- Department of General Practice, Shijiazhuang People’s Hospital, Shijiazhuang, China
Aims: Patients with coronary-artery disease (CAD) and type 2 diabetes mellitus (T2DM) are often in a hypercoagulable state and have an increased thrombosis risk. We aimed to evaluate the effects of sodium/glucose cotransporter 2 inhibitors (SGLT2is) on coagulation function and explore their potential role in regulating coagulation in these patients.
Methods: We conducted a retrospective cohort study between June 2020 and June 2024 in patients with CAD and T2DM. Eligible patients were assigned to either the SGLT2i or non-SGLT2i group. Clinical information, laboratory tests, and echocardiographic (EKG) examination results were retrieved. We performed inter- and intragroup comparisons of coagulation function measurements before and after treatment, and also conducted regression analysis to assess the impact of treatment on coagulation function.
Results: A total of 121 patients were included, with 49 and 72 in the SGLT2i and non-SGLT2i groups, respectively. After 30 days of treatment, antithrombin III (AT-III) activity increased by 5.39% (P = 0.026) in the SGLT2i group, but slightly decreased in the non-SGLT2i group. SGLT2 is also decreased D-dimer levels by 95 mg/L (group P = 0.051, group:time P = 0.075). Further regression analysis showed a significant interaction between group and time for AT-III and D-dimer (P = 0.026 and P = 0.039). Additionally, prothrombin time (PT) showed a slight increase after SGLT2i treatment.
Conclusion: SGLT2is could affect coagulation function by prolonging coagulation time and increasing anticoagulatory activity in patients with T2DM and CAD. These drugs could be used to ameliorate hypercoagulable states and reduce thrombosis risk in these patients.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a common disease. It is reported that up to 34.8% of T2DM patients have comorbid coronary-artery disease (CAD) (1). The presence of both CAD and T2DM can activate intrinsic- and extrinsic-coagulation pathways, elevate factor VIII (FVIII) levels, shorten activated partial thromboplastin time (APTT), and reduce the activity of anticoagulant proteins [protein C, protein S, and antithrombin (AT)], leading to a sustained hypercoagulable state and a high risk for thrombosis (2–4). Appropriate anticoagulant and antiplatelet treatments are required to effectively prevent thrombotic formation and cardiovascular (CV) complications in these patients with multiple comorbidities.
Sodium/glucose cotransporter 2 inhibitors (SGLT2is) are a class of antihyperglycemic agents. In addition to their glucose-lowering effects, SGLT2is can provide CV protection and reduce the incidence of major adverse cardiovascular events (MACEs) (5–7). These drugs are recommended in patients with T2DM and CAD to mitigate their CV risks (8). Several studies have demonstrated that SGLT2is can increase adenosine monophosphate (AMP)–activated protein kinase activity and enhance phosphorylation of endothelial nitric oxide synthase (eNOS) to directly regulate vascular homeostasis (9–11). In addition, the anti-inflammatory activities of SGLT2is alleviate endothelial inflammation and maintain endothelial integrity (12, 13). A recent study reported that SGLT2is could reduce mortality caused by CV disease (CVD), probably via their thrombosis-preventing effects (14). All of this evidence suggests a potential role for SGLT2is in modulating thrombosis, which might be beneficial to patients in hypercoagulable states. However, clinical research into the anticoagulatory role of SGLT2is in patients in hypercoagulable states, such as those with comorbid CAD and T2DM, is limited.
Therefore, we performed a retrospective study aiming to assess the effect of SGLT2is on coagulation function in patients with CAD and T2DM. Our objective was to provide an evidence-based strategy to optimize anticoagulant therapy, decrease disease complications, and improve quality of life (QoL) in these patients.
2 Methods
2.1 Study design and participant selection
This was a retrospective cohort study in patients who visited Shijiazhuang People's Hospital (Shijiazhuang, China) between June 2020 and June 2024. The study protocol was approved by the hospital's ethics committee (Approval No. 083), and the study was conducted in compliance with the ethical standards of the Declaration of Helsinki. Informed consent was waived due to the retrospective study design.
Inclusion criteria were patients (1) aged 18–90 years; (2) diagnosed with CAD based on typical angina symptoms, coronary angiography (CAG), and/or echocardiographic (EKG) results; and (3) with a diagnosis of T2DM per World Health Organization (WHO) criteria. Exclusion criteria were patients (1) using SGLT2is for at least 1 month before study initiation; (2) with severe hepatic or renal impairment [defined as alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >3× the upper limit of normal, or an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2]; (3) comorbidly using other antidiabetic agents [glucagon-like peptide-1 (GLP-1) receptor agonists] during the study period; (4) who were pregnant or lactating; (5) with malignancies, hematological disorders, or infections; (6) on long-term anticoagulant therapy (warfarin or heparin); (7) with psychiatric disorders, cognitive impairments, or any other conditions compromising their ability to follow the treatment regimen; or (8) who were allergic to SGLT2is and therefore discontinued the treatment. Patients with incomplete or missing data were also excluded.
2.2 Data collection
We reviewed patients' medical records and retrieved the following information: age, sex, height, weight, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Medications, including SGLT2is (dapagliflozin or empagliflozin 20 mg 1×/day), aspirin, clopidogrel or ticagrelor, statins or ezetimibe, angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), angiotensin receptor neprilysin inhibitors (ARNIs), beta blockers, diuretics, metformin, sulfonylureas, and insulin, were recorded.
We documented laboratory test results before and 1 month after SGLT2i treatment. Tests included coagulation markers such as APTT, antithrombin III (AT-III), prothrombin time (PT), prothrombin activity (PTA), international normalized ratio (INR), thrombin time (TT), fibrin degradation products (FDPs), and D-dimer. We also tested for fasting glucose (Glu), glycated hemoglobin (HbA1c), red blood cell count (RBC), hemoglobin (HGB), hematocrit (Hct), mean corpuscular volume (MCV), total platelet count (PLT), plateletcrit (PCT), mean platelet volume (MPV), low-density lipoprotein (LDL), high-density lipoprotein (HDL), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), total cholesterol (TC), triglycerides (TG), lipoprotein a [Lp(a)], ALT, AST, cholinesterase (ChE), alkaline phosphatase (AKP), gamma-glutamyl transferase (GGT), urea, uric acid (UA), and eGFR. Cardiac function was evaluated by EKG examination, and heart failure (HF) was classified as heart failure with reduced ejection fraction (HFrEF), mildly reduced ejection fraction (HFmEF), or preserved ejection fraction (HFpEF).
2.3 Statistical analysis
We performed data analysis using R software version 4.4.2 (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as means with standard deviation (SDs) or with interquartile ranges (IQRs), depending on the normality test results. Normality was assessed via the Kolmogorov–Smirnov test, and homogeneity of variances was assessed via Levene's test. For continuous variables that followed a normal distribution and had homogeneity of variance, repeated measures two-way ANOVA was used for between-group comparisons. For non-normally distributed or heteroscedastic data, if normality and homogeneity of variance were achieved after logarithmic transformation, repeated measures two-way ANOVA was applied; otherwise, the Scheirer–Ray–Hare test was used. Categorical variables were described using frequencies and percentages and compared using the χ2 test or Fisher's exact test. First, we compared the baseline characteristics between the SGLT2i and non-SGLT2i groups, after which we conducted an analysis of the changes in coagulation parameters before and after treatment within each group. Furthermore, regression analysis was performed to evaluate the impact of SGLT2i treatment on coagulation function. For continuous dependent variables that followed a normal distribution, a linear mixed-effects model (LMM) was applied, whereas for non-normally distributed continuous dependent variables, a generalized linear mixed-effects model (GLMM) was used.
3 Results
3.1 Participant characteristics
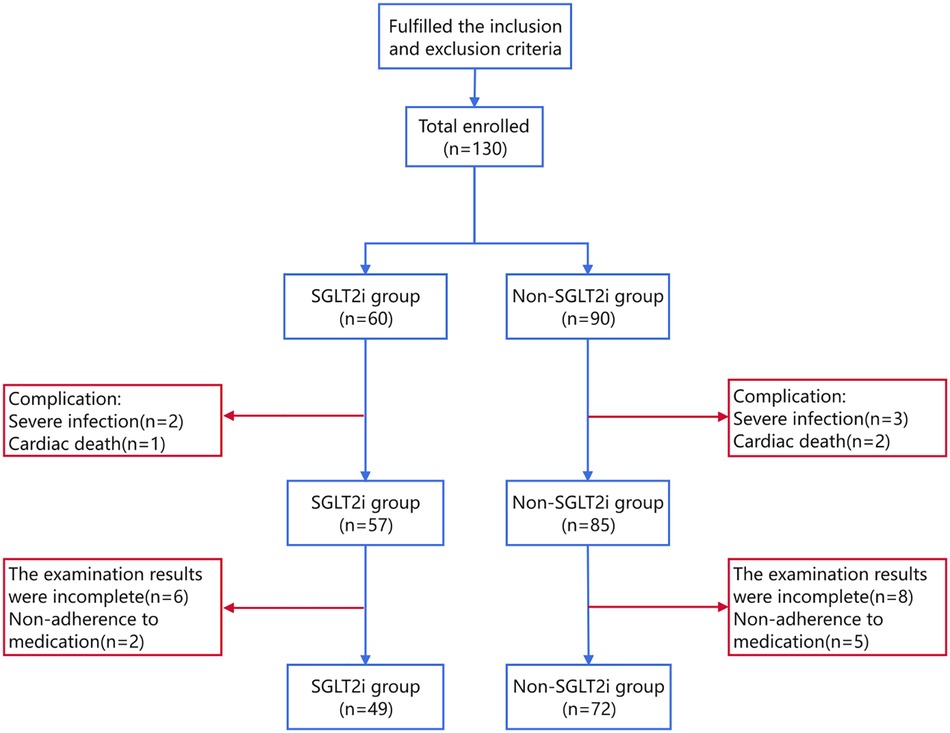
A total of 121 patients were included in the current study, with 49 and 72 in the SGLT2i and non-SGLT2i groups, respectively. The participant selection flowchart is shown in Figure 1.
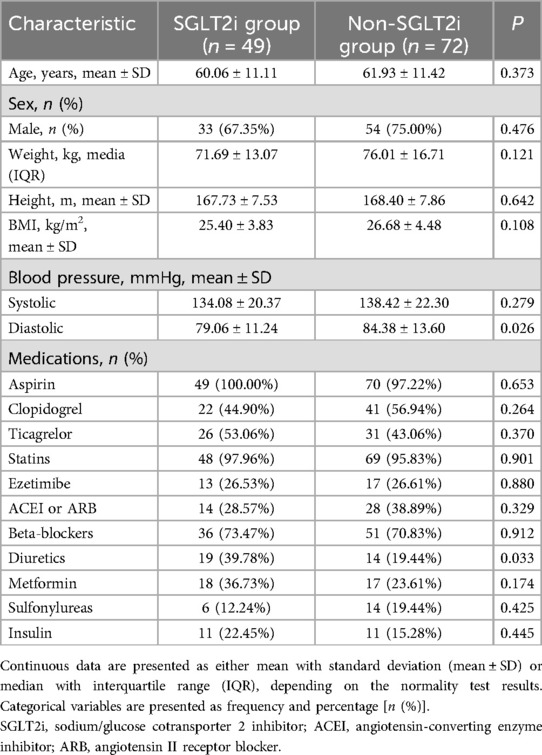
Comparisons of baseline clinical characteristics and medications between the two groups showed that only diuretic use had a statistically significant intergroup difference (Table 1). All other variables were comparable.
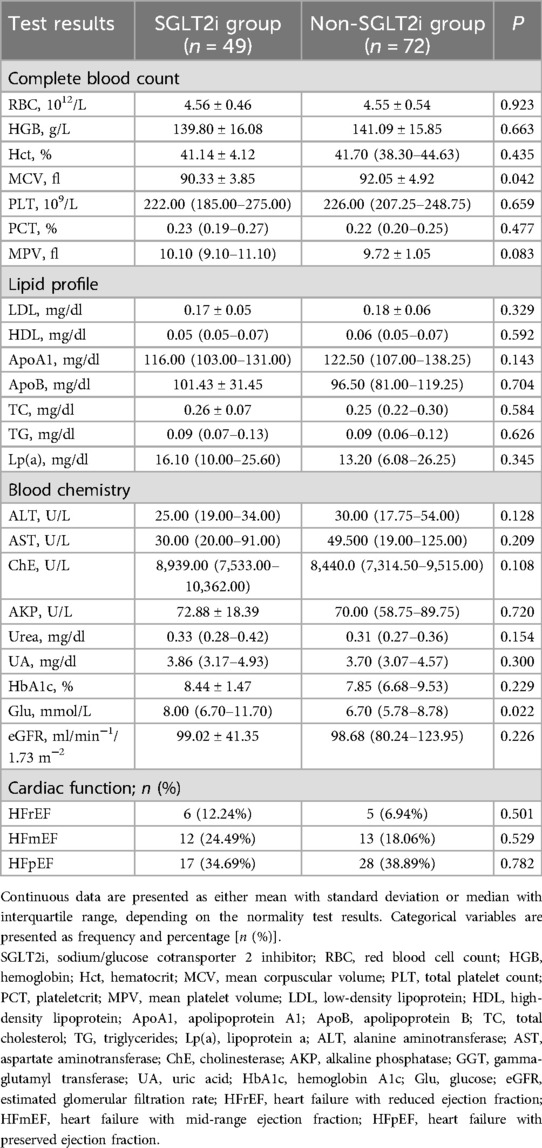
Baseline laboratory test and EKG result comparisons showed statistically significant intergroup differences in Glu and MCV levels (Table 2). Otherwise, all other measurements were similar between the groups.
3.2 Differential analysis of coagulation profile changes
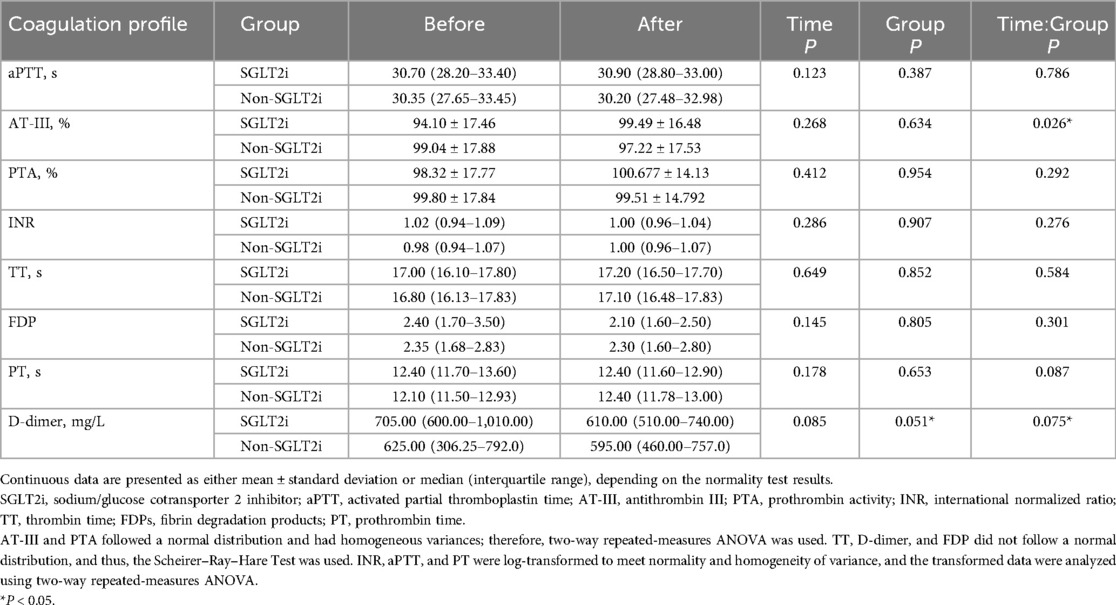
Since patients with T2DM and CAD often have abnormal coagulation profiles, we performed further analyses to examine coagulation profile changes following the SGLT2i treatment (Table 3, Figure 2).
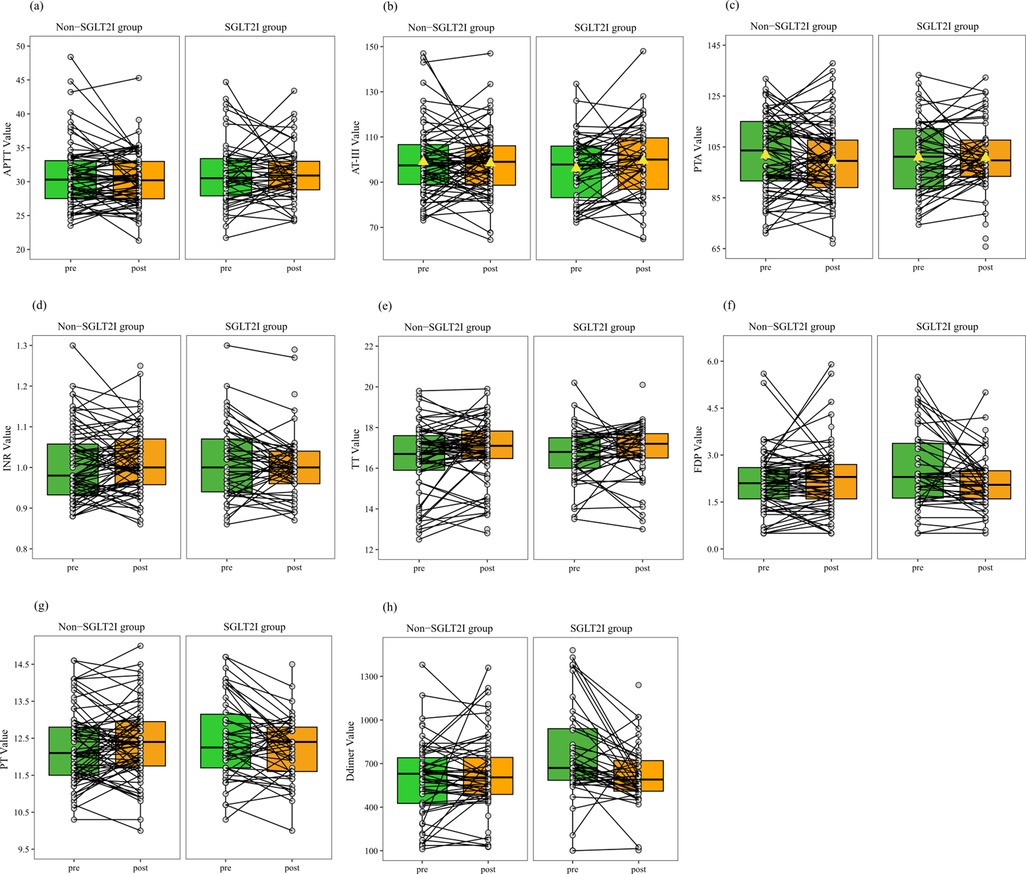
Figure 2. Paired line plots for stratified analysis comparing coagulation profiles before and after treatment between the SGLT2i and non-SGLT2i groups of patients stratified into the normal reference range subgroup. (a), aPTT; (b), AT-III; (c), D-dimer; (d), INR; (e), PTA; (f), TT; (g), FDP; (h), PT. (b) Unlike the non-SGLT2i group, the SGLT2i group exhibits an upward shift in the box plot, indicating a higher median after treatment. (c) Unlike the non-SGLT2i group, the SGLT2i group exhibits a downward shift in the box plot, suggesting a lower median after treatment. (a, d–h) Similar to the non-SGLT2i group, the SGLT2i group exhibits no significant change in the median or trend of the connected lines after treatment. (b, c) AT-III and PTA follow a normal distribution, with the mean represented by a triangle.
After 30 days of treatment, the mean AT-III level in the SGLT2i group increased from a baseline mean of 94.10% to 99.49%, reflecting a 5.39% increase. In the non-SGLT2i group, the AT-III level decreased from 99.04% before treatment to 97.22% after treatment. Repeated measures two-way ANOVA indicated a significant interaction effect between time and group (P = 0.026), suggesting that AT-III changes differed between the groups. However, the overall effect of time alone was not significant (P = 0.268), indicating that SGLT2i treatment could have a potential upregulating effect on AT-III.
The median D-dimer level in the SGLT2i group decreased from a baseline value of 705.00 mg/L to 610.00 mg/L, reflecting a reduction of 95 mg/L. In the non-SGLT2i group, the median D-dimer level decreased from 625.00 mg/L before treatment to 595.00 mg/L after treatment. In terms of the magnitude of the reduction, the decrease in the non-SGLT2i group was smaller than that in the SGLT2i group. Repeated measures two-way ANOVA showed that although statistical significance was not reached, the interaction effect between time and group (P = 0.075) and the overall effect of time alone (P = 0.085) both showed trends toward significance, especially the group effect (P = 0.051). These findings suggest that SGLT2i treatment may reduce D-dimer levels, although the effect did not reach statistical significance and further analysis of potential impacts is needed.
The median PT in the SGLT2i group remained unchanged at 12.40 s. In the non-SGLT2i group, the median PT increased from 12.10 s before treatment to 12.40 s after treatment. Repeated measures two-way ANOVA showed no significant interaction effect between time and group (P = 0.087), nor significant differences in the overall time effect (P = 0.178) or group effect (P = 0.653). These findings suggested that while PT remained largely unchanged with SGLT2i treatment, the interaction effect between time and group (P = 0.087) was close to being significant, indicating the need for further analysis of the potential impact of group.
For the remaining coagulation parameters, including aPTT, PTA, INR, TT, and FDP, no statistically significant differences were observed between the SGLT2i and non-SGLT2i groups. Repeated measures two-way ANOVA showed no significant time effect, group effect, or time– group interaction for these indicators (all P > 0.05). These results suggested that SGLT2i treatment did not significantly alter these coagulation markers over the study period.
3.3 Regression analysis of coagulation profile
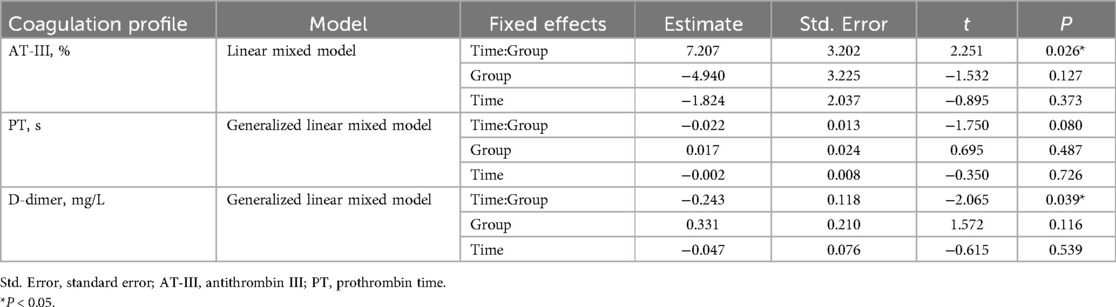
We conducted a regression analysis to evaluate the impact of SGLT2i treatment on AT-III, PT, and D-dimer. The results showed that the interaction between time and group significantly affected AT-III and D-dimer (P = 0.026 and P = 0.039, respectively). Specifically, the interaction coefficient for AT-III was positive (estimate = 7.207), indicating that the increase in AT-III levels over time was greater in the SGLT2i group than in the non-SGLT2i group. In contrast, the interaction coefficient for D-dimer was negative (estimate = −0.243), suggesting that D-dimer levels decreased more over time in the SGLT2i group than in the non-SGLT2i group. For PT, the estimated group effect of SGLT2i was 0.017, indicating that, on average, PT levels were slightly higher in the SGLT2i group than in the non-SGLT2i group, regardless of whether at baseline or one month after treatment. However, this difference was small and not statistically significant (P = 0.487). Additionally, the group effects of SGLT2i on AT-III and D-dimer were not statistically significant (Table 4, Figure 3).
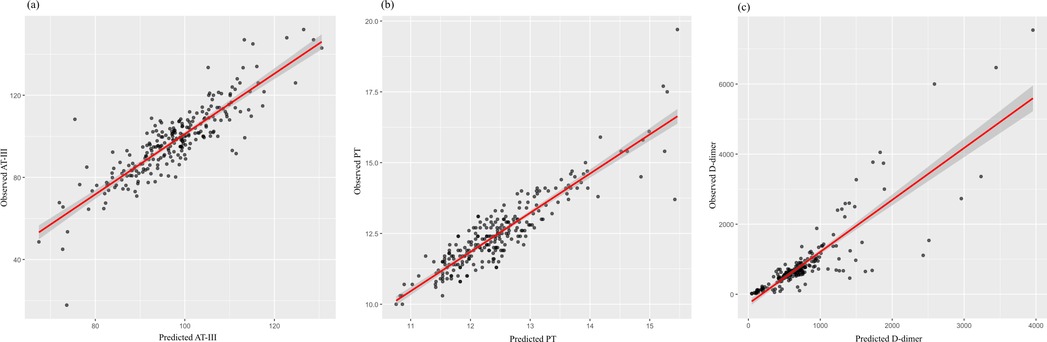
Figure 3. Scatter plots illustrating the correlation between the predicted and observed values of (a) antithrombin III (AT-III), (b) prothrombin time (PT), and (c) D-dimer. Each black dot represents an individual observation. The red line indicates the fitted regression line, with the shaded area representing the confidence interval. (a, b) The strong alignment along the diagonal suggests a good model fit for AT-III and PT predictions. (c) While most data points closely align with the regression line, a few extreme values suggest potential outliers or high variability in D-dimer predictions.
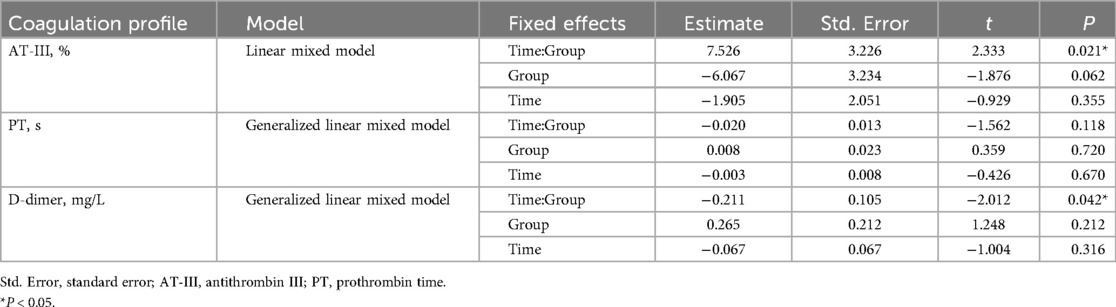
Because the observed difference in diuretic use between the groups at baseline (Table 1) could potentially confound the results, we also performed regression analyses using linear or generalized linear models, with diuretic use (yes/no) included as a covariate. After adjusting for this potential confounder, the results remained consistent and robust (Table 5, Figure 4).
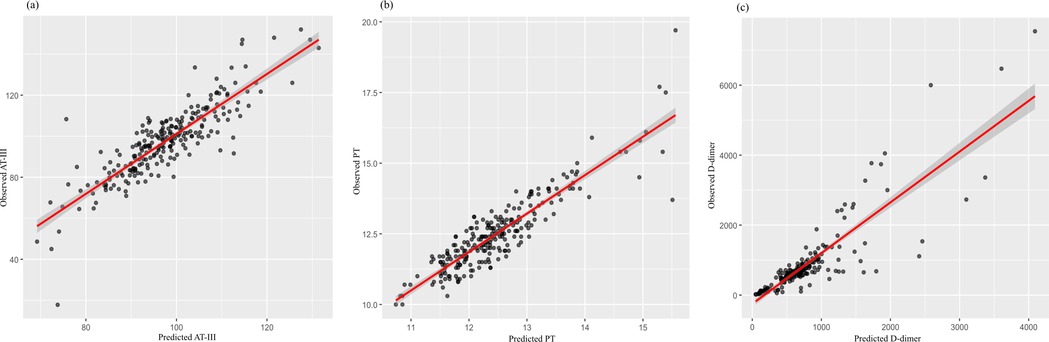
Figure 4. Scatter plots illustrating the correlation between predicted and observed values for (a) antithrombin III (AT-III), (b) prothrombin time (PT), and (c) D-dimer after adjusting for diuretic use (yes/no) in the regression models. Each black dot represents an individual observation. The red line denotes the fitted regression line, and the shaded area represents the 95% confidence interval. (a, b) The close alignment of data points along the diagonal line indicates a good model fit for AT-III and PT. (c) While most data points cluster around the regression line, a few extreme values suggest potential outliers or increased variability in D-dimer predictions.
4 Discussion
Patients with CAD and T2DM can be in hypercoagulable states and have a high risk for thrombotic formation, leading to serious complications. Herein, we showed for the first time that SGLT2is might exert anticoagulatory effects by increasing AT-III levels and prolonging PT in patients with CAD and T2DM. Our study results could provide a new evidence-based approach to alleviating hypercoagulable states and reducing thrombosis risk in these patients.
SGLT2is are a class of antihyperglycemic agents approved by the Food and Drug Administration (FDA) to treat patients with T2DM. Studies have shown that SGLT2 inhibitors could have CV protective functions, probably by reducing endothelial oxidative stress (OS) via inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation (15, 16), decreasing cardiac preload and afterload via diuretic and natriuretic actions (6), alleviating sodium and calcium overload in cardiomyocytes by inhibiting sodium/hydrogen exchanger-1 (NHE-1) (17), reducing myocardial fibrosis (18), and modulating adipokines and inflammatory mediators (19–21). In the current study, we provided evidence to show that SGLT2is could mitigate the hypercoagulable state, which might contribute to their CV protective functions.
Baseline analysis before SGLT2i treatment revealed significant differences in Glu levels, diuretic use, and MCV between the SGLT2i and non-SGLT2i groups, indicating baseline disparities in metabolic status, medication use, and hematological parameters. In diabetic patients, blood Glu levels are closely related to liver and kidney function as well as blood cell morphology, while the use of diuretics can further influence blood and coagulation profiles. These baseline differences might imply the reliability of subsequent analyses.
To address potential bias arising from baseline differences, we accounted for these variables in subsequent analyses. Adjustments for confounding factors were implemented to mitigate the effects of baseline disparities and treatment-related influences, thereby enhancing the rigor and reliability of the results.
Patients receiving SGLT2i treatment exhibited a significant reduction in D-dimer levels, while the increase in AT-III levels approached statistical significance. Both D-dimer and AT-III are key markers of coagulation regulation. D-dimer is a degradation product of fibrin and serves as a crucial indicator of a hypercoagulable state, reflecting increased thrombus formation and fibrinolytic system activity. AT-III is a key anticoagulant protein that enhances the anticoagulation system by inhibiting activated coagulation factors. The increase in AT-III levels suggests enhanced anticoagulant activity. Additionally, we observed a potential increasing trend in PT, further indicating a hypocoagulable state. Taken together, these findings support the potential role of SGLT2is in modulating coagulation function (Figure 5). In contrast, no significant changes were detected in the non-SGLT2i group, highlighting the distinct impact of SGLT2is on coagulation function.
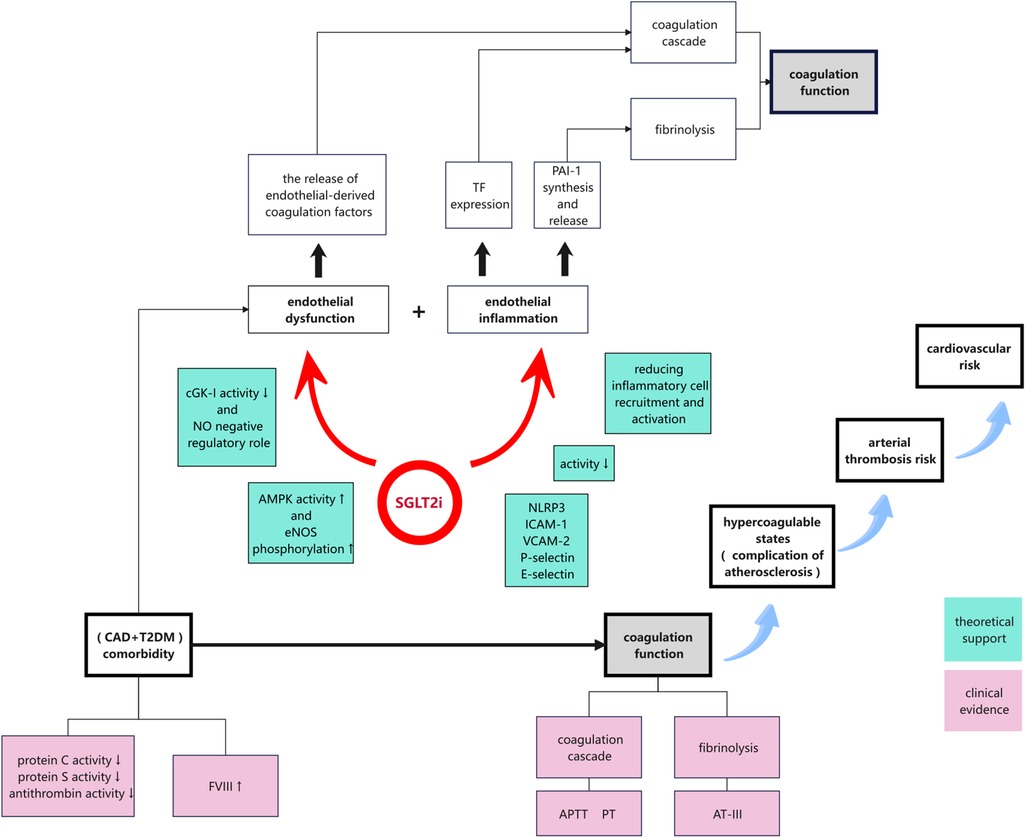
Figure 5. Proposed mechanisms by which SGLT2is modulated coagulation function in patients with CAD and T2DM via endothelial-function enhancement and anti-inflammatory effects. SGLT2is promoted nitric oxide production, suppressed the expression of inflammatory mediators, and reduced inflammatory-cell activation. These effects collectively mitigated the hypercoagulable state, decreased the risk of thrombosis, and could potentially reduce CV events in this high-risk patient population.
In our study, patients receiving SGLT2i treatment exhibited altered coagulation features that were characterized by a decreasing trend in D-dimer levels, but unchanged PT, suggesting that the extrinsic coagulation pathway was not significantly activated and that there were no thrombosis events. Notably, an increase in AT-III was also observed, indicating enhanced endogenous anticoagulant activity and a delayed intrinsic coagulation cascade. These findings suggest that SGLT2is might modulate the coagulation system to ultimately reduce thrombosis formation, possibly through the upregulation of endogenous anticoagulant mechanisms rather than by affecting the controlled activation of the extrinsic pathway.
In this study, although the observed coagulation profile changes caused by SGLT2is were relatively modest, they might still have indicated an effect of these drugs on coagulation function. While minor changes often have limited clinical effect in patients with a single disease, they can hold significant implications in those with multiple comorbidities. In patients with complicated disease backgrounds, subtle changes can reflect early pathological shifts and indicate underlying processes, and therefore they should not be overlooked. For example, Abdullah et al. reported that the hemostatic profiles of patients with CAD mirrored those of patients with venous thrombosis, with an elevated FVIII level contributing to a hypercoagulable state. In their study, linear-regression analysis showed a significant negative correlation between FVIII levels and aPTT in CAD patients (R2 = 10%, P < 0.0001), with each 1% increase in FVIII level associated with a 0.013-s reduction in aPTT [95% confidence interval (CI), −0.019 to −0.007] (2). An elevated FVIII level can accelerate activation of the intrinsic coagulation pathway and potentiate the extrinsic pathway, thereby increasing the risk of atherothrombosis. FVIII levels are markedly elevated in patients with comorbid CAD and T2DM, a rise that is inversely correlated with shortened aPTT. In the current study, after treatment with SGLT2is for 1 month, patients in the stratified normal-range subgroup had statistically significantly prolonged aPTT. This implied that SGLT2is exerted regulatory effects on coagulation function in patients with comorbidities, including comorbid CAD and T2DM.
Our findings suggested that SGLT2is might exert a unique anticoagulant regulatory effect in high-risk patients with both CAD and T2DM. Patients with multiple comorbidities often have chronic inflammation and hypercoagulability, requiring traditional anticoagulant therapy to carefully balance the risks of thrombosis and bleeding. By prolonging coagulation time and enhancing anticoagulant activity, SGLT2is might alleviate hypercoagulable states and reduce thrombosis risk without significantly increasing bleeding potential. This indicates that SGLT2is could serve as an adjunctive anticoagulant therapy in populations at high risk of MACEs and with multiple comorbidities, supporting more-personalized therapeutic strategies.
Furthermore, the impact of SGLT2is on coagulation function should not be considered an isolated finding, but instead as an integral component of their pleiotropic pharmacological profile. The mechanisms of these inhibitors include improved glycemic control, reduced vascular inflammation, altered coagulation pathways, and, more recently, anti-arrhythmic effects. Collectively, these effects suggest that SGLT2is may have broad therapeutic potential in patients with complex comorbidities. Recent studies have shown that SGLT2i use is associated with a reduced risk of atrial fibrillation and other arrhythmias, further supporting their potential role in rhythm stabilization (22, 23). Overall, these pleiotropic effects highlight the value of SGLT2is not only in metabolic and cardiovascular regulation, but also in the integrated management of thrombosis and arrhythmia. Future studies should explore the synergistic effects of SGLT2is and conventional anticoagulants in high-risk patient populations, with the goal of developing safer and more effective antithrombotic strategies.
Our study had several limitations, such as its retrospective study design and single-center nature. Additionally, the small sample size may limit the reliability of the analytical results. We studied coagulation profile changes after short-term (30-day) SGLT2i treatment. A long treatment duration might result in more-marked effects on coagulation profile. Many other factors, such as concomitant medication use and dietary patterns, could also affect coagulation function and coagulability state, but these factors could not be fully addressed in this retrospective study. Future multicenter studies with large sample sizes and long treatment durations are required to confirm our findings. Such studies will help assess whether the small changes in AT-III and D-dimer observed in the current study can be translated into significant therapeutic benefits, such as a reduction in thrombotic events or cardiovascular morbidity.
In conclusion, SGLT2is could prolong coagulation time and enhance anticoagulant activity, which affected coagulation function and contributed to CV protection in patients with CAD and T2DM. SGLT2is could be used to ameliorate hypercoagulable states and reduce thrombosis risk in such patients. Future research is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Shijiazhuang People's Hospital, affiliated with Shijiazhuang People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
XQ: Data curation, Formal analysis, Writing – original draft, Funding acquisition, Investigation, Software, Visualization. GS: Conceptualization, Writing – review & editing, Methodology, Project administration, Resources, Supervision, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Self-Financed Public Health Project under the S&T Program of Shijiazhuang (Project No. 231200403).
Acknowledgments
We thank the Self-Financed Public Health Project under the S&T Program of Shijiazhuang (Project No. 231200403) for its support of this study. We thank LetPub (https://www.letpub.com) for linguistic assistance and pre-submission expert review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dal Canto E, Ceriello A, Rydén L, Ferrini M, Hansen TB, Schnell O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. (2019) 26(2_suppl):25–32. doi: 10.1177/2047487319878371
2. Abdullah WZ, Moufak SK, Yusof Z, Mohamad MS, Kamarul IM. Shortened activated partial thromboplastin time, a hemostatic marker for hypercoagulable state during acute coronary event. Transl Res. (2010) 155(6):315–9. doi: 10.1016/j.trsl.2010.02.001
3. Ferrannini G, Manca ML, Magnoni M, Andreotti F, Andreini D, Latini R, et al. Coronary artery disease and type 2 diabetes: a proteomic study. Diabetes Care. (2020) 43(4):843–51. doi: 10.2337/dc19-1902
4. May JE, Moll S. How I treat unexplained arterial thrombosis. Blood. (2020) 136(13):1487–98. doi: 10.1182/blood.2019000820
5. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
6. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
7. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
8. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. (2023) 44(39):4043–140. doi: 10.1093/eurheartj/ehad192
9. Zhou L, Cryan EV, D'Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (Sglt1). J Cell Biochem. (2003) 90(2):339–46. doi: 10.1002/jcb.10631
10. Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity Index and kidney injury. Cardiovasc Diabetol. (2018) 17(1):108. doi: 10.1186/s12933-018-0750-8
11. Sayour AA, Korkmaz-Icöz S, Loganathan S, Ruppert M, Sayour VN, Oláh A, et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J Transl Med. (2019) 17(1):127. doi: 10.1186/s12967-019-1881-8
12. Wan Z, Wen W, Ren K, Zhou D, Liu J, Wu Y, et al. Involvement of NLRP3 inflammasome in the impacts of sodium and potassium on insulin resistance in normotensive Asians. Br J Nutr. (2018) 119(2):228–37. doi: 10.1017/s0007114517002926
13. Dmitrieva NI, Burg MB. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote atherosclerosis. PLoS One. (2015) 10(6):e0128870. doi: 10.1371/journal.pone.0128870
14. Shi L, Wei X, Luo J, Tu L. SGLT2 inhibition, venous thrombolism, and death due to cardiac causes: a mediation Mendelian randomization study. Front Cardiovasc Med. (2024) 11:1339094. doi: 10.3389/fcvm.2024.1339094
15. Khemais-Benkhiat S, Belcastro E, Idris-Khodja N, Park SH, Amoura L, Abbas M, et al. Angiotensin II-induced redox-sensitive SGLT1 and 2 expression promotes high glucose-induced endothelial cell senescence. J Cell Mol Med. (2020) 24(3):2109–22. doi: 10.1111/jcmm.14233
16. Miyata KN, Lo CS, Zhao S, Liao MC, Pang Y, Chang SY, et al. Angiotensin ii up-regulates sodium-glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice. Clin Sci (Lond). (2021) 135(7):943–61. doi: 10.1042/cs20210094
17. Chen S, Wang Q, Bakker D, Hu X, Zhang L, van der Made I, et al. Empagliflozin prevents heart failure through inhibition of the NHE1-NO pathway, independent of SGLT2. Basic Res Cardiol. (2024) 119:751–72. doi: 10.1007/s00395-024-01067-9
18. Ma H, Wu K, Dong F, Cai B, Wu D, Lu H. Effects of empagliflozin and dapagliflozin in alleviating cardiac fibrosis through SIRT6-mediated oxidative stress reduction. Sci Rep. (2024) 14:30764. doi: 10.1038/s41598-024-30764-0
19. Morciano C, Gugliandolo S, Capece U, Di Giuseppe G, Mezza T, Ciccarelli G, et al. SGLT2 inhibition and adipose tissue metabolism: current outlook and perspectives. Cardiovasc Diabetol. (2024) 23:39. doi: 10.1186/s12933-024-02539-x
20. Xu Y, Li L, Ji Y, Chen C, Lu Y, Zhang Y. Effects and mechanisms of SGLT2 inhibitors on the NLRP3 inflammasome, with a focus on atherosclerosis. Front Endocrinol (Lausanne). (2022) 13:992937. doi: 10.3389/fendo.2022.992937
21. Mashayekhi M, Safa BI, Gonzalez MSC, Kim SF, Echouffo-Tcheugui JB. Systemic and organ-specific anti-inflammatory effects of sodium-glucose cotransporter-2 inhibitors. Trends Endocrinol Metab. (2024) 35(5):425–38. doi: 10.1016/j.tem.2024.03.002
22. Duan HY, Barajas-Martinez H, Antzelevitch C, Hu D. The potential anti-arrhythmic effect of SGLT2 inhibitors. Cardiovasc Diabetol. (2024) 23:252. doi: 10.1186/s12933-024-02495-6
Keywords: sodium/glucose cotransporter 2 inhibitors, coronary-artery disease, type 2 diabetes mellitus, coagulation profile, activated partial thromboplastin time
Citation: Qin X and Song G (2025) Effects of sodium/glucose cotransporter 2 inhibitors on the coagulation profile in patients with coronary-artery disease and type 2 diabetes mellitus: a retrospective cohort study. Front. Cardiovasc. Med. 12:1588797. doi: 10.3389/fcvm.2025.1588797
Received: 6 March 2025; Accepted: 21 April 2025;
Published: 7 May 2025.
Edited by:
Gordana Krljanac, University of Belgrade, SerbiaReviewed by:
Ubaid Khan, King Edward Medical University, PakistanNicola Pierucci, Sapienza University of Rome, Italy
Fábio Oliveira, FAESC, Brazil
Copyright: © 2025 Qin and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: GuoBin Song, c29uZ2diMTMxQDE2My5jb20=
 XiaoYue Qin
XiaoYue Qin GuoBin Song
GuoBin Song