- 1Department of Plastic Surgery, The Fifth Clinical Medical College of Henan University of Chinese Medicine (Zhengzhou People’s Hospital), Zhengzhou, China
- 2Department of Cardiology, Henan Province Key Laboratory of Cardiac Injury and Repair, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Atrial fibrillation (AF) is a critical arrhythmia in hypertrophic cardiomyopathy (HCM), yet the role of right ventricular (RV) dysfunction in AF risk stratification remains underexplored. We aimed to evaluate the association between RV remodeling and incident AF in HCM patients.
Methods: This retrospective cohort study included 612 HCM patients who underwent cardiac magnetic resonance (CMR) at our institution (2016–2023). Incident AF was identified via electronic medical records or structured telephone interviews. RV function was assessed using CMR-derived parameters, including ejection fraction (RVEF), peak emptying rate (PER), and peak filling rate (PFR).
Results: Among 612 patients (66.1% male), 72 (11.8%) had preexisting AF, and 29 (5.4%) developed new-onset AF over a median follow-up of 3.3 years. Patients with AF (preexisting or new-onset) exhibited older age and impaired RV function at baseline, including reduced RVEF, PER, and PFR (P < 0.05 for all). Multivariable Cox regression identified age, left atrial diameter (LAD), RVEF, and RV-PFR as independent predictors of new-onset AF. Adding RVEF and RV-PFR to a clinical model (age, NYHA class III/IV, LAD) significantly improved risk stratification (NRI: 0.80, P < 0.01; IDI: 0.07, P < 0.01).
Conclusions: RV dysfunction is prevalent in HCM patients with AF and provides incremental prognostic value for predicting new-onset AF beyond traditional clinical markers. These findings underscore RV functional assessment as a critical tool in AF risk stratification for HCM patients.
1 Introduction
Hypertrophic cardiomyopathy (HCM), a genetic disorder characterized by unexplained myocardial hypertrophy, is associated with a substantially elevated risk of atrial fibrillation (AF), occurring in approximately 20% of patients—four times higher than the general population (1, 2). Traditional predictors such as left atrial (LA) enlargement, age, and New York Heart Association (NYHA) functional class form the basis of the HCM-AF risk score, yet its predictive accuracy remains limited (3).
The historical focus on left-sided cardiac remodeling in HCM has overshadowed the significance of RV involvement. Recent studies highlight that right ventricular (RV) dysfunction is not merely a secondary phenomenon but a critical contributor to adverse outcomes in HCM (4, 5). Mechanistically, RV dysfunction in HCM may arise from shared myopathic processes affecting both ventricles, ventricular interdependence due to septal hypertrophy, or secondary pulmonary hypertension (6). These pathways create a substrate for atrial stretch, neurohormonal activation, and arrhythmogenesis—factors central to AF development (7).
Notably, RV functional indices such as peak emptying rate (PER) and peak filling rate (PFR) offer unique insights into diastolic and systolic reserve, which may precede structural RV changes (8). In non-HCM cohorts, RV dysfunction independently predicts AF incidence, suggesting its role as a systemic marker of cardiopulmonary pathology (9). Despite these advances, the interplay between RV remodeling and AF in HCM remains poorly characterized, leaving a critical gap in risk stratification strategies.
This study investigates RV functional parameters derived from cardiac magnetic resonance (CMR) as novel biomarkers to redefine AF risk stratification in HCM, emphasizing the underappreciated role of the right ventricle.
2 Methods
We included patients with HCM who underwent CMR examinations in our hospital from 2016 to 2023. The diagnosis of HCM was based on either a genetic diagnosis of HCM and LV wall thickness of ≥13 mm, or non-familial HCM patients with a LV wall thickness ≥15 mm, but no other cause of hypertrophy identified. HCM patients with poor CMR image quality, a history of ablation for supraventricular arrhythmias, a history of septal myectomy and alcohol septal ablation and severe primary valvular heart disease were excluded.
This study was carried out in accordance with the Declaration of Helsinki.Written informed consent was obtained from every patient. The study protocol was approved by the ethics committee of the first affiliated hospital of Zhengzhou University.
2.1 CMR protocol
CMR imaging was performed using a Siemens Skyra (3.0 T) magnetic resonance imaging system equipped with body coils comprising 16 channels. During the imaging procedure, four MRI-compatible electrodes were affixed between the right first and second ribs and the left fifth and sixth ribs of the subjects. Electrocardiogram (ECG) signals were acquired to synchronize the scanning at a specific time point. The epicardial and endocardial boundaries of the left ventricle (LV) and right ventricle (RV) myocardium were meticulously delineated throughout the complete cardiac cycle on every cine short-axis image. This facilitated the assessment of LV and RV end-diastolic and end-systolic volumes (EDV and ESV), end-diastolic diameter (EDD), ejection fractions (EF), and myocardial mass. Myocardial mass was computed by multiplying the volume of the myocardium estimated at end-diastole by the specific gravity of the myocardium (1.05 g/ml). The indices for EDV, ESV and mass were indexed to the individual's body surface area.
Feature-tracking analysis was conducted using the CVI software (version 5.3.4, Circle Vascular Imaging), which offers precise bi-ventricular anatomical tracking capabilities. All post-processing analyses were performed by two independent cardiovascular imaging specialists with more than 5 years of dedicated CMR experience. Analysts were blinded to clinical outcomes and group assignments. The endocardial boundaries of left atrial (LA) was manually traced at the phase of the maximal LA volume before mitral valve opening and at the phase of the minimum LA volume after atrial contraction on 2-chamber and 4-chamber view. LAV max, LAV min and LAEF were derived from the CVI software. The indices for LAV max and LAV min were indexed to body surface area. RV global strain measurements were taken from the free wall. Global longitudinal strains (GLS) were derived from tracking the long horizontal axis cine images, while circumferential strains (GCS) and radial strains (GRS) were obtained from the short-axis cine images acquired through the standard CMR steady-state free precession sequence. The RV volume/time (V/t) and dV/dt curves were obtained plotting the cavity volumes over time (Figure 1). From the ventricular dV/dt curves, the peak during diastole was defined as the peak filling rate (PFR). PFR represents the maximum speed of RV filling. The peak during systole was defined as the peak empty rate (PER). PER represents the maximum speed of RV emptying. PFR and PER were normalized by the RV EDV, obtaining PFR index and PER index.
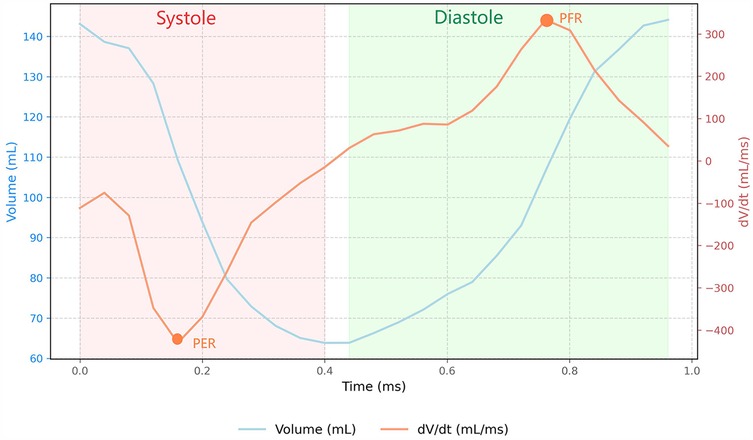
Figure 1. Right ventricular volume-time and dV/dt dynamics during cardiac cycle. The blue curve corresponds to the ventricular Volume-Time relationship, while the orange curve illustrates the corresponding dV/dt curve. The diastolic peak, identified as the peak filling rate (PFR), represents the maximum rate of ventricular filling during diastolic phase;The systolic peak, designated as the peak emptying rate (PER), indicates the maximum rate of ventricular volume reduction during systolic contraction.
2.2 Patients follow-up
The new-onset AF during follow-up was identified through a review of hospital electronic medical records or a telephone interview with each study subject. AF was defined as an irregular heart rhythm without distinct P-waves documented on ECG, Holter registration (if duration ≥30 s), including any form of AF (paroxysmal, persistent, permanent).
2.3 Statistic analysis
Continuous variables are presented as mean ± SD and were analyzed by Student t test. When continuous variables had a skewed distribution, they were shown as median and interquartile range and were analyzed by Mann–Whitney U test. Categorical variables are presented as number (%) and were analyzed by the χ2 test. Receiver operating characteristic curves were used to determine the optimal cutoff for the new-onset AF based on the Youden index. Event rates are plotted in Kaplan–Meier curves for new-onset AF, and groups were compared using the log-rank test. Variables with P < 0.05 at univariate Cox proportional hazards models were included as covariables in multivariable models. Although NYHA III/IV was insignificant in univariate Cox analysis, we still included it in multivariable analysis as a well-recognized variable to predict AF in previous studies (3, 4). Post-hoc power analysis demonstrated 82% power to detect the observed RV-PFR difference (α = 0.05), supporting the robustness of our findings despite cohort size constraints.
To assess the incremental value of RV dysfunction in addition to conventional risk factors for predicting adverse events, we calculated the improvement in global χ2 values. Reclassification of patients was calculated using the category-free versions of the net reclassification index (NRI) and integrated discrimination improvement (IDI). Statistical analyses were performed using R software (Version 4.3.1; Package survivalROC and survIDINRI; R foundation for Statistical Computing, Vienna, Austria).
3 Results
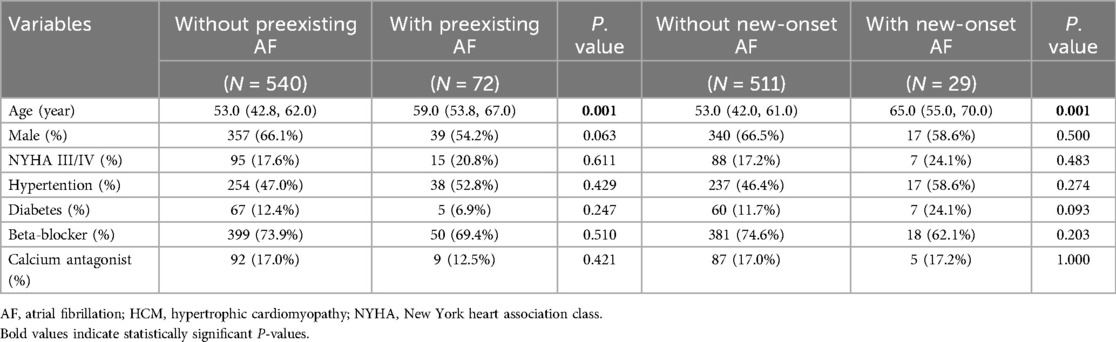
The present study included 612 subjects, containing 357 (66.1%) males, 72 (11.8%) patients with preexisting AF and 29 (5.4%) patients with new-onset AF during a median follow-up of 3.3 years (2.3–3.8years). The baseline characteristics were summarized in Table 1.
3.1 RV function between HCM patients with and without AF
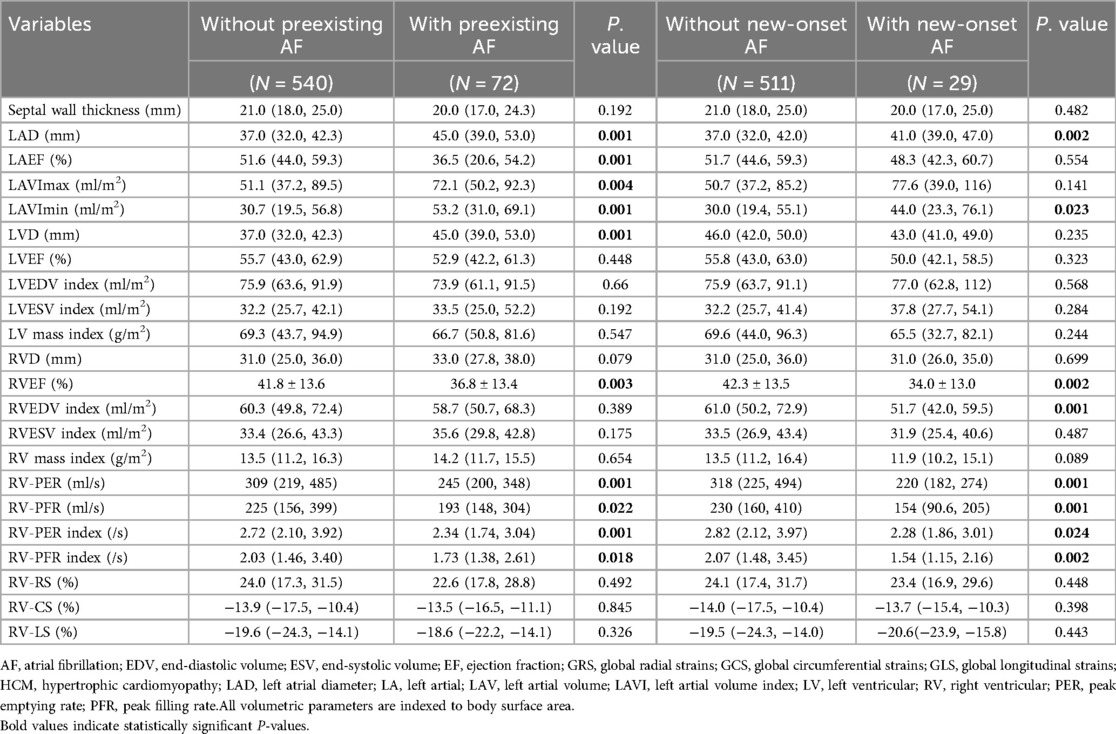
As showed in Table 2, in patients with preexisting AF, age and left atrial remodeling were significantly advanced compared to the non-AF group (all P < 0.05), with larger left atrial dimensions (LAD, LAVI max/min, all P < 0.01) and impaired LAEF (P = 0.001). Notably, this group exhibited pronounced RV dysfunction characterized by reduced systolic and diastolic performance (RVEF, P = 0.003; RV-PFR, P = 0.022), despite preserved RV size and strain parameters (P > 0.05).
Among patients developing new-onset AF during follow-up, similar patterns emerged but with earlier-stage alterations: elevated baseline LAD (P = 0.002) and LAVI min (P = 0.023) coexisted with preserved LAEF (P = 0.18). RV functional decline was more globally apparent, involving both contractile and volumetric parameters (RVEF, P = 0.002; RVEDV, P = 0.001), alongside markedly depressed peak filling/emptying rates (RV-PFR, P = 0.001; RV-PER, P = 0.001).
3.2 Predictors of new-onset AF
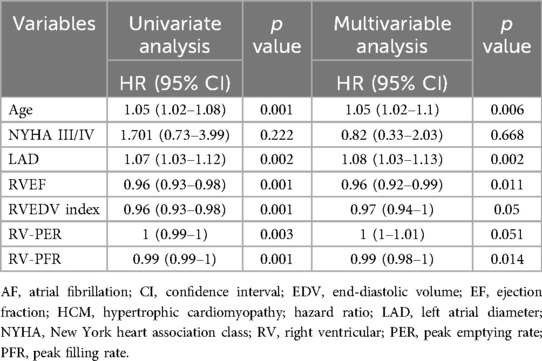
In univariate analysis (Table 3), age, LAD, NYHA III/IV, RVEF, RVEDV index, RV-PER and RV-PFR predicted the new-onset AF in HCM patients. However, only age, LAD, RVEF, RV-PFR remained significant in multivariable analysis.
The receiver operating characteristic analysis determined the optimal RVEF and RV-PFR cutoff was 43.1% and 210.9 ml/s respectively for predicting new-onset AF. Kaplan–Meier analyses of event-free survival for new-onset AF were shown in Figure 2. HCM patients with lower RVEF (<43.1%, Figure 2B) and lower RV-PFR (<210.9 ml/s, Figure 2A) were more likely to experience new-onset AF in the follow-up (both p < 0.05). In addition, HCM patients both with lower RVEF (<43.1%) and lower RV-PFR (<210.9 ml/s) showed the worst prognosis for new-onset AF (Figure 2C).
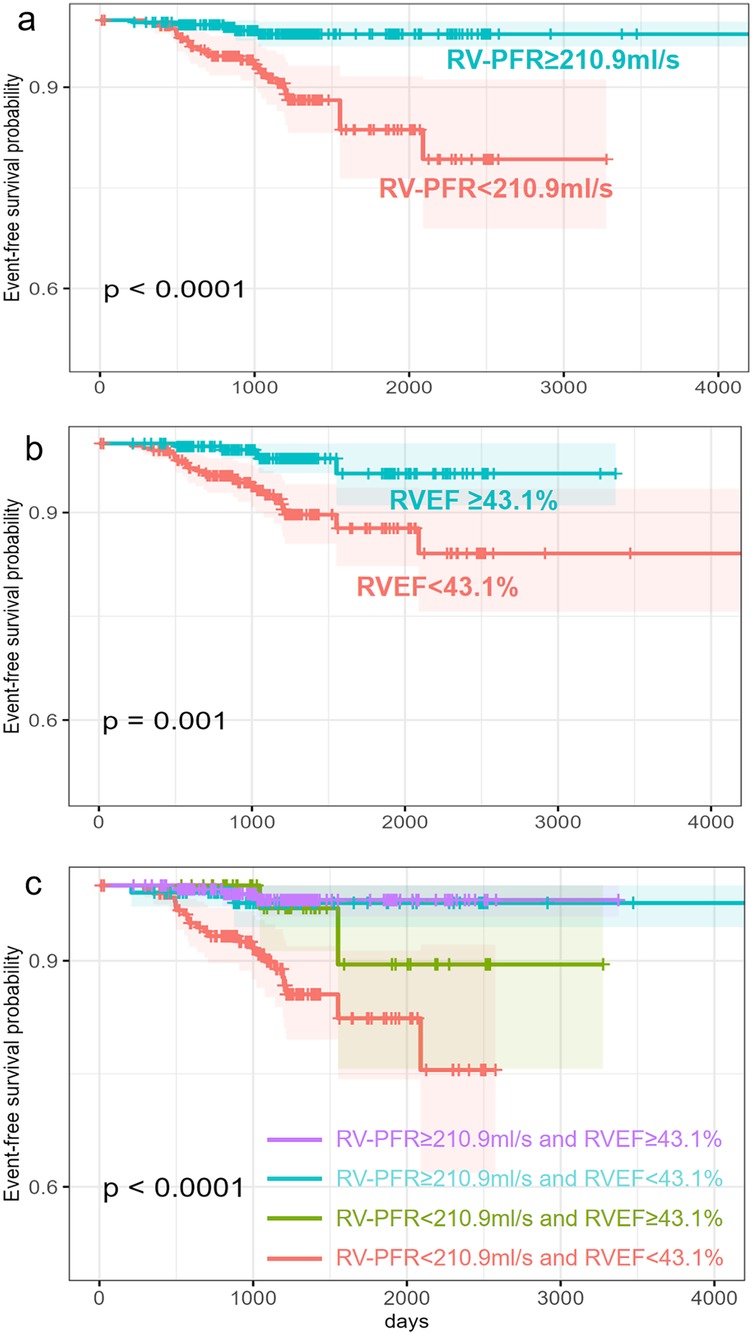
Figure 2. Kaplan–Merier survival analysis for new-onset AF in HCM patients. Patients are stratified by RVEF (b), RV-PFR (a) and both RVEF and RV-PFR (c).
3.3 Incremental prognostic value of RVEF and RV-PFR for new-onset AF
As showed in Figure 3, we added clinical variables, such as age, LAD, NYHA III/IV to basic model (global χ2, 18.21; C index 0.741). Addition of RVEF achieved an increase in the χ2 statistic (global χ2, 32.88; C index 0.799, p < 0.001; Table 4) and improved risk stratification [NRI, 0.6795 (0.3326–1.0264), p < 0.01; IDI, 0.0461 (0.0138–0.0784), p < 0.01]. Furthermore, adding RV-PFR achieved an additional increase in the χ2 statistic (global χ2 45.54; C index 0.819, p < 0.001) and greater reclassification of patients [NRI, 0.8029 (0.4959–1.1099), p < 0.01; IDI, 0.0724 (0.0322–0.1126), p < 0.01; Table 4].
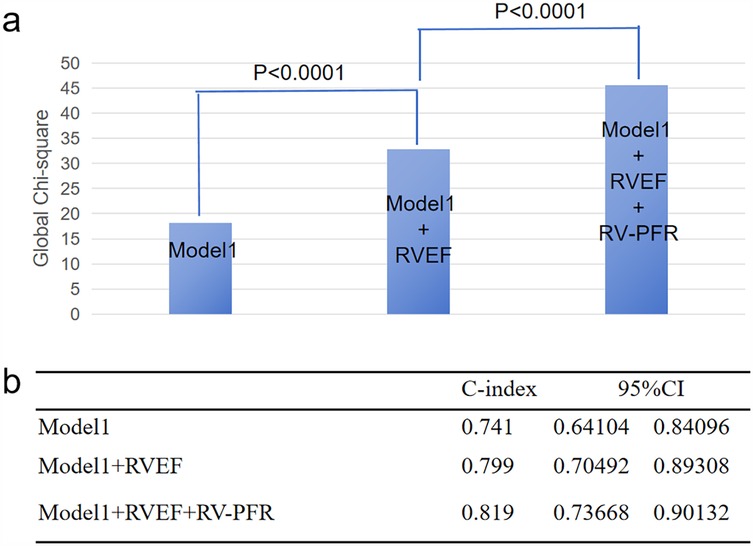
Figure 3. Incremental value of RVEF and RV-PFR for new-onset AF in HCM patients. The addition of RVEF and RV-PFR in sequence achieved incremental global χ2 (a) and C index (b) to known risk factors for the prediction of new-onset AF (age, NYHA class III/IV, LAD).
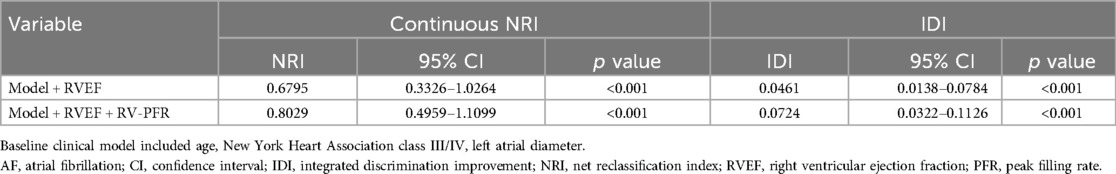
Table 4. Continuous NRI and IDI for new-onset AF by adding RVEF and RV-PFR to a model including clinical covariables.
4 Discussion
This study provides the a comprehensive analysis of the association between RV dysfunction and AF risk in patients with HCM. Our findings reveal three key insights: (1) RV functional impairment is prevalent in HCM patients with AF, (2) RVEF and RV-PFR independently predict new-onset AF, and (3) RV metrics significantly enhance AF risk stratification beyond conventional predictors. These discoveries fundamentally reshape our understanding of RV contributions to HCM pathophysiology.
4.1 RV functional assessment: a paradigm shift in HCM evaluation
Historically, RV function has been overshadowed by a predominant focus on LV pathology (10). Our findings challenge this LV-centric paradigm by demonstrating that 30% of HCM patients exhibit RV dysfunction—a prevalence mirroring recent CMR studies (11). Notably, Mahmod et al. (12) demonstrated that RV strain abnormalities precede LV systolic dysfunction in HCM, suggesting RV assessment could serve as an early disease marker. These observations position the RV not as a passive bystander, but as an active contributor to HCM progression. This RV involvement likely originates from three interconnected mechanisms: shared myopathic processes affecting both ventricles, ventricular interdependence due to septal hypertrophy, and secondary pulmonary hypertension (6, 13).
4.2 RV-PFR: overcoming limitations of conventional metrics
Current RV assessment tools present critical limitations. RVEF, while valuable for global systolic evaluation, proves load-dependent and insensitive to early dysfunction (14). Strain analysis suffers from modality-specific variability (15), and volumetric indices fail to capture functional decline. In this study RV-PFR quantifies the maximal early diastolic filling rate, reflecting ventricular compliance through direct measurement of pressure-driven atrial-ventricular gradients. Our results demonstrate that RV-PFR independently predicts new-onset AF (HR = 0.99, P = 0.014), with its addition to clinical models significantly improving reclassification accuracy (NRI = 0.80).This divergence may arise from their distinct pathophysiological correlates: RV-PFR directly capture pressure-mediated ventricular filling dynamics, whereas strain quantifies myocardial deformation magnitude. In HCM, early RV dysfunction manifests predominantly as impaired relaxation rather than contractile failure, favoring flow-derived metrics over deformation indices for AF prediction.
4.3 Mechanistic links between RV dysfunction and AF pathogenesis
The pathophysiological interplay between RV dysfunction and AF development in HCM involves a triad of interconnected mechanisms. Central to this relationship is hemodynamic coupling, where reduced RV peak filling rate directly elevates right atrial pressure through impaired ventricular compliance. This pressure overload induces atrial wall stretch, triggering electrical remodeling via calcium handling abnormalities and connexin dysregulation (16). Notably, this aligns with Chatterjee's observations in non-HCM populations, where RV dysfunction independently promoted AF initiation through similar pressure-mediated pathways (4). Neurohormonal activation further amplifies this process, as RV dysfunction correlates with elevated B-type natriuretic peptide (BNP) and pro-inflammatory cytokines such as IL-6 (17). These molecular mediators create a pro-fibrotic atrial microenvironment that disrupts intercellular conduction velocity and wavefront propagation. This mechanical strain propagates bidirectionally, exacerbating left atrial enlargement—the most robust traditional AF predictor (18, 19). Our multivariable analysis underscores this complex synergy, with RV-PFR (HR = 0.99) and left atrial diameter (HR = 1.08) operating through distinct but complementary pathways to drive AF progression.
4.4 Limitations and future directions
This study has limitations: (1) Retrospective, single-center design risks selection bias and underdetection of asymptomatic AF; (2) Small new-onset AF cohort (n = 29) limits multivariable model robustness; While the new-onset AF subgroup was modest (n = 29), post-hoc calculations indicated sufficient sensitivity (power = 82%) to identify RV dysfunction effects, reflecting the challenges of HCM cohort recruitment. (3) Lack of molecular data (e.g., genetic variants) to elucidate RV-AF pathways; (4) RV-PFR reproducibility across CMR platforms requires validation. Manual RV volumetric analysis remains time-intensive and lacks universal automation, limiting scalability. Future studies should employ prospective multicenter cohorts, integrate advanced imaging, and explore genotype-specific RV phenotypes.
5 Conclusions
RV dysfunction is prevalent in HCM patients with AF and provides incremental prognostic value for predicting new-onset AF beyond traditional clinical markers. These findings underscore RV functional assessment as a critical tool in AF risk stratification for HCM patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of the first affiliated hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition. XB: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Investigation, Methodology, Project administration. QA: Data curation, Formal analysis, Writing – original draft. YL: Data curation, Writing – review & editing. CL: Data curation, Writing – review & editing. DS: Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. (2022) 79(4):372–89. doi: 10.1016/j.jacc.2021.12.002
2. Alphonse P, Virk S, Collins J, Campbell T, Thomas SP, Semsarian C, et al. Prognostic impact of atrial fibrillation in hypertrophic cardiomyopathy: a systematic review. Clin Res Cardiol. (2021) 110(4):544–54. doi: 10.1007/s00392-020-01730-w
3. Carrick RT, Maron MS, Adler A, Wessler B, Hoss S, Chan RH, et al. Development and validation of a clinical predictive model for identifying hypertrophic cardiomyopathy patients at risk for atrial fibrillation: the HCM-AF score. Circ Arrhythm Electrophysiol. (2021) 14(6):e9796. doi: 10.1161/CIRCEP.120.009796
4. Chatterjee NA, Shah RV, Murthy VL, Praestgaard A, Shah SJ, Ventetuolo CE, et al. Right ventricular structure and function are associated with incident atrial fibrillation: MESA-RV study (multi-ethnic study of atherosclerosis-right ventricle). Circ Arrhythm Electrophysiol. (2017) 10(1):e004738. doi: 10.1161/CIRCEP.116.004738
5. Hiemstra YL, Debonnaire P, Bootsma M, Schalij MJ, Bax JJ, Delgado V, et al. Prevalence and prognostic implications of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am J Cardiol. (2019) 124(4):604–12. doi: 10.1016/j.amjcard.2019.05.021
6. Raman B, Smillie RW, Mahmod M, Chan K, Ariga R, Nikolaidou C, et al. Incremental value of left atrial booster and reservoir strain in predicting atrial fibrillation in patients with hypertrophic cardiomyopathy: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. (2021) 23(1):109. doi: 10.1186/s12968-021-00793-6
7. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2011) 58(25):2703–38. doi: 10.1016/j.jacc.2011.10.825
8. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. (2008) 117(13):1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584
9. Severino S, Caso P, Cicala S, Galderisi M, de Simone L, D'Andrea A, et al. Involvement of right ventricle in left ventricular hypertrophic cardiomyopathy: analysis by pulsed Doppler tissue imaging. Eur J Echocardiogr. (2000) 1(4):281–8. doi: 10.1053/euje.2000.0043
10. Mahmod M, Raman B, Chan K, Sivalokanathan S, Smillie RW, Abd Samat AH, et al. Right ventricular function declines prior to left ventricular ejection fraction in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. (2022) 24(1):36. doi: 10.1186/s12968-022-00868-y
11. Aziz EF, Kukin M, Javed F, Musat D, Nader A, Pratap B, et al. Right ventricular dysfunction is a strong predictor of developing atrial fibrillation in acutely decompensated heart failure patients, ACAP-HF data analysis. J Card Fail. (2010) 16(10):827–34. doi: 10.1016/j.cardfail.2010.05.004
12. Lee S, Park J, Uhm J, Kim JY, Pak H-N, Lee M-H, et al. Impact of atrial fibrillation on the clinical course of apical hypertrophic cardiomyopathy. Heart (British Cardiac Society). (2017) 103(19):1496–501. doi: 10.1136/heartjnl-2016-310720
13. Sørensen E, Myrstad M, Solberg MG, Øie E, Tveit A, Aarønæs M. Right heart structure and function in lifelong recreational endurance athletes with and without paroxysmal atrial fibrillation. J Am Soc Echocardiogr. (2022) 35(12):1259–68. doi: 10.1016/j.echo.2022.06.008
14. Ventetuolo CE, Lima JAC, Barr RG, Bristow MR, Bagiella E, Chahal H, et al. The renin-angiotensin system and right ventricular structure and function: the mesa-right ventricle study. Pulm Circ. (2012) 2(3):379–86. doi: 10.4103/2045-8932.101657
15. Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging. (2015) 8(12):1444–60. doi: 10.1016/j.jcmg.2015.11.001
16. Bi X, Song Y, Yang C, Song Y, Zhao S, Qiao S, et al. Sex differences in atrial remodeling and its relationship with myocardial fibrosis in hypertrophic obstructive cardiomyopathy. Front Cardiovasc Med. (2022) 9:947975. doi: 10.3389/fcvm.2022.947975
17. Ting P, Chou A, Wu VC-C, Tsai F-C, Chu J-J, Chen C-Y, et al. Relationship between right ventricular function and atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth. (2017) 31(5):1663–71. doi: 10.1053/j.jvca.2017.05.018
18. Kawut SM, Poor HD, Parikh MA, Hueper K, Smith BM, Bluemke DA, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol. (2014) 64(19):2000–9. doi: 10.1016/j.jacc.2014.07.991
19. Chahal H, Heckbert SR, Barr RG, Bluemke DA, Jain A, Habibi M, et al. Ability of reduced lung function to predict development of atrial fibrillation in persons aged 45–84 years (from the multi-ethnic study of atherosclerosis-lung study). Am J Cardiol. (2015) 115(12):1700–4. doi: 10.1016/j.amjcard.2015.03.018
Keywords: hypertrophic cardiomyopathy, right ventricular dysfunction, atrial fibrillation, cardiac magnetic resonance, prognosis
Citation: Li S, Bi X, An Q, Li Y, Li C and Shen D (2025) Right ventricular dysfunction improves prediction of atrial fibrillation in hypertrophic cardiomyopathy: a cardiac magnetic resonance study. Front. Cardiovasc. Med. 12:1587398. doi: 10.3389/fcvm.2025.1587398
Received: 4 March 2025; Accepted: 19 May 2025;
Published: 5 June 2025.
Edited by:
Ahmed Abuzaid, Providence Alaska Medical Center, United StatesReviewed by:
Samir Saha, Sundsvall Municipality, SwedenAli Bahbah, Minneapolis Heart Institute Foundation (MHIF), United States
Copyright: © 2025 Li, Bi, An, Li, Li and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuanye Bi, Nzg0Mjc0NzEwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Shengxu Li
Shengxu Li Xuanye Bi
Xuanye Bi Quanxu An2
Quanxu An2