Abstract
Background:
This study aimed to construct a prediction model for the occurrence of venous thromboembolism (VTE) in patients hospitalized with coronary heart disease (CHD) using machine learning algorithms.
Methods:
Clinical data were from the medical records of CHD patients admitted to tertiary hospitals in eastern Liaoning Province between 2019 and 2024. Five machine learning algorithms—random forest (RF), classification and regression tree (CART), logistic regression (LR), logistic regression + least absolute shrinkage and selection operator (LR + LASSO), and extreme gradient boosting (XGBoost)—were used to construct predictive models. The area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and accuracy were comparison metrics between different models.
Results:
A total of 3113 CHD inpatients were included in the study. In the internal validation set, XGBoost had the highest AUC (0.704), sensitivity (0.708), and accuracy (0.692), and RF had the highest specificity (0.706). In the time external validation set, LR + LASSO had the highest AUC (0.649), the highest specificity (0.683) for RF, and the highest sensitivity (0.682) and accuracy (0.656) for XGBoost. D-dimer, Age, and Neutrophil Count (NEUT) were the three most important relevant indicators.
Conclusion:
The prediction model based on machine learning algorithms for the occurrence of VTE in CHD inpatients has a specific diagnostic value. The prediction model constructed by LR + LASSO and XGBoost is more effective than the models constructed by other methods. The results of this study can provide research ideas for the clinical prevention and treatment of VTE events occurring in CHD inpatients.
Introduction
Coronary heart disease (CHD) seriously affects human health and imposes a heavy disease burden on the global healthcare system (1). From 2012 to 2020, the mortality rate among CHD patients in China has risen steadily each year. In 2020, the mortality rate for urban CHD patients in China was 126.91 per 100,000 population, while the rate for rural CHD patients reached as high as 135.88 per 100,000 population. CHD has become a significant cause of death among the Chinese population (2).
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common complication in hospitalized patients and the most common cardiovascular disease secondary to acute ST-elevated myocardial infarction (STEMI) and stroke (3). The estimated incidence of VTE in Europe and the United States is estimated to be 1–2/1,000 person-years (4). In Asia, which varies widely by age, gender, ethnicity, and medical conditions, the incidence of VTE is thought to be lower than in Europe and the United States (5). Some studies have reported an approximately 38-fold increased risk of VTE in hospitalized patients compared to ambulatory patients (6), and 25%–70% of VTE events are associated with hospitalization (7–9). Therefore, the occurrence of VTE in hospitalized patients is an important indicator of overall health (5).
There is a lack of epidemiological statistics on the occurrence of VTE events in CHD patients in China. According to the U.S. National Inpatient Sample Database, the incidence of VTE in hospitalized adult patients with STEMI in the U.S. from 2003 to 2013 was approximately 1%, of which STEMI patients who developed VTE accounted for 17.3% of the overall in-hospital mortality rate, which was significantly higher than that of those who did not develop VTE (8.9%). In addition, patients who developed VTE had higher hospitalization costs compared to those who did not develop VTE (10).
Higher morbidity, mortality, and healthcare expenditures warrant more attention to the occurrence of VTE events in CHD inpatients. Therefore, early detection and timely management of VTE in CHD inpatients is critical. Clinicians screen for VTE in hospitalized patients using the scores of Padua (11) and Caprini (12). Variables in these scoring systems mainly include active cancer, previous VTE, reduced activity, known thrombotic disease, trauma and/or surgery, age, cardiac and/or respiratory failure, acute myocardial infarction or ischemic stroke, acute infections and/or rheumatic diseases, clinical characteristics such as obesity and hormone therapy, body weight, and some serological and genetic markers (11, 13–16). However, the fact that some of these VTE risk factors used in the Padua and Caprini scores overlap with those of CHD and that some of the tests in the Padua and Caprini scores are not available in community health care facilities, as well as the variability in ethnogenetic characteristics and the social environment, make the existing scoring models not applicable to the entire population of patients with VTE (17).
Therefore, this study collected widely validated risk factors for VTE onset in clinical practice and reported risk factors for VTE in CHD patients as research variables. Concurrently, we gathered clinical characteristics and laboratory indicators of hospitalized CHD patients to construct the research dataset. Using this dataset as a platform, we employed various machine learning algorithms to develop predictive models for the occurrence of VTE in hospitalized patients with CHD. In order to improve the interpretability of the results, the machine learning algorithms in this study mainly included logistic regression (LR), logistic regression + least absolute shrinkage and selection operator (LR + LASSO), categorical regression tree (CART), random forest (RF), and extreme gradient boosting (XGBoost).
Methods
Data source
The data for this study were from the Benxi Central Hospital and the Benxi Clinical Biospecimen Repository in Liaoning Province. We collected data from patients hospitalized for CHD between 2019 and 2024. The Chinese Clinical Trial Register approved clinical study admission at https://www.chictr.org.cn/ with the registration number ChiCTR2400094214. The Ethics Committee of Benxi Central Hospital approved this study (20040809). The Ethics Committee of Benxi Central Hospital agreed to the application for exemption from informed consent for this study due to its retrospective nature, which did not require written informed consent. Patient records and information were anonymized and de-identified prior to analysis.
Definition
The diagnosis of VTE (ICD-10, I80) was based on the CHEST Guideline and Expert Panel Report 2016 (18) and the 2020 American Society of Hematology guidelines (19). All CHD patients (ICD10, I25) included in this study underwent limb venous vascular ultrasound, and three clinically experienced physicians confirmed the diagnosis of VTE based on vascular ultrasound.
The specific diagnostic methods were as follows: the images were initially interpreted by the reporting physician and subsequently reviewed by a physician with a higher title or seniority. Both physicians had undergone professional standardized training to minimize information bias. A vascular disease specialist manually reviewed all cases in which the imaging data confirmed the presence of VTE.
Inclusion and exclusion criteria
Inclusion criteria: (i) age ≥ 18 years; (ii) patients hospitalized with a diagnosis of CHD who underwent limb vascular ultrasound; (iii) data obtained between 2019 and 2024.
Exclusion criteria: (i) patients with >30% missing data on the main study variables (20). (ii) missing limb vascular ultrasound data. The study selection process was shown in Figure 1.
Figure 1
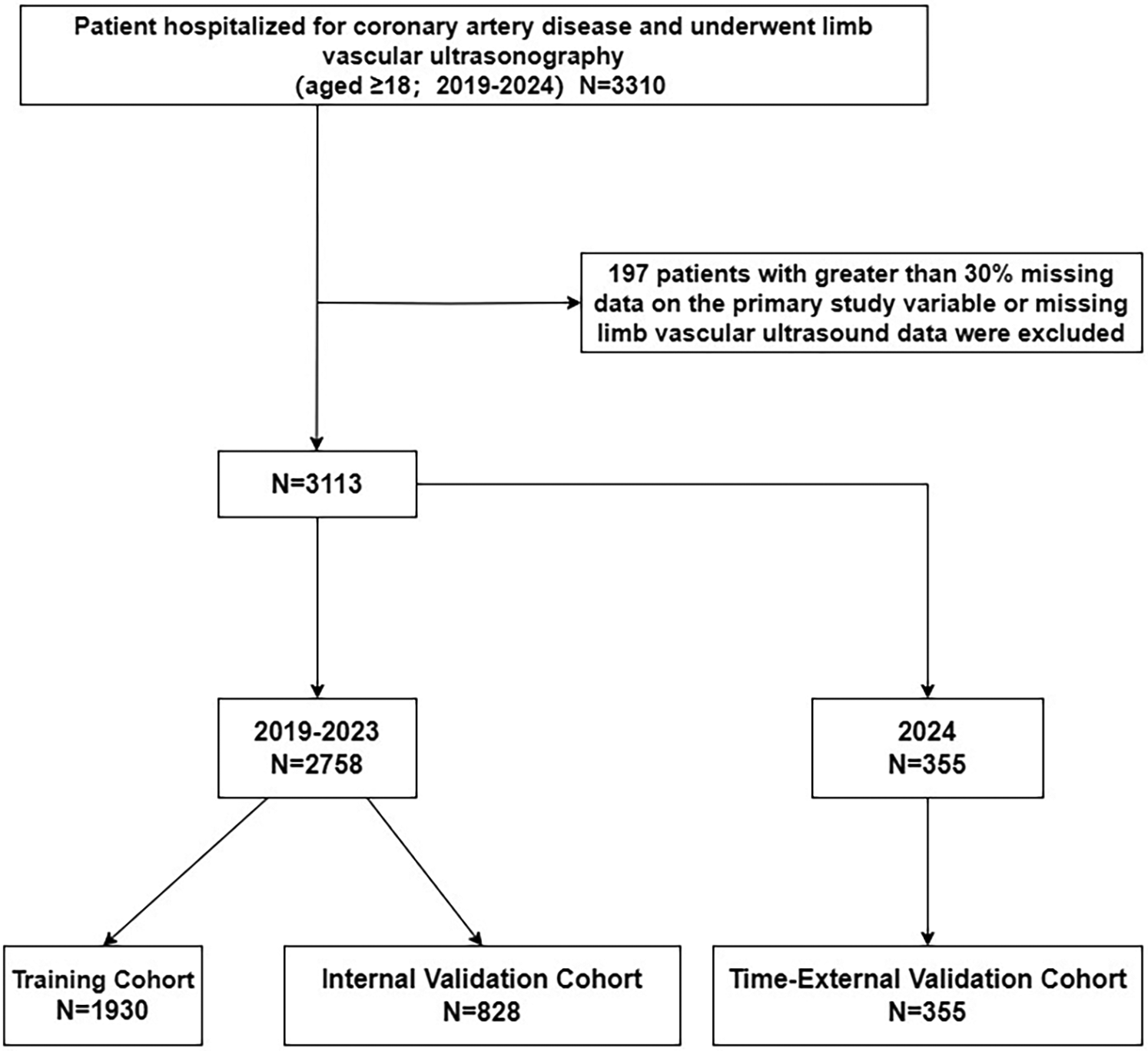
Flow of inclusions and exclusions.
Data collection
Based on previous studies, we selected 41 risk factors that may be associated with VTE in CHD inpatients. These risk factors were from variables previously employed in published risk assessment models, such as the Caprini and Padua scores (21). We had also collected several readily available tests in primary healthcare settings (11, 13).
Forty-one risk factors included: Gender, Active Cancer, Heart Failure, Stroke, Rheumatic Disease, Myocardial Infarction, Respiratory Failure, Lung Diseases, Infections, Blood Transfusion, Trauma, Limitations of limb movement, Dyspnea, Chest Pain, Age, Diastolic Blood Pressure (DBP), Systolic Blood Pressure (SBP), Pulse Rate (PR), Body Mass Index(BMI), Prothrombin Activity (PTA), Activated Partial Thromboplastin Time (APTT), Fibrinogen (Fib), Prothrombin Time (TT), D-dimer, Neutrophil Count (NEUT), Lymphocyte Count (LYM), Monocyte Count (MONO), Red Blood Cell Count (RBC), Hemoglobin (HGB), Red Blood Cell Distribution Width-Coefficient Of Variation (RDWCV), Red Blood Cell Distribution Width-Standard Deviation (RDWSD), Platelet Count (PLT), Uric Acid (UA), Direct Bilirubin (DBIL), Indirect Bilirubin (IBIL), Globulin (GLB), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Creatinine (CRE), Blood Urea Nitrogen (BUN) And Fasting Blood Glucose (FBG).
Statistical analyses
We used a multiple imputation method based on predicted mean matching to handle missing continuous variables (22). Predicted mean matching not only considered linear relationships between variables but also filled in missing values based on the distribution characteristics of the original data, ensuring that the distribution of the imputed data remains consistent with that of the original data. By comparing the description of the imputed data with the original data, we ensured that all imputed values were reasonable and accurate. The patients included in this study had a treatment duration spanning from 2019 to 2024, with all data sourced from the same center. There were sufficient cases for time-external validationto assess the model's stability over time (23). We divided the internal dataset (data from hospitalized patients between 2019 and 2023) and the time-external validation set (data from hospitalized patients after 2024) based on the patients' hospitalization duration (24). We split the internal dataset into a training set and an internal validation set in a 7:3 ratio (Figure 1). We used the training set to establish the predictive model. Then we validated and compared the final performance of each model in both the internal validation set and the time-external validation set. We used receiver operating characteristic (ROC) curves, area under the curve (AUC), sensitivity, specificity, and accuracy as comparison metrics to rank different models, with the AUC value being the most important ranking criterion. After selecting the optimal model, we ranked the variables in the model based on their importance to identify the key variables influencing the model, which were also important factors associated with VTE events in CHD patients hospitalized with VTE.
Continuous variables were expressed as median (interquartile range). Categorical variables were reported as percentage counts. Continuous variables were tested with the Mann–Whitney U test and categorical variables with the chi-square test. All statistical analyses were performed using two-sided tests, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 26.0 and R (version 4.4.2).
Results
Patient characteristics
This study included a total of 3,113 hospitalized patients with CHD, among whom 474 had VTE (prevalence rate of 15.2%). The data were divided into a training set (N = 1,930), an internal validation set (N = 828), and a time-external validation set (N = 355). There was no statistically significant difference between the data before and after interpolation, as shown in Table 1.
Table 1
| Variables | Before interpolation | After interpolation | P-values |
|---|---|---|---|
| Age (IQR; years) | 76.00 (67.00; 85.00) | 76.00 (67.00; 85.00) | 1.000 |
| DBP (IQR; mmHg) | 78.00 (70.00; 88.00) | 78.00 (69.00; 88.00) | 0.518 |
| SBP (IQR; mmHg) | 139.00 (124.00; 155.00) | 139.00 (123.00; 155.00) | 0.848 |
| PR (IQR; bpm) | 80.00 (71.00; 92.00) | 80.00 (70.00; 92.00) | 0.348 |
| BMI (IQR; kg/m²) | 24.20 (21.64; 26.87) | 24.03 (21.48; 26.67) | 0.105 |
| PTA (IQR; %) | 94.00 (84.00; 104.00) | 95.00 (84.00; 105.00) | 0.360 |
| APTT (IQR; s) | 36.90 (33.90; 40.50) | 36.80 (33.80; 40.40) | 0.409 |
| Fib (IQR; g/L) | 3.77 (3.05; 4.79) | 3.74 (3.05; 4.75) | 0.478 |
| TT (IQR; s) | 17.00 (16.20; 17.80) | 17.00 (16.20; 17.80) | 0.856 |
| D-dimer (IQR; mg/L) | 1.00 (0.46; 2.09) | 0.97 (0.46; 2.02) | 0.319 |
| NEUT (IQR; × 109/L) | 4.95 (3.62; 7.27) | 4.92 (3.60; 7.21) | 0.611 |
| LYM (IQR; × 109/L) | 1.36 (0.92; 1.86) | 1.36 (0.93; 1.85) | 0.939 |
| MONO (IQR; × 109/L) | 0.48 (0.36; 0.64) | 0.48 (0.36; 0.64) | 0.668 |
| RBC (IQR; × 1012/L) | 4.16 (3.65; 4.59) | 4.16 (3.66; 4.57) | 0.929 |
| HGB (IQR; g/L) | 126.00 (110.00; 140.00) | 126.00 (111.00; 139.00) | 0.871 |
| RDWCV (IQR; %) | 13.30 (12.70; 14.10) | 13.30 (12.60; 14.10) | 0.581 |
| RDWSD (IQR; fI) | 44.10 (41.80; 47.30) | 44.00 (41.80; 47.20) | 0.704 |
| PLT (IQR; × 109/L) | 201.00 (160.00; 253.00) | 200.00 (160.00; 251.00) | 0.684 |
| UA (IQR; μmol/L) | 346.00 (273.00; 445.00) | 345.00 (272.50; 442.00) | 0.858 |
| DBIL (IQR; μmol/L) | 3.00 (2.10; 4.70) | 3.00 (2.10; 4.70) | 0.597 |
| IBIL (IQR; μmol/L) | 6.30 (4.00; 9.10) | 6.30 (4.00; 8.90) | 0.733 |
| GLB (IQR; g/L) | 27.70 (25.00; 30.80) | 27.60 (24.80; 30.70) | 0.528 |
| ALT (IQR; U/L) | 17.00 (12.00; 26.00) | 18.00 (13.00; 26.00) | 0.614 |
| AST (IQR; U/L) | 21.00 (16.00; 29.00) | 21.00 (16.00; 29.00) | 0.877 |
| CRE (IQR; μmol/L) | 77.00 (62.00; 104.00) | 77.00 (62.00; 101.00) | 0.354 |
| BUN (IQR; mmol/L) | 6.93 (5.29; 9.47) | 6.85 (5.29; 9.09) | 0.267 |
| FBG (IQR; mmol/L) | 6.17 (5.14; 8.34) | 6.18 (5.14; 8.30) | 0.845 |
Comparison of continuous variables before and after interpolation.
DBP, diastolic blood pressure; SBP, systolic blood pressure; PR, pulse rate; BMI, body mass index; PTA, prothrombin activity; APTT, activated partial thromboplastin time; Fib, fibrinogen; TT, thrombin time; NEUT, neutrophil count; LYM, lymphocyte count; MONO, monocyte count; RBC, red blood cell count; HGB, hemoglobin; RDWCV, red blood cell distribution width-coefficient of variation; RDWSD, red blood cell distribution width-standard deviation; PLT, platelet count; UA, uric acid; DBIL, direct bilirubin; IBIL, indirect bilirubin; GLB, globulin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRE, creatinine; BUN, blood urea nitrogen; FBG, fasting blood glucose; IQR, interquartile range.
In the training set, there were 285 cases of VTE (prevalence of 14.8%) and 1,645 cases of non-VTE. We used PASS software to estimate the sample size based on the prevalence of the training set samples obtained. The number of samples in the training set of this study met the sample size requirements for constructing a prediction model.
The results of the training set were in Table 2. Compared with patients in the non-VTE group, patients in the VTE group had a higher proportion of females (P < 0.001), rheumatic diseases (P < 0.001), infections (P = 0.006), blood transfusions (P = 0.004), and dyspnea symptoms (P = 0.018).
Table 2
| Variables | VTE (N = 285) | Non-VTE (N = 1,645) | P-values |
|---|---|---|---|
| Gender/Female (n, %) | 160 (56.14%) | 721 (43.83%) | <0.001 |
| Active cancer (n, %) | 7 (2.46%) | 61 (3.71%) | 0.290 |
| Heart failure (n, %) | 167 (58.60%) | 911 (55.38%) | 0.313 |
| Stroke (n, %) | 61 (21.40%) | 331 (20.12%) | 0.619 |
| Rheumatic disease (n, %) | 6 (2.11%) | 25 (1.52%) | 0.468 |
| Myocardial infarction (n, %) | 62 (21.75%) | 411 (24.98%) | 0.242 |
| Respiratory failure (n, %) | 71 (24.91%) | 253 (15.38%) | <0.001 |
| Lung diseases (n, %) | 70 (24.56%) | 381 (23.16%) | 0.606 |
| Infections (n, %) | 161 (56.49%) | 784 (47.66%) | 0.006 |
| Blood transfusion (n, %) | 28 (9.82%) | 89 (5.41%) | 0.004 |
| Trauma (n, %) | 17 (5.96%) | 100 (6.08%) | 0.941 |
| Limb limitations (n, %) | 23 (8.07%) | 109 (6.63%) | 0.373 |
| Dyspnea (n, %) | 113 (39.65%) | 534 (32.46%) | 0.018 |
| Chest pain (n, %) | 19 (6.67%) | 170 (10.33%) | 0.054 |
| Age (IQR; years) | 81 (73.00, 86.00) | 75 (66.00, 84.00) | <0.001 |
| DBP (IQR; mmHg) | 77 (68.00, 88.00) | 78 (70.00, 87.00) | 0.336 |
| SBP (IQR; mmHg) | 136 (122.50, 155.00) | 140 (123.00, 155.00) | 0.207 |
| PR (IQR; bpm) | 82 (72.00, 97.50) | 80 (70.00, 92.00) | 0.017 |
| BMI (IQR; kg/m²) | 23.44 (20.76, 26.29) | 24.16 (21.56, 26.64) | 0.013 |
| PTA (IQR; %) | 92 (80.00, 100.00) | 96 (85.00, 106.00) | <0.001 |
| APTT (IQR; s) | 36.6 (33.60, 40.55) | 36.7 (33.90, 40.40) | 0.996 |
| Fib(IQR; g/L) | 3.88 (3.06, 4.86) | 3.68 (3.04, 4.69) | 0.317 |
| TT (IQR; s) | 17 (16.20, 17.90) | 17 (16.20, 17.80) | 0.940 |
| D-dimer (IQR; mg/L) | 1.93 (0.94, 4.99) | 0.89 (0.43, 1.88) | <0.001 |
| NEUT (IQR; × 109/L) | 5.59 (4.10, 8.48) | 4.77 (3.59, 6.83) | <0.001 |
| LYM (IQR; × 109/L) | 1.21 (0.82, 1.62) | 1.41 (0.96, 1.92) | <0.001 |
| MONO (IQR; × 109/L) | 0.48 (0.36, 0.66) | 0.48 (0.36, 0.63) | 0.934 |
| RBC (IQR; × 1012/L) | 4.02 (3.56, 4.46) | 4.18 (3.70, 4.60) | <0.001 |
| HGB (IQR; g/L) | 122 (107.00, 136.00) | 127 (112.00, 140.00) | <0.001 |
| RDWCV (IQR; %) | 13.5 (12.90, 14.60) | 13.2 (12.60, 13.90) | <0.001 |
| RDWSD (IQR; fI) | 44.8 (42.70, 48.95) | 43.8 (41.70, 47.00) | <0.001 |
| PLT (IQR; × 109/L) | 200 (161.00, 255.50) | 202 (160.00, 253.00) | 0.940 |
| UA (IQR; μmol/L) | 340 (272.50, 445.00) | 347 (273.00, 445.00) | 0.795 |
| DBIL (IQR; μmol/L) | 3.3 (2.10, 5.25) | 2.9 (2.10, 4.60) | 0.072 |
| IBIL (IQR; μmol/L) | 6.4 (3.90, 8.80) | 6.3 (4.00, 9.10) | 0.778 |
| GLB (IQR; g/L) | 27.9 (25.60, 31.55) | 27.4 (24.70, 30.60) | 0.008 |
| ALT (IQR; U/L) | 19 (13.50, 30.00) | 18 (13.00, 26.00) | 0.045 |
| AST (IQR; U/L) | 22 (16.00, 29.50) | 21 (16.00, 29.00) | 0.647 |
| CRE (IQR; μmol/L) | 78 (61.00, 102.00) | 77 (61.00, 101.50) | 0.592 |
| BUN (IQR; mmol/L) | 7.46 (5.39, 10.21) | 6.68(5.29, 8.99) | 0.011 |
| FBG(IQR; mmol/L) | 6.4(5.33, 8.87) | 6.15(5.12, 8.03) | 0.023 |
Univariate analyses of variables associated with VTE.
VTE, venous thromboembolism; limb limitations, Limitations of limb movement; DBP, diastolic blood pressure; SBP, systolic blood pressure; PR, pulse rate; BMI, body mass index; PTA, prothrombin activity; APTT, activated partial thromboplastin time; Fib, fibrinogen; TT, thrombin time; NEUT, neutrophil count; LYM, lymphocyte count; MONO, monocyte count; RBC, red blood cell count; HGB, hemoglobin; RDWCV, red blood cell distribution width-coefficient of variation; RDWSD, red blood cell distribution width-standard deviation; PLT, platelet count; UA, uric acid; DBIL, direct bilirubin; IBIL, Indirect Bilirubin; GLB, Globulin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRE, creatinine; BUN, blood urea nitrogen; FBG, fasting blood glucose; IQR, interquartile range.
Age (P < 0.001), PR (P = 0.017), D-dimer (P < 0.001), NEUT (P < 0.001), RDWSV (P < 0.001), RDWSD (P < 0.001), GLB (P = 0.008), ALT (P = 0.045), BUN (P = 0.011) and FBG (P = 0.023) were higher than those in the non-VTE group. The levels of BMI (P = 0.013), PTA (P < 0.001), LYM (P < 0.001), RBC (P < 0.001), and HGB (P < 0.001) were lower in the patients in the VTE group than those in the non-VTE group.
Predictive effects of different models
Five models, RF, CART, LR, LR + LASSO, and XGBoost, were used to model the prediction of VTE occurring in CHD inpatients. As shown in Figure 2, the ROC curves showed the performance results of the five different models for predicting VTE in the internal and time-external validation sets. The results showed that in the internal validation set, the XGBoost model had the best performance in predicting the occurrence of VTE events with an AUC value of 0.704. In the tine-external validation set, the LR + LASSO model performed best in predicting VTE events with an AUC value of 0.650.
Figure 2
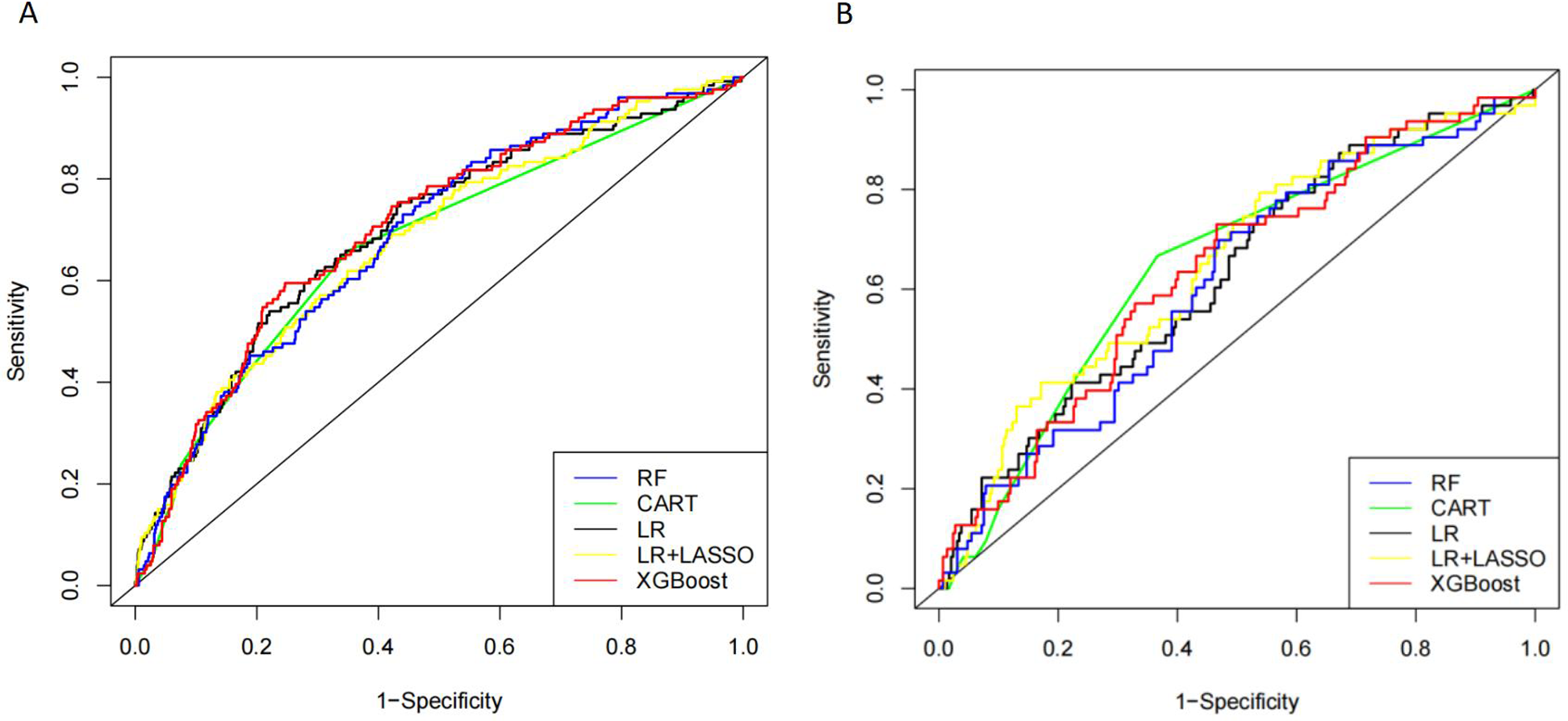
Receiver operating characteristic (ROC) curves of five different models in internal validation cohort (A) and time-external validation cohort (B).
As shown in Table 3, in the internal validation set, XGBoost had the highest AUC (0.704), sensitivity (0.708), and accuracy (0.692), and RF had the highest specificity (0.706). In the time external validation set, LR + LASSO had the highest AUC (0.649), the highest specificity (0.683) for RF, and the highest sensitivity (0.682) and accuracy (0.656) for XGBoost (Table 4).
Table 3
| Model | Sensitivity | Specificity | Accuracy | AUC (95% CI) |
|---|---|---|---|---|
| RF | 0.577 | 0.706 | 0.597 | 0.688 (0.639–0.737) |
| CART | 0.65 | 0.659 | 0.651 | 0.671 (0.622–0.721) |
| LR | 0.704 | 0.603 | 0.688 | 0.698 (0.647–0.749) |
| LR + LASSO | 0.697 | 0.571 | 0.678 | 0.679 (0.627–0.730) |
| XGBoost | 0.708 | 0.603 | 0.692 | 0.704 (0.655–0.754) |
Detailed performance metrics for the five models in internal validation.
LR, logistic regression; CART, classification and regression tree; RF, random forest; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Table 4
| Model | Sensitivity | Specificity | Accuracy | AUC (95% CI) |
|---|---|---|---|---|
| RF | 0.531 | 0.683 | 0.558 | 0.611 (0.536–0.685) |
| CART | 0.634 | 0.667 | 0.639 | 0.647 (0.578–0.715) |
| LR | 0.644 | 0.492 | 0.617 | 0.628 (0.553–0.702) |
| LR + LASSO | 0.603 | 0.54 | 0.592 | 0.649 (0.574–0.723) |
| XGBoost | 0.682 | 0.54 | 0.656 | 0.637 (0.563–0.710) |
Detailed performance metrics for the five models in external validation.
LR, logistic regression; CART, classification and regression tree; RF, random forest; AUC, area under the receiver operating characteristic curve; CI, confidence interval.
Table 5 presented the order of importance of the top five feature variables in the five models in the internal and time-external validation sets. As shown in Table 5, D-dimer, NEUT, and Age were the main features in the prediction model for the occurrence of VTE in CHD inpatients. Figure 3 showed the feature screening process for the LR + LASSO model, and Figure 4 showed the feature importance ranking for the XGBoost model.
Table 5
| Rank | XGBoost | LR + LASSO | LR | CART | RF |
|---|---|---|---|---|---|
| 1 | D-dimer | D-dimer | D-dimer | D-dimer | D-dimer |
| 2 | NEUT | Age | Age | GLB | NEUT |
| 3 | Age | NEUT | NEUT | Gender | Age |
| 4 | BUN | Gender | Gender | NEUT | BMI |
| 5 | FBG | RDWCV | MONO | Fib | BUN |
Ranks of feature importance in RF, XGBoost, LR, CART, and LR + LASSO for predicting VTE.
LR, logistic regression; RF, random forest; CART, classification and regression tree; VTE, venous thromboembolism; BMI, body mass index; Fib, fibrinogen; NEUT, neutrophil count; MONO, monocyte count; RDWCV, red blood cell distribution width-coefficient of variation; GLB, globulin; BUN, blood urea nitrogen; FBG, fasting blood glucose.
Figure 3
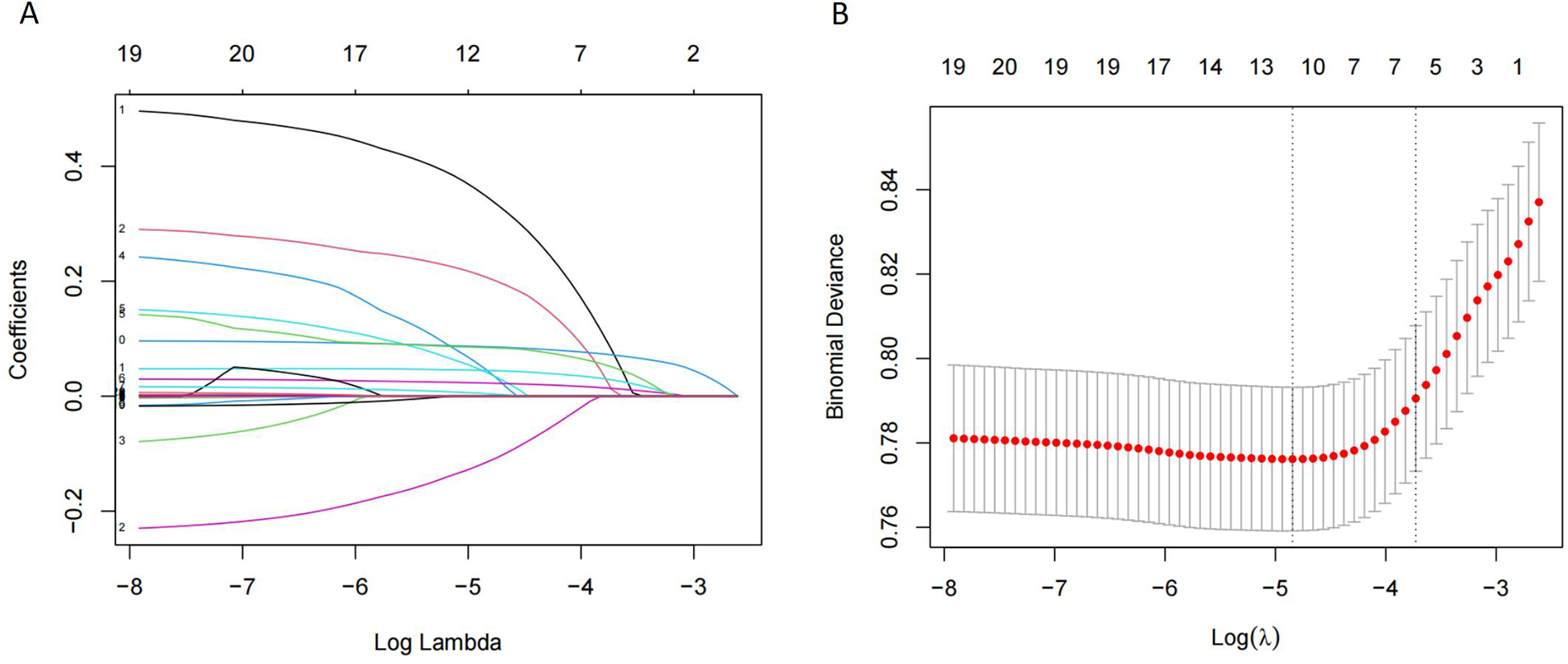
Features selection by LASSO. (A) LASSO coefficients profiles (y-axis) of the 20 features. The upper x-axis is the average numbers of predictors and the lower x-axis is the log (λ). (B) Ten-fold cross-validation for tuning parameter selection in the LASSO model.
Figure 4
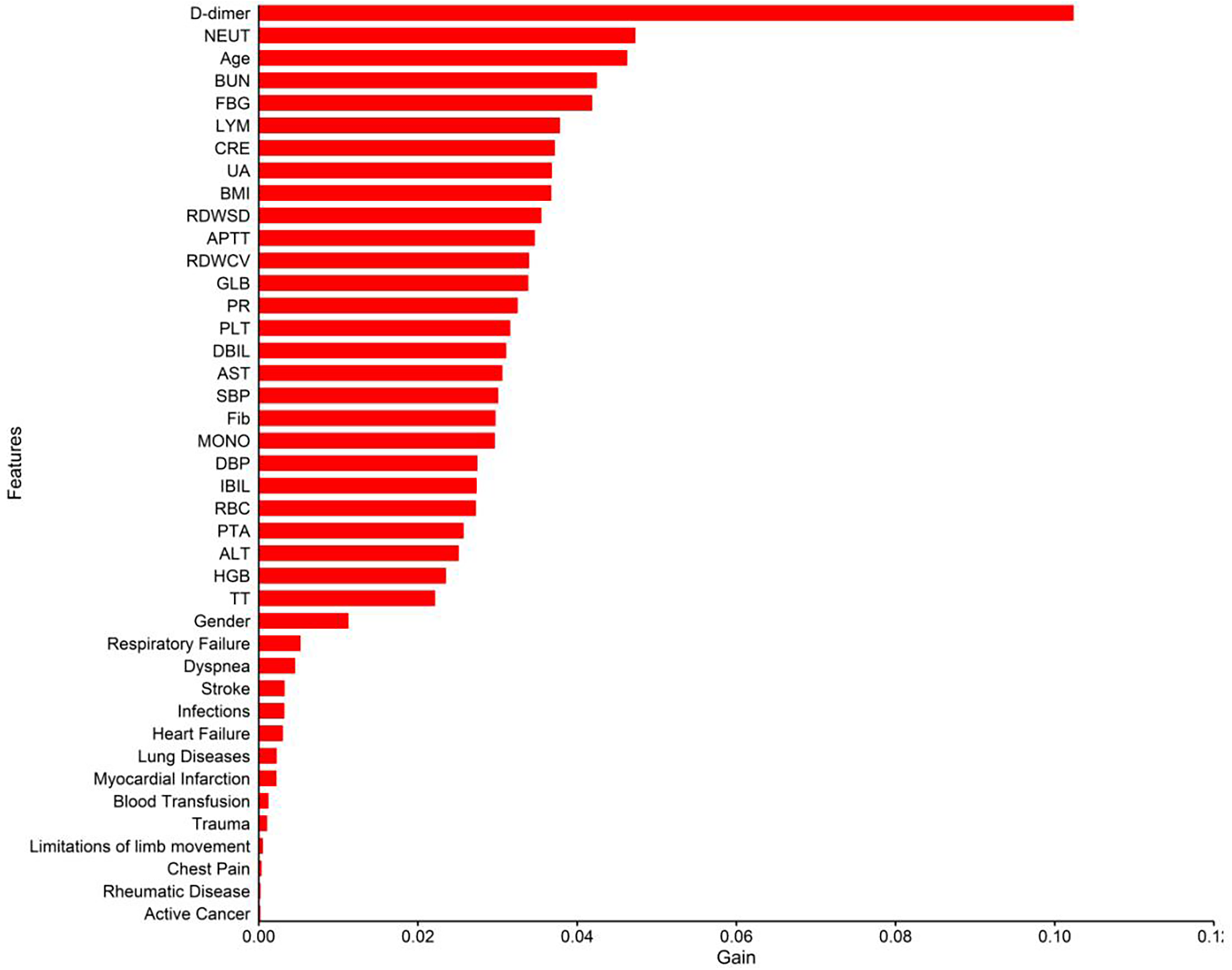
Importance analysis of indexes in XGBoost model.
Discussion
A total of 3,113 CHD inpatients were included in this study, and 41 possible factors associated with the occurrence of VTE were included. Five machine learning algorithms were used to construct predictive models, and the final performance of each model was validated and compared in an internal validation set and an time-external validation cohort. The results show that the prediction models constructed by XGBoost and LR + LASSO were more effective than those constructed by the other algorithms (Tables 3, 4) (Figure 2). D-dimer, NEUT, and Age were the most important correlates of VTE in CHD inpatients (Table 5) (Figure 4).
Machine learning algorithms are a powerful data analysis tool to help in clinical decision-making and disease prevention (25–27). RF is one of the most popular machine-learning techniques for prediction problems (28). RF belongs to the category of supervised machine learning and is widely used for classification and regression. It is an easy-to-use machine-learning algorithm that produces good results without hyperparameter tuning (29). CART is a binary recursive partitioning process capable of processing continuous and nominal attributes as objectives and predictions (30). It does not need to specify the association between the independent variables and the results. The constructed tree is automatically modified to reduce the effect of noise, and the validity of the nodes is determined for the final decision (31). Although both RF and CART are decision tree algorithms, they are prone to overfitting problems due to the depth and complexity of the tree, i.e., the model may over-capture specific rules of the training data, leading to a loss of generalization (32). LR is a commonly used machine learning model in medical research for assessing associations between one or more independent variables and a binary dependent variable (33). The disadvantages of this algorithm are that it requires a large amount of training data, and the interpretation of the results is more complex (34). LASSO is a prevalent machine learning model that performs variable selection and regularisation to improve the resulting model's prediction accuracy and interpretability (35). LR + LASSO incorporates regularisation on top of linear regression, and LASSO performs variable selection and model fitting (Figure 3). XGBoost is an excellent integrated learning algorithm (36). It improves the accuracy of the output by serial integration of decision tree models and has a strong learning ability. The complexity of the model is controlled by regularisation, which helps to prevent overfitting. The essence of XGBoost is to integrate multiple weak classifiers into a single strong classifier to improve prediction accuracy (37).
Previous VTE risk prediction models have also employed the five machine learning methods examined in this study. Among multiple models predicting VTE occurrence in intensive care units, RF demonstrated the best performance (38). In pediatric oncology studies, CART models successfully predicted VTE based on risk factors (39). In a prospective cohort study, researchers developed multiple VTE risk prediction models for patients with traumatic brain injury and found that LR performed better than other models (40). In a prognostic study of VTE in allogeneic transplant patients, LASSO was the optimal machine learning model (41). During the development and validation of machine learning models for predicting VTE in hospitalized cancer patients, the XGBoost model demonstrated superior performance compared with other machine learning approaches (42).
In this study, XGBoost achieved the best results in the internal validation set, and LR + LASSO achieved the best results in the time-external validation set. Based on the order of importance of the first five characteristic variables of the first two models in the validation set, we identified D-dimer, NEUT, and age as the most important correlates of the occurrence of VTE in CHD inpatients (Table 5, Figure 4).
In patients in the VTE group, the median D-dimer was 1.93 mg/L. In contrast, in patients in the non-VTE group, the median D-dimer was 0.89 mg/L, which was statistically different (P = 0.000). D-dimer is a fibrin degradation product, which is usually elevated in the context of DVT. D-dimer shows high sensitivity but low specificity in diagnosing VTE. It is often also elevated in inflammation, malignancy, and other systemic diseases and is a non-specific indicator (43). It has been shown that D-dimer levels are strongly associated with the development of VTE within specific patient groups. A study on risk factors for VTE in lung cancer patients found that a pre-chemotherapy D-dimer concentration of ≥1.44 mg/mL was significantly associated with the occurrence of VTE events in patients with non-small cell lung cancer (44). In another study evaluating the risk of VTE in COVID-19 patients, researchers developed a multivariate predictive model that incorporated D-dimer. This model confirmed the predictive value of D-dimer for VTE events in patients with infectious diseases (45). Our study found that plasma D-dimer levels in CHD patients who experienced VTE events were significantly higher than in those who did not develop VTE. This result suggested that plasma D-dimer levels in CHD patients could predict the occurrence of VTE events.
In the present study, the median age of the patients in the VTE group was significantly higher than that in the non-VTE group (81 vs. 75 years), with a statistically significant difference (Table 2). CHD is a common disease in the elderly population. Some studies have also shown that the incidence of VTE increases with age (21). We had similar findings in the present study. Increasing age increases the risk of VTE in CHD patients. Older patients with CHD are associated with more VTE-related risk factors, and plasma concentrations of coagulation factors increase with age (5). This change in risk factors and coagulation factors explains why age is an independent risk factor for VTE in CHD inpatients.
Another finding of this study was that the level of NEUT was significantly higher in VTE patients than in non-VTE patients (5.59 × 109/L vs. 4.77 × 109/L), with a statistically significant difference (Table 2). Multiple studies have investigated the correlation between NEUT and VTE events (46–48). In a study of sepsis patients, researchers found that neutrophil extracellular traps (NETs) can promote hypercoagulability in these patients (49). Additional research has also identified plasma citrullinated histone H3 (CitH3) as a biomarker for NET formation (50). Patients with symptomatic VTE exhibit elevated plasma CitH3 levels and accelerated thrombin kinetics (51). These findings suggest that NETs may serve as an intermediary link between NEUT and VTE development.In the present study, we found that NEUT levels could be a risk factor for the development of VTE in hospitalized patients with CHD. It is noteworthy that clinicians usually focus on the correlation between age, D-dimer, and VTE while ignoring the correlation between high NEUT levels and VTE events, especially for CHD inpatients. Clinicians should pay attention to NEUT levels in CHD hospitalized patients to prevent VTE in advance.
This study represented the first investigation into predictive models for the occurrence of VTE in hospitalized patients with CHD. Given the convenience and low cost of tests for indicators such as D-dimer and NEUT, which are readily accessible in primary healthcare settings (52, 53), machine learning models based on these variables demonstrate strong potential for widespread application. Future research will explore intervention thresholds for VTE in specific study populations to enhance the model's applicability (54). Of course, this study also had several limitations. Firstly, we did not analyze the data of patients with PE separately, mainly because the number of patients with a diagnosis of PE was small in this study. We found that all patients with PE had concomitant DVT, and therefore, we did not conduct a separate study on PE. Second, the optimal models obtained in this study were inconsistent between the internal and external validation sets. Although the performance of LR + LASSO in the external validation set was better than that of XGBoost, the number of samples in the external validation set in this study was significantly lower than in the internal validation set. Therefore, we believe that XGBoost may be more stable than the LR + LASSO method. Due to the small sample size of the external validation set, this study might be subject to selection bias, which could affect the model's generalizability. In the future, we will expand the sample size to improve model stability and increase the number of variables to make the machine learning model comparable to existing scoring systems (such as Padua and Caprini).
In summary, machine learning models (XGBoost and LR + LASSO) perform well in predicting VTE in hospitalized CHD patients using easily obtainable characteristics at admission. Machine learning algorithms can help clinicians screen for VTE and dynamically monitor changes in the condition of VTE-prone populations.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data for this study were obtained from the Benxi Central Hospital in Liaoning Province and the Benxi Clinical Biospecimen Bank. The dataset is a non-public use dataset due to the patient information involved. In this study, patient records and information were anonymized and de-identified prior to data analysis. Readers who wish to review and exchange data analyzed in this study may contact the corresponding author of this article. Requests to access these datasets should be directed to Hui He, Email: lnbxwxf@yeah.net.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Benxi Central Hospital/Benxi Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/ institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to its retrospective nature. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article due to its retrospective nature. Patient records and information were anonymized and de-identified prior to analysis.
Author contributions
YY: Formal analysis, Methodology, Project administration, Software, Writing – original draft. HY: Writing – original draft, Data curation, Funding acquisition, Investigation. WL: Funding acquisition, Investigation, Writing – original draft. ZY: Investigation, Writing – original draft, Validation. XW: Investigation, Funding acquisition, Supervision, Writing – review & editing. CL: Investigation, Data curation, Validation, Writing – original draft. YZ: Data curation, Writing – review & editing. JW: Data curation, Writing – review & editing. JY: Writing – review & editing, Investigation. HH: Writing – review & editing, Conceptualization, Formal analysis, Methodology, Validation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Benxi City 2023 Key Research & Development Plan Guidance Plan Project of China (No. 2023ZDJH006), the Benxi City 2023 Science &Technology Innovation Project of China (No. BKYW2303) and the Benxi City 2023 Science & Technology Innovation Project of China (No. BKYW2301).
Conflict of interest
Author HH was employed by company Shenyang Kati Health Consulting Co. LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Mensah GA Fuster V Murray CJL Roth GA Mensah GA Abate YH et al Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. (2023) 82(25):2350–473. 10.1016/j.jacc.2023.11.007
2.
Wang Z Ma L Liu M Fan J Hu S . Summary of the 2022 report on cardiovascular health and diseases in China. Chin Med J (Engl). (2023) 136(24):2899–908. 10.1097/CM9.0000000000002927
3.
Di Nisio M van Es N Büller HR . Deep vein thrombosis and pulmonary embolism. Lancet. (2016) 388(10063):3060–73. 10.1016/S0140-6736(16)30514-1
4.
Wendelboe AM Raskob GE . Global burden of thrombosis. Circ Res. (2016) 118(9):1340–7. 10.1161/CIRCRESAHA.115.306841
5.
Lutsey PL Zakai NA . Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. (2023) 20(4):248–62. 10.1038/s41569-022-00787-6
6.
Jordan Bruno X Koh I Lutsey PL Walker RF Roetker NS Wilkinson K et al Venous thrombosis risk during and after medical and surgical hospitalizations: the medical inpatient thrombosis and hemostasis (MITH) study. J Thromb Haemost. (2022) 20(7):1645–52. 10.1111/jth.15729
7.
Stubbs JM Assareh H Curnow J Hitos K Achat HM . Incidence of in-hospital and post-discharge diagnosed hospital-associated venous thromboembolism using linked administrative data. Intern Med J. (2018) 48(2):157–65. 10.1111/imj.13679
8.
Heit JA Spencer FA White RH . The epidemiology of venous thromboembolism. J Thromb Thrombolysis. (2016) 41(1):3–14. 10.1007/s11239-015-1311-6
9.
Cardoso L Krokoscz D Paiva E Furtado I Mattar J Sa M et al Results of a venous thromboembolism prophylaxis program for hospitalized patients. Vasc Health Risk Manag. (2016) 12:491–6. 10.2147/VHRM.S101880
10.
Al-Ogaili A Ayoub A Diaz Quintero L Torres C Fuentes HE Fugar S et al Rate and impact of venous thromboembolism in patients with ST-segment elevation myocardial infarction: analysis of the nationwide inpatient sample database 2003–2013. Vasc Med. (2019) 24(4):341–8. 10.1177/1358863X19833451
11.
Barbar S Noventa F Rossetto V Ferrari A Brandolin B Perlati M et al A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the padua prediction score. J Thromb Haemost. (2010) 8(11):2450–7. 10.1111/j.1538-7836.2010.04044.x
12.
Golemi I Salazar Adum JP Tafur A Caprini J . Venous thromboembolism prophylaxis using the caprini score. Dis Mon. (2019) 65(8):249–98. 10.1016/j.disamonth.2018.12.005
13.
Cronin M Dengler N Krauss ES Segal A Wei N Daly M et al Completion of the updated caprini risk assessment model (2013 version). Clin Appl Thromb Hemost. (2019) 25:1–10. 10.1177/1076029619838052
14.
Bo H Li Y Liu G Ma Y Li Z Cao J et al Assessing the risk for development of deep vein thrombosis among Chinese patients using the 2010 caprini risk assessment model: a prospective multicenter study. J Atheroscler Thromb. (2020) 27(8):801–8. 10.5551/jat.51359
15.
Chandra D Dabhi K Lester W . Are we assessing venous thromboembolism (VTE) risk appropriately for hospitalised medical patients? The national VTE risk assessment tool versus padua prediction score. Br J Haematol. (2020) 189(1):e16–8. 10.1111/bjh.16411
16.
Lavon O Tamir T . Evaluation of the padua prediction score ability to predict venous thromboembolism in Israeli non-surgical hospitalized patients using electronic medical records. Sci Rep. (2022) 12(1):6121. 10.1038/s41598-022-10209-9
17.
Yang Y Wang X Huang Y Chen N Shi J Chen T . Ontology-based venous thromboembolism risk assessment model developing from medical records. BMC Med Inform Decis Mak. (2019) 19(Suppl 4):151. 10.1186/s12911-019-0856-2
18.
Kearon C Akl EA Ornelas J Blaivas A Jimenez D Bounameaux H et al Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149(2):315–52. 10.1016/j.chest.2015.11.026
19.
Ortel TL Neumann I Ageno W Beyth R Clark NP Cuker A et al American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. (2020) 4(19):4693–738. 10.1182/bloodadvances.2020001830
20.
Chen Y Wang C Liu X Duan M Xiang T Huang H . Machine learning-based coronary heart disease diagnosis model for type 2 diabetes patients. Front Endocrinol (Lausanne). (2025) 16:1550793. 10.3389/fendo.2025.1550793
21.
Mugeni R Nkusi E Rutaganda E Musafiri S Masaisa F Lewis KL et al Proximal deep vein thrombosis among hospitalised medical and obstetric patients in Rwandan university teaching hospitals: prevalence and associated risk factors: a cross-sectional study. BMJ Open. (2019) 9(11):e032604. 10.1136/bmjopen-2019-032604
22.
Austin PC White IR Lee DS van Buuren S . Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol. (2021) 37(9):1322–31. 10.1016/j.cjca.2020.11.010
23.
Moons KG Kengne AP Grobbee DE Royston P Vergouwe Y Altman DG et al Risk prediction models: II. External validation, model updating, and impact assessment. Heart. (2012) 98(9):691–8. 10.1136/heartjnl-2011-301247
24.
Xu Q Peng Y Tan J Zhao W Yang M Tian J . Prediction of atrial fibrillation in hospitalized elderly patients with coronary heart disease and type 2 diabetes mellitus using machine learning: a multicenter retrospective study. Front Public Health. (2022) 10:842104. 10.3389/fpubh.2022.842104
25.
Khan A Qureshi M Daniyal M Tawiah K . A novel study on machine learning algorithm-based cardiovascular disease prediction. Health Soc Care Community. (2023) 2023(1):1. 10.1155/2023/1406060
26.
Li J Liu S Hu Y Zhu L Mao Y Liu J . Predicting mortality in intensive care unit patients with heart failure using an interpretable machine learning model: retrospective cohort study. J Med Internet Res. (2022) 24(8):e38082. 10.2196/38082
27.
Mirjalili SR Soltani S Heidari Meybodi Z Marques-Vidal P Kraemer A Sarebanhassanabadi M . An innovative model for predicting coronary heart disease using triglyceride-glucose index: a machine learning-based cohort study. Cardiovasc Diabetol. (2023) 22(1):200. 10.1186/s12933-023-01939-9
28.
Zhang H Zimmerman J Nettleton D Nordman DJ . Random forest prediction intervals. Am Stat. (2019) 74(4):392–406. 10.1080/00031305.2019.1585288
29.
Edeh MO Dalal S Obagbuwa IC Prasad BVVS Ninoria SZ Wajid MA et al Bootstrapping random forest and CHAID for prediction of white spot disease among shrimp farmers. Sci Rep. (2022) 12(1):20876. 10.1038/s41598-022-25109-1
30.
Steinberg D . CART: Classification and Regression Trees, in the top ten Algorithms in Data Mining. New York: Chapman and Hall/CRC (2009). p. 193–216.
31.
Abdalrada AS Abawajy J Al-Quraishi T Islam SMS . Machine learning models for prediction of co-occurrence of diabetes and cardiovascular diseases: a retrospective cohort study. J Diabetes Metab Disord. (2022) 21(1):251–61. 10.1007/s40200-021-00968-z
32.
Bengio Y Lodi A Prouvost A . Machine learning for combinatorial optimization: a methodological tour d’horizon. Eur J Oper Res. (2021) 290(2):405–21. 10.1016/j.ejor.2020.07.063
33.
Panda NR . A review on logistic regression in medical research. Natl J Commun Med. (2022) 13(4):265–70. 10.55489/njcm.134202222
34.
Dineva K Atanasova T . Systematic look at machine learning algorithms–advantages, disadvantages and practical applications. Int Multidiscip Sci GeoConf. (2020) 20(2.1):317–24. 10.5593/sgem2020/2.1/s07.041
35.
Turriziani L Currò A Gagliano A Di Rosa G Caccamo D Tonacci A et al A machine learning approach to the diagnosis of autism spectrum disorder and multi-systemic developmental disorder based on retrospective data and ADOS-2 score. Brain Sci. (2023) 13(6):883. 10.3390/brainsci13060883
36.
Ma M Zhao G He B Li Q Dong H Wang S et al XGBoost-based method for flash flood risk assessment. J Hydrol (Amst). (2021) 598:126382. 10.1016/j.jhydrol.2021.126382
37.
Deng X Ye A Zhong J Xu D Yang W Song Z et al Bagging–XGBoost algorithm based extreme weather identification and short-term load forecasting model. Energy Reports. (2022) 8:8661–867. 10.1016/j.egyr.2022.06.072
38.
Guan C Ma F Chang S Zhang J . Interpretable machine learning models for predicting venous thromboembolism in the intensive care unit: an analysis based on data from 207 centers. Critical Care. (2023) 27(1):406. 10.1186/s13054-023-04683-4
39.
McCarty KL Staggs VS Bolen EE Massey JK Amos LE . Venous Thromboembolism Prophylaxis in High-Risk Pediatric Oncology Patients. Washington, DC: American Society of Hematology (2022).
40.
Qi H Li L Fang J Pei T Li A Ding Z et al Development and validation of an interpretable machine learning model for predicting venous thromboembolism in ICU patients with traumatic brain injury: a multicenter study. World Neurosurg. (2025) 202:124399. 10.1016/j.wneu.2025.124399
41.
Deng RX Zhu XL Zhang AB He Y Fu HX Wang FR et al Machine learning algorithm as a prognostic tool for venous thromboembolism in allogeneic transplant patients. Transplant Cell Ther. (2023) 29(1):57.e1–57.e10. 10.1016/j.jtct.2022.10.007
42.
Meng L Wei T Fan R Su H Liu J Wang L et al Development and validation of a machine learning model to predict venous thromboembolism among hospitalized cancer patients. Asia Pac J Oncol Nurs. (2022) 9(12):100128. 10.1016/j.apjon.2022.100128
43.
Konstantinides SV Meyer G Becattini C Bueno H Geersing G-J Harjola V-P et al 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. (2020) 41(4):543–603. 10.1093/eurheartj/ehz405
44.
Hiraide M Shiga T Minowa Y Nakano Y Yoshioka H Suzuki K et al Identification of risk factors for venous thromboembolism and evaluation of Khorana venous thromboembolism risk assessment in Japanese lung cancer patients. J Cardiol. (2020) 75(1):110–4. 10.1016/j.jjcc.2019.06.013
45.
Li J Wang H Yin P Li D Wang D Peng P et al Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J Thromb Haemost. (2021) 19(4):1038–48. 10.1111/jth.15261
46.
Rattazzi M Villalta S Galliazzo S Del Pup L Sponchiado A Faggin E et al Low CD34(+) cells, high neutrophils and the metabolic syndrome are associated with an increased risk of venous thromboembolism. Clin Sci (Lond). (2013) 125(4):211–22. 10.1042/CS20120698
47.
Petito E Falcinelli E Paliani U Cesari E Vaudo G Sebastiano M et al Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis. (2021) 223(6):933–44. 10.1093/infdis/jiaa756
48.
Huang X He R Jiang Y Tang J Xu X Laoguo S et al Neutrophil extracellular traps: potential thrombotic markers and therapeutic targets in colorectal cancer. J Leukoc Biol. (2024) 117(3):1–9. 10.1093/jleuko/qiae235
49.
Yang S Qi H Kan K Chen J Xie H Guo X et al Neutrophil extracellular traps promote hypercoagulability in patients with sepsis. Shock. (2017) 47(2):132–9. 10.1097/SHK.0000000000000741
50.
Mauracher L-M Posch F Martinod K Grilz E Däullary T Hell L et al Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost. (2018) 16(3):508–18. 10.1111/jth.13951
51.
Navarro SM Thompson RJ MacArthur TA Spears GM Bailey KR Immermann JM et al Increased citrullinated histone h3 levels and accelerated thrombin kinetics in trauma patients who develop venous thromboembolism. Shock. (2024) 63(3):441–7. 10.1097/SHK.0000000000002526
52.
Wang Q Liu J Hu S Du J Zhou S Huang Z et al Establishment of reference intervals for complete blood count in healthy adults at different altitudes on the Western Sichuan Plateau. Front Med (Lausanne). (2025) 12:1586778. 10.3389/fmed.2025.1586778
53.
Ellis JE Johnston TW Craig D Scribner A Simon W Kirstein J . Performance evaluation of the quantitative point-of-care LumiraDx D-dimer test. Cardiol Ther. (2021) 10(2):547–59. 10.1007/s40119-021-00241-7
54.
Nwagha T Nweke M . Stratification of risk factors of lung cancer-associated venous thromboembolism and determining the critical point for preemptive intervention: a systematic review with meta-analysis. Clin Med Insights Oncol. (2023) 17:11795549231175221. 10.1177/11795549231175221
Summary
Keywords
coronary heart disease, venous thromboembolism, machine learning, prediction models, risk factors
Citation
Yang Y-J, Yan H-B, Liu W-T, Yang Z-C, Wang X-H, Liu C, Zhang Y-N, Wang J, Yao J-P and He H (2025) Application of machine learning to predict the occurrence of venous thromboembolism in patients hospitalized for coronary artery disease: a single-center retrospective study. Front. Cardiovasc. Med. 12:1610938. doi: 10.3389/fcvm.2025.1610938
Received
14 April 2025
Revised
10 November 2025
Accepted
17 November 2025
Published
28 November 2025
Volume
12 - 2025
Edited by
Rodrigo Assar, University of Chile, Chile
Reviewed by
Martins Nweke, University of Pretoria, South Africa
Wongwit Senavongse, Srinakharinwirot University—Ongkharak Campus, Thailand
Vera Sa-ing, King Mongkut's University of Technology North Bangkok, Thailand
Updates
Copyright
© 2025 Yang, Yan, Liu, Yang, Wang, Liu, Zhang, Wang, Yao and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hui He lnbxwxf@yeah.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.