- 1The First College of Clinical Medical Science, China Three Gorges University, Yichang, China
- 2Department of Critical Care Medicine, Yichang Central People’s Hospital, Yichang, China
- 3Yichang Sepsis Clinical Research Center, Yichang, Hubei, China
Background: The blood urea nitrogen to serum albumin ratio (BAR) has been identified as a novel indicator of both inflammatory and nutritional status, exhibiting a correlation with adverse cardiovascular outcomes.
Objective: To explore the association between the BAR and 28-day all-cause mortality in cardiac arrest patients who achieved return of spontaneous circulation (ROSC) and were admitted to the intensive care unit (ICU).
Methods: Data for patients with cardiac arrest were obtained from the Medical Information Mart for Intensive Care IV database. The outcome was 28-day all-cause mortality. Multivariable-adjusted Cox regression analysis, curve fitting, and threshold effects analysis were used to assess the relationship between the BAR and 28-day all-cause mortality in patients with cardiac arrest in the intensive care unit.
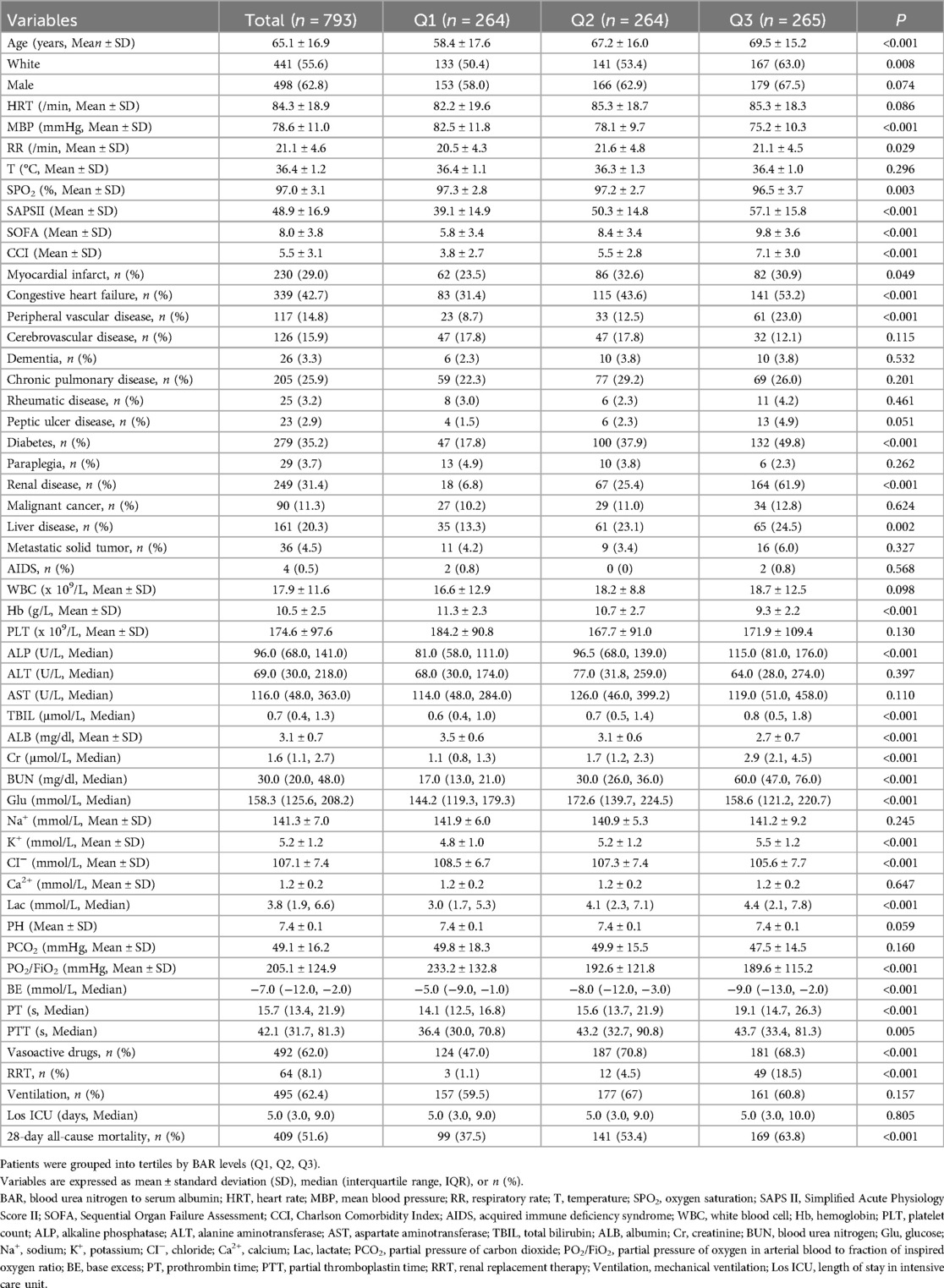
Result: A total of 793 patients were included and divided into tertiles based on the BAR (Q1, Q2, Q3); 8-day all-cause mortality rates were 37.5%, 53.4%, and 63.8%, respectively (P < 0.001). A higher BAR at initial admission was significantly associated with an increased 28-day all-cause mortality risk. Results from the adjusted Models 2, 3, 4, and 5 were consistent with those of Model 1. Subgroup analysis revealed no interactions in age, sex, renal disease, liver disease, vasoactive drug use, ventilation, race, aids, malignant cancer, diabetes, peptic ulcer disease, rheumatic disease, chronic pulmonary disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure and myocardial infarct between the BAR and 28-day all-cause mortality. Restricted cubic spline analysis revealed a nonlinear association between the BAR and 28-day all-cause mortality (P = 0.003). With BAR ≤ 17.981, each 1-unit increase in the BAR was associated with a 5.7% higher risk of death [95% CI (1.012–1.105), P < 0.05].
Conclusion: This study identified a non-linear relationship between the BAR and 28-day all-cause mortality in patients with cardiac arrest.
1 Introduction
Cardiac arrest is a major public health burden, as a leading cause of death and disability, with approximately 350,000 cardiac arrests occurring outside the hospital annually in the United States (1). With advances in treatment and supportive care, survival rates remain low (typically less than 10%), highlighting the urgent need for better prevention and emergency response strategies (2).
The sudden loss of effective circulation and respiratory function can lead to an immediate life-threatening situation, such as non-occlusive mesenteric ischemia and other circulatory hypoperfusion-related complications (3, 4), and timely intervention is crucial for improving patient outcomes. Despite advancements in emergency medical services and post-resuscitation care (5), the 28-day all-cause mortality rate among patients with cardiac arrest remains high (6). Therefore, a comprehensive understanding of 28-day mortality and its related factors may contribute to its prevention and control, thereby improving prognosis and quality of life among affected patients.
The blood urea nitrogen to serum albumin ratio (BAR) is a crucial blood measurement parameter that serves as a key indicator of physiological health (7). The BAR has an important role in assessing the overall well-being and functioning of the human body. This measure has shown superior predictive value for prognosis in various diseases, including sepsis, pneumonia, chronic obstructive pulmonary disease, and acute ischemic stroke (8–11). However, there is little scientific research on the correlation between the BAR and outcomes in patients with cardiac arrest. This study was undertaken to explore the prognostic value of the BAR for 28-day mortality among patients who experience cardiac arrest.
2 Materials and methods
2.1 Data source
This was a retrospective study based on data retrieved from the Medical Information Mart for Intensive Care IV (MIMIC-IV) v3.0 database, which includes critically ill patients admitted to an intensive care unit (ICU) or the emergency department of the Beth Israel Deaconess Medical Center (Boston, MA, USA) between 2008 and 2019. One of the authors passed the Collaborative Institutional Training Initiative program course required to access the database and obtained study approval from the Institutional Review Board of Beth Israel Deaconess Medical Center.
2.2 Study population
This study included adult patients admitted to the ICU after achieving return of spontaneous circulation (ROSC) following cardiac arrest (12). We included patients in the MIMIC-IV v2.2 database who were aged ≥18 years, admitted to ICU for the first time, and diagnosed with cardiac arrest at hospital admission according to diagnostic codes of the International Classification of Diseases Ninth Revision and Tenth Revision. The exclusion criteria were as follows: (I) not the first ICU admission; (II) age <18 years; (III) pregnancy or in the postpartum period; (IV) missing data for blood urea nitrogen (BUN) and serum albumin (ALB); (V) admission >24 h earlier; (VI) multiple admissions.
2.3 Extraction of variables
We used PostgreSQL version 10.13 to extract data from MIMIC-IV v2.2 regarding patients' baseline characteristics, including age, sex, comorbidities, and laboratory test results.
We extracted information from the first document containing data for vital signs and laboratory test data for patients with cardiac arrest who were admitted to the hospital. Demographic data included age, sex, and race. Vital signs data included body temperature (T), mean blood pressure (MBP), heart rate (HRT), respiratory rate (RR), and pulse oximetry-derived oxygen saturation (SPO2). Comorbid conditions included the Charlson Comorbidity Index (CCI), myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, diabetes, paraplegia, renal disease, malignant cancer, liver disease, metastatic solid tumor, and AIDS. Laboratory test results within the first 24 h were extracted from the time of admission to the ICU and included white blood cell count, hemoglobin (Hb), platelet count (PLT), alkaline phosphatase (ALP), alanine aminotransferase, aspartate aminotransferase, total bilirubin (TBIL), creatinine (Cr), glucose, sodium, potassium (K+), chloride (CI−), calcium, lactate (Lac), pH, partial pressure of carbon dioxide, partial pressure of oxygen/fraction of inspired oxygen, base excess (BE), prothrombin time (PT), and partial thromboplastin time (PTT). We also recorded scoring systems applied in the ICU including the Sequential Organ Failure Assessment (SOFA) score and Simplified Acute Physiology Score II (SAPS II). Therapy included the use of vasoactive drugs, mechanical ventilation, and renal replacement therapy (RRT) during hospitalization. The length of stay in the ICU and 28-day all-cause mortality were also recorded.
2.4 Statistical analysis
To minimize bias from missing data imputation, variables with ≥25% missing values were excluded; the remaining missing values were handled using multiple imputation (13). The number of imputations was five. Continuous variables were imputed using linear regression models through an iterative computational process to ensure stability. The final results were obtained by pooling estimates across multiply imputed datasets, providing unbiased statistical inference under the missing-at-random (MAR) assumption.
Continuous variables that exhibited a normal distribution are expressed as mean ± standard deviation; otherwise, these are expressed as median (interquartile range). Categorical variables are reported as frequency and percentage. To explore the association between different BAR levels and 28-day all-cause mortality, enrolled patients were categorized into three groups according to the tertile distribution of BAR values within the study cohort (with the 33rd and 66th percentiles used as cutoff points).
We used the t-test for continuous variables with a normal distribution, the Mann–Whitney U test for continuous variables with a skewed distribution, and the chi-squared test for categorical variables to analyze differences between the two groups.
Univariable and multivariable Cox regression analyses were performed to examine the relevance between an increased serum BAR and risk of 28-day all-cause mortality in the ICU. Restricted cubic splines analysis was applied to detect the association between the BAR and risk of 28-day all-cause mortality. Kaplan–Meier analysis was conducted to compare the cumulative survival rate between the high and low BAR groups using the log-rank test. The survival rates among different groups were then compared using log-rank tests.
Variables that were considered potential confounders based on the existing literature and clinical judgment were included. Five regression models were built, and the results are presented as hazard ratio (HR) with 95% confidence interval (CI). Model 1 was unadjusted. Model 2 adjusted for age and sex. Model 3 further adjusted for the Charlson Comorbidity Index, myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, diabetes, paraplegia, renal disease, malignant cancer, liver disease, metastatic solid tumor, and AIDS, based on Model 2. Model 4 further adjusted for white blood cell count, Hb, platelet count, ALP, alanine aminotransferase, aspartate aminotransferase, TBIL, Cr, glucose, sodium, K+, CI−, calcium, Lac, pH, partial pressure of carbon dioxide, partial pressure of oxygen/fraction of inspired oxygen, BE, PT, partial thromboplastin time, vasoactive drug use, RRT, mechanical ventilation, and length of ICU stay, based on Model 3. Model 5 further adjusted for HRT, MBP, RR, T, SPO2, SAPS II, and SOFA, based on Model 4.
Subgroup analyses were performed according to age, sex, renal disease, liver disease, vasoactive drugs, rrt, ventilation, race, aids, malignant cancer, paraplegia, diabetes, peptic ulcer disease, rheumatic disease, chronic pulmonary disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure and myocardial infarct. Data analyses were completed using Stata version 17.0 and IBM SPSS version 26 (IBM, Armonk, NY, USA). Statistical significance was defined as a two-tailed P-value <0.05.
3 Results
3.1 Baseline characteristics
A total of 793 patients were ultimately enrolled in the study (Figure 1). The enrolled patients were divided into three groups according to tertiles of the BAR, the Q1, Q2, and Q3 groups. The mean age of study participants was 65.1 ± 16.9 years, 498 (62.8%) participants were men, and the 28-day all-cause mortality rate among patients with cardiac arrest was 51.6% (409/793). Patients in the group with a higher BAR(Q3) were older, predominantly men, and had higher values for SAPS II, SOFA, the Charlson Comorbidity Index, ALP, TBIL, Cr, BUN, K+, Lac, BE, and PT, as well as a higher prevalence of renal disease, congestive heart failure, peripheral vascular disease, diabetes, renal disease, and liver disease. Additionally, the use of vasoactive drugs and RRT was more common. With increased BAR, the values for MBP, SPO2, CI−, PO2/FiO2, Hb, and ALB showed a tendency to decrease. The 28-day all-cause mortality showed a significant incremental trend between BAR groups. The difference in mortality rates between the three groups (Q1, Q2, Q3) was statistically significant (37.5% vs. 53.4% vs. 63.8%, respectively; P < 0.001), suggesting that the BAR is associated with the risk of 28-day all-cause mortality (Table 1).
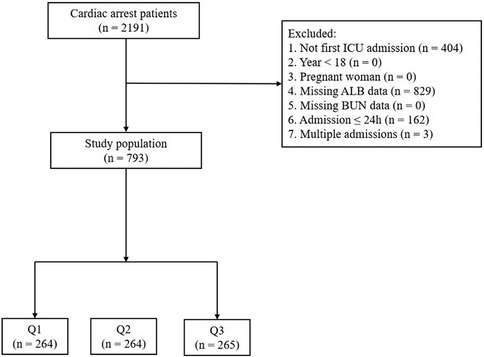
Figure 1. Flowchart of study participants. Patients were grouped into tertiles by BAR levels (Q1, Q2, Q3). BAR, blood urea nitrogen (BUN) to serum albumin (ALB) ratio; ICU, intensive care unit.
3.2 Univariate Cox regression analysis
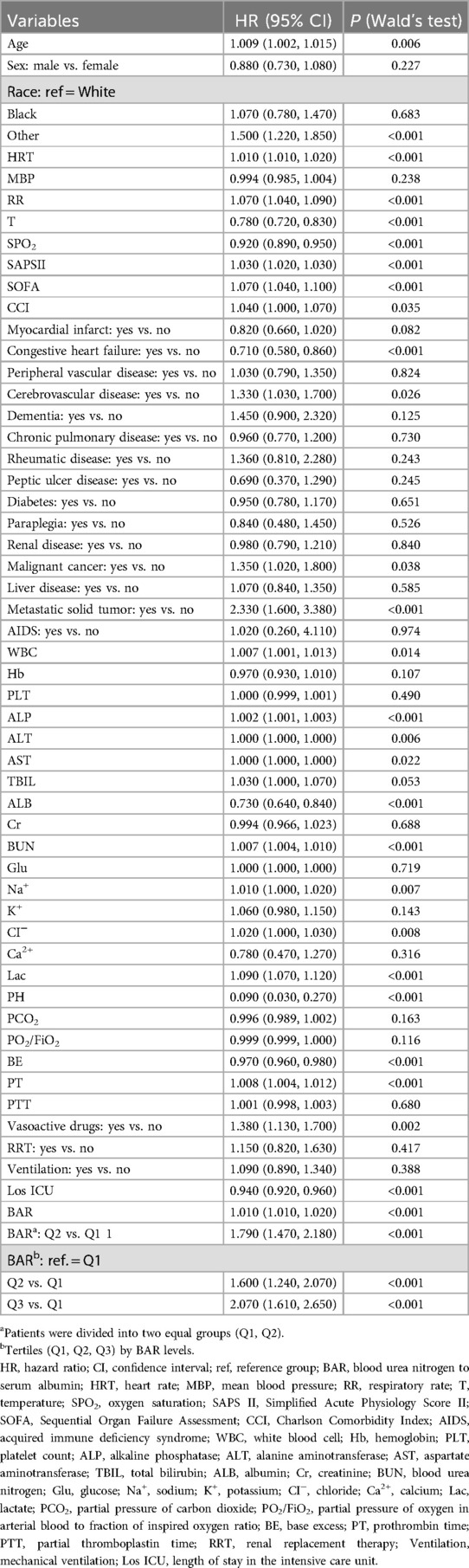
When the BAR was treated as a continuous variable, for each 1-unit increase in the BAR, the risk of mortality increased by 1% (HR = 1.010, 95% CI: 1.010–1.020, P < 0.001). Patients were divided into two equal groups (Q1, Q2) according to BAR value; the HR (Q1 for reference) was 1.79 (95% CI: 1.470–2.180, P < 0.001). Moreover, when patients were divided into three groups according to BAR value (Q1, Q2, Q3), the HR (Q2 vs. Q1) was 1.6 (95% CI: 1.240–2.070, P < 0.001) and further increased (Q3 vs. Q1) to 2.07 (95% CI: 1.610–2.650, P < 0.001). This indicates that the higher the BAR, the higher the risk of 28-day all-cause mortality among patients (Table 2).
3.3 Multivariate Cox regression analysis
The effect of the BAR on 28-day all-cause mortality was assessed using a Cox proportional hazard model. When the BAR was treated as a continuous variable, it was positively associated with 28-day all-cause mortality. As shown in Table 3, in the unadjusted Model 1, each unit increase in the BAR was associated with a 1% increase in the risk of 28-day all-cause mortality (HR = 1.010, 95% CI: 1.010–1.020, P < 0.001). In Model 2 (adjusted for age and sex), the results remained significant (P < 0.001). In Model 3 (Model 2, further adjusted for myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, diabetes, paraplegia, renal disease, malignant cancer, liver disease, metastatic solid tumor, and AIDS), the results remained significant (P < 0.001). In Model 4 (Model 3, further adjusted for white blood cell count, Hb, platelet count, ALP, alanine aminotransferase, aspartate aminotransferase, TBIL, Cr, glucose, sodium, K+, CI−, Lac, pH, partial pressure of carbon dioxide, partial pressure of oxygen/fraction of inspired oxygen, BE, PT, partial thromboplastin time, vasoactive drug use, RRT, mechanical ventilation, and length of ICU stay), the results remained significant (P < 0.001). In Model 5 (Model 4, further adjusted for HRT, MBP, RR, T, SPO2, SAPS II, and SOFA), the results showed significant differences (HR = 1.010, 95% CI: 1.000–1.020, P = 0.003).
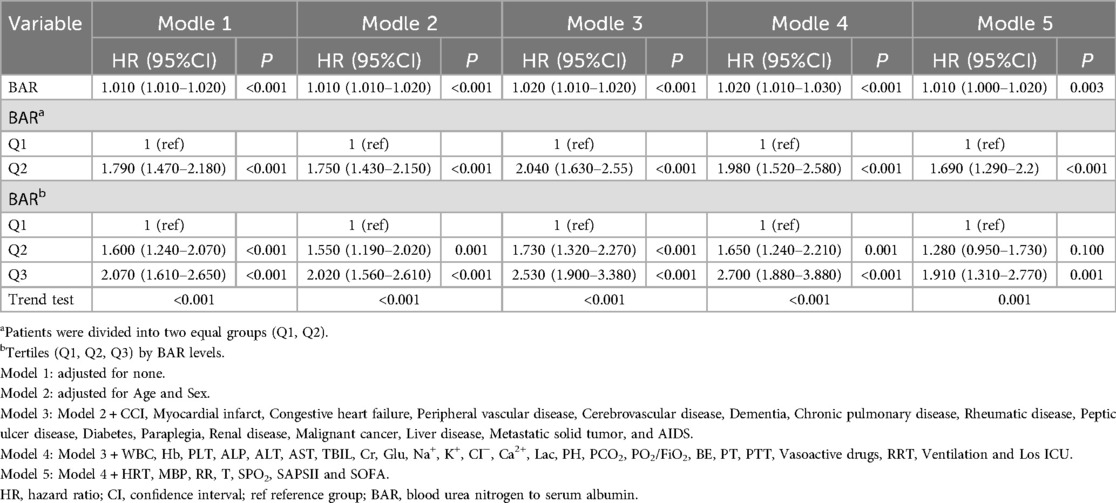
Table 3. Multivariate Cox regression analysis for 28-day all-cause mortality in patients with cardiac arrest.
Patients were divided into equal groups based on BAR values (Q1, Q2), where the Q1 group was used as the reference group; the results remained significant in the different models.
To further explore the effect of the BAR, patients were divided into three groups according to BAR values (Q1, Q2, Q3), with the Q1 group used as the reference. The results of Model 1 showed that higher initial admission in patients was associated with an increased 28-day all-cause mortality, with a gradual increase in the HR starting from Q2 (HR = 1.600, P < 0.001) to Q3 (HR = 2.070, P < 0.001). Overall, the adjusted Models 2, 3, 4, and 5 showed consistency with Model 1.
3.4 Subgroup and sensitivity analysis
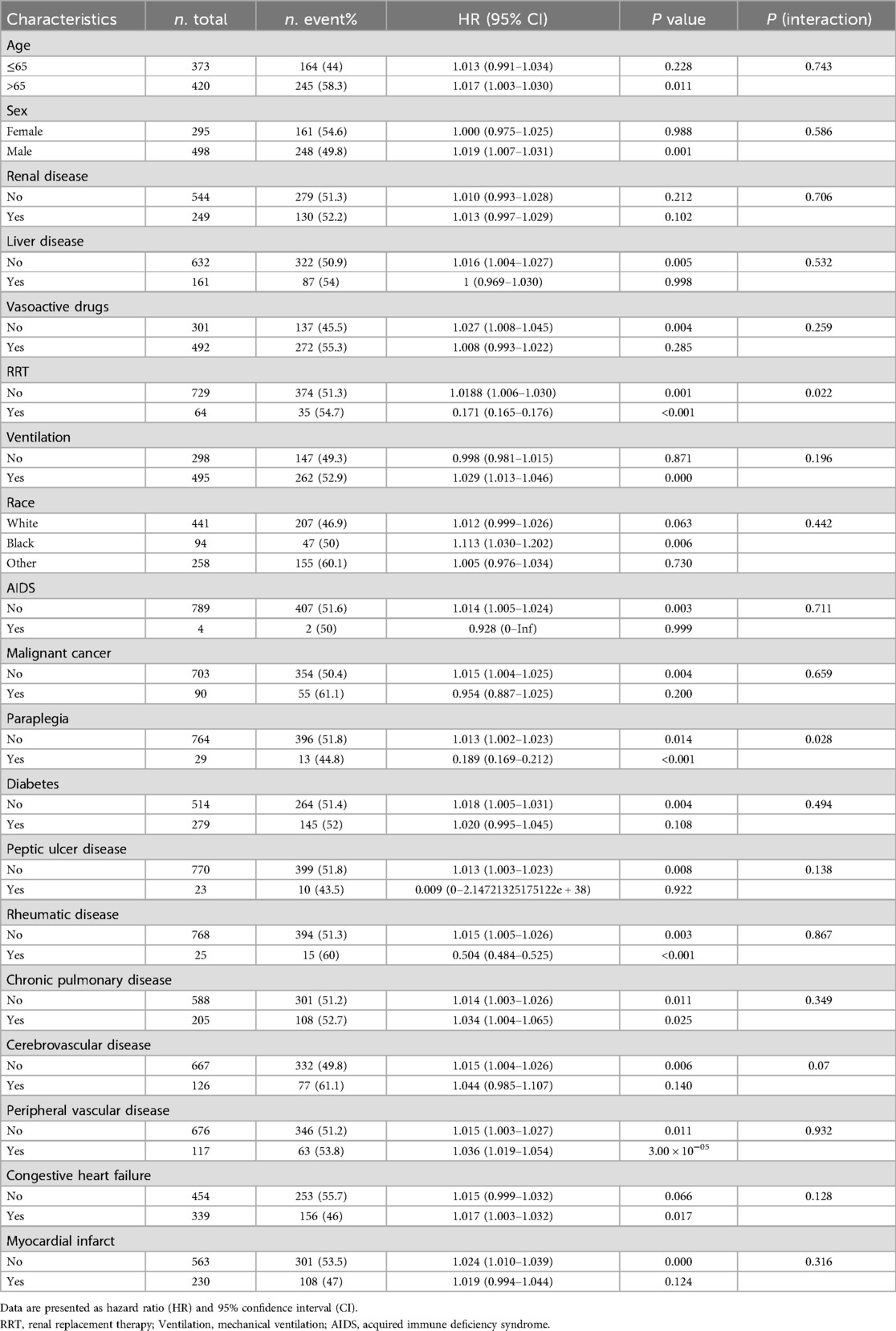
To further investigate the impact of other risk factors on the correlation of the BAR with 28-day all-cause mortality, subgroup analyses were carried out using the following variables in stratification: age, sex, renal disease, liver disease, vasoactive drugs, rrt, ventilation, race, aids, malignant cancer, paraplegia, diabetes, peptic ulcer disease, rheumatic disease, chronic pulmonary disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure and myocardial infarct. Additive interactions between the BAR and 28-day all-cause mortality were observed for rrt and paraplegia (P for interaction <0.05). However, significant interactions were not found for age, sex, renal disease, liver disease, vasoactive drugs, ventilation, race, aids, malignant cancer, diabetes, peptic ulcer disease, rheumatic disease, chronic pulmonary disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure and myocardial infarct.
In the sensitivity analyses, among patients aged ≤65 years, each one-unit increase in the BAR was positively associated with mortality risk, although this relationship did not reach statistical significance (HR = 1.013, p = 0.228). In contrast, among patients aged >65 years, the association was significant (HR = 1.017, p < 0.05). A significant positive correlation between BAR and mortality was observed in male patients, whereas no such association was found in female patients. In patients without liver disease, AIDS, malignant cancer, Diabetes, Peptic ulcer disease, Cerebrovascular disease, Peripheral vascular disease, Myocardial infarct and in those not receiving vasoactive drugs—higher BAR correlated significantly with increased mortality (p < 0.05). Similarly, patients undergoing mechanical ventilation and those with congestive heart failure exhibited a significant increase in mortality risk per unit increase in BAR (p < 0.05); In the non-RRT group, each one-unit increase in BAR was associated with a significant rise in mortality risk, whereas in the RRT group, each one-unit increase in BAR was associated with a significant reduced in mortality risk both subgroup differences reached statistical significance (p < 0.05). The findings in the paraplegia and rheumatic disease subgroups were also consistent with those observed in the RRT subgroup. In the Chronic pulmonary disease subgroup analysis, BAR was significantly associated with increased mortality risk in both patients without Chronic pulmonary disease (HR = 1.014, p < 0.05) and those with Chronic pulmonary disease (HR = 1.034, p < 0.05) (Table 4).
3.5 Nonlinear relationship analysis
After adjusting for the covariates in Model 5, the results of restricted cubic spline analysis showed a nonlinear relationship between the BAR and 28-day all-cause mortality (Figure 2). As shown in Table 5, with BAR ≤ 17.981, the 28-day all-cause mortality risk increased by 5.7% (95% CI: 1.012–1.105, P < 0.05) for every 1-unit increase in the BAR. With BAR > 17.981, there was a 1.7% (95% CI: 0.994–1.041, P > 0.05) increase in the 28-day all-cause mortality risk for every 1-unit increase in the BAR; although there was an upward trend, the data did not reach statistical significance.
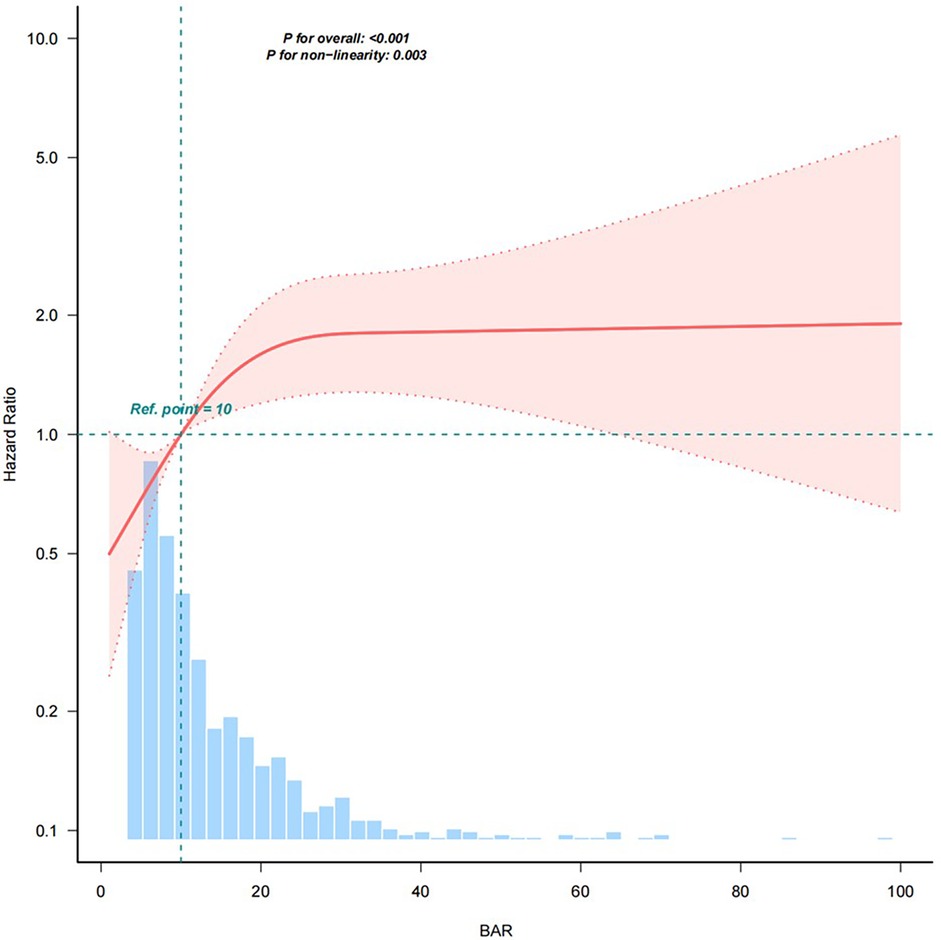
Figure 2. Restricted cubic spline plot of the relationship between the BAR and risk of 28-day all-cause mortality. The model was adjusted for Age, Sex, CCI, Myocardial infarct, Congestive heart failure, Peripheral vascular disease, Cerebrovascular disease, Dementia, chronic pulmonary disease, Rheumatic disease, Peptic ulcer disease, Diabetes, Paraplegia, Renal disease, Malignant cancer, Liver disease, Metastatic solid tumor, AIDS, WBC, Hb, PLT, ALP, ALT, AST, TBIL, Cr, Glu, Na+, K+, CI−, Ca2+, Lac, pH, PCO2, PO2/FiO2, BE, PT, PTT, Vasoactive drugs, RRT, Ventilation, Los ICU, HRT, MBP, RR, T, SPO2, SAPS II and SOFA. Solid lines represent the hazard ratio of 28-day all-cause mortality in patients with cardiac arrest; dotted lines represent the corresponding 95% confidence interval. Hazard ratio = 1 was set as the reference line (takes the upper limit of 100%). BAR, blood urea nitrogen to serum albumin ratio.
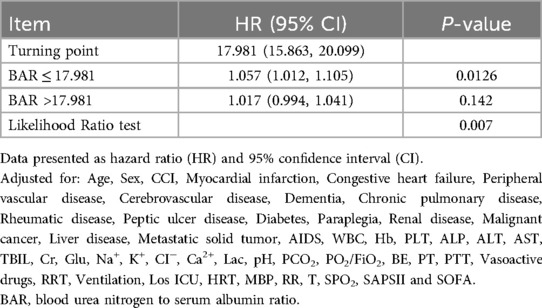
Table 5. Threshold effect analysis of the association between the BAR and 28-day all-cause mortality.
3.6 Kaplan–Meier survival curve analysis
The cumulative mortality rate was compared using Kaplan–Meier curves. Following restricted cubic splines analysis, patients were divided into tertiles by BAR values (Q1, Q2, Q3). The results showed that a higher BAR (Q3) was significantly associated with an enhanced risk of 28-day all-cause mortality, compared with other BAR groups (Figure 3).
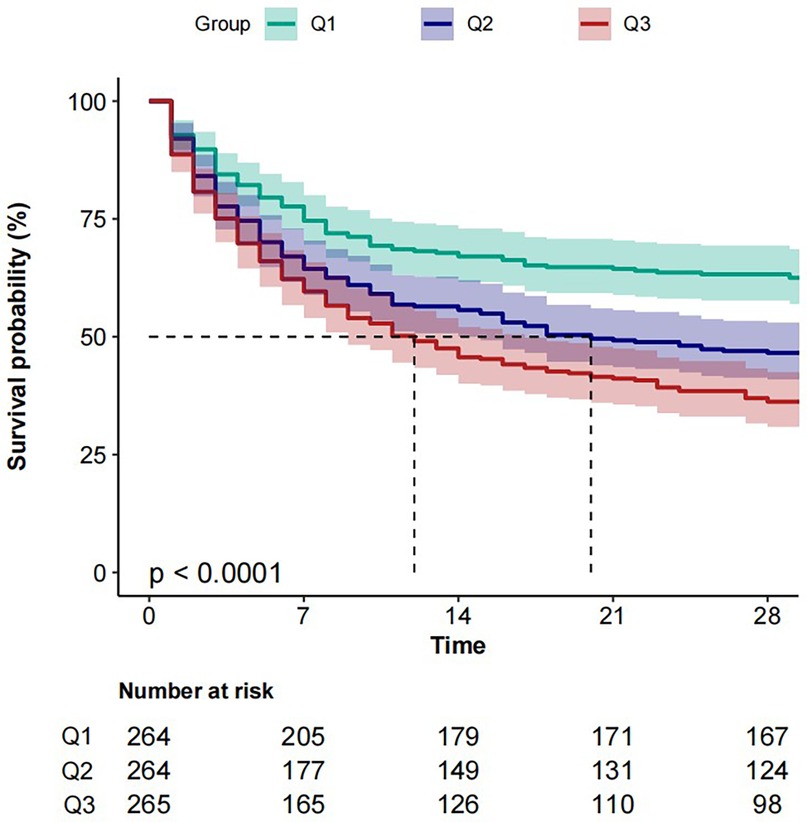
Figure 3. Kaplan–Meier analysis of 28-day all-cause mortality survival according to BAR group. Patients were grouped into tertiles by BAR levels (Q1, Q2, Q3). BAR, blood urea nitrogen to serum albumin ratio.
4 Discussion
In this retrospective cohort study, we first revealed a non-linear association between the BAR and 28-day all-cause mortality in patients with cardiac arrest. We found that BAR ≤ 17.981 was positively correlated with 28-day all-cause mortality. However, BAR > 17.981 showed no such correlation. This finding reveals a novel predictive indicator for risk stratification in patients with cardiac arrest, with important clinical implications.
BUN is the final product of protein metabolism, reflecting renal function and overall metabolic status (14), with prognostic value in heart failure surpassing that of the glomerular filtration rate and serum Cr (15, 16), and elevated levels being significantly associated with poor outcomes in cardiac arrest patients (17–20). Our analysis confirmed that BUN is an independent risk factor for 28-day mortality. As the BUN level increased, the rate of 28-day all-cause mortality showed an upward trend. ALB is the main protein in plasma, with physiological functions including anti-inflammatory and antioxidant effects, as well as the maintenance of plasma colloid osmotic pressure (21). Several studies have shown that hypoalbuminemia on admission is associated with increased in-hospital mortality (22–24), worse neurologic outcomes (24, 25), and a prolonged ICU stay (23). In this study, we also found that ALB levels in the non-survivor group were significantly lower than those in the survivor group.
Predicting the prognosis of patients with cardiac arrest using individual indicators has certain limitations. The BAR, a composite indicator composed of BUN and ALB, can comprehensively reflect the body's metabolic state, nutritional status, inflammatory response, and organ function. The BAR has been identified as an effective prognostic marker for various diseases (26–29). This study expands BAR's clinical utility by identifying its unique prognostic value in cardiac arrest patients. Cox regression analysis revealed that the BAR was positively correlated with 28-day all-cause mortality in patients with cardiac arrest, with a 1% increase in mortality risk for every 1-unit increase (HR = 1.010, 95% CI: 1.010–1.020, P < 0.001); this association remained consistent across multivariable-adjusted models. Kaplan–Meier curves showed that a higher BAR was significantly associated with an enhanced risk of 28-day all-cause mortality compared with other BAR groups. Huang et al. (30) observed that BAR ≥ 10.42 was significantly associated with long-term mortality in acute ischemic stroke. Wang et al. found that a BAR > 8.0 predicted 30-day and 360-day mortality in patients with sepsis (31); when BAR levels exceeded certain thresholds, mortality increased with higher BAR values. In this study, a similar trend was observed. When BAR levels decreased below 17.981, patient mortality increased; however, when BAR levels exceeded 17.981, no significant correlation was observed between the BAR and mortality.
The mechanism via which BAR increases the mortality risk in patients with cardiac arrest is not fully understood, but it may involve several potential mechanisms and related pathophysiological factors. Following cardiac arrest, the abrupt drop in cardiac output rapidly activates the renin–angiotensin–aldosterone system, sympathetic nervous system, and arginine vasopressin release (32), causing renal vasoconstriction, a reduced glomerular filtration rate (10), and enhanced sodium–water reabsorption, which markedly increases passive tubular BUN reabsorption (33). Another mechanism may involve post-resuscitation ischemia–reperfusion triggering systemic inflammation (34); cytokines disrupt the endothelium and increase permeability (34), resulting in albumin extravasation and disturbed renal hemodynamics that further elevate BUN (35). Simultaneously, the surge in reactive oxygen species induces oxidative stress, directly damaging cell membranes and proteins; additionally, reduced albumin—a key plasma antioxidant—impairs the body's defense against oxidative injury (36–38). Elevated BUN reflects renal insufficiency, metabolic disorders, and systemic inflammation, directly indicating the severity of disease. Reduced ALB reflects worsened nutritional status, uncontrolled inflammation, and impaired antioxidant defense mechanisms, directly increasing the risk of multi-organ failure. Subgroup analysis revealed an interaction between the BAR and 28-day all-cause mortality observed in RRT. This is because RRT is typically initiated in patients with severe renal dysfunction, where the clearance of BUN is compromised and the loss of ALB due to dialysis or ongoing inflammation is more pronounced.
On the basis of our threshold analysis, with BAR ≤ 17.981, each 1-unit increase was associated with a 5.7% rise in the 28-day all-cause mortality risk (HR = 1.057, P = 0.0126). Clinicians should implement daily BUN and albumin monitoring, optimize renal perfusion (maintaining MBP ≥ 65 mmHg), and initiate high-protein enteral nutrition within 24 h to correct hypoalbuminemia. Conversely, with BAR > 17.981 (HR = 1.017, P = 0.142), although further increases did not reach statistical significance, such patients remain at high overall risk and therefore require immediate multi-organ support (e.g., lung-protective mechanical ventilation, early continuous RRT), proactive complication and infection management, and multidisciplinary case reviews with structured prognostic discussions to guide precision care and timely decision-making.
Our study has several limitations. First, this study is the disproportionately high missingness rate of albumin (ALB) data, which exceeded the total enrolled cases due to inconsistent laboratory measurements across centers and retrospective data collection constraints. This degree of missingness may introduce bias despite multiple imputation, as the missing-at-random (MAR) assumption becomes less tenable when missingness exceeds 50% of observations (30, 38, 39). Consequently, conclusions derived from ALB-related analyses should be interpreted with caution. In addition, some critical variables such as the cause of cardiac arrest, response time, resuscitation time, temperature management, and body mass index (BMI) were not included in the analysis due to incomplete database records, Notably, BMI has been shown to independently predict short-term mortality in ICU populations (40). Future multicenter, large-sample, prospective studies are needed for further validation. Second, we only collected baseline indicators at admission and did not dynamically monitor the changes in BUN, ALB, and other indicators, which may affect the accuracy of prognostic assessment. Third, this study only describes the correlation between the BAR and 28-day prognosis in patients with cardiac arrest and does not establish a causal relationship.
5 Conclusion
In this study, we found that the relationship between the BAR and 28-day all-cause mortality in patients with cardiac arrest was non-linear. As a simple and readily available indicator, the BAR has potential clinical value in the prognostic assessment of patients with cardiac arrest.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Centre. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because This study was approved by the Institutional Review Boards of the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Centre and was exempt from informed consent.
Author contributions
GZ: Writing – original draft. YT: Writing – original draft. XL: Writing – review & editing, Formal analysis. YW: Writing – review & editing, Formal analysis. DW: Supervision, Writing – review & editing. ML: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Health Development Promotion Project—Adsorption Engineering Academic Construction Project-Emergency and Critical Care Research and Academic Enhancement Project (QS-XFGCJWZZ-0049), the special scientific research project of the Beijing Critical Care Ultrasound Research Association (2023-CCUSG-A-03), and the Medical health research project of Yichang (A24-2-011).
Acknowledgments
We thank Analisa Avila, MPH, ELS, of Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sharpe AR, Richardson K, Stanton M, Dang C, Feih J, Brazauskas R, et al. Lack of association of initial vasopressor dosing with survival and cardiac Re-arrest likelihood after return of spontaneous circulation. J Emerg Med. (2023) 65:e209–20. doi: 10.1016/j.jemermed.2023.05.002
2. Marijon E, Narayanan K, Smith K, Barra S, Basso C, Blom MT, et al. The lancet commission to reduce the global burden of sudden cardiac death: a call for multidisciplinary action. Lancet. (2023) 402:883–936. doi: 10.1016/S0140-6736(23)00875-9
3. Ryu SJ, Lee JH, Lee DH, Lee BK, Bae SJ, Choi YH, et al. The relationship between the ratio of urine osmolality to serum osmolality and neurological outcomes in out-of-hospital cardiac arrest patients. Rev Cardiovasc Med. (2024) 25:157. doi: 10.31083/j.rcm2505157
4. Smalcova J, Belohlavek J. Non-occlusive mesenteric ischemia in cardiac arrest patients. Rev Cardiovasc Med. (2023) 24:262. doi: 10.31083/j.rcm2409262
5. Penketh J, Nolan JP. In-hospital cardiac arrest: the state of the art. Crit Care. (2022) 26:376. doi: 10.1186/s13054-022-04247-y
6. Li P, Liang S, Wang L, Guan X, Wang J, Gong P. Predictive value of neutrophil extracellular trap components for 28-day all-cause mortality in patients with cardiac arrest: a pilot observational study. Shock Augusta Ga. (2023) 60:664–70. doi: 10.1097/SHK.0000000000002225
7. Milas GP, Issaris V, Papavasileiou V. Blood urea nitrogen to albumin ratio as a predictive factor for pneumonia: a meta-analysis. Respir Med Res. (2022) 81:100886. doi: 10.1016/j.resmer.2022.100886
8. Zou X-L, Feng D-Y, Wu W-B, Yang H-L, Zhang T-T. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med Clin (Barc). (2021) 157:219–25. doi: 10.1016/j.medcli.2020.06.060
9. Xia B, Song B, Zhang J, Zhu T, Hu H. Prognostic value of blood urea nitrogen-to-serum albumin ratio for mortality of pneumonia in patients receiving glucocorticoids: secondary analysis based on a retrospective cohort study. J Infect Chemother. (2022) 28:767–73. doi: 10.1016/j.jiac.2022.02.015
10. Zeng Z, Ke X, Gong S, Huang X, Liu Q, Huang X, et al. Blood urea nitrogen to serum albumin ratio: a good predictor of in-hospital and 90-day all-cause mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. (2022) 22:476. doi: 10.1186/s12890-022-02258-7
11. Li W, Huang Q, Zhan K. Association of serum blood urea nitrogen to albumin ratio with in-hospital mortality in patients with acute ischemic stroke: a retrospective cohort study of the eICU database. Balk Med J. (2024) 41:458–68. doi: 10.4274/balkanmedj.galenos.2024.2024-8-77
12. Lee DH, Lee BK, Ryu SJ. The association between troponin-I clearance after the return of spontaneous circulation and outcomes in out-of-hospital cardiac arrest patients. Rev Cardiovasc Med. (2024) 25:24. doi: 10.31083/j.rcm2501024
13. Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. (2016) 4:30. doi: 10.3978/j.issn.2305-5839.2015.12.63
14. Chen X, Zhou J, Wang R, Wang Y, Luo S, Yang J, et al. Blood urea nitrogen to albumin ratio predicts risk of acute kidney injury and in-hospital mortality associated with immunological and surgical diseases: a retrospective analysis of 1994 patients. Int Immunopharmacol. (2024) 143:113600. doi: 10.1016/j.intimp.2024.113600
15. Duan S, Li Y, Yang P. Predictive value of blood urea nitrogen in heart failure: a systematic review and meta-analysis. Front Cardiovasc Med. (2023) 10:1189884. doi: 10.3389/fcvm.2023.1189884
16. Liu H, Ishigami J, Mathews L, Konety S, Hall M, Chang PP, et al. Association of blood urea nitrogen with incident heart failure in the community—the atherosclerosis risk in communities (ARIC) study. Circ J. (2024). doi: 10.1253/circj.CJ-24-0502
17. Barros JCC, Ferreira GM, Souza IDA, Shalova A, Azevedo PS, Polegato BF, et al. Serum urea increase during hospital stay is associated with worse outcomes after in-hospital cardiac arrest. Am J Med Sci. (2024) 368:153–8. doi: 10.1016/j.amjms.2024.04.016
18. Schriefl C, Schwameis M, Ettl F, Poppe M, Clodi C, Mueller M, et al. Blood urea nitrogen kinetics in the early postcardiac arrest phase are associated with clinical outcome. Eur J Anaesthesiol. (2022) 39:405–7. doi: 10.1097/EJA.0000000000001572
19. Lee W-L, Lee F-K, Wang P-H. Blood urea nitrogen and creatinine in in-hospital cardiac arrest patients. J Chin Med Assoc. (2023) 86:1–2. doi: 10.1097/JCMA.0000000000000847
20. Nishioka N, Kobayashi D, Izawa J, Irisawa T, Yamada T, Yoshiya K, et al. Association between blood urea nitrogen to creatinine ratio and neurologically favourable outcomes in out-of-hospital cardiac arrest in adults: a multicentre cohort study. J Cardiol. (2023) 81:397–403. doi: 10.1016/j.jjcc.2022.11.009
21. Hryciw N, Joannidis M, Hiremath S, Callum J, Clark EG. Intravenous albumin for mitigating hypotension and augmenting ultrafiltration during kidney replacement therapy. Clin J Am Soc Nephrol. (2021) 16:820–8. doi: 10.2215/CJN.09670620
22. Kokulu K, Sert ET. The role of the lactate/albumin ratio in predicting survival outcomes in patients resuscitated after out-of-hospital cardiac arrest: a preliminary report. Am J Emerg Med. (2021) 50:670–4. doi: 10.1016/j.ajem.2021.09.059
23. Li Y, She Y, Mo W, Jin B, Xiang W, Luo L. Albumin level at admission to the intensive care unit is associated with prognosis in cardiac arrest patients. Cureus. (2021) 13(4):e14501. doi: 10.7759/cureus.14501
24. Lee H, Lee J, Shin H, Lim T-H, Jang B-H, Cho Y, et al. Association between early phase serum albumin levels and outcomes of post-cardiac arrest patients: a systematic review and meta-analysis. J Pers Med. (2022) 12:1787. doi: 10.3390/jpm12111787
25. Kim SH, Youn CS, Kim HJ, Choi SP. Prognostic value of serum albumin at admission for neurologic outcome with targeted temperature management after cardiac arrest. Emerg Med Int. (2019) 2019:1–7. doi: 10.1155/2019/6132542
26. Ge S, Li Y, Li R, Liu J, Zhang R, Fu H, et al. Blood urea nitrogen-to-albumin ratio as a new prognostic indicator of 1-year all-cause mortality in patients with IPF. Front Med. (2025) 11:1497530. doi: 10.3389/fmed.2024.1497530
27. Zhang S, Gao L, Zhao Z, Zhao Q, Yang T, Zeng Q, et al. Blood urea nitrogen to serum albumin ratio as a new indicator of disease severity and prognosis in idiopathic pulmonary artery hypertension. Respir Med. (2024) 227:107643. doi: 10.1016/j.rmed.2024.107643
28. Wang Y, Qin L, Zhang K, Zhang D, Wang W, Xu A, et al. Blood urea nitrogen to albumin ratio is a novel predictor of fatal outcome for patients with severe fever with thrombocytopenia syndrome. J Med Virol. (2024) 96:e29731. doi: 10.1002/jmv.29731
29. Liang Y, Zhou R, Jin C, Liang J, Wang X, Fan W, et al. Association between blood urea nitrogen/albumin and the incidence as well as progression of type 2 diabetes. Nutrients. (2024) 17:113. doi: 10.3390/nu17010113
30. Huang Y, Li Z, Wang J, Wang D, Yin X. Association of the blood urea nitrogen to serum albumin ratio and all-cause mortality in critical ill acute ischemic stroke patients: a retrospective cohort study of MIMIC-IV database 3.0. Front Nutr. (2025) 11:1509284. doi: 10.3389/fnut.2024.1509284
31. Wang Y, Gao S, Hong L, Hou T, Liu H, Li M, et al. Prognostic impact of blood urea nitrogen to albumin ratio on patients with sepsis: a retrospective cohort study. Sci Rep. (2023) 13:10013. doi: 10.1038/s41598-023-37127-8
32. Ye L, Shi H, Wang X, Duan Q, Ge P, Shao Y. Elevated blood urea nitrogen to serum albumin ratio is an adverse prognostic predictor for patients undergoing cardiac surgery. Front Cardiovasc Med. (2022) 9:888736. doi: 10.3389/fcvm.2022.888736
33. Wu Q, Zheng J, Lin J, Xie L, Tang M, Ke M, et al. Preoperative blood urea nitrogen-to-serum albumin ratio for prediction of in-hospital mortality in patients who underwent emergency surgery for acute type a aortic dissection. Hypertens Res. (2024) 47:1934–42. doi: 10.1038/s41440-024-01673-z
34. Taha Sert E, Kokulu K, Mutlu H, Gül M, Uslu Y. Performance of the systemic immune-inflammation index in predicting survival to discharge in out-of-hospital cardiac arrest. Resusc Plus. (2023) 14:100382. doi: 10.1016/j.resplu.2023.100382
35. Kim HH, Lee JH, Lee DH, Lee BK. Association between C-reactive protein-to-albumin ratio and 6-month mortality in out-of-hospital cardiac arrest. Acute Crit Care. (2022) 37:601. doi: 10.4266/acc.2022.00542
36. Cui K, Feng S, Mao Y, Luo H, Yang J, Xu R, et al. The association between blood urea nitrogen to albumin ratio and the 28 day mortality in tuberculosis patients complicated by sepsis. Sci Rep. (2024) 14:16430. doi: 10.1038/s41598-024-65622-z
37. Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu J, et al. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. (2024) 9:1–39. doi: 10.1038/s41392-023-01688-x
38. Yang F, Wang R, Lu W, Hu H, Li Z, Shui H. Prognostic value of blood urea nitrogen to serum albumin ratio for acute kidney injury and in-hospital mortality in intensive care unit patients with intracerebral haemorrhage: a retrospective cohort study using the MIMIC-IV database. BMJ Open. (2023) 13:e069503. doi: 10.1136/bmjopen-2022-069503
39. Shi Y, Duan H, Liu J, Shi X, Zhang Y, Zhang Q, et al. Blood urea nitrogen to serum albumin ratio is associated with all-cause mortality in patients with AKI: a cohort study. Front Nutr. (2024) 11:1353956. doi: 10.3389/fnut.2024.1353956
Keywords: cardiac arrest, blood urea nitrogen, serum albumin, mortality, prognosis
Citation: Zhou G, Tan Y, Li X, Wang Y, Wang D and Liu M (2025) Association of the blood urea nitrogen to serum albumin ratio and 28-day all-cause mortality in patients with cardiac arrest: a retrospective cohort study using the MIMIC-IV database. Front. Cardiovasc. Med. 12:1609059. doi: 10.3389/fcvm.2025.1609059
Received: 9 April 2025; Accepted: 26 May 2025;
Published: 6 June 2025.
Edited by:
Guo-wei Tu, Fudan University, ChinaCopyright: © 2025 Zhou, Tan, Li, Wang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Liu, MTM4ODY3MjY2ODBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Gaosheng Zhou
Gaosheng Zhou Yayuan Tan1,2,3,†
Yayuan Tan1,2,3,† Yixun Wang
Yixun Wang Min Liu
Min Liu