Abstract
Heart Failure with Preserved Ejection Fraction (HFpEF) is a heterogeneous syndrome characterized by systemic multiorgan dysfunction, and exercise rehabilitation has emerged as a promising non-pharmacological intervention. This review synthesizes current evidence on the pathophysiological mechanisms underlying exercise intolerance in HFpEF and evaluates the therapeutic efficacy of exercise-based interventions. Key mechanisms include myocardial stiffness due to chronic inflammation, coronary microvascular dysfunction, skeletal muscle mitochondrial impairment, and endothelial dysfunction. Clinical studies indicate that tailored exercise regimens (e.g., combined aerobic-resistance training) improve peak oxygen consumption, 6 min walking distance, and quality of life through multi-organ adaptations: enhanced cardiac output reserve, skeletal muscle metabolic remodeling, and reduced systemic inflammation. However, challenges persist in optimizing exercise prescriptions for phenotypically diverse HFpEF subpopulations (e.g., obese, elderly frail). Future research must prioritize phenotype-specific protocols, validate long-term outcomes (mortality, hospitalization), and integrate biomarkers (e.g., H2FPEF score) with digital health technologies to advance precision rehabilitation strategies. This review highlights the imperative for mechanistic insights to guide clinical translation in HFpEF management.
1 Introduction
Heart failure with preserved ejection fraction (HFpEF), is defined as a left ventricular ejection fraction (LVEF) ≥50% with accompanying symptoms and/or signs, in the presence of objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/raised LV filling pressures, including raised natriuretic peptides (1). HFpEF constituting nearly 50% of heart failure cases, is a multisystem disorder driven by aging, obesity, and metabolic dysfunction (1–3). Unlike heart failure with reduced ejection fraction (HFrEF), HFpEF involves systemic pathophysiology such as myocardial stiffness, skeletal muscle mitochondrial impairment, endothelial dysfunction, and neurohormonal activation, culminating in profound exercise intolerance and poor prognosis (4–6). Despite pharmacological advances, no therapies improve survival, underscoring the unmet need for effective interventions (7). Exercise rehabilitation emerges as a pivotal non-pharmacological strategy, demonstrating improvements in functional capacity of peak oxygen consumption (VO2peak), quality of life, and hemodynamic profiles through cardiac-skeletal muscle adaptations and anti-inflammatory effects (8, 9). However, evidence gaps persist: most trials focus on short-term outcomes (3–6 months), while impacts on mortality/hospitalization remain unproven (10, 11). HFpEF's heterogeneity, obese, elderly, or amyloidosis subphenotypes, demands precision approaches to optimize efficacy-safety balances (12, 13). This review synthesizes mechanisms of exercise intolerance, evaluates therapeutic evidence, and proposes a roadmap integrating phenomapping, digital monitoring, and tailored regimens to transform HFpEF rehabilitation from symptom management to disease modification.
2 Epidemiological characteristics of HFpEF
HFpEF constitutes approximately 50% of heart failure cases, with rising prevalence linked to aging, obesity, and metabolic comorbidities (2, 3). Large cohort studies show comparable HFpEF/HFrEF incidence (4). Women exhibit higher HFpEF risk, tied to estrogen signaling and pregnancy complications (e.g., preeclampsia) (5, 6).
Independent risk factors for HFpEF include advanced age, obesity, diabetes, hypertension, and atrial fibrillation(AF) (1). Current smoking shows dose-response HFpEF/HFrEF risk. Cessation reduces but residual risk persists decades post-cessation (7). Infertility history, also elevate HFpEF risk (8). Racial disparities are evident, with African American populations showing heightened left ventricular hypertrophy and concentric remodeling, predisposing them to HFpEF (9).
HFpEF manifests as a multisystem disorder, involving skeletal muscle dysfunction, peripheral vascular abnormalities, pulmonary congestion, renal impairment, and cerebral hemodynamic alterations (1). Comorbid cardiovascular conditions, including secondary tricuspid regurgitation (STR) and pulmonary hypertension (PH), are prevalent. Approximately 35% of severe STR cases are attributable to HFpEF, with concomitant STR increasing adverse event risks (10). HFpEF also correlates strongly with stroke; post-stroke patients exhibit elevated HFpEF hospitalization rates and cardiovascular event incidence (11).
3 Pathophysiological characteristics of HFpEF and mechanisms of exercise intolerance
3.1 Pathophysiological characteristics of HFpEF
The pathophysiological landscape of HFpEF is characterized by multisystem organ involvement, extending beyond cardiac dysfunction to encompass skeletal muscle metabolic derangements, pulmonary vascular congestion, renal impairment, peripheral endothelial dysfunction, and neurovascular dysregulation (2) (Figure 1). Central to its pathogenesis is the chronic low-grade inflammation and metabolic dysregulation. Obesity, diabetes, and hypertension drive visceral adipose tissue (VAT) and epicardial adipose tissue (EAT) expansion, which secretes proinflammatory cytokines [e.g., Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α)] and profibrotic mediators, ultimately inducing myocardial stiffness augmentation and diastolic impairment (12).
Figure 1
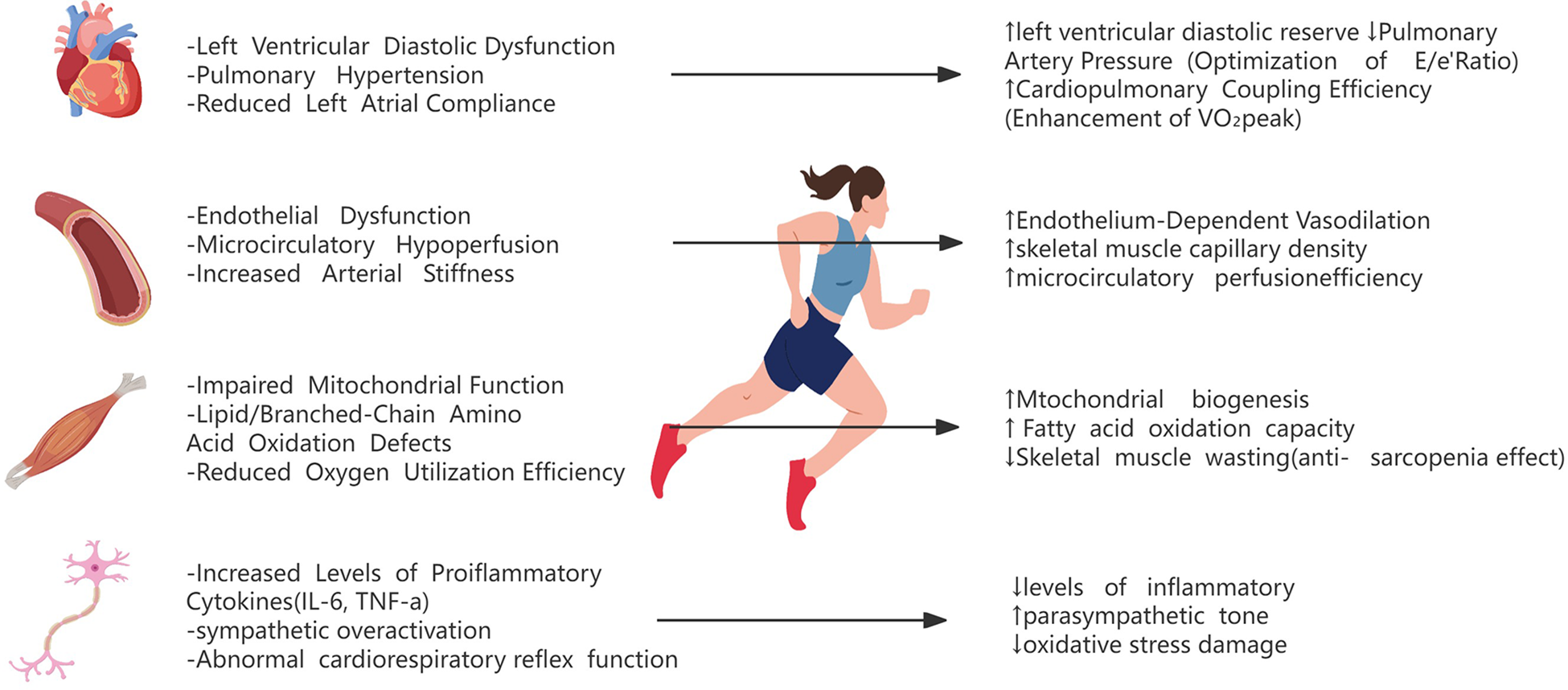
Pathophysiological mechanisms of exercise intolerance and exercise interventions in HFpEF.
Coronary microvascular dysfunction (CMD) affects 50% of HFpEF patients, driving myocardial ischemia, calcium mishandling, and impaired relaxation (13). A multicenter prospective cohort study demonstrated that 91% of HFpEF patients exhibited either epicardial coronary artery disease (CAD), CMD, or both. Among those without obstructive CAD, >80% displayed endothelium-independent or endothelium-dependent CMD (13).
Structural cardiac remodeling, including left ventricular hypertrophy (LVH) and left atrial myopathy, further typifies HFpEF. The hemodynamic hallmark of HFpEF, elevated left ventricular filling pressures and exertional intolerance, primarily stems from left ventricular diastolic dysfunction, arising from impaired relaxation kinetics due to dysregulated sarcoplasmic reticulum calcium reuptake (SERCA2a dysfunction) (14), cardiomyocyte hypertrophy with altered titin isoform expression, and extracellular matrix (ECM) remodeling via collagen crosslinking (15, 16), compounded by left atrial (LA) decompensation manifested as reduced LA compliance from interstitial fibrosis (17), impaired LA reservoir/conduit function (18), and diminished left atrial emptying fraction with compliance reduction—all correlating with elevated pulmonary capillary wedge pressure(PCWP) (19).
Notably, transthyretin amyloid cardiomyopathy (ATTR-CM) demonstrates high prevalence among elderly HFpEF cohorts, with cardiac amyloid deposition directly compromising diastolic mechanics through myocyte infiltration and restrictive physiology (19).
3.2 Mechanisms of exercise intolerance in HFpEF
The mechanistic basis of exercise intolerance in HFpEF arises from multilevel pathophysiological derangements, with skeletal muscle dysfunction constituting an essential component. Impaired skeletal muscle bioenergetics—characterized by reduced oxidative capacity, diminished mitochondrial content, and aberrant mitochondrial dynamics (fusion/fission imbalance)—significantly contributes to exertional limitation in elderly HFpEF cohorts (20). Skeletal muscle phenotype switching further exacerbates functional decline, evidenced by selective reduction of type I oxidative muscle fibers (reliant on mitochondrial ATP production) in HFpEF patients (21). Mitochondrial dysfunction manifests as network fragmentation, decreased mitochondrial cross-sectional area, and downregulation of fusion regulators (Mitofusin 1, Mitofusin 2, Optic atrophy 1), collectively impairing oxidative phosphorylation capacity (22, 23). These defects potentiate calcium mishandling, oxidative stress overload, and nitric oxide (NO) depletion, driving endothelial/cardiomyocyte uncoupling (24). Concomitant obesity-related myosteatosis and muscle atrophy further compromise oxygen utilization efficiency (25, 26).
Abnormal cardiopulmonary interactions exacerbate hemodynamic compromise through three interlinked mechanisms: (1) Diastolic reserve exhaustion during exertion elevates left atrial pressure, precipitating post-capillary PH via pulmonary venous congestion—a phenotype observed in approximately 80% of HFpEF patients (27, 28); (2) This PH-driven right ventricular afterload augmentation disrupts ventilation-perfusion (V/Q) matching through altered pulmonary vascular impedance and right-left ventricular interdependence (2, 19); (3) Concomitant inspiratory muscle weakness, independent of cardiac loading conditions, directly correlates with reduced exercise capacity by impairing respiratory pump efficiency and oxygen delivery (29).
Peripheral vascular dysfunction encompasses two interrelated pathological axes: firstly, endothelial-dependent vasodilatory impairment driven by reduced nitric oxide (NO) bioavailability restricts microvascular reserve capacity during exertion (30); concurrently, arterial stiffening—quantified invasively through elevated aortic impedance—exacerbates ventricular-arterial uncoupling, thereby diminishing peak oxygen consumption (VO2peak) (7, 31). Critically, exercise-induced exacerbation of arterial stiffness demonstrates a direct linear association with pathological increments in pulmonary capillary wedge pressure (PCWP) during exertion, thereby contributing to diminished peak oxygen uptake (VO2peak) through ventricular-arterial decoupling and impaired cardiopulmonary efficiency (32).
Autonomic dysregulation perpetuates this vicious cycle through three sequential pathological cascades: Initially, sympathetic nervous system and renin-angiotensin-aldosterone system overactivation initiates a maladaptive cascade—inducing vasoconstrictive responses and aldosterone-mediated myocardial fibrosis (33); subsequently, chronic norepinephrine excess triggers β-adrenergic receptor downregulation via GRK2-mediated desensitization, while concurrently promoting cardiomyocyte apoptosis through calcium/calpain pathway activation (34); compounding these effects, hypoxia-induced lipotoxic metabolites (e.g., free fatty acids) directly inhibit mitochondrial complex I/III activity, exacerbating oxidative phosphorylation failure during energy-demanding states (25, 35).
4 Evidence of efficacy of exercise rehabilitation on HFpEF
The pathophysiological mechanism of HFpEF involves multisystem abnormalities, providing potential targets for exercise-based rehabilitation interventions. Although randomized controlled trials (RCTs) directly evaluating exercise rehabilitation remain limited, accumulating evidence indirectly supports its clinical utility. In the ejection fraction subgroup analysis of the REHAB-HF trial, while the prespecified interaction test lacked statistical significance (interaction P > 0.1), the intervention demonstrated a clinically meaningful improvement trend favoring the HFpEF subgroup. Specifically, Short Physical Performance Battery (SPPB) scores in rehabilitation-treated HFpEF patients increased by +1.9 points from baseline at 3-month follow-up, surpassing improvements observed in HFrEF counterparts, suggesting enhanced responsiveness to multidisciplinary rehabilitation strategies in this population (36).
The phenotypic heterogeneity of HFpEF necessitates individualized comprehensive care. As a cornerstone of lifestyle modification, exercise rehabilitation potentiates pharmacotherapy through synergistic blood pressure reduction and glycemic control (2). Observational cohort data indicate substantially elevated post-hospitalization venous thromboembolism (VTE) risk in HFpEF (adjusted HR = 3.13), with exercise potentially mitigating this risk via hemodynamic optimization and coagulation cascade modulation (31). Furthermore, 62% of new-onset atrial fibrillation (AF) cases exhibit high-risk HFpEF phenotypes (stratified by H2FPEF score), where structured exercise may attenuate arrhythmic progression through atrial unloading mechanisms (2, 37).
Future research must focus on three priorities: (1) developing phenotype-specific exercise prescriptions by stratifying subtypes (e.g., obesity or arterial stiffness-predominant phenotypes) for tailored regimens (37); (2) validating biomarker-guided efficacy, emphasizing exercise-enhanced cardiac function (e.g., global longitudinal strain) and refining risk stratification using tools like the H2FPEF score (2, 4, 8, 31); and (3) addressing adherence challenges in frail elderly, particularly in those with PH or ATTR-CM (19). While exercise may improve HFpEF prognosis via multimodal mechanisms, large RCTs are needed for confirmation. Integrating H2FPEF risk models with imaging biomarkers (e.g., speckle-tracking echocardiography) will advance precision rehabilitation strategies (2, 4, 5, 31).
5 Multimodal mechanisms of exercise rehabilitation in ameliorating HFpEF
Exercise rehabilitation improves the pathophysiological status of HFpEF patients through the synergistic effect of the central and peripheral multiple systems. Regarding central mechanisms, patients with HFpEF exhibit compromised cardiopulmonary reserve capacity and impaired ventriculoarterial coupling. Regular aerobic training may mitigate exercise-induced elevation in left ventricular filling pressure and abnormal pulmonary vascular pressures through reducing resting heart rate and ameliorating hemodynamic derangements (2). Exercise training enhances exercise-related cardiac output through coordinated optimization of preload regulation (e.g., reduced PCWP) and increased cardiac index (CI) (38). In high-risk heart failure patients, exercise-trained cohorts demonstrated significant reductions in PCWP during mild exercise (25 W), while exhibiting increased CI from 2.9 to 3.4 L/min/m² (39). These hemodynamic adaptations are mechanistically linked to enhanced cardiac reserve capacity, potentially involving improved cardiomyocyte calcium handling and optimized ventriculoarterial coupling (40, 41). Concurrently, exercise training restores endothelium-dependent vasodilation capacity (manifested as 2.5%–4.1% improvement in flow-mediated dilation) and ameliorates peripheral vascular resistance (41, 42), mechanisms associated with enhanced nitric oxide bioavailability, attenuation of oxidative stress markers (e.g., malondialdehyde), and improved endothelial progenitor cell functionality (43).
The peripheral mechanism in HFpEF is fundamentally characterized by skeletal muscle structural degeneration and metabolic remodeling. In HFpEF patients, skeletal muscles consistently demonstrate three cardinal pathological features: a 20%–50% reduction in capillary density, impaired mitochondrial oxidative phosphorylation capacity, and dysregulated autophagic flux (44, 45). This myopathic phenotype manifests clinically as mitochondrial dysfunction coupled with microcirculatory disturbances, collectively contributing to diminished exercise tolerance. Notably, exercise-based rehabilitation has been shown to ameliorate peripheral oxygen utilization through dual mechanisms: enhancing skeletal muscle oxidative metabolic capacity and stimulating angiogenesis (2). At the systemic level, exercise exerts metabolic-inflammatory regulatory effects on core risk factors including obesity and insulin resistance. Specifically, it reduces visceral adiposity deposition, suppresses proinflammatory cytokine release (e.g., IL-6 and TNF-α), and improves both endothelial function and insulin sensitivity through pleiotropic pathways (2, 5).
The multi-system synergistic interactions confer substantial clinical benefits in HFpEF management. Exercise rehabilitation induces a 35–50 m improvement in 6 min walking distance and 15–20-point elevation in KCCQ scores, demonstrating both functional and quality-of-life enhancements (46, 47). Beyond physiological adaptations, the therapeutic effects involve psychoneuroendocrine modulation, including anxiety alleviation through autonomic nervous system rebalancing (evidenced by increased heart rate variability) and reinforcement of self-efficacy (46). Crucially, longitudinal exercise interventions reduce cardiovascular hospitalization rates by 20%–30%, achieved via multi-organ protective mechanisms: suppression of systemic inflammation (0.5–1.2 mg/dl decrease in high-sensitivity C-reactive protein), enhancement of vascular compliance (8%–12% increase in carotid artery distensibility), and optimization of cardiopulmonary coupling efficiency (48) (Figure 1).
6 Optimization strategies for exercise rehabilitation in HFpEF
The optimization of exercise rehabilitation in HFpEF necessitates individualized, multidimensional intervention strategies, and prioritizes phenotype-driven precision therapeutics. Regarding exercise modality selection, combined endurance-resistance training should be tailored to phenotypic characteristics: endurance training (e.g., walking, cycling) significantly enhances peak oxygen uptake (VO2peak), while resistance training improves peripheral metabolic capacity via skeletal muscle functional augmentation—particularly critical for elderly patients with sarcopenia (49, 50). Meta-analytic evidence demonstrates that combined training improves both 6-minute walking distance and diastolic function parameters (e.g., E/e’ ratio reduction) (49). While older female phenotypes emphasize resistance and balance training (51, 52). In HFpEF subgroups with PH or respiratory muscle weakness, low-intensity inspiratory muscle training coupled with functional electrical stimulation (FES) safely optimizes hemodynamics and exercise tolerance. Notably, while high-intensity interval training (HIIT) exhibits proven efficacy in HFrEF, its application in HFpEF requires meticulous intensity titration based on baseline cardiopulmonary exercise testing (CPET) metrics (e.g., anaerobic threshold, VO2peak) (53).
Optimizing exercise prescription necessitates a delicate balance between safety and therapeutic efficacy. Current evidence supports moderate-intensity exercise regimens (40%–80% heart rate reserve) administered 3–5 sessions per week with 30–60 minutes per session, demonstrating that sustained implementation (>12 weeks) yields significant improvements in peak oxygen uptake (VO2peak) (mean increase: +2.72 ml/kg/min; 95% CI: 2.1–3.3) and enhanced quality-of-life metrics (e.g., KCCQ score Δ+8–12 points) (54). For obese HFpEF phenotypes, aerobic exercise combined with caloric restriction (e.g., ≥200 min/week moderate activity) demonstrates synergistic metabolic benefits (55).
For patients with HFpEF and AF, exercise should prioritize heart rate control (50%–70% max HR) to prevent ventricular rate escalation. Structured aerobic training (150 min/week) improves QoL (↑20%–30%) and LV function (LVEF ↑3%–5%) despite AF-related limitations (56). Elderly females require fall risk mitigation and anticoagulant safety evaluation (warfarin/DOACs) (56, 57).
Hypertension exacerbates HFpEF via LV hypertrophy and stiffness. Exercise rehabilitation requires integration with antihypertensives (ARNIs/SGLT2i), low-sodium diet, and monitored aerobic training (brisk walking/swimming) to ↓ peripheral vascular resistance (58–60). The REHAB-HF trial showed 6-minute walking distance gains (30–50 m) and frailty risk reduction with multi-domain rehabilitation (61, 62).
Intensity stratification proves critical: low-intensity training (40%–60% peak heart rate) is prioritized for patients with multiple comorbidities or severe PH, whereas moderate-high intensity (60%–80%) targets those with preserved functional reserves (CPET-derived anaerobic threshold >11 ml/kg/min) (63). Implementation safeguards include real-time heart rate monitoring via wearable technology and periodic 6-minute Walk Test and CPET to dynamically adjust workloads—strategies shown to reduce exertional adverse events by 38%–45% in vulnerable subgroups (64, 65). Meanwhile, echocardiography can serve as a follow-up assessment after exercise training, providing objective evidence for functional improvement and prognostic evaluation in HFpEF patients by assessing changes in LA pressure and pulmonary artery pressure (66). Emerging protocols further incorporate intervalized resistance training (2–3 sets, 60%–80% 1RM) to counteract sarcopenic progression while maintaining hemodynamic stability.
Multimodal interventions (e.g., exercise combined with SGLT2 inhibitors or nutritional protocols) may yield synergistic therapeutic effects (67, 68). SGLT2 inhibitors alleviate symptoms such as dyspnea and fatigue, enhance physical activity capacity and quality of life (QoL) scores, significantly reduce blood pressure, and lower the risk of heart failure hospitalizations and cardiovascular mortality (69–71). Multimodal intervention synergism emerges when combining exercise with SGLT2 inhibitors (e.g., dapagliflozin 10 mg/day) or omega-3 fatty acid supplementation (4 g/day EPA/DHA), showing additive improvements in ventricular compliance (E/e’ Δ−1.8) and systemic inflammation (hs-CRP Δ−0.6 mg/L) (52, 67, 72). Additionally, Glucagon-Like Peptide-1 (GLP-1) receptor agonists, such as liraglutide, not only promote weight loss but also improve cardiometabolic parameters and may confer benefits for patients with HFpEF (73).
Adherence management is critical for HFpEF rehabilitation efficacy. Multicomponent strategies (health education, goal-setting, biosensors) sustain ≥120 min/week exercise adherence while reducing anxiety (46). Home-based achieves outcomes comparable to center-based programs with 30%–45% cost reduction (72). Group CBT alleviates psychological burdens (depression Δ−2.4, P < 0.01) (45). Gamified mHealth platforms may enhance engagement via real-time feedback.
7 Challenges and future directions in exercise rehabilitation for HFpEF
Although exercise rehabilitation for heart failure with preserved ejection fraction (HFpEF) has demonstrated clinical benefits, it continues to face multiple challenges. First, unlike HFrEF, HFpEF lacks exercise-induced improvements in hard endpoints like mortality or cardiovascular hospitalization (41). Current research predominantly focuses on short-term outcomes (3–6 months), such as enhanced exercise tolerance and quality-of-life metrics (74), but lacks evidence for long-term prognostic benefits (75). Secondly, the physiological mechanisms underlying exercise benefits remain partially elucidated. While exercise augments peak oxygen uptake and 6-minute walk capacity, its mechanistic interplay with left ventricular diastolic function,skeletal muscle mitochondrial biogenesis, and peripheral vascular adaptation requires deeper interrogation (41, 76, 77). Furthermore, HFpEF patients are predominantly elderly, female, and often present with multiple comorbidities (e.g., obesity, diabetes mellitus, atrial fibrillation, hypertension), necessitating phenotype-driven, personalized, and multidimensional therapeutic approaches. Multimodal regimens integrating aerobic, resistance, and HIIT training with caloric restriction, SGLT2 inhibitors, and GLP-1 receptor agonists may yield superior therapeutic outcomes.
Routine CPET faces logistical challenges, including limited availability, cost, and patient compliance. While CPET may serve as an optional adjunct in specialized cardiac rehabilitation centers, alternative assessments such as the 6-minute walk test can be prioritized in resource-limited settings (78).
Infrastructure gaps persist: 78% of trials are hospital-based, and home/community models show lower adherence (58% vs. 85%) (77, 79). Unresolved debates on exercise modality (HIIT vs. MICT), frequency (3–5 vs. 5–7 sessions/week), and duration (30–60 vs. 20–45 min/session) contribute to guideline adherence <40% in real-world settings (80).
Future HFpEF research must achieve dual breakthroughs in mechanistic elucidation and technological innovation. Firstly, core exercise-mediated mechanisms, peripheral endothelial function, skeletal muscle mitochondrial metabolism, and oxygen utilization, require validation via multimodal imaging (STE, CMR T1 mapping) and biomarkers (NT-proBNP) (41, 76, 81). Secondly, personalized rehabilitation protocols require phenotypic stratification integrating clinical profiles (inflammatory/metabolic biomarkers) and energy metabolism gene expression (82–84), combined with wearable biosensors and tele-rehab platforms for real-time monitoring (77, 79).
Multimodal approaches, including high-intensity interval training (HIIT), resistance/flexibility training, and home-based models, are pivotal for HFpEF rehabilitation. HIIT enhances peak oxygen uptake (VO2peak) but requires hemodynamic safety validation (PCWP <25 mmHg) in elderly patients (85, 86). While HIIT combined with resistance training benefits HFrEF (2), HFpEF evidence remains limited, necessitating supervised trials with rigorous monitoring. Resistance/flexibility training combats sarcopenia (85). Home-based programs (e.g., REACH-HFpEF) improve accessibility but lack long-term efficacy data (87). Interdisciplinary integration—combining Mediterranean diets, cognitive therapy, and AI-driven “exercise-pharmacology-behavior” networks (e.g., REVERSE-HFpEF trial)—shifts management from symptom relief to disease modification (80, 84).
Bridging evidence gaps necessitates large-scale trials assessing exercise impacts on mortality and rehospitalization (41, 74). Inclusive enrollment of underrepresented groups (women, octogenarians, multimorbid patients) is critical (75, 83). Only through interdisciplinary collaboration, precision phenotyping, and technological innovation can we overcome the therapeutic challenges of HFpEF, ultimately improving patients’ functional status and long-term prognosis.
8 Conclusion
HFpEF, a multisystem disorder, demands personalized rehabilitation. Exercise improves functional capacity (VO2peak), quality of life, and hemodynamics via cardiac-skeletal adaptations and anti-inflammatory effects, yet lacks robust mortality/hospitalization reduction. Heterogeneous subphenotypes (obese, hypertensive, AF, frail, amyloidosis) require precision strategies integrating phenomapping (H2FPEF), biomarkers, and digital tools. In the future, large trials validating hard endpoints, home-based multimodal interventions, and AI-driven dynamic dosing to transition from symptom relief to disease modification.
Statements
Author contributions
JF: Writing – original draft. ZW: Writing – review & editing, Conceptualization, Supervision. JY: Validation, Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al 2023 focused update of the 2021 esc guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 44(37):3627–39. 10.1093/eurheartj/ehad195
2.
Borlaug BA Sharma K Shah SJ Ho JE . Heart failure with preserved ejection fraction. J Am Coll Cardiol. (2023) 81(18):1810–34. 10.1016/j.jacc.2023.01.049
3.
Campbell P Rutten FH Lee MM Hawkins NM Petrie MC . Heart failure with preserved ejection fraction: everything the clinician needs to know. Lancet. (2024) 403(10431):1083–92. 10.1016/S0140-6736(23)02756-3
4.
Eckhardt CM Balte PP Barr RG Bertoni AG Bhatt SP Cuttica M et al Lung function impairment and risk of incident heart failure: the nhlbi pooled cohorts study. Eur Heart J. (2022) 43(23):2196–208. 10.1093/eurheartj/ehac205
5.
Reddy YNV Carter RE Sundaram V Kaye DM Handoko ML Tedford RJ et al An evidence-based screening tool for heart failure with preserved ejection fraction: the HFpEF-aba score. Nat Med. (2024) 30(8):2258–64. 10.1038/s41591-024-03140-1
6.
Khan SS Beach LB Yancy CW . Sex-based differences in heart failure. J Am Coll Cardiol. (2022) 79(15):1530–41. 10.1016/j.jacc.2022.02.013
7.
Ding N Shah AM Blaha MJ Chang PP Rosamond WD Matsushita K . Cigarette smoking, cessation, and risk of heart failure with preserved and reduced ejection fraction. J Am Coll Cardiol. (2022) 79(23):2298–305. 10.1016/j.jacc.2022.03.377
8.
Lau ES Wang D Roberts M Taylor CN Murugappan G Shadyab AH et al Infertility and risk of heart failure in the women’s health initiative. J Am Coll Cardiol. (2022) 79(16):1594–603. 10.1016/j.jacc.2022.02.020
9.
Chandra A Skali H Claggett B Solomon SD Rossi JS Russell SD et al Race- and gender-based differences in cardiac structure and function and risk of heart failure. J Am Coll Cardiol. (2022) 79(4):355–68. 10.1016/j.jacc.2021.11.024
10.
Naser JA Harada T Reddy YN Pislaru SV Michelena HI Scott CG et al Prevalence of HFpEF in isolated severe secondary tricuspid regurgitation. Jama Cardiol. (2025) 10(2):182. 10.1001/jamacardio.2024.3767
11.
Yang M Kondo T Butt JH Abraham WT Anand IS Desai AS et al Stroke in patients with heart failure and reduced or preserved ejection fraction. Eur Heart J. (2023) 44(31):2998–3013. 10.1093/eurheartj/ehad338
12.
Dronkers J van Veldhuisen DJ van der Meer P Meems LMG . Heart failure and obesity. J Am Coll Cardiol. (2024) 84(17):1666–77. 10.1016/j.jacc.2024.07.016
13.
Rush CJ Berry C Oldroyd KG Rocchiccioli JP Lindsay MM Touyz RM et al Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Jama Cardiol. (2021) 6(10):1130. 10.1001/jamacardio.2021.1825
14.
Hamo CE DeJong C Hartshorne-Evans N Lund LH Shah SJ Solomon S et al Heart failure with preserved ejection fraction. Nat Rev Dis Primers. (2024) 10(1):55. 10.1038/s41572-024-00540-y
15.
Aurigemma GP Gaasch WH . Clinical practice. Diastolic heart failure. N Engl J Med. (2004) 351(11):1097–105. 10.1056/NEJMcp022709
16.
Zile MR Brutsaert DL . New concepts in diastolic dysfunction and diastolic heart failure: part i: diagnosis, prognosis, and measurements of diastolic function. Circulation. (2002) 105(11):1387–93. 10.1161/hc1102.105289
17.
Melenovsky V Hwang S Redfield MM Zakeri R Lin G Borlaug BA . Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation Heart Fail. (2015) 8(2):295–303. 10.1161/CIRCHEARTFAILURE.114.001667
18.
Cameli M Lisi M Righini FM Massoni A Natali BM Focardi M et al Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. (2013) 111(4):595–601. 10.1016/j.amjcard.2012.10.049
19.
AbouEzzeddine OF Davies DR Scott CG Fayyaz AU Askew JW McKie PM et al Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. Jama Cardiol. (2021) 6(11):1267. 10.1001/jamacardio.2021.3070
20.
Molina AJ Bharadwaj MS Van Horn C Nicklas BJ Lyles MF Eggebeen J et al Skeletal muscle mitochondrial content, oxidative capacity, and mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. Jacc Heart Fail. (2016) 4(8):636–45. 10.1016/j.jchf.2016.03.011
21.
Saw EL Werner LD Zamani P Chirinos JA Valero-Munoz M Sam F . Skeletal muscle phenotypic switching in heart failure with preserved ejection fraction. Front Cardiovasc Med. (2022) 9:1016452. 10.3389/fcvm.2022.1016452
22.
Cui X Spanos M Zhao C Wan W Cui C Wang L et al Mitochondrial dysfunction in HFpEF: potential interventions through exercise. J Cardiovasc Transl Res. (2025) 18(2):442–56. 10.1007/s12265-025-10591-5
23.
Abudureyimu M Luo X Jiang L Jin X Pan C Yu W et al Fbxl4 protects against hfpef through drp1-mediated regulation of mitochondrial dynamics and the downstream serca2a. Redox Biol. (2024) 70:103081. 10.1016/j.redox.2024.103081
24.
Schiattarella GG Altamirano F Tong D French KM Villalobos E Kim SY et al Nitrosative stress drives heart failure with preserved ejection fraction. Nature. (2019) 568(7752):351–56. 10.1038/s41586-019-1100-z
25.
Huynh PM Wang F An YA . Hypoxia signaling in the adipose tissue. J Mol Cell Biol. (2024) 16(8):mjae039. 10.1093/jmcb/mjae039
26.
Hu Y Hai J Ti Y Kong B Yao G Zhao Y et al Adipose zfp36 protects against diet-induced obesity and insulin resistance. Metab Clin Exp. (2025) 164:156131. 10.1016/j.metabol.2024.156131
27.
Omote K Verbrugge FH Sorimachi H Omar M Popovic D Obokata M et al Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail. (2023) 25(2):185–96. 10.1002/ejhf.2747
28.
Lam CSP Roger VL Rodeheffer RJ Borlaug BA Enders FT Redfield MM . Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. (2009) 53(13):1119–26. 10.1016/j.jacc.2008.11.051
29.
Yamada K Kinugasa Y Sota T Miyagi M Sugihara S Kato M et al Inspiratory muscle weakness is associated with exercise intolerance in patients with heart failure with preserved ejection fraction: a preliminary study. J Card Fail. (2016) 22(1):38–47. 10.1016/j.cardfail.2015.10.010
30.
Upadhya B Haykowsky MJ Eggebeen J Kitzman DW . Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol. (2015) 12(3):294–304. 10.11909/j.issn.1671-5411.2015.03.013
31.
Fanola CL Norby FL Shah AM Chang PP Lutsey PL Rosamond WD et al Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. (2020) 75(2):148–58. 10.1016/j.jacc.2019.10.058
32.
Zern EK Ho JE Panah LG Lau ES Liu E Farrell R et al Exercise intolerance in heart failure with preserved ejection fraction: arterial stiffness and aabnormal left ventricular hemodynamic responses during exercise. J Card Fail. (2021) 27(6):625–34. 10.1016/j.cardfail.2021.02.011
33.
Zucker IH Xiao L Haack KKV . The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin Sci. (2014) 126(10):695–706. 10.1042/CS20130294
34.
Freeman K Lerman I Kranias EG Bohlmeyer T Bristow MR Lefkowitz RJ et al Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. (2001) 107(8):967–74. 10.1172/JCI12083
35.
Bahn YJ Wang Y Dagur P Scott N Cero C Long KT et al Tgf-β antagonism synergizes with pparγ agonism to reduce fibrosis and enhance beige adipogenesis. Mol Metab. (2024) 90:102054. 10.1016/j.molmet.2024.102054
36.
Chew DS Li Y Zeitouni M Whellan DJ Kitzman D Mentz RJ et al Economic outcomes of rehabilitation therapy in older patients with acute heart failure in the rehab-HF trial. Jama Cardiol. (2022) 7(2):140. 10.1001/jamacardio.2021.4836
37.
Naser JA Lee E Scott CG Kennedy AM Pellikka PA Lin G et al Prevalence and incidence of diastolic dysfunction in atrial fibrillation: clinical implications. Eur Heart J. (2023) 44(48):5049–60. 10.1093/eurheartj/ehad592
38.
Hieda M Sarma S Hearon CM MacNamara JP Dias KA Samels M et al One-year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage b heart failure with preserved ejection fraction. Circulation. (2021) 144(12):934–46. 10.1161/CIRCULATIONAHA.121.054117
39.
Gudmundsdottir HL Axelsson Raja A Rossing K Rasmusen H Snoer M Andersen LJ et al Exercise training in patients with hypertrophic cardiomyopathy without left ventricular outflow tract obstruction: a randomized clinical trial. Circulation. (2025) 151(2):132–44. 10.1161/CIRCULATIONAHA.124.070064
40.
Tucker WJ Lijauco CC Hearon CM Angadi SS Nelson MD Sarma S et al Mechanisms of the improvement in peak vo2 with exercise training in heart failure with reduced or preserved ejection fraction. Heart Lung Circ. (2018) 27(1):9–21. 10.1016/j.hlc.2017.07.002
41.
Sachdev V Sharma K Keteyian SJ Alcain CF Desvigne-Nickens P Fleg JL et al Supervised exercise training for chronic heart failure with preserved ejection fraction: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. (2023) 147(16):e699–715. 10.1161/CIR.0000000000001122
42.
Kitzman DW Brubaker PH Herrington DM Morgan TM Stewart KP Hundley WG et al Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. (2013) 62(7):584–92. 10.1016/j.jacc.2013.04.033
43.
Andrade DC Arce-Alvarez A Toledo C Diaz HS Lucero C Quintanilla RA et al Revisiting the physiological effects of exercise training on autonomic regulation and chemoreflex control in heart failure: does ejection fraction matter? Am J Physiol Heart Circ Physiol. (2018) 314(3):H464–74. 10.1152/ajpheart.00407.2017
44.
Matsiukevich D Kovacs A Li T Kokkonen-Simon K Matkovich SJ Oladipupo SS et al Characterization of a robust mouse model of heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. (2023) 325(2):H203–31. 10.1152/ajpheart.00038.2023
45.
Bowen TS Herz C Rolim NPL Berre AO Halle M Kricke A et al Effects of endurance training on detrimental structural, cellular, and functional alterations in skeletal muscles of heart failure with preserved ejection fraction. J Card Fail. (2018) 24(9):603–13. 10.1016/j.cardfail.2018.08.009
46.
Alonso WW Kupzyk KA Norman JF Lundgren SW Fisher A Lindsey ML et al The heart camp exercise intervention improves exercise adherence, physical function, and patient-reported outcomes in adults with preserved ejection fraction heart failure. J Card Fail. (2022) 28(3):431–42. 10.1016/j.cardfail.2021.09.003
47.
Jaconiano E Moreira-Gonçalves D . Unveiling the role of exercise training in targeting the inflammatory paradigm of heart failure with preserved ejection fraction: a narrative review. Heart Fail Rev. (2022) 27(1):163–90. 10.1007/s10741-021-10138-1
48.
Fujimoto N Prasad A Hastings JL Bhella PS Shibata S Palmer D et al Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. (2012) 164(6):869–77. 10.1016/j.ahj.2012.06.028
49.
Zhuang C Luo X Wang Q Wang W Sun R Zhang X et al The effect of exercise training and physiotherapy on diastolic function, exercise capacity and quality of life in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. Kardiol Pol. (2021) 79(10):1107–15. 10.33963/KP.a2021.0101
50.
Zhou M Li R Chen Y Gao Y Wei Y Lu M et al Impact of resistance exercise rehabilitation and whey protein supplementation in elderly patients with heart failure with preserved ejection fraction with sarcopenia: a study protocol for a randomised controlled trial. BMJ Open. (2022) 12(12):e66331. 10.1136/bmjopen-2022-066331
51.
Boulmpou A Theodorakopoulou MP Boutou AK Alexandrou M Papadopoulos CE Bakaloudi DR et al Effects of different exercise programs on the cardiorespiratory reserve in HFpEF patients: a systematic review and meta-analysis. Hellenic J Cardiol. (2022) 64:58–66. 10.1016/j.hjc.2021.10.003
52.
Wheat HL Fedson S Bozkurt B Josephson RA . Cardiac rehabilitation in heart failure: indications for exercise training based on heart failure phenotype. Prog Cardiovasc Dis. (2022) 70:16–21. 10.1016/j.pcad.2021.10.003
53.
Schindler MJ Adams V Halle M . Exercise in heart failure—what is the optimal dose to improve pathophysiology and exercise capacity?Curr Heart Fail Rep. (2019) 16(4):98–107. 10.1007/s11897-019-00428-z
54.
Pandey A Parashar A Kumbhani D Agarwal S Garg J Kitzman D et al Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circulation Heart Fail. (2015) 8(1):33–40. 10.1161/CIRCHEARTFAILURE.114.001615
55.
Kitzman DW Brubaker P Morgan T Haykowsky M Hundley G Kraus WE et al Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. (2016) 315(1):36–46. 10.1001/jama.2015.17346
56.
Alves LS Bocchi EA Chizzola PR Castro RE Salemi V de Melo M et al Exercise training in heart failure with reduced ejection fraction and permanent atrial fibrillation: a randomized clinical trial. Heart Rhythm. (2022) 19(7):1058–66. 10.1016/j.hrthm.2022.03.1217
57.
Pugliese NR Pellicori P Filidei F De Biase N Maffia P Guzik TJ et al Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: implications for future interventions. Cardiovasc Res. (2023) 118(18):3536–55. 10.1093/cvr/cvac133
58.
Habel N Infeld M Lustgarten D Meyer M . Atrial fibrillation and heart failure with preserved ejection fraction “twindemic"-shared root causes and treatment targets. Heart Rhythm. (2025) 22(5):1188–96. 10.1016/j.hrthm.2024.08.064
59.
Gao C Xiong Z Liu Y Wang M Wang M Liu T et al Glucagon receptor antagonist for heart failure with preserved ejection fraction. Circ Res. (2024) 135(5):614–28. 10.1161/CIRCRESAHA.124.324706
60.
Tang F Han H Fu S Liu Q Zhou S Huang J et al Nonpharmacological approaches to managing heart failure with preserved ejection fraction. Circ Heart Fail. (2024) 17(8):e11269. 10.1161/CIRCHEARTFAILURE.123.011269
61.
Laddu DR Ozemek C Sabbahi A Severin R Phillips SA Arena R . Prioritizing movement to address the frailty phenotype in heart failure. Prog Cardiovasc Dis. (2021) 67:26–32. 10.1016/j.pcad.2021.01.005
62.
Peters AE Kitzman DW Chen H Nelson MB Pastva AM Duncan PW et al Obesity status and physical rehabilitation in older patients hospitalized with acute HF: insights from rehab-HF. Jacc Heart Fail. (2022) 10(12):918–27. 10.1016/j.jchf.2022.07.008
63.
Ding R . Exercise-based rehabilitation for heart failure: clinical evidence. Adv Exp Med Biol. (2017) 1000:31. 10.1007/978-981-10-4304-8_3
64.
Tucker WJ Angadi SS Haykowsky MJ Nelson MD Sarma S Tomczak CR . Pathophysiology of exercise intolerance and its treatment with exercise-based cardiac rehabilitation in heart failure with preserved ejection fraction. J Cardiopulm Rehabil Prev. (2020) 40(1):9–16. 10.1097/HCR.0000000000000481
65.
Buber J Robertson HT . Cardiopulmonary exercise testing for heart failure: pathophysiology and predictive markers. Heart. (2023) 109(4):256–63. 10.1136/heartjnl-2021-319617
66.
Saito Y Obokata M Harada T Kagami K Sorimachi H Yuasa N et al Disproportionate exercise-induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: a non-invasive echocardiographic study. Eur J Heart Fail. (2023) 25(6):792–802. 10.1002/ejhf.2821
67.
Zhan Q Peng W Wang S Gao J . Heart failure with preserved ejection fraction: pathogenesis, diagnosis, exercise, and medical therapies. J Cardiovasc Transl Res. (2023) 16(2):310–26. 10.1007/s12265-022-10324-y
68.
Abdin A Böhm M Shahim B Karlström P Kulenthiran S Skouri H et al Heart failure with preserved ejection fraction epidemiology, pathophysiology, diagnosis and treatment strategies. Int J Cardiol. (2024) 412:132304. 10.1016/j.ijcard.2024.132304
69.
Zannad F Ferreira JP Pocock SJ Anker SD Butler J Filippatos G et al SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the emperor-reduced and dapa-HF trials. Lancet. (2020) 396(10254):819–29. 10.1016/S0140-6736(20)31824-9
70.
Usman MS Bhatt DL Hameed I Anker SD Cheng A Hernandez AF et al Effect of SGLT2 inhibitors on heart failure outcomes and cardiovascular death across the cardiometabolic disease spectrum: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. (2024) 12(7):447–61. 10.1016/S2213-8587(24)00102-5
71.
Kosiborod MN Angermann CE Collins SP Teerlink JR Ponikowski P Biegus J et al Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: results from the empulse trial. Circulation. (2022) 146(4):279–88. 10.1161/CIRCULATIONAHA.122.059725
72.
Molloy C Long L Mordi IR Bridges C Sagar VA Davies EJ et al Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Db Syst Rev. (2024) 3(3):CD3331. 10.1002/14651858.CD003331.pub6
73.
Ueno H Zhang W Nakazato M . Regulation of feeding and therapeutic application of bioactive peptides. Pharmacol Ther. (2022) 239:108187. 10.1016/j.pharmthera.2022.108187
74.
Molloy CD Long L Mordi IR Bridges C Sagar VA Davies EJ et al Exercise-based cardiac rehabilitation for adults with heart failure—2023 cochrane systematic review and meta-analysis. Eur J Heart Fail. (2023) 25(12):2263–73. 10.1002/ejhf.3046
75.
Eleyan L Gonnah AR Farhad I Labib A Varia A Eleyan A et al Exercise training in heart failure: current evidence and future directions. J Clin Med. (2025) 14(2):359. 10.3390/jcm14020359
76.
Haykowsky M Brubaker P Kitzman D . Role of physical training in heart failure with preserved ejection fraction. Curr Heart Fail Rep. (2012) 9(2):101–06. 10.1007/s11897-012-0087-7
77.
Taylor RS Dalal HM McDonagh S . The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol. (2022) 19(3):180–94. 10.1038/s41569-021-00611-7
78.
Martens P Augusto SJ Finet JE Tang W . Distinct impact of noncardiac comorbidities on exercise capacity and functional status in chronic heart failure. Jacc Heart Fail. (2023) 11(10):1365–76. 10.1016/j.jchf.2023.05.018
79.
Paleviciute E Simbelyte T Eichstaedt CA Benjamin N Egenlauf B Grunig E et al The effect of exercise training and physiotherapy on left and right heart function in heart failure with preserved ejection fraction: a systematic literature review. Heart Fail Rev. (2023) 28(1):193–206. 10.1007/s10741-022-10259-1
80.
Reddy YNV Rikhi A Obokata M Shah SJ Lewis GD AbouEzzedine OF et al Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. (2020) 22(6):1009–18. 10.1002/ejhf.1788
81.
Haykowsky MJ Brubaker PH John JM Stewart KP Morgan TM Kitzman DW . Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. (2011) 58(3):265–74. 10.1016/j.jacc.2011.02.055
82.
Cuesta-Vargas AI Fuentes-Abolafio IJ García-Conejo C Díaz-Balboa E Trinidad-Fernández M Gutiérrez-Sánchez D et al Effectiveness of a cardiac rehabilitation program on biomechanical, imaging, and physiological biomarkers in elderly patients with heart failure with preserved ejection fraction (HFpEF): funnel+study protocol. BMC Cardiovasc Disord. (2023) 23(1):1–550. 10.1186/s12872-023-03555-7
83.
Gou Q Zhao Q Dong M Liang L You H . Diagnostic potential of energy metabolism-related genes in heart failure with preserved ejection fraction. Front Endocrinol (Lausanne). (2023) 14:1296547. 10.3389/fendo.2023.1296547
84.
Zhang R Sun X Li Y He W Zhu H Liu B et al The efficacy and safety of sacubitril/valsartan in heart failure patients: a review. J Cardiovasc Pharmacol Ther. (2022) 27:2090350599. 10.1177/10742484211058681
85.
Haykowsky MJ Tomczak CR Scott JM Paterson DI Kitzman DW . Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). (2015) 119(6):739–44. 10.1152/japplphysiol.00049.2015
86.
Chen X Zhang T Hu X Wen Z Lu W Jiang W . High-intensity interval training programs versus moderate-intensity continuous training for individuals with heart failure: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2025) 106(1):98–112. 10.1016/j.apmr.2024.05.028
87.
Eyre V Lang CC Smith K Jolly K Davis R Hayward C et al Rehabilitation enablement in chronic heart failure—a facilitated self-care rehabilitation intervention in patients with heart failure with preserved ejection fraction (reach-HFpEF) and their caregivers: rationale and protocol for a single-centre pilot randomised controlled trial. Bmj Open. (2016) 6(10):e12853. 10.1136/bmjopen-2016-012853
Summary
Keywords
heart failure with preserved ejection fraction, pathophysiological mechanisms, exercise rehabilitation, exercise intolerance, therapeutic evidence, optimization strategies
Citation
Fang J, Wang Z and Yu J (2025) Advances in pathophysiological mechanisms and therapeutic efficacy of exercise rehabilitation in patients with heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 12:1598878. doi: 10.3389/fcvm.2025.1598878
Received
24 March 2025
Accepted
14 May 2025
Published
27 May 2025
Volume
12 - 2025
Edited by
Erberto Carluccio, Heart Failure Unit, Italy
Reviewed by
Samir Saha, Sundsvall Municipality, Sweden
Dejan Simonovic, Institute for Treatment and Rehabilitation Niska Banja, Serbia
Updates
Copyright
© 2025 Fang, Wang and Yu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Zhenhua Wang wzh0522@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.