, which can cause heterogeneous conduction in the atrium, leading to unidirectional conduction block and reentry, thereby triggering AF. At the same time, long-term AF can exacerbate atrial fibrosis, further promoting the progression and maintenance of AF, known as “AF promoting AF” (9, 10). Inflammation is involved in the pathological process of various cardiovascular diseases and is the main regulatory factor of repair response after cardiac injury (11, 12). A large amount of evidence supports the close relationship between inflammation and AF. The atrial electrophysiological and structural remodeling mediated by inflammatory response are important risk factors for inducing AF, and the activity of AF itself can also induce inflammatory response, forming the “AF promoting AF” cycle (13, 14). Since both fibrosis and inflammation play important roles in atrial remodeling, what is the relationship between the two? The crosstalk between fibroblasts and immunocytes demonstrates the interaction between fibrosis and inflammation, which together promote atrial remodeling, leading to the occurrence and persistence of AF (15). This article will review the roles of atrial fibrosis and inflammation in the pathophysiological mechanisms of AF, their relationship, and corresponding treatment methods to provide a theoretical basis for the clinical management of AF.
2 Atrial fibrosis and AF
2.1 Atrial fibrosis
Atrial remodeling plays a central role in the occurrence and development of AF, and atrial fibrosis is one of the key factors in atrial remodeling (16). Atrial fibrosis is a process of cardiac remodeling caused by the interaction of multiple neurohormonal mediators, characterized by abnormal activation, proliferation, and differentiation of cardiac fibroblasts, as well as excessive deposition of extracellular matrix (ECM) proteins (17). Fibroblasts are the main cells that regulate the synthesis and composition of ECM. Fibroblasts are the most numerous cells in the heart, accounting for approximately 75% of all heart cells (18). When various harmful stimuli cause myocardial injury, fibroblasts migrate to the damaged area, proliferate, and transform into the phenotype of myofibroblasts. The contractility of myofibroblasts is enhanced through the secretion of contractile proteins such as alpha-smooth muscle actin (α-SMA), which participate in cardiac injury repair. However, sustained damage may overactivate fibroblasts, causing them to continuously synthesize ECM, leading to excessive deposition of ECM, collagen proportional imbalance, especially the increase in the proportion of type I and III collagen, and disordered collagen alignment, ultimately developing into progressive fibrosis (19–21).
Myocardial fibrosis is divided into two different types, namely reparative fibrosis and interstitial fibrosis. Reparative fibrosis refers to the replacement of necrotic cardiomyocytes with fibrosis tissue, with the most obvious example being myocardial infarction (MI) scars. Interstitial fibrosis refers to the abnormal accumulation of ECM around the interstitium and blood vessels without significant cardiomyocyte loss, which is more common in non-ischemic cardiomyopathy (22–24).
Atrial fibrosis is typically considered a type of myocardial fibrosis. But in fact, this view is problematic. The experimental results on congestive heart failure (CHF) canine model indicated that the AF substrate of CHF was associated with widespread cell death (25) and fibrosis disruption of muscle bundle continuity (26), leading to longitudinal conduction disorders. Another study has shown that the thicker the left atrial interstitial collagen strands in patients with AF, the longer the duration of AF, and the faster the longitudinal conduction velocity. This suggested that the structure and severity of AF were related to atrial conduction abnormalities (27). Therefore, for atrial fibrosis that occurs in AF, these two different types of fibrosis may coexist.
2.2 Relationship between atrial fibrosis and AF
AF is a complex and progressive disease that requires triggering and susceptible substrates for its occurrence and maintenance. The current research has found that the triggering sites of AF mainly include the atrial sleeves of the pulmonary veins (PVs) and long-standing rotors with fibrillatory conduction. Intracellular Ca2+ handling and autonomic nerve activation can promote early afterdepolarization (EAD) and delayed afterdepolarization (DAD) activities, which induce ectopic focal discharges in PVs, leading to AF (28, 29). And the rotor is another possible trigger site for AF, which is composed of heterogeneity in the form of spatially distributed refractory gradients in the atrium. The waves emitted by the high-speed rotation of the rotor can cause turbulent electrical activation, manifested as fibrillatory conduction, thereby triggering AF (28, 30).
In addition, atrial fibrosis, as a susceptible substrate to AF, plays a crucial role in the sustained development of AF. The landmark DECAAF study, a multicenter prospective observational cohort investigation involving 260 patients with both paroxysmal and persistent AF, demonstrated a significant correlation between atrial fibrosis extent and AF recurrence risk. The quantitative assessment of atrial fibrosis in patients showed that for every 1% increase in fibrosis degree, the risk of AF recurrence increased by 6%. The degree of atrial fibrosis was an independent predictor of AF recurrence (31). Additionally, extensive preclinical studies using various animal models have further substantiated the pivotal role of atrial fibrosis in AF initiation and maintenance. Both HF and chronic mitral regurgitation (MR) dog models exhibited significant interstitial fibrosis, which induced and maintained AF by causing local conduction interference (32, 33). In a goat model with cardiac specific overexpression of transforming growth factor beta 1 (TGF-β1), increased atrial fibrosis, progressive P-wave prolongation, and slowed atrial conduction were observed, leading to increased AF susceptibility (34). Meanwhile, a study on a transgenic mouse model of atrial fibrosis induced by TGF-β1 overexpression demonstrated that fibrosis could enhance atrial conduction heterogeneity, making reentry more likely to occur, thereby promoting the progression and maintenance of AF (35). The above studies involving patients, large animal models, and transgenic animal models showed that atrial fibrosis increased AF susceptibility and the risk of AF recurrence. Atrial fibrosis can cause and maintain AF by altering the atrial conductibility, leading to local conduction block and reentry.
In fact, atrial fibrosis may also be a result of AF. Clinical pathological examinations reveal that approximately 17% of patients with lone AF demonstrate patchy fibrosis patterns on atrial biopsy (36). Additionally, experimental investigations utilizing canine rapid atrial pacing models revealed significantly augmented interstitial fibrosis in AF-induced animals relative to control cohorts (37). Similarly, a dog model study aimed at exploring the impact of AF on electrophysiology showed that AF without ventricular dysfunction lead to atrial fibrosis and increased susceptibility to AF, while AF with rapid ventricular response increased atrial and ventricular fibrosis (38). In summary, atrial fibrosis is both a triggering factor and a result of AF, playing a crucial role in its occurrence and sustained development.
2.3 Profibrotic substrate
Major contributors to advancing atrial fibrosis and their mechanistic pathways are summarized (Figure 1).
Figure 1
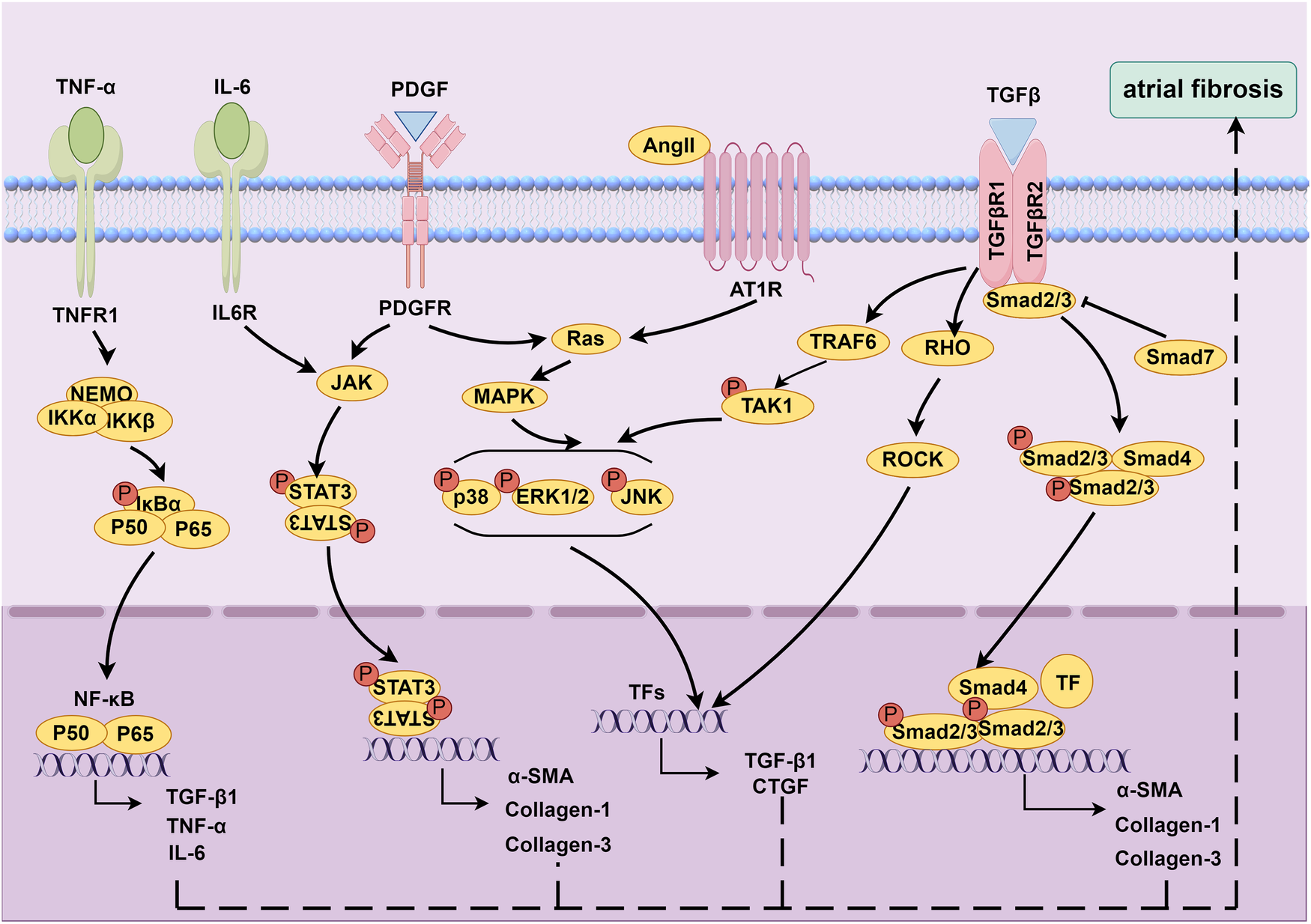
Molecular mechanisms underlying atrial fibrosis. Created using Figdraw, www.figdraw.com.
2.3.1 Renin-angiotensin-aldosterone system (RAAS)
RAAS is a hormone cascade reaction primarily responsible for regulating blood pressure and water-salt balance, maintaining homeostasis in the human body (39). RAAS is involved in the fibrosis process of various diseases, such as hypertension (40), CHF (41), and MI (42). Angiotensin II (Ang II) is a key molecule in this system and plays an important role in atrial fibrosis (39, 43, 44). A study suggested that the occurrence of atrial fibrosis in CHF dog models was associated with increased Ang II concentration (44). Moreover, a mouse model overexpressing angiotensin converting enzyme (ACE) showed atrial dilation, focal fibrosis, and AF (43). In addition, blocking the effect of Ang II with ACE inhibitors (ACEIs) can reduce atrial fibrosis (44, 45). Regarding the mechanism of Ang II promoting fibrosis, previous studies have confirmed that after binding to Angiotensin II type 1 receptor (AT1R), Ang II stimulated fibroblast proliferation and differentiation by activating the phosphorylation cascade of mitogen-activated protein kinase (MAPK) (46, 47). After activating the MARK cascade with Ang II, atrial fibrosis could be mediated by upregulating the expressions of TGF-β1 (48–52) and connective tissue growth factor (CTGF) (49, 53, 54).
2.3.2 TGF-β1
TGF-β1 is an important profibrotic cytokine. TGF-β1 can mediate the differentiation of fibroblasts into myofibroblasts and promote increased collagen secretion by activating Smad dependent or independent signaling pathways (7, 9, 55). In the classic Smad dependent signaling pathway, TGF-β1 binds to type I and type II receptors, activating downstream Smad2/3/4 proteins and promoting increased collagen secretion (56). TGF-β1 can also reduce the negative feedback regulation of TGF -β1/Smad signaling by inhibiting Smad7 (57, 58). The currently discovered Smad independent signaling pathways mainly include the MAPK/TGF-β1/tumor necrosis factor receptor associated factor 6 (TRAF6)/TGF-β activated kinase 1 (TAK1) signaling (59, 60) and TGF-β1/Ras homolog family member A (RhoA)/Rho-associated kinase (ROCK) (61).
2.3.3 Cytokines
Inflammatory response is closely linked to the formation of atrial fibrosis (7, 9, 55). Multiple inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin (IL) -1β, IL-2, IL-6, etc., can mediate the occurrence of atrial fibrosis. Liew et al. found that TNF-α activated fibroblasts and promoted collagen synthesis by activating the TGF-β signaling pathway and promoting the secretion of matrix metalloproteinases (MMP), thereby mediating the occurrence of atrial fibrosis in mice (62). Inhibition of the TNF-α/nuclear factor-kappaB (NF-κB)/TGF-β signaling pathway can effectively suppress myocardial fibrosis and cardiac remodeling, thereby attenuating the progression of AF (63). Meanwhile, the activation of signal transducer and activator of transcription 3 (STAT3) signaling pathway by IL-6 contributes to AF development through stimulating cardiac fibroblast activation (64). Chen et al. found that IL-6-miR-210 promoted the expressions of α-SMA, type I collagen, and type III collagen by targeting Foxp3, leading to atrial fibrosis (65). Studies have shown that epicardial adipose tissue (EAT) could secrete pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and monocyte chemoattractant protein-1 (MCP-1), which could trigger inflammation in adjacent atrial tissue through paracrine action, leading to atrial fibrosis (66–69).
2.3.4 PDGF
The platelet-derived growth factor (PDGF) family proteins are encoded by four genes, namely PDGF-A, PDGF-B, PDGF-C, and PDGF-D (70). PDGF can promote proliferation and differentiation of fibroblasts, and regulate ECM synthesis via various pathways, such as MAPK, Janus kinase (JAK)/STAT, and Ras/extracellular regulated protein kinase 1/2 (ERK1/2) (55). Different subtypes of PDGF are involved in the development of myocardial fibrosis. Studies have shown that PDGF-D promoted the proliferation and differentiation of rat cardiac fibroblasts, as well as the secretion of type I collagen, by mediating the activation of TGF-β1 signaling pathway, exerting a profibrotic effect (71). Cardiac fibrosis was observed in mice with cardiac specific overexpression of PDGF-A and PDGF-B (72). Liao et al. found that the expression of PDGF-A increased in mast cells in the atrium of mice with pressure-overloaded heart, promoting fibroblast proliferation and collagen synthesis, thereby promoting atrial fibrosis and enhancing susceptibility to AF (73). In the HF dogs induced by rapid ventricular pacing, the mRNA levels of PDGF subtypes A, C, and D in the left atrial (LA) fibroblasts increased, activating the JAK-STAT pathway, promoting ECM synthesis and LA fibrosis (74). In addition, in rat cardiac allografts, these PDGF subtypes mediated profibrotic effects by regulating the TGF-β1 signaling (75).
2.3.5 miRNA
Micro-ribonucleic acids (microRNAs, miRNAs or miRs) are a class of evolutionarily conserved non-coding small molecule RNAs, typically between 21 and 23 nucleotides in length, that can regulate gene expression at the translation level (76). Multiple studies have shown that miRNA plays an important role in atrial fibrosis and AF (22, 55, 77). Among them, miR-21 is a promising target that regulates AF and atrial fibrosis through multiple mechanisms. In a rat model of HF induced AF, the expression of atrial miR-21 was upregulated, and knocking it out could inhibit atrial fibrosis and AF development (78). The research of Adam et al. showed that compared with sinus rhythm (SR) population, miR-21 expression was upregulated in LA of AF patients. After Rac1 was activated by Ang II, the expressions of CTGF and lysyl oxidase increased, mediating the increase in miR-21 expression and the decrease in its downstream molecule Sprouty 1 (Spry 1, a protein that inhibits fibroblast proliferation) expression, leading to an increase in atrial collagen content and promoting fibrosis (79). Another study has shown that miR-21 was upregulated in fibroblasts of failing hearts and activated the ERK-MAPK signaling pathway by inhibiting Spry1, thereby promoting fibroblast proliferation and interstitial fibrosis (80). He et al. found that in a rabbit model of AF induced by rapid atrial pacing, miR-21 could also reduce the inhibitory feedback regulation of TGF-β1/Smad signaling by mediating Smad7 specific degradation, thereby promoting the development of atrial fibrosis in AF (81). In addition, in a rat model of sterile pericarditis, STAT3 and miR-21 formed a feedback loop, promoting fibroblast proliferation and increasing ECM synthesis, thereby increasing AF susceptibility (64). Other miRNAs are also involved in the process of atrial fibrosis.
For example, elevated miR-486-5p levels were detected in AF patients and correlated with increased left atrial fibrosis occurrence (82). The downregulation of miR-26 regulated the inward-rectifier potassium current in fibroblasts by increasing KCNJ2 expression, thereby promoting fibroblast proliferation and AF (83, 84). Wang et al.'s study showed that downregulation of miR-27b inhibited the Smad2/3 signaling by targeting ALK5, thereby improving Ang II induced atrial fibrosis and AF (85). MiR-29b may be involved in atrial fibrosis. In a canine model of CHF induced by rapid ventricular pacing, miR-29b expression was reduced in atrial tissue and atrial fibroblasts, accompanied by increased ECM expression in fibroblasts (86). MiR-30 and miR-133 could reduce collagen production and inhibit cardiac fibrosis by downregulating CTGF (87).
3 Inflammation and AF
3.1 Relationship between inflammation and AF
Numerous studies have shown that inflammation is involved in the occurrence and development of various cardiovascular diseases (88, 89). Regarding the link between inflammation and AF, Bruins et al. first discovered that C-reactive protein (CRP) level in patients with coronary artery disease (CAD) was associated with arrhythmia after revascularization (90). Afterwards, Chung et al. also found that serum CRP levels in patients with AF were higher than those in patients with SR, and CRP levels in patients with persistent AF were higher than those in patients with paroxysmal AF (91). Both studies suggest that inflammatory response is closely related to AF. With the continuous exploration of the relationship between inflammation and AF, the causal relationship between the two is gradually becoming clear.
3.1.1 Pathological mechanisms of inflammation promoting AF
Inflammatory response triggers and maintains AF by altering the electrophysiology and structure of atrial tissue, leading to atrial electrical and structural remodeling (13, 14, 92).
3.1.1.1 Electrical remodeling mechanisms
Regarding atrial electrical remodeling, multiple studies have shown that various inflammatory factors, such as TNF (93–96) and PDGF (97), as well as NLRP3 inflammasome (98–100), can induce atrial electrical remodeling by inducing abnormal calcium processing, triggering abnormal PV electrical activity, shortening the atrial action potential duration, leading to inflammation related AF. Moreover, the abnormal expression and distribution of atrial connexin 40 (Cx40) and Cx43 caused by inflammatory response can induce atrial heterogeneous conduction, which is an important factor in increasing susceptibility to AF (101). Studies have shown that TNF-α (102) and IL-6 (103) can cause downregulation of Cx40 and Cx43 expression, leading to abnormal atrial conduction and inducing atrial electrical remodeling. In addition, NF-κB, a transcription factor that regulates the expression of multiple inflammatory cytokines, can induce downregulation of Na+ channel expression by binding to Na+ channel promoter region, leading to atrial electrical remodeling in AF (104).
3.1.1.2 Structural remodeling mechanisms
In terms of atrial structural remodeling, various inflammation associated cytokines, such as TNF-α (62), IL-6 (65), PDGF (73, 105), galectin-3 (106), etc., can also induce the occurrence and development of AF by promoting atrial fibrosis. TNF-α induces atrial fibrosis and alters Cx40 expression by regulating the TGF-β/Smad signaling, activating fibroblasts, and promoting MMP secretion, thereby promoting the development of AF in mice (62). IL-6 can also activate the TGF-β/Smad signaling pathway, leading to cardiac fibrosis (107). In addition, a large number of immunocytes in atrial tissue can also mediate the profibrotic process (7, 108). After cardiac injury, macrophages can induce the migration, proliferation, and activation of fibroblasts, and promote ECM synthesis by producing various pro-inflammatory cytokines (such as TNF-α and IL-6), profibrotic cytokines (such as TGF-β and PDGF), and profibrotic proteases (such as MMP and chymase), thereby exerting profibrotic effects (109–111). Similarly, studies have shown that neutrophils, T cells, and mast cells also participate in the profibrotic process (108, 110).
3.1.2 Feedback mechanisms by which AF exacerbates inflammation
Conversely, AF can also induce inflammation, thereby further promoting the development of AF (13, 14, 112). A prospective study on patients with persistent AF found that after restoring and maintaining SR, the levels of high-sensitivity CRP (hs-CRP) in AF patients were significantly reduced [0.10 (SD 0.06) mg/dl vs. 0.29 (SD 0.13) mg/d1, p < 0.001] (113). Another case-control study of AF patients also showed that the levels of CRP (3.1 mg/dl vs. 1.7 mg/dl) and IL-6 (2.3 ng/ml vs. 1.5 ng/ml) were higher during AF than during SR (114). In addition, a prospective study on patients with atrial flutter also found that after radical ablation, the levels of CRP (6.28 mg/L vs. 2.92 mg/L, p = 0.028) and IL-6 (p = 0.002) in patients with atrial flutter significantly decreased (115). The above clinical studies all indicate that AF is the cause of inflammation, not the result.
In a rapid atrial pacing induced AF dog model, we observed elevated levels of TNF-a, IL-6, and CRP, shortened effective refractory period, and increased AF susceptibility (116, 117). The anti-inflammatory effect of prednisone could effectively reverse this process and significantly shorten the AF duration (118). However, the specific mechanism by which AF leads to inflammation is currently unclear. Some studies suggested that AF may trigger calcium overload in atrial myocytes, leading to programmed cell death and the release of danger-associated molecular patterns (DAMPs) to activate low-grade inflammatory responses to repair cell damage (112, 119). A study evaluating the relationship between cell free DNA (cfDNA) and AF found that in the AF HL-1 cell model, unmethylated mitochondrial cfDNA (mt-cfDNA) promoted the expression of IL-1β and IL-6, indicating that AF could induce systemic inflammation through cfDNA (120). Further in-depth research is needed on the molecular mechanisms underlying AF induced inflammation.
From this, it can be concluded that inflammation may lead to AF, and AF can also promote inflammation, forming a vicious cycle.
3.2 Systemic inflammation and AF
Many systemic diseases are associated with low-grade inflammation, which may be the source of AF associated inflammation (5, 7, 112).
3.2.1 Severe sepsis
The incidence rate of AF in sepsis patients is high (121–123). Meierhrich et al. found that CRP levels in septic shock patients remained consistently and significantly elevated before the onset of AF, which can prove that systemic inflammation is an important factor in triggering AF (122).
3.2.2 Chronic inflammatory diseases
The risk of AF was significantly increased in patients with rheumatoid arthritis (RA) (124–126). Although the underlying mechanism of RA induced AF is complex, the key factor is still systemic inflammatory response. Systemic inflammation activation can not only produce substrates for promoting AF by accelerating the development of ischemic heart disease (IHD) and CHF, but also directly trigger AF by altering atrial electrophysiology (127). A clinical study involving over 20,000 patients with autoimmune rheumatic disease (ARD) showed that high CRP level was an independent predictor of AF in ARD patients (HR 1.75, 95%CI 1.07–2.86, p = 0.04), indicating that the risk of AF in ARD patients was influenced by inflammatory responses (128). In addition, it was found in a rat model of RA that the inducibility and duration of AF were obviously increased, and the AF duration was significantly positively correlated with serum IL-6 and TNF-α levels, indicating that RA related systemic inflammation was associated with increased susceptibility to AF (129). Psoriatic patients, especially those with psoriatic arthritis, have an increased risk of developing AF (130, 131). The risk of AF was also significantly increased in patients with inflammatory bowel disease (IBD) (132, 133). A study has found that the P-wave dispersion in IBD patients, a risk factor for the development of AF, was significantly higher than that in healthy individuals (134). Another study has shown that atrial electrical conduction was delayed in IBD patients, and chronic inflammation activation might induce electrophysiological and structural changes in atrial tissue, which is the main factor leading to slowed atrial conduction velocity (135).
3.2.3 Hypertension
Hypertension is an independent risk factor for AF (136–138). Ang II is a key molecule in the RAAS system and a major mediator of hypertensive vasoconstriction. It can trigger systemic inflammatory response by stimulating the production of inflammatory cytokines, activating immunocytes, and promoting immunocyte recruitment (139). Hypertension related inflammation can induce atrial electrical and structural remodeling, thereby triggering and maintaining AF. In hypertensive sheep and rat models, an increase in atrial inflammatory infiltration was observed, which was associated with the occurrence of atrial fibrosis and remodeling (140, 141). The pathogenesis of hypertension related inflammation induced AF needs further clarification.
3.2.4 Metabolic disorders
Obesity is an important risk factor for new-onset AF in the general population and patients after cardiac surgery (142–144). Obesity can not only induce immunocyte activation and infiltration into adipose tissue (145–147), but also promote the secretion of a large number of inflammatory cytokines (148, 149). The resulting low-grade systemic inflammatory response may lead to the occurrence and development of AF (150, 151). Diabetes is also an important risk factor for AF (152, 153). Inflammation in the context of diabetes can participate in atrial electrical and structural remodeling, thus inducing AF (152, 154).
3.2.5 CAD
CAD is an important risk factor for AF (155). Some studies suggested that chronic low-grade inflammatory response caused by CAD may be a triggering factor for AF. Stellos et al. found that there were differences in the expressions of platelet-bound stromal cell-derived factor-1 (SDF-1) and plasma SDF-1 between AF patients and SR population in CAD patients, and SDF-1 was associated with inflammatory cell recruitment (156). A clinical study found that IL-6 upregulation was significantly associated with the occurrence of AF in CAD patients, indicating that IL-6 is an important biomarker for CAD associated AF (157).
3.2.6 Cardiac surgery and ablation
The systemic inflammatory response after cardiac surgery and radiofrequency catheter ablation is associated with the occurrence and recurrence of AF (158). The ARMYDA-3 study showed that postoperative high CRP level in patients receiving cardiac surgery was associated with an increased risk of AF (159). Another clinical study showed that IL-2 level in patients undergoing coronary artery bypass grafting (CABG) was associated with early postoperative AF (160). In a dog model of cardiac surgery induced inflammation, it was observed that the degree of atrial inflammation was associated with the inhomogeneity of atrial conduction and increased AF duration, which may be a factor in the early postoperative AF (161). In addition, multiple studies on the recurrence of AF after catheter ablation have confirmed that inflammatory biomarkers can serve as predictive factors for early recurrence of AF (162–164).
3.3 Inflammatory markers and AF
Inflammatory markers can predict the risk of AF and the prognosis of AF after cardioversion or ablation (165–167).
3.3.1 CRP
CRP is an acute inflammatory protein commonly used as a biomarker for infection and inflammation in clinical practice (168, 169). CRP, including hs-CRP, is currently one of the most extensively studied inflammatory biomarkers for AF. Chung et al. found that compared to individuals with SR, CRP levels were elevated in patients with AF, and CRP levels were higher in patients with persistent AF than in patients with paroxysmal AF (91). Another study by Chung et al. showed that CRP is not only associated with the presence of AF, but can also predict the risk of AF in the future (170). Studies have shown that elevated CRP was significantly associated with an increased risk of mortality in patients with AF (171). CRP can also predict the risk of recurrence of AF after electrical cardioversion, catheter ablation, or cardiac surgery (166, 167, 172–174). In addition, hs-CRP is also associated with the occurrence and persistence of AF. Studies have shown that hs-CRP level is an independent predictor of successful AF cardioversion and SR maintenance after cardioversion (175, 176).
3.3.2 Interleukins
Interleukin is a type of cytokine secreted by lymphocytes, macrophages, and other cells, which plays an important role in inflammatory responses (177). Among them, IL-6 has been relatively extensively studied in the field of AF. It was found that the increase of IL-6 was related to the increase of incidence of AF (157). Elevated IL-6 was significantly associated with increased risk of mortality in patients with AF (171). In addition, an increase in IL-6 was also associated with the prothrombotic state of AF (178). There is evidence to suggest that IL-6 could be used to predict the risk of AF after CABG (179) and the risk of AF recurrence after catheter ablation (166). Amdur et al. also found that plasma IL-6 level was an independent predictor of AF in patients with chronic kidney disease (CKD) (180). Other interleukins have also been shown to be associated with the occurrence and development of AF. Hak et al. found a direct correlation between serum IL-2 levels and AF after CABG, and IL-2 could serve as a predictive indicator for early AF after CABG (160). Moreover, serum IL-2 level could be used to predict the risk of AF recurrence after cardioversion or ablation (181, 182). Li et al. found that the level of IL-8 in the serum of patients with AF was elevated (183). Liuba et al. found that plasma IL-8 levels in the femoral vein, right atrium, and coronary sinus were elevated in patients with permanent AF compared to those with paroxysmal AF (184). Studies have shown that IL-8 was a predictive factor for new-onset AF in CAD patients after CABG (185–187). In addition, there is evidence to suggest that IL-1, IL-10, IL-18, etc. are also associated with AF (92, 112).
3.3.3 TNF-α
TNF-α is a multifunctional pro-inflammatory cytokine that plays an important role in local and systemic inflammatory responses (188). Compared with individuals with SR, patients with AF had elevated levels of TNF-α (189), and the same phenomenon has also been observed in the context of valvular disease (190). In addition, the levels of TNF-α increased sequentially in patients with paroxysmal, persistent, and permanent AF (183). The above studies all indicate a close correlation between TNF-α levels and AF.
3.3.4 Immunocyte population
White blood cell (WBC) count and neutrophil-to-lymphocyte ratio (NLR) are also common biomarkers of AF inflammation. Weymann et al. found that both WBC count and NLR were potential predictors for new-onset and recurrent AF (191). The Framingham Heart Study results showed a significant correlation between an increase in WBC count and AF events (192). Studies have shown that an increase in WBC count was an independent predictive factor of AF after cardiac surgery (193–195). In addition, after electrical cardioversion for persistent AF, the WBC count of patients maintaining SR was significantly reduced compared to those with early AF recurrence (196). And NLR can not only predict the risk of new-onset AF, but also predict the risk of recurrence of AF after cardiac surgery, radiofrequency ablation, and cardioversion (197–199).
3.3.5 Others
MCP-1 is also an important pro-inflammatory cytokine that plays a crucial role in the occurrence and development of inflammation (200). Studies have shown that MCP-1 level was significantly increased in patients with AF (183, 201). Myeloproxidase (MPO) is a heme-containing protease secreted by neutrophils, which can participate in regulating the body's inflammatory response (202). There were studies confirming that patients with high MPO levels in paroxysmal AF had an increased risk of AF recurrence after catheter ablation (164, 203). Heat shock protein (HSP) is an important molecular chaperone protein in the body that can exert anti-inflammatory effects to protect the body from inflammatory damage (204). Currently, research has found that HSP27 and HSP70 can predict postoperative AF recurrence, and their mechanisms may be related to inflammation (205–207).
4 Relationship between atrial fibrosis and inflammation
As the two main factors that induce and maintain AF, atrial fibrosis and inflammation are closely related (208, 209). As mentioned earlier, various pro-inflammatory cytokines and activated immunocytes can mediate the occurrence of atrial fibrosis through multiple mechanisms (7, 62, 108). Activated cardiac fibroblasts during fibrosis can also enhance local inflammatory responses by releasing inflammation associated cytokines and growth factors, and recruiting and activating more immunocytes (210, 211). At the level of molecular mechanism, the crosstalk between fibroblasts and immunocytes provides a good explanation for the self-sustaining relationship between fibrosis and inflammation: in damaged hearts, inflammatory cells can trigger the proliferation and differentiation of fibroblasts into myofibroblasts by releasing a large amount of inflammatory mediators; Conversely, myofibroblasts can also produce a large amount of collagen and chemokines, which further activate inflammatory cells and attract other immunocytes to enhance cardiac inflammatory response (15, 212, 213). In addition, there is clinical evidence supporting the view that there is a link between atrial fibrosis and inflammation. A study on evaluating left atrial remodeling in non-valvular AF showed that compared with SR patients, AF patients had significantly higher levels of NLR and hs-CRP, and NLR showed a highly significant correlation with LA volume index, indicating that AF inflammatory markers were associated with atrial remodeling (214). From this, it can be seen that inflammation leads to atrial fibrosis, and atrial fibrosis enhances local inflammatory response, forming a vicious cycle that synergistically increases the risk of AF (Figure 2).
Figure 2
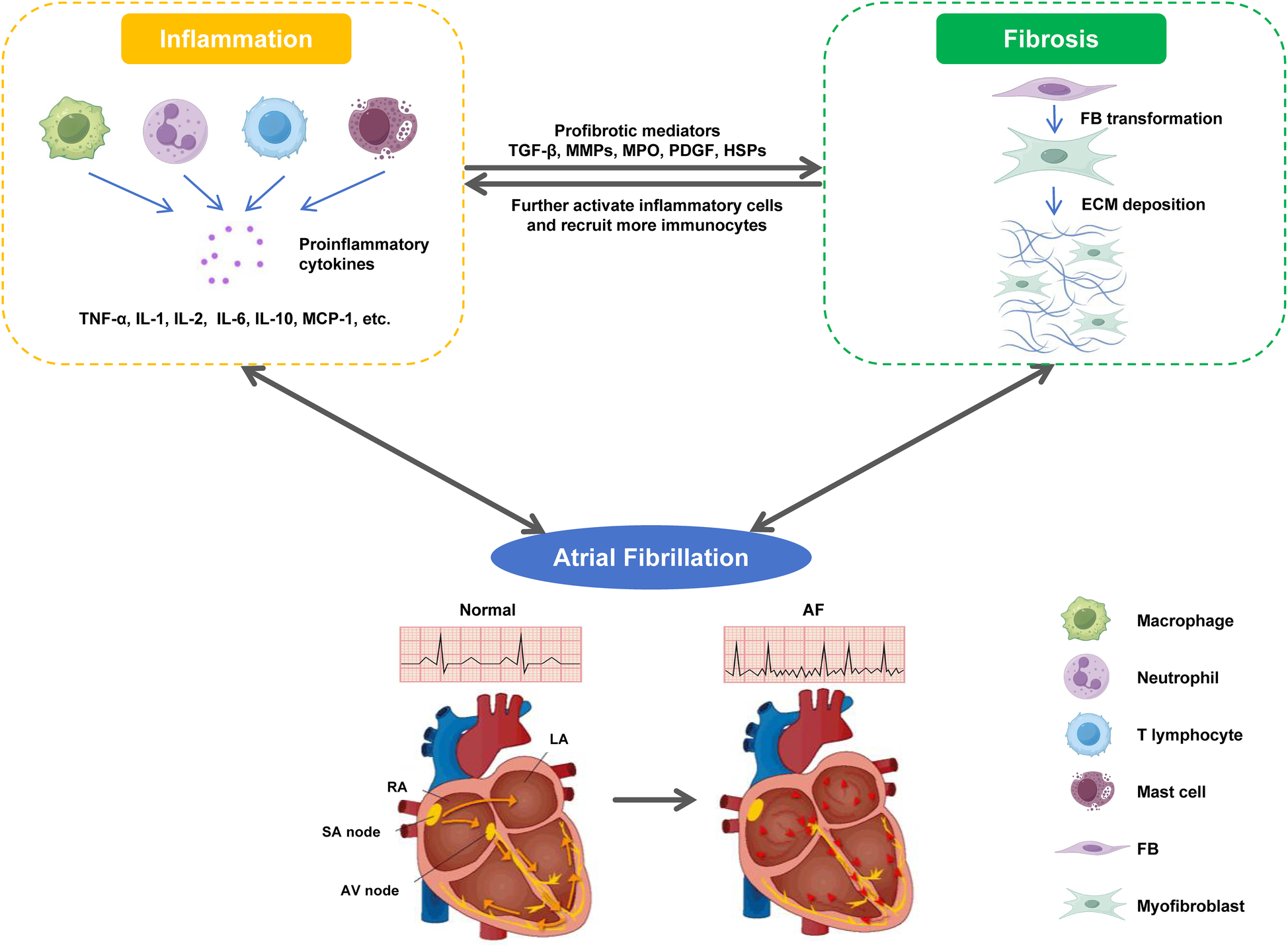
The malignant cycle between fibrosis and inflammation. In damaged heart, inflammatory cells trigger the phenotypic transformation of FBs into myofibroblasts by releasing a large amount of pro-inflammatory cytokines; Conversely, myofibroblasts produce a large amount of collagen and chemokines, which further activate inflammatory cells, and recruit and activate more immunocytes to enhance the cardiac inflammatory response. The malignant cycle between fibrosis and inflammation jointly triggers and maintains AF.
Current studies have demonstrated that both atrial fibrosis and inflammatory responses are significantly associated with the risk of AF recurrence after ablation therapy (215, 216). From a pathophysiological perspective, the vicious cycle formed between inflammatory mediators and fibrotic progression may serve as a critical underlying factor contributing to poor clinical outcomes. A deeper understanding of this interaction mechanism may hold important clinical significance for the future development of multi-target combination therapeutic strategies (such as combined anti-inflammatory and anti-fibrotic therapies), potentially offering novel treatment approaches to improve long-term prognosis in patients with AF.
5 Detection tool for atrial fibrosis
We have previously pointed out that atrial fibrosis is the core pathophysiologic basis for the occurrence and maintenance of AF. Mechanistically, atrial fibrosis constitutes a potential substrate for arrhythmogenesis in AF, which leads to slowing and blocking of electrical conduction, increasing conduction heterogeneity, and formation of reentrant circuits, creating conditions for arrhythmia (217, 218). The triggered activities and arrhythmogenic substrates may interact to jointly promote the occurrence and persistence of AF (9, 55). Clinical evidence also demonstrated that the presence and severity of atrial fibrosis are closely related to poor clinical outcomes in AF patients. A retrospective study found that more severe LA fibrosis significantly increased the risk of stroke and transient ischemic attack in AF patients (219). Moreover, for AF patients undergoing catheter ablation, the degree of LA fibrosis was positively correlated with increased risk of recurrent arrhythmia and increased demand for repeat ablation (31, 220–222). Therefore, how to accurately detect and quantify atrial fibrosis has become an important issue in clinical diagnosis and treatment of AF. Currently, the main means used in clinical practice to detect atrial fibrosis include direct detection of fibrosis using late gadolinium enhancement (LGE) displayed by cardiac magnetic resonance (CMR), as well as indirect detection of fibrosis using low voltage areas (LVA) on electroanatomic mapping (EAM) and LA strain measured by speckle tracking echocardiography (STE) (9, 223).
5.1 LGE magnetic resonance imaging (LGE MRI)
The LGE displayed by CMR has long been proven to be useful for quantifying the degree of LA fibrosis in AF patients (224, 225). The visualization principle of atrial fibrosis area is based on altered washout kinetics of gadolinium. Compared with normal myocardial tissue, gadolinium accumulates in the fibrotic area, resulting in high enhancement in this area, while healthy tissue appears as non-enhanced images (226). The degree of atrial fibrosis was quantified using the Utah classification system proposed by Marrouche et al., and divided into four stages based on the proportion of gadolinium enhancement amount to LA wall volume: stage 1 (<10%), stage 2 (≥10% ≤20%), stage 3 (≥ 20% ≤30%), and stage 4 (≥30%) (31). As an evaluation indicator of atrial fibrosis, LGE detected by CMR and LVA on EAM have mutually confirmed (224, 227). Compared with echocardiography and EAM, CMR exhibits unique advantages in evaluating LA fibrosis. It is less likely to be affected by wall tracing errors (strain and strain rate obtained through echocardiography) and tissue contact (EAM), and can more comprehensively capture potential fibrotic lesions (228, 229). However, the clinical popularization of CMR technology faces practical obstacles. Some medical institutions lack MRI equipment or physicians with CMR expertise, greatly limiting the widespread application of this technology (230). Therefore, under limited conditions, applying indirect evaluation methods such as echocardiography and EAM to detect LA fibrosis is a more clinically feasible alternative.
5.2 EAM
The presence of LVA on EAM is considered as a surrogate for the detection of LA fibrosis (9, 223). LA voltage maps are created through thousands of voltage points mapped onto the atrial endocardium's geometric model.LA bipolar voltage amplitude measured by voltage maps is taken to define LVA, characterizing LA fibrosis (231). LVA is usually defined as a bipolar voltage amplitude of less than 0.5 mV (232, 233). However, the voltage threshold of LVA has not been histologically validated (231). Although there is a lack of histological evidence linking LVA to LA fibrosis, previous studies have revealed a high consistency between LVA displayed on EAM and fibrosis areas quantified by LGE MRI (224, 234, 235). It is known that the voltage mapping collected by EAM has some limitations, mainly because the voltage signals change with changes in cycle length and direction of wavefront caused by electrode position, size, and spacing, as well as tissue contact (229, 236).
Substrate mapping based solely on LA LVA measured by EAM cannot fully and accurately quantify LA fibrosis. Regarding this, some scholars have proposed the concept of LA spatial entropy (LASE) measured by EAM, attempting to further characterize LA fibrosis. In the field of cardiac research, entropy can be used to analyze the homogeneity of cardiac tissue and predict related cardiac events (237). There are currently research reports on Shannon entropy, a signal amplitude distribution index, which can be used to measure signal complexity of atrial electrograms, assist AF rotor mapping, assess the nature of AF rotors (238–240). The concept of entropy can also be applied to LA electrical activities. If the amplitude range of atrial voltage is uniform, then entropy will be high. On the contrary, if there is fibrosis present, the distribution of electrical activities will be uneven, leading to skewed probability distribution and a decrease in entropy (237). Gigli et al. demonstrated on this basis that LASE can clearly distinguish between paroxysmal and persistent AF, as well as normal and abnormal LA fibrotic substrates, and is independent of heart rhythm during map collection (241). LASE is a highly sensitive and specific measurement tool that can serve as an auxiliary tool for predicting fibrosis substrates based on EAM.
5.3 Two-dimensional (2D) STE
LA strain has been widely recognized as a key indicator for evaluating LA myocardiac deformation (242), and its measurement can be achieved through feasible and reproducible 2D STE (243). In recent years, further research has found that LA strain can also serve as an emerging tool for evaluating LA function (244, 245). Scholars have confirmed that in AF patients, the degree of LA wall fibrosis displayed by LGE MRI was negatively correlated with LA longitudinal strain and strain rate measured by 2D STE (246, 247). In addition, studies have pointed out that cine CMR can also be used for myocardiac feature tracking to quantify LA longitudinal strain and strain rate (248, 249). It should be noted that LA strain and strain rate as alternative indicators for evaluating LA fibrosis also has some drawbacks, such as vendor dependence in LA strain measurement, lack of recognized LA strain reference values, technical bottlenecks in STE image acquisition, etc. (226, 250–252).
6 Strategies for treating AF
Targeted intervention in fibrosis and inflammation is a promising treatment strategy for AF, mainly including catheter ablation, RAAS inhibition, anti-inflammatory therapy, lifestyle changes and risk factor management.
6.1 Catheter ablation
In recent years, catheter ablation has been increasingly used in the clinical treatment of AF and is the most effective means of rhythm control for AF (253). Pulmonary vein isolation (PVI) is the foundation of catheter ablation. Although a simple PVI strategy can treat most patients with paroxysmal AF, patients with persistent AF who receive catheter ablation therapy face problems such as recurrent arrhythmias after ablation, low long-term success rates, and the need for repeat ablation (254–256).
Atrial fibrosis is an important predictor of poor response to PVI ablation for AF (31, 257). There are significant differences in the localization and degree of LA fibrosis among AF patients, which can serve as individual fingerprints reflecting potential arrhythmogenic substrates. Therefore, accurate localization and quantification of atrial fibrosis may provide strong support for personalized ablation strategies in AF patients (258). We have previously described in detail the techniques used clinically to detect atrial fibrosis, including LGE MRI, EAM, and STE. These tools can be used to supplement PVI strategies to improve the effectiveness of catheter ablation. Some research reported that targeted therapy for atrial fibrosis detected by LGE MRI is a novel custom-tailored ablation strategy for treating recurrent arrhythmia after AF ablation (258, 259). Many researchers have demonstrated that fibrotic substrate modification based on LA LVA detected by EAM is a new assistant technology for PVI ablation. Compared with AF patients who only received PVI ablation, patients who received further LVA guided substrate modification had a significantly lower recurrence rate of AF (260, 261). This indicates that this personalized arrhythmogenic substrate modification can effectively improve the prognosis of PVI ablation in AF patients. Kottkamp et al. applied a patient-tailored modification strategy targeting fibrotic substrates to AF patients undergoing catheter ablation: box isolation of fibrotic areas. This strategy provides a new treatment option for AF patients undergoing simple PVI ablation by performing circumferential isolation on EAM characterized fibrotic substrates (LVA: <0.5 mV) (262). Clarifying the individual distribution and quantity of LA fibrotic substrates may provide personalized ideas for the prevention, diagnosis, and treatment of AF patients. For example, the burden of LA fibrosis in AF patients can be included in the AF risk stratification and staging system. The substrate modification targeting LA fibrosis can be applied as a supplementary strategy for PVI ablation. However, these ideas need to be confirmed and validated for their effectiveness in prospective, multi-center, randomized clinical studies.
Interestingly, the clinical outcomes of AF ablation show significant sex differences. Compared with male AF patients, women who undergo catheter ablation have a higher risk of arrhythmia recurrence, lower rates of arrhythmia free survival, and increased risks of postoperative complications and rehospitalization (263–265). These observations suggest that the AF mechanism may vary by gender. LGE MRI showed that women had a greater burden of atrial fibrosis compared to men (266). A histological analysis involving fibrosis markers also showed that women had a higher degree of atrial fibrosis than men (267). In addition, LA LVA measured by EAM was more likely to occur in females than males, which was a powerful predictor of AF recurrence after ablation (268). Based on this, Wong et al. demonstrated significant sex differences in atrial electrophysiology in AF patients using high-density EAM, characterized by female AF patients having more advanced atrial substrates, including lower voltage, slower conduction velocity, and a higher proportion of complex fractionated potentials (269). The above research provides important reference for conducting gender specific risk stratification and developing personalized ablation strategies in clinical practice, helping optimize the diagnosis and treatment protocols for AF patients of different genders.
6.2 RAAS inhibition
As discussed earlier, RAAS activation can promote the formation of atrial fibrosis (270–272). Multiple studies have shown that ACEIs, angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs) reduce the progression of atrial fibrosis by inhibiting RAAS activation, thereby treating AF (37, 44, 273–277). In addition, RAAS activation has also been shown to be closely related to inflammation (278–280). Studies have shown that ACEI/ARB can effectively reduce levels of pro-inflammatory cytokines (281, 282), which may be a mechanism for treating AF (283). Sacubitril/valsartan (Sc/Pal) is currently a relatively new drug for treating HF (284). Sc/Pal can simultaneously antagonize angiotensin receptors and neprilysins, exerting anti-inflammatory, anti-fibrotic, and anti-hypertrophic effects by blocking AT1R and inhibiting natriuretic peptide degradation (285–288). It was showed that Sc/Pal can improve left atrial and left atrial appendage function in patients with AF and pressure-overloaded mice, which may be a new approach for treating atrial remodeling and AF (289).
6.3 Anti-inflammatory therapy
Multiple clinical studies have confirmed that some anti-inflammatory drugs, such as steroids (290–294), colchicine (295–299), and statins (300–305), can effectively prevent the recurrence of AF after ablation or cardioversion, as well as new-onset AF after cardiac surgery. In addition, drugs targeting inflammatory cytokines are gradually being applied in cardiovascular and cerebrovascular diseases (306–309). However, potential risks in clinical application require vigilance. A case report documented recurrent AF episodes in a multiple sclerosis patient following high-dose methylprednisolone (a glucocorticoid) treatment (310). While colchicine reduces AF recurrence rates, it increases gastrointestinal adverse effects (311). Although these anti-inflammatory drugs show promise in cardiovascular disease treatment, their precise efficacy in AF management requires further research validation. Clinical practice should incorporate individualized risk-benefit assessments based on patient characteristics. Future studies need to further elucidate drug mechanisms and optimize treatment protocols to achieve safe and effective personalized therapy.
6.4 Lifestyle changes and risk factor management
Inflammation related risk factors of AF include obesity, lack of exercise, hypertension, diabetes, sleep apnea, smoking/drinking habits (138, 312). Multiple studies have confirmed that managing the above risk factors can effectively prevent the occurrence of AF, help AF patients reduce the burden of AF symptoms, maintain SR, and reduce AF recurrence (313–317).
7 Conclusion
The pathophysiology of AF is very complex, and atrial fibrosis and inflammation play key roles in it. Atrial fibrosis and inflammation are simultaneous and mutually reinforcing processes in the occurrence and development of AF, and they synergistically promote atrial remodeling, leading to the occurrence and persistence of AF. A better understanding of the role, characteristics, and mechanisms of atrial fibrosis and inflammation during AF may help identify new clinical biomarkers and develop new, personalized, and more effective treatments for AF.
Statements
Author contributions
ZP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. YR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ZY: Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
2D, two-dimensional; α-SMA, alpha-smooth muscle actin; ACE, angiotensin converting enzyme; ACEIs, angiotensin converting enzyme inhibitors; AF, atrial fibrillation; Ang II, angiotensin II; ARBs, angiotensin II receptor blockers; ARD, autoimmune rheumatic disease; AT1R, angiotensin II type 1 receptor; AV node, atrioventricular node; CABG, coronary artery bypass grafting; CAD, coronary artery disease; cfDNA, cell free DNA; CHF, congestive heart failure; CKD, chronic kidney disease; CMR, cardiac magnetic resonance; CRP, C-reactive protein; CTGF, connective tissue growth factor; Cx40, connexin 40; DAD, delayed afterdepolarization; DAMPs, danger-associated molecular patterns; EAD, early afterdepolarization; EAM, electroanatomic mapping; EAT, epicardial adipose tissue; ECM, extracellular matrix; ERK1/2, extracellular regulated protein kinase 1/2; FB, fibroblast; HF, heart failure; hs-CRP, high-sensitivity C-reactive protein; HSP, heat shock protein; IκBα, inhibitor of kappa B alpha; IBD, inflammatory bowel disease; IHD, ischemic heart disease; IKKα, inhibitor of nuclear factor kappa-B kinase alpha; IKKβ, inhibitor of nuclear factor kappa-B kinase beta; IL -1β, interleukin 1 beta; IL-2, interleukin 2; IL-6, interleukin 6; IL6R, interleukin-6 receptor; JAK, Janus kinase; LA, left atrial; LASE, left atrial spatial entropy; LGE, late gadolinium enhancement; LVA, low voltage areas; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; MI, myocardial infarction; MMP, matrix metalloproteinases; MPO, myeloproxidase; MR, mitral regurgitation; MRAs, mineralocorticoid receptor antagonists; MRI, magnetic resonance imaging; mt-cfDNA, mitochondrial cell free DNA; NEMO, NF-κB essential modulator; NF-κB, nuclear factor-kappaB; NLR, neutrophil-to-lymphocyte ratio; PDGF, platelet-derived growth factor; PDGFR, platelet-derived growth factor receptor; PVI, pulmonary vein isolation; PVs, pulmonary veins; RA, right atrium; RAAS, renin-angiotensin-aldosterone system; RhoA, Ras homolog family member A; ROCK, Rho-associated kinase; SA node, sinoatrial node; Sc/Pal, Sacubitril/valsartan; SDF-1, stromal cell-derived factor-1; SR, sinus rhythm; Spry 1, Sprouty 1; STAT3, signal transducer and sctivator of transcription 3; STE, speckle tracking echocardiography; TAK1, transforming growth factor beta activated kinase 1; TF, transcription factor; TGF-β1, transforming growth factor beta 1; TGFβR1, transforming growth factor beta receptor 1; TNF-α, tumor necrosis factor alpha; TNFR1, tumor necrosis factor receptor 1; TRAF6, tumor necrosis factor receptor associated factor 6; WBC, white blood cell.
References
1.
Lippi G Sanchis-Gomar F Cervellin G . Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. (2021) 16:217–21. 10.1177/1747493019897870
2.
Du X Guo L Xia S Du J Anderson C Arima H et al Atrial fibrillation prevalence, awareness and management in a nationwide survey of adults in China. Heart. (2021) 107:535–41. 10.1136/heartjnl-2020-317915
3.
Chung MK Refaat M Shen WK Kutyifa V Cha YM Di Biase L et al Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. (2020) 75:1689–713. 10.1016/j.jacc.2020.02.025
4.
Madhavan M Graff-Radford J Piccini JP Gersh BJ . Cognitive dysfunction in atrial fibrillation. Nat Rev Cardiol. (2018) 15:744–56. 10.1038/s41569-018-0075-z
5.
Andrade J Khairy P Dobrev D Nattel S . The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. (2014) 114:1453–68. 10.1161/CIRCRESAHA.114.303211
6.
Hu M Han Y Zhao W Chen W . Long-term cost-effectiveness comparison of catheter ablation and antiarrhythmic drugs in atrial fibrillation treatment using discrete event simulation. Value Health. (2022) 25:975–83. 10.1016/j.jval.2021.10.014
7.
Sagris M Vardas EP Theofilis P Antonopoulos AS Oikonomou E Tousoulis D . Atrial fibrillation: pathogenesis, predisposing factors, and genetics. Int J Mol Sci. (2021) 23:6. 10.3390/ijms23010006
8.
Iwasaki YK Nishida K Kato T Nattel S . Atrial fibrillation pathophysiology: implications for management. Circulation. (2011) 124:2264–74. 10.1161/CIRCULATIONAHA.111.019893
9.
Ma J Chen Q Ma S . Left atrial fibrosis in atrial fibrillation: mechanisms, clinical evaluation and management. J Cell Mol Med. (2021) 25:2764–75. 10.1111/jcmm.16350
10.
Lau DH Linz D Schotten U Mahajan R Sanders P Kalman JM . Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ. (2017) 26:887–93. 10.1016/j.hlc.2017.05.119
11.
Prabhu SD Frangogiannis NG . The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res. (2016) 119:91–112. 10.1161/CIRCRESAHA.116.303577
12.
Berg AH Scherer PE . Adipose tissue, inflammation, and cardiovascular disease. Circ Res. (2005) 96:939–49. 10.1161/01.RES.0000163635.62927.34
13.
Ihara K Sasano T . Role of inflammation in the pathogenesis of atrial fibrillation. Front Physiol. (2022) 13:862164. 10.3389/fphys.2022.862164
14.
Korantzopoulos P Letsas KP Tse G Fragakis N Goudis CA Liu T . Inflammation and atrial fibrillation: a comprehensive review. J Arrhythm. (2018) 34:394–401. 10.1002/joa3.12077
15.
Van Linthout S Miteva K Tschope C . Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. (2014) 102:258–69. 10.1093/cvr/cvu062
16.
Sohns C Marrouche NF . Atrial fibrillation and cardiac fibrosis. Eur Heart J. (2020) 41:1123–31. 10.1093/eurheartj/ehz786
17.
Ding Y Wang Y Zhang W Jia Q Wang X Li Y et al Roles of biomarkers in myocardial fibrosis. Aging Dis. (2020) 11:1157–74. 10.14336/AD.2020.0604
18.
Yue L Xie J Nattel S . Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. (2011) 89:744–53. 10.1093/cvr/cvq329
19.
Han M Zhou B . Role of cardiac fibroblasts in cardiac injury and repair. Curr Cardiol Rep. (2022) 24:295–304. 10.1007/s11886-022-01647-y
20.
Plikus MV Wang X Sinha S Forte E Thompson SM Herzog EL et al Fibroblasts: origins, definitions, and functions in health and disease. Cell. (2021) 184:3852–72. 10.1016/j.cell.2021.06.024
21.
Frangogiannis NG . Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Aspects Med. (2019) 65:70–99. 10.1016/j.mam.2018.07.001
22.
Li G Yang J Zhang D Wang X Han J Guo X . Research progress of myocardial fibrosis and atrial fibrillation. Front Cardiovasc Med. (2022) 9:889706. 10.3389/fcvm.2022.889706
23.
Lopez B Ravassa S Moreno MU Jose GS Beaumont J Gonzalez A et al Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat Rev Cardiol. (2021) 18:479–98. 10.1038/s41569-020-00504-1
24.
Burstein B Nattel S . Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. (2008) 51:802–9. 10.1016/j.jacc.2007.09.064
25.
Hanna N Cardin S Leung TK Nattel S . Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. (2004) 63:236–44. 10.1016/j.cardiores.2004.03.026
26.
Burstein B Comtois P Michael G Nishida K Villeneuve L Yeh YH et al Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. (2009) 105:1213–22. 10.1161/CIRCRESAHA.108.183400
27.
Krul SP Berger WR Smit NW van Amersfoorth SC Driessen AH van Boven WJ et al Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2015) 8:288–95. 10.1161/CIRCEP.114.001752
28.
Atienza F Martins RP Jalife J . Translational research in atrial fibrillation: a quest for mechanistically based diagnosis and therapy. Circ Arrhythm Electrophysiol. (2012) 5:1207–15. 10.1161/CIRCEP.111.970335
29.
Haissaguerre M Jais P Shah DC Takahashi A Hocini M Quiniou G et al Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659–66. 10.1056/NEJM199809033391003
30.
Narayan SM Krummen DE Rappel WJ . Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol. (2012) 23:447–54. 10.1111/j.1540-8167.2012.02332.x
31.
Marrouche NF Wilber D Hindricks G Jais P Akoum N Marchlinski F et al Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. (2014) 311:498–506. 10.1001/jama.2014.3
32.
Guerra JM Everett THT Lee KW Wilson E Olgin JE . Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. (2006) 114:110–8. 10.1161/CIRCULATIONAHA.105.606251
33.
Li D Fareh S Leung TK Nattel S . Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. (1999) 100:87–95. 10.1161/01.cir.100.1.87
34.
Polejaeva IA Ranjan R Davies CJ Regouski M Hall J Olsen AL et al Increased susceptibility to atrial fibrillation secondary to atrial fibrosis in transgenic goats expressing transforming growth factor-beta1. J Cardiovasc Electrophysiol. (2016) 27:1220–9. 10.1111/jce.13049
35.
Verheule S Sato T Everett TT Engle SK Otten D Rubart-von der Lohe M et al Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. (2004) 94:1458–65. 10.1161/01.RES.0000129579.59664.9d
36.
Frustaci A Chimenti C Bellocci F Morgante E Russo MA Maseri A . Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. (1997) 96:1180–4. 10.1161/01.cir.96.4.1180
37.
Li Y Li W Yang B Han W Dong D Xue J et al Effects of cilazapril on atrial electrical, structural and functional remodeling in atrial fibrillation dogs. J Electrocardiol. (2007) 40(100):e101–106. 10.1016/j.jelectrocard.2006.04.001
38.
Avitall B Bi J Mykytsey A Chicos A . Atrial and ventricular fibrosis induced by atrial fibrillation: evidence to support early rhythm control. Heart Rhythm. (2008) 5:839–45. 10.1016/j.hrthm.2008.02.042
39.
Atlas SA . The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. (2007) 13:9–20. 10.18553/jmcp.2007.13.s8-b.9
40.
Brilla CG Pick R Tan LB Janicki JS Weber KT . Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. (1990) 67:1355–64. 10.1161/01.res.67.6.1355
41.
Weber KT Brilla CG Janicki JS . Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. (1993) 27:341–8. 10.1093/cvr/27.3.341
42.
Hanatani A Yoshiyama M Kim S Omura T Toda I Akioka K et al Inhibition by angiotensin II type 1 receptor antagonist of cardiac phenotypic modulation after myocardial infarction. J Mol Cell Cardiol. (1995) 27:1905–14. 10.1016/0022-2828(95)90013-6
43.
Xiao HD Fuchs S Campbell DJ Lewis W Dudley SC Jr Kasi VS et al Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. (2004) 165:1019–32. 10.1016/S0002-9440(10)63363-9
44.
Li D Shinagawa K Pang L Leung TK Cardin S Wang Z et al Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. (2001) 104:2608–14. 10.1161/hc4601.099402
45.
Sakabe M Fujiki A Nishida K Sugao M Nagasawa H Tsuneda T et al Enalapril prevents perpetuation of atrial fibrillation by suppressing atrial fibrosis and over-expression of connexin43 in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Pharmacol. (2004) 43:851–9. 10.1097/00005344-200406000-00015
46.
Sugden PH Clerk A . “Stress-responsive” mitogen-activated protein kinases (c-jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. (1998) 83:345–52. 10.1161/01.res.83.4.345
47.
Dostal DE Hunt RA Kule CE Bhat GJ Karoor V McWhinney CD et al Molecular mechanisms of angiotensin II in modulating cardiac function: intracardiac effects and signal transduction pathways. J Mol Cell Cardiol. (1997) 29:2893–902. 10.1006/jmcc.1997.0524
48.
Li L Fan D Wang C Wang JY Cui XB Wu D et al Angiotensin II increases periostin expression via ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. (2011) 91:80–9. 10.1093/cvr/cvr067
49.
Tsai CT Tseng CD Hwang JJ Wu CK Yu CC Wang YC et al Tachycardia of atrial myocytes induces collagen expression in atrial fibroblasts through transforming growth factor beta1. Cardiovasc Res. (2011) 89:805–15. 10.1093/cvr/cvq322
50.
Tokuda K Kai H Kuwahara F Yasukawa H Tahara N Kudo H et al Pressure-independent effects of angiotensin II on hypertensive myocardial fibrosis. Hypertension (Dallas, Tex.: 1979). (2004) 43:499–503. 10.1161/01.HYP.0000111831.50834.93
51.
Naito T Masaki T Nikolic-Paterson DJ Tanji C Yorioka N Kohno N . Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am J Physiol Renal Physiol. (2004) 286:F278–287. 10.1152/ajprenal.00139.2003
52.
Kupfahl C Pink D Friedrich K Zurbrugg HR Neuss M Warnecke C et al Angiotensin II directly increases transforming growth factor beta1 and osteopontin and indirectly affects collagen mRNA expression in the human heart. Cardiovasc Res. (2000) 46:463–75. 10.1016/s0008-6363(00)00037-7
53.
Ko WC Hong CY Hou SM Lin CH Ong ET Lee CF et al Elevated expression of connective tissue growth factor in human atrial fibrillation and angiotensin II-treated cardiomyocytes. Circ J. (2011) 75:1592–600. 10.1253/circj.cj-10-0892
54.
Liu B Yu J Taylor L Zhou X Polgar P . Microarray and phosphokinase screenings leading to studies on ERK and JNK regulation of connective tissue growth factor expression by angiotensin II 1a and bradykinin B2 receptors in Rat1 fibroblasts. J Cell Biochem. (2006) 97:1104–20. 10.1002/jcb.20709
55.
Li CY Zhang JR Hu WN Li SN . Atrial fibrosis underlying atrial fibrillation (review). Int J Mol Med. (2021) 47:9. 10.3892/ijmm.2020.4842
56.
Evans RA Tian YC Steadman R Phillips AO . TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of smad proteins. Exp Cell Res. (2003) 282:90–100. 10.1016/s0014-4827(02)00015-0
57.
He X Gao X Peng L Wang S Zhu Y Ma H et al Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific arkadia-mediated downregulation of Smad7. Circ Res. (2011) 108:164–75. 10.1161/CIRCRESAHA.110.234369
58.
Gramley F Lorenzen J Koellensperger E Kettering K Weiss C Munzel T . Atrial fibrosis and atrial fibrillation: the role of the TGF-beta1 signaling pathway. Int J Cardiol. (2010) 143:405–13. 10.1016/j.ijcard.2009.03.110
59.
Zhang D Chen X Wang Q Wu S Zheng Y Liu X . Role of the MAPKs/TGF-beta1/TRAF6 signaling pathway in postoperative atrial fibrillation. PLoS One. (2017) 12:e0173759. 10.1371/journal.pone.0173759
60.
Gu J Liu X Wang QX Tan HW Guo M Jiang WF et al Angiotensin II increases CTGF expression via MAPKs/TGF-beta1/TRAF6 pathway in atrial fibroblasts. Exp Cell Res. (2012) 318:2105–15. 10.1016/j.yexcr.2012.06.015
61.
Liu LJ Yao FJ Lu GH Xu CG Xu Z Tang K et al The role of the rho/ROCK pathway in ang II and TGF-beta1-induced atrial remodeling. PLoS One. (2016) 11:e0161625. 10.1371/journal.pone.0161625
62.
Liew R Khairunnisa K Gu Y Tee N Yin NO Naylynn TM et al Role of tumor necrosis factor-alpha in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ J. (2013) 77:1171–9. 10.1253/circj.cj-12-1155
63.
Fu H Li G Liu C Li J Wang X Cheng L et al Probucol prevents atrial remodeling by inhibiting oxidative stress and TNF-alpha/NF-kappaB/TGF-beta signal transduction pathway in alloxan-induced diabetic rabbits. J Cardiovasc Electrophysiol. (2015) 26:211–22. 10.1111/jce.12540
64.
Huang Z Chen XJ Qian C Dong Q Ding D Wu QF et al Signal transducer and activator of transcription 3/MicroRNA-21 feedback loop contributes to atrial fibrillation by promoting atrial fibrosis in a rat Sterile pericarditis model. Circ Arrhythm Electrophysiol. (2016) 9:e003396. 10.1161/CIRCEP.115.003396
65.
Chen Y Chang G Chen X Li Y Li H Cheng D et al IL-6-miR-210 suppresses regulatory T cell function and promotes atrial fibrosis by targeting Foxp3. Mol Cells. (2020) 43:438–47. 10.14348/molcells.2019.2275
66.
Abe I Teshima Y Kondo H Kaku H Kira S Ikebe Y et al Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. (2018) 15:1717–27. 10.1016/j.hrthm.2018.06.025
67.
Packer M . Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. (2018) 71:2360–72. 10.1016/j.jacc.2018.03.509
68.
Iacobellis G Barbaro G . The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. (2008) 40:442–5. 10.1055/s-2008-1062724
69.
Mazurek T Zhang L Zalewski A Mannion JD Diehl JT Arafat H et al Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. (2003) 108:2460–6. 10.1161/01.CIR.0000099542.57313.C5
70.
Fredriksson L Li H Eriksson U . The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. (2004) 15:197–204. 10.1016/j.cytogfr.2004.03.007
71.
Zhao T Zhao W Chen Y Li VS Meng W Sun Y . Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts. Am J Physiol Heart Circ Physiol. (2013) 304:H1719–1726. 10.1152/ajpheart.00130.2013
72.
Gallini R Lindblom P Bondjers C Betsholtz C Andrae J . PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp Cell Res. (2016) 349:282–90. 10.1016/j.yexcr.2016.10.022
73.
Liao CH Akazawa H Tamagawa M Ito K Yasuda N Kudo Y et al Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. (2010) 120:242–53. 10.1172/JCI39942
74.
Chen Y Surinkaew S Naud P Qi XY Gillis MA Shi YF et al JAK-STAT signalling and the atrial fibrillation promoting fibrotic substrate. Cardiovasc Res. (2017) 113:310–20. 10.1093/cvr/cvx004
75.
Tuuminen R Nykanen AI Krebs R Soronen J Pajusola K Keranen MA et al PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol. (2009) 29:691–8. 10.1161/ATVBAHA.108.178558
76.
Luo X Yang B Nattel S . MicroRNAs and atrial fibrillation: mechanisms and translational potential. Nat Rev Cardiol. (2015) 12:80–90. 10.1038/nrcardio.2014.178
77.
Nattel S . Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. (2017) 3:425–35. 10.1016/j.jacep.2017.03.002
78.
Cardin S Guasch E Luo X Naud P Le Quang K Shi Y et al Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol. (2012) 5:1027–35. 10.1161/CIRCEP.112.973214
79.
Adam O Lohfelm B Thum T Gupta SK Puhl SL Schafers HJ et al Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. (2012) 107:278. 10.1007/s00395-012-0278-0
80.
Thum T Gross C Fiedler J Fischer T Kissler S Bussen M et al MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. (2008) 456:980–4. 10.1038/nature07511
81.
He X Zhang K Gao X Li L Tan H Chen J et al Rapid atrial pacing induces myocardial fibrosis by down-regulating Smad7 via microRNA-21 in rabbit. Heart Vessels. (2016) 31:1696–708. 10.1007/s00380-016-0808-z
82.
Zhang F Geng L Zhang J Han S Guo M Xu Y et al miR-486-5p diagnosed atrial fibrillation, predicted the risk of left atrial fibrosis, and regulated angiotensin II-induced cardiac fibrosis via modulating PI3K/Akt signaling through targeting FOXO1. Mol Cell Biochem. (2025) 480:1077–87. 10.1007/s11010-024-05027-8
83.
Qi XY Huang H Ordog B Luo X Naud P Sun Y et al Fibroblast inward-rectifier potassium current upregulation in profibrillatory atrial remodeling. Circ Res. (2015) 116:836–45. 10.1161/CIRCRESAHA.116.305326
84.
Luo X Pan Z Shan H Xiao J Sun X Wang N et al MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J Clin Invest. (2013) 123:1939–51. 10.1172/JCI62185
85.
Wang Y Cai H Li H Gao Z Song K . Atrial overexpression of microRNA-27b attenuates angiotensin II-induced atrial fibrosis and fibrillation by targeting ALK5. Hum Cell. (2018) 31:251–60. 10.1007/s13577-018-0208-z
86.
Dawson K Wakili R Ordog B Clauss S Chen Y Iwasaki Y et al MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. (2013) 127:1466–75. 1475e1461–1428. 10.1161/CIRCULATIONAHA.112.001207
87.
Duisters RF Tijsen AJ Schroen B Leenders JJ Lentink V van der Made I et al miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. (2009) 104:170–8, 176p following 178. 10.1161/CIRCRESAHA.108.182535
88.
Chen R Zhang H Tang B Luo Y Yang Y Zhong X et al Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. (2024) 9:130. 10.1038/s41392-024-01840-1
89.
Soysal P Arik F Smith L Jackson SE Isik AT . Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. (2020) 1216:55–64. 10.1007/978-3-030-33330-0_7
90.
Bruins P te Velthuis H Yazdanbakhsh AP Jansen PG van Hardevelt FW de Beaumont EM et al Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. (1997) 96:3542–8. 10.1161/01.cir.96.10.3542
91.
Chung MK Martin DO Sprecher D Wazni O Kanderian A Carnes CA et al C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. (2001) 104:2886–91. 10.1161/hc4901.101760
92.
Zhou X Dudley SC Jr . Evidence for inflammation as a driver of atrial fibrillation. Front Cardiovasc Med. (2020) 7:62. 10.3389/fcvm.2020.00062
93.
Choi EK Chang PC Lee YS Lin SF Zhu W Maruyama M et al Triggered firing and atrial fibrillation in transgenic mice with selective atrial fibrosis induced by overexpression of TGF-beta1. Circ J. (2012) 76:1354–62. 10.1253/circj.cj-11-1301
94.
Kao YH Chen YC Cheng CC Lee TI Chen YJ Chen SA . Tumor necrosis factor-alpha decreases sarcoplasmic reticulum Ca2+-ATPase expressions via the promoter methylation in cardiomyocytes. Crit Care Med. (2010) 38:217–22. 10.1097/CCM.0b013e3181b4a854
95.
Lee SH Chen YC Chen YJ Chang SL Tai CT Wongcharoen W et al Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. (2007) 80:1806–15. 10.1016/j.lfs.2007.02.029
96.
Saba S Janczewski AM Baker LC Shusterman V Gursoy EC Feldman AM et al Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. (2005) 289:H1456–1467. 10.1152/ajpheart.00733.2004
97.
Musa H Kaur K O'Connell R Klos M Guerrero-Serna G Avula UM et al Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart Rhythm. (2013) 10:1044–51. 10.1016/j.hrthm.2013.03.014
98.
Heijman J Muna AP Veleva T Molina CE Sutanto H Tekook M et al Atrial myocyte NLRP3/CaMKII nexus forms a substrate for postoperative atrial fibrillation. Circ Res. (2020) 127:1036–55. 10.1161/CIRCRESAHA.120.316710
99.
Wu X Liu Y Tu D Liu X Niu S Suo Y et al Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J Cardiovasc Transl Res. (2020) 13:731–40. 10.1007/s12265-020-09965-8
100.
Yao C Veleva T Scott L Jr Cao S Li L Chen G et al Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. (2018) 138:2227–42, 10.1161/CIRCULATIONAHA.118.035202
101.
Ryu K Li L Khrestian CM Matsumoto N Sahadevan J Ruehr ML et al Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. (2007) 293:H1231–1241. 10.1152/ajpheart.00607.2006
102.
Sawaya SE Rajawat YS Rami TG Szalai G Price RL Sivasubramanian N et al Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. (2007) 292:H1561–1567. 10.1152/ajpheart.00285.2006
103.
Lazzerini PE Laghi-Pasini F Acampa M Srivastava U Bertolozzi I Giabbani B et al Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J Am Heart Assoc. (2019) 8:e011006. 10.1161/JAHA.118.011006
104.
Shang LL Sanyal S Pfahnl AE Jiao Z Allen J Liu H et al NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. (2008) 294:C372–379. 10.1152/ajpcell.00186.2007
105.
Burstein B Libby E Calderone A Nattel S . Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. (2008) 117:1630–41. 10.1161/CIRCULATIONAHA.107.748053
106.
Kang Q Li X Yang M Fernando T Wan Z . Galectin-3 in patients with coronary heart disease and atrial fibrillation. Clin Chim Acta. (2018) 478:166–70. 10.1016/j.cca.2017.12.041
107.
Ma F Li Y Jia L Han Y Cheng J Li H et al Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. (2012) 7:e35144. 10.1371/journal.pone.0035144
108.
Zaidi Y Aguilar EG Troncoso M Ilatovskaya DV DeLeon-Pennell KY . Immune regulation of cardiac fibrosis post myocardial infarction. Cell Signal. (2021) 77:109837. 10.1016/j.cellsig.2020.109837
109.
Lafuse WP Wozniak DJ Rajaram MVS . Role of cardiac macrophages on cardiac inflammation, fibrosis and tissue repair. Cells. (2020) 10:51. 10.3390/cells10010051
110.
Wagner MJ Khan M Mohsin S . Healing the broken heart; the immunomodulatory effects of stem cell therapy. Front Immunol. (2020) 11:639. 10.3389/fimmu.2020.00639
111.
Toba H Cannon PL Yabluchanskiy A Iyer RP D’Armiento J Lindsey ML . Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol. (2017) 312:H375–83. 10.1152/ajpheart.00633.2016
112.
Hu YF Chen YJ Lin YJ Chen SA . Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12:230–43. 10.1038/nrcardio.2015.2
113.
Kallergis EM Manios EG Kanoupakis EM Mavrakis HE Kolyvaki SG Lyrarakis GM et al The role of the post-cardioversion time course of hs-CRP levels in clarifying the relationship between inflammation and persistence of atrial fibrillation. Heart. (2008) 94:200–4. 10.1136/hrt.2006.108688
114.
Marcus GM Smith LM Ordovas K Scheinman MM Kim AM Badhwar N et al Intracardiac and extracardiac markers of inflammation during atrial fibrillation. Heart Rhythm. (2010) 7:149–54. 10.1016/j.hrthm.2009.10.004
115.
Marcus GM Smith LM Glidden DV Wilson E McCabe JM Whiteman D et al Markers of inflammation before and after curative ablation of atrial flutter. Heart Rhythm. (2008) 5:215–21. 10.1016/j.hrthm.2007.10.007
116.
Zhao Q Zhang S Zhao H Zhang S Dai Z Qian Y et al Median nerve stimulation prevents atrial electrical remodelling and inflammation in a canine model with rapid atrial pacing. Europace. (2018) 20:712–8. 10.1093/europace/eux003
117.
Mahood WH . The letter from Utrecht. JAMA. (1992) 268:791. 10.1001/jama.1992.03490060127049
118.
Shiroshita-Takeshita A Brundel BJ Lavoie J Nattel S . Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc Res. (2006) 69:865–75. 10.1016/j.cardiores.2005.11.028
119.
Patel P Dokainish H Tsai P Lakkis N . Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. (2010) 21:1064–70. 10.1111/j.1540-8167.2010.01774.x
120.
Yamazoe M Sasano T Ihara K Takahashi K Nakamura W Takahashi N et al Sparsely methylated mitochondrial cell free DNA released from cardiomyocytes contributes to systemic inflammatory response accompanied by atrial fibrillation. Sci Rep. (2021) 11:5837. 10.1038/s41598-021-85204-7
121.
Kuipers S Klein Klouwenberg PM Cremer OL . Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. (2014) 18:688. 10.1186/s13054-014-0688-5
122.
Meierhenrich R Steinhilber E Eggermann C Weiss M Voglic S Bogelein D et al Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. (2010) 14:R108. 10.1186/cc9057
123.
Christian SA Schorr C Ferchau L Jarbrink ME Parrillo JE Gerber DR . Clinical characteristics and outcomes of septic patients with new-onset atrial fibrillation. J Crit Care. (2008) 23:532–6. 10.1016/j.jcrc.2007.09.005
124.
Bandyopadhyay D Banerjee U Hajra A Chakraborty S Amgai B Ghosh RK et al Trends of cardiac complications in patients with rheumatoid arthritis: analysis of the United States national inpatient sample; 2005–2014. Curr Probl Cardiol. (2021) 46:100455. 10.1016/j.cpcardiol.2019.100455
125.
Ungprasert P Srivali N Kittanamongkolchai W . Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. (2017) 20:434–41. 10.1111/1756-185X.12820
126.
Bacani AK Crowson CS Roger VL Gabriel SE Matteson EL . Increased incidence of atrial fibrillation in patients with rheumatoid arthritis. Biomed Res Int. (2015) 2015:809514. 10.1155/2015/809514
127.
Lazzerini PE Capecchi PL Laghi-Pasini F . Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur Heart J. (2017) 38:1717–27. 10.1093/eurheartj/ehw208
128.
Baek YS Kim TH Uhm JS Kim JY Pak HN Lee MH et al Prevalence and the clinical outcome of atrial fibrillation in patients with autoimmune rheumatic disease. Int J Cardiol. (2016) 214:4–9. 10.1016/j.ijcard.2016.03.083
129.
Dai H Wang X Yin S Zhang Y Han Y Yang N et al Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc. (2017) 6:e007320. 10.1161/JAHA.117.007320
130.
Chiu HY Chang WL Huang WF Wen YW Tsai YW Tsai TF . Increased risk of arrhythmia in patients with psoriatic disease: a nationwide population-based matched cohort study. J Am Acad Dermatol. (2015) 73:429–38. 10.1016/j.jaad.2015.06.023
131.
Ahlehoff O Gislason GH Jorgensen CH Lindhardsen J Charlot M Olesen JB et al Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish nationwide cohort study. Eur Heart J. (2012) 33:2054–64. 10.1093/eurheartj/ehr285
132.
Choi YJ Choi EK Han KD Park J Moon I Lee E et al Increased risk of atrial fibrillation in patients with inflammatory bowel disease: a nationwide population-based study. World J Gastroenterol. (2019) 25:2788–98. 10.3748/wjg.v25.i22.2788
133.
Kristensen SL Lindhardsen J Ahlehoff O Erichsen R Lamberts M Khalid U et al Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace. (2014) 16:477–84. 10.1093/europace/eut312
134.
Dogan Y Soylu A Eren GA Poturoglu S Dolapcioglu C Sonmez K et al Evaluation of QT and P wave dispersion and mean platelet volume among inflammatory bowel disease patients. Int J Med Sci. (2011) 8:540–6. 10.7150/ijms.8.540
135.
Efe TH Cimen T Ertem AG Coskun Y Bilgin M Sahan HF et al Atrial electromechanical properties in inflammatory bowel disease. Echocardiography. (2016) 33:1309–16. 10.1111/echo.13261
136.
Elliott AD Middeldorp ME Van Gelder IC Albert CM Sanders P . Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. (2023) 20:404–17. 10.1038/s41569-022-00820-8
137.
Hendriks JM Gallagher C Middeldorp ME Lau DH Sanders P . Risk factor management and atrial fibrillation. Europace. (2021) 23:ii52–60. 10.1093/europace/euaa346
138.
Chung MK Eckhardt LL Chen LY Ahmed HM Gopinathannair R Joglar JA et al Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. (2020) 141:e750–72. 10.1161/CIR.0000000000000748
139.
Harrison DG Guzik TJ Lob HE Madhur MS Marvar PJ Thabet SR et al Inflammation, immunity, and hypertension. Hypertension (Dallas, Tex.: 1979). (2011) 57:132–40. 10.1161/HYPERTENSIONAHA.110.163576
140.
Lau DH Shipp NJ Kelly DJ Thanigaimani S Neo M Kuklik P et al Atrial arrhythmia in ageing spontaneously hypertensive rats: unraveling the substrate in hypertension and ageing. PLoS One. (2013) 8:e72416. 10.1371/journal.pone.0072416
141.
Lau DH Mackenzie L Kelly DJ Psaltis PJ Brooks AG Worthington M et al Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. (2010) 7:1282–90. 10.1016/j.hrthm.2010.05.010
142.
Baek YS Yang PS Kim TH Uhm JS Park J Pak HN et al Associations of abdominal obesity and new-onset atrial fibrillation in the general population. J Am Heart Assoc. (2017) 6:e004705. 10.1161/JAHA.116.004705
143.
Zacharias A Schwann TA Riordan CJ Durham SJ Shah AS Habib RH . Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. (2005) 112:3247–55. 10.1161/CIRCULATIONAHA.105.553743
144.
Wang TJ Parise H Levy D D’Agostino RB Sr Wolf PA Vasan RS et al Obesity and the risk of new-onset atrial fibrillation. JAMA. (2004) 292:2471–7. 10.1001/jama.292.20.2471
145.
Kim D Kim J Yoon JH Ghim J Yea K Song P et al CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. (2014) 57:1456–65. 10.1007/s00125-014-3237-5
146.
Lumeng CN DelProposto JB Westcott DJ Saltiel AR . Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. (2008) 57:3239–46. 10.2337/db08-0872
147.
Lumeng CN Bodzin JL Saltiel AR . Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. (2007) 117:175–84. 10.1172/JCI29881
148.
Mohamed-Ali V Goodrick S Rawesh A Katz DR Miles JM Yudkin JS et al Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. (1997) 82:4196–200. 10.1210/jcem.82.12.4450
149.
Hotamisligil GS Arner P Caro JF Atkinson RL Spiegelman BM . Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. (1995) 95:2409–15. 10.1172/JCI117936
150.
Nalliah CJ Sanders P Kottkamp H Kalman JM . The role of obesity in atrial fibrillation. Eur Heart J. (2016) 37:1565–72. 10.1093/eurheartj/ehv486
151.
Poirier P Giles TD Bray GA Hong Y Stern JS Pi-Sunyer FX et al Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the obesity committee of the council on nutrition, physical activity, and metabolism. Circulation. (2006) 113:898–918. 10.1161/CIRCULATIONAHA.106.171016
152.
Karam BS Chavez-Moreno A Koh W Akar JG Akar FG . Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. (2017) 16:120. 10.1186/s12933-017-0604-9
153.
Huxley RR Filion KB Konety S Alonso A . Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. (2011) 108:56–62. 10.1016/j.amjcard.2011.03.004
154.
Goudis CA Korantzopoulos P Ntalas IV Kallergis EM Liu T Ketikoglou DG . Diabetes mellitus and atrial fibrillation: pathophysiological mechanisms and potential upstream therapies. Int J Cardiol. (2015) 184:617–22. 10.1016/j.ijcard.2015.03.052
155.
Sinno H Derakhchan K Libersan D Merhi Y Leung TK Nattel S . Atrial ischemia promotes atrial fibrillation in dogs. Circulation. (2003) 107:1930–6. 10.1161/01.CIR.0000058743.15215.03
156.
Stellos K Rahmann A Kilias A Ruf M Sopova K Stamatelopoulos K et al Expression of platelet-bound stromal cell-derived factor-1 in patients with non-valvular atrial fibrillation and ischemic heart disease. J Thromb Haemost. (2012) 10:49–55. 10.1111/j.1538-7836.2011.04547.x
157.
Marcus GM Whooley MA Glidden DV Pawlikowska L Zaroff JG Olgin JE . Interleukin-6 and atrial fibrillation in patients with coronary artery disease: data from the heart and soul study. Am Heart J. (2008) 155:303–9. 10.1016/j.ahj.2007.09.006
158.
Galea R Cardillo MT Caroli A Marini MG Sonnino C Narducci ML et al Inflammation and C-reactive protein in atrial fibrillation: cause or effect? Tex Heart Inst J. (2014) 41:461–8. 10.14503/THIJ-13-3466
159.
Patti G Chello M Candura D Pasceri V D’Ambrosio A Covino E et al Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (atorvastatin for reduction of MYocardial dysrhythmia after cardiac surgery) study. Circulation. (2006) 114:1455–61. 10.1161/CIRCULATIONAHA.106.621763
160.
Hak L Mysliwska J Wieckiewicz J Szyndler K Siebert J Rogowski J . Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J Interferon Cytokine Res. (2009) 29:327–32. 10.1089/jir.2008.0082.2906
161.
Ishii Y Schuessler RB Gaynor SL Yamada K Fu AS Boineau JP et al Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. (2005) 111:2881–8. 10.1161/CIRCULATIONAHA.104.475194
162.
Korodi S Toganel R Benedek T Hodas R Chitu M Ratiu M et al Impact of inflammation-mediated myocardial fibrosis on the risk of recurrence after successful ablation of atrial fibrillation—the FIBRO-RISK study: protocol for a non-randomized clinical trial. Medicine (Baltimore). (2019) 98:e14504. 10.1097/MD.0000000000014504
163.
Lim HS Schultz C Dang J Alasady M Lau DH Brooks AG et al Time course of inflammation, myocardial injury, and prothrombotic response after radiofrequency catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2014) 7:83–9. 10.1161/CIRCEP.113.000876
164.
Richter B Gwechenberger M Socas A Zorn G Albinni S Marx M et al Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol. (2012) 101:217–25. 10.1007/s00392-011-0383-3
165.
Jabati S Fareed J Liles J Otto A Hoppensteadt D Bontekoe J et al Biomarkers of inflammation, thrombogenesis, and collagen turnover in patients with atrial fibrillation. Clin Appl Thromb Hemost. (2018) 24:718–23. 10.1177/1076029618761006
166.
Wu N Xu B Xiang Y Wu L Zhang Y Ma X et al Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. (2013) 169:62–72. 10.1016/j.ijcard.2013.08.078
167.
Liu T Li L Korantzopoulos P Goudevenos JA Li G . Meta-analysis of association between C-reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am J Cardiol. (2008) 101:1749–52. 10.1016/j.amjcard.2008.02.066
168.
Dix C Zeller J Stevens H Eisenhardt SU Shing K Nero TL et al C-reactive protein, immunothrombosis and venous thromboembolism. Front Immunol. (2022) 13:1002652. 10.3389/fimmu.2022.1002652
169.
Sproston NR Ashworth JJ . Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. 10.3389/fimmu.2018.00754
170.
Aviles RJ Martin DO Apperson-Hansen C Houghtaling PL Rautaharju P Kronmal RA et al Inflammation as a risk factor for atrial fibrillation. Circulation. (2003) 108:3006–10. 10.1161/01.CIR.0000103131.70301.4F
171.
Hijazi Z Aulin J Andersson U Alexander JH Gersh B Granger CB et al Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. (2016) 102:508–17. 10.1136/heartjnl-2015-308887
172.
Jiang Z Dai L Song Z Li H Shu M . Association between C-reactive protein and atrial fibrillation recurrence after catheter ablation: a meta-analysis. Clin Cardiol. (2013) 36:548–54. 10.1002/clc.22157
173.
Liu T Li G Li L Korantzopoulos P . Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta-analysis. J Am Coll Cardiol. (2007) 49:1642–8. 10.1016/j.jacc.2006.12.042
174.
Zarauza J Rodriguez Lera MJ Farinas Alvarez C Hernando JP Ceballos B Gutierrez B et al [Relationship between C-reactive protein level and early recurrence of atrial fibrillation after electrical cardioversion]. Rev Esp Cardiol. (2006) 59:125–9. 10.1157/13084639
175.
Acampa M Lazzerini PE Guideri F Tassi R Lo Monaco A Martini G . Inflammation and atrial electrical remodelling in patients with embolic strokes of undetermined source. Heart Lung Circ. (2019) 28:917–22. 10.1016/j.hlc.2018.04.294
176.
Watanabe E Arakawa T Uchiyama T Kodama I Hishida H . High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol. (2006) 108:346–53. 10.1016/j.ijcard.2005.05.021
177.
Mizel SB . The interleukins. FASEB J. (1989) 3:2379–88. 10.1096/fasebj.3.12.2676681
178.
Conway DS Buggins P Hughes E Lip GY . Relationship of interleukin-6 and C-reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. (2004) 43:2075–82. 10.1016/j.jacc.2003.11.062
179.
Kaireviciute D Blann AD Balakrishnan B Lane DA Patel JV Uzdavinys G et al Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb Haemost. (2010) 104:122–7. 10.1160/TH09-12-0837
180.
Amdur RL Mukherjee M Go A Barrows IR Ramezani A Shoji J et al Interleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. PLoS One. (2016) 11:e0148189. 10.1371/journal.pone.0148189
181.
Cabrera-Bueno F Medina-Palomo C Ruiz-Salas A Flores A Rodriguez-Losada N Barrera A et al Serum levels of interleukin-2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine. (2015) 73:74–8. 10.1016/j.cyto.2015.01.026
182.
Rizos I Tsiodras S Rigopoulos AG Dragomanovits S Kalogeropoulos AS Papathanasiou S et al Interleukin-2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine. (2007) 40:157–64. 10.1016/j.cyto.2007.08.013
183.
Li J Solus J Chen Q Rho YH Milne G Stein CM et al Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. (2010) 7:438–44. 10.1016/j.hrthm.2009.12.009
184.
Liuba I Ahlmroth H Jonasson L Englund A Jonsson A Safstrom K et al Source of inflammatory markers in patients with atrial fibrillation. Europace. (2008) 10:848–53. 10.1093/europace/eun111
185.
Anatolevna RO Veniaminovich FO Mikhaylovich KS . Predictors of new-onset atrial fibrillation in elderly patients with coronary artery disease after coronary artery bypass graft. J Geriatr Cardiol. (2016) 13:444–9. 10.11909/j.issn.1671-5411.2016.05.017
186.
Wu ZK Laurikka J Vikman S Nieminen R Moilanen E Tarkka MR . High postoperative interleukin-8 levels related to atrial fibrillation in patients undergoing coronary artery bypass surgery. World J Surg. (2008) 32:2643–9. 10.1007/s00268-008-9758-7
187.
Ishida K Kimura F Imamaki M Ishida A Shimura H Kohno H et al Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. (2006) 29:501–5. 10.1016/j.ejcts.2005.12.028
188.
Kalliolias GD Ivashkiv LB . TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. (2016) 12:49–62. 10.1038/nrrheum.2015.169
189.
Deng H Xue YM Zhan XZ Liao HT Guo HM Wu SL . Role of tumor necrosis factor-alpha in the pathogenesis of atrial fibrillation. Chin Med J. (2011) 124:1976–82. 10.3760/cma.j.issn.0366-6999.2011.13.010
190.
Qu YC Du YM Wu SL Chen QX Wu HL Zhou SF . Activated nuclear factor-kappaB and increased tumor necrosis factor-alpha in atrial tissue of atrial fibrillation. Scand Cardiovasc J. (2009) 43:292–7. 10.1080/14017430802651803
191.
Weymann A Ali-Hasan-Al-Saegh S Sabashnikov A Popov AF Mirhosseini SJ Liu T et al Prediction of new-onset and recurrent atrial fibrillation by complete blood count tests: a comprehensive systematic review with meta-analysis. Med Sci Monit Basic Res. (2017) 23:179–222. 10.12659/msmbr.903320
192.
Rienstra M Sun JX Magnani JW Sinner MF Lubitz SA Sullivan LM et al White blood cell count and risk of incident atrial fibrillation (from the Framingham heart study). Am J Cardiol. (2012) 109:533–7. 10.1016/j.amjcard.2011.09.049
193.
Fontes ML Amar D Kulak A Koval K Zhang H Shi W et al Increased preoperative white blood cell count predicts postoperative atrial fibrillation after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. (2009) 23:484–7. 10.1053/j.jvca.2009.01.030
194.
Amar D Goenka A Zhang H Park B Thaler HT . Leukocytosis and increased risk of atrial fibrillation after general thoracic surgery. Ann Thorac Surg. (2006) 82:1057–61. 10.1016/j.athoracsur.2006.03.103
195.
Lamm G Auer J Weber T Berent R Ng C Eber B . Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth. (2006) 20:51–6. 10.1053/j.jvca.2005.03.026
196.
Korantzopoulos P Kolettis TM Kountouris E Siogas K Goudevenos JA . Variation of inflammatory indexes after electrical cardioversion of persistent atrial fibrillation. Is there an association with early recurrence rates?Int J Clin Pract. (2005) 59:881–5. 10.1111/j.1368-5031.2005.00569.x
197.
Shao Q Chen K Rha SW Lim HE Li G Liu T . Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. (2015) 46:199–206. 10.1016/j.arcmed.2015.03.011
198.
Karavelioglu Y Karapinar H Yuksel M Memic K Sarak T Kurt R et al Neutrophil to lymphocyte ratio is predictor of atrial fibrillation recurrence after cardioversion with amiodarone. Clin Appl Thromb Hemost. (2015) 21:5–9. 10.1177/1076029613518368
199.
Bhat T Teli S Rijal J Bhat H Raza M Khoueiry G et al Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11:55–9. 10.1586/erc.12.159
200.
Deshmane SL Kremlev S Amini S Sawaya BE . Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. (2009) 29:313–26. 10.1089/jir.2008.0027
201.
Lackermair K Clauss S Voigt T Klier I Summo C Hildebrand B et al Alteration of endothelin 1, MCP-1 and chromogranin A in patients with atrial fibrillation undergoing pulmonary vein isolation. PLoS One. (2017) 12:e0184337. 10.1371/journal.pone.0184337
202.
Aratani Y . Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. (2018) 640:47–52. 10.1016/j.abb.2018.01.004
203.
Li SB Yang F Jing L Ma J Jia YD Dong SY et al Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation. J Investig Med. (2013) 61:722–7. 10.2310/JIM.0b013e3182857fa0
204.
Knowlton AA . NFkappab, heat shock proteins, HSF-1, and inflammation. Cardiovasc Res. (2006) 69:7–8. 10.1016/j.cardiores.2005.10.009
205.
Hu YF Yeh HI Tsao HM Tai CT Lin YJ Chang SL et al Electrophysiological correlation and prognostic impact of heat shock protein 27 in atrial fibrillation. Circ Arrhythm Electrophysiol. (2012) 5:334–40. 10.1161/CIRCEP.111.965996
206.
Mandal K Torsney E Poloniecki J Camm AJ Xu Q Jahangiri M . Association of high intracellular, but not serum, heat shock protein 70 with postoperative atrial fibrillation. Ann Thorac Surg. (2005) 79:865–71; discussion 871. 10.1016/j.athoracsur.2004.08.018
207.
St Rammos K Koullias GJ Hassan MO Argyrakis NP Voucharas CG Scarupa SJ et al Low preoperative HSP70 atrial myocardial levels correlate significantly with high incidence of postoperative atrial fibrillation after cardiac surgery. Cardiovasc Surg. (2002) 10:228–32. 10.1016/s0967-2109(01)00138-7
208.
Harada M Nattel S . Implications of inflammation and fibrosis in atrial fibrillation pathophysiology. Card Electrophysiol Clin. (2021) 13:25–35. 10.1016/j.ccep.2020.11.002
209.
Thomas TP Grisanti LA . The dynamic interplay between cardiac inflammation and fibrosis. Front Physiol. (2020) 11:529075. 10.3389/fphys.2020.529075
210.
Dean RA Cox JH Bellac CL Doucet A Starr AE Overall CM . Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, -7, -8, and -13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. (2008) 112:3455–64. 10.1182/blood-2007-12-129080
211.
Van den Steen PE Proost P Wuyts A Van Damme J Opdenakker G . Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. (2000) 96:2673–81. 10.1182/blood.V96.8.2673
212.
Frangogiannis NG . The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. (2014) 11:255–65. 10.1038/nrcardio.2014.28
213.
Song J Wu C Zhang X Sorokin LM . In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol. (2013) 190:401–10. 10.4049/jimmunol.1202286
214.
Sonmez O Ertem FU Vatankulu MA Erdogan E Tasal A Kucukbuzcu S et al Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med Sci Monit. (2014) 20:463–70. 10.12659/MSM.890635
215.
Pavlovic M Apostolovic S Stokanovic D Momcilovic S Jevtovic-Stoimenov T Zdravkovic SC et al The association between galectin-3 and hs-CRP and the clinical outcome after non-ST-elevation myocardial infarction with preexisting atrial fibrillation. Sci Rep. (2017) 7:15106. 10.1038/s41598-017-15265-0
216.
Akoum N Wilber D Hindricks G Jais P Cates J Marchlinski F et al MRI assessment of ablation-induced scarring in atrial fibrillation: analysis from the DECAAF study. J Cardiovasc Electrophysiol. (2015) 26:473–80. 10.1111/jce.12650
217.
Xintarakou A Tzeis S Psarras S Asvestas D Vardas P . Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. Europace. (2020) 22:342–51. 10.1093/europace/euaa009
218.
Nguyen TP Qu Z Weiss JN . Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol. (2014) 70:83–91. 10.1016/j.yjmcc.2013.10.018
219.
King JB Azadani PN Suksaranjit P Bress AP Witt DM Han FT et al Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. (2017) 70:1311–21. 10.1016/j.jacc.2017.07.758
220.
Chelu MG King JB Kholmovski EG Ma J Gal P Marashly Q et al Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J Am Heart Assoc. (2018) 7:e006313. 10.1161/JAHA.117.006313
221.
Moteleb A Zarif JK Ali AN . Incidence of atrial fibrosis in non-valvular atrial fibrillation patients and its impact on recurrence after pulmonary vein antral isolation. J Atr Fibrillation. (2018) 11:1773. 10.4022/jafib.1773
222.
Luetkens JA Wolpers AC Beiert T Kuetting D Dabir D Homsi R et al Cardiac magnetic resonance using late gadolinium enhancement and atrial T1 mapping predicts poor outcome in patients with atrial fibrillation after catheter ablation therapy. Sci Rep. (2018) 8:13618. 10.1038/s41598-018-31916-2
223.
Quah JX Dharmaprani D Tiver K Lahiri A Hecker T Perry R et al Atrial fibrosis and substrate based characterization in atrial fibrillation: time to move forwards. J Cardiovasc Electrophysiol. (2021) 32:1147–60. 10.1111/jce.14987
224.
Oakes RS Badger TJ Kholmovski EG Akoum N Burgon NS Fish EN et al Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. (2009) 119:1758–67. 10.1161/CIRCULATIONAHA.108.811877
225.
Peters DC Wylie JV Hauser TH Kissinger KV Botnar RM Essebag V et al Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. (2007) 243:690–5. 10.1148/radiol.2433060417
226.
Olsen FJ Bertelsen L de Knegt MC Christensen TE Vejlstrup N Svendsen JH et al Multimodality cardiac imaging for the assessment of left atrial function and the association with atrial arrhythmias. Circ Cardiovasc Imaging. (2016) 9:e004947. 10.1161/CIRCIMAGING.116.004947
227.
Khurram IM Beinart R Zipunnikov V Dewire J Yarmohammadi H Sasaki T et al Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. (2014) 11:85–92. 10.1016/j.hrthm.2013.10.007
228.
Jellis CL Klein AL . Heart failure with preserved ejection fraction: do you know your left atrial strain?Circ Cardiovasc Imaging. (2016) 9:e004521. 10.1161/CIRCIMAGING.116.004521
229.
Josephson ME Anter E . Substrate mapping for ventricular tachycardia: assumptions and misconceptions. JACC Clin Electrophysiol. (2015) 1:341–52. 10.1016/j.jacep.2015.09.001
230.
Vijayakumar S Kholmovski EG Haslam MM Burgon N Marrouche NF . Dependence of image quality of late gadolinium enhancement MRI of left atrium on number of patients imaged: results of multi-center trial DECAAF. J Cardiovasc Magn Reson. (2014) 16:P146. 10.1186/1532-429X-16-S1-P146
231.
Sim I Bishop M O’Neill M Williams SE . Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol. (2019) 56:213–27. 10.1007/s10840-019-00537-8
232.
Saghy L Callans DJ Garcia F Lin D Marchlinski FE Riley M et al Is there a relationship between complex fractionated atrial electrograms recorded during atrial fibrillation and sinus rhythm fractionation? Heart Rhythm. (2012) 9:181–8. 10.1016/j.hrthm.2011.09.062
233.
Teh AW Kistler PM Lee G Medi C Heck PM Spence SJ et al The relationship between complex fractionated electrograms and atrial low-voltage zones during atrial fibrillation and paced rhythm. Europace. (2011) 13:1709–16. 10.1093/europace/eur197
234.
Jadidi AS Cochet H Shah AJ Kim SJ Duncan E Miyazaki S et al Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol. (2013) 62:802–12. 10.1016/j.jacc.2013.03.081
235.
Spragg DD Khurram I Zimmerman SL Yarmohammadi H Barcelon B Needleman M et al Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. (2012) 9:2003–9. 10.1016/j.hrthm.2012.08.039
236.
Wong GR Nalliah CJ Lee G Voskoboinik A Prabhu S Parameswaran R et al Dynamic atrial substrate during high-density mapping of paroxysmal and persistent AF: implications for substrate ablation. JACC Clin Electrophysiol. (2019) 5:1265–77. 10.1016/j.jacep.2019.06.002
237.
Wu KC Chrispin J . More than meets the eye: cardiac magnetic resonance image entropy and ventricular arrhythmia risk prediction. JACC Cardiovasc Imaging. (2022) 15:793–5. 10.1016/j.jcmg.2022.01.012
238.
Hwang M Song JS Lee YS Li C Shim EB Pak HN . Electrophysiological rotor ablation in in-silico modeling of atrial fibrillation: comparisons with dominant frequency, shannon entropy, and phase singularity. PLoS One. (2016) 11:e0149695. 10.1371/journal.pone.0149695
239.
Ganesan AN Kuklik P Lau DH Brooks AG Baumert M Lim WW et al Bipolar electrogram shannon entropy at sites of rotational activation: implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. (2013) 6:48–57. 10.1161/CIRCEP.112.976654
240.
Ng J Borodyanskiy AI Chang ET Villuendas R Dibs S Kadish AH et al Measuring the complexity of atrial fibrillation electrograms. J Cardiovasc Electrophysiol. (2010) 21:649–55. 10.1111/j.1540-8167.2009.01695.x
241.
Gigli L Preda A Coluzzi D Sartore M Vila M Carbonaro M et al Left atrial spatial entropy: a novel tool for electrophysiological substrate characterization in atrial fibrillation. Front Physiol. (2024) 15:1474568. 10.3389/fphys.2024.1474568
242.
Badano LP Kolias TJ Muraru D Abraham TP Aurigemma G Edvardsen T et al Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600. 10.1093/ehjci/jey042
243.
Cameli M Caputo M Mondillo S Ballo P Palmerini E Lisi M et al Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. (2009) 7:6. 10.1186/1476-7120-7-6
244.
Lenart-Migdalska A Kaznica-Wiatr M Drabik L Knap K Smas-Suska M Podolec PP et al Assessment of left atrial function in patients with paroxysmal, persistent, and permanent atrial fibrillation using two-dimensional strain. J Atr Fibrillation. (2019) 12:2148. 10.4022/jafib.2148
245.
Saraiva RM Demirkol S Buakhamsri A Greenberg N Popovic ZB Thomas JD et al Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. (2010) 23:172–80. 10.1016/j.echo.2009.11.003
246.
Kuppahally SS Akoum N Badger TJ Burgon NS Haslam T Kholmovski E et al Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. (2010) 160:877–84. 10.1016/j.ahj.2010.07.003
247.
Kuppahally SS Akoum N Burgon NS Badger TJ Kholmovski EG Vijayakumar S et al Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. (2010) 3:231–9. 10.1161/CIRCIMAGING.109.865683
248.
Peters DC Duncan JS Grunseich K Marieb MA Cornfeld D Sinusas AJ et al CMR-verified lower LA strain in the presence of regional atrial fibrosis in atrial fibrillation. JACC Cardiovasc Imaging. (2017) 10:207–8. 10.1016/j.jcmg.2016.01.015
249.
Kowallick JT Kutty S Edelmann F Chiribiri A Villa A Steinmetz M et al Quantification of left atrial strain and strain rate using cardiovascular magnetic resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson. (2014) 16:60. 10.1186/s12968-014-0060-6
250.
Wang Y Li Z Fei H Yu Y Ren S Lin Q et al Left atrial strain reproducibility using vendor-dependent and vendor-independent software. Cardiovasc Ultrasound. (2019) 17:9. 10.1186/s12947-019-0158-y
251.
Pathan F D'Elia N Nolan MT Marwick TH Negishi K . Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. (2017) 30:59–70.e58. 10.1016/j.echo.2016.09.007
252.
Demir M Aktas I Camci S . Left atrial mechanical function and stiffness in patients with atrial septal aneurysm: a speckle tracking study. Cardiol J. (2015) 22:535–40. 10.5603/CJ.a2015.0033
253.
Anderson JL Halperin JL Albert NM Bozkurt B Brindis RG Curtis LH et al Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2013) 61:1935–44. 10.1016/j.jacc.2013.02.001
254.
Holmqvist F Kesek M Englund A Blomstrom-Lundqvist C Karlsson LO Kenneback G et al A decade of catheter ablation of cardiac arrhythmias in Sweden: ablation practices and outcomes. Eur Heart J. (2019) 40:820–30. 10.1093/eurheartj/ehy709
255.
Sultan A Luker J Andresen D Kuck KH Hoffmann E Brachmann J et al Predictors of atrial fibrillation recurrence after catheter ablation: data from the German ablation registry. Sci Rep. (2017) 7:16678. 10.1038/s41598-017-16938-6
256.
Brooks AG Stiles MK Laborderie J Lau DH Kuklik P Shipp NJ et al Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm. (2010) 7:835–46. 10.1016/j.hrthm.2010.01.017
257.
McGann C Akoum N Patel A Kholmovski E Revelo P Damal K et al Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. (2014) 7:23–30. 10.1161/CIRCEP.113.000689
258.
Boyle PM Zghaib T Zahid S Ali RL Deng D Franceschi WH et al Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng. (2019) 3:870–9. 10.1038/s41551-019-0437-9
259.
Fochler F Yamaguchi T Kheirkahan M Kholmovski EG Morris AK Marrouche NF . Late gadolinium enhancement magnetic resonance imaging guided treatment of post-atrial fibrillation ablation recurrent arrhythmia. Circ Arrhythm Electrophysiol. (2019) 12:e007174. 10.1161/CIRCEP.119.007174
260.
Yagishita A Gimbel JR DE Oliveira S Manyam H Sparano D Cakulev I et al Long-term outcome of left atrial voltage-guided substrate ablation during atrial fibrillation: a novel adjunctive ablation strategy. J Cardiovasc Electrophysiol. (2017) 28:147–55. 10.1111/jce.13122
261.
Yamaguchi T Tsuchiya T Nakahara S Fukui A Nagamoto Y Murotani K et al Efficacy of left atrial voltage-based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. (2016) 27:1055–63. 10.1111/jce.13019
262.
Kottkamp H Berg J Bender R Rieger A Schreiber D . Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. (2016) 27:22–30. 10.1111/jce.12870
263.
Cheng X Hu Q Gao L Liu J Qin S Zhang D . Sex-related differences in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. (2019) 21:1509–18. 10.1093/europace/euz179
264.
Cheung JW Cheng EP Wu X Yeo I Christos PJ Kamel H et al Sex-based differences in outcomes, 30-day readmissions, and costs following catheter ablation of atrial fibrillation: the United States nationwide readmissions database 2010–14. Eur Heart J. (2019) 40:3035–43. 10.1093/eurheartj/ehz151
265.
Kuck KH Brugada J Furnkranz A Chun KRJ Metzner A Ouyang F et al Impact of female sex on clinical outcomes in the FIRE AND ICE trial of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2018) 11:e006204. 10.1161/CIRCEP.118.006204
266.
Cochet H Mouries A Nivet H Sacher F Derval N Denis A et al Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. (2015) 26:484–92. 10.1111/jce.12651
267.
Li Z Wang Z Yin Z Zhang Y Xue X Han J et al Gender differences in fibrosis remodeling in patients with long-standing persistent atrial fibrillation. Oncotarget. (2017) 8:53714–29. 10.18632/oncotarget.16342
268.
Kim DY Kim YG Choi HY Choi YY Boo KY Lee KN et al Sex-related differences in left atrial low-voltage areas according to CHA(2)DS(2)-VA scores among patients with atrial fibrillation. J Clin Med. (2022) 11:3111. 10.3390/jcm11113111
269.
Wong GR Nalliah CJ Lee G Voskoboinik A Chieng D Prabhu S et al Sex-related differences in atrial remodeling in patients with atrial fibrillation: relationship to ablation outcomes. Circ Arrhythm Electrophysiol. (2022) 15:e009925. 10.1161/CIRCEP.121.009925
270.
Jansen HJ Mackasey M Moghtadaei M Belke DD Egom EE Tuomi JM et al Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillation. J Mol Cell Cardiol. (2018) 124:12–25. 10.1016/j.yjmcc.2018.09.011
271.
Sanders P Elliott AD Linz D . Upstream targets to treat atrial fibrillation. J Am Coll Cardiol. (2017) 70:2906–8. 10.1016/j.jacc.2017.10.043
272.
Shahid F Lip GYH Shantsila E . Renin-angiotensin blockade in atrial fibrillation: where are we now?J Hum Hypertens. (2017) 31:425–6. 10.1038/jhh.2017.6
273.
Takemoto Y Ramirez RJ Kaur K Salvador-Montanes O Ponce-Balbuena D Ramos-Mondragon R et al Eplerenone reduces atrial fibrillation burden without preventing atrial electrical remodeling. J Am Coll Cardiol. (2017) 70:2893–905. 10.1016/j.jacc.2017.10.014
274.
Swedberg K Zannad F McMurray JJ Krum H van Veldhuisen DJ Shi H et al Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (eplerenone in mild patients hospitalization and SurvIval study in heart failure) study. J Am Coll Cardiol. (2012) 59:1598–603. 10.1016/j.jacc.2011.11.063
275.
Nomura M Kawano T Nakayasu K Nakaya Y . The effects of losartan on signal-averaged P wave in patients with atrial fibrillation. Int J Cardiol. (2008) 126:21–7. 10.1016/j.ijcard.2007.03.106
276.
Kumagai K Nakashima H Urata H Gondo N Arakawa K Saku K . Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. (2003) 41:2197–204. 10.1016/s0735-1097(03)00464-9
277.
Goette A Staack T Rocken C Arndt M Geller JC Huth C et al Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. (2000) 35:1669–77. 10.1016/s0735-1097(00)00611-2
278.
Liang Z Shi XM Liu LF Chen XP Shan ZL Lin K et al Renal denervation suppresses atrial fibrillation in a model of renal impairment. PLoS One. (2015) 10:e0124123. 10.1371/journal.pone.0124123
279.
Benigni A Cassis P Remuzzi G . Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. (2010) 2:247–57. 10.1002/emmm.201000080
280.
Korantzopoulos P Goudevenos JA . Aldosterone signaling in atrial fibrillation another piece in the puzzle of atrial remodeling. J Am Coll Cardiol. (2010) 55:771–3. 10.1016/j.jacc.2009.10.032
281.
Manabe S Okura T Watanabe S Fukuoka T Higaki J . Effects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertension. J Cardiovasc Pharmacol. (2005) 46:735–9. 10.1097/01.fjc.0000185783.00391.60
282.
Takeda T Hoshida S Nishino M Tanouchi J Otsu K Hori M . Relationship between effects of statins, aspirin and angiotensin II modulators on high-sensitive C-reactive protein levels. Atherosclerosis. (2003) 169:155–8. 10.1016/s0021-9150(03)00158-8
283.
Xu W Yang YM Zhu J Wu S Wang J Zhang H et al Impact of renin-angiotensin-aldosterone-system inhibitor drugs on mortality in patients with atrial fibrillation and hypertension. BMC Cardiovasc Disord. (2022) 22:141. 10.1186/s12872-022-02580-2
284.
McMurray JJ Packer M Desai AS Gong J Lefkowitz MP Rizkala AR et al Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371:993–1004. 10.1056/NEJMoa1409077
285.
Hubers SA Brown NJ . Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. (2016) 133:1115–24. 10.1161/CIRCULATIONAHA.115.018622
286.
Fujita S Shimojo N Terasaki F Otsuka K Hosotani N Kohda Y et al Atrial natriuretic peptide exerts protective action against angiotensin II-induced cardiac remodeling by attenuating inflammation via endothelin-1/endothelin receptor A cascade. Heart Vessels. (2013) 28:646–57. 10.1007/s00380-012-0311-0
287.
Hayashi D Kudoh S Shiojima I Zou Y Harada K Shimoyama M et al Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mitogen-activated protein kinase phosphatase-1. Biochem Biophys Res Commun. (2004) 322:310–9. 10.1016/j.bbrc.2004.07.119
288.
Kapoun AM Liang F O’Young G Damm DL Quon D White RT et al B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. (2004) 94:453–61. 10.1161/01.RES.0000117070.86556.9F
289.
Suo Y Yuan M Li H Zhang Y Li Y Fu H et al Sacubitril/valsartan improves left atrial and left atrial appendage function in patients with atrial fibrillation and in pressure overload-induced mice. Front Pharmacol. (2019) 10:1285. 10.3389/fphar.2019.01285
290.
Ishii Y Schuessler RB Gaynor SL Hames K Damiano RJ Jr . Postoperative atrial fibrillation: the role of the inflammatory response. J Thorac Cardiovasc Surg. (2017) 153:1357–65. 10.1016/j.jtcvs.2016.12.051
291.
Kim YR Nam GB Han S Kim SH Kim KH Lee S et al Effect of short-term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. (2015) 8:1366–72. 10.1161/CIRCEP.115.002957
292.
Liu C Wang J Yiu D Liu K . The efficacy of glucocorticoids for the prevention of atrial fibrillation, or length of intensive care unite or hospital stay after cardiac surgery: a meta-analysis. Cardiovasc Ther. (2014) 32:89–96. 10.1111/1755-5922.12062
293.
Halonen J Halonen P Jarvinen O Taskinen P Auvinen T Tarkka M et al Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA. (2007) 297:1562–7. 10.1001/jama.297.14.1562
294.
Prasongsukarn K Abel JG Jamieson WR Cheung A Russell JA Walley KR et al The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: a prospective randomized trial. J Thorac Cardiovasc Surg. (2005) 130:93–8. 10.1016/j.jtcvs.2004.09.014
295.
Lennerz C Barman M Tantawy M Sopher M Whittaker P . Colchicine for primary prevention of atrial fibrillation after open-heart surgery: systematic review and meta-analysis. Int J Cardiol. (2017) 249:127–37. 10.1016/j.ijcard.2017.08.039
296.
Salih M Smer A Charnigo R Ayan M Darrat YH Traina M et al Colchicine for prevention of post-cardiac procedure atrial fibrillation: meta-analysis of randomized controlled trials. Int J Cardiol. (2017) 243:258–62. 10.1016/j.ijcard.2017.04.022
297.
Verma S Eikelboom JW Nidorf SM Al-Omran M Gupta N Teoh H et al Colchicine in cardiac disease: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. (2015) 15:96. 10.1186/s12872-015-0068-3
298.
Deftereos S Giannopoulos G Kossyvakis C Efremidis M Panagopoulou V Kaoukis A et al Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. (2012) 60:1790–6. 10.1016/j.jacc.2012.07.031
299.
Imazio M Brucato A Ferrazzi P Rovere ME Gandino A Cemin R et al Colchicine reduces postoperative atrial fibrillation: results of the colchicine for the prevention of the postpericardiotomy syndrome (COPPS) atrial fibrillation substudy. Circulation. (2011) 124:2290–5. 10.1161/CIRCULATIONAHA.111.026153
300.
An J Shi F Liu S Ma J Ma Q . Preoperative statins as modifiers of cardiac and inflammatory outcomes following coronary artery bypass graft surgery: a meta-analysis. Interact Cardiovasc Thorac Surg. (2017) 25:958–65. 10.1093/icvts/ivx172
301.
Yan P Dong P Li Z Cheng J . Statin therapy decreased the recurrence frequency of atrial fibrillation after electrical cardioversion: a meta-analysis. Med Sci Monit. (2014) 20:2753–8. 10.12659/MSM.891049
302.
Pinho-Gomes AC Reilly S Brandes RP Casadei B . Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Signal. (2014) 20:1268–85. 10.1089/ars.2013.5542
303.
Reilly SN Jayaram R Nahar K Antoniades C Verheule S Channon KM et al Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. (2011) 124:1107–17. 10.1161/CIRCULATIONAHA.111.029223
304.
Liu T Li L Korantzopoulos P Liu E Li G . Statin use and development of atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials and observational studies. Int J Cardiol. (2008) 126:160–70. 10.1016/j.ijcard.2007.07.137
305.
Siu CW Lau CP Tse HF . Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol. (2003) 92:1343–5. 10.1016/j.amjcard.2003.08.023
306.
Su JH Luo MY Liang N Gong SX Chen W Huang WQ et al Interleukin-6: a novel target for cardio-cerebrovascular diseases. Front Pharmacol. (2021) 12:745061. 10.3389/fphar.2021.745061
307.
Everett BM Cornel JH Lainscak M Anker SD Abbate A Thuren T et al Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. (2019) 139:1289–99. 10.1161/CIRCULATIONAHA.118.038010
308.
Ridker PM Everett BM Thuren T MacFadyen JG Chang WH Ballantyne C et al Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. 10.1056/NEJMoa1707914
309.
Kleveland O Kunszt G Bratlie M Ueland T Broch K Holte E et al Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J. (2016) 37:2406–13. 10.1093/eurheartj/ehw171
310.
Pavicic T Ruska B Adamec I Habek M . Recurrent atrial fibrillation after pulse corticosteroid treatment for a relapse of multiple sclerosis. Mult Scler Relat Disord. (2019) 32:30–2. 10.1016/j.msard.2019.04.022
311.
Wang X Peng X Li Y Lin R Liu X Ruan Y et al Colchicine for prevention of post-cardiac surgery and post-pulmonary vein isolation atrial fibrillation: a meta-analysis. Rev Cardiovasc Med. (2022) 23:387. 10.31083/j.rcm2312387
312.
Du X Dong J Ma C . Is atrial fibrillation a preventable disease?J Am Coll Cardiol. (2017) 69:1968–82. 10.1016/j.jacc.2017.02.020
313.
Rienstra M Hobbelt AH Alings M Tijssen JGP Smit MD Brugemann J et al Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. (2018) 39:2987–96. 10.1093/eurheartj/ehx739
314.
Hong KL Glover BM . The impact of lifestyle intervention on atrial fibrillation. Curr Opin Cardiol. (2018) 33:14–9. 10.1097/HCO.0000000000000470
315.
Lau DH Nattel S Kalman JM Sanders P . Modifiable risk factors and atrial fibrillation. Circulation. (2017) 136:583–96. 10.1161/CIRCULATIONAHA.116.023163
316.
Pathak RK Middeldorp ME Lau DH Mehta AB Mahajan R Twomey D et al Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. (2014) 64:2222–31. 10.1016/j.jacc.2014.09.028
317.
Abed HS Wittert GA Leong DP Shirazi MG Bahrami B Middeldorp ME et al Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. (2013) 310:2050–60. 10.1001/jama.2013.280521
Summary
Keywords
atrial fibrillation, fibrillation, atrial, inflammation, fibrosis, atrial fibrosis
Citation
Pang Z, Ren Y and Yao Z (2025) Interactions between atrial fibrosis and inflammation in atrial fibrillation. Front. Cardiovasc. Med. 12:1578148. doi: 10.3389/fcvm.2025.1578148
Received
17 February 2025
Accepted
26 June 2025
Published
10 July 2025
Volume
12 - 2025
Edited by
Sebastian Clauss, Ludwig Maximilian University of Munich, Germany
Reviewed by
Weichieh Lee, Chi Mei Medical Center, Taiwan
Alberto Preda, Niguarda Ca’ Granda Hospital, Italy
Updates
Copyright
© 2025 Pang, Ren and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zhuhua Yao yaozhuhua021@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.