Abstract
Background:
Acute myocardial infarction (AMI) often leads to heart failure with reduced ejection fraction (HFrEF), with some patients showing recovery of left ventricular ejection fraction (HFrecEF) over time. This study aimed to evaluate the prognostic differences between persistent HFrEF and HFrecEF.
Methods:
This prospective cohort study included AMI patients with reduced LVEF (<40%) at admission. LVEF was reassessed one month later to classify patients into persistent HFrEF (LVEF <40%) or HFrecEF, defined as follow-up LVEF >40% with an absolute increase of ≥10% from baseline, in accordance with recent consensus definitions. Outcomes included cardiovascular mortality and/or rehospitalization for heart failure. Predictors of LVEF recovery were also analyzed.
Results:
Of the 679 patients analyzed, 373 (55%) had persistent HFrEF, while 306 (45%) transitioned to HFrecEF. Patients with HFrecEF were younger, had fewer comorbidities, and were more likely to receive renin-angiotensin system (RAS) inhibitors and β-blockers.Cardiovascular mortality was significantly lower in the HFrecEF group (3.3% vs. 8.3%; adjusted HR 0.37, 95% CI: 0.18–0.77, p = 0.007), as was the rate of heart failure rehospitalization (6.2% vs. 10.2%; adjusted HR 0.60, 95% CI: 0.35–1.05, p = 0.074). Independent predictors of LVEF recovery included younger age, beta-blocker use, and RAS inhibitor use.
Conclusion:
This study emphasizes the critical role of transitioning from persistent HFrEF to HFrecEF in improving clinical outcomes for AMI patients. Tailored management approaches, combined with routine echocardiographic monitoring and adherence to optimal medical therapy, are essential for optimizing patient care and long-term prognosis.
Introduction
Heart failure with reduced ejection fraction (HFrEF) is a common complication of acute myocardial infarction (AMI), driven by significant myocardial injury and subsequent ventricular remodeling (1, 2). While many patients experience persistent ventricular dysfunction, others achieve substantial recovery of left ventricular ejection fraction (LVEF), a condition termed heart failure with recovered ejection fraction (HFrecEF) (2). This phenomenon has garnered increasing clinical attention due to its implications for long-term outcomes and management strategies (3, 4).
Despite the improved prognosis associated with HFrecEF, the underlying mechanisms facilitating LVEF recovery remain poorly understood. Factors such as age, baseline LVEF, adherence to guideline-directed medical therapy (GDMT), and comorbidities have been proposed as potential contributors (2, 4, 5). Moreover, the prognostic disparities between patients with persistent HFrEF and HFrecEF highlight the need for a nuanced approach to post-AMI care.
This study aims to investigate the prognostic differences between persistent HFrEF and HFrecEF following AMI. By identifying predictors of LVEF recovery and examining associated clinical outcomes, we seek to inform strategies for optimizing management and improving long-term survival in this population.
Methods
Study design and population
This prospective, multicenter registry-based cohort study was conducted at Gyeongsang National University Changwon Hospital and Gyeongsang National University Hospital (Jinju), which share standardized clinical protocols and a unified electronic data management system (6) (NCT04650529). Consecutive patients with significant CAD who underwent PCI (Jinju and Changwon) between January 2010 and November 2020 were enrolled in this registry, which evaluated multiple vascular, hemostatic, and physiological parameters, if available.
This prospective cohort study included patients admitted for AMI, who had LVEF <40% at admission. Follow-up echocardiography was performed at 1 month to classify patients into persistent HFrEF (LVEF <40%) and HFrecEF, defined as follow-up LVEF >40% with an absolute increase of ≥10% from baseline, in accordance with recent consensus definitions (7).
Data collection and outcomes
Clinical, laboratory, and echocardiographic data were collected using a standardized case report form by trained study coordinators at each center. All data were prospectively recorded based on a unified study protocol. Additional information was obtained from hospital electronic medical records or by contacting the principal investigators when necessary. Outcomes of interest included cardiovascular mortality and/or rehospitalization for heart failure, confirmed through medical records or telephone contact with patients or family members.
Statistical analysis
Statistical analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Categorical variables were compared using chi-square tests or Fisher's exact tests, as appropriate. Continuous variables were analyzed using independent t-tests or Mann–Whitney U-tests, based on data distribution. Survival analyses were performed using Kaplan–Meier methods with log-rank tests to compare survival curves. Multivariate Cox proportional hazards regression models were used to identify predictors of outcomes, adjusting for potential confounders such as age, sex, and comorbidities. To identify clinical predictors of LVEF recovery at 1-month follow-up, we first performed univariate logistic regression analyses using baseline demographic, clinical, and treatment variables. Variables with p-values < 0.05 in univariate analysis were considered candidates for multivariable modeling. A multivariable logistic regression model was constructed using backward stepwise selection to determine independent predictors of LVEF recovery (HFrecEF). Variables considered for inclusion in the model were based on clinical relevance and included age, sex, height, weight, hypertension, diabetes mellitus, current smoking, chronic kidney disease, LAD-PCI, complex PCI, and use of discharge medications (angiotensin blockade, beta-blockers, calcium channel blockers). The final model retained only variables that remained statistically significant (p < 0.05).
Results
Study flow
Figure 1 illustrates the study flow diagram. Of 2,631 AMI patients undergoing PCI, 904 had reduced LVEF at admission. After excluding those with preserved LVEF (1,727), unmeasured LVEF at 1 month (82), and major clinical events during the first month (143), 679 patients remained, classified into persistent HFrEF (373) and HFrecEF (306).
Figure 1
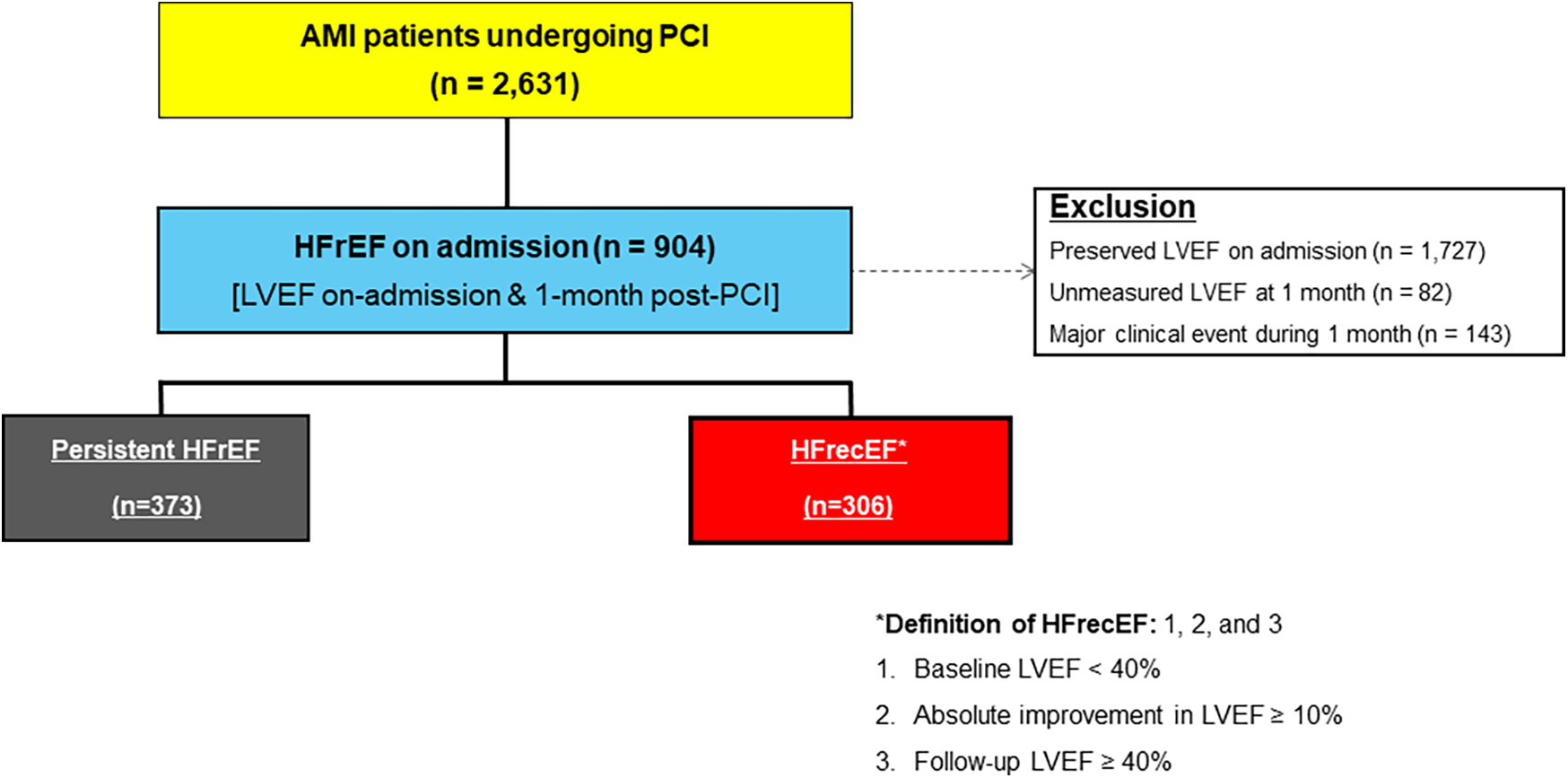
Study flow diagram. AMI, acute myocardial infarction; HFrecEF, heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; PCI, percutaneous coronary intervention.
Baseline characteristics
Patients with HFrecEF were younger (66.4 ± 12.5 years vs. 69.2 ± 12.8 years, p < 0.001) and had higher on-admission LVEF (34.1 ± 6.3% vs. 35.7 ± 4.8%, p < 0.001). Beta-blockers (85.9% vs. 77.2%, p = 0.013) and RAS inhibitor (85.0% vs. 74.8%, p < 0.001) were higher in they were more in HFrecEF group. At the 1-month follow-up, their LVEF further improved to 56.1 ± 4.7%, compared to 42.5 ± 5.6% in the persistent HFrEF group. HFrecEF patients also exhibited fewer comorbidities, including hypertension (41.8% vs. 55.5%, p < 0.001) and chronic kidney disease (21.6% vs. 27.9%, p < 0.001) (Table 1).
Table 1
| Variables | Persistent HFrEF (n = 373) | HFrecEF (n = 306) | P-value |
|---|---|---|---|
| LV ejection fraction, % | |||
| On-admission | 34.1 ± 6.3 | 35.7 ± 4.8 | <0.001 |
| 1-month follow-up | 42.5 ± 5.6 | 56.1 ± 4.7 | |
| Index presentation, n (%) | 0.001 | ||
| Non-ST-segment elevation MI | 164 (44.0) | 145 (47.4) | |
| ST-segment elevation MI | 209 (56.0) | 161 (52.6) | |
| Age, years | 69.2 ± 12.8 | 66.4 ± 12.5 | <0.001 |
| Male, n (%) | 250 (67.0) | 214 (69.9) | 0.005 |
| Body mass index, kg/m² | 23.4 ± 3.7 | 23.6 ± 3.7 | <0.001 |
| Previous history, n (%) | |||
| Previous PCI | 29 (7.8) | 11 (3.6) | 0.010 |
| Previous stroke | 28 (7.5) | 23 (7.5) | 0.371 |
| Risk factor, n (%) | |||
| Hypertension | 207 (55.5) | 128 (41.8) | <0.001 |
| Diabetes mellitus | 119 (31.9) | 95 (31.0) | <0.001 |
| Dyslipidemia | 200 (53.6) | 193 (63.1) | 0.005 |
| Smoking | 149 (39.9) | 130 (42.5) | 0.034 |
| Chronic kidney disease | 104 (27.9) | 66 (21.6) | <0.001 |
| Anemia | 113 (30.3) | 75 (24.5) | <0.001 |
| Laboratory measurements | |||
| White blood cell, ×103/mm3 | 11.4 ± 4.4 | 10.7 ± 3.5 | <0.001 |
| Hemoglobin, g/dl | 13.1 ± 2.0 | 13.5 ± 2.2 | <0.001 |
| Platelet, ×103/mm3 | 253.9 ± 79.0 | 258.2 ± 85.3 | 0.020 |
| Glomerular filtration rate, ml/min/1.73m2 | 77.6 ± 34.2 | 83.5 ± 35.8 | <0.001 |
| Total cholesterol, mg/dl | 186.2 ± 47.3 | 191.1 ± 48.2 | 0.036 |
| HbA1c, % | 6.48 ± 1.39 | 6.66 ± 1.37 | 0.002 |
| Procedural characteristics | |||
| Culprit lesion | <0.001 | ||
| Left main coronary artery | 10 (2.7) | 9 (2.9) | |
| Left anterior descending artery | 241 (64.6) | 199 (65.0) | |
| Left circumflex artery | 75 (20.1) | 65 (21.2) | |
| Right coronary artery | 115 (30.8) | 109 (35.6) | |
| Multivessel disease, n (%) | 211 (56.6) | 158 (51.6) | 0.001 |
| Concomitant medications, n (%) | |||
| Aspirin | 370 (99.2) | 304 (99.3) | 0.927 |
| P2Y12 receptor inhibition | 0.007 | ||
| Clopidogrel | 302 (81.0) | 256 (83.7) | |
| Prasugrel | 13 (3.5) | 8 (2.6) | |
| Ticagrelor | 53 (14.2) | 38 (12.4) | |
| Beta blocker | 288 (77.2) | 263 (85.9) | 0.013 |
| Angiotensin blockade | 279 (74.8) | 260 (85.0) | <0.001 |
| Statin | 356 (95.4) | 297 (97.1) | 0.114 |
Baseline characteristics of study groups.
Values are mean ± SD or n (%). MI, myocardial infarction; HFrecEF, heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction; HbA1c, hemoglobin A1c; PCI, percutaneous coronary intervention.
Predictors of LVEF recovery
Logistic regression analysis identified younger age (OR 0.98, p = 0.022), beta-blocker use (OR 1.60, p = 0.039), and RAS inhibitor use (OR 1.66, p = 0.022) as independent predictors of LVEF recovery (Table 2).
Table 2
| Univariate | Multiple (Final model) | ||||
|---|---|---|---|---|---|
| Parameter | Odds Ratio | p | Odds Ratio | Standard Error | p |
| Age | 0.99 | 0.091 | 0.98 | 0.01 | 0.022 |
| Sex | 0.88 | 0.644 | – | – | – |
| Height | 1.00 | 0.875 | – | – | – |
| Body weight | 1.00 | 0.666 | – | – | – |
| Hypertension | 0.62 | 0.009 | – | – | – |
| Diabetes mellites | 1.11 | 0.598 | – | – | – |
| Current smoker | 0.80 | 0.263 | – | – | – |
| Chronic kidney disease | 0.80 | 0.281 | – | – | – |
| LAD PCI | 0.85 | 0.366 | – | – | – |
| Complex PCI | 0.86 | 0.587 | – | – | – |
| Angiotensin blockade | 1.67 | 0.019 | 1.66 | 0.22 | 0.022 |
| Beta blocker | 1.64 | 0.030 | 1.60 | 0.23 | 0.039 |
| Calcium channel blocker | 0.68 | 0.500 | – | – | – |
The independent predictors of recovered LV function after AMI in the logistic regression analyses.
LAD, left anterior descending artery; PCI, percutaneous coronary intervention.
Clinical outcomes
Kaplan–Meier curves (Figure 2) demonstrate significantly better outcomes in the HFrecEF group. The composite outcome of all-cause death or heart failure readmission occurred in 7.8% of HFrecEF patients compared to 13.9% in the persistent HFrEF group (adjusted HR 0.55, 95% CI: 0.34–0.90, p = 0.018). HFrecEF patients also had lower rates of all-cause death (3.3% vs. 8.3%, adjusted HR 0.37, 95% CI: 0.18–0.77, p = 0.007). While HF readmission rates were lower in HFrecEF (6.2% vs. 10.2%), the adjusted model showed a trend towards significance (adjusted HR 0.60, 95% CI: 0.35–1.05, p = 0.074) (Table 3).
Figure 2
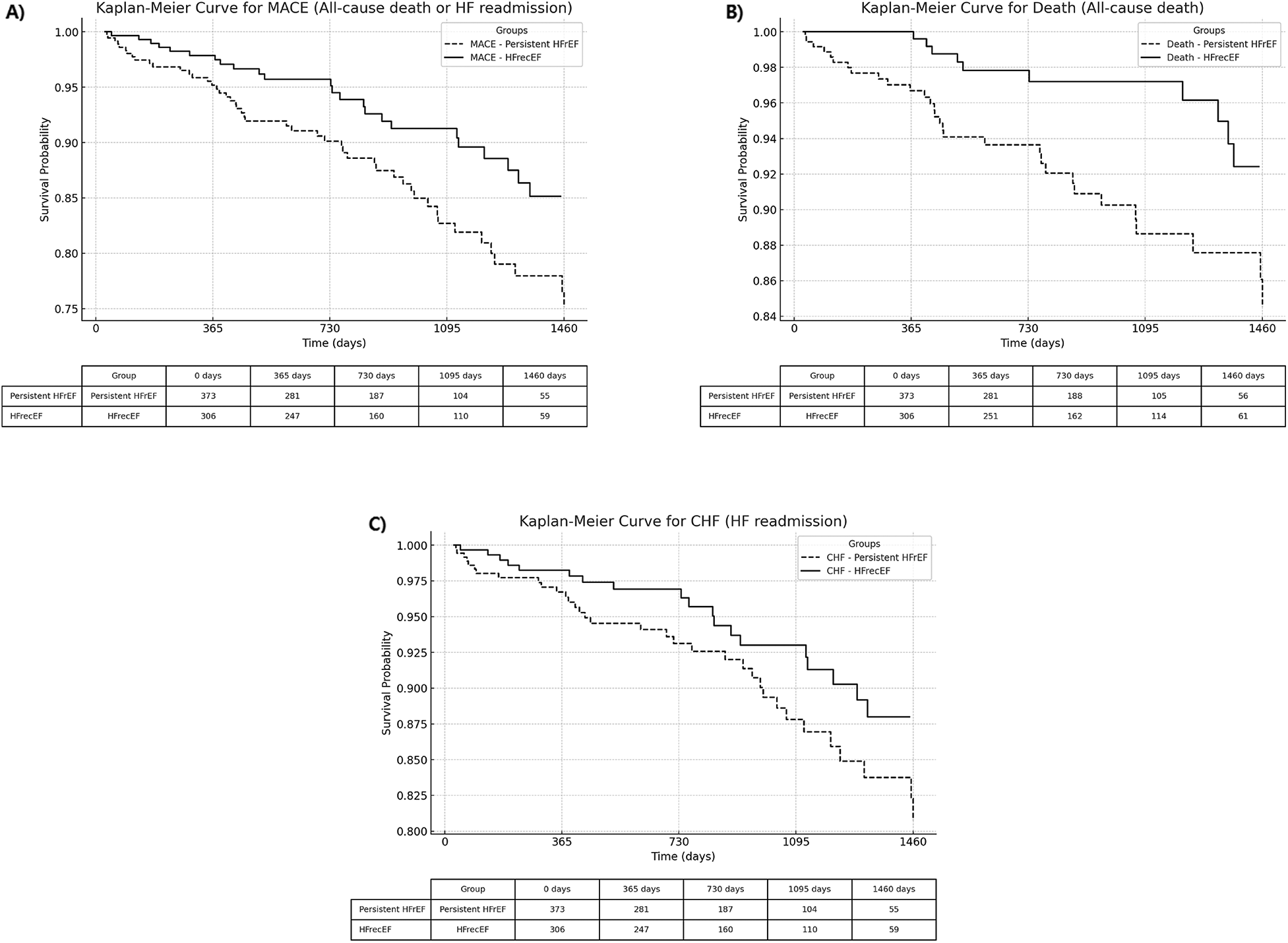
Kaplan–Meier curves comparing adverse events between HFrecEF and persistent HFrEF groups. (A) All-cause death or heart failure readmission; (B) All-cause death; (C) Heart failure readmission. Solid lines represent the HFrecEF group; dashed lines represent the persistent HFrEF group. HFrecEF, heart failure with recovered ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 3
| Events | Rates | Unadjusted model | Adjusted model* | |||||
|---|---|---|---|---|---|---|---|---|
| Persistent HFrEF (n = 373) | HFrecEF (n = 306) | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| All-cause death or HF readmission | 52 (13.9%) | 24 (7.8%) | 0.50 | 0.31–0.81 | 0.005 | 0.55 | 0.34–0.90 | 0.018 |
| : All-cause death | 31 (8.3%) | 10 (3.3%) | 0.35 | 0.17–0.71 | 0.004 | 0.37 | 0.18–0.77 | 0.007 |
| : HF readmission | 38 (10.2%) | 19 (6.2%) | 0.54 | 0.31–0.93 | 0.028 | 0.60 | 0.35–1.05 | 0.074 |
Clinical outcomes according to recovered LVEF after AMI.
Adjusted for index STEMI presentation, age, gender, BMI, smoking, DM, hypertension, dyslipidemia, CKD, multivessel disease.
Values are n (%). CI, confidence interval; HR, hazard ratio.
Discussion
This study adds to the growing body of evidence on the prognostic significance of early LVEF recovery following AMI by comparing clinical outcomes between patients with persistent HFrEF and those with HFrecEF. Patients with HFrecEF demonstrated more favorable outcomes, including lower rates of cardiovascular mortality and heart failure rehospitalization. These findings support the clinical relevance of early post-AMI echocardiographic reassessment and may inform future strategies for risk stratification and individualized management across different heart failure phenotypes.The results highlight that the importance of adhering to guideline-directed medical therapy (GDMT) and serial assessment of left ventricular function by echocardiography can be used to predict the clinical outcome of patients underwent PCI due to ACS (8, 9).
Younger age and the use of beta-blockers and RAS inhibitors emerged as pivotal predictors of LVEF recovery, offering actionable insights into optimizing care for at-risk populations (2). This aligns with prior evidence suggesting that early initiation of GDMT, combined with consistent patient adherence, can significantly enhance outcomes in heart failure patients (8, 9).
Despite these advancements, persistent HFrEF remains a challenging phenotype, characterized by higher risks and poor outcomes. Comprehensive, individualized approaches combining pharmacological and non-pharmacological strategies are needed to address this group effectively. Device-based therapies, such as implantable cardioverter defibrillators (ICDs) or cardiac resynchronization therapy (CRT), may offer additional benefits for patients with severe ventricular dysfunction (7, 9). Moreover, lifestyle interventions, including dietary optimization and supervised exercise programs, could further improve functional status and quality of life (10, 11).
These findings underline the heterogeneity in heart failure phenotypes and the necessity for precision medicine in this domain. Future research should focus on exploring novel pharmacological agents, such as SGLT2 inhibitors, which have shown promise in recent trials for heart failure management (7, 9, 12). Furthermore, long-term studies investigating the durability of LVEF recovery and its impact on survival will be crucial for advancing care in this population. Younger age and the use of beta-blockers and RAS inhibitors emerged as pivotal predictors of LVEF recovery, offering actionable insights into optimizing care for at-risk populations. Although younger age was identified as a predictor of LVEF recovery, this could also underscore the importance of early detection, close follow-up, and the optimization of medical therapy in elderly patients, who may have more a limited potential for spontaneous recovery. Strategies such as comprehensive geriatric assessment, frailty screening, and enhanced support for medication adherence may contribute to improve outcomes in this population.
Nonetheless, a significant proportion of patients continue to experience persistent HFrEF, highlighting an unmet clinical need. Comprehensive, individualized approaches combining pharmacological and non-pharmacological strategies are needed to address this group effectively. These findings underline the heterogeneity in heart failure phenotypes and the necessity for precision medicine in this domain.
Limitations
This study has certain limitations. First, as a single-cohort study, residual confounding cannot be ruled out. Factors such as infarct size, extent of coronary artery disease, prior revascularization history, adherence to medications, and socioeconomic variables were not fully captured in our dataset and may influence the observed associations. Second, the exclusion of patients with unmeasured LVEF or major clinical events may have resulted in a selection bias And due to the nature of our registry, written informed consent was obtained only from patients who survived the initial 1-month post-AMI period without major events, in accordance with IRB requirements. As a result, patients with early death or major complications were not enrolled, which may introduce selection bias and limit the generalizability of our findings. Third, our dataset lacked comprehensive information on the use of mineralocorticoid receptor antagonists (MRAs) and sodium-glucose co-transporter 2 (SGLT2) inhibitors. As a result, our analysis could not fully assess the implementation of contemporary guideline-directed medical therapy (GDMT), and the findings regarding pharmacologic treatment are primarily limited to RAS inhibitors and β-blockers. Fourth, interobserver variability in echocardiographic assessments could influence group classification.
Finally, although the study used a 1-month follow-up period in accordance with routine post-AMI clinical practice, we acknowledge that this time frame may not fully capture the extent or durability of left ventricular reverse remodeling, which can evolve over 3 to 6 months. Future studies incorporating serial echocardiographic evaluations over longer follow-up periods and multicenter validation are warranted to confirm and extend our findings.
Conclusion
In conclusion, this study highlights the critical prognostic differences between persistent HFrEF and HFrecEF following AMI. The transition to HFrecEF is associated with significantly better outcomes, driven by predictors such as younger age and the use of beta-blockers and RAS inhibitors. These findings underscore the importance of regular echocardiographic monitoring and tailored therapeutic strategies to optimize recovery.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The institutional review board of Gyeongsang National University Changwon Hospital approved this study: NCT04650529. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
JJ: Writing – original draft, Data curation, Conceptualization. JL: Formal analysis, Data curation, Conceptualization, Writing – original draft. YS: Writing – review & editing, Investigation, Formal analysis. Y-LK: Conceptualization, Writing – original draft, Data curation. GY: Methodology, Writing – original draft, Conceptualization, Data curation. JB: Methodology, Investigation, Project administration, Writing – original draft. Y-HC: Formal analysis, Data curation, Writing – original draft. CK: Writing – original draft, Data curation, Investigation, Conceptualization. MK: Methodology, Formal analysis, Writing – original draft. K-HK: Investigation, Writing – original draft, Formal analysis. JP: Writing – original draft, Data curation. J-YH: Formal analysis, Data curation, Writing – original draft, Visualization, Validation. Y-HJ: Project administration, Writing – original draft, Methodology. J-HA: Writing – review & editing, Methodology, Writing – original draft, Investigation, Data curation, Resources, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Gerber Y Weston SA Berardi C McNallan SM Jiang R Redfield MM et al Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. (2013) 178:1272–80. 10.1093/aje/kwt109
2.
Kim KA Kim SH Lee KY Yoon AH Hwang B Choo EH et al Predictors and long-term clinical impact of heart failure with improved ejection fraction after acute myocardial infarction. J Am Heart Assoc. (2024) 13:e034920. 10.1161/JAHA.124.034920
3.
Wilcox JE Fang JC Margulies KB Mann DL . Heart failure with recovered left ventricular ejection fraction: JACC Scientific Expert Panel. J Am Coll Cardiol. (2020) 76:719–34. 10.1016/j.jacc.2020.05.075
4.
Hammer Y Yosef M Khalatbari S Aaronson KD . Heart failure with recovered ejection fraction in patients with nonischemic cardiomyopathy: characteristics, outcomes, and long-term follow-up. J Card Fail. (2023) 29:1593–602. 10.1016/j.cardfail.2023.06.022
5.
Coiro S Girerd N Rossignol P Bauersachs J Pitt B Fay R et al Association of digitalis treatment with outcomes following myocardial infarction in patients with heart failure or evidence of left ventricular dysfunction: an analysis from the high-risk myocardial infarction database initiative. Clin Res Cardiol. (2017) 106:722–33. 10.1007/s00392-017-1116-z
6.
Kwon O Ahn J-H Koh J-S Park Y Hwang SJ Tantry US et al Platelet-fibrin clot strength and platelet reactivity predicting cardiovascular events after percutaneous coronary interventions. Eur Heart J. (2024) 45:2217–31. 10.1093/eurheartj/ehae296
7.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. 10.1093/eurheartj/ehab368
8.
Yamaguchi T Kitai T Miyamoto T Kagiyama N Okumura T Kida K et al Effect of optimizing guideline-directed medical therapy before discharge on mortality and heart failure readmission in patients hospitalized with heart failure with reduced ejection fraction. Am J Cardiol. (2018) 121:969–74. 10.1016/j.amjcard.2018.01.006
9.
Patel J Rassekh N Fonarow GC Deedwania P Sheikh FH Ahmed A et al Guideline-directed medical therapy for the treatment of heart failure with reduced ejection fraction. Drugs. (2023) 83:747–59. 10.1007/s40265-023-01887-4
10.
Wenger NK . Lifestyle interventions to improve exercise tolerance in obese older patients with heart failure and preserved ejection fraction. JAMA. (2016) 315:31–3. 10.1001/jama.2015.17347
11.
Lee VYJ Houston L Perkovic A Barraclough JY Sweeting A Yu J et al The effect of weight loss through lifestyle interventions in patients with heart failure with preserved ejection fraction-A systematic review and meta-analysis of randomised controlled trials. Heart Lung Circ. (2024) 33:197–208. 10.1016/j.hlc.2023.11.022
12.
Matsukawa R Okahara A Tokutome M Itonaga J Koga E Hara A et al A scoring evaluation for the practical introduction of guideline-directed medical therapy in heart failure patients. ESC Heart Fail. (2023) 10:3352–63. 10.1002/ehf2.14524
Summary
Keywords
AMI, HFREF, HFrecEF, prognosis, predictors
Citation
Jang JY, Lee JM, Shin Y, Kim Y-L, Yu G, Bae JS, Cho Y-H, Kwak CH, Kang MG, Kim K-H, Park JR, Hwang J-Y, Jeong Y-H and Ahn J-H (2025) Prognostic differences between persistent HFrEF and HFrecEF following acute myocardial infarction. Front. Cardiovasc. Med. 12:1597947. doi: 10.3389/fcvm.2025.1597947
Received
22 March 2025
Accepted
02 July 2025
Published
17 July 2025
Volume
12 - 2025
Edited by
Ting-Yung Chang, Taipei Veterans General Hospital, Taiwan
Reviewed by
Cheng-I. Wu, Taipei Veterans General Hospital, Taiwan
Cheng Hwee Soh, Baker Heart and Diabetes Institute, Australia
Updates
Copyright
© 2025 Jang, Lee, Shin, Kim, Yu, Bae, Cho, Kwak, Kang, Kim, Park, Hwang, Jeong and Ahn.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jong-Hwa Ahn jonghwaahn@naver.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.