Abstract
Cardio-kidney metabolic (CKM) syndrome represents a complex and circular interplay of cardiovascular, renal, and metabolic dysfunctions, significantly contributing to global morbidity and mortality. This expert opinion synthesizes insights from a panel of Italian specialists in cardiology, nephrology, and diabetology, advocating for a holistic and integrated framework for CKM management. The recommendations underline the critical need for early identification and stratification of CKM stages, fostering an interdisciplinary approach that bridges specialties and ensures comprehensive care. Emphasizing innovative pathways for collaboration, including dynamic referral protocols, telemedicine, and shared decision-making, the proposed strategies aim to overcome structural and organizational barriers in healthcare. By promoting a unified approach, the framework seeks to streamline CKM care, enhance communication among specialists, and improve the coordination of services. This holistic strategy represents a pivotal step toward mitigating disease progression, improving clinical outcomes, and enhancing the quality of life for patients with CKM syndrome.
1 Introduction
The Cardiovascular-Kidney-Metabolic (CKM), also referred to as Cardio-Renal-Metabolic (CRM) syndrome, represents a complex and interdependent interplay between cardiovascular, renal, and metabolic systems, primarily encompassing heart failure, chronic kidney disease (CKD), and type 2 diabetes (T2D). Dysfunction in one system triggers or worsens dysfunction in others, perpetuating a self-reinforcing cycle that elevates morbidity and mortality globally (1–11). Cardiovascular disease (CVD) remains a major driver of this burden, and the global prevalence of CKM-related comorbidities is estimated at 25%–30%, highlighting its widespread burden and clinical seriousness (7, 12).
In response to the growing severity of CKM syndrome, the American Heart Association (AHA) recently proposed a position statement and a CKM classification system to stratify patients and facilitate early interventions (1, 2). This framework defines CKM syndrome as simultaneous dysfunction across CV, renal, and metabolic systems, categorizing the syndrome from early-stage dysfunction to advanced, irreversible organ damage. It emphasizes timely detection and holistic strategies to mitigate disease progression (1, 2, 8, 13).
This paper aims to address the unmet clinical needs of CKM syndrome and proposes integrated care solutions tailored to its systemic nature. Italian experts in cardiology, nephrology, and diabetology have collaborated to identify gaps in CKM management, focusing on early diagnosis, prognosis, and therapeutic approaches. The second core objective was to establish a standard and unified communication protocol among specialists, enabling a more seamless and collaborative approach to CKM care. This integrated approach seeks to improve outcomes by addressing comorbidities holistically and advancing care coordination between general practitioners (GPs), cardiologists, nephrologists, and diabetologists (1–4).
2 The pathophysiologic background of CKM
CKM syndrome is characterized by a complex, bidirectional interplay among cardiovascular, kidney, and metabolic systems, forming a self-perpetuating cycle of organ damage. Dysfunction in one system exacerbates pathology in the others, accelerating disease progression and increasing mortality risk (1, 2, 5–7, 13).
Chronic inflammation is a central driver, linking metabolic syndrome, CVD, and kidney impairment. Elevated levels of pro-inflammatory markers, such as high-sensitivity C-reactive protein and interleukin-6, reflect an underlying inflammatory state that contributes to endothelial dysfunction and atherosclerosis through mechanisms involving cytokine signaling, oxidative stress, and immune cell activation. These processes precipitate myocardial infarction and ischemic stroke. Inflammation also exacerbates insulin resistance, glucose dysregulation, and oxidative stress, accelerating diabetic kidney disease, heart failure (HF), and vascular stiffness, which further impair renal and cardiovascular function (1, 2, 5–7, 13, 14). Endothelial dysfunction amplifies this cycle through reduced nitric oxide availability, increased vasoconstriction, and glomerular sclerosis, resulting in proteinuria and albuminuria, markers of kidney damage that worsen cardiovascular strain. Neurohormonal Activation, driven by renin–angiotensin–aldosterone system (RAAS) and sympathetic nervous system dysregulation, contributes to vasoconstriction, sodium retention, fibrosis, and maladaptive cardiac remodeling, perpetuating systemic organ dysfunction (1, 2, 5–7, 13, 14).
The pathophysiology and progression of CKM syndrome should be best understood as an evolving continuum rather than a static or linear process. Without intervention, early metabolic disturbances and initial organ damage progress to CKD, cardiac dysfunction, and worsening insulin resistance, ultimately leading to irreversible damage in advanced stages, such as HF and end-stage kidney disease. Understanding CKM as a progressive trajectory emphasizes the need for early, system-wide interventions to disrupt this cycle before irreversible damage occurs.
3 Staging and risk stratification in CKM patients
In light of these considerations, the current staging of CKM proposed by the AHA should be reconsidered. While the AHA model categorized CKM syndrome into five distinct stages (stages 0–4), each of these stages has been analyzed and redefined to incorporate novel insights and perspectives. The AHA classification provides a detailed framework; however, in daily clinical practice, its multiple intermediary stages may sometimes delay the recognition of disease progression or create inconsistencies in patient management. To enhance clinical applicability, this reexamination has led to a more practical and holistic approach, consolidating these stages into two broad categories: early-stage disease and advanced disease, emphasizing proactive intervention from the earliest signs of dysfunction, and ensuring timely prevention and management while maintaining a dynamic, risk-based approach aligned with actual organ involvement
3.1 Early stages: from risk factors to subclinical changes
The early stages involve patients at risk or with early organ damage but without overt clinical manifestations.
“Stage 0” includes individuals with no cardiovascular, renal, or metabolic risk factors and no evidence of organ damage. At this stage, maintaining a healthy lifestyle is essential to prevent the onset of risk factors and disease progression.
“Stage 1” comprises patients presenting with early metabolic risk factors, including abdominal obesity, hypertension, or dyslipidemia. Unlike the AHA classification, which defines stage 1 solely by excess adiposity—likely reflecting the epidemiological characteristics of the US population, where obesity is a predominant concern—our approach incorporates additional metabolic risk factors. While obesity is also a significant concern in Italy, this broader perspective allows for a more comprehensive metabolic risk assessment, facilitating early identification of high-risk individuals. Recognizing these factors at an earlier stage enables timely interventions through lifestyle modifications, weight management, and metabolic control, ultimately reducing cardiovascular and renal complications.
“Stage 2” represents a later phase in which metabolic risk factors become more pronounced. Patients may exhibit impaired glucose tolerance, recently diagnosed diabetes, resistant hypertension (requiring at least two antihypertensive drugs plus a diuretic), or CKD with eGFR >45 ml/min/1.73 m2. Persistent albuminuria and metabolic abnormalities like hypertriglyceridemia may also appear. These patients are at high risk of progression and require intensive interventions targeting glycemic control, lipid management, anti-albuminuric strategies, and blood pressure optimization.
3.2 Advanced disease: from subclinical to overt organ dysfunction
Advanced stages indicate significant organ involvement and clinical manifestations.
“Stage 3” is characterized by subclinical organ dysfunction, meaning damage occurs in the absence of symptoms and involves multiple systems. Cardiovascular markers include coronary calcification, elevated BNP/NT-proBNP or hs-troponin, ejection fraction (EF) < 50%, moderate-to-severe diastolic dysfunction, and carotid atheromasia. Kidney involvement includes severe CKD (eGFR 30–45 ml/min/1.73 m2), rapid progression (eGFR decline of 7–8 ml/year), nephropathy diagnosis, and Urine Albumin-to-Creatinine Ratio (UACR) > 300 mg/g. In this stage of CKM syndrome, a holistic approach becomes essential to recognize and address cardiac, renal, and metabolic complications.
“Stage 4” represents the most severe phase of CKM syndrome, where patients exhibit overt clinical symptoms and advanced organ damage, including end-stage kidney disease, advanced HF, or acute cardiovascular events, including myocardial infarction or acute coronary syndrome. The complexity of these cases requires shared holistic management as an alternative to the traditional referrals where the primary specialist is selected based on the patient's predominant comorbidity.
3.3 The dynamic referral throughout the CKM continuum
To enhance adherence to recommendations for CKM management, it is essential to adopt a holistic approach where all clinicians are equipped to address key parameters across disciplines from early to late stages (15–17). Cardiologists, nephrologists, diabetologists and GPs must develop a shared understanding of overlapping conditions, including diabetes, CKD, and CVD. This integrated knowledge enables tailored interventions aligned with the patient's disease severity and clinical needs.
GPs play a pivotal role in early CKM identification and management (1, 2, 18). They are uniquely positioned to screen for risk factors such as hypertension, obesity, and elevated glucose levels. Routine assessments, including blood pressure, serum creatinine, UACR, and glycated hemoglobin (HbA1c), allow GPs to early detection of asymptomatic CKM indicators and timely interventions. Additionally, GPs guide high-risk individuals to appropriate lifestyle changes, emphasizing weight management, diet, and exercise. When risk factors are identified, or disease progression becomes evident, GPs are also responsible for referring patients to specialists for advanced care. These dynamic referrals typically involve a non-permanent evaluation, where specialists provide recommendations for additional tests and long-term management strategies. Specialists' expertise is essential for managing CKM's complex interplay between cardiovascular, kidney, and metabolic health. In cardiology, it is essential to evaluate target organ damage (e.g., left ventricular hypertrophy), systolic and diastolic dysfunctions, and subclinical cardiovascular changes. Furthermore, it is of utmost importance to stress the relevance of strict control of CV risk, which is worldwide suboptimal (19). In nephrology, it is important to confirm CKD diagnoses by using repeated UACR and creatinine tests over three months. Persistent morphological abnormalities, microhematuria, or proteinuria further guide nephrology evaluations. Monitoring of creatinine is mandatory to assess the impact of cardiological therapies on renal function. Managing diabetes necessitates an individualized approach to optimize care. If diabetes is diagnosed during cardiological or nephrological monitoring, a diabetological evaluation is necessary.
This holistic approach ensures a comprehensive CKM management strategy, with each specialist contributing to slowing disease progression and improving patient outcomes. Harmonizing diagnostic approaches across disciplines is critical to the early identification of high-risk CKM patients and tailored interventions.
4 The key diagnostic tools for the holistic approach to CKM patients
Several key biomarkers and diagnostic tests are critical for detecting early signs of organ damage in CKM patients, including subclinical stages. In nephrology, the Kidney Disease Improving Global Outcomes (KDIGO) classification offers a robust framework for assessing kidney function (Figure 1) (9). By combining eGFR and UACR, the KDIGO heat map stratifies patients by their global risk, including all-cause death, CKD progression and fatal and non-fatal cardiovascular events. Persistent albuminuria, even with normal eGFR, may indicate subclinical kidney damage and necessitates timely intervention. These assessments, often performed by GPs, are essential for early detection and effective risk stratification. Regular eGFR and UACR evaluations guide treatment decisions according to disease severity (20). Additionally, venous bicarbonate measurement is a valuable tool for detecting metabolic acidosis, a common CKD complication linked to muscle wasting, inflammation, and cardiovascular risk. Correcting acidosis may slow CKD progression and improve outcomes. Additionally, bioimpedance analysis provides insights into body composition, hydration, and muscle mass, aiding in nutritional interventions and optimizing care for patients at risk of sarcopenia and cardiovascular complications.
Figure 1
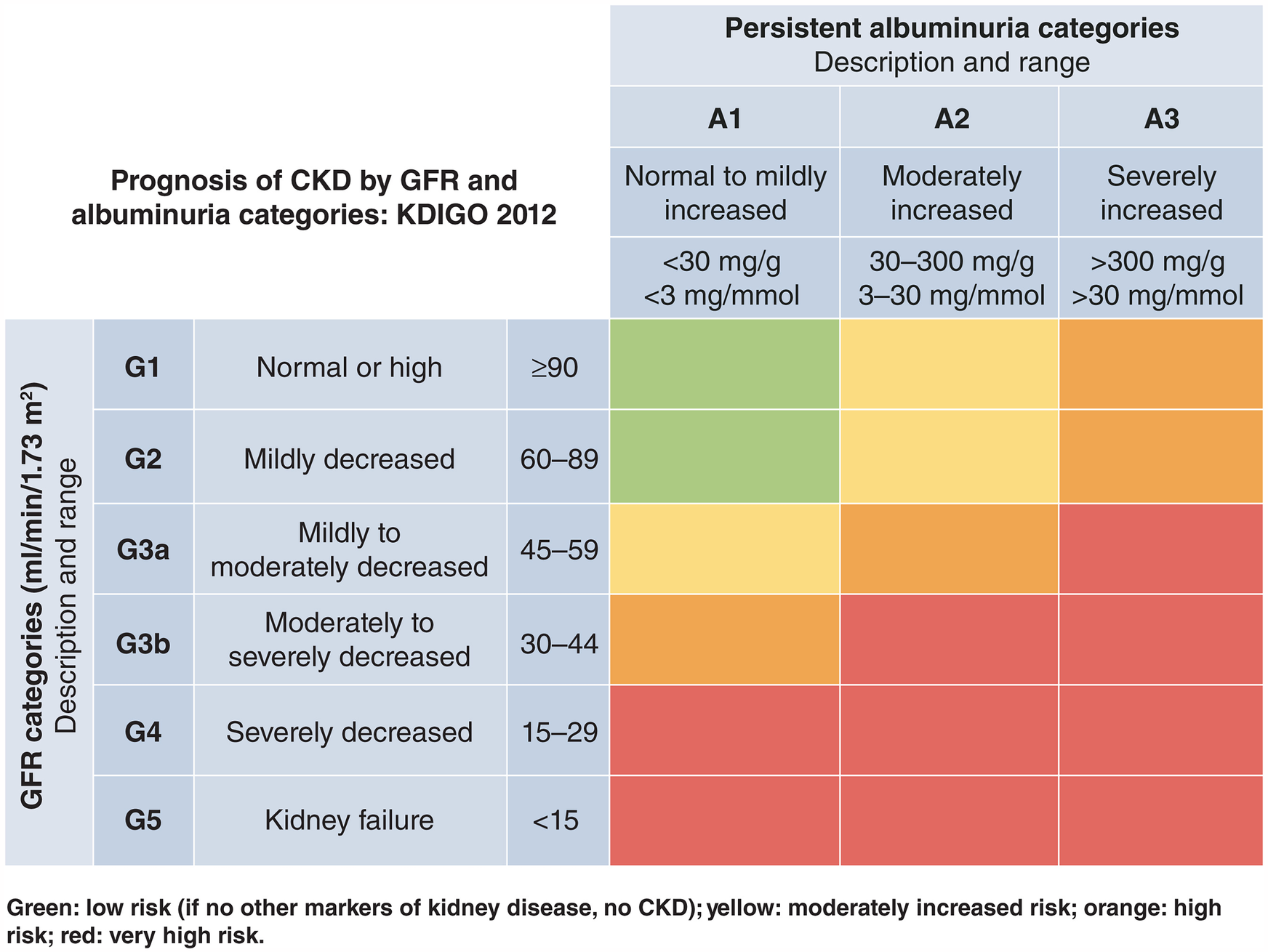
KDIGO heat map for assessing renal function and kidney damage. Source: Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. 2024 (9). Reproduced from Elsevier under a Creative Commons (CC BY-NC-ND 4.0) licence.
In cardiology, diagnostic tests such as ECG, natriuretic peptides (BNP/NT-proBNP), and high-sensitivity troponin (Hs-troponin) provide strong prognostic value. Elevated natriuretic peptide levels correlate with an increased risk of heart failure and help identify at-risk patients for early therapeutic intervention (21–24). Additionally, BNP/NT-proBNP evaluation should guide the echocardiogram in diabetic patients as part of the second-level screening, and both systolic and diastolic function should be evaluated, as alterations in these parameters are critical indicators of cardiovascular involvement in CKM syndrome (25). Hs-troponin is a valuable marker of myocardial injury, but its interpretation must always consider the clinical context. A single elevation in Hs-troponin does not necessarily indicate an acute coronary syndrome and should be assessed alongside serial measurements, ECG findings, and echocardiographic parameters to guide appropriate management and avoid unnecessary emergency department visits (26).
Metabolic evaluations, including blood glucose and glycated hemoglobin (HbA1c), provide vital insights into glycemic control, a key factor in slowing vascular damage and reducing cardiovascular and renal complications. Lipid profiling, blood pressure monitoring, and kidney damage assessments are also critical. In obese patients, additional tests, such as transaminases and color Doppler ultrasound of the supra-aortic trunks, further refine risk assessment.
Together, these diagnostic tests enable early detection of subclinical organ damage, risk stratification, and implementation of targeted interventions, ultimately slowing CKM progression and improving patient outcomes.
5 CKM stages and the role of an integrated holistic approach
The CKM staging approach facilitates identifying individuals at different levels of syndromic severity, thereby providing windows for preventive action to halt or reverse disease progression. Early detection in the preclinical phase is a primary goal, aiming to delay or avert clinical cardiovascular and kidney complications (1, 2). Active screening for CKM risk factors across the lifespan is recommended to improve prevention strategies, with screening frequency tailored to the CKM stage (1, 2).
In stages 0 and 1, the main clinical parameters include blood pressure, waist circumference, ECG, urine tests, creatinine and transaminases, in addition to blood glucose, HbA1c, and lipid profile assessments (HDL included), which collectively highlight patients at high metabolic or cardiovascular risk. GPs play a central role in identifying early abnormalities and initiating treatment, such as antihypertensives for resistant hypertension or lipid-lowering therapy for dyslipidemia. Referrals to specialists are indicated for resistant hypertension, CKD, albuminuria, newly diagnosed diabetes, or evidence of advanced disease.
Stage 2 focuses on detecting early organ stress. Primary kidney indicators include either low eGFR or increased UACR. In cases of kidney abnormalities, a kidney ultrasound is required for a comprehensive evaluation and refining diagnosis. Cardiologic assessments, including ECG, echocardiography, and BNP/NT-proBNP levels, aid in identifying subclinical cardiac damage. Monitoring HbA1c, assessing glucose intolerance, and performing Doppler ultrasound of the lower limbs are particularly relevant for diabetic patients (9). Specialists provide advanced care when required, while many patients can remain under GP supervision with periodic specialist input.
In stage 3, cardiologic findings include left ventricular hypertrophy, ejection fraction <50%, diastolic dysfunction, elevated BNP/NT-proBNP or Hs-troponin, carotid atheromas, and ECG abnormalities. Nephrologic indicators include eGFR 30–45 ml/min/1.73 m2 and/or UACR >300 mg/g. Patients with glomerulonephritis and/or fast progressors (eGFR reduction >7–8 ml/min per year) should remain under nephrologist care even with an eGFR >45 ml/min/1.73 m2. Diabetologic complications include retinopathy, microangiopathy, diabetic foot ulcers, or metabolic decompensation. At this stage, an integrated, holistic approach and close collaboration among specialists is crucial. Specialists must monitor parameters across systems, such as cardiologists evaluating kidney indicators (eGFR, UACR) and nephrologists considering cardiac biomarkers (BNP/NT-proBNP, Hs-troponin). These diagnostic tests should be repeated biannually to guide individualized care.
At stage 4, the cardiologist plays a central role, particularly for those with HF, myocardial infarction, or acute coronary syndrome. Close follow-up every 2–3 months, or more frequently during exacerbations, is critical to monitor disease progression and adjust treatment. For kidney failure patients (eGFR <15 ml/min/1.73 m2), the nephrologist becomes the primary case manager, supported by the cardiologist in addressing cardiovascular complications. At this stage, the nephrologist must also collaborate with the patient to decide on the most appropriate kidney replacement therapy and plan the necessary interventions, such as creating vascular access, placing a peritoneal catheter, or enrolling the patient on a waitlist for preemptive kidney transplantation. The diabetologist plays a key role in ensuring metabolic stability and glycemic control, with follow-ups approximately every 6 months.
A structured, multidisciplinary collaboration is crucial to ensure effective CKM management. While GPs play a central role in early detection and risk stratification, a shared-care model involving cardiologists, nephrologists, and diabetologists becomes essential as the disease progresses. The coordination of care should be dynamic, adapting to the predominant organ dysfunction: in early stages, GPs manage preventive strategies and referrals; in intermediate stages, specialists collaborate through periodic assessments; and in advanced CKM, the primary specialist (typically a cardiologist or nephrologist) leads the therapeutic approach.
Table 1 provides an updated summary of the main characteristics and key diagnostic tools for a holistic approach to CKM patients.
Table 1
| Stage | Patient characteristics | Who is responsible | Examinations | Referral criteria |
|---|---|---|---|---|
| Stage 0 | Individuals without cardiovascular, renal, or metabolic risk factors and no signs of organ damage | GP | Periodic monitoring of basic metabolic parameters (glucose, lipid profile, blood pressure), BMI, abdominal circumference | Not applicable (routine monitoring for primary prevention) |
| Stage 1 | Patients with initial metabolic risk factors, including abdominal obesity, hypertension, or dyslipidemia | GP | Glucose, glycated hemoglobin, lipid profile (including HDL), creatinine, urine tests, ECG, abdominal circumference, BMI, transaminases | Nephrologist: CKD diagnosis Cardiologist: no response to therapy (2 treatment classes), abnormal ECG Diabetologist: pre-diabetes according to guidelines |
| Stage 2 | Patients with glucose intolerance, newly diagnosed diabetes, resistant hypertension (at least 2 drugs + 1 diuretic), CKD with eGFR >45 ml/min/1.73 m2 | GP ↔ Specialist (mainly diabetologist) | ECG, peptides, echocardiogram, renal ultrasound, supra-aortic trunks and lower limb Doppler ultrasound | Presence of characteristics of subsequent stages |
| Stage 3 | C: Left ventricular hypertrophy, EF < 50%, moderate-to-severe diastolic dysfunction, elevated BNP, carotid atheromasia K: eGFR between 30 and 45 ml/min/1.73 m2, fast progressor (eGFR decline >7–8 ml/min/year), nephropathy diagnosis M: Retinopathy, microangiopathy, ulceration, inadequate glycemic control, UACR >300 mg/g |
Multidisciplinary team | Clinical assessment—at least semi-annual monitoring | Holistic multidisciplinary management |
| Stage 4 | Advanced renal-cardiovascular disease: eGFR <30 ml/min/1.73 m2, heart failure, AMI, ACS | Cardiologist/Nephrologist with diabetologist support | Frequent monitoring | eGFR <15 ml/min/1.73 m2 |
Summary of updated staging; main characteristics and key diagnostic tools for a holistic approach to CKM patients.
GP, general practitioner; AMI, acute myocardial infarction; ACS, acute coronary syndrome.
6 Challenges and solutions for implementing a holistic approach in the management of CKM patients in Italy
The management of CKM syndrome in Italy faces several structural and organizational barriers. A primary challenge is the limited integration of the CKM concept, encompassing cardiology, nephrology, and diabetology into clinical practice. Specialists often work in “silos”, resulting in fragmented care and inconsistent treatment strategies (1). Secondly, the lack of a shared clinical language complicates information sharing, resulting in redundant or conflicting diagnostic and therapeutic plans and ultimately affecting care quality and efficiency (27).
6.1 Structural and organizational barriers
Chronic patient management is frequently assigned to hospitals or specialized facilities, while territorial healthcare systems in Italy often lack centralized frameworks or a holistic approach for integrated care pathways. The healthcare system predominantly focuses on acute care, with insufficient support for chronic conditions. Limited access to advanced diagnostic tools for GPs hinders early detection and referral, fragmenting the care process. Inadequate reimbursement for essential tests, such as BNP/NT-proBNP and Hs-troponin, further constrains their use. Obesity, a key component of CKM, is often managed in private settings, creating disparities in access to care.
6.2 Shortage of specialists and limited referral pathways
A significant shortage of specialists, particularly nephrologists and diabetologists, exacerbates challenges in patient management, especially in less urbanized areas or regions with limited access to specialized care. Referrals between specialists are often limited to complex or acute cases, limiting opportunities for early intervention.
6.3 Limitations in guideline support
Current guidelines, including those provided by KDIGO, the American Diabetes Association and the European Society of Cardiology, do not adequately support a holistic approach to CKM management. They focus on risk stratification for individual conditions, such as diabetes, kidney disease, or CVD, overlooking the syndromic nature of CKM. This underscores the need for integrated guidelines addressing CKM's interconnected pathophysiology.
6.4 Disease-specific challenges in CKM management
6.4.1 Diabetologic challenges
The shortage of diabetologists in Italy often results in suboptimal care for many diabetes patients, a core component of CKM. Furthermore, reluctance to prescribe therapies requiring frequent monitoring, such as hypoglycemic therapies, hampers effective risk evaluation, often underestimated in outpatient settings by both GPs and diabetologists.
6.4.2 Cardiologic challenges
Cardiologic care for CKM patients often falls short in addressing critical risk factors such as weight and blood pressure. HF management overly focuses on left ventricular ejection fraction, neglecting diastolic function, leaving many HF patients with preserved ejection fraction unidentified. The lack of dedicated care pathways for patients with hypertension, atherosclerosis, or post-myocardial infarction results in insufficient follow-up and fragmented care. Additionally, biomarkers like Hs-troponin and BNP/NT-proBNP remain underutilized due to reimbursement limitations.
6.4.3 Nephrologic challenges
Shared CKM management is hindered by the limited presence of nephrologists in territorial healthcare settings, as most nephrology care is hospital-based. There is significant confusion in the use of urinary tests; microalbuminuria is often conflated with proteinuria, and the UACR is rarely measured despite its predictive value, complicating the identification of fast progressors. Prescriptive restrictions, such as combining GLP-1 receptor agonists and SGLT2 inhibitors, limit therapeutic options for patients with declining kidney function, complicating the identification of fast progressors.
6.5 Proposed solutions to improve a holistic approach in the management of CKM patients in Italy
To address these challenges, the advisory board identified key strategies to improve CKM management:
6.5.1 Enhancing communication and coordination
The use of telemedicine and teleconsultations was proposed as a promising solution to bridge gaps between specialists and GPs, facilitating more consistent and integrated CKM care (28, 29). Telemedicine enables real-time discussions, coordinated diagnostics, and timely adjustments in treatment plans, particularly for rural or underserved patients. Additionally, remote monitoring tools for blood pressure, glucose levels, and weight can minimize the need for frequent in-person visits, ensuring timely interventions (30, 31).
6.5.2 Developing shared management models
Establishing a shared management framework between GPs and specialists can improve care continuity. GPs would handle initial screenings, risk monitoring, and preventive measures, while specialists would intervene when CKM indicators emerge (1, 2).
6.5.3 Promoting a holistic culture and standardizing referral protocols and guidelines
Adopting a holistic diagnostic-therapeutic approach is essential to improving clinical outcomes in CKM patients. Specialists and GPs must understand key parameters across all CKM domains to ensure cohesive, patient-centered care. Integrated referral protocols and treatment guidelines can promote consistent management and reduce care variability (27).
6.5.4 Improving access to diagnostics and therapies
Expanding reimbursement criteria for therapies and diagnostic tools, such as Hs-troponin and BNP/NT-proBNP tests, as well as simplifying the tie-consuming procedures for prescription of the newest agents, could enhance outcomes. Advocacy efforts to demonstrate their cost-effectiveness and long-term benefits are crucial.
By addressing these barriers and implementing the proposed solutions, Italy's healthcare system can advance toward a more holistic CKM approach, improving outcomes and quality of life for patients.
7 Therapeutic overview
In managing CKM syndrome, several pharmacological classes effectively address overlapping cardiovascular, renal, and metabolic risks. Guidelines emphasize a multidisciplinary and personalized treatment approach tailored to patients' clinical profiles (32).
Sodium–glucose co-transporter 2 (SGLT2) inhibitors are a breakthrough therapy, providing cardiovascular, renal, and metabolic benefits, including reduced HF hospitalizations, slower renal disease progression, and cardiovascular protection, even in non-diabetic patients (33–38). Their organ-protective effects make them suitable for a broad range of CKM patients. Glucagon-like peptide-1 receptor agonists, used for glycemic control, also provide cardiovascular benefits such as weight loss and reduced atherosclerotic risk (39, 40). RAAS inhibitors remain essential, offering blood pressure control, reduced proteinuria, and organ protection. Mineralocorticoid Receptor Antagonists support heart failure management but require monitoring for hyperkalemia (41). Emerging therapies, including nonsteroidal mineralocorticoid receptor antagonists, a combination of SGLT2 inhibitors with GLP-1 receptor agonists, and combined GLP-1/GIP receptor agonists, provide further options to address the multifaceted CKM features (32). Therapy should align with the CKM stage and comorbidities, ensuring efficacy and safety.
8 Conclusions and recommendations
Effective CKM syndrome management requires a holistic, interdisciplinary approach that integrates cardiology, nephrology, and diabetology to address the complex interplay of organ dysfunctions. While the AHA guidelines provide a useful framework, they may not fully account for variations in epidemiology and healthcare structures across different regions. A broader classification incorporating additional clinical parameters is needed to improve early risk stratification and patient management.
A structured, multidisciplinary model supported by shared protocols, telemedicine, and dynamic referral pathways can enhance coordination between specialists and primary care, improving clinical outcomes. Addressing structural and organizational healthcare system challenges is essential to ensure equitable access to early diagnosis, advanced therapies, and integrated care. A tailored, patient-centered approach that recognizes regional and systemic healthcare disparities is crucial for mitigating disease progression, improving quality of life, and reducing the global burden of CKM syndrome.
Statements
Data availability statement
All data are available from the corresponding author upon reasonable request.
Author contributions
MI: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. GG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. RM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. DP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. RT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors did not receive payment related to the development of the manuscript. Medical writing assistance was funded by Boehringer Ingelheim.
Acknowledgments
The concept of this manuscript was discussed during a round table organized and funded by Boehringer Ingelheim, Italy. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations but had no input and influence in the content during the development of the manuscript. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Medical writing assistance was provided by Mattia Zamboni and Aashni Shah (Polistudium SRL, Milan, Italy).
Conflict of interest
Giuseppe Grandaliano has been a member of advisory board for Astellas, Novartis, Bayer, Stada, Lilly, Boehringer Ingelheim, Astrazeneca and received fees for lectures from Astellas, Bayer, Novartis, Astrazeneca, Boehringer Ingelheim; Roberto Minutolo has been a member of Advisory Boards for Amgen, Astellas, Bayer and has received fees for lectures from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Vifor Pharma; Mauro Gori has been a member of the advisory board for Amgen, Bayer, and received consulting fees from Amgen, AstraZeneca, Bayer, Vifor Pharma, Menarini, Boehringer Ingelheim, and Eli Lilly; Roberto Trevisan has been a member of the advisory board for Novo, Bayer, and received consulting fees from Novo, AstraZeneca, Bayer, Boehringer Ingelheim, and Eli Lilly; Massimo Iacoviello has been consultant for Boehringer Ingelheim, Lilly, Novartis, and has received fees for lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Neopharmed Gentili, Novo Nordisk.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ndumele CE Rangaswami J Chow SL Neeland IJ Tuttle KR Khan SS et al Cardiovascular-kidney-metabolic health: a presidential advisory from the American Heart Association. Circulation. (2023) 148:1606–35. 10.1161/CIR.0000000000001184
2.
Ndumele CE Rangaswami J Chow SL Neeland IJ Tuttle KR Khan SS et al A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. (2023) 148:1636–64. 10.1161/CIR.0000000000001186
3.
ElSayed NA Aleppo G Aroda VR Bannuru RR Brown FM Bruemmer D et al 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. (2023) 46:S19–40. 10.2337/dc23-S002
4.
Zoccali C Zannad F . Refocusing cardio-renal problems: the cardiovascular-kidney-metabolic syndrome and the chronic cardiovascular-kidney disorder. Nephrol Dial Transplant. (2024) 39:1378–80. 10.1093/ndt/gfae086
5.
Larkin H . Here’s what to know about cardiovascular-kidney-metabolic syndrome, newly defined by the AHA. JAMA. (2023) 330:2042–3. 10.1001/jama.2023.22276
6.
Rangaswami J Bhalla V Blair JEA Chang TI Costa S Lentine KL et al Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. (2019) 139:e840–78. 10.1161/CIR.0000000000000664
7.
Kittelson KS Junior AG Fillmore N da Silva Gomes R . Cardiovascular-kidney-metabolic syndrome—an integrative review. Prog Cardiovasc Dis. (2024) 87:26–36. 10.1016/j.pcad.2024.10.012
8.
Malik S Wong ND Franklin SS Kamath TV L'Italien GJ Pio JR et al Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. (2004) 110:1245–50. 10.1161/01.CIR.0000140677.20606.0E
9.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105(4S):S117–314. 10.1016/j.kint.2023.10.018
10.
Garg PK Biggs ML Carnethon M Ix JH Criqui MH Britton KA et al Metabolic syndrome and risk of incident peripheral artery disease: the cardiovascular health study. Hypertension. (2014) 63:413–9. 10.1161/HYPERTENSIONAHA.113.01925
11.
Powell-Wiley TM Poirier P Burke LE Després JP Gordon-Larsen P Lavie CJ et al Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 143:e984–e1010. 10.1161/CIR.0000000000000973
12.
Ostrominski JW Arnold SV Butler J Fonarow GC Hirsch JS Palli SR et al Prevalence and overlap of cardiac, renal, and metabolic conditions in US adults, 1999–2020. JAMA Cardiol. (2023) 8:1050–60. 10.1001/jamacardio.2023.3241
13.
Marassi M Fadini GP . The cardio-renal-metabolic connection: a review of the evidence. Cardiovasc Diabetol. (2023) 22:195. 10.1186/s12933-023-01937-x
14.
Ismail Y Kasmikha Z Green HL McCullough PA . Cardio-renal syndrome type 1: epidemiology, pathophysiology, and treatment. Semin Nephrol. (2012) 32:18–25. 10.1016/j.semnephrol.2011.11.003
15.
Narain R Bijman L Joshi H Chen M . Novel multidisciplinary cardiometabolic clinic in a UK tertiary cardiology centre: early activity, interventions and potential for cardiovascular risk optimization. Eur Heart J. (2021) 42:ehab724.2631. 10.1093/eurheartj/ehab724.2631
16.
Anderegg MD Gums TH Uribe L MacLaughlin EJ Hoehns J Bazaldua OV et al Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. (2018) 38:309–18. 10.1002/phar.2083
17.
Katz IJ Pirabhahar S Williamson P Raghunath V Brennan F O’Sullivan A et al Iconnect CKD—virtual medical consulting: a web-based chronic kidney disease, hypertension and diabetes integrated care program. Nephrology (Carlton). (2018) 23:646–52. 10.1111/nep.13070
18.
Kushner PR Cavender MA Mende CW . Role of primary care clinicians in the management of patients with type 2 diabetes and cardiorenal diseases. Clin Diabetes. (2022) 40:401–12. 10.2337/cd21-0119
19.
Makover ME Surma S Banach M Toth PP . Eliminating atherosclerotic cardiovascular disease residual risk. Eur Heart J. (2023) 44:4731–3. 10.1093/eurheartj/ehad446
20.
Grams ME Coresh J Matsushita K Ballew SH Sang Y Surapaneni A et al Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. (2023) 330:1266–77. 10.1001/jama.2023.17002
21.
Jehn S Mahabadi AA Pfohl C Vogel L Al-Rashid F Luedike P et al BNP and NT-proBNP thresholds for the assessment of prognosis in patients without heart failure. JACC Adv. (2023) 2:100688. 10.1016/j.jacadv.2023.100688
22.
McCullough PA Nowak RM McCord J Hollander JE Herrmann HC Steg PG et al B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: an analysis from the breathing not properly (BNP) multinational study. Circulation. (2002) 106:416–22. 10.1161/01.CIR.0000025242.79963.4C
23.
Daniels LB Maisel AS . Natriuretic peptides. J Am Coll Cardiol. (2007) 50:2357–68. 10.1016/j.jacc.2007.09.021
24.
Omland T de Lemos JA Sabatine MS Christophi CA Rice MM Jablonski KA et al A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. (2009) 361:2538–47. 10.1056/NEJMoa0805299
25.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. 10.1016/j.echo.2014.10.003
26.
Gori M Gupta DK Claggett B Selvin E Folsom AR Matsushita K et al Natriuretic peptide and high-sensitivity troponin for cardiovascular risk prediction in diabetes: the atherosclerosis risk in communities (ARIC) study. Diabetes Care. (2016) 39:677–85. 10.2337/dc15-1760
27.
Cosentino F Grant PJ Aboyans V Bailey CJ Ceriello A Delgado V et al 2019 ESC guidelines on diabetes, Pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2020) 41:255–323. 10.1093/eurheartj/ehz486
28.
Anawade PA Sharma D Gahane S . A comprehensive review on exploring the impact of telemedicine on healthcare accessibility. Cureus. (2024) 16:e55996. 10.7759/cureus.55996
29.
Haleem A Javaid M Singh RP Suman R . Telemedicine for healthcare: capabilities, features, barriers, and applications. Sens Int. (2021) 2:100117. 10.1016/j.sintl.2021.100117
30.
Serrano LP Maita KC Avila FR Torres-Guzman RA Garcia JP Eldaly AS et al Benefits and challenges of remote patient monitoring as perceived by health care practitioners: a systematic review. Perm J. (2023) 27:100–11. 10.7812/TPP/23.022
31.
Thomas EE Taylor ML Banbury A Snoswell CL Haydon HM Gallegos Rejas VM et al Factors influencing the effectiveness of remote patient monitoring interventions: a realist review. BMJ Open. (2021) 11:e051844. 10.1136/bmjopen-2021-051844
32.
Handelsman Y Anderson JE Bakris GL Ballantyne CM Bhatt DL Bloomgarden ZT et al DCRM 2.0: multispecialty practice recommendations for the management of diabetes, cardiorenal, and metabolic diseases. Metab Clin Exp. (2024) 159:155931. 10.1016/j.metabol.2024.155931
33.
Packer M Anker SD Butler J Filippatos G Pocock SJ Carson P et al Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383:1413–24. 10.1056/NEJMoa2022190
34.
Verma S McMurray JJV . SGLT2 Inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. (2018) 61:2108–17. 10.1007/s00125-018-4670-7
35.
Anker SD Butler J Filippatos G Ferreira JP Bocchi E Böhm M et al Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. 10.1056/NEJMoa2107038
36.
McGuire DK Zinman B Inzucchi SE Wanner C Fitchett D Anker SD et al Effects of empagliflozin on first and recurrent clinical events in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a secondary analysis of the EMPA-REG OUTCOME trial. Lancet Diabetes Endocrinol. (2020) 8(12):949–59. 10.1016/S2213-8587(20)30344-2
37.
Zinman B Wanner C Lachin JM Fitchett D Bluhmki E Hantel S et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. 10.1056/NEJMoa1504720
38.
Mann JFE Ørsted DD Brown-Frandsen K Marso SP Poulter NR Rasmussen S et al Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. (2017) 377:839–48. 10.1056/NEJMoa1616011
39.
Wanner C Inzucchi SE Lachin JM Fitchett D von Eynatten M Mattheus M J et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. (2016) 375:323–34. 10.1056/NEJMoa1515920
40.
Tsapas A Karagiannis T Kakotrichi P Avgerinos I Mantsiou C Tousinas G et al Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. (2021) 23(9):2116–24. 10.1111/dom.14451
41.
Ferreira JP Pitt B Zannad F . Mineralocorticoid receptor antagonists in heart failure: an update. Circ Heart Fail. (2024) 17:e011629. 10.1161/CIRCHEARTFAILURE.124.011629
Summary
Keywords
cardio-kidney metabolic syndrome, cardio-renal metabolic syndrome, cardiovascular-kidney metabolic syndrome, CKM syndrome, CRM syndrome, cardiovascular, renal and metabolic disorders, chronic kidney disease
Citation
Iacoviello M, Gori M, Grandaliano G, Minutolo R, Pitocco D and Trevisan R (2025) A holistic approach to managing cardio-kidney metabolic syndrome: insights and recommendations from the Italian perspective. Front. Cardiovasc. Med. 12:1583702. doi: 10.3389/fcvm.2025.1583702
Received
26 February 2025
Accepted
24 March 2025
Published
08 April 2025
Volume
12 - 2025
Edited by
Manfredi Tesauro, University of Rome Tor Vergata, Italy
Reviewed by
Annalisa Noce, University of Rome Tor Vergata, Italy
Francesca Schinzari, Agostino Gemelli University Polyclinic (IRCCS), Italy
Updates
Copyright
© 2025 Iacoviello, Gori, Grandaliano, Minutolo, Pitocco and Trevisan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Massimo Iacoviello massimo.iacoviello@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.