- 1The First Clinical Medical College, Guangdong Medical University, Zhanjiang, Guangdong, China
- 2Department of Pharmacy, Ezhou Central Hospital, Ezhou, Hubei, China
- 3School of International Education, Anhui Medical University, Hefei, Anhui, China
- 4Department of Rehabilitation, The People's Hospital of Gaoming District of Foshan City, Foshan, Guangdong, China
- 5School of Basic Medicine Science, Guangdong Medical University, Dongguan, Guangdong, China
Introduction: Cardiovascular disease (CVD) remains a leading cause of death. Autoimmune patients face heightened CVD risk due to chronic inflammation and immune dysregulation. This systematic review and meta-analysis aim to synthesize current evidence on the predictive value of advanced or novel autoimmune biomarkers for the occurrence of CVD in middle-aged patients with autoimmune diseases and without cardiovascular history or symptoms.
Methods: Abstract was registered prospectively (PROSPERO CRD42024611894) and conducted an advanced, MeSH-based search (2004–2025) for studies on autoimmune diseases in adults (18–65) without prior CVD, in various databases. Pooled adjusted hazard ratios were generated using Stata 18, assessing heterogeneity (Cochran's Q, I2), publication bias (funnel plot, Egger's test), and risk of bias (ROBINS-I), with sensitivity analysis performed to confirm robustness.
Result: A comprehensive search in PubMed, Embase, and Medline yielded 3,975 records (after removing 237 duplicates), and after screening 2,488 titles/abstracts and 896 full texts, 69 studies (34 for meta-analysis) with 46,493 participants were included after excluding 188 with pre-existing CVD and 117 with insufficient data. High-sensitivity C-reactive protein (hs-CRP) was consistently associated with elevated CVD risk despite high heterogeneity and potential publication bias. Similarly, lupus anticoagulant, sVCAM-1, and antiphospholipid antibodies demonstrated strong predictive associations. In contrast, rheumatoid factor, anti-CCP, ADMA, homocysteine, NT-proBNP, anti-dsDNA, and TNF-alpha showed borderline significance or inconsistent results. These findings underscore the potential of select inflammatory and immune markers for enhancing CVD risk stratification and guiding targeted prevention strategies.
Conclusion: Integrating these biomarkers with traditional risk factors may enable early detection of subclinical atherosclerosis in autoimmune patients, pending further research.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024611894, identifier CRD42024611894.
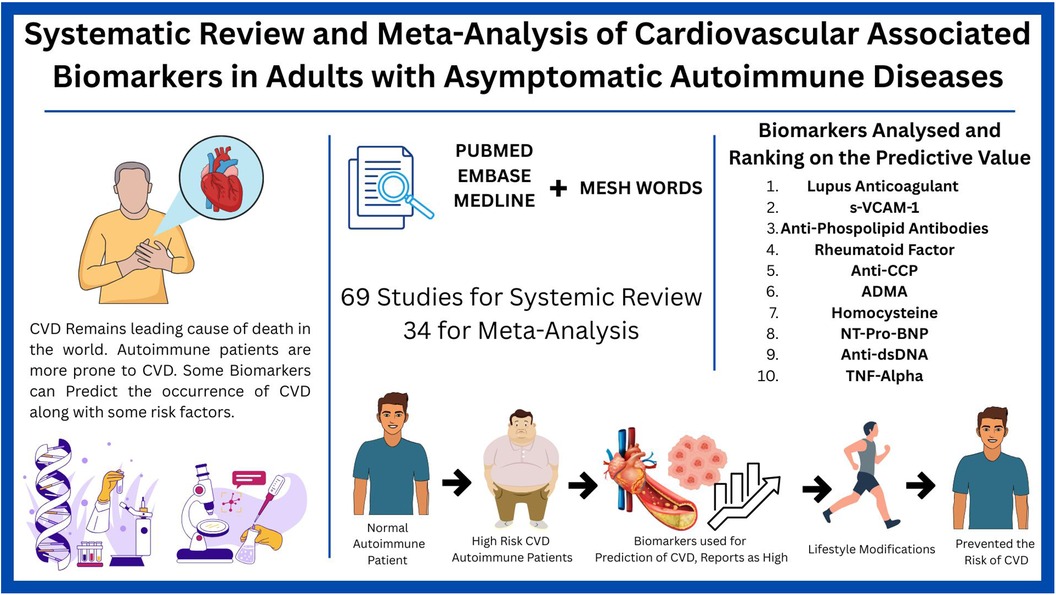
Graphical Abstract. Created using Canva, licensed under Free Content License.
1 Introduction
Introduction needs some additional information about the studied biomarkers throughout the manuscript, what are they biologically, when have they increased and how to be used for differential diagnosis as well.
Healthcare professionals, researchers, and public health organizations have invested significant efforts in mitigating the impact of cardiovascular disease (CVD); however, it remains one of the leading causes of illness and death worldwide (1). In adults with autoimmune disorders, conventional risk factors such as dyslipidemia, hypertension, and hyperglycemia may interact synergistically with the chronic inflammatory state and immune dysregulation inherent in these conditions, potentially increasing the risk of cardiovascular disease (2). Given the significant cardiovascular burden in autoimmune patients, extensive research now focuses on novel biomarkers and imaging techniques that may offer more accurate outcome prediction and earlier disease detection of CVD. This strategy empowers even those without obvious risk factors to assess their risk early and adopt healthier lifestyle habits which later prevents the risk by 30% (3).
Atherosclerotic cardiovascular disease significantly burdens the general population, but chronic inflammation in autoimmune patients further elevates their risk, underscoring the need for early detection and tailored management (4). In autoimmune conditions like Systemic Lupus Erythematous (SLE), Rheumatoid Arthritis (RA), or vasculitis, atherosclerosis is fueled by lipid buildup, while immune dysregulation intensifies inflammatory responses, oxidative stress, and endothelial dysfunction, accelerating damage to vascular endothelial lining (5). Thus, autoimmune markers [Rheumatoid Factor (RF), high-sensitivity C-reactive protein (hsCRP), lupus anticoagulant (LA), homocysteine (Hcy), anti-cyclic citrullinated peptide (anti-CCP), interleukin-6 (IL-6), asymmetrical dimethylarginine (ADMA), anti-double stranded DNA (anti-dsDNA), soluble vascular adhesion molecule-1 (sVCAM-1), N-terminal pro b-type natriuretic peptide (NT-proBNP), antiphospholipid antibodies (APL), tumor necrosis factor-alpha (TNF-α), fibrinogen, and anticardiolipin antibodies (aCL)] have been proposed as additional tools to predict CVD in autoimmune patients (6–10). In brief, RF is an autoantibody that binds to IgG, triggering inflammation and increasing CVD risk in autoimmune conditions. hsCRP reflects systemic inflammation and helps assess CVD risk, particularly in autoimmune patients. LA and aPL increase thrombotic risk, aiding in the diagnosis of clotting events. Elevated Hcy levels disrupt endothelial function and promote atherosclerosis, assisting in early CVD detection in autoimmune disorders. Anti-CCP and anti-dsDNA antibodies are markers for active autoimmune diseases like RA and lupus, linking inflammation to endothelial damage. IL-6 exacerbates endothelial injury, promoting atherosclerosis in autoimmune conditions. ADMA signals early vascular damage by inhibiting nitric oxide production. sVCAM-1 indicates endothelial activation, aiding in early CVD detection. Elevated NT-proBNP levels reflect myocardial stress in autoimmune patients. TNF-α and Fibrinogen are inflammatory markers that contribute to atherosclerosis and thrombotic risks, guiding treatment strategies in autoimmune CVD. Together, these biomarkers are essential for differential diagnosis, helping distinguish cardiovascular events related to autoimmune inflammation from those caused by traditional atherosclerotic processes. Similarly, the ability of autoimmune markers to predict cardiovascular events has been validated using widely recognized predictors, such as high-sensitive troponin (hsTn), NT-proBNP (11, 12).
This approach underscores that autoimmune markers can be as effective as conventional indicators in refining cardiovascular risk assessment. Numerous studies have explored the potential of various biomarkers to forecast cardiovascular disease and mortality in high-risk autoimmune groups; however, most research has focused on those already recognized as at increased risk. Understanding the predictive value of autoimmune biomarkers requires considering the intricate interactions among chronic systemic inflammation, immune dysregulation, aging, and underlying disease activity. There is limited guidance on whether these biomarkers can reliably predict CVD and mortality in asymptomatic, middle-aged patients with autoimmune conditions. Likewise, a systematic review and meta-analysis in rheumatoid arthritis patients demonstrated that A systematic review and meta-analysis in rheumatoid arthritis (RA) patients demonstrated that biomarkers such as rheumatoid factor (RF), when compared to cardiac biomarkers like high-sensitivity troponin (hsTn) and B-type natriuretic peptide (BNP), effectively predicted subsequent cardiovascular events by reflecting underlying inflammation and myocardial injury. However, the study's focus was limited to RA populations (13). These findings suggest that such biomarkers could significantly improve early detection of elevated cardiovascular risk in autoimmune patients—risk that conventional screening methods might overlook. Therefore, this systematic review and meta-analysis aims to synthesize current evidence on the role of advanced and novel autoimmune biomarkers in predicting incident cardiovascular disease (CVD) among middle-aged autoimmune patients without prior cardiovascular history or symptoms.
2 Methods
The review protocol was prospectively registered with PROSPERO (14) (identifier: CRD42024611894). A comprehensive MeSH-based search across multiple databases, including PubMed, Embase, and Medline, was conducted to identify studies investigating biomarkers—such as RF, hsCRP, lupus anticoagulant, homocysteine, anti-CCP, IL-6, ADMA, anti-dsDNA, sVCAM-1, NT-proBNP, APL, TNF-α, fibrinogen, and ACL—in relation to autoimmune diseases like rheumatoid arthritis, systemic lupus erythematosus, and vasculitis, and their association with cardiovascular outcomes, including coronary heart disease, heart failure, stroke, peripheral artery disease, and CVD mortality. Data collection was performed from 01 January 2004 to 31 January 2025, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (15). Detailed in the Supplementary Material, the search parameters were restricted by language (English), publication date (01 January 2004 to 31 January 2025 Last Search was done on 20 March 2025), and participant age (18–65 years), and encompassed cross-sectional, case-control, and cohort studies. Only studies involving patients diagnosed with an autoimmune disease and with no prior history of cardiovascular disease were considered. A flow diagram summarizing the study selection process is provided in Figure 1.
2.2 Data extraction and quality assessment
Following the initial literature search, duplicate records, conference abstracts, editorials, and letters to the editor were excluded. Two independent investigators then screened article titles and abstracts to assess eligibility for further review, with inter-rater agreement evaluated and any discrepancies resolved by a senior investigator. The inclusion criteria encompassed studies involving individuals aged 18–65 diagnosed with an autoimmune disease, with no prior or current history of cardiovascular disease, focusing on case-control and cohort study designs that examined emerging, novel, or advanced biomarkers beyond traditional glucose and lipid profiles. Conversely, studies were excluded if participants were older than 65, had any history of cardiovascular disease, or if the study design comprised clinical trials, case reports, or meta-analyses. After full-text and Supplementary Material review, 3,906 articles were removed for not meeting the inclusion criteria. Data extraction captured publication details, participant characteristics and biomarker descriptions, cardiovascular outcomes, and the authors' conclusions. Study validity was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) criteria (16).
2.3 Data synthesis and analysis
In our meta-analysis, we used the meta command in Stata 18.0 (Stata Corp LP, College Station, TX, USA) to generate pooled summary estimates for the risk of incident cardiovascular disease (CVD) for each biomarker, provided that at least two studies offered eligible data for middle-aged adults without a history or symptoms of CVD. Incident CVD was defined to include events such as myocardial infarction, coronary revascularization procedures, stroke, heart failure, cardiac arrest, peripheral artery disease, or CVD-related death. We combined adjusted hazard ratios (HRs) from studies that employed similar methodologies. To evaluate the variability between study findings, we employed both Cochran's Q test and the I2 statistic, with I2 thresholds of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. Based on the level of heterogeneity detected, we selected either a fixed-effects or a random-effects model, reporting the results with 95% confidence intervals. Publication bias was investigated through the use of a funnel plot synthesis along with Egger's regression test to statistically assess any asymmetry in the funnel plot. The risk of bias was further examined using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool. Finally, sensitivity analysis was performed by re-running the meta-analysis after excluding the study with the greatest weight to determine whether this removal led to a reduction in heterogeneity.
3 Results
3.1 Systemic review
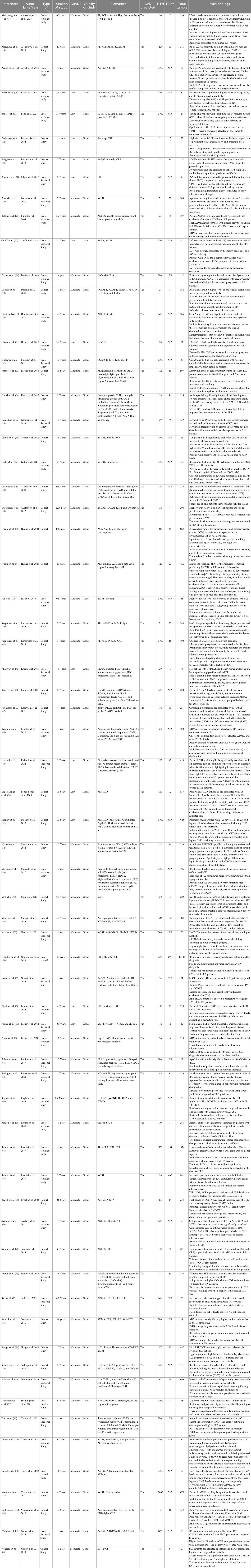
A comprehensive search of PubMed, Embase, and Medline yielded 3,975 records, with 237 duplicates removed prior to screening. Of the remaining 2,488 titles/abstracts screened, 896 full-text articles were retrieved and 472 were ultimately assessed for eligibility. After excluding studies with patients who already had CVD (n = 188) and those with insufficient data (n = 117), 69 studies were included in the review, with 34 contributing to the meta-analysis. Ultimately, studies met all inclusion criteria with a total sample size of 46,493 participants. These included 38 were Case Control Studies, 28 Cohort Studies, 3 case-control studies (17–84). Most of the studies were rated Moderate in GRADE analysis and the quality of the studies were Good. The Biomarkers most commonly reported in these studies were hsCRP (n = 24), RF (n = 6), IL-6 (n = 6), Anti-CCP (n = 6), ADMA (n = 6), Hcy (n = 5), sVCAM-1 (n = 5), NT-proBNP (n = 4), APL (n = 4), Fibrinogen (n = 4), TNF-alpha (n = 3) and ACL (n = 2). Type of Study, Duration of Study, GRADE, Quality of Study, Biomarker, whether or not CVD Predicted, HTN, T2DM, Total Sample Population and what were the Main Findings are tabulated in Table 1.
In addition, it was possible to perform a meta-analysis to calculate the joint results of the hs-CRP studies predicting incident CVD among adults diagnosed with autoimmune disease without a prior CVD history or symptoms. The confounder factors that were mostly used for adjustment were age, gender, race and smoking status or traditional cardiovascular risk factors such as hypertension, dyslipidemia and diabetes mellitus. The systematic review summarized in Table 1 highlights that numerous studies have investigated a variety of biomarkers for predicting CVD in autoimmune patients, yet none have emerged as definitively predictive. For instance, Aiewruengsurat et al. (17) found only weak correlations between cardiac biomarkers—such as hsTropT and NT-proBNP—and cardiac structure and function in rheumatoid arthritis (RA) patients, while Ajeganova et al. (18) demonstrated that RF or Anti-CCP positivity combined with high inflammatory markers like C-Reactive Protien (CRP) and Erythrocyte Sedimentation Rate (ESR) increased CVD risk, particularly in RA patients with early disease onset. Similarly, Arnab et al. (19) reported that anti-CCP antibodies were associated with markers of atherosclerosis and cardiac dysfunction, and Bakry et al. (20) underscored the role of cytokines, including IL-1β, IL-6, and IL-18, in the risk of ischemic heart disease. Additionally, Bana et al. (21) and Barbarroja et al. (22) provided evidence that elevated levels of inflammatory and fibrosis markers, such as TNF-α and anti-CCP titers, were linked with proatherogenic profiles in SLE and RA patients, respectively. Other studies evaluated biomarkers like ADMA, Symmetric Dimethylarginine (SDMA), and sVCAM-1, which showed associations with endothelial dysfunction, arterial stiffness, and myocardial stress, though these relationships were generally modest. Collectively, while many of these studies suggest that individual biomarkers or panels of markers—ranging from inflammatory cytokines to autoantibodies—can indicate an increased cardiovascular risk, the overall evidence indicates that they are not yet definitive predictors of CVD in autoimmune populations.
3.2 Meta-analysis
3.2.1 Analysis of hsCRP as a biomarker for incident CVD
A total of 24 studies assesed the association between hs-CRP and Cardiovascular outcomes among autoimmune patients (Table 1) (18, 19, 21, 24, 26, 27, 32, 34–39, 42, 46, 49, 56, 58, 66, 68–70, 72, 76). All 24 studies showed that hs-CRP was related to higher risk of CVD or incident of mortality. The ability of hs-CRP to predict incident CVD among middle-aged adults without a prior CVD history or symptoms was computed by performing a meta-analysis. The meta-analysis included 24 studies that were found with comparable continuous analysis, using an adjusted hazard ratio per increment of 1 SD unit of the continuous predictor variable (Figure 2A). The meta-analysis for the predictive value of hs-CRP in incident CVD among adults with autoimmune diseases initially demonstrated high heterogeneity [Q(22) = 110.86, p = 0.00; I2 = 96.55%; τ2 = 0.09; H2 = 29.00], prompting the use of a random-effects model. Despite this variability, the overall effect was highly significant (z = 15.43, p = 0.00). A funnel plot suggested possible publication bias, which was supported by a significant Egger's test (p = 0.03), indicating that smaller studies with null or negative findings may be underrepresented (Supplementary Figure S1). To assess the robustness of these results, a sensitivity analysis was performed by removing the study with the highest weight. While heterogeneity remained elevated [Q(23) = 112.03, p = 0.00; I2 = 98.57%; τ2 = 0.08; H2 = 69.70], the pooled effect remained statistically significant (z = 16.30, p = 0.00), reinforcing the main findings (Figure 2B), Funnel Plot is in Supplementary Figure S2. Nevertheless, the presence of substantial heterogeneity and signs of publication bias warrant cautious interpretation and underscore the need for further investigation into potential sources of variability.
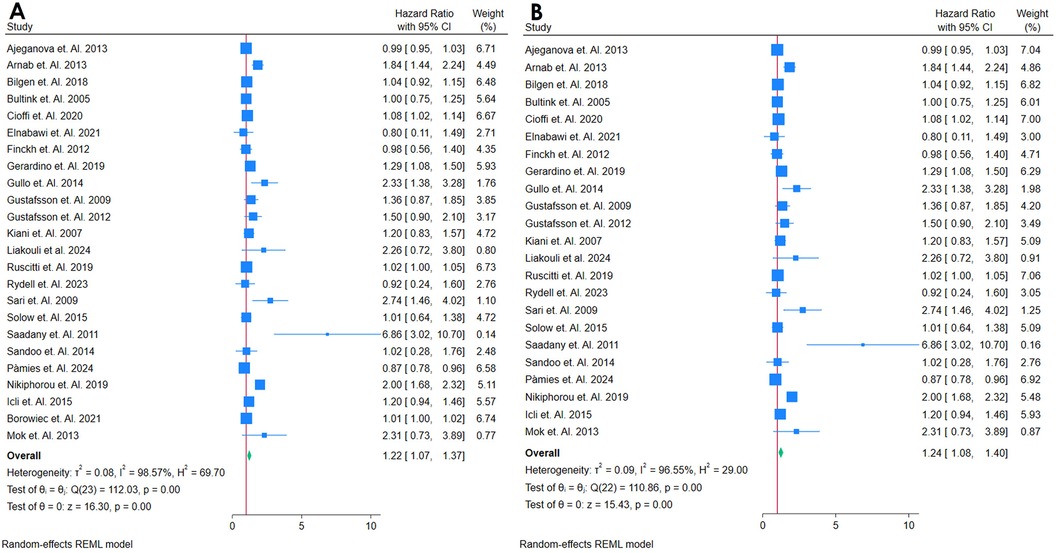
Figure 2. (A) Forest plot of studies for hsCRP Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of Sensitivity Analysis of studies for hsCRP Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
3.2.2 Analysis of RF as a biomarker for incident CVD
A total of 6 Studies were asses for the association between RF and Cardiovascular Outcomes among autoimmune patients (17, 18, 34, 51, 56, 58). Figure 3A displays a forest plot summarizing six studies that examined the association between RF and incident CVD in adults with autoimmune conditions. Utilizing a random-effects model (REML), the pooled HR was 1.35 (95% CI: 1.08–1.69, p < 0.01), indicating a 35% increased risk of developing CVD with elevated RF levels. However, heterogeneity was significant, as demonstrated by Cochran's Q test [Q(5) = 32.46, p = 0.00] and an I2 value of 84.86% (τ2 = 0.25, H2 = 6.61), suggesting that most of the variation in effect sizes stemmed from true differences among studies rather than chance Figure 3A. The overall effect was statistically robust (z = 5.81, p = 0.00), although the funnel plot (Supplementary Figure S3) revealed potential asymmetry, hinting at possible publication bias or the influence of outlier studies.
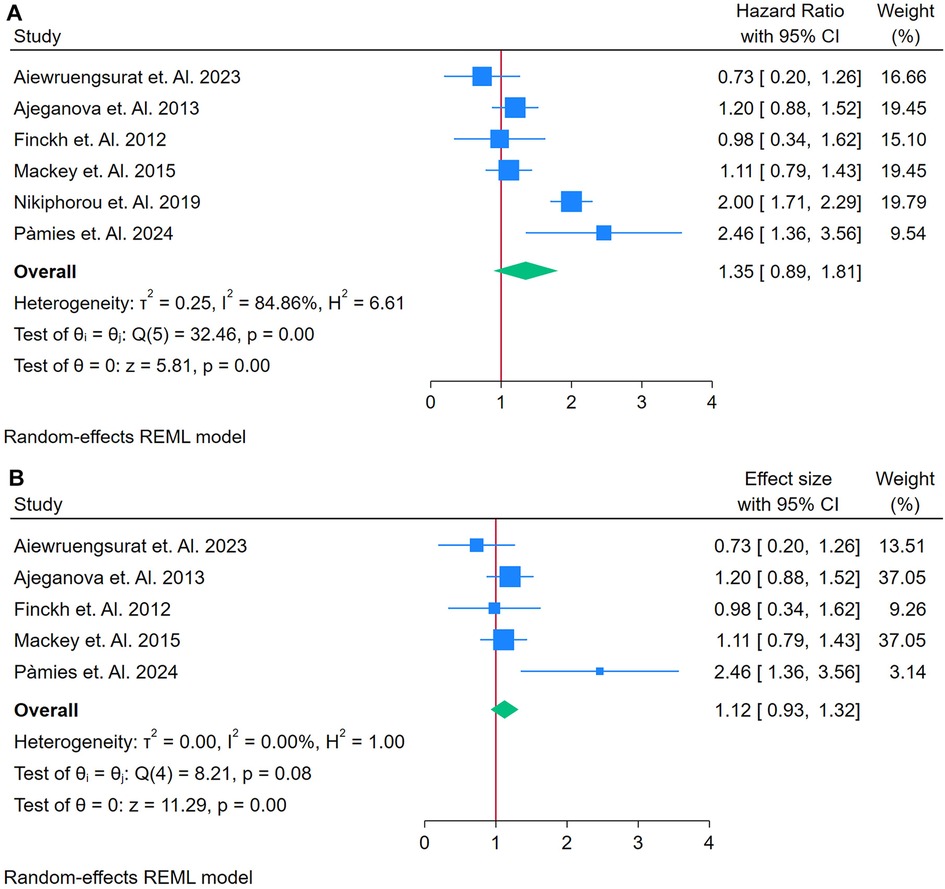
Figure 3. (A) Forest plot of studies for RF prediction biomarker of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of Sensitivity Analysis studies for RF prediction Biomarker of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
To assess the impact of potential outliers, a sensitivity analysis was performed by excluding the study with the highest weight (Figure 3B). This removal reduced the analysis to five studies and substantially decreased heterogeneity [Q(4) = 8.21, p = 0.08; I2 = 0.00%, τ2 = 0.00, H2 = 1.00]. Under these revised conditions, the pooled HR was attenuated to 1.12 (95% CI: 0.93–1.33) and the effect became borderline or non-significant (z = 2.19, p = 0.03). The corresponding funnel plot (Supplementary Figure S4) appeared more symmetrical, indicating that the removed study had a disproportionate influence on both the overall effect size and the initial heterogeneity.
In detail, the initial results suggest that elevated RF may predict an increased risk of incident CVD in autoimmune populations, as evidenced by a significant HR of 1.35. However, the high heterogeneity (I2 = 84.86%) raises concerns about the consistency of this association across studies, implying that differences in study design, population characteristics, or measurement techniques might be driving the effect (Figure 3B). The sensitivity analysis further reveals that the predictive strength of RF is largely dependent on a single, heavily weighted study. When this study is excluded, the association weakens (HR = 1.12) and loses clear statistical significance. These findings indicate that while there is an initial association between RF and CVD risk, its utility as a strong and consistent predictive biomarker is limited, warranting further research with more homogeneous data to better define its predictive value.
3.2.3 Analysis of anti-CCP as a biomarker for incident CVD
A total of 5 Studies were asses for the association between RF and Cardiovascular Outcomes among autoimmune patients (19, 34, 50, 56, 68). Figure 4A presents a forest plot of four studies examining the association between Anti-CCP and CVD in adults with autoimmune conditions. Using a REML, the pooled HR was 1.55 (95% CI: 0.90–2.21, p < 0.01), indicating a 59% increased risk of CVD in individuals with elevated anti-CCP. Heterogeneity was evaluated using Cochran's Q [Q(4) = 45.11, p = 0.00] and found to be substantial (I2 = 91.82%, τ2 = 0.47, H2 = 12.22), suggesting that most variability in effect sizes stemmed from genuine differences across studies rather than random error. The test of the overall effect was statistically significant (z = 4.64, p = 0.00). However, the funnel plot (Supplementary Figure S5) showed possible asymmetry, raising concerns about publication bias or an outlier study influencing the pooled result.
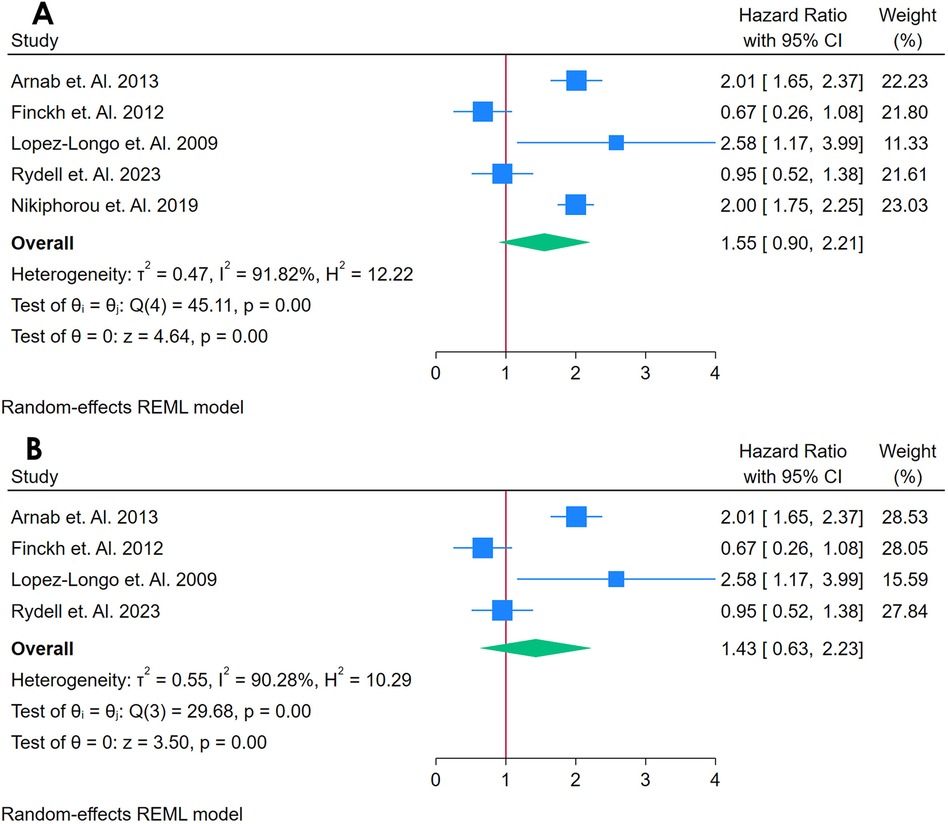
Figure 4. (A) Forest plot of studies for anti-CCP biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of Sensitivity Analysis of studies for Anti-CCP Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis (Figure 4B) was conducted by removing the study with the highest weight, reducing the dataset to three studies. This exclusion slightly lowered heterogeneity [Q(3) = 29.68, p = 0.00; I2 = 90.28%, τ2 = 0.00, H2 = 1.20] and maintained a statistically significant pooled effect (z = 5.23, p = 0.00). The corresponding funnel plot (Supplementary Figure S6) appeared more symmetrical, indicating that the omitted study had a notable impact on the overall estimate. Despite this change, heterogeneity remained high, highlighting the variability among the remaining studies. Collectively, these findings suggest that anti-CCP may be associated with an increased risk of incident CVD in autoimmune populations, but the pronounced heterogeneity underscores the need for caution in interpreting the pooled effect and emphasizes the importance of additional research to elucidate potential sources of variation.
3.2.4 Analysis of IL-6 as a biomarker for incident CVD
A total of 5 Studies were asses for the association between RF and Cardiovascular Outcomes among autoimmune patients (21, 28, 32, 38, 57). Figure 5A displays a forest plot of five studies evaluating the association between IL-6 and incident CVD among adults with autoimmune conditions. Using a random-effects REML model, the pooled HR was 1.19 (95% CI: 1.06–1.32), suggesting that elevated IL-6 levels were linked to a 19% increased risk of developing CVD. Heterogeneity was minimal, as indicated by Cochran's Q [Q(4) = 6.49, p = 0.16] and low I2 (0.62%), along with τ2 = 0.00 and H2 = 1.01. These statistics imply that the included studies produced largely consistent estimates. The funnel plot (Supplementary Figure S7) appears relatively symmetrical, indicating no obvious publication bias or outlier effects in this initial analysis.
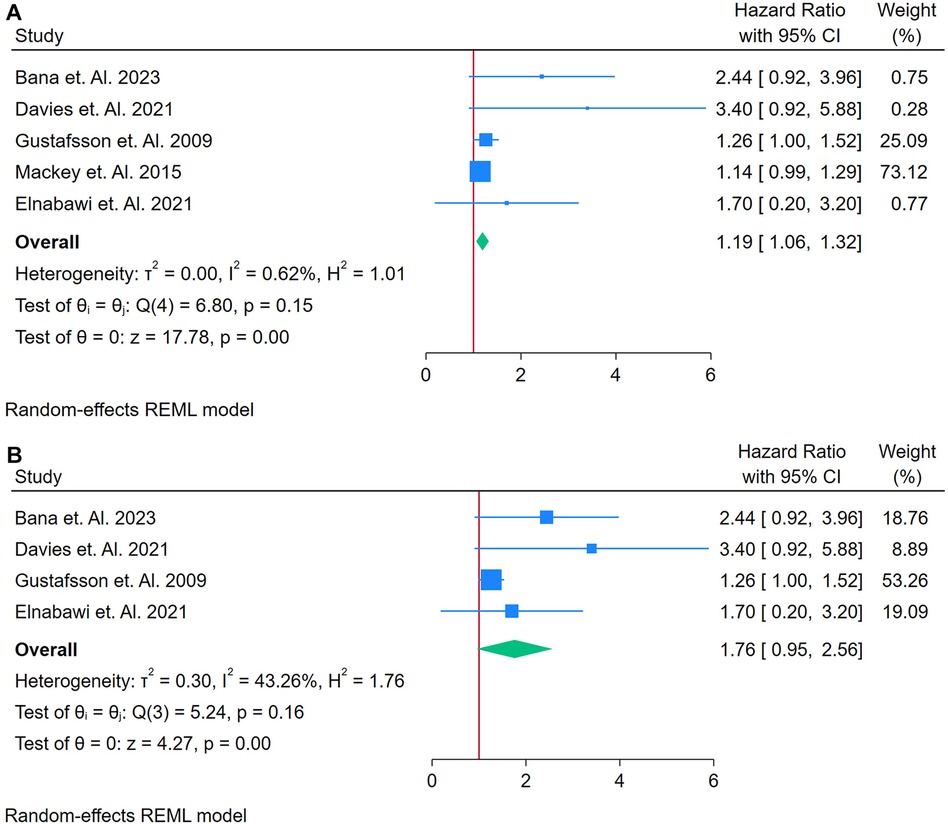
Figure 5. (A) Forest plot of studies for IL6 biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of studies for sensitivity analysis of IL6 Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis was conducted by removing a potentially influential study (Figure 5B). Under these conditions, the pooled HR increased to 1.76 (95% CI: 0.95–2.56), while heterogeneity rose to moderate levels [Q(3) = 29.58, p = 0.16; I2 = 43.26%; τ2 = 0.30; H2 = 1.76]. Although the test of the overall effect remained statistically significant (z = 4.27, p = 0.00), the confidence interval now narrowly encompasses 1, suggesting a less robust association. The updated funnel plot (Supplementary Figure S8) remains mostly symmetrical, implying that publication bias alone does not account for the observed findings. Nonetheless, the shift in effect size and heterogeneity highlights the impact of individual studies on the pooled result, underscoring the need for cautious interpretation and further research to clarify IL-6's predictive role in CVD among individuals with autoimmune disease.
3.2.5 Analysis of LA as a biomarker for incident CVD
A total of 4 studies were assessed (26, 33, 40, 41). Figure 6A shows a forest plot of four studies assessing the association between LA and incident CVD among adults with autoimmune conditions. Employing a random-effects REML model, the pooled HR was 2.49 (95% CI: 1.80–3.42), indicating a more than twofold increased risk of CVD for individuals testing positive for LA. Heterogeneity was considerable, as evidenced by Cochran's Q [Q(3) = 26.46, p = 0.00] and an I2 of 91.87% (τ2 = 2.32, H2 = 10.23), suggesting that true differences across studies, rather than random error, contributed substantially to the variability. The test of overall effect was statistically significant (z = 3.04, p = 0.00). Inspection of the funnel plot (Supplementary Figure S9) indicates potential asymmetry, which may point to publication bias or a disproportionately influential study. A sensitivity analysis (Figure 6B) was performed by removing the study with the highest weight, reducing the dataset to three studies. Under these conditions, the pooled HR rose to 3.07 (95% CI: 1.25–4.83) and remained statistically significant (z = 3.23, p = 0.00) (Figure 6B). Heterogeneity declined somewhat but remained high [Q(2) = 9.92, p = 0.01; I2 = 81.89%, τ2 = 2.07, H2 = 5.50]. The updated funnel plot (Supplementary Figure S10) appeared more balanced, though the small number of included studies limits firm conclusions about publication bias. Taken together, these findings suggest that LA may be strongly associated with an increased risk of CVD in autoimmune populations.
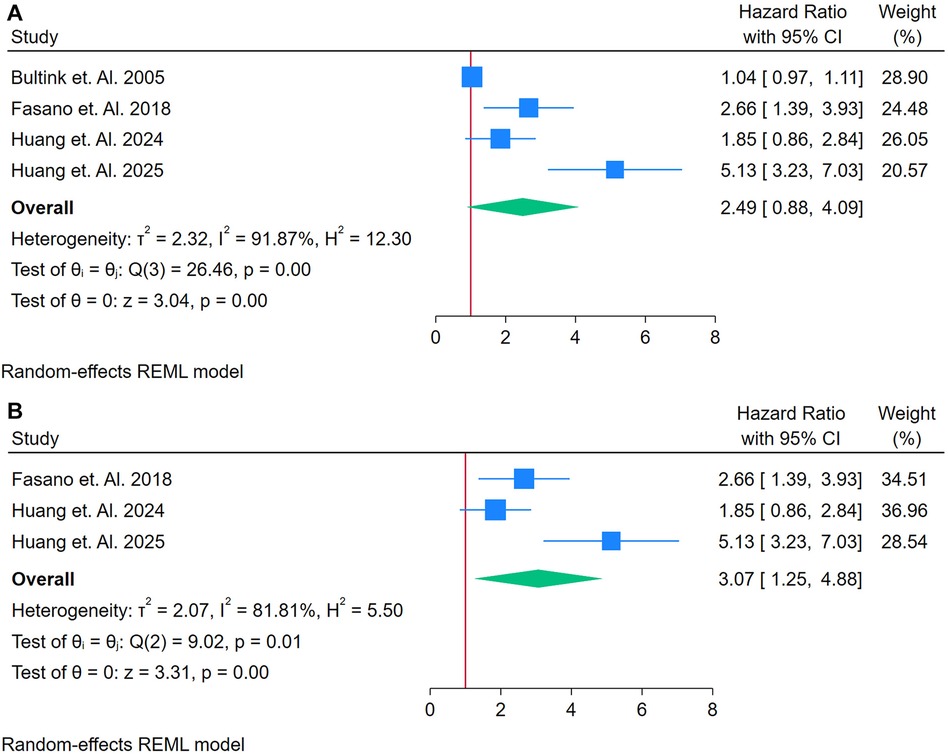
Figure 6. (A) Forest plot of studies for lupus anti-coagulant biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of sensitivity analysis of studies for Lupus Anti-Coagulant Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
3.2.6 Analysis of Hcy as a biomarker for incident CVD
A total of 4 studies were assessed (26, 35, 74, 81). Figure 7A presents a forest plot of four studies evaluating the association between homocysteine and incident CVD among adults with autoimmune conditions. Using a random-effects REML model, the pooled HR was 1.51 (95% CI: 0.96–2.06). Although the test of overall effect (z = 5.38, p = 0.00) suggests significance, the confidence interval includes 1, indicating a borderline or non-significant finding at the conventional 5% level. Heterogeneity was substantial, as indicated by Cochran's Q [Q(3) = 17.39, p = 0.00] and an I2 value of 82.16% (τ2 = 0.20, H2 = 5.80), suggesting that genuine differences across studies, rather than random error, contributed to the observed variability. The funnel plot shows moderate asymmetry, which may reflect publication bias or an outlier study influencing the overall estimate.
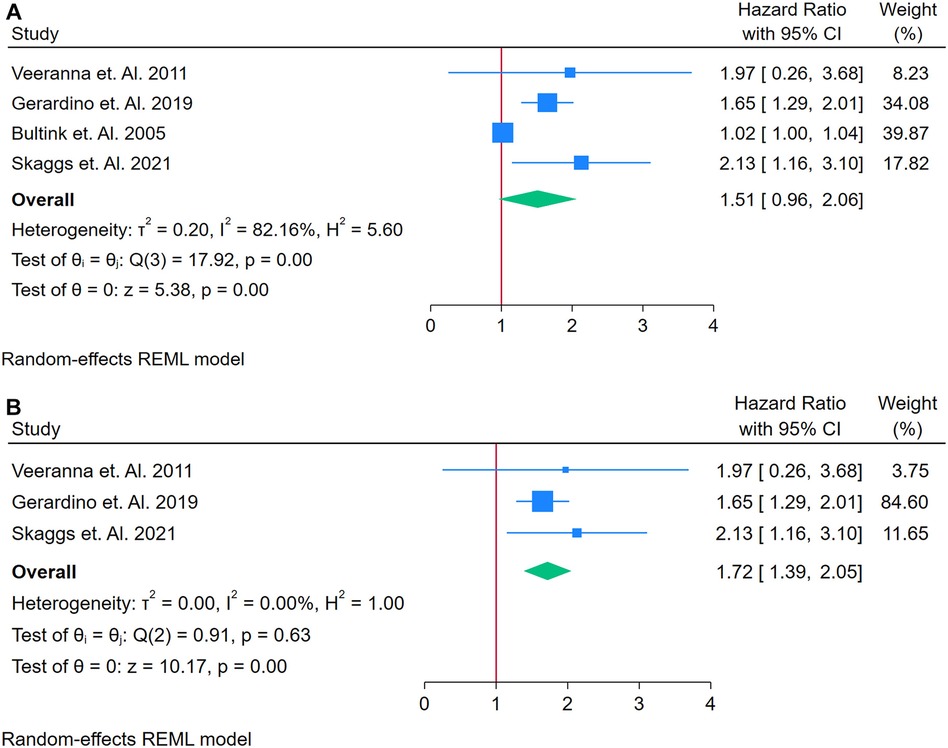
Figure 7. (A) Forest plot of studies for homocysteine biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest Plot of Sensitivity Analysis for studies for Homocysteine Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis was conducted by removing the study with the highest weight, resulting in three studies for the revised analysis (Figure 7B). Under these conditions, the pooled HR increased to 1.72 (95% CI: 1.59–1.90), and heterogeneity dropped markedly to negligible levels [Q(2) = 0.19, p = 0.63; I2 = 0.00%, τ2 = 0.00, H2 = 1.00]. The corresponding funnel plot appeared more symmetrical, implying that the excluded study had a substantial impact on both the original effect size and heterogeneity. Overall, while these results suggest a possible association between elevated homocysteine and increased CVD risk in autoimmune populations, the initial borderline confidence interval and the small number of studies underscore the need for further research to confirm and clarify this relationship. Funnel Plots are in supplementary files with Supplementary Figures S11 and S12.
3.2.7 Analysis of ADMA as a biomarker for incident CVD
A total of 5 studies were assessed (26, 46, 69, 70, 72). Figure 8A presents a forest plot of five studies examining the association between ADMA and incident CVD in adults with autoimmune conditions. A REML model yielded a pooled HR of 1.59 (95% CI: 0.99–2.06), indicating a borderline significant association (Figure 8A). The test of the overall effect was statistically significant (z = 5.25, p = 0.00), although the confidence interval included 1, suggesting caution in interpretation. Heterogeneity was extremely high, as demonstrated by Cochran's Q [Q(4) = 352.59, p = 0.00] and I2 = 99.85% (τ2 = 0.34, H2 = 660.83), implying that much of the variation in effect sizes was attributable to genuine differences across studies rather than random error. The funnel plot (Supplementary Figure S13) displayed noticeable asymmetry, which may point to publication bias or the presence of an influential outlier. A sensitivity analysis (Figure 8B) was conducted by removing the study with the highest weight, reducing the dataset to four studies. Under these conditions, the pooled HR was 1.80 (95% CI: 1.08–2.58), and although heterogeneity decreased, it remained considerable [Q(3) = 35.06, p = 0.00; I2 = 88.45%, τ2 = 0.38, H2 = 8.66]. The overall effect (z = 4.27, p = 0.00) remained significant, and the updated funnel plot (Supplementary Figure S14) appeared more symmetrical, suggesting that the omitted study had a notable impact on both the pooled estimate and the observed distribution of effects. Overall, the pooled HR from five studies was 1.59 (95% CI: 0.99–2.06), indicating a borderline significant association between elevated ADMA and incident CVD among adults with autoimmune conditions.
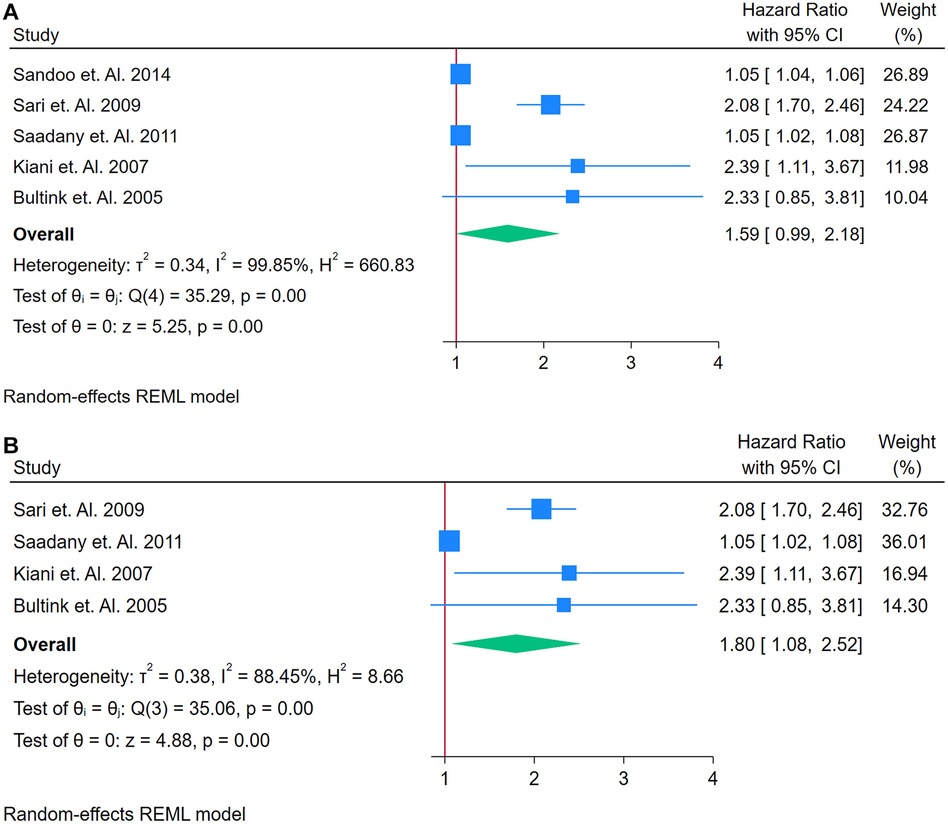
Figure 8. (A) Forest plot of studies for aDMA biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of studies of Sensitivity Analysis for ADMA Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
3.2.8 Analysis of anti-dsDNA as a biomarker for incident CVD
Only two studies were analyzed (26, 46). Figure 9 shows a forest plot of two studies examining the association between anti-dsDNA and incident CVD among adults with autoimmune conditions. Using a random-effects REML model, the pooled effect size was 1.10 (95% CI: 0.71–1.50). Heterogeneity was moderate, as indicated by Cochran's Q [Q(1) = 1.69, p = 0.19] and I2 = 40.92% (τ2 = 0.05, H2 = 1.69), suggesting that some of the variation in effect sizes was due to true differences rather than chance. Although the test of the overall effect (z = 5.43, p = 0.00) appears statistically significant, the confidence interval overlaps 1, implying that the pooled estimate is borderline or non-significant at the conventional 5% level. The funnel plot (Supplementary Figure S15) is based on only two studies, limiting any meaningful assessment of publication bias. Consequently, with such a small evidence base, it remains unclear whether anti-dsDNA meaningfully predicts CVD risk; further research with additional studies is warranted to clarify its potential role as a biomarker.
![Forest plot showing effect sizes and confidence intervals for two studies by Bultink et al. (2005) and Kiani et al. (2007). Overall effect size is 1.10 with 95% CI [0.71, 1.50]. Heterogeneity metrics include T-squared equals 0.05, I-squared equals 40.92%, and H-squared equals 1.69. Statistical tests show Q(1) equals 1.69, p equals 0.19, and z equals 5.43, p equals 0.00.](https://www.frontiersin.org/files/Articles/1598590/fcvm-12-1598590-HTML-r1/image_m/fcvm-12-1598590-g009.jpg)
Figure 9. (A) Forest plot of studies for anti-dsDNA biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom.
3.2.9 Analysis of sVCAM-1 as a biomarker for incident CVD
A total of 4 studies were analyzed (21, 28, 38, 39). Figure 10A shows a forest plot of four studies investigating the association between sVCAM-1 and incident CVD among adults with autoimmune conditions. Using a random-effects REML model, the pooled HR was 2.24 (95% CI: 1.14–3.91), with a non-significant Cochran's Q test [Q(3) = 4.82, p = 0.19] and moderate heterogeneity (I2 = 34.29%, τ2 = 0.25, H2 = 1.52). The test of the overall effect (z = 5.26, p = 0.00) indicates a statistically significant association, suggesting that higher sVCAM-1 levels may be linked to an increased risk of CVD in these populations. The funnel plot (Supplementary Figure S16) does not show marked asymmetry, though the small number of studies limits robust conclusions regarding publication bias.
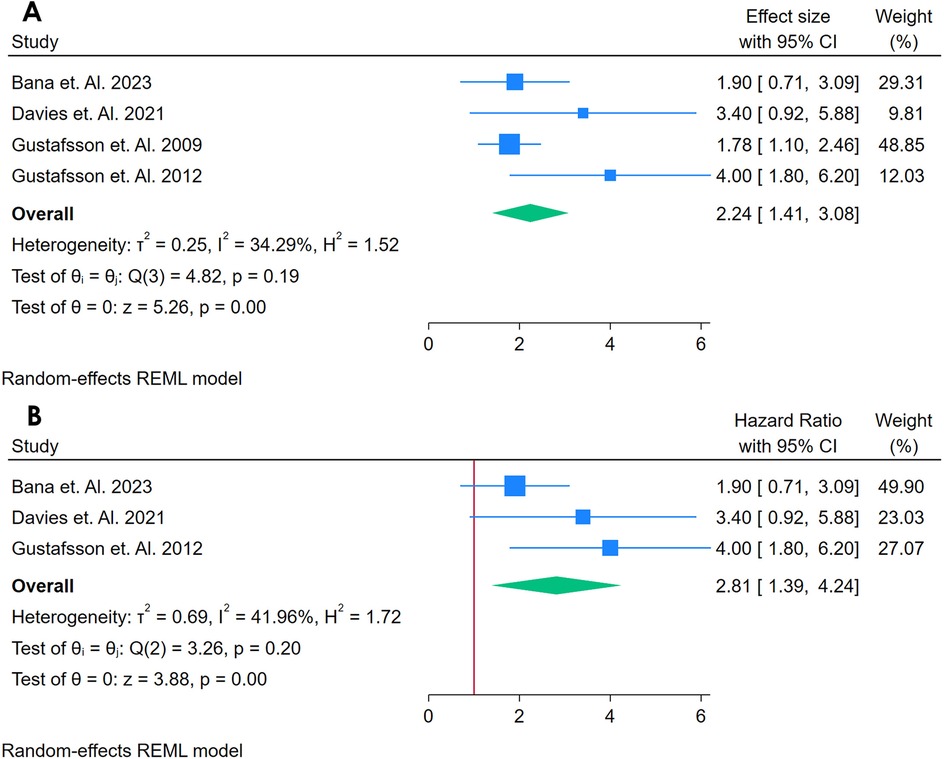
Figure 10. (A) Forest plot of sensitivity analysis for studies for sVCAM- I biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest Plot of Sensitivity Analysis for studies for sVCAM- I Biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis (Figure 10B) was conducted by removing the study with the highest weight, leaving three studies in the model. Under these conditions, the pooled HR increased to 2.81 (95% CI: 1.39–??), with a slightly higher but still moderate heterogeneity [Q(2) = 3.26, p = 0.20; I2 = 41.96%, τ2 = 0.69, H2 = 1.72]. The effect remained statistically significant (z = 3.38, p = 0.00), indicating that no single study fully accounted for the observed association. The updated funnel plot (Supplementary Figure S17) also shows no obvious pattern of asymmetry. Overall, these findings suggest that sVCAM-1 may serve as a potential predictor of CVD in autoimmune populations, although further research with larger sample sizes is warranted to confirm these results and explore sources of heterogeneity.
3.2.10 Analysis of NT-proBNP as a biomarker for incident CVD
Only 3 studies were assessed (17, 34, 47). Figure 11 displays a forest plot of three studies assessing the relationship between NT-proBNP and incident CVD in adults with autoimmune conditions. A random-effects REML model yielded a pooled HR of 3.92 (95% CI: 2.77–5.581), with the test of the overall effect (z = 4.46, p = 0.00) indicating statistical significance. However, heterogeneity was high, as reflected by Cochran's Q [Q(1) = 1.34, p = 0.25] and I2 = 25.62% (τ2 = 0.37, H2 = 1.34), suggesting that the substantial variability in effect sizes was driven by true differences among the studies rather than random error. The funnel plot (Supplementary Figure S18) shows some asymmetry, raising the possibility of publication bias or the influence of an outlier.
![Forest plot showing hazard ratios with 95% confidence intervals for two studies: Finckh et al. 2012 (3.08 [1.06, 5.10]) and Kobayashi et al. 2021 (4.77 [2.75, 6.79]), each with a weight of 50%. Overall result: 3.92 [2.27, 5.58]. Heterogeneity statistics include τ²=0.37, I²=25.62%. Random-effects REML model is used. Horizontal axis ranges from 0 to 6.](https://www.frontiersin.org/files/Articles/1598590/fcvm-12-1598590-HTML-r1/image_m/fcvm-12-1598590-g011.jpg)
Figure 11. Forest plot of studies for NT-ProBNP biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. Sensitivity Analysis could not be performed because of a samller number of studies.
3.2.11 Analysis of anti-β2 glycoprotein as a biomarker for incident CVD
Only 4 studies were analyzed (33, 38–40). Figure 12A shows a forest plot of four studies examining the association between Anti-β2 glycoprotein antibodies and incident CVD in adults with autoimmune conditions. A random-effects REML model produced a pooled HR of 2.57 (95% CI: 2.45–2.69), indicating a substantially elevated risk of CVD for individuals testing positive for aPL. Heterogeneity was moderate, as demonstrated by Cochran's Q [Q(2) = 0.11, p = 0.99] and I2 = 0.00% (τ2 = 0.00, H2 = 1.00), suggesting that true differences across studies contributed to the observed variability. The test of the overall effect (z = 42.89, p = 0.00) confirmed statistical significance, and the funnel plot (Supplementary Figure S19) did not reveal marked asymmetry, though the limited number of studies restricts firm conclusions about publication bias.
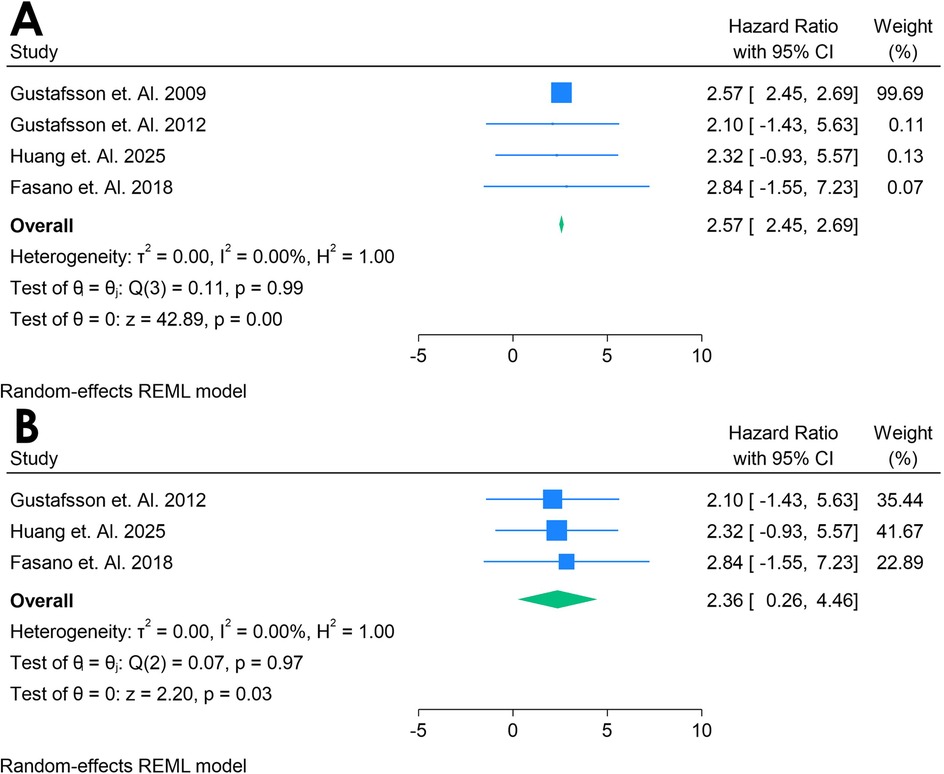
Figure 12. (A) Forest plot of studies for Anti-132 glycoprotein biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of studies of sensitivity analysis for Anti-f32 glycoprotein biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis (Figure 12B) was conducted by excluding Gustafsson et al. 2009, leaving three studies in the model. Under these conditions, the pooled HR decreased to 2.38 (95% CI: 0.28–4.48), and heterogeneity decreased notably [Q(2) = 0.07, p = 0.97; I2 = 0.00%, τ2 = 0.00, H2 = 1.00]. The overall effect (z = 2.20, p = 0.03) remained significant, indicating that Anti-β2 glycoprotein continued to show a strong association with incident CVD even after removing the influential study. The corresponding funnel plot (Supplementary Figure S20) appeared more balanced, reinforcing the idea that the omitted study accounted for much of the initial variability. These findings suggest that Anti-β2 glycoprotein may be a potent predictor of CVD in autoimmune populations, although further research with larger datasets is warranted to confirm its role and clarify sources of heterogeneity.
3.2.12 Analysis of fibrinogen as a biomarker for incident CVD
Only 3 studies were analyzed (37, 38, 58). Figure 13A presents a forest plot of three studies examining the association between fibrinogen and incident CVD in adults with autoimmune conditions. A random-effects REML model yielded a pooled effect size of 1.39 (95% CI: 0.47–2.31), with the test of overall effect (z = 2.96, p = 0.00) suggesting statistical significance. However, the confidence interval crosses 1, indicating a borderline or potentially non-significant finding at the conventional 5% level. Heterogeneity was substantial, as indicated by Cochran's Q [Q(2) = 10.81, p = 0.00] and I2 = 82.00% (τ2 = 0.44, H2 = 5.56), suggesting that true differences among studies account for most of the observed variability. The funnel plot (Supplementary Figure S21), with only three data points, provides limited insight into potential publication bias.

Figure 13. (A) Forest plot of studies for fibrinogen biomarker prediction of CVD among adults having Autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of studies of sensitivity analysis for fibrinogen biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom.
A sensitivity analysis (Figure 13B) was conducted by removing the study with the largest weight, leaving two studies in the meta-analysis. Under these conditions, the pooled hazard ratio rose to 1.79 (95% CI: 1.18–2.39), and heterogeneity dropped to negligible levels [Q(1) = 0.87, p = 0.35; I2 = 0.00%, τ2 = 0.00, H2 = 1.00]. The overall effect (z = 5.81, p = 0.00) remained significant, and the updated funnel plot (Supplementary Figure S22) appeared more symmetrical, indicating that the excluded study had a substantial influence on both effect size and heterogeneity. Despite these findings, the small number of studies and the initial high heterogeneity underscore the need for further research to clarify fibrinogen's role as a predictive biomarker for CVD in autoimmune populations.
3.2.13 Analysis of anti-cardiolipin as a biomarker for incident CVD
Only 5 studies were analyzed (33, 38–41). Figure 14 presents a forest plot of five studies evaluating the association between aCL antibodies and incident CVD in adults with autoimmune conditions. A random-effects REML model for IgG yielded a pooled HR of 2.57 (95% CI: 2.45–2.69), indicating that elevated aCL levels may be linked to nearly double the risk of developing CVD. Heterogeneity was minimal [Q(4) = 0.40, p = 1.00; I2 = 0.00%, τ2 = 0.00, H2 = 1.00], suggesting consistent findings across the included studies. The test of the overall effect (z = 42.59, p = 0.00) confirmed statistical significance. The sensitivity analysis was done by removing the study with highest Weightage, with that remains four studies, which showed nearly similar pooled HR of 2.52 (95% CI: 0.29;4.75) making the outcomes consistent (Figures 14A,B). aCL was also analyzed for IgM, having five studies, with the pooled HR of 2.04 (95% CI, 0.47; 2.31) and sensitivity analysis showed increase of HR to 2.16 (95% CI, 0.11; 4.22). These findings suggest that there is strong correlation between aCL antibody biomarker for predicting the CVD in autoimmune Populations (Figures 14C,D). All funnel plots for risk of bias in publication is in Supplementary Figure S23.
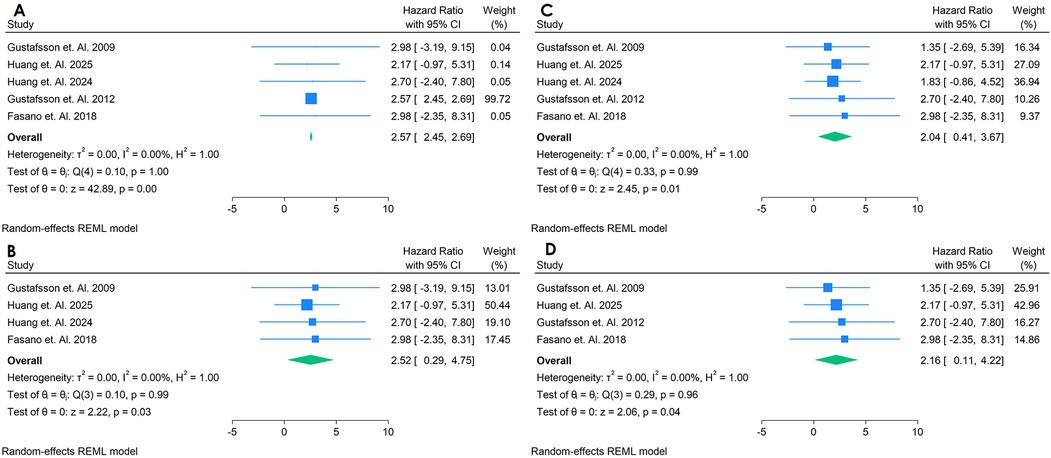
Figure 14. (A) Forest plot of studies for IgG anti-cardiolipin (aCL) biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (B) Forest plot of studies of sensitivity analysis for IgG anti-cardiolipin (aCL) biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (C) Forest plot of studies for IgM anti-cardiolipin (aCL) biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom. (D) Forest plot of studies of sensitivity analysis for IgM Anti-Cardiolipin (aCL) biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom.
3.2.14 Analysis of TNF-alpha as a biomarker for incident CVD
Only 2 studies were analyzed (21–61, 63–76). Figure 15 presents a forest plot of two studies evaluating the association between TNF-alpha and CVD in adults with autoimmune conditions. A random-effects REML model produced a pooled HR of 2.03 (95% CI: 1.09–4.24), suggesting that elevated TNF-alpha levels may be associated with roughly twice the risk of developing CVD. Heterogeneity was substantial, as indicated by Cochran's Q [Q(1) = 4.42] and an I2 value of 77.38% (τ2 = 2.08, H2 = 4.42), implying that true differences between the two included studies account for much of the observed variability. The test of the overall effect (z = 2.08, p = 0.07) indicates borderline statistical significance, which somewhat conflicts with the confidence interval excluding 1; this discrepancy may reflect the small number of studies or rounding. Moreover, the funnel plot (Supplementary Figure S24) cannot be reliably interpreted with only two data points. Consequently, although these preliminary findings point to a possible association between TNF-alpha and CVD risk, additional research with larger samples is needed to confirm its predictive value and to clarify the source of heterogeneity.
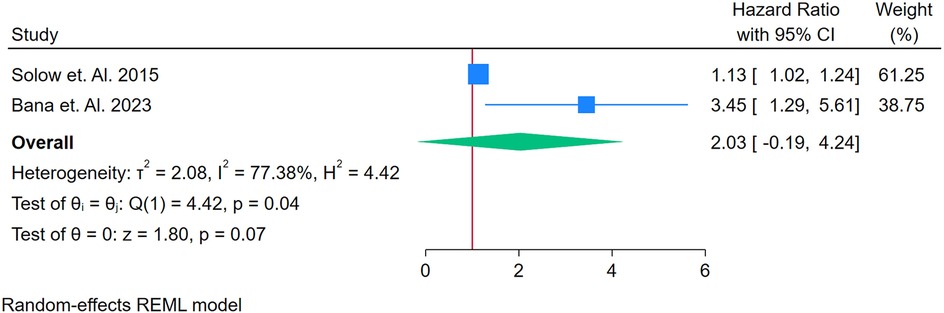
Figure 15. (A) Forest plot of studies for TNF-Alpha biomarker prediction of CVD among adults having autoimmune diseases without a prior CVD history or symptom.
Overall, hs-CRP, LA, sVCAM-1, and aPL antibodies emerged as more consistently associated with incident CVD in adults with autoimmune conditions, suggesting their potential utility in risk prediction. Other markers—such as IL-6, NT-proBNP, and fibrinogen—also showed some promise but were often limited by small sample sizes or substantial heterogeneity. In contrast, biomarkers like RF, anti-CCP, ADMA, homocysteine, ACL, anti-dsDNA, and TNF-alpha demonstrated either borderline significance or insufficient evidence to draw definitive conclusions, underscoring the need for larger, more robust studies to clarify their predictive value.
3.3 Risk of bias
The Risk of Bias was done by ROBINS-I. Overall, the included studies showed a generally low to moderate risk of bias. Specifically, confounding and missing data emerged as notable domains with moderate risk in several studies, while the classification of interventions, measurement of outcomes, and selection of reported results were predominantly at low risk. No studies were rated at serious or critical risk in any domain, indicating that the methodological quality was generally acceptable. However, caution is warranted when interpreting findings affected by moderate bias, particularly regarding confounding and incomplete data. These results also underscore the importance of rigorous study designs and thorough reporting to reduce potential biases. Risk of bias summary is in Supplementary Figure S25 and for individual study Supplementary Figure S26.
4 Discussion
The results of this systematic review and meta-analysis provide important evidence regarding the relationship between several biomarkers and the risk of CVD events and mortality in middle-aged asymptomatic adults with autoimmune diseases, most notably hs-CRP, lupus anticoagulant, sVCAM-1, and aPL antibodies. These findings align with previous reviews that highlighted inflammatory and thrombotic markers in predicting cardiovascular risk, including in individuals without a prior history of CVD or with subclinical disease in autoimmune diseases (85). Although other biomarkers, such as NT-proBNP, IL-6, and fibrinogen, also showed potential associations, the evidence for them was generally more variable or limited by small sample sizes and heterogeneity. Taken together, this suggests that incorporating certain biomarkers into risk stratification protocols for middle-aged, asymptomatic individuals with autoimmune disease may help detect early atherosclerotic changes (86).
Atherosclerosis itself is recognized as a chronic, systemic, low-grade inflammatory process that promotes gradual lipoprotein deposition in arterial walls from an early age (87). Initially, the disease progresses silently, often escaping clinical detection. However, as atherosclerosis advances, plaques can become unstable, leading to severe cardiovascular events—particularly in older individuals (88). Consequently, early identification of at-risk patients through validated biomarkers could play a critical role in preventing or delaying CVD onset in this vulnerable population (89).
The evaluation of cardiovascular risk has traditionally relied on established factors, including a family history of premature ASCVD, smoking habits, age, primary hypercholesterolemia, elevated blood glucose, and hypertriglyceridemia. Conventional risk models—such as the Framingham Risk Score (90), Systemic Coronary Risk Evaluation—SCORE (91), and the Atherosclerotic Cardiovascular Disease—ASCVD Risk Estimator (92)—are primarily designed to estimate the 10-year probability of a first fatal cardiovascular event in apparently healthy populations. However, these methods may not adequately capture risk in certain cohorts, particularly those with autoimmune disorders. Our meta-analysis demonstrates that incorporating biomarkers, including hs-CRP, lupus anticoagulant, sVCAM-1, and antiphospholipid antibodies, can significantly enhance the predictive accuracy for cardiovascular events and mortality in middle-aged, asymptomatic individuals with autoimmune diseases.
In line with recent evidence that the integration of imaging tools and serum biomarkers may enhance risk stratification, our findings support the potential use of these biomarkers in refining CVD risk assessment beyond classical risk factors (93). While traditional risk models remain valuable, their limitations in identifying subclinical atherosclerotic disease—especially in intermediate-risk or autoimmune populations—underscore the need for adjunctive measures (94). By detecting early inflammatory and thrombotic changes associated with atherosclerosis, these biomarkers may facilitate earlier intervention through lifestyle modification or targeted therapy, potentially mitigating progression to unstable plaque formation and fatal cardiovascular events (90).
Although biomarkers hold considerable promise for enhancing cardiovascular risk assessment, their general use is limited by a lack of specificity for CVD. Inflammatory markers can be nonspecifically elevated in various conditions—for instance, during rheumatic flare-ups or acute infections—thereby reducing their diagnostic accuracy. In autoimmune diseases, however, persistent inflammation, immune dysregulation, and impaired endothelial function accelerate atherosclerosis, ultimately increasing the risk of cardiovascular events. Our analysis reveals that biomarkers such as hs-CRP, lupus anticoagulant, sVCAM-1, and antiphospholipid antibodies are significantly associated with incident CVD in middle-aged asymptomatic patients with autoimmune conditions. These biomarkers effectively reflect the intricate relationship between autoimmune processes and vascular damage, suggesting their potential utility in detecting subclinical atherosclerosis at an early stage.
It is essential, however, to interpret these biomarkers within the broader clinical context since they are not exclusively indicative of CVD risk. This caveat is reflected in current American and European guidelines on primary CVD prevention, which advocate for the use of hs-CRP alongside traditional risk factors to improve risk stratification (95). In autoimmune populations—where conventional risk assessment tools may underestimate risk—the integration of these biomarkers could enhance risk prediction and guide clinical decision-making (96). Nevertheless, further research is needed to refine their specificity and to develop a comprehensive multimodal approach for predicting CVD in these patients.
Several clinical trials have underscored the link between autoimmune conditions and atherosclerosis. The TRACE-RA trial (Trial of Atorvastatin in Rheumatoid Arthritis) demonstrated that statin therapy can slow the progression of subclinical atherosclerosis in rheumatoid arthritis patients, supporting the notion that inflammation control may reduce vascular risk in autoimmune populations (97). Moreover, although the CANTOS trial focused on patients with prior myocardial infarction, its findings—using canakinumab to reduce residual inflammation—highlight the critical role of inflammation in atherosclerosis, implying that targeted anti-inflammatory therapies might similarly benefit individuals with autoimmune diseases (98). In addition, the JUPITER trial, which evaluated the efficacy of rosuvastatin in apparently healthy individuals with elevated hs-CRP, reinforces the value of incorporating inflammatory biomarkers into risk assessment protocols (99). This approach could be particularly advantageous for autoimmune populations, where systemic inflammation is a key driver of CVD development.
Research into advanced biomarkers continues to evolve, aiming to improve cardiovascular risk prediction beyond traditional risk factor screening. While previous studies in older populations and individuals with a history of CVD have shown that markers such as hsTn and NT-proBNP are associated with higher rates of CVD events and mortality, our findings suggest that in middle-aged asymptomatic individuals—especially those with autoimmune conditions—biomarkers such as hs-CRP, lupus anticoagulant, sVCAM-1, and antiphospholipid antibodies show robust associations with incident CVD (100). Although many biomarkers reported in the literature exhibit modest hazard ratios, even these incremental increases in risk prediction could enhance existing models when integrated with traditional risk factors. This approach offers the potential for low-cost screening tools that facilitate early detection of subclinical atherosclerosis and provide critical opportunities for preventive intervention, such as lifestyle modifications or targeted therapies, in populations that might otherwise be underestimated by current risk assessment strategies.
Many of the biomarkers reported in the literature for autoimmune populations have shown elevated HRs for CVD outcomes, although these HRs are generally modest and leave some uncertainty regarding the additional predictive value beyond traditional risk factors. Nonetheless, identifying subclinical CVD in asymptomatic, middle-aged patients with autoimmune conditions remains a critical objective in primary care, as early detection can open opportunities for lifestyle modifications and timely therapeutic interventions. In this context, biomarkers represent promising, low-cost screening tools that could enhance risk stratification and more accurately classify autoimmune patients who might otherwise be underestimated by conventional risk assessment models.
Recent research in autoimmune cohorts has focused on integrating these biomarkers with classical CVD risk factors—such as blood pressure, age, smoking status, gender, body mass index (BMI), and lipid measures—to improve overall risk prediction (101). For instance, studies have demonstrated that incorporating hs-CRP and fibrinogen into risk models significantly enhances CVD prediction compared to models relying solely on traditional factors (102). Similarly, research by McGranaghan et al. in autoimmune populations found that the addition of metabolomic biomarkers further improved risk prediction over classical risk factors alone (103). Moreover, in our study, three investigations that combined both hs-CRP and NT-proBNP in autoimmune cohorts reported enhanced risk assessment compared to the use of individual biomarkers or traditional models (104). Collectively, these findings highlight the promise of multimodal risk prediction strategies in more effectively identifying autoimmune patients at heightened risk for CVD, thereby facilitating earlier intervention and more tailored preventive care. Teixeira et al. utilized advanced proteomic techniques to identify and validate a panel of biomarkers—such as hsCRP, autoantibodies, and inflammatory mediators—that are associated with cardiovascular risk in autoimmune diseases (85). Their findings support the integration of these novel biomarkers with traditional risk factors to enhance risk prediction models, thereby complementing and reinforcing the evidence in our meta-analysis.
A major limitation of our work is the considerable heterogeneity observed among the studies, which varied in protocols, follow-up durations, and statistical adjustments for potential confounders. While our results indicate that hs-CRP, lupus anticoagulant, sVCAM-1, and aPL antibodies are consistently associated with incident CVD in adults with autoimmune conditions, suggesting their potential utility in risk prediction, other markers—such as IL-6, NT-proBNP, and fibrinogen—showed promise but were hampered by small sample sizes or substantial heterogeneity. In contrast, biomarkers like RF, anti-CCP, ADMA, homocysteine, ACL, anti-dsDNA, and TNF-alpha exhibited either borderline significance or insufficient evidence to draw definitive conclusions. Although we conducted comprehensive searches across all relevant databases, these methodological limitations and the variability among studies underscore the need for larger, more robust, and standardized investigations to confirm the predictive value of these biomarkers in this specific population.
5 Conclusion
Atherosclerosis originates early in life as a subtle, chronic inflammatory process that eventually leads to clinical CVD and heightens the risk of acute coronary events. Our systematic review and meta-analysis indicate that certain serum biomarkers may enhance risk assessment in middle-aged, asymptomatic individuals with autoimmune disorders. Specifically, hs-CRP, lupus anticoagulant, sVCAM-1, and antiphospholipid antibodies consistently correlate with future CVD events, suggesting they could be valuable for detecting subclinical atherosclerosis. Other markers, including IL-6, NT-proBNP, and fibrinogen, have also shown potential, although their predictive capabilities are often constrained by small sample sizes and significant variability among studies.
Despite exhaustive searches across several databases, inconsistencies in study methodologies, variations in follow-up periods, and differing adjustments for confounding factors—such as statin usage, body mass index, and lifestyle behaviors—make it difficult to reach definitive conclusions. Consequently, more prolonged and larger-scale investigations are essential to confirm these findings and fine-tune risk prediction models. Ultimately, the integration of these biomarkers with traditional risk factors could lead to more effective early detection and preventive measures, potentially alleviating the overall burden of CVD in this particularly vulnerable autoimmune cohort before broad clinical guidelines are established.
Data availability statement
The datasets presented in this article are not readily available because it was generated through a systematic review and meta-analysis, which required extensive effort in literature search, screening, data extraction, and quality assessment. The dataset contains structured information derived from published studies, some of which may have restrictions on redistribution of raw data due to copyright or publisher agreements. To ensure appropriate academic use, transparency, and correct interpretation, the dataset is made available on request to bona fide researchers rather than placed openly in the public domain. This also helps safeguard against misuse, misinterpretation, or incomplete citation of the work. Requests to access the datasets should be directed toaGFyc2hhd2FyZGhhbnJhbXRla2UyMEBnbWFpbC5jb20=.
Author contributions
WU: Software, Writing – original draft, Investigation, Data curation, Resources, Formal analysis, Visualization, Funding acquisition, Project administration, Conceptualization, Supervision, Writing – review & editing, Methodology, Validation. LH: Writing – review & editing, Data curation, Supervision, Funding acquisition, Visualization, Software. HR: Visualization, Formal analysis, Project administration, Writing – original draft, Funding acquisition, Investigation, Methodology, Software, Data curation, Writing – review & editing, Supervision, Validation, Resources, Conceptualization. JY: Writing – review & editing, Investigation, Project administration, Software, Supervision, Funding acquisition, Writing – original draft, Methodology, Validation, Visualization, Data curation, Conceptualization, Resources, Formal analysis. ZH: Software, Writing – review & editing, Resources, Funding acquisition, Writing – original draft, Formal analysis, Validation, Visualization, Data curation, Supervision, Investigation, Conceptualization, Project administration, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1598590/full#supplementary-material
Abbreviations
CVD, cardiovascular diseases; ROBINS, I—Risk of Bias in Non-Randomized Studies of Interventions; hsCRP, High Sensitive C-Reactive Protien; sVCAM, serum levels of soluble vascular cell adhesion molecule-1; Anti-CCP, Anti–cyclic citrullinated peptide; ADMA, Asymmetric Dimethylarginine; NT-ProBNP, N-terminal pro b-type natriuretic peptide; Anti dsDNA, Anti-double stranded DNA; TNF alpha, Tumour Necrosis Factor alpha; SLE, Systemic Lupus Erythematous; RA, Rheumatoid Factor; hsTn, High Sensitivity Troponin; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; HR, Hazard ratios; Hcy, Homocysteine; IL6, Interleukin 6; aPL, Antiphospholipid Antibodies; RF, Rheumatoid Factor; aCL, Anti-Citrullinated Protein Antibody.
References
1. Inam M, Samad Z, Vaughan EM, Almas A, Hanif B, Minhas AM, et al. Global cardiovascular research: gaps and opportunities. Curr Cardiol Rep. (2023) 25(12):1831–1838. doi: 10.1007/s11886-023-01996-2
2. Porsch F, Binder CJ. Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2024) 21(11):780–807. doi: 10.1038/s41569-024-01045-7
3. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9
4. Moran CA, Collins LF, Beydoun N, Mehta PK, Fatade Y, Isiadinso I, et al. Cardiovascular implications of immune disorders in women. Circ Res. (2022) 130(4):593–610. doi: 10.1161/circresaha.121.319877
5. Napiórkowska-Baran K, Schmidt O, Szymczak B, Lubański J, Doligalska A, Bartuzi Z. Molecular linkage between immune system disorders and atherosclerosis. Curr Issues Mol Biol. (2023) 45(11):8780–8815. doi: 10.3390/cimb45110552
6. Ekelund C, Dereke J, Nilsson C, Landin-Olsson M. Are soluble E-selectin, ICAM-1, and VCAM-1 potential predictors for the development of diabetic retinopathy in young adults, 15-34 years of age? A Swedish prospective cohort study. PLoS One. (2024) 19(6):e0304173. doi: 10.1371/journal.pone.0304173
7. Balaji D, Mohanasundaram K, Gopalakrishnan KV, Suthakaran PK. Anti-carbamylated protein antibodies positivity in rheumatoid arthritis and its association with rheumatoid factor and anti-cyclic citrullinated protein antibodies. Cureus. (2024) 16(7):e63652. doi: 10.7759/cureus.63652
8. Yan J, Yang S, Han L, Ba X, Shen P, Lin W, et al. Dyslipidemia in rheumatoid arthritis: the possible mechanisms. Front Immunol. (2023) 14:1254753. doi: 10.3389/fimmu.2023.1254753
9. Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. (1998) 188(5):985–90. doi: 10.1084/jem.188.5.985
10. Li LL, Luan ZQ, Tan Y, Wang H, Yu XJ, Qu Z, et al. Anti-complement factor H (CFH) autoantibodies could delay pristane-induced lupus nephritis. Immunol Res. (2023) 71(6):849–859. doi: 10.1007/s12026-023-09396-y
11. Garcia-Peña AA, Mariño A, Muñoz-Velandia OM, Saa-González D. Survival differences according to baseline characteristics of patient with advanced heart failure treated with levosimendan. SAGE Open Med. (2025) 13:20503121251357357. doi: 10.1177/20503121251357357
12. Ishigami J, Kim Y, Sang Y, Menez SP, Grams ME, Skali H, et al. High-Sensitivity cardiac troponin, natriuretic peptide, and long-term risk of acute kidney injury: the atherosclerosis risk in communities (ARIC) study. Clin Chem. (2021) 67(1):298–307. doi: 10.1093/clinchem/hvaa288
13. Borra SR, Panjiyar BK, Panicker SS, Danduboyina A. Role of cardiac biomarkers in the evaluation of rheumatoid arthritis: a systematic review. Cureus. (2023) 15(10):e47416. doi: 10.7759/cureus.47416
14. Ramteke H, Khan R, Thomas IJ. The role of advanced lipoproteins and novel biomarkers in forecasting cardiovascular events in asymptomatic populations: a systematic review. PROSPERO (2024). Available online at: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024611894 (Accessed March 04, 2025).
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
16. Prasad M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin Epidemiology Glob Health. (2024) 25:101484. doi: 10.1016/j.cegh.2023.101484
17. Aiewruengsurat D, Phongnarudech T, Liabsuetrakul T, Nilmoje T. Correlation of rheumatoid and cardiac biomarkers with cardiac anatomy and function in rheumatoid arthritis patients without clinically overt cardiovascular diseases: a cross-sectional study. Int J Cardiol Heart Vasc. (2022) 44:101161. doi: 10.1016/j.ijcha.2022.101161
18. Ajeganova S, Andersson ML, Frostegård J, Hafström I. Disease factors in early rheumatoid arthritis are associated with differential risks for cardiovascular events and mortality depending on age at onset: a 10-year observational cohort study. J Rheumatol. (2013) 40(12):1958–66. doi: 10.3899/jrheum.130365
19. Arnab B, Biswadip G, Arindam P, Shyamash M, Anirban G, Rajan P. Anti-CCP antibody in patients with established rheumatoid arthritis: does it predict adverse cardiovascular profile? J Cardiovasc Dis Res. (2013) 4(2):102–6. doi: 10.1016/j.jcdr.2012.09.003
20. El Bakry SA, Fayez D, Morad CS, Abdel-Salam AM, Abdel-Salam Z, ElKabarity RH, et al. Ischemic heart disease and rheumatoid arthritis: do inflammatory cytokines have a role? Cytokine. (2017) 96:228–233. doi: 10.1016/j.cyto.2017.04.026
21. Bana TM, Shey M, Samuels P, Jermy S, Kraus S, Ntusi N. Ab0648 cytokine and cardiovascular biomarker profiles in South African systemic lupus erythematosus patients with subclinical cardiovascular involvement characterised by multimodal imaging. Ann Rheum Dis. (2023) 82(Suppl 1):1526–7. doi: 10.1136/annrheumdis-2023-eular.5313
22. Barbarroja N, Pérez-Sanchez C, Ruiz-Limon P, Castro-Villegas C, Aguirre MA, Carretero R, et al. Anticyclic citrullinated protein antibodies are implicated in the development of cardiovascular disease in rheumatoid arthritis. Arterioscler Thromb Vasc Biol. (2014) 34(12):2706–16. doi: 10.1161/ATVBAHA.114.304475
23. Bengtsson C, Ohman ML, Nived O, Rantapää Dahlqvist S. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus. (2012) 21(4):452–9. doi: 10.1177/0961203311425524
24. Apraş Bilgen Ş, Kalyoncu U, Erden A, Canpolat U, Kılıç L, Karadağ Ö, et al. Assessment of subclinical atherosclerosis in psoriatic arthritis patients without clinically overt cardiovascular disease or traditional atherosclerosis risk factors. Turk Kardiyol Dern Ars. (2018) 46(5):358–365. doi: 10.5543/tkda.2018.36169
25. Borowiec A, Kowalik I, Chwyczko T, Jankowski J, Kandyba P, Życińska K. Predictors of cardiovascular events in patients with primary systemic vasculitis: a 5 years prospective observational study. Eur J Intern Med. (2021) 91:70–74. doi: 10.1016/j.ejim.2021.05.016
26. Bultink IE, Teerlink T, Heijst JA, Dijkmans BA, Voskuyl AE. Raised plasma levels of asymmetric dimethylarginine are associated with cardiovascular events, disease activity, and organ damage in patients with systemic lupus erythematosus. Ann Rheum Dis. (2005) 64(9):1362–5. doi: 10.1136/ard.2005.036137
27. Cioffi G, Viapiana O, Orsolini G, Ognibeni Sonographer F, Dalbeni A, Gatti D, et al. Left ventricular hypertrophy predicts poorer cardiovascular outcome in normotensive normoglycemic patients with rheumatoid arthritis. Int J Rheum Dis. (2021) 24(4):510–518. doi: 10.1111/1756-185X.14082
28. Davies R, Williams J, Sime K, Jin HS, Thompson C, Jordan L, et al. The role of interleukin-6 trans-signalling on cardiovascular dysfunction in inflammatory arthritis. Rheumatology (Oxford). (2021) 60(6):2852–2861. doi: 10.1093/rheumatology/keaa725
29. Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther. (2005) 7(3):R634–43. doi: 10.1186/ar1717
30. Dimitroulas T, Hodson J, Sandoo A, Smith J, Kitas GD. Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylarginines and systemic inflammation. Arthritis Res Ther. (2017) 19(1):32. doi: 10.1186/s13075-017-1232-1
31. Divard G, Abbas R, Chenevier-Gobeaux C, Chanson N, Escoubet B, Chauveheid MP, et al. High-sensitivity cardiac troponin T is a biomarker for atherosclerosis in systemic lupus erythematous patients: a cross-sectional controlled study. Arthritis Res Ther. (2017) 19(1):132. doi: 10.1186/s13075-017-1352-7
32. Elnabawi YA, Garshick MS, Tawil M, Barrett TJ, Fisher EA, Lo Sicco K, et al. Ccl20 in psoriasis: a potential biomarker of disease severity, inflammation, and impaired vascular health. J Am Acad Dermatol. (2021) 84(4):913–920. doi: 10.1016/j.jaad.2020.10.094
33. Fasano S, Margiotta DP, Gualtierotti R, Corrado A, Berardicurti O, Iacono D, et al. The incidence of cardiovascular events in Italian patients with systemic lupus erythematosus is lower than in North European and American cohorts: implication of disease-associated and traditional risk factors as emerged by a 16-year retrospective GIRRCS study: GIRRCS = Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale. Medicine (Baltimore). (2018) 97(15):e0370. doi: 10.1097/MD.0000000000010370
34. Finckh A, Courvoisier DS, Pagano S, Bas S, Chevallier-Ruggeri P, Hochstrasser D, et al. Evaluation of cardiovascular risk in patients with rheumatoid arthritis: do cardiovascular biomarkers offer added predictive ability over established clinical risk scores? Arthritis Care Res (Hoboken). (2012) 64(6):817–25. doi: 10.1002/acr.21631
35. Pocovi-Gerardino G, Correa-Rodríguez M, Rubio JC, Fernández RR, Amada MM, Caparros MC, et al. The relationships of high-sensitivity C-reactive protein and homocysteine levels with disease activity, damage accrual, and cardiovascular risk in systemic lupus erythematosus. Biol Res Nurs. (2020) 22(2):169–177. doi: 10.1177/1099800419889192
36. Mok CC, Birmingham DJ, Ho LY, Hebert LA, Rovin BH. High-sensitivity C-reactive protein, disease activity, and cardiovascular risk factors in systemic lupus erythematosus. Arthritis Care Res (Hoboken). (2013) 65(3):441–7. doi: 10.1002/acr.21841
37. Lo Gullo A, Mandraffino G, Imbalzano E, Mamone F, Aragona CO, D'Ascola A, et al. Toll-like receptor 3 and interleukin 1β expression in CD34+ cells from patients with rheumatoid arthritis: association with inflammation and vascular involvement. Clin Exp Rheumatol. (2014) 32(6):922–9.25436985
38. Gustafsson J, Gunnarsson I, Börjesson O, Pettersson S, Möller S, Fei GZ, et al. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus—a prospective cohort study. Arthritis Res Ther. (2009) 11(6):R186. doi: 10.1186/ar2878
39. Gustafsson JT, Simard JF, Gunnarsson I, Elvin K, Lundberg IE, Hansson LO, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther. (2012) 14(2):R46. doi: 10.1186/ar3759
40. Huang C, Li Y, Wang Z, Lin S, Zhao JL, Wang Q, et al. Predicting the risk of cardiovascular and cerebrovascular event in systemic lupus erythematosus: a Chinese SLE treatment and research group study XXVI. RMD Open. (2024) 10(3):e004425. doi: 10.1136/rmdopen-2024-004425
41. Huang C, Ding Y, Chen Z, Wu L, Wei W, Zhao C, et al. Future atherosclerotic cardiovascular disease in systemic lupus erythematosus based on CSTAR (XXVIII): the effect of different antiphospholipid antibodies isotypes. BMC Med. (2025) 23(1):8. doi: 10.1186/s12916-024-03843-9
42. Icli A, Cure E, Cure MC, Uslu AU, Balta S, Mikhailidis DP, et al. Endocan levels and subclinical atherosclerosis in patients with systemic lupus erythematosus. Angiology. (2016) 67(8):749–55. doi: 10.1177/0003319715616240
43. Karpouzas G, Ormseth S, Hernandez E, Budoff M. Highly-Sensitive cardiac troponin-I and Beta-2-glycoprotein-I IgA antibodies may guide atherosclerosis screening and surveillance in rheumatoid arthritis. Med Res Arch. (2023) 11(1). doi: 10.18103/mra.v11i1.3536
44. Karpouzas GA, Papotti B, Ormseth SR, Palumbo M, Hernandez E, Adorni MP, et al. Changes in serum cholesterol loading capacity are linked to coronary atherosclerosis progression in rheumatoid arthritis. RMD Open. (2024) 10(4):e004991. doi: 10.1136/rmdopen-2024-004991
45. Khairy N, Ezzat Y, Naeem N, Taha R, Wesam R. Atherosclerosis biomarkers in female systemic lupus erythematosus patients with and without cardiovascular diseases. Egypt Rheumatol. (2017) 39(1):7–12. doi: 10.1016/j.ejr.2016.03.003
46. Kiani AN, Mahoney JA, Petri M. Asymmetric dimethylarginine is a marker of poor prognosis and coronary calcium in systemic lupus erythematosus. J Rheumatol. (2007) 34(7):1502–5.17611963
47. Kobayashi M, Ferreira MB, Costa RQ, Fonseca T, Oliveira JC, Marinho A, et al. Circulating biomarkers and cardiac structure and function in rheumatoid arthritis. Front Cardiovasc Med. (2021) 8:754784. doi: 10.3389/fcvm.2021.754784
48. Kwaśny-Krochin B, Głuszko P, Undas A. Plasma asymmetric dimethylarginine in active rheumatoid arthritis: links with oxidative stress and inflammation. Pol Arch Med Wewn. (2012) 122(6):270–6. doi: 10.20452/pamw.1277
49. Liakouli V, Verde I, Ruscitti P, Di Vico C, Ruggiero A, Mauro D, et al. Clinical and subclinical atherosclerosis in patients with systemic sclerosis: an observational, multicentre study of GIRRCS (gruppo italiano di ricerca in reumatologia clinica e sperimentale). Clin Exp Rheumatol. (2024) 42(8):1645–1655. doi: 10.55563/clinexprheumatol/zr8j5p
50. López-Longo FJ, Oliver-Miñarro D, de la Torre I, González-Díaz de Rábago E, Sánchez-Ramón S, Rodríguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. (2009) 61(4):419–24. doi: 10.1002/art.24390
51. Mackey RH, Kuller LH, Deane KD, Walitt BT, Chang YF, Holers VM, et al. Rheumatoid arthritis, anti-cyclic citrullinated peptide positivity, and cardiovascular disease risk in the Women’s health initiative. Arthritis Rheumatol. (2015) 67(9):2311–22. doi: 10.1002/art.39198
52. McMahon M, Skaggs BJ, Grossman JM, Sahakian L, Fitzgerald J, Wong WK, et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. (2014) 66(1):130–9. doi: 10.1002/art.38204
53. Vázquez-Del Mercado M, Gomez-Bañuelos E, Chavarria-Avila E, Cardona-Muñoz E, Ramos-Becerra C, Alanis-Sanchez A, et al. Disease duration of rheumatoid arthritis is a predictor of vascular stiffness: a cross-sectional study in patients without known cardiovascular comorbidities: a STROBE-compliant article. Medicine (Baltimore). (2017) 96(33):e7862. doi: 10.1097/MD.0000000000007862
54. Mongin D, Pagano S, Lamacchia C, Juillard C, Antinori-Malaspina P, Dan D, et al. Anti-apolipoprotein A-1 IgG, incident cardiovascular events, and lipid paradox in rheumatoid arthritis. Front Cardiovasc Med. (2024) 11:1386192. doi: 10.3389/fcvm.2024.1386192
55. Mansour A, Ibrahim MT, EIshahawy EM, Mahmoud A, Nasr R. Biomarkers of myocardial injury in lupus nephritis. J Egypt Soc Nephrol Transplant. (2021) 21(2):80. doi: 10.4103/jesnt.jesnt_27_20
56. Nikiphorou E, de Lusignan S, Mallen CD, Khavandi K, Bedarida G, Buckley CD, et al. Cardiovascular risk factors and outcomes in early rheumatoid arthritis: a population-based study. Heart. (2020) 106(20):1566–1572. doi: 10.1136/heartjnl-2019-316193
57. Nowak B, Madej M, Łuczak A, Małecki R, Wiland P. Disease activity, oxidized-LDL fraction and anti-oxidized LDL antibodies influence cardiovascular risk in rheumatoid arthritis. Adv Clin Exp Med. (2016) 25(1):43–50. doi: 10.17219/acem/29847
58. Pàmies A, Llop D, Ibarretxe D, Rosales R, Girona J, Masana L, et al. Enhanced association of novel cardiovascular biomarkers fetuin-A and catestatin with serological and inflammatory markers in rheumatoid arthritis patients. Int J Mol Sci. (2024) 25(18):9910. doi: 10.3390/ijms25189910
59. Parker B, Al-Husain A, Pemberton P, Yates AP, Ho P, Gorodkin R, et al. Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus. Ann Rheum Dis. (2014) 73(6):1144–50. doi: 10.1136/annrheumdis-2012-203028
60. Perna M, Roman MJ, Alpert DR, Crow MK, Lockshin MD, Sammaritano L, et al. Relationship of asymmetric dimethylarginine and homocysteine to vascular aging in systemic lupus erythematosus patients. Arthritis Rheum. (2010) 62(6):1718–22. doi: 10.1002/art.27392
61. Robinson G, Radziszewska A, Wincup C, Ciurtin C, Ioannou Y, Torra IP, et al. Op0148 metabolomics in juvenile-onset SLE: identifying new biomarkers to predict cardiovascular risk. Ann Rheum Dis. (2019) 78:149–150. doi: 10.1136/annrheumdis-2019-eular.4067
62. Wright J. Functional independence measure. In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer (2011). p. 1112–3. doi: 10.1007/978-0-387-79948-3_1810
63. Rodrigues P, Ferreira B, Fonseca T, Costa RQ, Cabral S, Pinto JL, et al. Subclinical ventricular dysfunction in rheumatoid arthritis. Int J Cardiovasc Imaging. (2020) 37(3):847–859. doi: 10.1007/s10554-020-02057-3.hal-03009505
64. Roghani SA, Shamsi A, Jalili C, Jalili F, Lotfi R, Garman N, et al. Interleukin-6 positively correlates with cardiovascular disease predictor algorithms and biomarker in rheumatoid arthritis patients. J Cell Mol Med. (2024) 28(16):e70028. doi: 10.1111/jcmm.70028
65. Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. (2005) 46(1):194–9. doi: 10.1161/01.HYP.0000168055.89955.db
66. Ruscitti P, Margiotta DPE, Macaluso F, Iacono D, D'Onofrio F, Emmi G, et al. Subclinical atherosclerosis and history of cardiovascular events in Italian patients with rheumatoid arthritis: results from a cross-sectional, multicenter GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Medicine (Baltimore). (2017) 96(42):e8180. doi: 10.1097/MD.0000000000008180
67. Ruscitti P, Cipriani P, Liakouli V, Iacono D, Pantano I, Margiotta DPE, et al. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther. (2019) 21(1):204. doi: 10.1186/s13075-019-1975-y
68. Rydell E, Jacobsson LT, Saxne T, Turesson C. Cardiovascular disease risk in early rheumatoid arthritis: the impact of cartilage oligomeric matrix protein (COMP) and disease activity. BMC Rheumatol. (2023) 7(1):43. doi: 10.1186/s41927-023-00367-2
69. Saadany HE, El-Sergany M, Kasem E, El-Batch MM, Zakaria SS, Mourad H, et al. Biochemical and genetic risk factors for atherosclerosis in systemic lupus erythematosus. Egypt Rheumatol. (2011) 33(1):35–43. doi: 10.1016/j.ejr.2010.11.001
70. Sandoo A, Dimitroulas T, Hodson J, Smith JP, Douglas KM, Kitas GD. Cumulative inflammation associates with asymmetric dimethylarginine in rheumatoid arthritis: a 6 year follow-up study. Rheumatology. (2014) 54(7):1145–52. doi: 10.1093/rheumatology/keu349
71. Santos MJ, Carmona-Fernandes D, Canhão H, Canas da Silva J, Fonseca JE, Gil V. Early vascular alterations in SLE and RA patients–a step towards understanding the associated cardiovascular risk. PLoS One. (2012) 7(9):e44668. doi: 10.1371/journal.pone.0044668
72. Sari I, Kebapcilar L, Alacacioglu A, Bilgir O, Yildiz Y, Taylan A, et al. Increased levels of asymmetric dimethylarginine (ADMA) in patients with ankylosing spondylitis. Intern Med. (2009) 48(16):1363–8. doi: 10.2169/internalmedicine.48.2193
73. Şentürk T, Yılmaz N, Sargın G, Köseoğlu K, Yenisey Ç. Relationship between asymmetric dimethylarginine and endothelial dysfunction in patients with rheumatoid arthritis. Eur J Rheumatol. (2016) 3(3):106–108. doi: 10.5152/eurjrheum.2016.15096
74. Skaggs BJ, Grossman J, Sahakian L, Perry L, FitzGerald J, Charles-Schoeman C, et al. A panel of biomarkers associates with increased risk for cardiovascular events in women with systemic lupus erythematosus. ACR Open Rheumatol. (2021) 3(4):209–220. doi: 10.1002/acr2.11223
75. Södergren A, Karp K, Bengtsson C, Möller B, Rantapää-Dahlqvist S. Wållberg-Jonsson S. Biomarkers associated with cardiovascular disease in patients with early rheumatoid arthritis. PLoS One. (2019) 14(8):e0220531. doi: 10.1371/journal.pone.0220531
76. Solow EB, Yu F, Thiele GM, Sokolove J, Robinson WH, Pruhs ZM, et al. Vascular calcifications on hand radiographs in rheumatoid arthritis and associations with autoantibodies, cardiovascular risk factors and mortality. Rheumatology (Oxford). (2015) 54(9):1587–95. doi: 10.1093/rheumatology/kev027
77. Svenungsson E, Jensen-Urstad K, Heimbürger M, Silveira A, Hamsten A, de Faire U, et al. Risk factors for cardiovascular disease in systemic lupus erythematosus. Circulation. (2001) 104(16):1887–93. doi: 10.1161/hc4101.097518
78. Tam LS, Fan B, Li EK, Thomas GN, Yim SF, Haines CJ, et al. Patients with systemic lupus erythematosus show increased platelet activation and endothelial dysfunction induced by acute hyperhomocysteinemia. J Rheumatol. (2003) 30(7):1479–84.12858444
79. Patiño-Trives AM, Pérez-Sánchez C, Pérez-Sánchez L, Luque-Tévar M, Ábalos-Aguilera MC, Alcaide-Ruggiero L, et al. Anti-dsDNA antibodies increase the cardiovascular risk in systemic lupus erythematosus promoting a distinctive immune and vascular activation. Arterioscler Thromb Vasc Biol. (2021) 41(9):2417–2430. doi: 10.1161/ATVBAHA.121.315928
80. Turiel M, Atzeni F, Tomasoni L, de Portu S, Delfino L, Bodini BD, et al. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford). (2009) 48(7):834–9. doi: 10.1093/rheumatology/kep082
81. Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, et al. Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol. (2011) 58(10):1025–33. doi: 10.1016/j.jacc.2011.05.028
82. Vuilleumier N, Bas S, Pagano S, Montecucco F, Guerne PA, Finckh A, et al. Anti-apolipoprotein A-1 IgG predicts major cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. (2010) 62(9):2640–50. doi: 10.1002/art.27546
83. wahab MA, Laban AE, Hasan AA, Darweesh AF. The clinical value of anti-cyclic citrullinated peptide (anti-ccp) antibodies and insulin resistance (IR) in detection of early and subclinical atherosclerosis in rheumatoid arthritis (RA). Egypt Heart J. (2016) 68(2):109–16. doi: 10.1016/j.ehj.2015.01.001
84. Wigren M, Svenungsson E, Mattisson IY, Gustafsson JT, Gunnarsson I, Zickert A, et al. Cardiovascular disease in systemic lupus erythematosus is associated with increased levels of biomarkers reflecting receptor-activated apoptosis. Atherosclerosis. (2018) 270:1–7. doi: 10.1016/j.atherosclerosis.2018.01.022
85. Karakasis P, Patoulias D, Stachteas P, Lefkou E, Dimitroulas T, Fragakis N. Accelerated atherosclerosis and management of cardiovascular risk in autoimmune rheumatic diseases: an updated review. Curr Probl Cardiol. (2023) 48(12):101999. doi: 10.1016/j.cpcardiol.2023.101999
86. Wong ND, Budoff MJ, Ferdinand K, Graham IM, Michos ED, Reddy T, et al. Atherosclerotic cardiovascular disease risk assessment: an American society for preventive cardiology clinical practice statement. Am J Prev Cardiol. (2022) 10:100335. doi: 10.1016/j.ajpc.2022.100335
87. Gusev E, Sarapultsev A. Atherosclerosis and inflammation: insights from the theory of general pathological processes. Int J Mol Sci. (2023) 24(9):7910. doi: 10.3390/ijms24097910
88. Noothi SK, Ahmed MR, Agrawal DK. Residual risks and evolving atherosclerotic plaques. Mol Cell Biochem. (2023) 478(12):2629–2643. doi: 10.1007/s11010-023-04689-0
89. Wong YK, Tse HF. Circulating biomarkers for cardiovascular disease risk prediction in patients with cardiovascular disease. Front Cardiovasc Med. (2021) 8:713191. doi: 10.3389/fcvm.2021.713191
90. Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, et al. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. (2004) 94(1):20–4. doi: 10.1016/j.amjcard.2004.03.023
91. Tomasik T, Krzysztoń J, Dubas-Jakóbczyk K, Kijowska V, Windak A. The systematic coronary risk evaluation (SCORE) for the prevention of cardiovascular diseases. Does evidence exist for its effectiveness? A systematic review. Acta Cardiol. (2017) 72(4):370–379. doi: 10.1080/00015385.2017.1335052
92. Osoro I, Kumar R, Sharma A. Ten-year risk assessment for cardiovascular diseases using ASCVD risk estimator plus: outcomes from hypertension and diabetes patients. Diabetol Metab Syndr. (2023) 15(1):216. doi: 10.1186/s13098-023-01170-2
93. Tamarappoo BK, Lin A, Commandeur F, McElhinney PA, Cadet S, Goeller M, et al. Machine learning integration of circulating and imaging biomarkers for explainable patient-specific prediction of cardiac events: a prospective study. Atherosclerosis. (2021) 318:76–82. doi: 10.1016/j.atherosclerosis.2020.11.008
94. Leite JMRS, Pereira JL, Damasceno NRT, Soler JMP, Fisberg RM, Rogero MM, et al. Association of dyslipidemia with single nucleotide polymorphisms of the cholesteryl ester transfer protein gene and cardiovascular disease risk factors in a highly admixed population. Clin Nutr ESPEN. (2023) 58:242–252. doi: 10.1016/j.clnesp.2023.10.002
95. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 Aha/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation. (2023) 148(9):e9–119. doi: 10.1161/CIR.0000000000001168 (Erratum in: Circulation. 2023 Sep 26;148(13):e148. doi: 10.1161/CIR.0000000000001183. Erratum in: Circulation. 2023 Dec 5;148(23):e186. doi: 10.1161/CIR.0000000000001195. PMID: 37471501).37471501
96. Anyfanti P, Dara A, Angeloudi E, Bekiari E, Dimitroulas T, Kitas GD. Monitoring and managing cardiovascular risk in immune mediated inflammatory diseases. J Inflamm Res. (2021) 14:6893–6906. doi: 10.2147/JIR.S276986
97. Greco D, Gualtierotti R, Agosti P, Adorni MP, Ingegnoli F, Rota M, et al. Anti-atherogenic modification of Serum lipoprotein function in patients with rheumatoid arthritis after tocilizumab treatment, a pilot study. J Clin Med. (2020) 9(7):2157. doi: 10.3390/jcm9072157
98. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377(12):1119–1131. doi: 10.1056/NEJMoa1707914
99. Ridker PM, JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. (2003) 108(19):2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6
100. Echouffo-Tcheugui JB, Zhang S, Daya N, McEvoy JW, Tang O, Juraschek SP, et al. NT-proBNP and all-cause and cardiovascular mortality in US adults: a prospective cohort study. J Am Heart Assoc. (2023) 12(11):e029110. doi: 10.1161/JAHA.122.029110
101. Amaya-Amaya J, Montoya-Sánchez L, Rojas-Villarraga A. Cardiovascular involvement in autoimmune diseases. BioMed Res Int. (2014) 2014:367359. doi: 10.1155/2014/367359
102. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. (2020) 4:100130. doi: 10.1016/j.ajpc.2020.100130
103. McGranaghan P, Saxena A, Rubens M, Radenkovic J, Bach D, Schleußner L, et al. Predictive value of metabolomic biomarkers for cardiovascular disease risk: a systematic review and meta-analysis. Biomarkers. (2020) 25(2):101–11. doi: 10.1080/1354750x.2020.1716073
Keywords: autoimmune disease, cardiovascular disease, advanced biomarkers, prediction, atherosclerosis
Citation: U W, Hong L, Ramteke HD, Yang J and He Z (2025) Systematic review and meta-analysis of cardiovascular associated biomarkers in adults with asymptomatic autoimmune diseases. Front. Cardiovasc. Med. 12:1598590. doi: 10.3389/fcvm.2025.1598590
Received: 23 March 2025; Accepted: 8 September 2025;
Published: 16 October 2025.
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Polona Žigon, University Medical Centre Ljubljana, SloveniaHasan Alghetaa, University of Baghdad, Iraq
Copyright: © 2025 U, Hong, Ramteke, Yang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei He, aGV6aGl3ZWlAZ2RtdS5lZHUuY24=
†These authors share first authorship
 Waiseng U
Waiseng U Lin Hong2,†
Lin Hong2,† Harshawardhan Dhanraj Ramteke
Harshawardhan Dhanraj Ramteke Jiaen Yang
Jiaen Yang