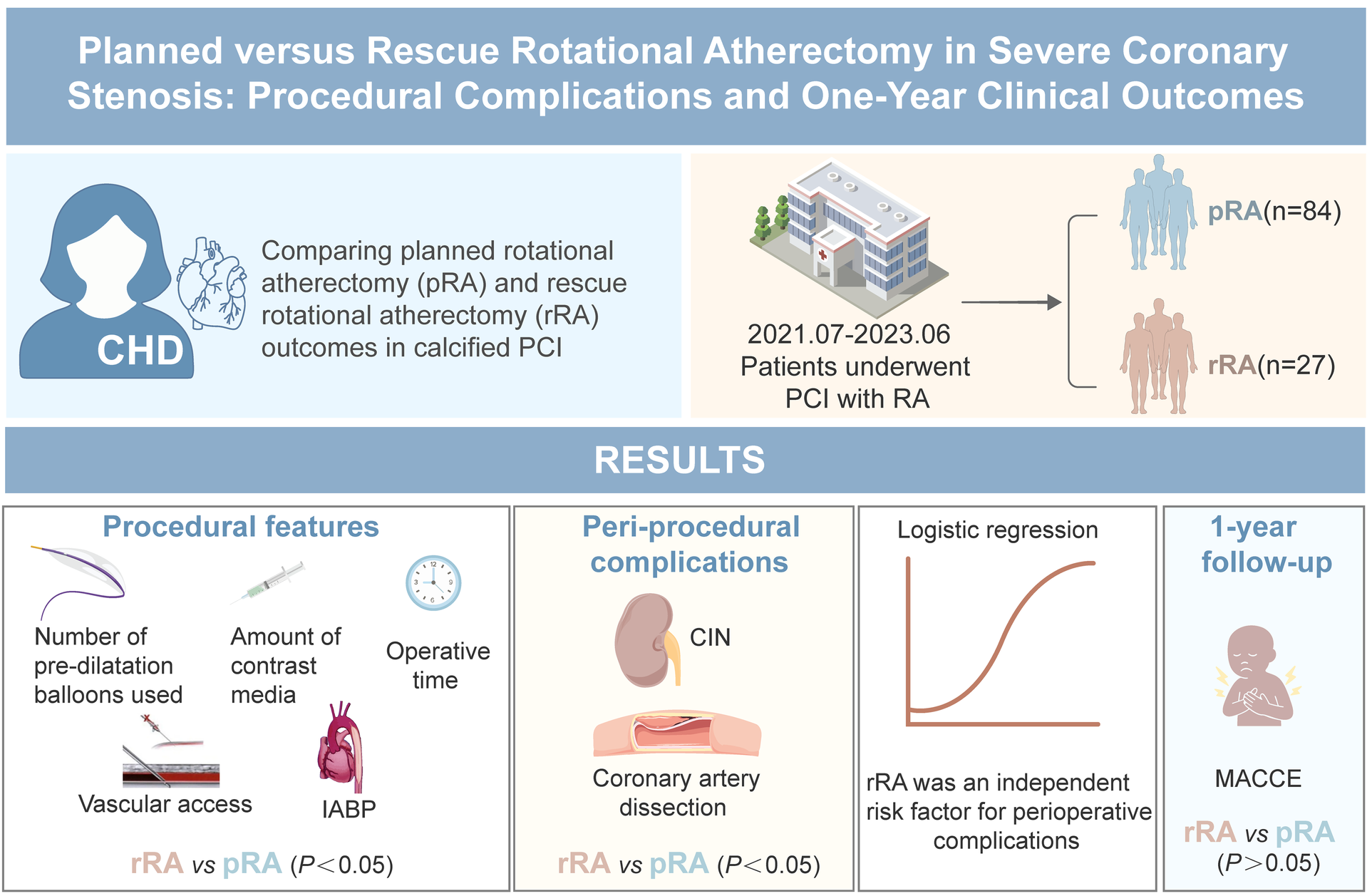
Abstract
Objective:
Current guidelines recommend rotational atherectomy (RA) as a rescue treatment for calcified or fibrotic lesions that cannot be fully expanded before stent implantation. Present study compared the procedural and one-year clinical outcome of planned (pRA) or rescue RA (rRA) for patients undergoing percutaneous coronary intervention with severe coronary stenosis and calcification.
Methods:
A total of 111 consecutive patients who underwent RA at the Fourth Hospital of Wuhan from July 2021 to June 2023 were enrolled. The general clinical data, coronary artery lesion characteristics, procedural characteristics, complication rate and major cerebral and cardiovascular event [MACCE, cardiac death, acute myocardial infarction (AMI), target vessel revascularization or acute ischemic stroke] rate at one year after procedure were compared between the two groups.
Results:
According to the timing of initiation of RA, patients were stratified into pRA group (n = 84) or rRA group (n = 27). Baseline clinical characteristics were similar between the two groups. The number of stents implanted was similar in the two groups. The rRA group required more pre—dilation balloons (1.7 ± 0.7 vs. 3.4 ± 0.5, P < 0.001), exhibited a higher rate of coronary artery dissection (29.6% vs. 7.1%, P = 0.02) and consumed a larger volume of contrast (189.8 ± 59 ml vs. 139.9 ± 46 ml, P < 0.001). Additionally, the incidence of contrast—induced nephropathy was significantly greater in the rRA group (29.6% vs. 9.5%, P = 0.01), and the procedure duration was markedly longer in this group compared to the pRA group (91.5 ± 24.3 min vs. 77.9 ± 25.2 min, P < 0.001). Multivariable logistic regression identified rRA as an independent predictor of periprocedural complications (adjusted OR = 2.83; 95% CI:1.01–7.99; P = 0.048). However, 1-year MACCE rates showed no intergroup difference (pRA 3.7% vs. rRA 4.8%; P = 1.00). No significant difference in the secondary endpoints of non-cardiac death, angina pectoris, heart failure, and cardiovascular rehospitalization were observed between the two groups.
Conclusion:
rRA is related with higher procedural complication rates, procedure time, and contrast agent dose compared with pRA, but has similar low MACCE rate as pRA at one year after procedure.
Graphical Abstract
Introduction
Coronary atherosclerotic heart disease (CAD) has become one of the major diseases that endanger people's health worldwide. With the development of interventional treatment technology, percutaneous coronary intervention (PCI) has become the most widely used treatment method for CAD, but severe coronary artery calcification (CAC) lesions remain as difficult challenge for the success of PCI (1–4). PCI in severe calcified coronary artery stenosis is often linked with serious problems such as low success rate, high incidence of complications, high incidence of postoperative restenosis and cardiac events, and poor prognosis (2, 5). For CAC, rotational atherectomy (RA) can reduce procedural complications and improve the success rate of PCI (6, 7). RA for CAC is usually divided into the following two situations: (1) severe calcification is visualized by coronary angiography or intravascular ultrasound (IVUS), RA is decided as a pre-treatment method for the lesion, which is planned rotational atherectomy (pRA); (2) RA was used as an “emergency rescue” procedure in case of difficulties occurred in balloon or stent delivery, or when the balloon cannot be effectively expanded or the stent fails to pass, which is rescue rotational atherectomy (rRA) (8).
The European Society of Cardiology guidelines for coronary artery revascularization highlight the necessity of using RA in patients with significant stenosis and calcified lesions (9). Due to the limitations of coronary angiography for calcified lesions, especially calcified nodules, it is sometimes difficult to judge whether it is necessary to start RA to pretreat calcified lesions. Sometimes, it is necessary to switch to rRA during the preparation of routine balloon dilatation before attempting coronary stent implantation. Studies have shown that pRA can reduce the incidence of adverse cardiovascular events at 1 year compared with rRA (8). This benefit may be related to the ability to perform effective balloon dilatation, reduce intraoperative complications, and obtain sufficient lumen area after pRA. However, some studies have shown that pRA does not improve the clinical efficacy of patients compared with rRA when the balloon cannot be fully expanded (10, 11). Therefore, it is of clinical significance to compare the impact of pRA and rRA on procedural and clinical outcomes. This study compared the procedural outcome and clinical outcome at 1 year after pRA or rRA in a realworld patient cohort.
Materials and methods
Study subjects
This study was a single-center, retrospective study. A total of 111 patients who underwent RA in the Department of Cardiology of our hospital from July 2021 to June 2023 were enrolled. These patients had a preoperative diagnosis of stable angina, unstable angina, or non-ST-segment elevation myocardial infarction. All patients signed the informed consent before PCI. Exclusion criteria: (1) acute ST-segment elevation myocardial infarction; (2) bridging vessel lesions; (3) acute thrombotic lesions; (4) in-stent restenosis lesions.
Indications for intracoronary Ra
The diagnosis of coronary artery calcification mainly relies on imaging methods, and the commonly used methods are coronary artery CT examination, coronary angiography and IVUS. The main indications include (12): (1) severe calcification, that is, clear coronary artery calcification shadows can be seen before contrast injection; (2) when coronary angiography cannot determine whether the lesion vessel is severely calcified, IVUS is used to determine the degree of coronary artery calcification. Lesions with a range of >270° are severely calcified; (3) Calcified lesions that cannot be fully pre-dilated by the balloon.
Clinical and angiographic data
(1) Clinical related indicators: sex, age, cardiovascular risk factors were noted, renal function, blood lipids, serum troponin I (TnI) and creatine kinase-isoenzyme (CK-MB) were measured at admission and 24 h after RA. Left ventricular ejection fraction (LVEF) was measured by echocardiography at admission. (2) Coronary angiography related indicators including severity of coronary artery lesions were analyzed. (3) Surgical operation related indicators including target vessel of RA, diameter of RA head, number of balloons used before stent placement, number of stents, total length of stents, operation time, amount of contrast agent used and incidence of intraoperative related complications were analyzed.
Revascularization methods and processes
PCI was performed through the radial artery, brachial artery or femoral artery, and unfractionated heparin 70–100 U/kg was given before PCI. The Boston Scientific Rotablator TM was used for RA, and the RA head was Rota Link TM (diameters: 1.25 mm, 1.50 mm, and 1.75 mm, respectively). The size of the RA head was selected to have a ratio of 0.5–0.6 between the RA head diameter and the vessel diameter, and the RA speed was 140,000–160,000 rpm, and each RA lasted 10–15 s. During RA, continuous intracoronary infusion of a mixture containing unfractionated heparin and nitroglycerin was used. The success of RA was defined as complete balloon dilatation of the target lesion after RA. Before RA, all patients received 300 mg aspirin and an oral loading dose of a P2Y12 inhibitor (clopidogrel or ticagrelor). At discharge, all patients received aspirin (100 mg daily) plus clopidogrel (75 mg daily) or ticagrelor (90 mg twice daily) for at least 1 year.
Perioperative complications
The occurrence of perioperative complications of RA was recorded, including complications directly related to RA and complications not directly related to RA. Complications directly related to RA included: slow coronary artery blood flow or no reflow, coronary artery spasm, severe coronary artery dissection, coronary artery perforation or cardiac tamponade, RA head incarceration that could not be evacuated, and severe bradycardia. Severe coronary artery dissection mainly refers to type C or above dissection defined by the National Institute of Heart, Lung, and Blood Diseases of the United States (13). Complications not directly related to RA refer to PCI- related complications, such as target lesion side branch occlusion, puncture site hematoma, and contrast-induced nephropathy. Angiographic success was defined as final residual stenosis <30% and TIMI blood flow grade III. Perioperative myocardial infarction was defined as TnI exceeding 5 times the upper reference limit within 48 h and new ECG changes or imaging evidence after PCI (14).
Clinical follow-up
Patients were clinically followed up at 1, 3, 6, and 12 months, and then every 6 months after PCI. The primary endpoint was major adverse cardiovascular and cerebrovascular events (MACCE) were defined as cardiac death, spontaneous myocardial infarction (MI), ischemic stroke, and repeat target vessel/leision revascularization (TVR/TLR). The secondary endpoints were the composite of non-cardiac death, angina pectoris, heart failure and cardiovascular rehospitalization. All endpoints were defined according to the standardized criteria proposed by the Cardiovascular Trials Initiative (15). Deaths were categorized as cardiac or noncardiac, with deaths of unknown etiology classified as cardiac deaths. Cardiac death was defined as death due to cardiovascular causes, including acute myocardial infarction, sudden cardiac death, heart failure (HF), stroke, complications from cardiovascular procedures, cardiovascular hemorrhage, and other cardiovascular-related etiologies. The clinical definition of Ml denotes the presence of acute myocardial injury detected by abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischaemia. Stroke was defined on the basis of the presence of acute infarction as demonstrated by imaging or based on the persistence of symptoms. TVR was defined as any repeat PCI or coronary artery bypass grafting (CABG) of the target vessel. Target lesion revascularization (TLR) was defined as any repeat revascularization of the target lesion with PCI or CABG for restenosis or other complications, which was defined as the treatment segment from 5 mm proximal to 5 mm distal to the stent.
Statistical analysis
All data were processed using SPSS V.26.0 (IBM SPSS). Normally distributed quantitative data were expressed as x ± s, and the t test was used for inter-group comparison. Non-normally distributed data were expressed as median (interquartile range), and the rank sum test was used for inter-group comparison. Enumeration data were expressed as percentage (%), and the Chi-square (χ2) test or Fisher's exact probability test was used for inter-group comparison. P < 0.05 was considered statistically significant. The effects of various clinical data, including hypertension, diabetes, heart failure, previous PCI, IABP, performance of rRA and preoperative diagnosis on periprocedural complications were evaluated using the Logistic regression module, and the 95% confidence interval (CI) of the odds ratio (odds ratio, OR) and P < 0.05 were considered statistically significant.
Results
Comparison of baseline data between the two groups
According to the timing of RA, patients were divided into the pRA group (n = 84) or the rRA group (n = 27) (Figure 1). As shown in Table 1, patients in the pRA group had a higher proportion of dialysis (23.3% vs. 9.5%, P = 0.01) and unstable angina (89% vs. 47.6%, P = 0.04) (Table 1) than the rRA group, and the rRA group had a higher proportion of non-STEMI (52.4% vs. 11%, P = 0.02). There were no significant differences in other baseline clinical characteristics such as sex, age, body mass index, cardiovascular risk factors, cardiac function, and previous medical history (myocardial infarction, PCI) (all P > 0.05).
Figure 1
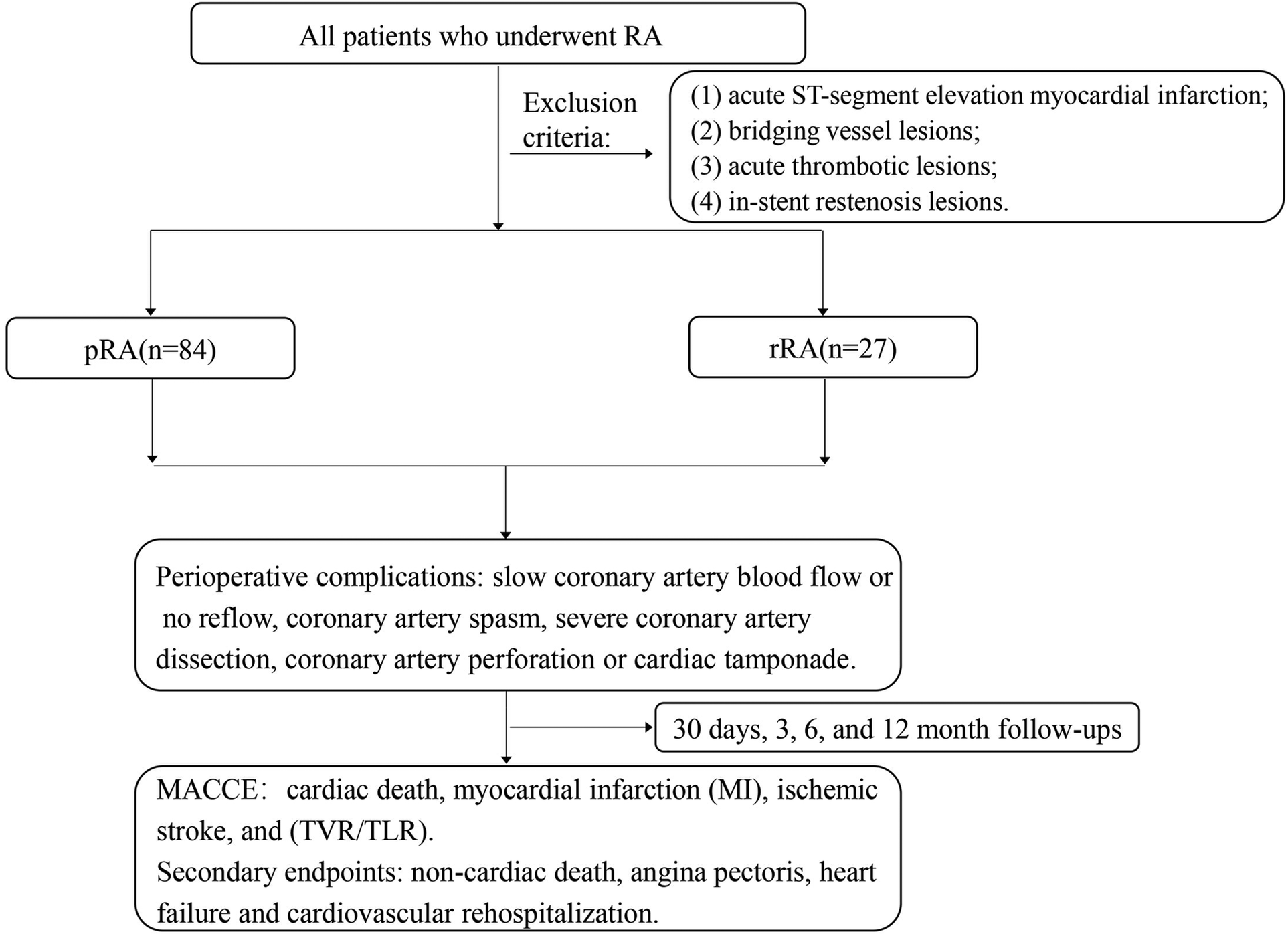
RA, rotational atherectomy; pRA, planned rotational atherectomy; rRA, rescue rotational atherectomy; MACCE, major adverse cardiovascular and cerebrovascular events.
Table 1
| Variable | pRA group (n = 84) | rRA group (n = 27) | P |
|---|---|---|---|
| Age, years old | 69 ± 9 | 69 ± 12 | 0.92 |
| Male, n (%) | 51 (60.7) | 12 (44.4) | 0.14 |
| Previous myocardial infarction, n (%) | 3 (3.6) | 2 (7.4) | 0.76 |
| Previous PCI, n (%) | 13 (15.5) | 6 (22.2) | 0.42 |
| Hypertension, n (%) | 75 (89.3) | 23 (85.2) | 0.82 |
| Diabetes, n (%) | 39 (46.4) | 13 (48.1) | 0.88 |
| Hyperlipidemia, n (%) | 20 (23.8) | 5 (18.5) | 0.57 |
| EF (mean ± s, %) | 58.4 ± 6.3 | 56.2 ± 7.6 | 0.13 |
| NT-proBNP (mean ± s, pg/ml) | 1,868.7 ± 714.2 | 4,026.3 ± 1,385.7 | 0.15 |
| NYHA, n (%) | |||
| I | 53 (63.1) | 20 (53.9) | 0.30 |
| II | 20 (23.8) | 5 (18.5) | 0.57 |
| III | 8 (9.5) | 4 (14.8) | 0.44 |
| IV | 3 (3.6) | 4 (14.8) | 0.10 |
| HF, n (%) | |||
| HFpEF | 24 (28.6) | 8 (29.6) | 0.92 |
| HFmrEF | 5 (6.0) | 3 (11.1) | 0.64 |
| HFrEF | 2 (2.4) | 2 (7.4) | 0.53 |
| Smoking, n (%) | 23 (27.4) | 4 (14.8) | 0.28 |
| Renal insufficiency (GFR < 80) | 40 (47.6) | 12 (44.4) | 0.77 |
| Unstable angina, n (%) | 71 (84.5) | 17 (63) | 0.04 |
| Non-STEMI, n (%) | 10 (11.9) | 10(37) | 0.02 |
Baseline characteristics of patients undergoing RA.
RA, rotational atherectomy; pRA, planned rotational atherectomy; rRA, rescue rotational atherectomy; PCI, percutaneous coronary intervention; EF, ejection fraction; Non-STEMI, non-ST-segment elevation myocardial infarction; NYHA, New York Heart Association; HFpEF, Heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Comparison of lesion characteristics and procedural features between the two groups
As shown in Table 2, there were no significant differences in target vessels, lesion classification, brachial artery access diameter, femoral artery access diameter, RA head diameter, number of stents implanted, and intraoperative IVUS use between the two groups (P > 0.05). The average diameter of the RA head was also similar between the two groups (1.47 ± 0.08 mm vs. 1.38 ± 0.16 mm, P = 0.91). The chronic occlusion rate in the rRA group was higher than that in the pRA group (7.1% vs. 22.2%, P = 0.03). In terms of PCI approach, radial artery route was more often used in the pRA group (95.2% vs. 81.5%, P = 0.02), while femoral artery route was more often used in the rRA group (1.2% vs. 11.1%, P = 0.02). The use of IABP during PCI was higher in the rRA group than in the pRA group (19% vs. 51.9%, P < 0.001). The number of pre-dilatation balloons used in the pRA group was significantly lower than that in the rRA group [(1.7 ± 0.7) vs. (3.4 ± 0.5), P < 0.001]. In the rRA group, the operation time was longer [(91.5 ± 24.3) min vs. (77.9 ± 25.2) min, P < 0.001], and the amount of contrast medium used was larger [(189.8 ± 59) ml vs. (139.9 ± 46) ml, P < 0.001].
Table 2
| Variable | pRA group (n = 84) | rRA group (n = 27) | P |
|---|---|---|---|
| Target blood vessels | |||
| LM | 8 (9.5) | 5 (18.5) | 0.21 |
| LAD | 67 (82.7) | 17 (68) | 0.11 |
| LCX | 4 (4.8) | 2 (7.4) | 0.60 |
| RCA | 10 (11.9) | 6 (22.2) | 0.18 |
| Chronic occlusive disease | 6 (7.1) | 6 (22.2) | 0.03 |
| Lesion classification | 0.88 | ||
| Single Vessel Disease | 8 (9.5) | 1 (3.7) | 0.58 |
| Multivessel disease | 68 (81) | 21 (77.8) | 0.72 |
| Surgical approach | |||
| Radial artery | 80 (95.2) | 22 (81.5) | 0.02 |
| Brachial artery | 3 (3.6) | 2 (7.4) | 0.40 |
| Femoral artery | 1 (1.2) | 3 (11.1) | 0.02 |
| IABP, n (%) | 16 (19) | 14 (51.9) | <0.001 |
| Intraoperative use of IVUS | 28 (33.4) | 10 (37) | 0.82 |
| Operation time | 77.9 ± 25.2 | 91.5 ± 24.3 | 0.01 |
| Contrast agent dosage | 139.9 ± 46 | 189.8 ± 59 | <0.001 |
| Grinding head diameter | 1.47 ± 0.08 | 1.38 ± 0.16 | 0.91 |
| Number of pre-dilation balloons used | 1.7 ± 0.8 | 3.4 ± 1.5 | <0.001 |
| Number of brackets | 1.7 ± 0.8 | 1.7 ± 0.7 | 0.51 |
Analysis of the characteristics of coronary artery lesions and interventional treatment in two groups of patients.
pRA, planned rotational atherectomy; rRA, rescue rotational atherectomy; LM, left main artery; LAD, descending anterior artery; LCX, circumflex coronary artery; RCA, right coronary artery; IABP, intraaortic balloon pump. IVUS, intravascular ultrasound.
Analysis of influencing factors of peri-procedural complications
Table 3 showed that incidence of coronary artery dissection and contrast-induced nephropathy was significantly higher rRA group than in the pRA group (both P < 0.05). There were no significant differences in other peri-procedural complications (coronary spasm, slow blood flow and no reflow, bradycardia, side branch occlusion and periprocedural myocardial infarction) between the two groups. Peri-procedural complications following coronary RA were designated as the dependent variable, and potential predictors of complications between groups, including hypertension, diabetes, heart failure, previous PCI, IABP, performance of rRA and preoperative diagnosis were included as independent variables. Logistic regression analysis revealed that performance of rRA (OR = 2.834; 95% CI: 1.006–7.986) was an independent predictor of periprocedural complications after RA (P < 0.05) (Table 3).
Table 3
| Influencing factors | β | SE | WaldX2 | OR(95%CI) | P |
|---|---|---|---|---|---|
| Diabetes | 0.569 | 0.449 | 1.607 | 1.766 (0.733–4.254) | 0.205 |
| Heart Failure | 0.493 | 0.523 | 0.890 | 1.638 (0.588–4.563) | 0.345 |
| Hypertension | 1.830 | 1.104 | 2.748 | 6.232 (0.716–54.217) | 0.097 |
| Previous PCI | −0.692 | 0.666 | 1.081 | 0.500 (0.136–1.846) | 0.299 |
| IABP | −0.078 | 0.563 | 0.019 | 0.925 (0.307–2.788) | 0.890 |
| rRA | 1.658 | 0.528 | 3.877 | 2.834 (1.006–7.986) | 0.049 |
| UA | −0.179 | 0.632 | 0.080 | 0.836 (0.242–2.889) 0.777 |
Multifactorial logistic regression model of peri-procedural complications risk.
PCI, percutaneous coronary intervention; IABP, intraaortic balloon pump; rRA, rescue rotational atherectomy; UA, unstable angina.
MACCE during hospitalization and at one year after PCI between the two groups
All patients completed 1-year follow-up, and MACCE occurred in 4 patients in pRA group and 1 patient in the rRA group (P = 0.67). The secondary endpoints were similar between the two groups (22% vs. 33%, P = 0.27). There were 4 cases of non cardiac death in both in the pRA group and the rRA group (P = 0.18), all related to pulmonary infection (Table 4).
Table 4
| Parameter | pRA group(n = 84) | rRA group(n = 27) | P |
|---|---|---|---|
| Perioperative complications | |||
| Coronary artery dissection | 6 (7.1) | 8 (29.6) | 0.02 |
| Coronary artery spasm | 17 (20.2) | 4 (14.8) | 0.53 |
| Slow flow and no-reflow | 5 (6.0) | 2 (7.4) | 0.79 |
| Severe bradycardia | 11 (13.1) | 3 (11.1) | 0.79 |
| Side branch occlusion | 2 (2.4) | 1 (3.7) | 0.71 |
| Periprocedural myocardial infarction | 7 (8.4) | 3 (11.1) | 0.97 |
| Contrast-induced nephropathy | 8 (9.5) | 8 (29.6) | 0.01 |
| The incidence of MACCE during hospitalization | 0 (0.0) | 1 (9.5) | 0.67 |
| MACCE 1 year after PCI | 4 (4.8) | 1 (3.7) | 1.00 |
| Secondary endpoints | 19 (22.6) | 9 (33.3) | 0.27 |
| Unstable angina | 13 (15.5) | 6 (22.2) | 0.42 |
| Arrhythmias | 2 (2.4) | 0 (0.0) | 1.00 |
| Heart failure | 6 (7.1) | 2 (7.4) | 0.96 |
| Cardiovascular readmission | 16 (19) | 8 (29.6) | 0.25 |
| Non-cardiac death | 4(2.4) | 4(7.4) | 0.18 |
Perioperative complications and 1-year clinical outcomes in both groups.
RA, rotational atherectomy; pRA, planned rotational atherectomy; rRA, rescue rotational atherectomy; MACCE, major adverse cardiovascular and cerebrovascular events.
Discussion
Our findings can be summarized as follows: despite increased peri-procedural complications in the rRA group, the long-term MACCE rate is similarly low as the pRA group. Therefore, rRA is a feasible and similar safe procedure as pRA in the long-term for patients undergoing selective PCI. Thus, there is no need to hesitate the application of rRA in indicated patients based on present results. Clearly, options are required to reduce the peri-procedural complications in rRA patients. Our real-world results are in line with previous meta-analysis (16).
rRA is known to be related to the deviation in the initial judgment of the severity of calcification or fibrous lesions based on coronary angiography. Most of the time, rRA is used after conventional pretreatment of the lesions fails. This transformation is mostly passive, as on the one hand increases the operation time, use of contrast agents and interventional instruments (17). On the other hand failure of the lesion by conventional preconditioning may increase the difficulty and risk of subsequent rotational milling treatment, especially the effect of excessive balloon preexpansion force on the localization of the lesion. Higher balloon pre-dilatation pressure is often required before rRA, and higher pre-dilatation pressure may lead to coronary artery entrapment or even vessel rupture, and even though balloon dilatation appears to be adequate, the stent may still have difficulty in reaching the lesion site. In the contrast, pRA can be used to pre-treat heavy calcified plaques and calcified nodules with RA, which changes the compliance of calcified plaques and facilitates the subsequent interventions, and can effectively avoid the complications associated with forcible pushing of balloons and stents or high-pressure dilation of balloons and stents.
Our results are consistent with above observations. In this study, we found that the percentage of patients in the pRA who developed entrapment was not high, and in fact vascular entrapment was conversely more common when repeated balloon dilatation of the lesion was used (especially for calcified lesions). The number of balloons used for predilatation of lesions (P < 0.001), procedure time (P < 0.05), and contrast dosage (P < 0.001) were significantly lower than in patients treated with rRA after predilatation failure. The reason for this may be that direct high-speed RA of the calcified lesion site makes the serious calcified nodules or calcified plaques removed by the RA size, and the modified and polished wall is relatively conducive to the passage of the balloon or stent, and the IVUS examination found that the calcified ring in the lumen was interrupted to varying degrees after RA, so that the balloon could more easily dilate the lesion, and it was not necessary to use more pre-dilatation balloon, which not only reduced the occurrence of complications, such as entrapment, but also made the procedure smoother. This reduces complications such as entrapment, and also results in a smoother procedure with shorter operative time and lower intraoperative contrast dosage.
Despite the known disadvantage during peri-procedural period (10, 18), the long-term outcome of rRA remains as the research focus of clinical research. We compared the MACCE rate between pRA and rRA, which was similarly low in the two groups (4.8% and 3.7%, P > 0.05). The rates of secondary endpoints, the composite of non-cardiac death, angina pectoris, heart failure and cardiovascular rehospitalization, are also similar between the two groups. Recent studies suggest that periprocedural myocardial infarction (MI) has prognostic significance in non-ST-segment elevation myocardial infarction (NSTEMI) (19). However, this association was not observed in our study, potentially because NSTEMI patients constituted only 18% of the cohort. Furthermore, the lack of correlation between higher procedural complications and MACCE in the rRA group may be explained by the following mechanisms: (i) All periprocedural complications were promptly managed through timely interventions, thereby mitigating the risk of complication-related adverse outcomes. (ii) Patients undergoing rRA inherently exhibited higher baseline risks due to complex coronary anatomy and procedural complexity. Nevertheless, RA-mediated plaque modification significantly improved stent expansion and wall apposition, consequently reducing risks of in-stent thrombosis or restenosis and counterbalancing the long-term prognostic impact of rRA. (iii) Intensified postprocedural antithrombotic regimens (e.g., dual antiplatelet therapy with adjunctive anticoagulation), implemented in response to intraprocedural thrombotic risks, further attenuated long-term event risks in the rRA group.
The clinical implications of these findings are twofold. First, meticulous preprocedural evaluation of PCI candidates should include advanced imaging to characterize high-risk lesions. For instance:
IVUS quantifies calcification thickness (threshold >0.5 mm indicating potential need for RA) and arc curvature. Optical coherence tomography (OCT) detects calcified nodules and microdissections. This imaging-guided approach allows selection of lesions with low likelihood of successful balloon predilation, thereby reducing the requirement for rRA. Second, an pRA first strategy should be prioritized over rRA to minimize periprocedural complications. Second, while pRA remains the preferred approach for severely calcified lesions, rRA demonstrates comparable long-term clinical outcomes and can be safely utilized as a bailout strategy when required by procedural complexity.
Study limitations
This study is a single-centre retrospective study. The sample size is limited, which may bias the results. Multicentre randomized prospective controlled studies with large sample sizes are still needed for further verification of present results.
Conclusion
Rescue RA is related with higher procedural complication rates, procedure time, and contrast agent dose compared with pRA, but has similar low MACCE rate as pRA at one year after procedure. Thus, our results indicate that rescue RA remains as a feasible clinical PCI strategy in indicated patients without safety concern in terms of long-term outcome.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Wuhan Fourth Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL: Writing – review & editing, Project administration, Conceptualization, Supervision, Methodology, Writing – original draft, Data curation, Investigation, Visualization, Resources, Formal analysis, Validation, Funding acquisition, Software. LW: Software, Data curation, Writing – original draft. YL: Writing – original draft. YG: Writing – review & editing. LH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Hubei Provincial Natural Science Foundation of China (2022CFB512).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Warth DC Leon MB O'Neill W Zacca N Polissar NL Buchbinder M . Rotational atherectomy multicenter registry: acute results, complications and 6-month angiographic follow-up in 709 patients. J Am Coll Cardiol. (1994) 24:641–8. 10.1016/0735-1097(94)90009-4
2.
Tran T Brown M Lasala J . An evidence-based approach to the use of rotational and directional coronary atherectomy in the era of drug-eluting stents: when does it make sense?Catheter Cardiovasc Interv. (2008) 72:650–62. 10.1002/ccd.21676
3.
Kuriyama N Kobayashi Y Yamaguchi M Shibata Y . Usefulness of rotational atherectomy in preventing polymer damage of everolimus-eluting stent in calcified coronary artery. JACC Cardiovasc Interv. (2011) 4:588–9. 10.1016/j.jcin.2010.11.017
4.
Hodgson JM Stone GW Lincoff AM Klein L Walpole H Bottner R et al Late stent thrombosis: considerations and practical advice for the use of drug-eluting stents: a report from the society for cardiovascular angiography and interventions drug-eluting stent task force. Catheter Cardiovasc Interv. (2007) 69:327–33. 10.1002/ccd.21093
5.
Tomey MI Kini AS Sharma SK . Current status of rotational atherectomy. JACC Cardiovasc Interv. (2014) 7:345–53. 10.1016/j.jcin.2013.12.196
6.
Clavijo LC Steinberg DH Torguson R Kuchulakanti PK Chu WW Fournadjiev J et al Sirolimus-eluting stents and calcified coronary lesions: clinical outcomes of patients treated with and without rotational atherectomy. Catheter Cardiovasc Interv. (2006) 68:873–8. 10.1002/ccd.20615
7.
Barbato E Carrie D Dardas P Fajadet J Gaul G Haude M et al European expert consensus on rotational atherectomy. EuroIntervention. (2015) 11:30–6. 10.4244/EIJV11I1A6
8.
Kawamoto H Latib A Ruparelia N Boccuzzi GG Pennacchi M Sardella G et al Planned versus provisional rotational atherectomy for severe calcified coronary lesions: insights from the rotate multi-center registry. Catheter Cardiovasc Interv. (2016) 88:881–9. 10.1002/ccd.26411
9.
Abdel-Wahab M Richardt G Joachim Buttner H Toelg R Geist V Meinertz T et al High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized rotaxus (rotational atherectomy prior to taxus stent treatment for complex native coronary artery disease) trial. JACC Cardiovasc Interv. (2013) 6:10–9. 10.1016/j.jcin.2012.07.017
10.
Allali A Abdel-Wahab M Sulimov DS Jose J Geist V Kassner G et al Comparison of bailout and planned rotational atherectomy for heavily calcified coronary lesions: a single-center experience. J Interv Cardiol. (2017) 30:124–33. 10.1111/joic.12361
11.
Qi Z Zheng H Wei Z Dai Q Xie J Wang L et al Short-term and long-term outcomes of bailout versus planned coronary rotational atherectomy. Rev Cardiovasc Med. (2020) 21:309–14. 10.31083/j.rcm.2020.02.36
12.
Aldrich RF Brensike JF Battaglini JW Richardson JM Loh IK Stone NJ et al Coronary calcifications in the detection of coronary artery disease and comparison with electrocardiographic exercise testing. Results from the national heart, lung, and blood institute’s type ii coronary intervention study. Circulation. (1979) 59:1113–24. 10.1161/01.CIR.59.6.1113
13.
Dorros G Cowley MJ Simpson J Bentivoglio LG Block PC Bourassa M et al Percutaneous transluminal coronary angioplasty: report of complications from the national heart, lung, and blood institute ptca registry. Circulation. (1983) 67:723–30. 10.1161/01.CIR.67.4.723
14.
Thygesen K Alpert JS Jaffe AS Chaitman BR Bax JJ Morrow DA et al Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. 10.1016/j.jacc.2018.08.1038
15.
Hicks KA Tcheng JE Bozkurt B Chaitman BR Cutlip DE Farb A et al 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Am Coll Cardiol. (2015) 66(4):403–69. 10.1016/j.jacc.2014.12.018
16.
Schwarz K Lovatt S Borovac JA Parasuraman S Kwok CS . Planned versus bailout rotational atherectomy: a systematic review and meta-analysis. Cardiovasc Revasc Med. (2022) 39:45–51. 10.1016/j.carrev.2021.09.013
17.
Arora S Panaich SS Patel N Patel NJ Savani C Patel SV et al Coronary atherectomy in the United States (from a nationwide inpatient sample). Am J Cardiol. (2016) 117:555–62. 10.1016/j.amjcard.2015.11.041
18.
de Waha S Allali A Buttner HJ Toelg R Geist V Neumann FJ et al Rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: two-year clinical outcome of the randomized rotaxus trial. Catheter Cardiovasc Interv. (2016) 87:691–700. 10.1002/ccd.26290
19.
Armillotta M Bergamaschi L Paolisso P Belmonte M Angeli F Sansonetti A et al Prognostic relevance of type 4a myocardial infarction and periprocedural myocardial injury in patients with non-ST-segment-elevation myocardial infarction. Circulation. (2025) 151:760–72. 10.1161/CIRCULATIONAHA.124.070729
Summary
Keywords
planned rotational atherectomy, rescue rotational atherectomy, outcome, severe calcified lesions, major adverse cardiac and cerebrovascular events (MACCE)
Citation
Liu X, Wan L, Liu Y, Gu Y and Hu L (2025) Planned vs. rescue rotational atherectomy in severe coronary calcification: procedural complications and one-year clinical outcomes. Front. Cardiovasc. Med. 12:1599091. doi: 10.3389/fcvm.2025.1599091
Received
24 March 2025
Accepted
04 June 2025
Published
19 June 2025
Volume
12 - 2025
Edited by
Saraschandra Vallabhajosyula, Brown University, United States
Reviewed by
Zoltan Ruzsa, University of Szeged, Hungary
Matteo Armillotta, University of Bologna, Italy
Updates
Copyright
© 2025 Liu, Wan, Liu, Gu and Hu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Liqun Hu 13986227811@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.