Abstract
Atherosclerosis (AS) is a major cause of cardiovascular disease morbidity and mortality, characterized by lipid accumulation, oxidative stress, and chronic inflammation. Natural organic sulfur compounds (OSCs), especially those derived from garlic (Allium sativum), have therapeutic value in slowing the course of AS. We systematically evaluate the mechanisms by which OSCs exert their anti-atherogenic effects, focusing on lipid metabolism regulation, antioxidant defense, anti-inflammatory responses, endothelial protection, and antibacterial activity. Key signaling pathways, including Nrf2/ARE, RhoA/ROCK, AMPK/SREBP-1c/SREBP-2, and PCSK9-LDLR, are highlighted as critical mediators of these effects. Preclinical and clinical investigations show that OSCs significantly reduce plasma cholesterol, suppress oxidative stress, and attenuate inflammatory cascades. However, challenges such as variable bioavailability and the absence of standardized formulations limit their clinical application. Future research should focus on clinical trials to establish efficacy, improve bioavailability, and create standardized formulations of OSCs for cardiovascular disease prevention and management.
1 Introduction
Cardiovascular disease (CVD) persists as the predominant cause of mortality across all disease categories in China. Notably, since 2020, CVD mortality rates in rural regions have consistently exceeded those in urban areas. Statistical data from 2024 indicate that the age-standardized CVD mortality rate reached 298.42 per 100,000 population in rural areas, compared to 264.84 per 100,000 in urban areas (1). This epidemiological pattern underscores the significant public health burden imposed by CVD, with atherosclerosis (AS) constituting the fundamental pathological mechanism underlying most CVD cases (2).
The development and progression of AS are intrinsically associated with plasma lipid levels, particularly total cholesterol (TC) and triglycerides (TG) concentrations.
Pathophysiological studies have demonstrated that elevated plasma lipid levels facilitate the infiltration and deposition of lipids within the arterial intima, subsequently triggering smooth muscle cell proliferation and foam cell formation—the hallmark cellular events in atherogenesis (3). Contemporary research has identified multiple risk factors contributing to AS pathogenesis, including but not limited to hypertension, diabetes mellitus, hyperlipidemia, obesity, tobacco use, and microbial infections. Recent investigations, illustrated in Figure 1, show that the complicated pathophysiology of AS involves numerous connected pathways, including oxidative stress, lipid accumulation, inflammatory response, and endothelial injury (4–6).
Figure 1
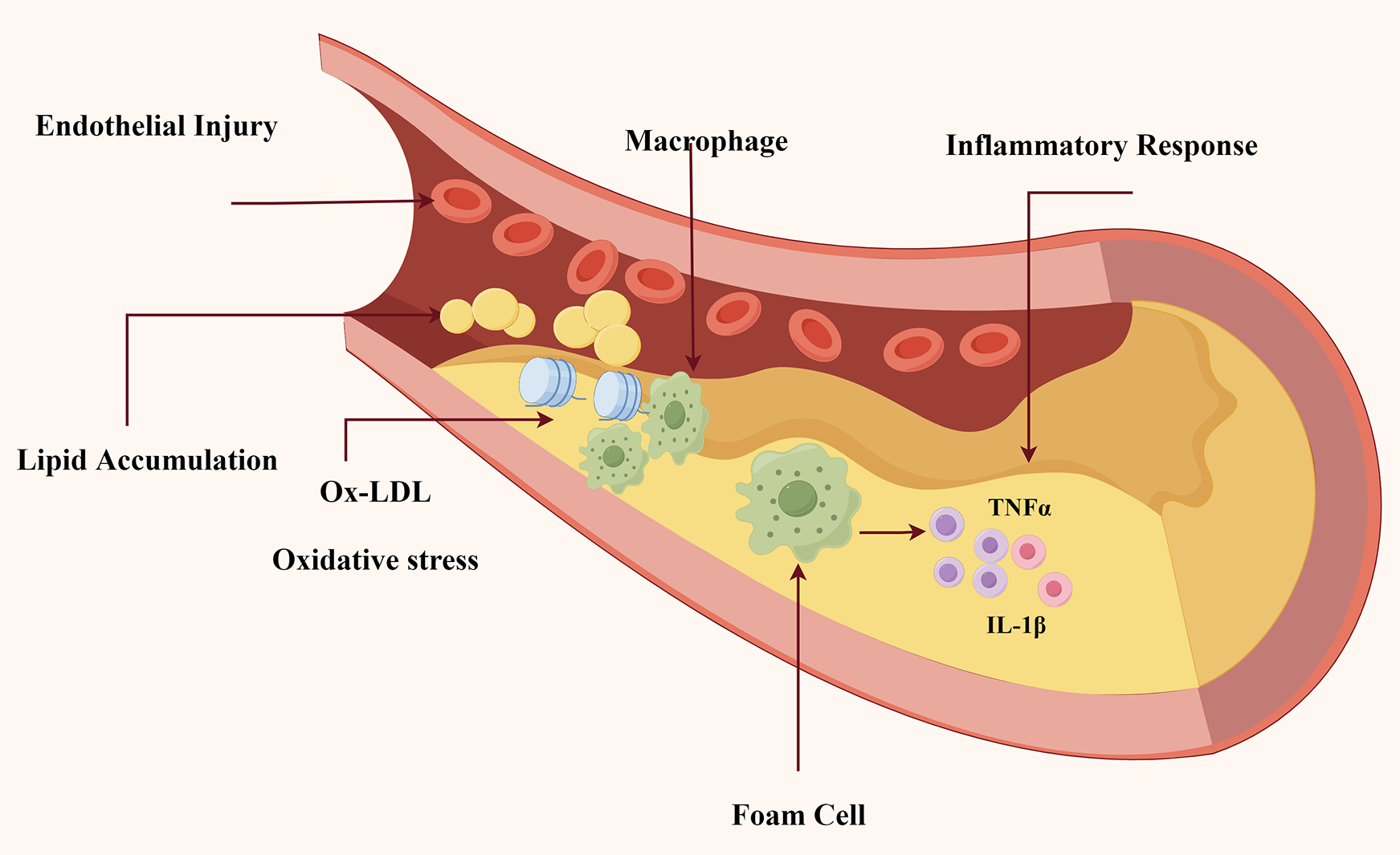
The underlying pathogenesis in atherosclerosis. As indicated in Figure. This schematic diagram depicts the essential pathophysiological processes involved in the beginning and development of AS: (1) Injury to endothelial cells initiates atherosclerotic plaque formation; (2) Lipid accumulation; and (3) Ox-LDL infiltration and macrophage recruitment; (4) Macrophages transform into foam cells and release pro-inflammatory cytokines (TNF-α, IL-1β), leading to persistent inflammation and plaque instability.
Natural organosulfur compounds (OSCs), a class of bioactive molecules with both hydrophilic and lipophilic properties, are predominantly distributed in plant species belonging to the Cruciferae and Liliaceae families (7). Among these, garlic (Allium sativum) has attracted particular attention from the scientific community due to its rich OSC content. Biochemical analyses have identified over 30 distinct OSCs in garlic, with the majority being enzymatically derived from L-cysteine sulfoxides and γ-glutamyl-L-cysteine peptides through various biosynthetic pathways (8).
Freeze-dried preparations of wild garlic (Allium ursinum) cloves have been shown to contain a diverse array of OSCs, with alliin being the predominant form stored in the cytoplasmic compartment of intact cells. The mechanical disruption of garlic cloves triggers the enzymatic conversion of alliin by alliinase, leading to the formation of allicin, which is responsible for the characteristic pungent aroma (9). Historical records from traditional Chinese medicine document the use of raw garlic for the prevention and treatment of atherosclerotic conditions.
Modern epidemiological evidence supports this traditional knowledge, demonstrating an inverse correlation between the consumption of fruits and vegetables and cardiovascular disease risk (10, 11). This relationship has been further substantiated by a longitudinal study involving 1,226 Australian women aged over 70 years, which revealed that increased consumption of cruciferous and liliaceous vegetables (≥75 g/day) was associated with reduced mortality from atherosclerotic cardiovascular disease over a 15-year follow-up period (12).
This review synthesizes current scientific literature to investigate the anti-atherosclerotic properties of OSCs and their underlying molecular mechanisms. Meanwhile, the purpose of this review is to summarize the current evidence on OSC-mediated anti-atherosclerotic mechanisms, addressing gaps in clinical translation and proposing future research directions.
2 Sources and classification of natural OSCs
Organosulfur compounds (OSCs) represent a distinct class of phytochemicals characterized by the presence of sulfur-containing functional groups within their molecular architecture. These bioactive compounds are predominantly concentrated in garlic bulbs, with particularly high levels being detected in this plant species (13). Although garlic is the primary source of OSCs, other important dietary sources include the Cruciferae (broccoli, Brussels sprouts) and Liliaceae (onions, leeks) families. For instance: Broccoli contains sulforaphane (SFN), a strong lipophilic OSC with anti-inflammatory as well as antioxidant properties. Similarly, onions contain allicin, diallyl disulfide (DADS), diallyl trisulfide (DATS), and other sulfur compounds that have been associated with cardiovascular benefits to health. Based on their physicochemical properties, OSCs can be categorized into two primary groups: lipophilic (fat-soluble) and hydrophilic (water-soluble) compounds, as shown in Figure 2.
Figure 2
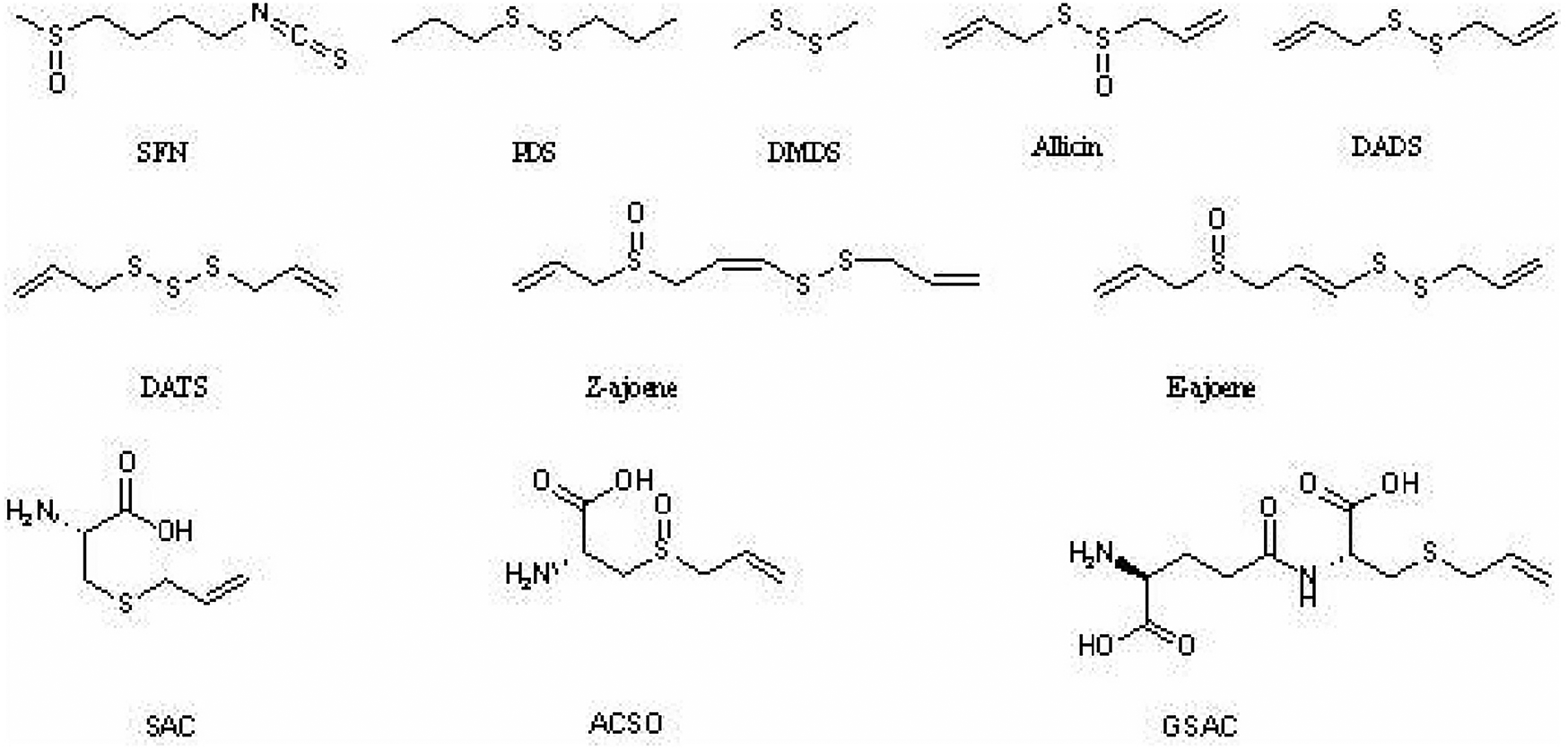
Chemical structure of natural organosulfur compounds. Figure shows the chemical structure of lipophilic (fat-soluble) and hydrophilic (water-soluble) natural organosulfur compounds with anti-atherosclerotic properties: (1) Lipophilic OSCs: sulforaphane (SFN), allicin, dimethyl disulfide (DMDS), propyl disulfide (PDS), diallyl disulfide (DADS), diallyl trisulfide (DATS), cis-ajoene (Z-ajoene), and trans-ajoene (E-ajoene); (2) Hydrophilic OSCs: S-allyl-L-cysteine (SAC), S-allyl-L-cysteine-sulfoxide (ACSO), and γ-L-glutamyl-S-allyl-L-cysteine (GSAC).
The lipophilic fraction of OSCs with demonstrated anti-atherosclerotic activity includes several well-characterized compounds: sulforaphane (SFN), allicin, dimethyl disulfide (DMDS), propyl disulfide (PDS), diallyl disulfide (DADS), diallyl trisulfide (DATS), cis-ajoene (Z-ajoene), and trans-ajoene (E-ajoene). Conversely, the hydrophilic OSC group with anti-atherogenic properties comprises S-allyl-L-cysteine (SAC), S-allyl-L-cysteine-sulfoxide (ACSO), and γ-L-glutamyl-S-allyl-L-cysteine (GSAC). Extensive research has been conducted on the anti-atherogenic mechanisms of lipophilic OSCs, particularly allicin and SFN. Similarly, water-soluble OSCs, including SAC, S-ethyl-L-cysteine (SEC), ACSO, and GSAC, have demonstrated significant pharmacological potential through multiple mechanisms, such as lipid-lowering, antioxidant, and anti-inflammatory effects (14, 15).
3 Biological functions of natural organosulfur compounds in anti-atherosclerosis
3.1 Lipid-lowering activity
Dyslipidemia, characterized by abnormal elevations in plasma lipid concentrations, particularly total cholesterol (TC) and low-density lipoprotein (LDL), represents a well-established risk factor for the development of atherosclerosis (AS) (16, 17). This pathological condition is specifically defined by increased LDL concentrations coupled with decreased high-density lipoprotein (HDL) levels. Recent pharmacological studies have demonstrated that garlic oil nanoemulsion exhibits significant lipid-lowering effects in hyperlipidemic Wistar rat models (18, 19).
The cholesterol-reducing properties of aged garlic extract (AGE) have been consistently observed in both human clinical trials and animal studies. Mechanistic investigations using Sprague-Dawley (SD) rats have revealed that this hypocholesterolemic effect is primarily mediated through the inhibition of hepatic cholesterol biosynthesis pathways (20). in vitro experimental data have further demonstrated that water-soluble OSCs, particularly SAC, effectively suppress cholesterol synthesis (21). The integration of in vitro and in vivo findings suggests that SAC may represent the primary bioactive component responsible for AGE-mediated plasma cholesterol reduction (22, 23).
Furthermore, allicin has been shown to ameliorate lipid metabolic disorders in HepG2 cells induced by 1,3-dichloropropanol exposure, as evidenced by significant reductions in both TG and TC levels (24). The molecular mechanisms underlying these effects appear to involve the modulation of key signaling pathways, including AMPK-SREBPs and PKA-CREB (25). Complementary research by Ma et al. (26) has revealed that DADS suppresses lipoprotein (a) [Lp(a)] expression in HepG2 cells through the MEK1-ERK1/2-ELK-1 signaling cascade.
Current research indicates that OSCs like allicin and DADS modulate the lipid metabolic process of HepG2 cells via pathways such as AMPK-SREBPs and MEK1-ERK1/2-ELK-1, resulting in effective lipid-lowering effects. Clinical investigations have demonstrated the cholesterol-lowering effects of AGE, and in vivo and in vitro experiments have confirmed that SAC is the fundamental bioactive component responsible for AGE-mediated plasma cholesterol reduction. The absence of standardized formulations and variable bioavailability of OSCs, however, makes OSCs difficult to use in treating AS. To improve practical applicability, future research should focus on nanocarrier-based drug delivery technologies and statin combination therapy.
3.2 Antioxidant activity
Oxidative stress represents a state of persistent cellular oxidative damage in biological systems, resulting from either excessive production of reactive oxygen species (ROS) or diminished antioxidant defense capacity. Clinical studies have demonstrated that individuals with chronic metabolic disorders, including AS, hypertension, hyperlipidemia, and diabetes mellitus, exhibit increased susceptibility to AS progression when experiencing systemic oxidative stress (27, 28). These findings underscore the pivotal role of oxidative stress in the pathogenesis of AS.
Under normal physiological conditions, ROS serve essential signaling functions at regulated concentrations. However, oxidative stress ensues when ROS production exceeds the cellular antioxidant capacity. Experimental evidence indicates that allicin (0.3–10 μmol/L) confers concentration-dependent protection against H2O2-induced cytotoxicity in H9C2 cardiomyocytes (29). Although allicin demonstrates limited 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, its cytoprotective effects appear to be mediated through the reduction of intracellular ROS accumulation, as proposed by Ma et al. (30). Complementary research by Wang et al. has reported that water-soluble OSCs, specifically SAC, ACSO, and GSAC, exhibit significant antioxidant properties through DPPH radical scavenging and ferrous ion chelation mechanisms (31).
Pathophysiological investigations have revealed that under hyperoxic conditions, LDL undergoes oxidation to form oxidized LDL (ox-LDL), which exerts cytotoxic effects on vascular endothelial cells. The resulting endothelial dysfunction subsequently accelerates LDL oxidation, thereby establishing a vicious cycle that promotes atherogenesis (32, 33). Recent research have identified that DADS and DATS protect endothelial nitric oxide synthase (eNOS) activity against ox-LDL-induced damage, potentially through modulation of the PI3 K/PKB signaling pathway and inhibition of eNOS degradation (34).
Chronic hyperglycemia-induced oxidative stress represents a significant contributor to endothelial dysfunction and atherogenesis (35). Preclinical studies have shown that DATS administration significantly reduces malondialdehyde (MDA) and ROS levels while enhancing mitochondrial superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity (36). Furthermore, DATS has been shown to improve mitochondrial respiratory function, suggesting its potential therapeutic application in oxidative stress-related pathologies, including AS, diabetes mellitus, and neurodegenerative disorders.
In summary, OSCs reduce oxidative stress through a variety of approaches, including ROS suppression, DPPH radical scavenging, ferrous ion chelation, and upregulation of endogenous antioxidant enzymes such as SOD and GSH-Px. In particular, DADS and DATS protect endothelial cells from ox-LDL-induced damage. A suitable dose of antioxidant will be required for future OSCs investigation.
3.3 Anti-inflammatory activity
The understanding of AS pathogenesis has evolved significantly from the traditional view of passive lipid accumulation in arterial walls to the current recognition of AS as a chronic inflammatory disease. This paradigm shift is supported by the identification of various inflammatory mediators and immune cells that contribute to the development and progression of atherosclerotic plaques (37).
Emerging evidence suggests that SAC exhibits anti-inflammatory properties through multiple mechanisms. Experimental studies have demonstrated that SAC may prevent skeletal muscle atrophy by modulating the expression of specific pro-inflammatory factors (38). Furthermore, SAC has been shown to mitigate lipopolysaccharide (LPS)-induced inflammation in 3T3-L1 adipocytes through upregulation of anti-inflammatory gene expression and metabolic modulation (39, 40).
The anti-inflammatory effects of OSCs extend beyond SAC. Research has revealed that (Z, E)-ajoene and its sulfonylated derivatives inhibit LPS-induced production of nitric oxide (NO) and prostaglandin E2 (PGE2) by suppressing inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression (41). This effect is mediated through the inhibition of NF-κB activation and reduction of p38 and ERK phosphorylation. Similarly, DATS has been shown to attenuate cytokine production and inflammatory mediator release, including interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), iNOS, and COX-2, through the blockade of NF-κB and MAPK signaling pathways (42).
Complementary studies by Chu et al. using an LPS-induced RAW 264.7 macrophage model demonstrated that DADS, DMDS, and propyl disulfide (PDS) effectively suppress NO and PGE2 synthesis, thereby limiting LPS-induced inflammatory responses (43, 44). Collectively, both in vitro and in vivo studies indicate that OSCs exert their anti-inflammatory effects primarily through the inhibition of NF-κB and MAPK signaling pathways, as well as the suppression of inflammatory mediators such as IL-6, IL-10, and TNF-α.
3.4 Protective effect on endothelial cells
Vascular endothelial cells play a crucial role in maintaining vascular homeostasis by regulating vascular smooth muscle cell proliferation and migration, while simultaneously modulating inflammatory responses and thrombotic processes. These protective functions are essential for maintaining vascular integrity (45). Disruption of endothelial function initiates a cascade of pathophysiological events that contribute to the development and progression of AS.
Experimental studies have demonstrated that allicin exerts protective effects against oxidized low-density lipoprotein (ox-LDL)-induced injury in human umbilical vein endothelial cells (HUVECs) through the inhibition of apoptotic pathways and attenuation of oxidative stress. Clinical investigations by Liu et al. have further revealed that allicin administration significantly reduces plasma homocysteine (Hcy) levels in patients with coronary heart disease (46). Elevated Hcy concentrations, particularly in the range of 10–1,000 μmol/L, have been shown to negatively correlate with HUVEC viability, with concentrations reaching 1,000 μmol/L resulting in significant endothelial cell dysfunction and apoptosis (47, 48).
DATS has emerged as a potential therapeutic agent through its ability to release hydrogen sulfide (H2S), a gaseous signaling molecule. Under physiological conditions, DATS-mediated H2S release enhances vascular responses following ischemic events, primarily through increased bioavailability of H2S and subsequent activation of endothelial nitric oxide synthase (eNOS) (49, 50). As the third endogenous gasotransmitter following nitric oxide (NO) and carbon monoxide (CO), H2S has been demonstrated to promote endothelial cell proliferation and migration at physiological concentrations (10–20 μmol/L). However, this stimulatory effect transitions to inhibition at elevated concentrations (500–1,000 μmol/L) (51, 52).
In conclusion, OSCs, such as allicin and DATS, protect vascular endothelial cells via three different processes: (1) suppression of apoptotic pathways, (2) lowering of plasma Hcy levels, and (3) H2S-mediated activation of eNOS function. Future research should focus on recognizing the therapeutic potential of OSCs-derived H2S donors in endothelial protection and their function in preventing AS development.
3.5 Antibacterial activity
According to epidemiological studies, a variety of bacterial and viral infections have been causally linked to the development of AS. Lin et al. identified pathogenic microorganisms in both human atherosclerotic tissues and animal models of AS (53).
Porphyromonas gingivalis (P. gingivalis), a key pathogen implicated in periodontitis, has been demonstrated to promote lipid accumulation in the vascular wall and contribute to the development of AS through multiple molecular mechanisms (54, 55). in vitro studies investigating the antibacterial efficacy of allicin against oral pathogens associated with dental caries and periodontitis have revealed that allicin, at a concentration of 2,400 μg/ml, significantly inhibits the growth of P. gingivalis (56).
Quorum sensing (QS), a bacterial cell-to-cell communication system, represents a promising target for anti-pathogenic interventions. Recent research has identified that Z-ajoene, a compound containing disulfide linkages, exhibits significant QS inhibitory properties (57). Moreover, DADS, another organosulfur compound, has demonstrated potent antibacterial activity against clinically relevant pathogens, including Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli (58). These findings highlight the potential of OSCs as therapeutic agents targeting both bacterial growth and virulence mechanisms.
In overall, DADS has broad-spectrum antibacterial activity, while Z-ajoene has considerable QS inhibitory properties, and allicin has a significant antibacterial effect on P. gingivalis, a periodontal pathogen implicated in AS pathogenesis through chronic infection. However, allicin's poor in vivo stability restricts its clinical application. The combination of stable analogues (for instance, allicin-loaded nanoparticles) and QS inhibitors may enhance pharmacological efficacy.
Up to now, the anti-atherosclerotic effects of OSCs, including their classification (lipophilic/hydrophilic), biological functions, mechanisms, study models, and key findings have been summarized in Table 1.
Table 1
| Compound | Class | Biological function | Mechanism of action | Study model | Key findings | Reference |
|---|---|---|---|---|---|---|
| Allicin | Lipophilic | Lipid-lowering | Inhibits hepatic cholesterol synthesis via AMPK-SREBP pathway | HepG2 cells, SD rats | Reduces TC, TG levels; activates AMPK | (24, 25) |
| SAC (S-allyl-L-cysteine) | Hydrophilic | Lipid-lowering | Suppresses cholesterol synthesis | in vitro, clinical trials | Primary bioactive component in AGE for cholesterol reduction | (21–23) |
| DADS (diallyl disulfide) | Lipophilic | Lipid-lowering | Suppresses Lp(a) via MEK1-ERK1/2-ELK-1 pathway | HepG2 cells | Reduces lipoprotein(a) expression | (26) |
| DATS (diallyl trisulfide) | Lipophilic | Antioxidant | Increases SOD, GSH-Px; reduces MDA, ROS | Preclinical models | Protects against ox-LDL-induced endothelial damage | (34, 36) |
| Allicin | Lipophilic | Antioxidant | Activates Nrf2/ARE pathway | H9C2 cells | Reduces H2O2-induced oxidative stress | (29, 30) |
| SAC | Hydrophilic | Antioxidant | DPPH radical scavenging, ferrous ion chelation | in vitro | Direct antioxidant activity | (31) |
| Z-ajoene | Lipophilic | Antibacterial | Quorum sensing inhibition | in vitro | Inhibits bacterial communication | (57) |
| Allicin | Lipophilic | Antibacterial | Growth inhibition of P. gingivalis | in vitro | 2,400 μg/ml inhibits periodontal pathogen | (56) |
| DADS | Lipophilic | Antibacterial | Broad-spectrum activity | in vitro | Effective against S. aureus, P. aeruginosa | (58) |
| Alliin | Hydrophilic | Lipid regulation | Activates AMPK/SREBP-2/LDLR pathway | HepG2 cells, SD rats | Reduces plasma LDL-C levels | (73, 77) |
| SFN (sulforaphane) | Lipophilic | Anti-inflammatory | Inhibits RhoA/ROCK/NF-κB pathway | Endothelial cells | Reduces ICAM-1 expression | (67, 68) |
Anti-atherosclerotic effects of natural organosulfur compounds.
4 Associated signaling pathways of natural organosulfur compounds in anti-atherosclerosis
4.1 The Nrf2/ARE signaling pathway
The Nrf2/ARE signaling pathway is crucial in the prevention and treatment of various diseases, including anti-atherosclerosis, anti-inflammation, and anti-oxidation (59). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key activator of the antioxidant responsive element (ARE) (60, 61). ARE is located in the upstream regulatory region of several protective genes and functions primarily by inducing the production of protective proteins through the coordination of these genes, thereby mitigating cellular damage caused by ROS.
Allicin could reduce lipopolysaccharide (LPS)-induced vascular injury in HUVECs (62). The protective mechanism of allicin is closely associated with its anti-oxidative stress and anti-inflammatory properties. Specifically, allicin was found to activate Nrf2, thereby protecting against LPS-induced vascular injury and attenuating vascular inflammation (63). Furthermore, studies have shown that organosulfur compounds such as SAC can directly activate Nrf2 to exert antioxidant effects (64). Other organic sulfides, such as alliin, competitively inhibit the expression of ARE genes due to the presence of cysteine groups in their structure, which interact with Nrf2, thereby playing an antioxidant role.
4.2 The rhoA/ROCK signaling pathway
The RhoA/ROCK signaling pathway is integral to both the initial stages of AS, particularly endothelial cell dysfunction, and the later stages involving the formation and rupture of atherosclerotic plaques (65). This pathway regulates the expression of endothelial nitric oxide synthase (eNOS), which decreases with increased eNOS mRNA stability, thereby protecting endothelial function from damage. Additionally, the RhoA/ROCK pathway enhances endothelial permeability by directly phosphorylating myosin light chain (MLC) or inhibiting myosin light chain phosphatase (MLCP) activity (66). It also mediates the proliferation and migration of vascular smooth muscle cells (VSMCs).
SFN inhibits the NF-κB DNA-binding protein and downregulates TNF-α-mediated intercellular adhesion molecule-1 (ICAM-1) expression in endothelial cells by inhibiting the RhoA/ROCK/NF-κB signaling pathway (67). Consequently, SFN plays a multifaceted role in inhibiting inflammation within atherosclerotic lesions, highlighting its potential in the prevention and treatment of AS and other inflammatory diseases (68).
4.3 The AMPK/SREBP-1c/SREBP-2 signaling pathway
Adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) is phosphorylated and activated by upstream kinases under various physiological and pathological conditions (69, 70). AMPK activation inhibits ROS production induced by mitochondrial dysfunction and endoplasmic reticulum stress, reduces the generation of pro-inflammatory factors caused by dyslipidemia and hyperglycemia, and prevents vascular endothelial dysfunction by increasing nitric oxide (NO) bioavailability (71, 72). Therefore, AMPK activation has emerged as a significant target for the prevention and treatment of AS.
Alliin can activate AMPK and regulate the expression of its downstream targets, sterol regulatory element-binding protein-1c (SREBP-1c) and sterol regulatory element-binding protein-2 (SREBP-2), thereby reducing triglyceride and cholesterol accumulation in HepG2 cells induced by 1,3-dichloro-2-propanol (1,3-DCP) (73). SREBP-1c, primarily expressed in the liver, intestines, and adipose tissue, regulates triglyceride and cholesterol synthesis. Overactivation of SREBP-1c can lead to metabolic diseases such as obesity and fatty liver. Sangeetha et al. confirmed that alliin inhibits SREBP-1c expression, reducing lipid accumulation in hepatocytes induced by free fatty acids (74). SREBP-2 is a key transcription factor responsible for regulating cholesterol synthesis. When cholesterol levels in the endoplasmic reticulum decrease, SREBP-2 is activated, increasing low-density lipoprotein receptor (LDLR) synthesis in the Golgi apparatus and promoting cholesterol uptake into cells, thereby reducing plasma LDL-cholesterol levels (75, 76). Studies have shown that alliin significantly reduces plasma LDL-cholesterol levels in high-fat diet-fed Sprague-Dawley (SD) rats by activating the SREBP-2/LDLR pathway (77).
4.4 The PCSK9-LDLR signaling pathway
Proprotein convertase subtilisin/kexin type 9 (PCSK9), identified in the early 2000s, is a critical regulator of LDL-cholesterol metabolism due to its ability to degrade LDL receptors (LDLRs) and clear circulating LDL-cholesterol (78, 79). PCSK9 degrades LDLR through both extracellular and intracellular pathways. In the extracellular pathway, PCSK9 binds to LDLR on the cell surface, forming a PCSK9-LDLR complex that is directed to lysosomes for degradation (80). In the intracellular pathway, PCSK9 binds directly to LDLR in the Golgi apparatus, inducing its degradation. Dyslipidemia, particularly elevated LDL-cholesterol levels, is a primary factor in the development and progression of AS. The level of LDL-cholesterol in the body is mainly determined by the number of LDLRs in the liver and the activity of PCSK9. Increased PCSK9 activity promotes LDLR degradation, leading to higher circulating LDL-cholesterol levels. A European study found that only 43% of patients using statins achieved the goal of reducing LDL-cholesterol levels (81). Consequently, PCSK9 inhibitors have been extensively studied as a novel therapeutic target for lowering LDL-cholesterol levels in patients with hypercholesterolemia (82).
Ahmad et al. investigated the components of proprotein convertase subtilisin/kexin 9 - garlic-derived organic sulfides (PCSK9-OSCs) and discovered that alliin is the most potent inhibitor of PCSK9 activity (83). Since PCSK9 is known to degrades LDLR, the discovery of alliin's inhibitory effect on PCSK9 activity implies that it has the potential to lower circulating LDL cholesterol levels. This is especially important for patients who are intolerant of statins or do not achieve adequate cholesterol reduction with ezetimibe combination therapy. The anti-AS effects of alliin, mediated through the AMPK/SREBP-1c/SREBP-2 signaling pathway and the PCSK9-LDLR signaling pathways, are illustrated in Figure 3.
Figure 3
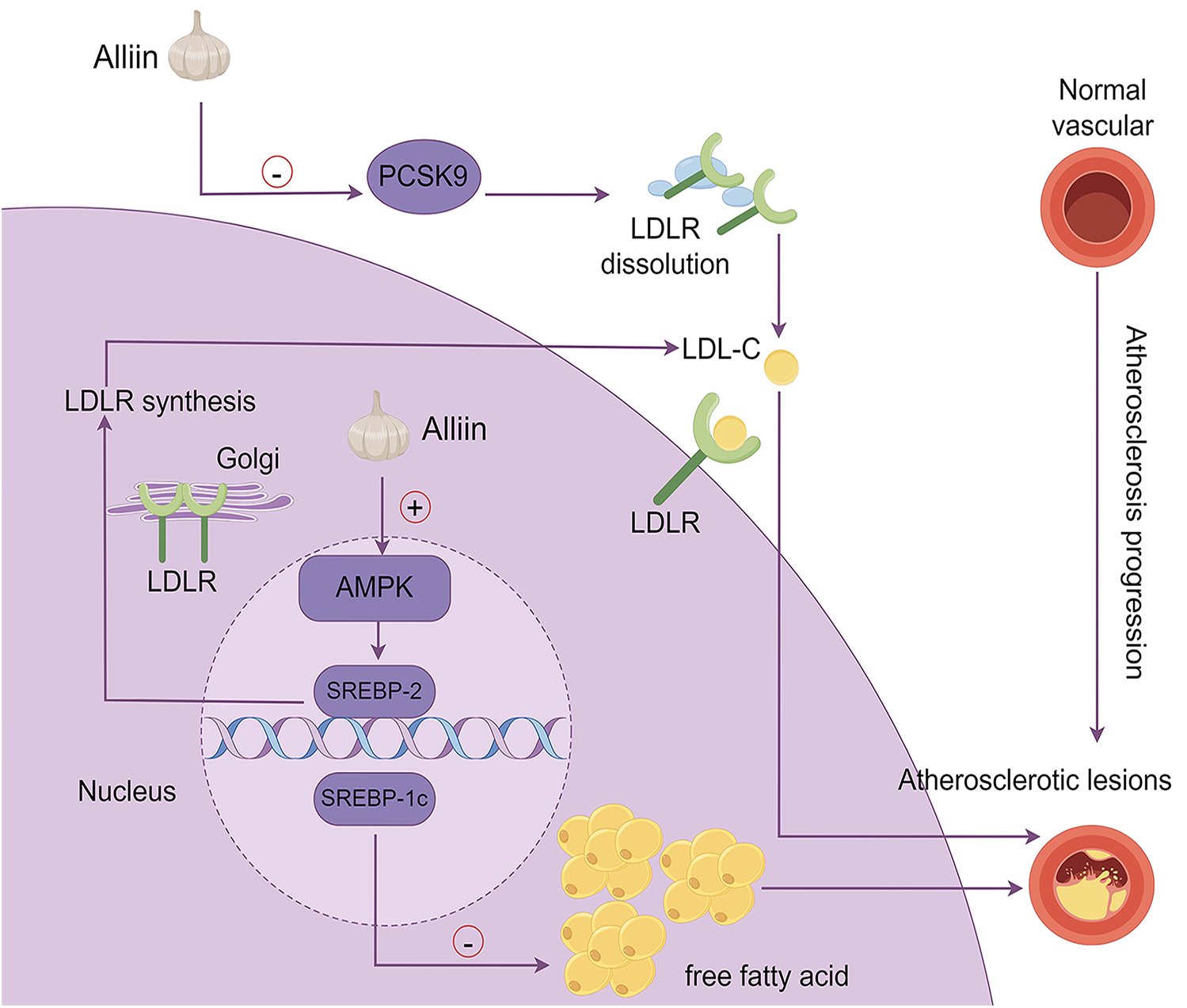
The mechanism by which alliin exerts its anti-aS effects. This diagram summarizes the molecular pathways through which alliin mitigates atherosclerosis: (1) PCSK9-LDLR pathway: alliin suppresses PCSK9 activity, preventing LDL receptor dissolution and lowering circulating LDL-cholesterol; (2) AMPK/SREBP-2 pathway: alliin activates AMPK, which regulates SREBP-2, leading to increased LDLR synthesis in the Golgi apparatus and enhanced cellular cholesterol uptake, thereby reducing plasma LDL-cholesterol levels; (3) AMPK/SREBP-1c pathway: alliin suppresses SREBP-1c expression, reducing lipid accumulation in hepatocytes induced by free fatty acids.
To summarize, OSCs contribute to anti-atherosclerotic effects via modulating numerous crucial signaling pathways, primarily the four core mechanisms listed above, shown in Figure 4: (1) The Nrf2/ARE pathway represents an antioxidant pathway. OSCs, such as allicin and SAC, can directly activate Nrf2 resulting in antioxidant effects. (2) OSCs including SFN inhibit the RhoA/ROCK pathway, leading to improved endothelial function and reduced inflammation by downregulating NF-κB. (3) The AMPK/SREBP-1c/SREBP-2 pathway is modulated by alliin, reducing lipid accumulation and cholesterol synthesis while promoting LDLR expression. (4) Alliin targets the PCSK9-LDLR pathway, inhibiting PCSK9 activity, limiting LDLR degradation and decreasing circulating LDL cholesterol. These multitarget mechanisms emphasize OSCs' potential for preventing AS by focusing on lipid metabolism, oxidative stress, inflammation, and endothelial dysfunction.
Figure 4
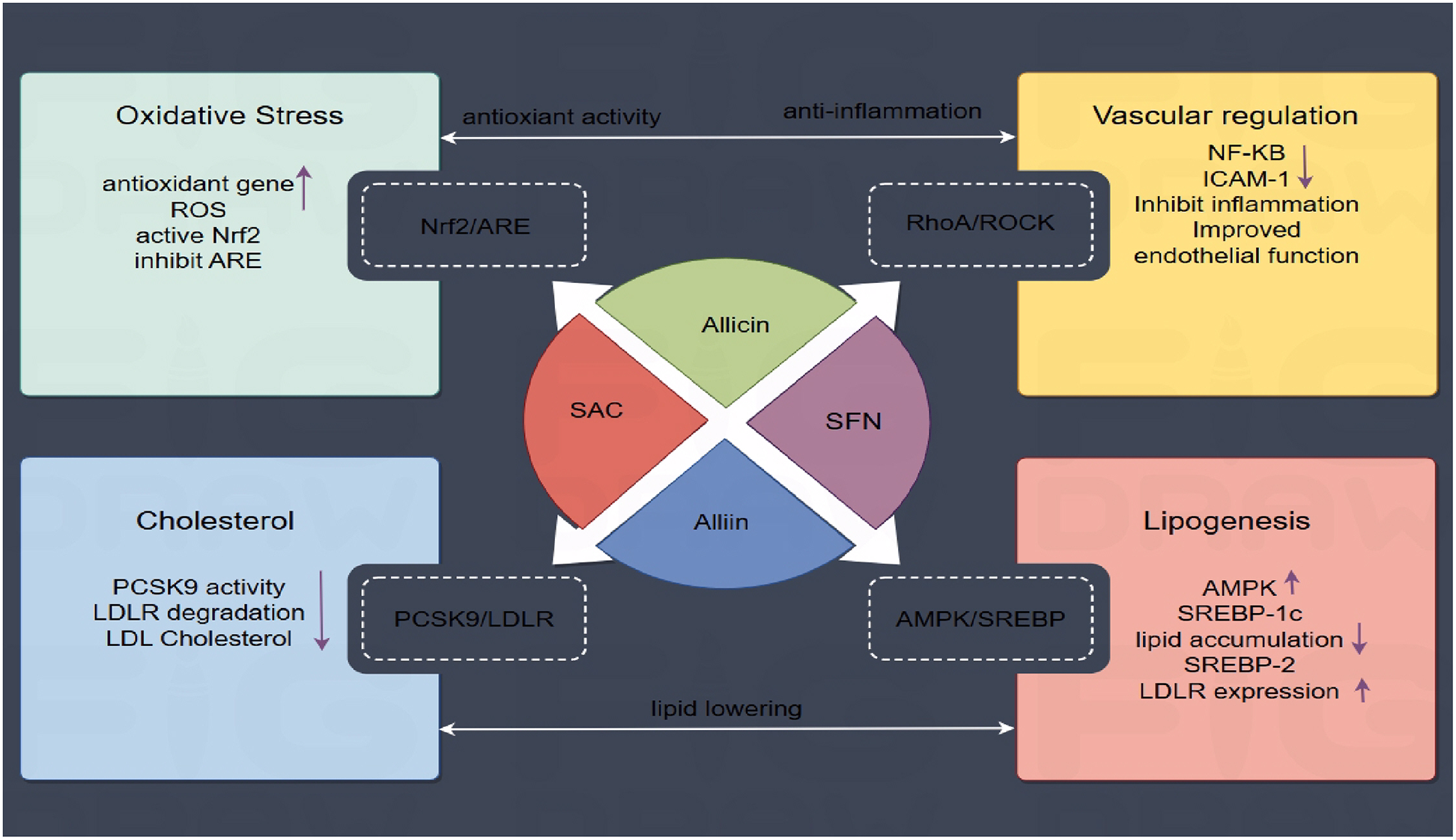
OSCs-Mediated signaling pathways in atherosclerosis prevention. OSCs regulate AS processes via various pathways. Four main mechanisms are shown: (1) Nrf2/ARE-mediated antioxidant response; (2) RhoA/ROCK vascular regulation; (3) AMPK/SREBP-controlled lipid metabolism; and (4) PCSK9 suppression of LDLR degradation.
5 Conclusion
Natural organosulfur compounds, notably garlic derivatives, have been shown to have anti-atherogenic effects on several targets, including lipid metabolism, antioxidant and anti-inflammatory activity, endothelial function protection, and antibacterial capabilities. Their regulatory role on critical signaling pathways such as Nrf2, RhoA/ROCK, AMPK, and PCSK9 emphasizes their therapeutic potential. While preclinical evidence is robust, clinical translation remains hindered by challenges such as variable bioavailability, lack of standardized formulations, and insufficient large-scale human trials. Future research should concentrate on clinical trials to determine efficacy, increase bioavailability, and develop standardized OSCs formulations for cardiovascular disease prevention and management.
Statements
Author contributions
YuT: Data curation, Conceptualization, Methodology, Supervision, Investigation, Writing – original draft, Writing – review & editing, Software. DL: Investigation, Writing – original draft, Conceptualization, Writing – review & editing, Software. YiT: Writing – original draft, Conceptualization, Writing – review & editing, Software. JW: Writing – review & editing, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Changshu Health Commission Science and Technology Program (CSWSQ202203) and Changshu Science and Technology Program (CY202301, CS202124).
Acknowledgments
We are grateful to North Sichuan Medical College for their assistance with our work, as well as Professor Chun-yang Zhou and Chun-yan Yang for their advice on rewriting the essay. Figure support was provided by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Goldsborough E Osuji N Blaha MJ . Assessment of cardiovascular disease risk: a 2022 update. Endocrinol Metab Clin North Am. (2022) 51(3):483–509. 10.1016/j.ecl.2022.02.005
2.
Libby P . The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. 10.1038/s41586-021-03392-8
3.
Zhu Y Xian X Wang Z Bi Y Chen Q Han X et al Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. (2018) 8(3):80. 10.3390/biom8030080
4.
Riccardi G Giosuè A Calabrese I Vaccaro O . Dietary recommendations for prevention of atherosclerosis. Cardiovasc Res. (2022) 118(5):1188–204. 10.1093/cvr/cvab173
5.
Falk E . Pathogenesis of atherosclerosis. J Am Coll Cardiol. (2006) 47(8):7–12. 10.1016/j.jacc.2005.09.068
6.
Pedro-Botet J Climent E Benaiges D . Atherosclerosis and inflammation. New therapeutic approaches. Med Clin (Barc). (2020) 155(6):256–62. 10.1016/j.medcli.2020.04.024
7.
Rouf R Uddin SJ Sarker DK Islam MT Ali ES Shilpi JA et al . Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: a systematic update of pre-clinical and clinical data. Tre44444nds Food Sci Technol. (2020) 104:219–34. 10.1016/j.tifs.2020.08.006
8.
Chen K Nakasone Y Yi S Ibrahim HR Sakao K Hossain MA et al Natural garlic organosulfur compounds prevent metabolic disorder of lipid and glucose by increasing gut commensal Bacteroides acidifaciens. J Agric Food Chem. (2022) 70(19):5829–37. 10.1021/acs.jafc.2c00555
9.
Petropoulos S Di Gioia F Ntatsi G . Vegetable organosulfur compounds and their health promoting effects. Curr Pharm Des. (2017) 23(19):2850–75. 10.2174/1381612823666170111100531
10.
Miekus N Marszalek K Podlacha M Iqbal A Puchalski C Swiergiel AH . Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules. (2020) 25(17):3804. 10.3390/molecules25173804
11.
Patiño-Morales CC Jaime-Cruz R Sánchez-Gómez C Corona JC Hernández-Cruz EY Kalinova-Jelezova I et al Antitumor effects of natural compounds derived from Allium sativum on neuroblastoma: an overview. Antioxidants (Basel). (2021) 11(1):48. 10.3390/antiox11010048
12.
Schepetkin IA Kirpotina LN Khlebnikov AI Balasubramanian N Quinn MT . Neutrophil immunomodulatory activity of natural organosulfur compounds. Molecules. (2019) 24(9):1809. 10.3390/molecules24091809
13.
Sharma K Rani V . Organosulfur compounds in aged garlic extract ameliorate glucose induced diabetic cardiomyopathy by attenuating oxidative stress, cardiac fibrosis, and cardiac apoptosis. Cardiovasc Hematol Agents Med Chem. (2023) 22(1):66–82. 10.2174/1871525721666230223145218
14.
Imaizumi VM Laurindo LF Manzan B Guiguer EL Oshiiwa M Otoboni AMMB et al Garlic: a systematic review of the effects on cardiovascular diseases. Crit Rev Food Sci Nutr. (2023) 63(24):6797–819. 10.1080/10408398.2022.2043821
15.
Rauf A Abu-Izneid T Thiruvengadam M Imran M Olatunde A Shariati MA et al Garlic (Allium sativum L.): its chemistry, nutritional composition, toxicity, and anticancer properties. Curr Top Med Chem. (2022) 22(11):957–72. 10.2174/1568026621666211105094939
16.
Xiang Q Tian F Xu J Du X Zhang S Liu L . New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol Rev Camb Philos Soc. (2022) 97(5):1844–67. 10.1111/brv.12866
17.
Mszar R Katz ME Grandhi GR Osei AD Gallo A Blaha MJ . Subclinical atherosclerosis to guide treatment in dyslipidemia and diabetes mellitus. Curr Atheroscler Rep. (2024) 26(6):217–30. 10.1007/s11883-024-01202-w
18.
Jain RC Konar DB . Effect of garlic oil in experimental cholesterol atherosclerosis. Atherosclerosis. (1978) 29(2):125–9. 10.1016/0021-9150(78)90002-3
19.
Jain RC Konar DB . Letter: garlic oil in experimental atherosclerosis. Lancet. (1976) 1(7965):918. 10.1016/s0140-6736(76)92149-8
20.
Vezza T Guillamón E García-García J Baños A Mut-Salud N García-López JD et al LDL-cholesterol-lowering effects of a dietary supplement containing onion and garlic extract used in healthy volunteers. Nutrients. (2024) 16(16):2811. 10.3390/nu16162811
21.
Valls RM Companys J Calderón-Pérez L Salamanca P Pla-Pagà L Sandoval-Ramírez BA et al Effects of an optimized aged garlic extract on cardiovascular disease risk factors in moderate hypercholesterolemic subjects: a randomized, crossover, double-blind, sustained and controlled study. Nutrients. (2022) 14(3):405. 10.3390/nu14030405
22.
Woo HJ Cha GS Kang MJ Kyung KH . Assessment of standardization of domestic commercial black garlic extract for S-allyl-l-cysteine and S-1-propenyl-l-cysteine. Food Sci Biotechnol. (2022) 31(2):253–60. 10.1007/s10068-021-01028-1
23.
Yamaguchi Y Kumagai H . Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-L-cysteine sulfoxide. Exp Ther Med. (2020) 19(2):1528–35. 10.3892/etm.2019.8385
24.
Lu J Cheng B Fang B Meng Z Zheng Y Tian X et al Protective effects of allicin on 1,3-DCP-induced lipid metabolism disorder in HepG2 cells. Biomed Pharmacother. (2017) 96:1411–7. 10.1016/j.biopha.2017.10.125
25.
Ba L Gao J Chen Y Qi H Dong C Pan H et al Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine. (2019) 58:152765. 10.1016/j.phymed.2018.11.025
26.
Ma X Liu Y Tan Y Qu K He X Zhang H et al Diallyl disulphide inhibits apolipoprotein(a) expression in HepG2 cells through the MEK1-ERK1/2-ELK-1 pathway. Lipids Health Dis. (2017) 16(1):223. 10.1186/s12944-017-0616-1
27.
Batty M Bennett MR Yu E . The role of oxidative stress in atherosclerosis. Cells. (2022) 11(23):3843. 10.3390/cells11233843
28.
Violi F Pignatelli P Valeriani E . Oxidative stress and atherosclerosis: basic and clinical open issues. Kardiol Pol. (2024) 82(7-8):689–91. 10.33963/v.phj.100994
29.
Chan JY Tsui HT Chung IY Chan RY Kwan YW Chan SW . Allicin protects rat cardiomyoblasts (H9c2 cells) from hydrogen peroxide-induced oxidative injury through inhibiting the generation of intracellular reactive oxygen species. Int J Food Sci Nutr. (2014) 65(7):868–73. 10.3109/09637486.2014.925428
30.
Ma L Chen S Li S Deng L Li Y Li H . Li S, et al. Effect of allicin against ischemia/hypoxia-induced H9c2 myoblast apoptosis via eNOS/NO pathway-mediated antioxidant activity. Evid Based Complement Alternat Med. (2018) 2018:3207973. 10.1155/2018/3207973
31.
Wang WD Sun YE Chen HW . Characterization of antioxidant activity of sulfur compounds in black garlic. Cell Mol Biol (Noisy-le-grand). (2018) 64(12):76–80. 10.14715/cmb/2018.64.12.15
32.
Hartley A Haskard D Khamis R . Oxidized LDL and anti-oxidized LDL antibodies in atherosclerosis—novel insights and future directions in diagnosis and therapy. Trends Cardiovasc Med. (2019) 29(1):22–6. 10.1016/j.tcm.2018.05.010
33.
Khatana C Saini NK Chakrabarti S Saini V Sharma A Saini RV et al Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid Med Cell Longev. (2020) 2020:5245308. 10.1155/2020/5245308
34.
Lei YP Liu CT Sheen LY Chen HW Lii CK . Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low-density lipoprotein. Mol Nutr Food Res. (2010) 54:42–52. 10.1002/mnfr.200900278
35.
Yan LJ . Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. (2014) 2014:137919. 10.1155/2014/137919
36.
Hsieh DJ Ng SC Zeng RY Padma VV Huang CY Kuo WW Diallyl trisulfide (DATS) suppresses AGE-induced cardiomyocyte apoptosis by targeting ROS-mediated PKCδ activation. Int J Mol Sci. (2020) 21(7):2608. 10.3390/ijms21072608
37.
Raggi P Genest J Giles JT Rayner KJ Dwivedi G Beanlands RS et al Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. (2018) 276:98–108. 10.1016/j.atherosclerosis.2018.07.014
38.
Gupta P Dutt V Kaur N Kalra P Gupta S Dua A et al S-allyl cysteine: a potential compound against skeletal muscle atrophy. Biochim Biophys Acta Gen Subj. (2020) 1864(10):129676. 10.1016/j.bbagen.2020.129676
39.
Manoonphol K Suttisansanee U Promkum C Butryee C . Effect of thermal processes on S-allyl cysteine content in black garlic. Foods. (2023) 12(6):1227. 10.3390/foods12061227
40.
Li N Chen K Dong H Yang J Yoshizawa M Kagami H et al Alliin inhibits adipocyte differentiation by downregulating akt expression: implications for metabolic disease. Exp Ther Med. (2021) 21(6):563. 10.3892/etm.2021.9995
41.
Lee DY Li H Lim HJ Lee HJ Jeon R Ryu JH . Anti-inflammatory activity of sulfur-containing compounds from garlic. J Med Food. (2012) 15(11):992–9. 10.1089/jmf.2012.2275
42.
Jiang X Zhu X Liu Y Zhou N Zhao Z Lv H . Diallyl trisulfide and its active metabolite allyl methyl sulfone attenuate cisplatin-induced nephrotoxicity by inhibiting the ROS/MAPK/NF-κB pathway. Int Immunopharmacol. (2024) 127:111373. 10.1016/j.intimp.2023.111373
43.
Liu KL Chen HW Wang RY Lei YP Sheen LY Lii CK . DATS Reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-κB activation in RAW 264.7 macrophages. J Agric Food Chem. (2006) 54(9):3472–8. 10.1021/jf060043k
44.
Chu CC Wu WS Shieh JP Chu HL Lee CP Duh PD . The anti-inflammatory and vasodilating effects of three selected dietary organic sulfur compounds from Allium species. J Funct Biomater. (2017) 8(1):5. 10.3390/jfb8010005
45.
Lin X Ouyang S Zhi C Li P Tan X Ma W et al Focus on ferroptosis, pyroptosis, apoptosis and autophagy of vascular endothelial cells to the strategic targets for the treatment of atherosclerosis. Arch Biochem Biophys. (2022) 715:109098. 10.1016/j.abb.2021.109098
46.
Liu DS Wang SL Li JM Liang ES Yan MZ Gao W . Allicin improves carotid artery intima-media thickness in coronary artery disease patients with hyperhomocysteinemia. Exp Ther Med. (2017) 14(2):1722–6. 10.3892/etm.2017.4698
47.
Zhang Y Ouyang J Zhan L Li Y Li S He Y et al Autophagy in homocysteine—induced HUVEC senescence. Exp Ther Med. (2023) 26(1):354. 10.3892/etm.2023.12053
48.
Borkowska A Ziolkowski W Kaczor K Herman-Antosiewicz A Knap N Wronska A et al Homocysteine-induced decrease in HUVEC cells’ resistance to oxidative stress is mediated by Akt-dependent changes in iron metabolism. Eur J Nutr. (2021) 60(3):1619–31. 10.1007/s00394-020-02360-8
49.
Tsai CY Wen SY Shibu MA Yang YC Peng H Wang B et al Diallyl trisulfide protects against high glucose-induced cardiac apoptosis by stimulating the production of cystathionine gamma-lyase-derived hydrogen sulfide. Int J Cardiol. (2015) 195:300–10. 10.1016/j.ijcard.2015.05.111
50.
Zhu X Lu R Zhang G Fan L Zhan Y Chen G et al Diallyl trisulfide attenuates alcohol-induced hepatocyte pyroptosis via elevation of hydrogen sulfide. Biosci Biotechnol Biochem. (2022) 86(11):1552–61. 10.1093/bbb/zbac149
51.
Kolluru GK Shackelford RE Shen X Dominic P Kevil CG . Sulfide regulation of cardiovascular function in health and disease. Nat Rev Cardiol. (2023) 20(2):109–25. 10.1038/s41569-022-00741-6
52.
Song Y Xu Z Zhong Q Zhang R Sun X Chen G . Sulfur signaling pathway in cardiovascular disease. Front Pharmacol. (2023) 14:1303465. 10.3389/fphar.2023.1303465
53.
Lin Y Wang J Bu F Zhang R Wang J Wang Y et al Bacterial extracellular vesicles in the initiation, progression and treatment of atherosclerosis. Gut Microbes. (2025) 17(1):2452229. 10.1080/19490976.2025.2452229
54.
Ruan Q Guan P Qi W Li J Xi M Xiao L et al Porphyromonas gingivalis regulates atherosclerosis through an immune pathway. Front Immunol. (2023) 14:1103592. 10.3389/fimmu.2023.1103592
55.
Ding LY Liang LZ Zhao YX Yang YN Liu F Ding QR et al Porphyromonas gingivalis-derived lipopolysaccharide causes excessive hepatic lipid accumulation via activating NF-κB and JNK signaling pathways. Oral Dis. (2019) 25(7):1789–97. 10.1111/odi.13153
56.
Bachrach G Jamil A Naor R Tal G Ludmer Z Steinberg D . Garlic allicin as a potential agent for controlling oral pathogens. J Med Food. (2011) 14(11):1338–43. 10.1089/jmf.2010.0165
57.
Vadekeetil A Kaur G Chhibber S Harjai K . Applications of thin-layer chromatography in extraction and characterisation of ajoene from garlic bulbs. Nat Prod Res. (2015) 29(8):768–71. 10.1080/14786419.2014.981815
58.
Bastaki SMA Ojha S Kalasz H Adeghate E . Chemical constituents and medicinal properties of Allium species. Mol Cell Biochem. (2021) 476(12):4301–21. 10.1007/s11010-021-04213-2
59.
Shaw P Chattopadhyay A . Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J Cell Physiol. (2020) 235(4):3119–30. 10.1002/jcp.29219
60.
Adelusi TI Du L Hao M Zhou X Xuan Q Apu C et al Keap1/Nrf2/ARE signaling unfolds therapeutic targets for redox imbalanced-mediated diseases and diabetic nephropathy. Biomed Pharmacother. (2020) 123:109732. 10.1016/j.biopha.2019.109732
61.
Lu MC Ji JA Jiang ZY You QD . The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev. (2016) 36(5):924–63. 10.1002/med.21396
62.
Sun F Xu K Zhou J Zhang W Duan G Lei M . Allicin protects against LPS-induced cardiomyocyte injury by activating Nrf2-HO-1 and inhibiting NLRP3 pathways. BMC Cardiovasc Disord. (2023) 23(1):410. 10.1186/s12872-023-03442-1
63.
Li CL Liu XH Qiao Y Ning LN Li WJ Sun YS et al Allicin alleviates inflammation of diabetic macroangiopathy via the Nrf2 and NF-κB pathway. Eur J Pharmacol. (2020) 876:173052. 10.1016/j.ejphar.2020.173052
64.
Shi H Jing X Wei X Perez RG Ren M Zhang X et al S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J Neurochem. (2015) 133(2):298–308. 10.1111/jnc.12986
65.
Ming XF Barandier C Viswambharan H Kwak BR Mach F Mazzolai L et al Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. (2004) 110(24):3708–14. 10.1161/01.CIR.0000142867.26182.32
66.
Surendran A Forbes Dewey C Jr Low BC Tucker-Kellogg L . A computational model of mutual antagonism in the mechano-signaling network of RhoA and nitric oxide. BMC Mol Cell Biol. (2021) 22(Suppl 1):47. 10.1186/s12860-021-00383-5
67.
Hung CN Huang HP Wang CJ Liu KL Lii CK . Sulforaphane inhibits TNF-α-induced adhesion molecule expression through the Rho A/ROCK/NF-κB signaling pathway. J Med Food. (2014) 17(10):1095–102. 10.1089/jmf.2013.2901
68.
Treasure K Harris J Williamson G . Exploring the anti-inflammatory activity of sulforaphane. Immunol Cell Biol. (2023) 101(9):805–28. 10.1111/imcb.12686
69.
Carling D . AMPK Signalling in health and disease. Curr Opin Cell Biol. (2017) 45:31–7. 10.1016/j.ceb.2017.01.005
70.
Lin SC Hardie DG . AMPK: sensing glucose as well as cellular energy status. Cell Metab. (2018) 27(2):299–313. 10.1016/j.cmet.2017.10.009
71.
Kim J Yang G Kim Y Kim J Ha J . AMPK Activators: mechanisms of action and physiological activities. Exp Mol Med. (2016) 48(4):e224. 10.1038/emm.2016.16
72.
Zhao Y Hu X Liu Y Dong S Wen Z He W et al ROS Signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. (2017) 16(1):79. 10.1186/s12943-017-0648-1
73.
Lu J Cheng B Meng Z Fang B Li T Sun M et al Alliin attenuates 1,3-dichloro-2-propanol-induced lipogenesis in HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. Life Sci. (2018) 195:19–24. 10.1016/j.lfs.2017.12.040
74.
Sangeetha T Darlin Quine S . Preventive effect of S-allyl cysteine sulphoxide (Alliin) on mitochondrial dysfunction in normal and isoproterenol induced cardiotoxicity in male Wistar rats: a histopathological study. Mol Cell Biochem. (2009) 328(1-2):1–8. 10.1007/s11010-009-0066-9
75.
Kartawijaya M Han HW Kim Y Lee SM . Genistein upregulates LDLR levels via JNK-mediated activation of SREBP-2. Food Nutr Res. (2016) 60:31120. 10.3402/fnr.v60.31120
76.
Sobati S Shakouri A Edalati M Mohammadnejad D Parvan R Masoumi J et al PCSK9: a key target for the treatment of cardiovascular disease (CVD). Adv Pharm Bull. (2020) 10(4):502–11. 10.34172/apb.2020.062
77.
Nasim SA Dhir B Samar F Rashmi K Mahmooduzzafa Mujib A . Sulphur treatment alters the therapeutic potency of alliin obtained from garlic leaf extract. Food Chem Toxicol. (2009) 47(4):888–92. 10.1016/j.fct.2009.01.024
78.
Hummelgaard S Vilstrup JP Gustafsen C Glerup S Weyer K . Targeting PCSK9 to tackle cardiovascular disease. Pharmacol Ther. (2023) 249:108480. 10.1016/j.pharmthera.2023.108480
79.
Lambert G Sjouke B Choque B Kastelein JJ Hovingh GK . The PCSK9 decade. J Lipid Res. (2012) 53(12):2515–24. 10.1194/jlr.R026658
80.
Katzmann JL Laufs U . PCSK9-directed Therapies: an update. Curr Opin Lipidol. (2024) 35(3):117–25. 10.1097/MOL.0000000000000919
81.
Marcellaud E Jost J Tchalla A Magne J Aboyans V . Statins in primary prevention in people over 80 years. Am J Cardiol. (2023) 187:62–73. 10.1016/j.amjcard.2022.10.015
82.
Melendez QM Krishnaji ST Wooten CJ Lopez D . Hypercholesterolemia: the role of PCSK9. Arch Biochem Biophys. (2017) 625-626:39–53. 10.1016/j.abb.2017.06.001
83.
Ahmad P Alvi SS Waiz M Khan MS Ahmad S Khan MS . Naturally occurring organosulfur compounds effectively inhibits PCSK-9 activity and restrict PCSK-9-LDL-receptor interaction via in-silico and in vitro approach. Nat Prod Res. (2024) 38(22):3924–33. 10.1080/14786419.2023.2269465
Summary
Keywords
organosulfur compounds, garlic, atherosclerosis, lipid metabolism, oxidative stress, anti-Inflammatory agents, endothelial protection, PCSK9 inhibitors
Citation
Tang Y, Lv D, Tao Y and Wang J (2025) The therapeutic effects of natural organosulfur compounds on atherosclerosis and their potential mechanisms: a comprehensive review. Front. Cardiovasc. Med. 12:1599154. doi: 10.3389/fcvm.2025.1599154
Received
27 March 2025
Accepted
16 June 2025
Published
01 July 2025
Corrected
10 December 2025
Volume
12 - 2025
Edited by
Professor Xiaofeng Yang, Temple University, United States
Reviewed by
Xin-Fang Leong, National University of Malaysia, Malaysia
Baonian Liu, Shanghai University of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Tang, Lv, Tao and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Yijing Tao 18800299601@163.com Juan Wang 13862240766@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.