Abstract
Objective:
This study aimed to systematically identify risk factors for urinary catheter-related hematuria in patients with acute myocardial infarction (AMI). By integrating logistic regression and decision tree models, we sought to develop actionable strategies for risk stratification and complication prevention.
Methods:
A retrospective analysis of 209 AMI patients was conducted to evaluate predictors of hematuria, including demographics, coagulation indices (INR, platelets), and procedural variables. Logistic regression and decision tree (CART algorithm) models were employed to identify risk factors and their interactions. Model performance was assessed using ROC-AUC, sensitivity, and specificity.
Results:
The incidence of catheter-related hematuria was 32.5%. Both models identified persistent agitation during catheter indwelling and PLT ≤ 246 as common predictors. The logistic regression model specifically identified Gender (OR = 0.202), patient awareness of catheter purpose and precautions (OR = 0.470), and emergency catheter placement (OR = 2.257) as significant factors. The decision tree model uniquely identified INR > 0.955 and repeated complaints of urethral pain as predictors.
Conclusion:
Hematuria in AMI patients results from coagulation dysfunction, procedural trauma, and behavioral factors. The combined use of logistic regression and decision trees enhances risk stratification. Clinical strategies should prioritize gentle catheterization, dynamic coagulation monitoring, and patient education to reduce complications.
1 Background
According to the Global Burden of Disease Study 2021, acute myocardial infarction (AMI) is the leading cause of death worldwide, with approximately 8.6 million deaths per year, accounting for 44.7% of total deaths from cardiovascular diseases (1).While contemporary guidelines emphasize rapid re-vascularization and anti-thrombotic therapy (2, 3), yet critical iatrogenic risks like catheter-related hematuria remain unaddressed in these guidelines.
At present, nursing studies for AMI patients mostly focus on the construction and optimization of early rehabilitation interventions (4), psychosocial support (5), and standardized complication prevention and control pathways (6). However, despite the important clinical value of indwelling urinary catheterization in the management of critically ill patients, there are still significant gaps in systematic studies on complications associated with indwelling urinary catheterization in AMI patients. Existing literature shows that complications such as hematuria and urinary tract infection may occur during indwelling urinary catheterization, but relevant studies are mostly limited to common inpatients (7, 8). It is worth noting that the risk of catheterism-related hematuria in AMI patients may be significantly higher than that of the general population due to special pathophysiological states such as high-intensity anticoagulation and antiplatelet therapy and hemodynamic instability (9). Hematuria not only exacerbates patient distress but may necessitate anticoagulant cessation, jeopardizing reperfusion efficacy (10). Notably, the 2023 ESC (2) and 2025 ACC/AHA (3) lack recommendations on catheter-complication prevention.
Conventional statistical approaches (e.g., logistic regression) often fail to capture clinically critical interactions, particularly in AMI patients where anticoagulant-ischemia-procedure interactions dominate hematuria pathogenesis. Decision tree models address this by identifying threshold-based risk strata (11), but their utility in AMI-specific iatrogenic injury prediction remains untested.
Therefore, this study pursues dual objectives: (1) Primarily, to identify clinically modifiable risk factors for catheter-related hematuria in AMI patients through multidimensional analysis of demographic, procedural, and laboratory variables. (2) Secondarily, to compare the predictive performance and complementary value of logistic regression vs. decision tree models for clinical risk stratification.
We hypothesize that machine learning approaches more effectively characterize nonlinear risk interactions specific to AMI pathophysiology.
2 Methods
2.1 Study design and participants
A convenience sample of 209 AMI patients admitted to a tertiary hospital cardiovascular center (December 2020–December 2022) was retrospectively analyzed. Inclusion criteria: (1) AMI diagnosis per Chinese guidelines; (2) ST-elevation myocardial infarction; (3) indwelling catheterization during hospitalization. Exclusion criteria: (1) preexisting hepatic/hematologic disorders, malignancy, or non-AMI anticoagulant use; (2) coma. This study was approved by the Institutional Review Board of Heyuan People's Hospital (Approval No. 2021-RE-026). Written informed consent was obtained from all individual participants or their legal guardians prior to data collection.
2.2 Variables and measurements
The binary outcome was hematuria occurrence (yes/no). Hematuria was objectively defined as the presence of ≥5 red blood cells per high-power field (RBCs/HPF) on microscopic examination of a centrifuged urine specimen (12). Any documented episode of gross hematuria in the medical record was also considered a positive outcome. Twenty-four covariates spanning demographics, urological history, catheterization procedural details, and laboratory indices (e.g., PLT, INR, PT) were evaluated. To ensure methodological rigor, we operationalized key variables through standardized definitions detailed in Table 1. This standardization enabled consistent data collection across clinical scenarios. According to the method of sample size calculation, the sample size was obtained from 5 to 10 times of the number of variables (15), and the sample size was calculated to be 120–240 cases.
Table 1
| Variable | Definition | Assessment method |
|---|---|---|
| Emergency catheter placement | Order-to-insertion time ≤10 min | Electronic Medical Record timestamp audit |
| Acute agitation during catheter insertion | RASS≥+2 (13) OR volitional catheter contact (from urethral meatus contact to balloon inflation) | Real-time nurse observation |
| Indwelling catheterization process smoothly | Catheter placement achieving all of the following: (1) first-attempt success (2) no procedural interruption >30 s (3) nurse-rated smoothness of “smooth” or “very smooth” | Nurse documentation and post-procedure questionnaire |
| Persistent agitation during catheter indwelling | ≥2 episodes of RASS ≥+2 in 24 h OR volitional catheter contact (post-inflation to removal) | Real-time nurse observation |
| Urinary fixed properly | Zero hazardous tension: (1) No visible urethral meatus traction; (2) Drainage bag weight fully supported (not dependent on tubing). | Bedside nurses assessment per 8 h |
| Repeated complaints of urethral pain | Frequency & Severity: (1) ≥3 discrete episodes in 24 h; (2) Each episode: Numeric Rating Scale (NRS) ≥4 (14) | NRS screening per 8 h |
| Patient was aware of the purpose and precautions of the indwelling catheter | The patient demonstrated two components: (1) Correct language expression of preventive measures; (2) Display of protective measures and hazard identification | Knowledge assessment and behavioral observation |
Operational definitions of key variables.
2.3 Bias mitigation
To address limitations of the retrospective design, we implemented a tripartite bias mitigation protocol: (1) Procedural standardization was enforced using the CAUTI bundle guidelines (16), ensuring consistent catheter insertion/maintenance techniques across all cases; (2) Blinding of outcome assessors to predictor variable status prevented detection bias during hematuria adjudication; (3) Multivariable adjustment for clinically relevant confounders (age and renal function) minimized residual confounding in regression models.
2.4 Statistical analysis
Complete variable assignment details (including coding logic for all 24 covariates) are documented in Supplementary Table S1. Continuous variables were compared via t-tests; categorical variables via χ2 or Fisher's exact tests.
All candidate variables with a univariate P < 0.1 were entered into the logistic regression and decision tree model. Multicollinearity was assessed using the variance inflation factor (VIF), with a threshold of VIF >5 indicating severe collinearity. Due to severe collinearity between INR and PT (VIF >10), one of them had to be excluded from the multivariate logistic regression model to ensure stability and interpretability. We opted to retain PT and exclude INR for the following reasons: (1) PT, as a direct measure of clotting time in seconds, offers more intuitive clinical interpretability in our STEMI cohort managed primarily with antiplatelet therapy; (2) PT represents the fundamental measurement from which INR is derived, making it a more robust and statistically stable choice for regression analysis. It is important to note that the clinically relevant information contained in INR was subsequently captured by the decision tree model, which identified a threshold of INR >0.955, thus demonstrating the complementary nature of our two-model approach.
Decision tree modeling employed the Classification and Regression Trees (CART) algorithm with Gini impurity minimization as the splitting criterion. Key parameters included: (1) Maximum tree depth: 5; (2) Minimum cases in parent node: 50; (3) Minimum cases in child node: 5; (4) Pruning via cost-complexity pruning (α = 0.01) and 10-fold cross-validation. Model performance was assessed via ROC-AUC, sensitivity, specificity, and Youden's index.
3 Result
3.1 Basic characteristics
Among 209 AMI patients with indwelling urinary catheters, hematuria occurred in 68 cases (32.5%). As detailed in Table 2, significant risk factors encompassed behavioral aspects such as persistent agitation during catheterization (57.97% vs. 20.00%, P < 0.001), emergency catheter placement (45.24% vs. 24.00%, P = 0.001), and repeated urethral pain complaints (52.94% vs. 22.70%, P < 0.001), demographic differences with higher incidence in males (39.13% vs. 19.72% in females, P = 0.005), as well as coagulation indicator characterized by elevated platelets (P = 0.003) and lower INR (P = 0.094). No significant associations were observed for age, cystitis history, catheter material, or thrombolytic therapy (P > 0.1) (17).
Table 2
| Categories | Total | No hematuria (n = 141) | Hematuria (n = 68) | χ 2/t | P |
|---|---|---|---|---|---|
| n (%) or Mean ± SD | n (%) or Mean ± SD | n (%) or Mean ± SD | |||
| Age | 2.131 | 0.345 | |||
| <60 | 49 (23.44) | 33 (67.35) | 16 (32.65) | ||
| 60–75 | 81 (38.76) | 59 (72.84) | 22 (27.16) | ||
| >75 | 79 (37.80) | 49 (62.03) | 30 (37.97) | ||
| Gender | 8.048 | 0 . 005 | |||
| Male | 138 (66.03) | 84 (60.87) | 54 (39.13) | ||
| Female | 71 (33.97) | 57 (80.28) | 14 (19.72) | ||
| Cystitis | 0.300 | 0.584 | |||
| No | 158 (75.6) | 105 (66.46) | 53 (33.54) | ||
| Yes | 51 (24.4) | 36 (70.59) | 15 (29.41) | ||
| History of hematuria (last six months) | 0.493 | 0.483 | |||
| No | 147 (70.33) | 97 (65.99) | 50 (34.01) | ||
| Yes | 62 (29.67) | 44 (70.97) | 18 (29.03) | ||
| History of urethral surgery | 0.489 | 0.484 | |||
| No | 148 (70.81) | 102 (68.92) | 46 (31.08) | ||
| Yes | 61 (29.19) | 39 (63.93) | 22 (36.07) | ||
| Urinary tract infections | 0.002 | 0.966 | |||
| No | 151 (72.25) | 102 (67.55) | 49 (32.45) | ||
| Yes | 58 (27.75) | 39 (67.24) | 19 (32.76) | ||
| Urinary systemmatic calculi | 1.238 | 0.266 | |||
| No | 127 (60.77) | 82 (64.57) | 45 (35.43) | ||
| Yes | 82 (39.23) | 59 (71.95) | 23 (28.05) | ||
| Urinary tube specification | 1 | ||||
| 16Fr | 203 (97.13) | 137 (67.49) | 66 (32.51) | ||
| <16Fr | 6 (2.87) | 4 (66.67) | 2 (33.33) | ||
| Emergency catheter placement | 10.324 | 0 . 001 | |||
| No | 125 (59.81) | 95 (76.00) | 30 (24.00) | ||
| Yes | 84 (40.19) | 46 (54.76) | 38 (45.24) | ||
| Catheter material | 1.221 | 0.269 | |||
| Silica gel | 128 (61.24) | 90 (70.31) | 38 (29.69) | ||
| Latex | 81 (38.76) | 51 (62.96) | 30 (37.04) | ||
| Acute agitation during catheter insertion | 0.365 | 0.546 | |||
| No | 162 (77.51) | 111 (68.52) | 51 (31.48) | ||
| Yes | 47 (22.49) | 30 (63.83) | 17 (36.17) | ||
| Indwelling catheterization process smoothly | 1.301 | 0.254 | |||
| No | 101 (48.33) | 72 (71.29) | 29 (28.71) | ||
| Yes | 108 (51.67) | 69 (63.89) | 39 (36.11) | ||
| Persistent agitation during catheter indwelling | 30.36 | 0 . 000 | |||
| No | 140 (66.99) | 112 (80.00) | 28 (20.00) | ||
| Yes | 69 (33.01) | 29 (42.03) | 40 (57.97) | ||
| Urinary fixed properly | 1.229 | 0.268 | |||
| No | 119 (56.94) | 84 (70.59) | 35 (29.41) | ||
| Yes | 90 (43.06) | 57 (63.33) | 33 (36.67) | ||
| Repeated complaints of urethral pain | 19.120 | 0 . 000 | |||
| No | 141 (67.46) | 109 (77.30) | 32 (22.70) | ||
| Yes | 68 (32.54) | 32 (47.06) | 36 (52.94) | ||
| Patient was aware of the purpose and precautions of the indwelling catheter | 6.745 | 0.009 | |||
| No | 93 (44.50) | 54 (58.06) | 39 (41.94) | ||
| Yes | 116 (55.50) | 87 (75.00) | 29 (25.00) | ||
| Thrombolytic therapy | 0.005 | 0.944 | |||
| No | 139 (66.51) | 94 (44.98) | 45 (21.53) | ||
| Yes | 70 (33.49) | 47 (22.49) | 23 (11.00) | ||
| Treatment | 1.183 | 0.553 | |||
| Primary PCI | 121 (57.89) | 78 (64.46) | 43 (35.54) | ||
| Post-thrombolysis PCI | 70 (33.5) | 50 (71.43) | 20 (28.57) | ||
| Post-thrombolysis CAG | 18 (8.6) | 13 (72.22) | 5 (27.78) | ||
| PLT (*109) | 236.172 ± 78.382 | 213.118 ± 63.941 | 247.291 ± 82.392 | 3.01 | 0 . 003 |
| PT(s) | 13.409 ± 1.968 | 13.989 ± 3.108 | 13.129 ± 0.942 | −2.231 | 0 . 029 |
| INR | 1.061 ± 0.589 | 1.202 ± 1.014 | 0.993 ± 0.094 | −1.699 | 0 . 094 |
| APTT(s) | 42.036 ± 34.376 | 45.535 ± 38.725 | 40.348 ± 32.081 | −1.022 | 0.308 |
| FIB | 5.55 ± 19.961 | 4.09 ± 1.435 | 6.25 ± 24.279 | 0.731 | 0.466 |
| TT | 24.02 ± 32.267 | 27.60 ± 38.777 | 22.30 ± 28.601 | −1.114 | 0.267 |
General and hematuria-related characteristics of the study participants (N = 209).
Percentages in No Hematuria and Hematuria columns represent proportions within each category.
Bold values indicate P < 0.1.
P < 0.05 was considered statistically significant.
CAG, coronary angiography; PCI, percutaneous coronary intervention bold; PLT, platelet count; PT, prothrombin time; INR, International Normalized Ratio; APTT, activated partial thromboplastin time; FIB, fibrinogen; TT, thrombin time.
3.2 Logistic regression analysis of factors affecting hematuria in patients with AMI during indwelling urinary catheterization
Using whether hematuria occurred as the dependent variable (coded as 0 = no, 1 = yes), variables with statistical significance in univariate analysis (P < 0.1) were included in the logistic regression model. The results of multivariate logistic regression analysis showed that emergency catheter placement, persistent agitation during catheter indwelling, and repeated complaints of urethral pain were risk factors for hematuria. Gender, patient awareness of the purpose and precautions of the indwelling catheter, PLT (*109), and INR were protective factors for hematuria, as detailed in Table 3.
Table 3
| Variate | B | S.E | Wald | OR (95% CI) | P |
|---|---|---|---|---|---|
| Gender | −1.597 | 0.456 | 12.245 | 0.202 (0.083–0.495) | 0.000 |
| Emergency catheter placement | 0.814 | 0.373 | 4.757 | 2.257 (1.086–4.692) | 0.029 |
| Persistent agitation during catheter indwelling | 1.617 | 0.411 | 15.461 | 5.039 (2.250–11.285) | 0.000 |
| Repeated complaints of urethral pain | 1.15 | 0.405 | 8.067 | 3.158 (1.428–6.983) | 0.005 |
| Patient was aware of the purpose and precautions of the indwelling catheter | −0.755 | 0.378 | 3.996 | 0.470 (0.224–0.985) | 0.046 |
| PLT (×109/L) | −0.008 | 0.003 | 7.181 | 0.992 (0.987–0.998) | 0.007 |
| PT(s) | 0.407 | 0.142 | 8.153 | 1.502 (1.136–1.986) | 0.004 |
| Constant | −5.049 | 2.021 | 6.243 | 0.006 | 0.012 |
Logistic regression analysis of hematuria during catheter indentation in patients with acute myocardial infarction.
INR was excluded from the final model due to severe multicollinearity with PT (see Methods for details).
3.3 Performance metrics and decision tree architecture of prediction models
3.3.1 Head-to-head performance metrics
As detailed in Table 4 and Figure 1, both models demonstrated robust predictive capability for hematuria. The logistic regression model achieved higher sensitivity (86.8% vs. 80.9%), while the decision tree model offered superior specificity (76.6% vs. 66.0%). The decision tree also yielded a marginally higher area under the ROC curve (AUC = 0.845, 95% CI: 0.789–0.900) compared to the logistic regression model (AUC = 0.825, 95% CI: 0.766–0.883), a difference that was statistically significant.
Table 4
| Model | AUC | S.E. | 95% CI | P |
|---|---|---|---|---|
| Logistic | 0.825 | 0.03 | 0.766–0.883 | 0.000 |
| Decision Tree | 0.845 | 0.028 | 0.789–0.9 | 0.000 |
Comparison of analysis effect between logistic regression model and decision tree model.
Figure 1
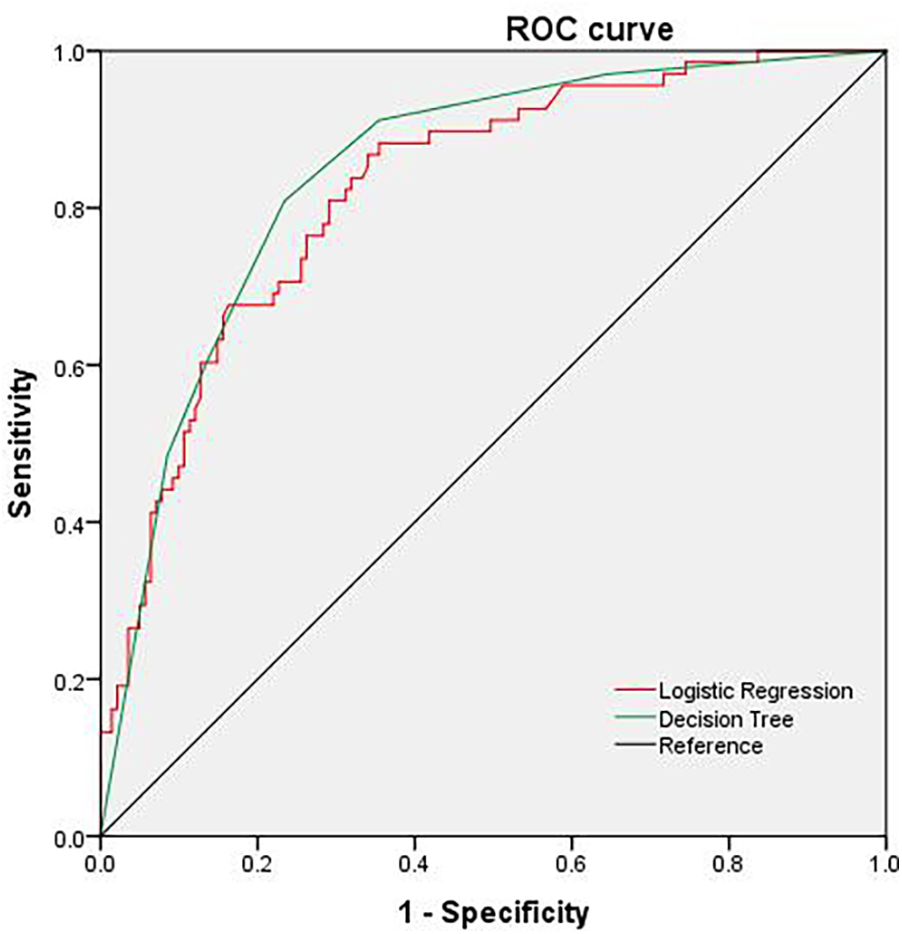
ROC curve predicted by decision tree and logistic regression model.
3.3.2 Decision tree architecture and risk stratification
Beyond conventional metrics, the decision tree provided clinically actionable, granular risk stratification that logistic regression cannot directly offer. The CRT algorithm (Figure 2) identified persistent agitation during catheter indwelling as the primary split node (Gini importance = 41.2%), establishing it as the most influential predictor. The model generated three distinct risk stratification pathways: (1) High-risk pathway (n = 30, 14.4% of cohort): Patients with persistent agitation and INR >0.955 had a 73.3% probability of hematuria. (2) Intermediate-risk pathway (n = 28, 13.4% of cohort): Patients without agitation but with PLT ≤ 246 × 109/L and INR >1.055 had a 50.0% probability of hematuria. (3) Low-risk pathway (n = 151, 72.2% of cohort): Patients without agitation, with PLT >246 × 109/L, and no repeated urethral pain had a 3.8% probability of hematuria.
Figure 2
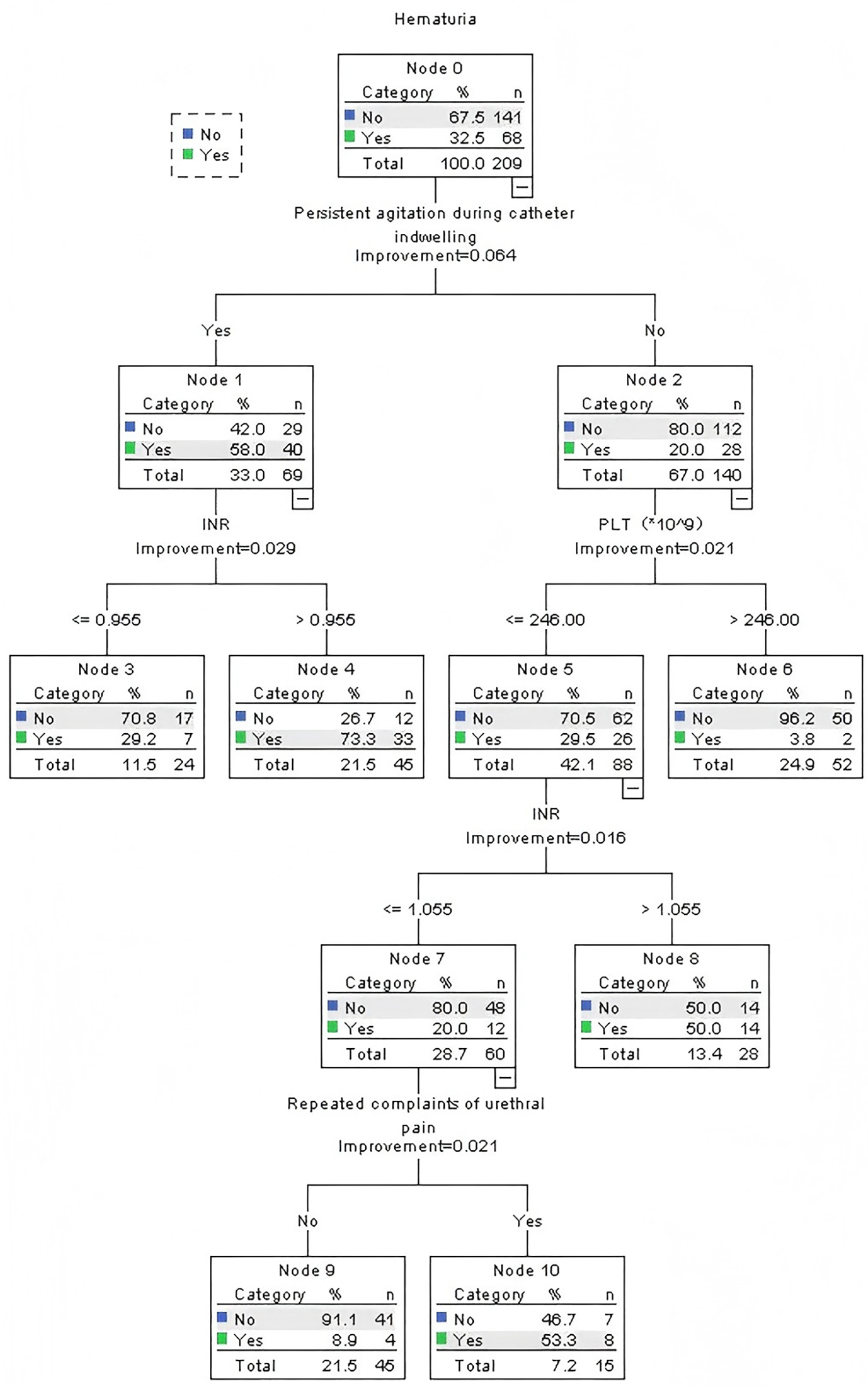
Classification and regression tree model (N = 209).
The relative importance of the predictors, as quantified by the normalized Gini decrease, was: persistent agitation (41.2%) > INR (28.5%) > platelet count (19.7%) > repeated urethral pain (10.6%).
4 Discussion
4.1 Key findings and mechanistic interpretations
The primary objective of this study was to identify clinically modifiable risk factors for catheter-related hematuria in AMI patients. Our analysis reveals a multifactorial etiology, wherein the interplay of coagulation dysfunction, procedural trauma, and behavioral factors creates a distinct high-risk phenotype. The incidence of catheter-related hematuria in this AMI cohort was 32.5%, which is strikingly elevated compared to the rate of 8%–15% reported in general inpatient populations (7, 18). This disparity can be attributed to the unique pathophysiological profile of AMI patients, which is characterized by a high-risk interplay of coagulation impairment, procedural vulnerability, and hemodynamic compromise. For instance, reduced cardiac output in AMI may lead to bladder microcirculation disorders, and resultant mucosal ischemia could potentially exacerbate mechanical injury from catheter friction (19). The modifiable risk factors identified in this study can be conceptually organized into three key mechanistic domains.
4.1.1 Procedural and behavioral factors: the most potent predictors
-
Persistent Agitation: Logistic regression analysis showed that the odds ratio (OR) for persistent agitation during catheter indwelling was 5.586 (P < 0.001). The decision tree model further identified it as the primary split node (Gini index = 0.32), contributing 41% of the model's explanatory power, underscoring the critical role of minimizing mechanical trauma through adequate sedation or non-pharmacological calming techniques. The pathological mechanism lies in the fact that agitation caused by pain or anxiety can exacerbate mechanical friction between the catheter and the urethral mucosa, directly damaging vascular endothelial cells (7). For high-risk patients (e.g., those with an pain score >4), it is recommended to administer a low dose of dexmedetomidine 10 min before catheterization. Low-dose dexmedetomidine can significantly reduce the incidence of postoperative catheter-related bladder discomfort (CRBD). Randomized controlled trials have shown that dexmedetomidine reduces the incidence of Catheter-related Bladder Discomfort (CRBD) by 55% (20), visualized procedural education or adequate communication can reduce the risk of catheter-related complications (7).
-
Emergency Catheter Placement: Emergency procedures significantly increased the risk (OR = 2.257, P = 0.029). Emergency operations often lead to insufficient lubrication and over-rapid balloon inflation (21) due to time constraints, increasing the risk of mucosal tearing (16). The European Association of Urology (EAU 2023) states that catheters must be adequately lubricated to minimize urethral damage during emergency catheterization. Sterile water-based lubricants should be preferred (22).
-
Patient Awareness: Patient awareness of the purpose and precautions of the indwelling catheter was a significant protective factor, associated with a 53% reduction in the risk of hematuria (OR = 0.470, P = 0.046). This presents a low-cost, high-impact intervention strategy. While the decision tree does not reflect such social behavioral factors because it focuses more on the stratification of physiological indicators.
4.1.2 Coagulation dysfunction: complementary insights from two modelling approaches
Our analysis robustly validates coagulation dysfunction as a central mechanism for hematuria, with logistic regression and decision tree models providing distinct yet complementary evidence.
The logistic regression model, isolating independent effects, identified Prolonged Prothrombin Time (PT) as a significant linear risk factor (OR = 1.502, P = 0.004), quantitatively confirming that anticoagulation therapy steadily increases hemorrhagic risk. The decision tree, designed to capture thresholds and interactions, pinpointed INR >0.955 as a critical classifier for high-risk patients, specifically when combined with patient agitation.
The use of PT in regression and INR in the tree is not a discrepancy but a reflection of their mechanistic redundancy and model purposes. PT provides the general, adjusted effect of coagulopathy, while INR provides a specific, clinically actionable threshold for rapid triage. Together, they offer a complete picture: coagulopathy is a fundamental driver of risk, and its danger is acutely amplified in agitated patients even at a marginally elevated INR.For high-risk patients with both elevated INR (>0.95) and decreased PLT (<150 × 109/L), a combined intervention strategy is required: ① Dynamic Monitoring: Use thromboelastography (TEG) to assess overall coagulation function, replacing single INR or PLT measurements. Adjust the intensity of anticoagulation dynamically based on the R value of TEG (8–12 min) to reduce the risk of excessive inhibition of coagulation factors (23). ② Stratified Management: When PLT <150 × 109/L, increase the prophylactic platelet transfusion threshold from 10 × 109/L to 50 × 109/L (24).
4.1.3 Demographic and other factors
Gender paradox: This study found that the risk of catheter-related hematuria in male patients was significantly lower than that in females (OR = 0.202, P < 0.001), which contrasts with the conventional anatomical view that male catheterization carries higher inherent risk. While this observed association requires further confirmation, we hypothesize that it could be explained by a combination of behavioral and pathophysiological factors, rather than anatomy alone. Several non-mutually exclusive hypotheses could be proposed to explain this finding: (1) Potential Operator Bias: Informal interviews with nursing staff suggested a perception of higher risk during male catheterization, which might lead to more cautious techniques (25) (e.g., gentler insertion, more generous lubrication) that could mitigate trauma. However, this was not objectively measured in our study. (2) Potential Anatomical and Pharmacological Interactions: The shorter female urethra could potentially be more susceptible to irritation from catheter movement (26). Furthermore, existing literature indicates that female AMI patients on clopidogrel may have a higher incidence of hyperplatelet reactivity (HPR) and an increased risk of mild bleeding events compared to males (27). It is plausible that the confluence of a more sensitive urethral anatomy and a sex-specific pharmacological profile could contribute to the elevated risk observed in females. The hypotheses outlined above regarding operator behavior and sex-specific pharmacodynamics are speculative and require rigorous validation in future prospective studies.
4.2 Synergistic model integration for enhanced clinical stratification
The integrative use of logistic regression and decision tree models provides a comprehensive and clinically actionable framework for hematuria risk stratification in AMI patients. While both models demonstrated robust predictive performance, they offer distinct and complementary insights: logistic regression quantifies the independent net effect of key variables (e.g., the protective role of patient education), while the decision tree model reveals critical interaction effects and threshold-based clinical pathways, enabling rapid triage. To translate these insights into bedside practice, we propose a sequential clinical workflow:
The process begins with rapid triage using the decision tree model (Figure 2). Clinicians can initially assess for Persistent Agitation to immediately identify the highest-risk cohort. For non-agitated patients, subsequent evaluation of Platelet Count and INR further stratifies them into intermediate- or low-risk pathways. This entire process leverages readily available data to assign a risk level rapidly.
This initial triage then directly informs targeted, model-guided interventions. For patients in the high-risk pathway, the logistic regression model underscores the critical need to mitigate Emergency Catheter Placement (OR = 2.257) and aggressively manage Persistent Agitation (OR = 5.039). For those at intermediate risk, the strong protective effect of Patient Awareness (OR = 0.470) highlights patient education as a key modifiable intervention. Furthermore, a signal like Repeated Urethral Pain across both models should trigger enhanced monitoring for complications.
This synergistic approach—using the decision tree for efficient stratification and the regression model for depth of intervention—bridges the gap between prediction and prevention, offering a pragmatic blueprint for clinical implementation.
4.3 Limitations
This study has the following limitations: Firstly, the single-center, retrospective design may lead to selection bias. Its research results still need to be validated in prospective, multi-center cohorts to enhance its universality. Secondly, our analysis did not stratify the risk of hematuria by clinical Settings (such as ICU and general wards), which may affect the occurrence and detection of hematuria.
5 Conclusion
This study systematically identified the risk profile for catheter-related hematuria in AMI patients using both logistic regression and decision tree models. Logistic regression highlighted the impact of behavioral and demographic factors such as gender, emergency catheterization, and patient education, while the decision tree model revealed critical interactions between coagulation function and procedural behavior. The complementary strengths of these models provide a comprehensive framework for clinical risk assessment, bridging “independent risk weights” with “stratified clinical pathways”. We recommend their combined use to optimize risk stratification and guide personalized intervention strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Heyuan People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – review & editing, Project administration, Methodology, Writing – original draft. SH: Writing – review & editing, Investigation, Methodology, Project administration, Writing – original draft, Data curation. DP: Conceptualization, Methodology, Writing – review & editing, Investigation, Funding acquisition, Data curation. JH: Data curation, Investigation, Writing – review & editing, Methodology. YC: Methodology, Investigation, Data curation, Writing – review & editing. LY: Data curation, Methodology, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Heyuan City Social Development Science and Technology Plan Project (211216101475213), Research Project of Guangdong Nurses Association (gdshsxh2021a011), Medical Science and Technology Research Foundation of Guangdong Province (A2022508).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1597796/full#supplementary-material
References
1.
Vos T Lim SS Abbafati C Abbas KM Abbasi M Abbasifard M et al Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396(10258):1204–22. 10.1016/S0140-6736(20)30925-9
2.
Byrne RA Rossello X Coughlan JJ Barbato E Berry C Chieffo A et al 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. 10.1093/eurheartj/ehad191
3.
Kumbhani DJ Cibotti-Sun M Moore MM . 2025 acute coronary syndromes guideline-at-a-glance. J Am Coll Cardiol. (2025) 85(22):2128–34. 10.1016/j.jacc.2025.01.018
4.
Piepoli MF Hoes AW Agewall S Albus C Brotons C Catapano AL et al 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J. (2016) 37(29):2315–81. 10.1093/eurheartj/ehw106
5.
Davidson KW Rieckmann N Clemow L Schwartz JE Shimbo D Medina V et al Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. (2010) 170(7):600–8. 10.1001/archinternmed.2010.29
6.
Gulati M Levy PD Mukherjee D Amsterdam E Bhatt DL Birtcher KK et al 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 78(22):e187–285. 10.1016/j.jacc.2021.07.053
7.
Saint S Greene MT Krein SL Rogers MAM Ratz D Fowler KE et al A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. (2016) 374(22):2111–9. 10.1056/NEJMoa1504906
8.
Titsworth WL Hester J Correia T Reed R Williams M Guin P et al Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. (2012) 116(4):911–20. 10.3171/2011.11.JNS11974
9.
Petrovic L Chhabra L . Selecting a treatment modality in acute coronary syndrome. (2025). PMID: 31334993
10.
Lopes RD Heizer G Aronson R Vora AN Massaro T Mehran R et al Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. (2019) 380(16):1509–24. 10.1056/NEJMoa1817083
11.
Rajkomar A Dean J Kohane I . Machine learning in medicine. N Engl J Med. (2019) 380(14):1347–58. 10.1056/NEJMra1814259
12.
Hk W Wd H Jw H . Hematuria—clinical Methods: The History, Physical, and Laboratory Examinations. John Wiley & Sons, Inc. (1990). PMID: 21250137
13.
Sessler CN Gosnell MS Grap MJ Brophy GM O'Neal PV Keane KA et al The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. (2002) 166(10):1338–44. 10.1164/rccm.2107138
14.
Waldman SD . Pain Management, 2nd ed. Philadelphia, PA: Elsevier/Saunders (2011). p. 1445.
15.
Peduzzi P Concato J Kemper E Holford TR Feinstein AR . A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. (1996) 49(12):1373–9. 10.1016/S0895-4356(96)00236-3
16.
Gould CV Umscheid CA Agarwal RK Kuntz G Pegues DA . Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. (2010) 31(4):319–26. 10.1086/651091
17.
Hosmer DW Jr Lemeshow S Sturdivant RX . Applied Logistic Regression, 3rd ed. Wiley (2013).
18.
Hollingsworth JM Rogers MAM Krein SL Hickner A Kuhn L Cheng A et al Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med. (2013) 159(6):401–10. 10.7326/0003-4819-159-6-201309170-00006
19.
Camici PG D'Amati G Rimoldi O . Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. (2015) 12(1):48–62. 10.1038/nrcardio.2014.160
20.
Zhang T Li H Lin C An R Lin W Tan H et al Effects of an intraoperative intravenous Bolus Dose of Dexmedetomidine on postoperative catheter-related bladder discomfort in male patients undergoing transurethral resection of bladder tumors: a randomized, double-blind, controlled trial. Eur J Clin Pharmacol. (2024) 80(3):465–74. 10.1007/s00228-024-03625-5
21.
Feneley RC Hopley IB Wells PN . Urinary catheters: history, current status, adverse events and research agenda. J Med Eng Technol. (2015) 39(8):459–70. 10.3109/03091902.2015.1085600
22.
Urology EAO. EAU Guidelines on Urological Trauma. [EB/OL]. Available online at:https://uroweb.org/guidelines/urological-trauma (Accessed March 18, 2025).
23.
Levine GN Ali MN Schafer AI . Antithrombotic therapy in patients with acute coronary syndromes. Arch Intern Med. (2001) 161(7):937–48. 10.1001/archinte.161.7.937
24.
Kaufman RM Djulbegovic B Gernsheimer T Kleinman S Tinmouth AT Capocelli KE et al Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. (2015) 162(3):205–13. 10.7326/M14-1589
25.
Catheterization CGOU. Centers for Disease Control and Prevention. [EB/OL]. Available online at:https://www.cdc.gov/infectioncontrol/guidelines/cauti/index.html (Accessed March 18, 2025).
26.
Mitchell M Hill B . Urinary catheters: PART 2 catheterisation in males and females. Br J Nurs. (2018) 27(22):1306–10. 10.12968/bjon.2018.27.22.1306
27.
Gasecka A Zimodro JM Appelman Y . Sex differences in antiplatelet therapy: state-of-the art. Platelets. (2023) 34(1):2176173. 10.1080/09537104.2023.2176173
Summary
Keywords
acute myocardial infarction, hematuria, logistic regression, decision tree, machine learning
Citation
Zhang J, Huang SB, Peng DN, Huang J, Chen YJ and Yang LL (2025) Risk factors for hematuria during indwelling urinary catheterization in acute myocardial infarction: a comparative analysis using logistic regression and decision tree. Front. Cardiovasc. Med. 12:1597796. doi: 10.3389/fcvm.2025.1597796
Received
21 March 2025
Revised
06 October 2025
Accepted
04 November 2025
Published
14 November 2025
Volume
12 - 2025
Edited by
Siyi He, General Hospital of Western Theater Command, China
Reviewed by
Jinrui Dong, Tianjin University, China
Nismat Javed, Mount Sinai Morningside-BronxCare, United States
Updates
Copyright
© 2025 Zhang, Huang, Peng, Huang, Chen and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jia Zhang 562232317@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.