- 1Department of Cardiology, First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 2Department of Cardiology, The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu, China
- 3Department of Cardiology, The Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
Background: The inflammatory burden index (IBI) is a novel and useful inflammatory marker. However, the association between IBI and new-onset atrial fibrillation (NOAF) in patients with ST-segment elevation myocardial infarction (STEMI) remains unclear. This study focuses on exploring the predictive ability of IBI for NOAF after percutaneous coronary intervention (PCI) in STEMI patients.
Materials and methods: This study is a single-center retrospective observational study. Patients diagnosed with STEMI and undergoing primary PCI between October 2022 and February 2025 were continuously enrolled. All enrolled patients received continuous electrocardiogram (ECG) monitoring (>72 h) and were grouped according to whether NOAF occurred during hospitalization. Logistic regression analysis was used to identify potential risk factors for NOAF. Meanwhile, restricted cubic spline (RCS) analysis was employed to thoroughly investigate the possible dose-response relationship between IBI and NOAF.
Results: A total of 696 STEMI patients were finally included in this study. The incidence of NOAF during hospitalization was 62/696 (8.9%). After adjusting for potential confounding factors, the results of multivariate logistic regression analysis showed that left ventricular ejection fraction (OR = 0.928, 95% CI: 0.895–0.962), age (OR = 1.048, 95% CI: 1.022–1.075), and IBI (OR = 1.007, 95% CI: 1.003–1.011) were independent factors for NOAF in STEMI patients (P < 0.05). RCS results suggested that there was a non-linear dose-response relationship between IBI and NOAF. After integrating IBI, the ability of the new model to predict NOAF was significantly improved (NRI = 0.617, 95% CI: 0.360–0.873, P < 0.01; IDI = 0.026, 95% CI: 0.007–0.046, P = 0.008).
Conclusions: Elevated IBI is an independent risk factor for NOAF after PCI in STEMI patients. Integrating IBI can improve the risk stratification for NOAF in STEMI patients.
Background
ST-segment elevation myocardial infarction (STEMI) is one of the major diseases threatening human health (1). Percutaneous coronary intervention (PCI) has currently become an important treatment modality for STEMI, which can significantly improve the prognosis of patients. However, STEMI patients still face numerous complications after PCI. Among them, new-onset atrial fibrillation (NOAF) is one of the most common complications (2, 3). NOAF can significantly increase the risk of adverse events such as thrombus formation, heart failure, and sudden cardiac death (3). More problematically, when atrial fibrillation (AF) coexists with STEMI, the condition becomes more complicated, and there are many dilemmas in the treatment process (4). Therefore, exploring the risk factors of NOAF and establishing a precise and effective risk stratification system are of great significance for improving the prognosis of STEMI patients.
Inflammation plays an important role in the occurrence and development of STEMI and AF. During the onset of STEMI, from the unstable rupture of coronary atherosclerotic plaques to myocardial ischemia-reperfusion injury, the inflammatory response persists throughout and plays a crucial role (5). Similarly, in the pathogenesis of AF, inflammation is considered one of the important factors triggering and maintaining atrial remodeling (6). In recent years, the inflammatory burden index (IBI), as an emerging inflammatory marker, has been proven to be closely associated with various diseases (7–9). However, the relationship between IBI and NOAF in STEMI patients remains unclear. This study aims to explore the relationship between IBI and NOAF in STEMI patients, with the hope of providing valuable references for clinical practice.
Material and methods
Study population
This single-center, retrospective observational study included consecutive patients diagnosed with STEMI (10) at the First People's Hospital of Lianyungang between October 2022 and February 2025. The study protocol was approved by the institutional review board of the First People's Hospital of Lianyungang (KY−20250213001-01). Given the no risk to participants, the requirement for written informed consent was waived. Inclusion criteria were: successful primary PCI within 12 h of symptom onset with post-procedural TIMI grade 3 flow; continuous electrocardiographic (ECG) monitoring for (>72 h) during hospitalization; and availability of complete clinical data. Exclusion criteria included: age <18 years; prior AF or atrial tachycardia; prior myocardial infarction; severe renal insufficiency (GFR < 30 ml/min/1.73 m2); malignancy or inflammatory diseases (any acute or chronic inflammatory condition within 3 months prior to enrollment that could affect inflammatory biomarkers, including autoimmune diseases, chronic inflammatory conditions, and moderate-to-severe infections); severe valvular heart disease; and thyroid dysfunction. In total, 696 patients were included in the analysis, of whom 62 developed NOAF (Figure 1).
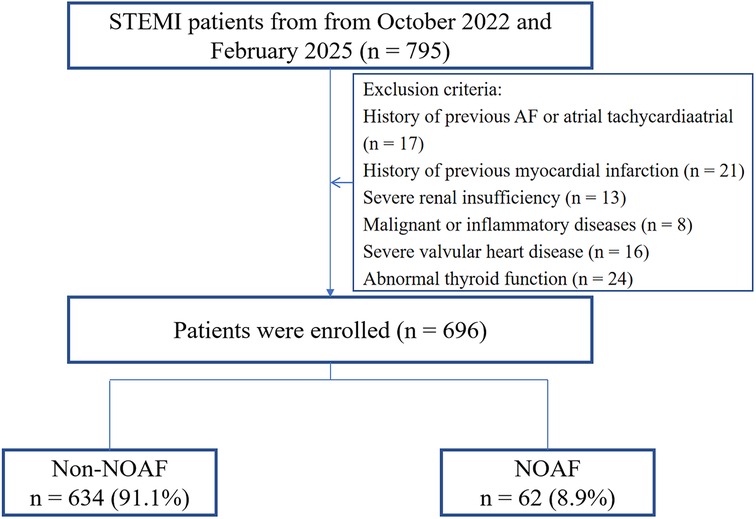
Figure 1. Study flowchart. STEMI ST-segment elevation myocardial infarction; NOAF, new-onset atrial fibrillation.
Clinical data collection
Baseline clinical data were collected for all patients, including sex, age, body mass index (BMI), smoking status, and medical history. Lymphocyte count, neutrophil count, and C-reactive protein (CRP) level were measured before PCI. The neutrophil-to-lymphocyte ratio (NLR) was defined as the ratio of neutrophil to lymphocyte counts, and IBI was defined as the product of CRP and NLR (7–9). During hospitalization, fasting venous blood samples were obtained for laboratory testing, including blood lipids and glucose. The peak values of Troponin T (TnI) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) were recorded. AF was defined as an arrhythmia documented on a single-lead ECG lasting (≥30 s) or on a 12-lead ECG, characterized by the absence of discrete P waves, replacement by fibrillatory waves of variable amplitude, morphology, and cycle length, and completely irregular RR intervals. NOAF was defined as the first occurrence of AF after admission in patients without a prior history of AF (11). The infarct-related artery (IRA) was determined by coronary angiography (CAG).
Statistical analysis
Statistical analyses were performed using SPSS (version 27.0; Chicago, USA) and R (version 4.3.1; R Foundation for Statistical Computing). The Kolmogorov–Smirnov test was used to assess normality. Normally distributed continuous variables are presented as mean ± standard deviation (SD) and compared using Student's t test. Non-normally distributed continuous variables are presented as median (Q25, Q75) and compared using the Mann–Whitney U test. Variables with (P < 0.05) in univariable analyses or deemed clinically relevant for NOAF were entered into a multivariable logistic regression model using stepwise forward method. Restricted cubic splines (RCS) were used to explore the dose–response relationship between IBI and NOAF. Receiver operating characteristic (ROC) curves, net reclassification improvement (NRI), and integrated discrimination improvement (IDI) were used to evaluate the incremental discriminative ability of IBI for NOAF. A two-sided (P < 0.05) was considered statistically significant.
Results
Baseline characteristics of the study population
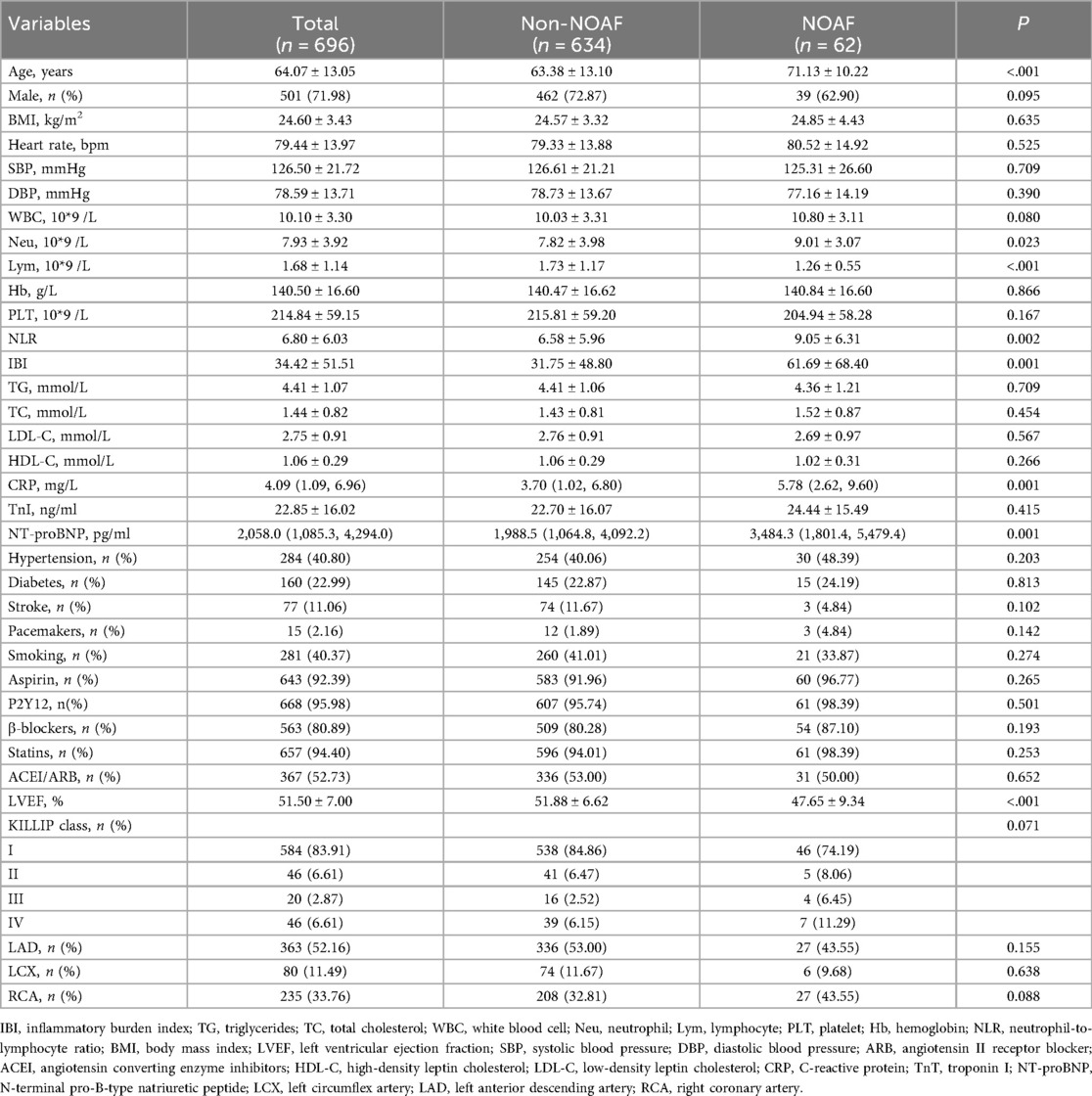
A total of 696 patients were included; of these, 62 (8.9%) developed NOAF. Baseline characteristics are summarized in Table 1. Compared with patients without NOAF, those with NOAF were older (71.13 ± 10.22 years vs. 63.38 ± 13.10 years, P < 0.001). The left ventricular ejection fraction (LVEF) was significantly lower in the NOAF group than in the non-NOAF group (47.65 ± 9.34% vs. 51.88 ± 6.62%, P < 0.001). Laboratory findings showed that the levels of NT-proBNP, neutrophil count, CRP, NLR, and IBI in the NOAF group were higher than those in the non-NOAF group (P < 0.005), while the level of lymphocyte count was lower (P < 0.001).
Univariate and multivariate logistic regression analysis
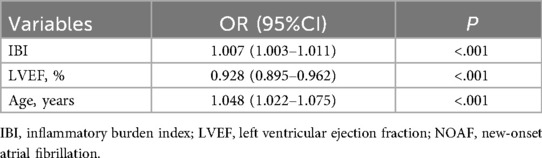
Univariable analyses were performed for all variables listed in Supplementary Table S1. Age, CRP, NT-proBNP, LVEF, Killip class >1, IBI, NLR, neutrophil count, and lymphocyte count were each significantly associated with NOAF (P < 0.05). Variables with (P < 0.05) in univariable analyses or of clinical relevance were entered into multivariable logistic regression. In the final model, LVEF (OR = 0.928, 95% CI: 0.895–0.962), age (OR = 1.048, 95% CI: 1.022–1.075), and IBI (OR = 1.007, 95% CI: 1.003–1.011) were independently associated with NOAF (Table 2). RCS analysis suggested a non-linear dose–response relationship between IBI and NOAF (Figure 2).
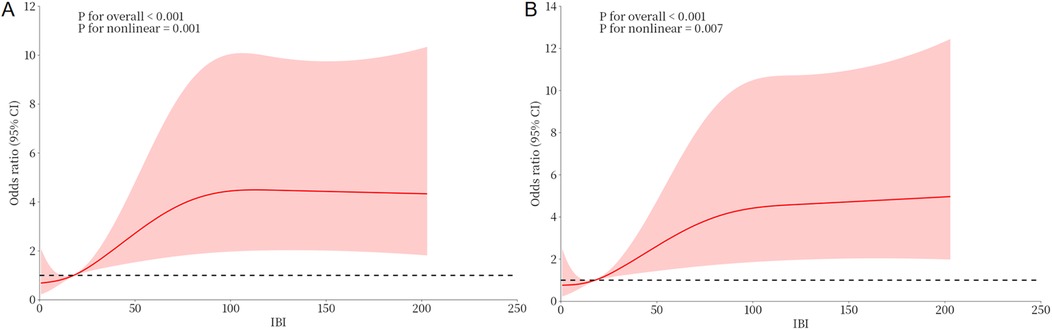
Figure 2. Dose-response relationship between IBI and NOAF from RCS analysis. (A) Unadjusted dose-response relationship between IBI and NOAF; (B) Adjusted dose-response relationship between IBI and NOAF. IBI, inflammatory burden index; NOAF, new-onset atrial fibrillation.
Discriminatory accuracy and reclassification accuracy of IBI for NOAF
ROC analysis showed that IBI demonstrated moderate discriminatory ability for NOAF (AUC = 0.690, 95% CI: 0.620–0.761). The optimal cutoff was 36.85, yielding a sensitivity of 59.7% and a specificity of 73.7% (Figure 3).
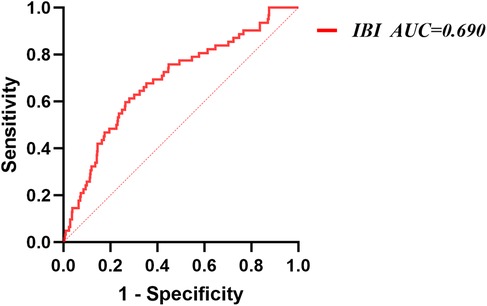
Figure 3. Receiver operating characteristic analysis (ROC) of IBI for NOAF. IBI, inflammatory burden index; NOAF, new-onset atrial fibrillation.
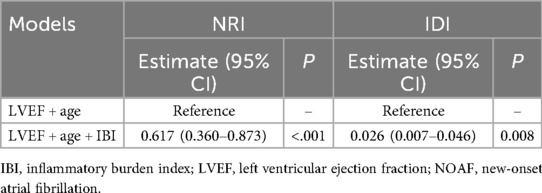
A traditional model was constructed based on the independent risk factors identified by the multivariate analysis, including age, and LVEF. ROC analysis results showed that the AUC of the traditional model for predicting NOAF during hospitalization was 0.719. After adding IBI, the AUC increased to 0.751. DeLong's test indicated that this improvement was statistically significant (Z = 2.203, P = 0.028) (Figure 4). Adding IBI also significantly improved reclassification and overall discrimination (NRI = 0.617, 95% CI: 0.360–0.873, P < 0.01; IDI = 0.026, 95% CI: 0.007–0.046, P = 0.008) (Table 3).
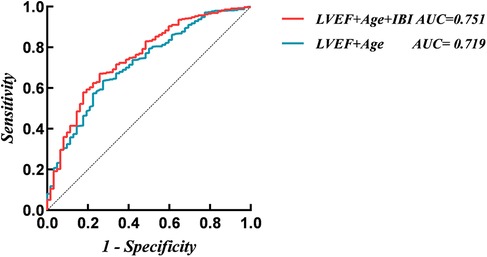
Figure 4. Receiver operating characteristic analysis (ROC) of models for new-onset atrial fibrillation. IBI, inflammatory burden index; LVEF, left ventricular ejection fraction.
Discussion
The main findings of this study were as follows. First, elevated IBI was an independent risk factor for NOAF in STEMI patients. Second, there was a non-linear dose-response relationship between the IBI and NOAF. Third, integration of IBI could improve the risk stratification for NOAF in STEMI patients.
Inflammation is a fundamental pathological process shared by STEMI and AF (5, 6). Previous studies have shown that NLR and CRP, as key indicators of cardiovascular risk, can effectively enhance the risk stratification assessment of patients with various cardiovascular diseases (12). Recently, the IBI, as an emerging inflammatory marker, has been widely used in the risk stratification of cancer patients (7–9). Compared with single inflammatory markers, the IBI, which is calculated from neutrophils, lymphocytes, and CRP through a specific formula, can provide a more stable and accurate assessment of inflammation, thus more precisely reflecting the body's inflammatory state and predicting the prognosis (8, 9, 13). In a cross-sectional study involving 15,325 American adults, Yu et al. found that the IBI level was independently associated with the prevalence of cardiovascular disease (CVD) and was closely related to the occurrence and development of CVD (7). In another multicenter study, the results indicated that the IBI was positively correlated with the risk of poor prognosis in patients with acute stroke (8). However, the relationship between IBI and NOAF after PCI in STEMI patients remains unclear. Our study found that in STEMI patients, elevated IBI was an independent risk factor for NOAF after PCI, and there was a non-linear dose-response relationship between them. CRP, as a traditional inflammatory marker, plays an important role in predicting the onset and prognosis of cardiovascular and cerebrovascular diseases (14, 15). Neutrophils and lymphocytes, as the two main types of peripheral inflammatory cells and important components of the immune system, play an indispensable role in the occurrence, development, and subsequent pathological processes of inflammation (16–18). During the acute phase of STEMI, the body is in a stress state. At this time, the elevated levels of catecholamines and cortisol lead to a decrease in lymphocyte count and an increase in neutrophil count and CRP level (19). A large number of inflammatory cells infiltrate the atrial tissue and release various cytokines and inflammatory mediators. These mediators can alter the function of ion channels, creating conditions for the occurrence of reentrant arrhythmias (20, 21). Additionally, the inflammatory state indicated by an elevated IBI may activate the sympathetic nervous system or inhibit the vagus nervous system (22, 23). Sympathetic nerve excitation can increase the automaticity of atrial myocytes and shorten the effective refractory period of the atria, making the atria more prone to fibrillation (24, 25). A decrease in vagal tone weakens its protective effect on the cardiac rhythm (26). The imbalance between the two provides a favorable neuroregulatory environment for the occurrence of AF (24, 26). The IBI analyzes the balance between acute inflammation and immune-mediated inflammation by comprehensively considering CRP and NLR, thus presenting a relatively complete picture of the body's pro-inflammatory and immune states. This may, to some extent, explain the relevant results of our study.
In addition, the results of our study are consistent with previous research findings (27, 28), further confirming that age and LVEF are also independent risk factors for NOAF in patients with STEMI. After incorporating the IBI into the traditional risk model, the predictive ability of the model for NOAF was significantly improved. In STEMI complicated with NOAF, accurate identification of risk factors is crucial for early intervention and improving prognosis. Incorporating IBI into the category of risk factors for NOAF helps clinicians more comprehensively and accurately assess the risk of NOAF after PCI in STEMI patients, providing additional information for the formulation of individualized prevention and treatment strategies. In clinical practice, for STEMI patients with high IBI, low LVEF, and advanced age, extended rhythm monitoring may be considered. In our study, the IBI cut-off was 36.85. To enhance clinical practicality, a more pragmatic stratification approach could be adopted in practice (e.g., using rounded thresholds such as >35 or >40) to guide enhanced patient monitoring. Moreover, it is important to emphasize that this cut-off requires external validation in independent cohorts to assess its generalizability and robustness.
Limitations
This study has several limitations that need to be addressed. First, it is a single-center retrospective study, and the generalization of its conclusions may require validation through more multicenter randomized controlled trials. Second, in our study, other comorbidities or acute conditions may influence inflammatory markers and increase the risk of NOAF. As this is a retrospective study, although we controlled for several factors, residual confounding may persist and could introduce potential bias. Third, although the results of this study confirm the association between the IBI and NOAF in patients with STEMI, the specific underlying mechanism may need further basic research for clarification. Fourth, due to the lack of long-term electrocardiogram monitoring of patients before admission, some previous histories of atrial fibrillation, especially cryptogenic atrial fibrillation, may have been overlooked. Fifth, NOAF during the chronic phase after discharge following STEMI is also an important topic that merits attention. However, we currently lack follow-up data in this area, and more targeted future studies are warranted.
Conclusions
Elevated IBI is an independent risk factor for NOAF after PCI in STEMI patients. Integration of IBI can improve the risk stratification for NOAF in STEMI patients. IBI may serve as a simple tool to identify high-risk patients, thereby supporting the implementation of intensified monitoring strategies and individualized management in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the study protocol was approved by the institutional review board of the First People's Hospital of Lianyungang (KY-20250213001-01). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin given the low risk to patients.
Author contributions
KL: Writing – review & editing, Writing – original draft. ZT: Writing – original draft, Writing – review & editing. GL: Writing – review & editing, Writing – original draft. ML: Writing – original draft, Writing – review & editing. JY: Writing – review & editing, Writing – original draft. LZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Lianyungang Municipal Health Commission (No. 202009, 202412).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1599152/full#supplementary-material
References
1. Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E, et al. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. (2019) 5(1):39. doi: 10.1038/s41572-019-0090-3
2. Chen L, Zhang M, Chen W, Li Z, Wang Y, Liu D, et al. Cardiac MRI left atrial strain associated with new-onset atrial fibrillation in patients with ST-segment elevation myocardial infarction. J Magn Reson Imaging. (2023) 58(1):135–44. doi: 10.1002/jmri.28491
3. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
4. Jortveit J, Sandberg EL, Pripp AH, Halvorsen S. Time trends in adherence to guideline recommendations for anticoagulation therapy in patients with atrial fibrillation and myocardial infarction. Open Heart. (2022) 9(1):e001934. doi: 10.1136/openhrt-2021-001934
5. Dong Z, Hou L, Luo W, Pan LH, Li X, Tan HP, et al. Myocardial infarction drives trained immunity of monocytes, accelerating atherosclerosis. Eur Heart J. (2024) 45(9):669–84. doi: 10.1093/eurheartj/ehad787
6. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
7. Yu F, Peng J. Association between inflammatory burden Index and cardiovascular disease in adult Americans: evidence from NHANES 2005–2010. Heliyon. (2024) 10(18):e38273. doi: 10.1016/j.heliyon.2024.e38273
8. Du M, Xu L, Zhang X, Huang X, Cao H, Qiu F, et al. Association between inflammatory burden Index and unfavorable prognosis after endovascular thrombectomy in acute ischemic stroke. J Inflamm Res. (2023) 16:3009–17. doi: 10.2147/JIR.S419087
9. Song Z, Lin F, Chen Y, Li T, Li R, Lu J, et al. Inflammatory burden index: association between novel systemic inflammatory biomarkers and prognosis as well as in-hospital complications of patients with aneurysmal subarachnoid hemorrhage. J Inflamm Res. (2023) 16:3911–21. doi: 10.2147/JIR.S416295
10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72(18):2231–64. doi: 10.1016/j.jacc.2018.08.1038
11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
12. Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11(1):55–9. doi: 10.1586/erc.12.159
13. Xie H, Ruan G, Ge Y, Zhang Q, Zhang H, Lin S, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. (2022) 41(6):1236–43. doi: 10.1016/j.clnu.2022.04.019
14. Guedeney P, Claessen BE, Kalkman DN, Aquino M, Sorrentino S, Giustino G, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. (2019) 73(19):2401–9. doi: 10.1016/j.jacc.2019.01.077
15. Shacham Y, Leshem-Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clin Exp Nephrol. (2015) 19(5):838–43. doi: 10.1007/s10157-014-1071-1
16. Zhang B, Lin L, Yuan F, Song G, Chang Q, Wu Z, et al. Clinical application values of neutrophil-to-lymphocyte ratio in intracranial aneurysms. Aging (Albany NY). (2021) 13(4):5250–62. doi: 10.18632/aging.202445
17. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. (2018) 9:754. doi: 10.3389/fimmu.2018.00754
18. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. (2005) 6(10):775–86. doi: 10.1038/nrn1765
19. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of the joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction [published correction appears in J am coll cardiol 2001 mar 1;37(3):973]. J Am Coll Cardiol. (2000) 36(3):959–69. doi: 10.1016/s0735-1097(00)00804-4
20. Boos CJ. Infection and atrial fibrillation: inflammation begets AF. Eur Heart J. (2020) 41(10):1120–2. doi: 10.1093/eurheartj/ehz953
21. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60(22):2263–70. doi: 10.1016/j.jacc.2012.04.063
22. Bazoukis G, Stavrakis S, Armoundas AA. Vagus nerve stimulation and inflammation in cardiovascular disease: a state-of-the-art review. J Am Heart Assoc. (2023) 12(19):e030539. doi: 10.1161/JAHA.123.030539
23. McAllen RM, McKinley MJ, Martelli D. Reflex regulation of systemic inflammation by the autonomic nervous system. Auton Neurosci. (2022) 237:102926. doi: 10.1016/j.autneu.2021.102926
24. Khan AA, Lip GYH, Shantsila A. Heart rate variability in atrial fibrillation: the balance between sympathetic and parasympathetic nervous system. Eur J Clin Invest. (2019) 49(11):e13174. doi: 10.1111/eci.13174
25. Kusayama T, Douglas A 2nd, Wan J, Doytchinova A, Wong J, Mitscher G, et al. Skin sympathetic nerve activity and ventricular rate control during atrial fibrillation. Heart Rhythm. (2020) 17(4):544–52. doi: 10.1016/j.hrthm.2019.11.017
26. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. (2014) 114(9):1500–15. doi: 10.1161/CIRCRESAHA.114.303772
27. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. (2009) 30(9):1038–45. doi: 10.1093/eurheartj/ehn579
28. Elshaer F, Alsaeed AH, Alfehaid SN, Alshahrani AS, Alduhayyim AH, Alsaleh AM. Incidence, clinical predictors, and clinical effect of new-onset atrial fibrillation in myocardial infarction patients: a retrospective cohort study. Saudi Med J. (2022) 43(8):933–40. doi: 10.15537/smj.2022.43.8.20220349
Keywords: inflammation response, inflammatory burden index, atrial fibrillation, ST-segment elevation myocardial infarction, risk stratification
Citation: Liu K, Tao Z, Li G, Li M, Yin J and Zhou L (2025) Predictive value of inflammatory burden index for new-onset atrial fibrillation in STEMI patients. Front. Cardiovasc. Med. 12:1599152. doi: 10.3389/fcvm.2025.1599152
Received: 24 March 2025; Accepted: 4 September 2025;
Published: 16 September 2025.
Edited by:
Alexandre Almorad, University Hospital Brussels, BelgiumReviewed by:
Mario Matta, AOU Città della Salute e della Scienza, ItalyVedran Pasara, University Hospital Centre Zagreb, Croatia
Copyright: © 2025 Liu, Tao, Li, Li, Yin and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayu Yin, NzgyMjQwMzkzQHFxLmNvbQ==; Lei Zhou, emhvdWxlaUBuam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Kun Liu
Kun Liu Zhiwen Tao1,†
Zhiwen Tao1,† Jiayu Yin
Jiayu Yin Lei Zhou
Lei Zhou