Abstract
Objectives:
Arterial hypertension plays a significant role in promoting organ damage and the development of atherosclerosis. The neutrophil–lymphocyte ratio (NLR) is an accessible and cost-effective biomarker that has been strongly associated with adverse outcomes in patients with coronary artery disease and chronic heart failure. The aim of this study was to evaluate the clinical utility of NLR as a surrogate biomarker of subclinical atherosclerotic damage in patients with essential hypertension.
Methods:
From January 2024 to November 2024, we consecutively enrolled 346 patients with essential hypertension. For all patients, we collected medical history, anthropometric data, biochemical analyses, and subclinical organ damage, including 24-h urinary excretion of microalbuminuria, carotid intima–media thickness, and transthoracic echocardiography. We excluded patients with arterial hypertension, coronary artery disease, or cerebrovascular or peripheral artery disease.
Results:
In our study, we found that patients with higher NLR were associated with high blood pressure values, the use of more than three antihypertensive medications, and a higher prevalence of dyslipidemia and obstructive sleep apnea syndrome. Moreover, elevated NLR values correlated with a higher prevalence of subclinical organ damage (left cardiac ventricular mass, carotid atherosclerosis, and increased microalbuminuria).
Conclusions:
Our study shows that in patients with essential hypertension, NLR is significantly correlated with some cardiovascular comorbidities and subclinical organ damage.
Introduction
Cardiovascular diseases (CVDs), including coronary artery disease (CAD), heart failure, and stroke, remain the leading causes of morbidity and mortality worldwide. In this context, arterial hypertension plays a significant role in promoting the development of atherosclerosis and CVDs (1–3). Arterial hypertension strongly drives the progressive inflammatory processes underlying atherosclerosis, interacting with both innate and adaptive immunity at the vascular level and in target organs (4, 5).
While traditional inflammatory markers such as C-reactive protein (CRP) and IL-6 have been extensively studied, more accessible and cost-effective biomarkers like the neutrophil–lymphocyte ratio (NLR) have gained increasing attention (6). NLR, calculated from routine blood counts, provides a simple measure of systemic inflammation. Neutrophils and lymphocytes are key components of the immune response (7). Neutrophils are primarily involved in acute inflammation and are often elevated in response to infection, stress, or tissue injury. In contrast, lymphocytes represent the adaptive immune response and tend to decrease under chronic stress and inflammatory conditions. Thus, the NLR reflects the balance between these two immune cell populations. A high NLR suggests a shift toward pro-inflammatory processes, which may contribute to endothelial dysfunction and arterial plaque formation, promoting the progression of atherosclerosis (8).
An elevated NLR has been associated with poor outcomes in various CVDs, representing a potential tool for risk stratification (9). Numerous studies have confirmed a strong association between elevated NLR and adverse outcomes in patients with CAD. A high NLR has been linked to greater severity of coronary artery stenosis, worse outcomes following percutaneous coronary intervention, and an increased risk of major adverse cardiovascular events, including myocardial infarction and death (10). A recent meta-analysis highlighted that patients with higher NLRs are more likely to experience recurrent cardiovascular events after acute coronary syndrome (ACS), as well as increased mortality and hospitalization rates related to acute and chronic heart failure (11). The inflammatory environment in heart failure, driven by immune dysregulation and oxidative stress, may be reflected by elevated NLRs, making it a valuable marker of disease. Similarly, in patients with peripheral artery disease (PAD), a high NLR has been correlated with poor outcomes. The chronic inflammatory state in PAD, characterized by vascular inflammation and immune cell activation, is reflected in increased NLRs, suggesting its potential utility in predicting disease progression and related complications (12).
However, limited data are available in the literature regarding the usefulness of NLR in patients with essential arterial hypertension, particularly among those without a history of major cardiovascular events.
The aim of the present study was to evaluate the clinical utility of NLR in patients with essential hypertension, particularly in relation to the evaluation of cardiovascular comorbidities and subclinical atherosclerotic damage.
Methods
From January 2024 to December 2024, 346 patients (186 men and 160 women) affected by essential hypertension were enrolled at the Center of Arterial Hypertension, Policlinico Umberto I Hospital, University Sapienza, Rome, Italy. All patients underwent anthropometric measurements, fasting venous blood sampling, 24-h urine collection, carotid intima–media thickness (cIMT) assessment, and transthoracic echocardiography.
This study was conducted in accordance with the guidelines of the Declaration of Helsinki II and was approved by the local ethical committee. The study design was clearly written in layperson language and provided to all study participants. Written informed consent was obtained from all patients. Clinical data were obtained as part of routine clinical practice and approved by the Local Ethical Committee of the Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, “Sapienza” University of Rome, Italy (date of approval: December 19, 2023).
Anthropometric measurements
Anthropometric data were collected from all participants. Standing height was measured on barefoot to the nearest 0.5 cm. Weight was measured in light clothing using a platform scale, accurate to the nearest 200 g, with the scale standardized to 0 before each use. Waist circumference was measured to the nearest 0.1 cm using a standard tape placed over the abdomen at the narrowest diameter between the costal margin and the iliac crest. Hip circumference was measured to the nearest 0.1 cm using a non-stretchable standard tape. Measurements were taken over light clothing at the level of the greater trochanter (usually representing the widest diameter around the buttocks). For both waist and hip measurements, the tape was kept horizontal and just tight enough to allow the insertion of a little finger beneath it. Two measurements were taken, and the average value was used for this analysis. Body mass index (BMI) was calculated as weight/(height)2.
Blood arterial pressure assessment and essential hypertension definition
Office blood pressure (BP) was measured using a standard aneroid sphygmomanometer after participants had been seated for 5 min. Systolic blood pressure (SBP) was recorded at the first sound on deflation of the cuff (Korotkoff phase I), and diastolic BP (DBP) was recorded at the complete disappearance of Korotkoff sounds (phase V). Essential hypertension was defined as a BP of 140/90 mmHg or more in three consecutive measurements or in patients receiving antihypertensive therapy. Secondary causes of arterial hypertension were excluded after specific evaluation on the basis of clinical, laboratory, hormonal, and imaging examinations (13). Individuals with a clinical history, clinical symptoms, or electrocardiographic, echocardiographic, or angiographic signs of coronary artery disease, heart failure, cardiomyopathies, or valvular or pericardial diseases were excluded. Individuals with a history of cerebrovascular or peripheral artery disease, hepatic disease, or drug abuse were excluded.
Measurement of carotid intima–media thickness
A Hewlett-Packard Sonor 5500 Ultrasound system (Hewlett-Packard, Andover, Massachusetts, USA), equipped with a 3.11-MHz real-time B-mode scanner, was used for carotid imaging. The right common carotid artery (CCA) was examined with participants turning their heads 45° to the left. High-resolution images were analyzed to calculate the cIMT, defined as the thickness of the vascular intima–media complex, measured at five consecutive regions of the CCA wall spaced every 4–5 mm, beginning near the bifurcation. For each individual, the cIMT value was calculated as the average of five measurements from the left and five from the right carotid artery. The mean common carotid diameter was defined as the distance across the media–adventitia interface from the near to the far wall and was calculated automatically by averaging measurements taken at 0.1 cm intervals over a 1-cm segment.
Assessment of echocardiography variables
Echocardiography was performed by expert cardiologists using a General Electric Vivid 7 ultrasound machine (General Electric Medical Systems, Horten, Norway) with a 2.5-MHz transducer and an Aplio CV Toshiba system with a 3-MHz transducer, according to the American Society of Echocardiography guidelines (14). Left ventricular (LV) end-diastolic and end-systolic diameters, as well as wall thickness, were assessed using M-mode. LV ejection fraction and fractional shortening were measured in biplane 2D mode using Simpson's method. LV mass was estimated using the Devereux formula and normalized by height (in meters) (LVMi) raised to the 2.7 power to avoid underestimation in overweight or obese patients. Left ventricular hypertrophy (LVH) was determined as detailed in the Supplemental Methods, defined as LV mass/height ≥50 g/m2.7 for men and ≥47 g/m2.7 for women, following recent recommendations. LV geometry was examined using the four-tiered classification of LVH based on concentricity (defined as relative wall thickness ≥0.42) and LV end-diastolic volume (considered increased when LV end-diastolic volume/body surface area ≥74 mL/m2 in men and 61 mL/m2 in women). The intra-observer variation coefficient of echocardiography parameters was ≈4% for M-mode measurements and within 10% for 2D and Doppler-derived variables. Bland–Altman plots were used to verify the reproducibility of echocardiographic measurements and exclude systematic biases.
Chronic kidney disease (CKD) assessment
Chronic renal disease was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, calculated using the CKD-EPI formula, or persistently elevated 24-h albumin urinary excretion (15).
Statistical analysis
All data are presented as mean ± standard deviation. Differences between means were assessed using Student's t-test or the Mann–Whitney U-test for non-normally distributed data in two-sample comparisons and by one-way analysis of variance with the Fisher least significant difference post hoc test for multiple comparisons. χ2 statistics were used to assess differences between categorical variables. Relationships between continuous variables were assessed by calculating the Pearson correlation coefficient or the Spearman rank correlation coefficient, as appropriate. Univariable logistic regression analysis was performed to evaluate the association between NLR and each established endpoint. Furthermore, we compared the predictive performance of NLR, fasting plasma glucose, creatinine, CRP, and uric acid using receiver operating characteristic (ROC) curves, with the area under the curve (AUC) estimated as a continuous variable for each marker. All tests were two-tailed, and analyses were performed using SPSS, version 25.0 (IBM). p values <0.05 were considered statistically significant.
Results
In the study, we enrolled 346 consecutive patients with essential hypertension (mean age 53.4 ± 14.8 years; 46% women) (Table 1). In our cohort, the mean BMI was 26.8 ± 4.8 kg/m2, with mean SBP and DBP values of 141 ± 20 mmHg and 87 ± 12 mmHg, respectively. When stratifying the population into tertiles of NLR, we observed that patients in the third tertile were older (50.1 ± 15.7 vs. 58.7 ± 13.3 years, respectively; p < 0.001) and exhibited higher SBP (136 ± 18 vs. 143 ± 20 mmHg, p < 0.02) and DBP (85 ± 12 vs. 86 ± 12 mmHg, p < 0.01). No significant differences were observed for sex, BMI, and heart rate (HR) in all groups. Hypertensive patients in the third NLR tertile showed significantly higher serum creatinine levels (0.92 ± 0.16 vs. 0.99 ± 0.34 mg/dL, p = 0.04), while no significant differences were found in glucose blood levels or serum levels of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol (Table 2). Regarding comorbidities, patients in the third NLR tertile were more frequently affected by dyslipidemia (49%) and OSAS (12.2%) and were treated with more than three antihypertensive medications (27%) compared to those in the first NLR tertile (37%, 7.8%, and 10%, respectively; p < 0.05) (Figure 1).
Table 1
| Enrolled patients | Age | M/F | BMI | SBP | DBP | HR |
|---|---|---|---|---|---|---|
| (years) | (%) | (kg/m2) | (mmHg) | (mmHg) | (bpm) | |
| All patients (n=346) |
53.4 ± 14.8 | 54/46 | 26.8 ± 4.8 | 141 ± 20 | 87 ± 12 | 68 ± 11 |
| First tertile NLR (n=115) |
50.1 ± 15.7 | 47/53 | 26.6 ± 4.8 | 136 ± 18 | 85 ± 12 | 68 ± 11 |
| Second tertile NLR (n=116) |
51.2 ± 13.6 | 58/42 | 27.1 ± 5.3 | 143 ± 21 | 90 ± 13 | 67 ± 10 |
| Third tertile NLR (n=115) |
58.7 ± 13.3* | 58/42 | 26.6 ± 4.2 | 143 ± 20* | 86 ± 12* | 70 ± 12 |
|
p-Value Third vs. first tertile |
<0.001 | n.s. | n.s. | <0.02 | <0.01 | n.s. |
Anthropometric measurements in enrolled patients, distinguished by NLR tertiles.
M/F, male/female ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Bold values report statistically significant comparisons.
*3rd vs 1st tertile <0.04.
Table 2
| Enrolled patients | Creatinine | Glycaemia | LDL-C | HDL-C | Trgls | Uric acid | Microalbuminuria | VGF | NLR |
|---|---|---|---|---|---|---|---|---|---|
| (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mg/dL) | (mL/min/ 1.73 m2) | ||
| All patients (n=346) |
0.96 ± 0.26 | 94.3 ± 7.2 | 105.5 ± 13.3 | 51.6 ± 14.3 | 103 ± 20 | 5.61 ± 0.46 | 47.9 ± 9.3 | 81.6 ± 13.6 | 2.4 ± 1.2 |
| First tertile NLR (n=115) |
0.92 ± 0.16 | 93.9 ± 10.6 | 109.4 ± 18.8 | 52.1 ± 13.9 | 106.6 ± 29 | 5.68 ± 0.60 | 13.3 ± 3 | 84.6 ± 17 | 1.4 ± 0.2 |
| Second tertile NLR (n=116) |
0.96 ± 0.22 | 92.3 ± 9.8 | 101.8 ± 14 | 49.5 ± 12.8 | 104.6 ± 22 | 5.75 ± 0.43 | 45.3 ± 22 | 81.5 ± 13 | 2.2 ± 0.2 |
| Third tertile NLR (n=115) |
0.99 ± 0.34* | 96.5 ± 11.6 | 105.5 ± 16.4 | 53.2 ± 15.8 | 96.8 ± 2 | 5.61 ± 0.47 | 87.3 ± 3* | 78.7 ± 11* | 3.6 ± 0.6* |
|
p-value Third vs. first tertile |
0.04 | n.s. | n.s. | n.s. | n.s. | n.s. | 0.05 | 0.05 | <0.001 |
Biochemical parameters of enrolled patients, distinguished by NLR tertiles.
LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; Trgls, triglycerides; VGF, velocity glomerular filtration.
Bold values report statistically significant comparisons.
*3rd vs 1st tertile <0.04.
Figure 1
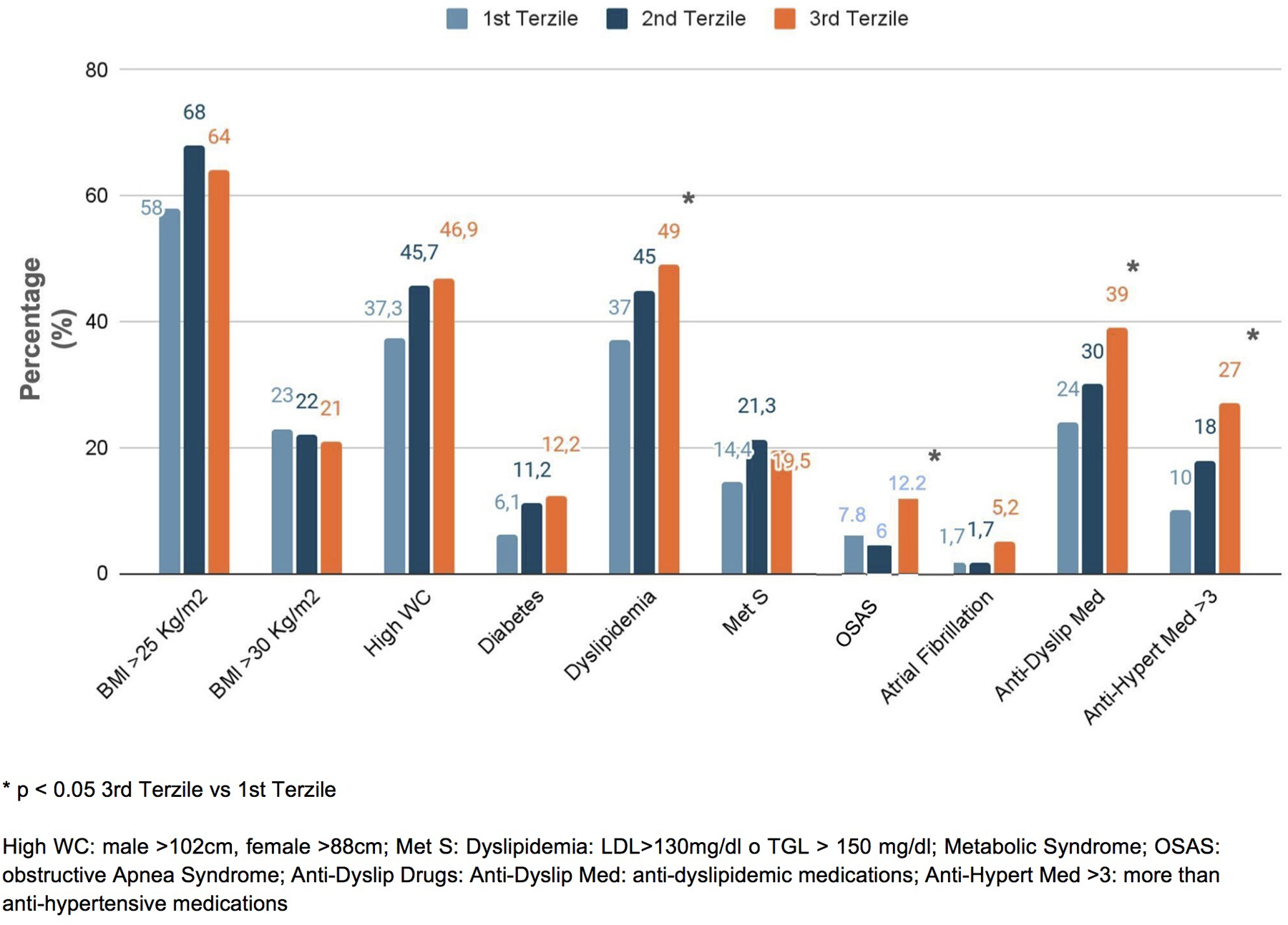
Prevalence of comorbidities in the enrolled patients, distinguished into tertiles of NLR.
In terms of organ damage, patients with higher NLRs exhibited a greater prevalence of subclinical cardiovascular changes. Specifically, those in the third tertile had a higher left ventricular mass index (LVMi) (89.4 ± 12 g/m²) and carotid intima–media thickness (cIMT) (0.83 ± 0.15 mm) compared to the first tertile (83.3 ± 14 g/m² and 0.78 ± 0.16 mm, respectively; p < 0.05). Additionally, carotid plaques were more frequent in the third tertile (41.7%) than in the first and second tertiles (29.6% and 28.4%, respectively; p < 0.05) (Figure 2). Furthermore, patients in the third NLR tertile showed significantly reduced kidney function, as indicated by a lower eGFR (78.7 ± 11 mL/min/1.73 m2) and higher 24-h urinary excretion of microalbuminuria (80.7 ± 13 mg/dL) compared to patients in the first NLR tertile (84.6 ± 17 mL/min/1.73 m2 and 13.5 ± 7 mg/dL, respectively; p < 0.05) (Figure 3).
Figure 2
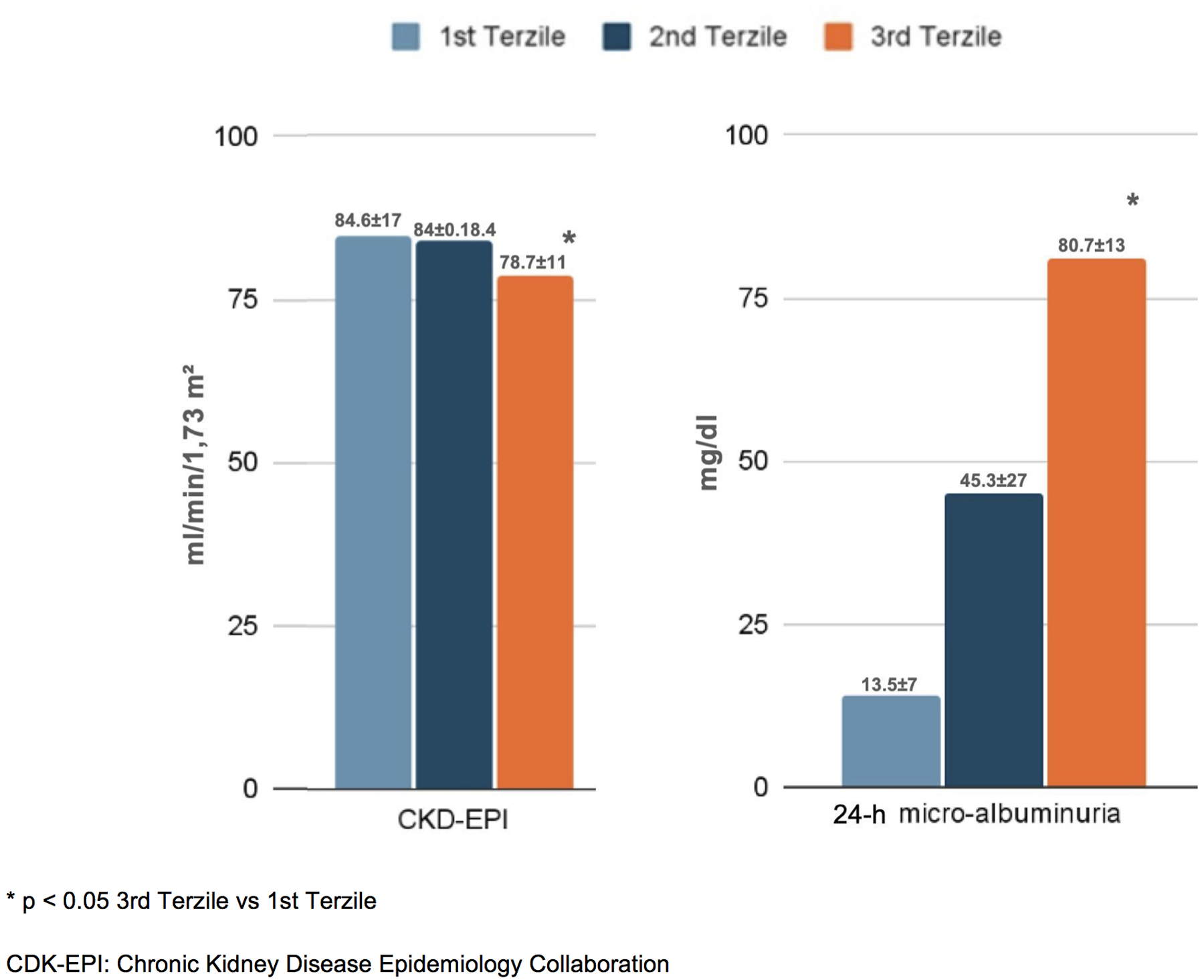
Cardiac and vascular damage in the enrolled patients, distinguished into tertiles of NLR.
Figure 3
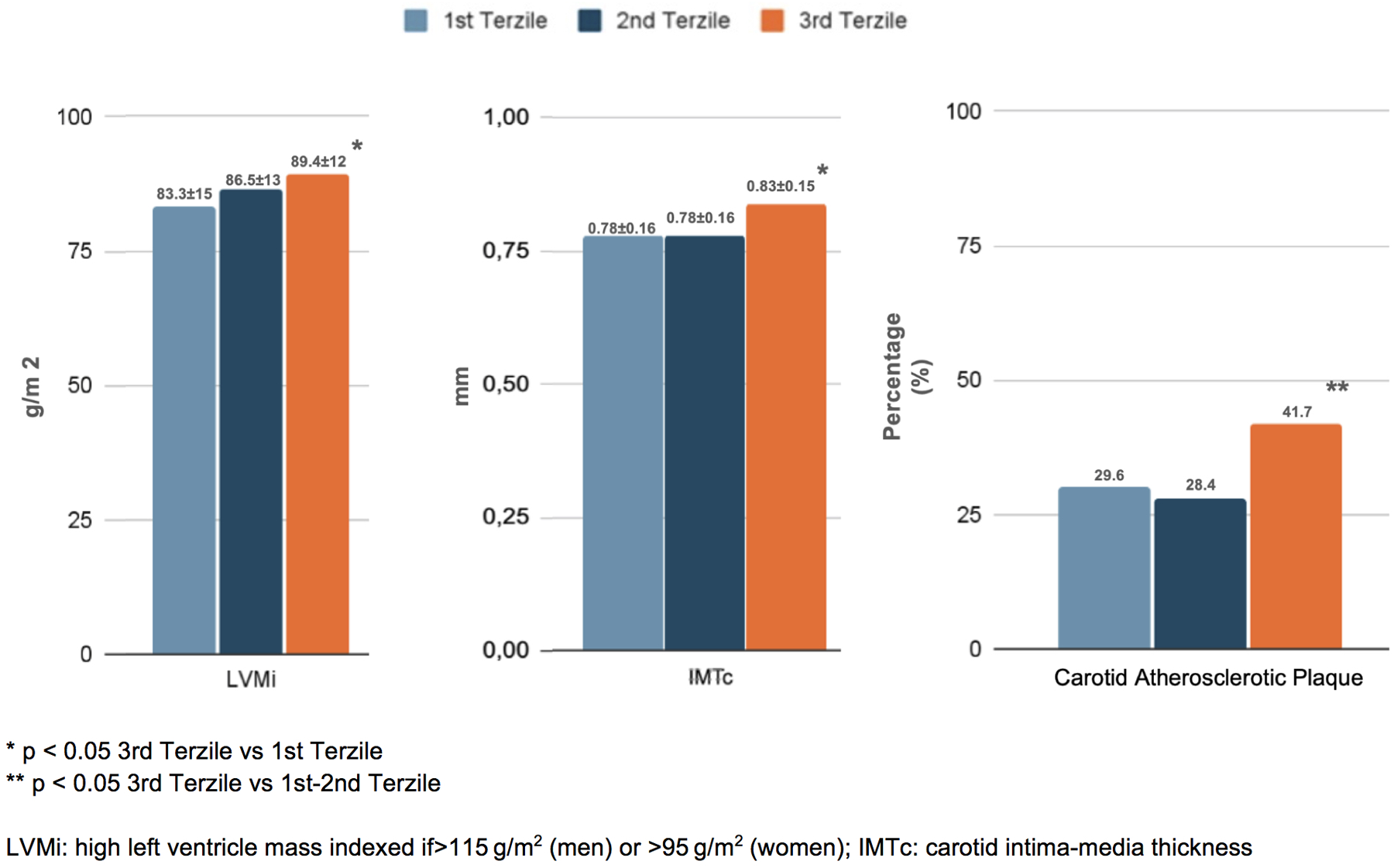
Renal damage in the enrolled patients, distinguished into tertiles of NLR.
We then conducted a univariable regression analysis to assess the association between NLR and study parameters. A higher NLR was strongly associated with increased LVMi [odds ratio (OR) 1.432, 95% confidence interval (95% CI) 1.080–1.896, p = 0.012], eGFR < 60 mL/min (OR 1.283, 95% CI 1.029–1.599, p = 0.027), cIMT > 0.9 mm (OR 1.266, 95% CI 1.040–1.543, p = 0.019), and the presence of carotid plaques (OR 1.307, 95% CI 1.072–1.594, p = 0.011) (Table 3).
Table 3
| Endpoint | Odds ratio | 95% CI | p-Value |
|---|---|---|---|
| LVMi high | 1.432 | 1.080–1.896 | 0.012 |
| eGFR < 60 mL/min | 1.283 | 1.029–1.599 | 0.027 |
| cIMT > 0.9 mm | 1.266 | 1.040–1.543 | 0.019 |
| Carotid atherosclerosis | 1.307 | 1.072–1.594 | 0.011 |
| CVD risk | 1.369 | 1.081–1.733 | 0.009 |
Univariate analysis of the association between NLR, target organ damage, and cardiovascular risk assessment.
CVD risk: at least one cardiovascular risk factor (smoking, dyslipidemia, hypertension, diabetes mellitus, obesity).
Finally, when evaluating different parameters, including glycemia, CRP, plasma uric acid, and creatinine, we found that NLR showed a stronger ability to identify the coexistence of at least two cardiovascular risk factors (AUC 0.591; p = 0.020) (Figure 4) and patients taking ≥3 antihypertensive medications or those with the presence of atherosclerotic plaques (AUC 0.634; p = 0.001) (Figure 5).
Figure 4
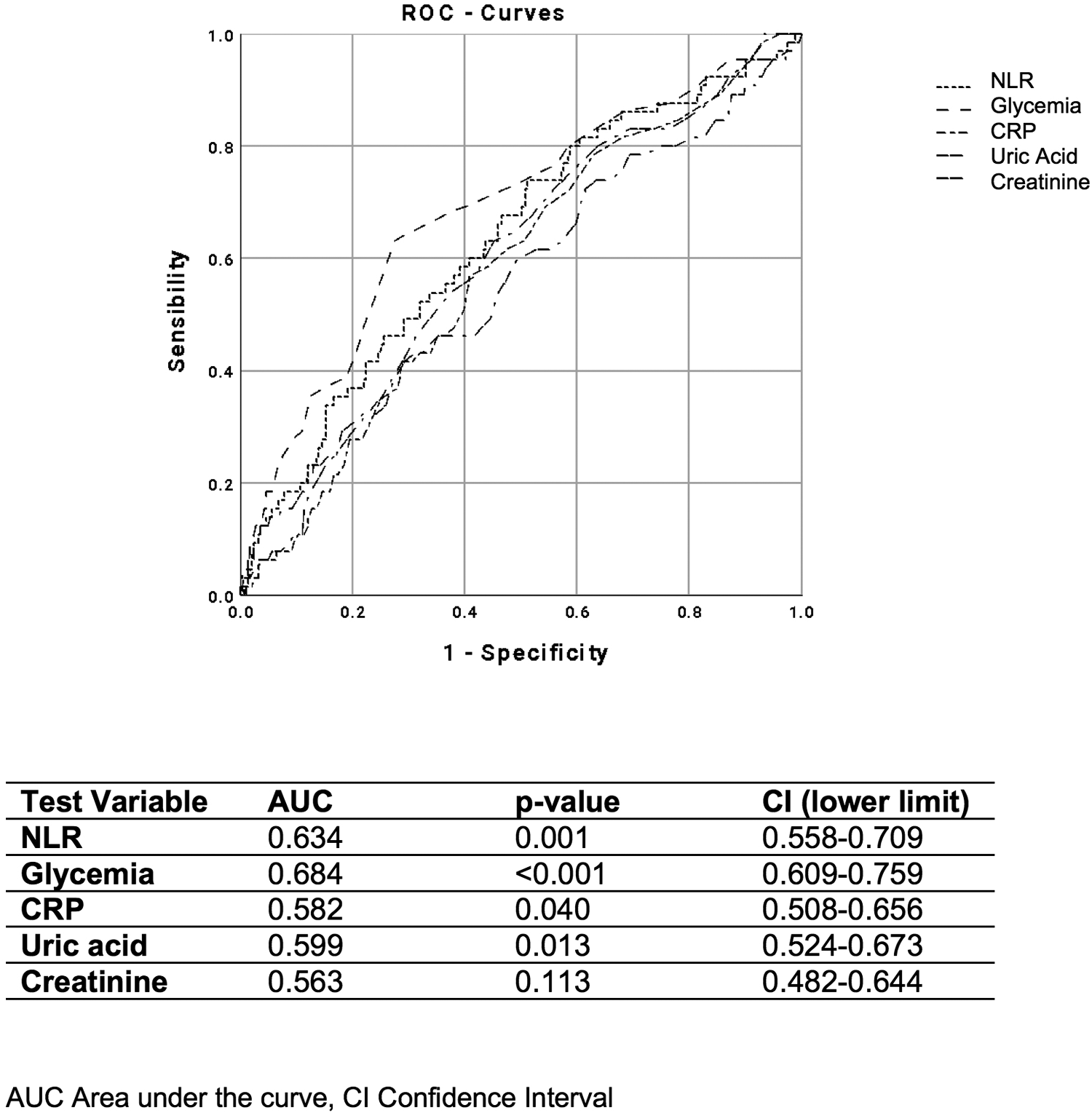
ROC curves of NLR, glycemia, CRP, plasma uric acid, and creatinine, for distinguishing the presence of two cardiovascular risk factors.
Figure 5
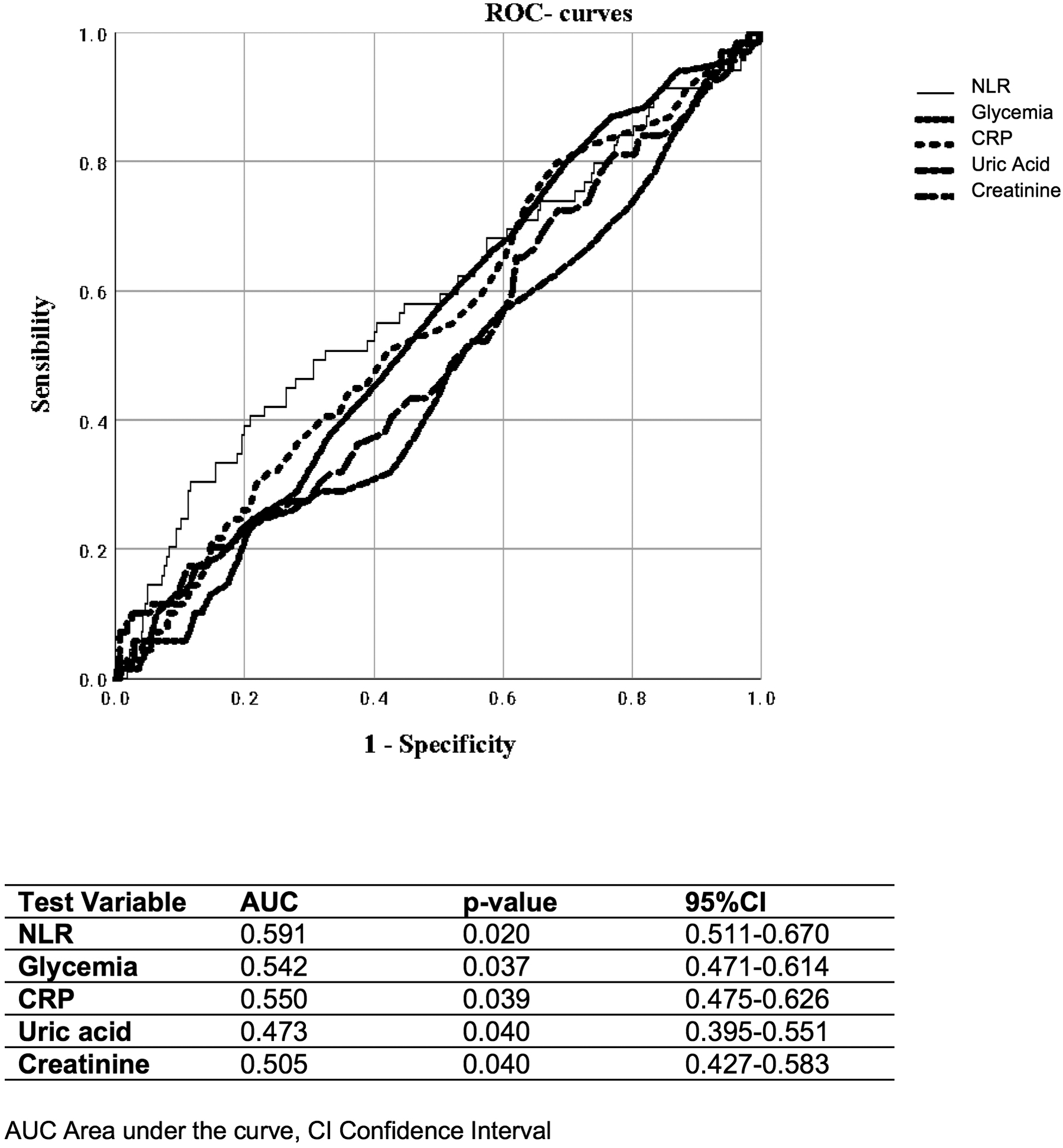
ROC curves of NLR, glycemia, C-reactive protein (CRP), plasma uric acid, and creatinine, for distinguishing patients with ≥3 antihypertensive drugs.
Discussion
The NLR is a readily available and inexpensive index calculated from blood routine examinations and is considered a novel inflammatory biomarker that reflects two complementary immune pathways: the adaptive immune response mediated by lymphocytes (16) and the innate immune response mediated by neutrophils, which are responsible for non-specific inflammation reactions (7).
Several researchers have explored the clinical utility of NLR in assessing atherosclerotic complications, including carotid atherosclerotic plaques (17), mixed and non-calcified plaques in the coronary arteries (18), and the severity of coronary atherosclerosis (19). A systematic review reported that a high NLR was significantly associated with the risk of CAD (OR 1.62), ACS (OR 1.64), stroke (OR 2.36), and composite cardiovascular events (OR 3.86), underscoring its potential as a marker of CVD complications (20).
Furthermore, NLR has been significantly associated with several cardiometabolic conditions, including a “non-dipping pattern” in hypertensive patients (21–23), metabolic syndrome (24), and the coexistence of comorbidities such as heart disease, cancer, and diabetes (25). The pathophysiological relationship between NLR and atherosclerotic events is confirmed by the results of different intervention studies on large populations, such as the CANTOS, JUPITER, SPIRE-1/2, and CIRT trials (26). Data from the Rotterdam Study (2002–2014) showed that NLR levels were higher in men, older individuals, smokers, and those with lower socioeconomic status, diabetes, a history of cancer, or previous CVDs. After multivariate analysis, elevated NLRs were independently and significantly associated with an increased risk of all-cause mortality (HR 1.64) and cardiovascular mortality (HR 1.92) (27).
In this study conducted on patients with essential hypertensive without a history of CVD events, we observed that elevated NLRs were associated with several comorbidities and subclinical atherosclerotic complications. Specifically, higher NLR was associated with elevated arterial blood pressure, a higher prevalence of patients treated with more than three antihypertensive medications, and a higher prevalence of dyslipidemia. Atherosclerosis is a degenerative process characterized by enhanced chronic inflammation; in this regard, NLR is closely related to the chronic inflammatory state. NLR is involved in the regulation of arterial function and the progression of atherosclerosis through interactions with the endothelium, platelets, and neutrophil infiltration (28, 29).
In this research, we observed a higher prevalence of visceral obesity and OSAS in patients with elevated NLR, both conditions characterized by enhanced chronic inflammation. Visceral adiposity is closely related to subclinical inflammation and CVDs. As regards, Bagyura et al. evaluated the association between NLR and coronary artery calcium score (CACS), finding a close interaction between tertiles of visceral adiposity, NLR, and CACS (30, 31). Regarding OSAS, Uygur et al. investigated the association between NLR and the severity of OSAS, finding significant associations between NLR and apnea–hypopnea index (r: 0.448), mean SaO2 (r: 0.341), and oxygen desaturation index (r: 0.327) (32). Several studies have shown that levels of inflammatory markers, including CRP, IL-6, and tumor necrosis factor, are elevated in patients with OSAS (33). It is well established that endothelial dysfunction caused by inflammatory processes plays a key role in the development of coronary artery disease, atherosclerosis, and other cardiovascular complications in patients with OSAS (34). Dysregulation of neutrophil apoptosis and increased expression of adhesion molecules may contribute significantly to the atherosclerotic process of OSAS (35).
While previous studies have demonstrated an association between NLR and the development of major atherosclerotic complications (i.e., coronary arteriopathy or peripheral arteriopathy), our study focused on patients with essential hypertensive without previous CVD events. We found that elevated NLRs were significantly correlated with a higher prevalence of cardiovascular remodeling [LVMi, cIMT, and atherosclerotic plaques] and chronic kidney disease (reduced glomerular filtration and increased microalbuminuria).
cIMT is a widely recognized method for CVD risk stratification. NLR has been evaluated as an independent risk factor for the development of asymptomatic atherosclerosis in populations with or without DM. In prediabetic and diabetic groups, studies conducted by Lee and Li compared patients with normal cIMT (cIMT <0.9 mm) to those with elevated cIMT (≥1 mm), finding that the latter group had higher mean NLR values, which were significantly correlated with age, HbA1c, and systolic blood pressure (36, 37). Several studies have reported increased levels of pro-inflammatory cytokines in prediabetic and diabetic patients (38), finding that persistent hyperglycemia continuously activates neutrophils, resulting in infiltration and damage of vascular endothelial cells (39). In contrast, lymphocytopenia is considered an inflammatory marker, particularly in conditions with increased corticosteroid levels in response to stress, which are associated with increased inflammatory reactions and lymphocyte apoptosis (40).
The relationship between type 2 DM and chronic inflammatory state is bidirectional. Type 2 DM is a itself chronic inflammatory condition, characterized by increased differentiation of monocytes into macrophages (41); however, on the other hand, the chronic inflammatory state promotes insulin resistance and the development of type 2 DM through altered signaling of inflammatory molecules (i.e., IL-6) in the liver (41).
In our study, increased cardiac remodeling and vascular damage were associated with higher pressure overload, which correlated significantly with NLR levels. Arterial hypertension is characterized by a progressive inflammatory process, involving the accumulation of innate and adaptive immune cells at vascular level and in the interstitium of affected organs (4). NLRs above 2.7 have been related to greater blood pressure variability and a more frequent non-dipping pattern, suggesting that this marker is an indicator of increased risk of related adverse cardiovascular events in hypertensive patients (42, 43). In a study on patients in secondary prevention, NLR, BNP, and CRP levels were higher in eccentric and concentric LVH compared to those without LVH (44). Other studies on asymptomatic patients have highlighted a higher prevalence of subclinical organ damage. Karagöz et al. reported greater diastolic dysfunction in patients with elevated NLRs (45), while studies on pediatric subjects with essential hypertension have shown that NLR is a useful marker of arterial damage/stiffness, correlating with diastolic, systolic, and mean blood pressure, as well as with PWV (46).
A limitation of the present study is the absence of additional biochemical markers of chronic inflammation (i.e., IL-6 and other cytokines) and specific subclasses of inflammatory cells, which could be potentially explored in future research.
In conclusion, this present study shows that in patients affected by arterial hypertension, evaluated in primary prevention, the NLR—an easily obtainable, repeatable, and low-cost marker—is significantly correlated with a higher prevalence of several comorbidities and elevated blood pressure. Moreover, it has a predictive ability for subclinical organ damage at the cardiac, vascular, and renal levels, making it a valuable surrogate marker of atherosclerotic damage.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Local Ethical Committee of the Department of Clinical, Internal, Anesthesiological and Cardiovascular Sciences, “Sapienza” University of Rome, Italy (date of approval: December 19, 2023). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LP: Conceptualization, Writing – original draft. FC: Writing – review & editing, Methodology, Resources. LC: Methodology, Software, Writing – original draft. DM: Writing – review & editing, Conceptualization, Formal analysis. AC: Conceptualization, Methodology, Writing – original draft. AS: Writing – original draft. EA: Resources, Writing – review & editing. LM: Data curation, Software, Writing – original draft. CL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Mancia G Kreutz R Brunström M Burnier M Grassi G Januszewicz A et al 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: endorsed by the International Society of Hypertension (ISH) and the European Renal Association. J Hypertens. (2023) 41(12):1874–2071. 10.1097/HJH.0000000000003480
2.
Sehestedt T Hansen TW Li Y Richart T Boggia J Kikuya M et al Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. (2011) 34(6):714–21. 10.1038/hr.2011.6
3.
Cheng H Zhong W Li H Wang L He C Huang L et al The association between neutrophil-to-lymphocyte ratio, atherogenic index of plasma, and cardiovascular disease incidence. Mediators Inflamm. (2025) 2025(1):3302911. 10.1155/mi/3302911
4.
Tsuda K . A link between white blood cell count and blood pressure levels. Hypertens Res. (2024) 47(2):537–9. 10.1038/s41440-023-01528-z
5.
Li S Zhang Y Wei W . Association between complete blood count-derived inflammatory biomarkers and renal failure: a cross-sectional study from NHANES 2007–2020. BMJ Open. (2025) 15:e103381. 10.1136/bmjopen-2025-103381
6.
Efros O Halevi TB Meisel E Soffer S Barda N Cohen O et al The prognostic role of neutrophil-to-lymphocyte ratio in patients hospitalized with acute pulmonary embolism. J Clin Med. (2021) 10(18):4058. 10.3390/jcm10184058
7.
Tamhane UU Aneja S Montgomery D Rogers EK Eagle KA Gurm HS . Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. (2008) 102(6):653–7. 10.1016/j.amjcard.2008.05.006
8.
Libby P Hansson GK . Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res. (2015) 116(2):307–11. 10.1161/CIRCRESAHA.116.301313
9.
Joshi A Bhambhani A Barure R Gonuguntla S Sarathi V Attia AM et al Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as markers of stable ischemic heart disease in diabetic patients: an observational study. Medicine (Baltimore). (2023) 102(5):E32735. 10.1097/MD.0000000000032735
10.
Dong CH Wang ZM Chen SY . Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta-analysis. Clin Biochem. (2018) 52(2017):131–6. 10.1016/j.clinbiochem.2017.11.008
11.
Delcea C Adrian Buzea C Dobrev D Andrei Dan G . Prognostic roles of neutrophil–lymphocyte, monocyte-lymphocyte and platelet-lymphocyte ratios for long-term all-cause mortality in heart failure. IJC Hear Vasc. (2024) 54:101502. 10.1016/j.ijcha.2024.101502
12.
Khanzadeh M Babadi S Ghaedi A Meidani FZ Rahmati R Aminizadeh S et al A systematic review on the role of neutrophil to lymphocyte ratio in limb ischemia. Ann Vasc Surg. (2025) 111:1–12. 10.1016/j.avsg.2024.09.065
13.
Concistrè A Petramala L Bisogni V Mezzadri M Olmati F Saracino V et al Subclinical atherosclerosis due to increase of plasma aldosterone concentrations in essential hypertensive individuals. J Hypertens. (2019) 37(11):2232–9. 10.1097/HJH.0000000000002170
14.
Lang RM Badano LP Victor MA Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. 10.1016/j.echo.2014.10.003
15.
Lameire NH Levin A Kellum JA Cheung M Jadoul M Winkelmayer WC et al Harmonizing acute and chronic kidney disease definition and classification: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. (2021) 100(3):516–26. 10.1016/j.kint.2021.06.028
16.
Bhat T Teli S Rijal J Bhat H Raza M Khoueiry G et al Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11(1):55–9. 10.1586/erc.12.159
17.
Corriere T Di Marca S Cataudella E Pulvirenti A Alaimo S Stancanelli B et al Neutrophil-to-lymphocyte ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr Metab Cardiovasc Dis. (2018) 28(1):23–7. 10.1016/j.numecd.2017.10.022
18.
Li T Gu C Wang F Lv B Zhang C Peng R et al Association of neutrophil–lymphocyte ratio and the presence of noncalcified or mixed coronary atherosclerotic plaques. Angiology. (2018) 69(3):256–63. 10.1177/0003319717718330
19.
Erturk M Cakmak HA Surgit O Celik O Aksu HU Akgul O et al The predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. (2014) 64(5):371–6. 10.1016/j.jjcc.2014.02.019
20.
Angkananard T Anothaisintawee T McEvoy M Attia J Thakkinstian A . Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int. (2018) 2018:2703518. 10.1155/2018/2703518
21.
Demir M . The relationship between neutrophil lymphocyte ratio and non-dipper hypertension. Clin Exp Hypertens. (2013) 35(8):570–3. 10.3109/10641963.2013.764893
22.
Günlü S Kayan F Karahan MZ . Comparison of diagnostic values of monocyte-lymphocyte ratio, neutrophil-lymphocyte ratio, red cell distribution width-lymphocyte ratio, and systemic inflammatory index in predicting patients with non-dipper hypertension. Interdiscip Med J. (2024) 15(51):27–33. 10.17944/interdiscip.1312657
23.
Chi X Bi Q You L Zhou Y Zhao C . Predictive value of NLR for the occurrence and clinical outcomes of hypertension: a systematic review and meta-analysis. Biomark Med. (2025) 19(16):783–91. 10.1080/17520363.2025.2542111
24.
Bahadır A Baltacı D Türker Y Türker Y Iliev D Öztürk S et al Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anadolu Kardiyol Derg. (2015) 15(10):816–22. 10.5152/akd.2014.5787
25.
Howard R Scheiner A Kanetsky PA Egan KM . Sociodemographic and lifestyle factors associated with the neutrophil-to-lymphocyte ratio. Ann Epidemiol. (2019) 38:11–21.e6. 10.1016/j.annepidem.2019.07.015
26.
Adamstein NH MacFadyen JG Rose LM Glynn RJ Dey AK Libby P et al The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. (2021) 42(9):896–903. 10.1093/eurheartj/ehaa1034
27.
Fest J Ruiter TR Groot Koerkamp B Rizopoulos D Ikram MA van Eijck CHJ et al The neutrophil-to-lymphocyte ratio is associated with mortality in the general population: the Rotterdam study. Eur J Epidemiol. (2019) 34(5):463–70. 10.1007/s10654-018-0472-y
28.
Li Y Chen X Huang L Lu J . Association between neutrophil–lymphocyte ratio and arterial stiffness in patients with acute coronary syndrome. Biosci Rep. (2019) 39(5):1–7. 10.1042/BSR20190015
29.
Haybar H Pezeshki SMS Saki N . Evaluation of complete blood count parameters in cardiovascular diseases: an early indicator of prognosis?Exp Mol Pathol. (2019) 110:104267. 10.1016/j.yexmp.2019.104267
30.
Bagyura Z Kiss L Lux Á Csobay-Novák C Jermendy ÁL Polgár L et al Neutrophil-to-lymphocyte ratio is an independent risk factor for coronary artery disease in central obesity. Int J Mol Sci. (2023) 24(8):1–11. 10.3390/ijms24087397
31.
Carollo C Sorce A Cirafici E Caimi G Ciuppa ME Mul G . Silent inflammation, loud consequences: decoding NLR across renal, cardiovascular and metabolic disorders. Int J Mol Sci. (2025) 26(17):8256. 10.3390/ijms26178256
32.
Uygur F Tanriverdi H Aktop Z Erboy F Altinsoy B Damar M et al The neutrophil-to-lymphocyte ratio in patients with obstructive sleep apnoea syndrome and its relationship with cardiovascular disease. Hear Lung J Acute Crit Care. (2016) 45(2):121–5. 10.1016/j.hrtlng.2016.01.002
33.
Yokoe T Minoguchi K Matsuo H Oda N Minoguchi H Yoshino G et al Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. (2003) 107(8):1129–34. 10.1161/01.CIR.0000052627.99976.18
34.
Ryan S Taylor CT McNicholas WT . Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. (2005) 112(17):2660–7. 10.1161/CIRCULATIONAHA.105.556746
35.
Dyugovskaya L Polyakov A Lavie P Lavie L . Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med. (2008) 177(5):544–54. 10.1164/rccm.200705-675OC
36.
Lee D Park MJ Kim MY Cho JJ Yoon JL . The correlation between carotid IntimaMedia thickness and neutrophil to lymphocyte ratio in prediabetes patients. Korean J Fam Med. (2021) 42(6):464–70. 10.4082/kjfm.21.0070
37.
Li X Shen J Lu Z Chen M Fang X Wang G . High neutrophil-to-lymphocyte ratio is associated with increased carotid artery intima-media thickness in type 2 diabetes. J Diabetes Investig. (2017) 8(1):101–7. 10.1111/jdi.12541
38.
Piepoli MF Hoes AW Agewall S Albus C Brotons C Catapano AL et al 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. (2016) 37(29):2315–81. 10.1093/eurheartj/ehw106
39.
van Oostrom AJ van Wijk JP Sijmonsma TP Rabelink TJ Castro Cabezas M . Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth J Med. (2004) 62(9):320–5.
40.
Hotchkiss RS Karl IE . The pathophysiology and treatment of sepsis. N Engl J Med. (2003) 348(2):138–50. 10.1056/NEJMra021333
41.
Gkrania-Klotsas E Ye Z Cooper AJ Sharp SJ Luben R Biggs ML et al Differential white blood cell count and type 2 diabetes: systematic review and meta-analysis of cross-sectional and prospective studies. PLoS One. (2010) 5(10):e13405. 10.1371/journal.pone.0013405
42.
Drugescu A Roca M Zota IM Costache AD Leon-Constantin MM Gavril OI et al Relationships between easily available biomarkers and non-dipper blood pressure pattern in patients with stable coronary artery disease. Life. (2023) 13(3):640. 10.3390/life13030640
43.
Sarejloo S Dehesh M Fathi M Khanzadeh M Lucke-Wold B Ghaedi A et al Meta-analysis of differences in neutrophil to lymphocyte ratio between hypertensive and non-hypertensive individuals. BMC Cardiovasc Disord. (2023) 23(1):1–16. 10.1186/s12872-023-03304-w
44.
Yu X Xue Y Bian B Wu X Wang Z Huang J et al NLR—;A simple indicator of inflammation for the diagnosis of left ventricular hypertrophy in patients with hypertension. Int Heart J. (2020) 61(2):373–9. 10.1536/ihj.19-138
45.
Karagöz A Vural A Günaydın ZY Bektaş O Gül M Çelik A et al The role of neutrophil to lymphocyte ratio as a predictor of diastolic dysfunction in hypertensive patients. Eur Rev Med Pharmacol Sci. (2015) 19(3):433–40.
46.
Skrzypczyk P Zacharzewska A Szyszka M Ofiara A Pańczyk-Tomaszewska M . Arterial stiffness in children with primary hypertension is related to subclinical inflammation. Cent Eur J Immunol. (2021) 46(3):336–43. 10.5114/ceji.2021.109156
Summary
Keywords
Neutrophil–lymphocyte ratio, essential arterial hypertension, subclinical organ damage, immune system, organ damage
Citation
Petramala L, Circosta F, Cremonesi L, Menichelli D, Cimò A, Servello A, Anastasi E, Marino L and Letizia C (2025) Neutrophil–lymphocyte ratio and subclinical atherosclerosis in essential hypertensive patients. Front. Cardiovasc. Med. 12:1579930. doi: 10.3389/fcvm.2025.1579930
Received
19 February 2025
Accepted
07 October 2025
Published
31 October 2025
Volume
12 - 2025
Edited by
Guido Iaccarino, Federico II University Hospital, Italy
Reviewed by
Caterina Carollo, University of Palermo, Italy
Serhat Günlü, Diyarbakır Gazi Yaşargil Training and Research Hospital, Türkiye
Updates
Copyright
© 2025 Petramala, Circosta, Cremonesi, Menichelli, Cimò, Servello, Anastasi, Marino and Letizia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Luca Marino luca.marino@uniroma1.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.