Abstract
Introduction:
Evaluation of left ventricular (LV) global systolic function is clinically important for patients with atrial fibrillation (AF); however, the rhythm irregularity inherent to AF poses challenges for assessing novel LV systolic function parameters, such as global myocardial work (MW). This study aimed to validate the feasibility of using the single index beat method to quantify LV MW during echocardiography in patients with AF, compared with the traditional 10-beat average method.
Methods:
A prospective study was performed in 120 patients with AF at the time of the index echocardiography. Global longitudinal strain was assessed using speckle tracking echocardiography from a triplane dataset, followed by MW analysis to calculate global myocardial work index (GWI), global constructive work (GCW), global wasted work (GWW), and global work efficiency (GWE). A total of 10 consecutive beats were evaluated, with both the average value and the maximal difference among the 10 beats recorded. The index beat was defined as on in which the ratio of the preceding to the pre-preceding R-R interval was approximately 1 (0.96–1.04). MW parameters from the index beat were extracted for analysis. Inter-method consistency was assessed using the intra-class correlation coefficient (ICC) with a single-rater, absolute agreement, two-way random effects model. Inter- and intra-observer reproducibility was also assessed.
Results:
Global MW derived from the index beat was comparable with the average of 10 beats: GWI, 1,157.19 ± 416.83 vs. 1,188.98 ± 452.96 mmHg% (p < 0.05); GCW, 1,721.46 ± 524.69 vs. 1,732.46 ± 524.24 mmHg% (p > 0.05); GWW, 237.95 (183.60) vs. 207.50 (207.25) mmHg% (p < 0.001); and GWE, 85.80% (11.05) vs. 86.50% (12.75) (p < 0.001). Consistency analysis showed that ICCs for all assessed MW parameters were >0.87. Satisfactory inter- and intra-observer reproducibility of the measurements by the index beat method was also found.
Conclusions:
Global MW measured using the index beat method demonstrated good agreement with the average over 10 beats in patients with AF, supporting its reliability as a surrogate for the traditional method in clinical practice.
Introduction
Atrial fibrillation (AF) is currently the most common sustained arrhythmia (1). It is associated with increased cardiovascular mortality and morbidity (2), and accurate evaluation of left ventricular (LV) global systolic function using echocardiography is clinically significant (3). However, it is also challenging due to the constantly changing LV contractile ability with varying cardiac cycle length that is characteristic of AF (4). Previously, LV global longitudinal strain (GLS) measured across 17 segments using two-dimensional (2D) speckle tracking echocardiography (STE) has been validated as a more reliable and sensitive parameter of LV systolic dysfunction than single-sectional GLS or traditional LV ejection fraction (EF) (5–7). More recently, non-invasive global myocardial work (MW) parameters derived from overall GLS have been proposed to overcome the limitation of strain's dependence on afterload (8), and have demonstrated better diagnostic and prognostic ability compared with GLS (9, 10). AF has been associated with subclinical myocardial dysfunction, as demonstrated by myocardial work parameters in a population-based cohort of AF patients compared with healthy individuals (11, 12). However, existing literature on the use of MW in AF remains limited, primarily due to the beat-by-beat variability of LV systolic function. Nowadays, triplane echocardiography allows real-time demonstration of three apical views simultaneously, making it possible to obtain a reliable overall GLS for each heartbeat in AF. Given that MW is a derivative of GLS, it is reasonable to expect its feasibility in this setting. Current guidelines acknowledge the inter-beat variability in LV systolic performance during AF and recommend averaging at least five beats for quantification as the standard method (13), with the use of a “‘representative beat”‘ considered only “‘acceptable.”‘ However, growing evidence supports the reliability of the index beat method for evaluating LV systolic function in AF (14–17). Therefore, the present study aimed to validate the reliability of the index beat method compared with the average-beat method for assessing LV MW in patients with AF using triplane echocardiography.
Methods
Study population
A prospective observational study was conducted between June 2021 and June 2024 (ChiCTR2100050725). A total of 158 consecutive patients in AF during echocardiographic examination were initially enrolled according to the pre-specified protocol, regardless of AF type or duration. Rhythm was confirmed by a simultaneously recorded surface electrocardiogram and supported by a single positive peak of the diastolic mitral flow spectrum with variable duration, velocity range, and intervals. Exclusion criteria were as follows: more than two non-visualized segments (n = 15), complete atrioventricular block (n = 3), frequent premature ventricular beats (n = 8), and massive pericardial effusion or clinical suspicion of constrictive pericarditis (n = 3). Upon analysis, patients were also excluded if no beat met the definition of the index beat (n = 9). Figure 1 shows the patient enrollment flowchart. To enhance real-world applicability, the exclusion criteria were kept narrow; thus, patients with structural heart disease, including severe valvar disease, congenital heart disease, or reduced EF, were included. In total, 120 AF patients were finally enrolled. The study was approved by our institutional review board (reference no. 21281-0-02) and written informed consent was obtained from all participants.
Figure 1
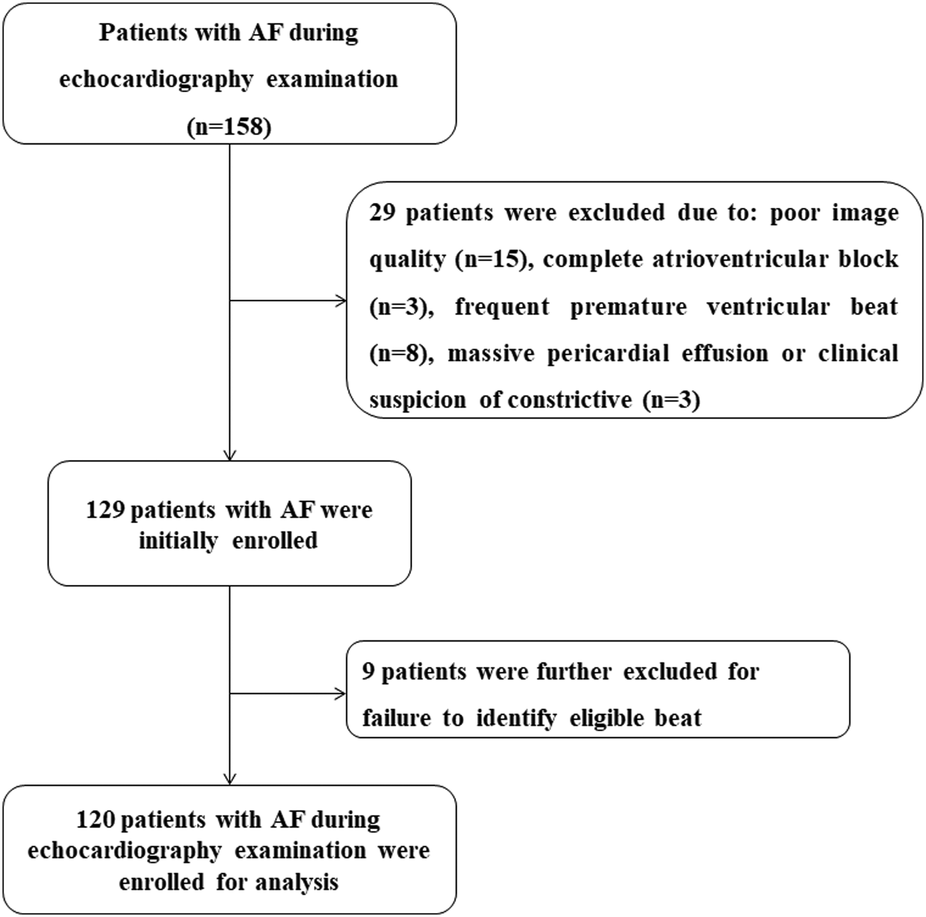
Flowchart of patient enrollment.
Clinical data
Patient demographics and clinical characteristics were recorded. All medications remained unchanged during the study.
Echocardiography
Patients were scanned in the left supine position with a commercially available ultrasound system (Vivid E9; GE Vingmed Ultrasound AS, Horten, Norway). All patients underwent comprehensive 2D echocardiography (2DE) with a M5S transducer (1–5 MHz), followed by triplane echocardiography with a 3 V transducer (1.7–3.3 MHz). Brachial blood pressure was measured after examination (averaged over three measurements). With the M5S transducer, standard 2D, color, pulsed, and continuous wave Doppler images were acquired according to the guidelines (18). LV apical two-, three-, and four-chamber views were sequentially and respectively acquired. With 3 V probe and the triplane echocardiography mode, apical two-, three-, and four-chamber views focusing on LV was demonstrated simultaneously in one ultrasonic view (Figure 2A). Care was taken to ensure that all 17 LV segments were visualized without apical foreshortening, and the image frame rates were maintained in the range of 40–80 Hz. At least 10 cycles were stored for analysis, consistent with previously described methodology (17).
Figure 2
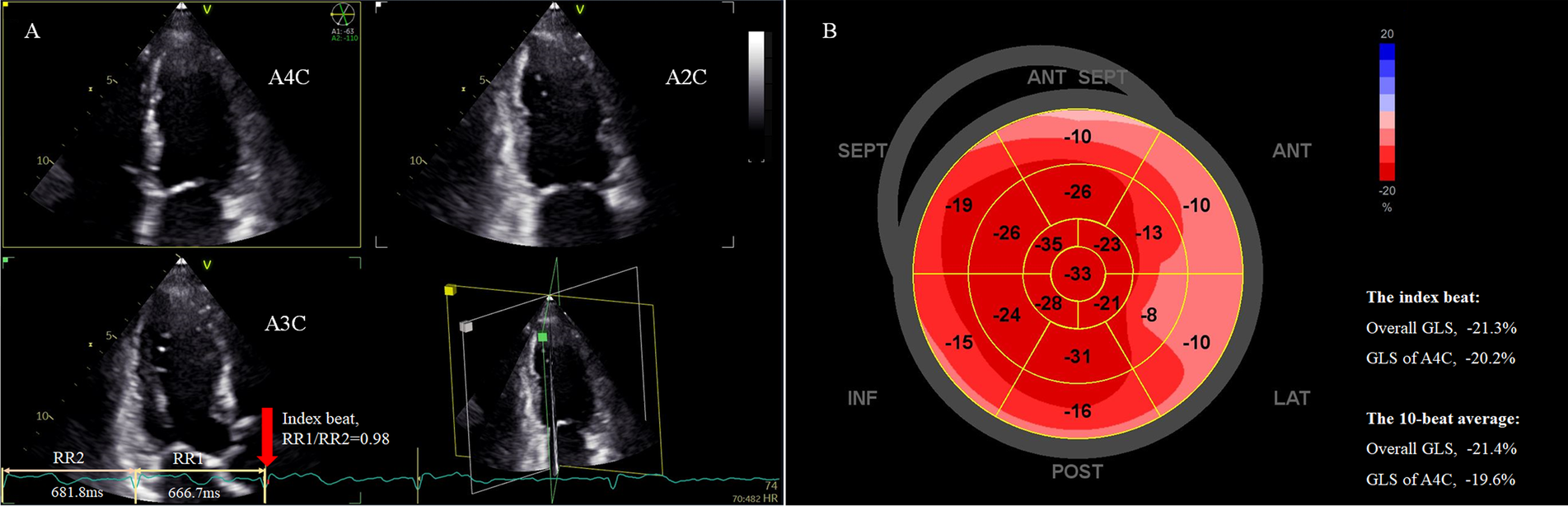
Assessment of LV overall GLS in a patient with persistent AF using the index beat. (A) Strain analysis was performed based on triplane echocardiography showing simultaneous apical two-, three-, and four-chamber views. The index beat (red arrow) was defined as the beat with an almost equal preceding (RR1) and pre-preceding (RR2) R-R intervals. (B) The bull's-eye plot of regional GLS. The overall GLS value and GLS of the atrial four-chamber view of the index beat closely matched the 10-beat average value. AF, atrial fibrillation; GLS, global longitudinal strain; LV, left ventricle.
Conventional 2DE measurements were performed by a single expert echocardiographer (L-YK) following the guidelines (18). LV volume, EF, and left atrial volume were measured using the uniplane Simpson's method from the apical four-chamber view only, as myocardial contractility varied beat-by-beat and biplane summation could not accurately represent the true systolic status of any beat (4, 19).
STE analysis was performed using a commercially available software (EchoPAC V204, GE). Strain was calculated as the percentage change in end-systolic length/initial length. GLS was measured from standard apical two-, three-, and four-chamber views of the triplane dataset (Figure 2). The bull’s eye of segmental peak longitudinal strain with sectional GLS, overall GLS of 17 segments, and heart rate for each beat could be obtained (Figure 2B). Measurements were performed beat-by-beat for 10 consecutive beats, and mean apical four-chamber GLS and overall GLS over these 10 beats were calculated for analysis, as these parameters are most frequently used in clinical practice. For each heartbeat, the R-R interval (ms) was calculated as 60,000 divided by the heart rate (bpm), the latter obtained automatically from the analysis software. For example, if the heart rate for a selected cycle was 60 bpm, the R-R interval would be 60,000/60 = 1,000 ms. The index beat was defined as the beat with nearly equal preceding (RR1) and pre-preceding (RR2) intervals (Figure 2A). Because a ratio of RR1/RR2 exactly equal 1.0 is difficult to obtain in AF, we accepted a range of 0.96–1.04 as eligible (17). The GLS values for both the apical four-chamber view and overall GLS from the index beat were extracted for analysis. The echocardiographer performing the initial measurements was blinded to both the average MW over 10 beats and the index beat for each patient. An index beat-derived overall GLS was considered representative if its absolute difference from the 10-beat average was within 5%.
Quantification of MW was performed using the same software package following each GLS. It was also evaluated beat-by-beat. Strain and pressure data were synchronized by aligning the valvular event times (20). The mitral and aortic valve opening and closure was determined from the parasternal long axis view. The result of MW analysis for each heartbeat was presented as a figure with four panels (Figure 3). A non-invasive pressure strain loop was then derived, with the right lower point indicating mitral closure when the strain value was 0. Its area was an index of global MW. Segmental LV MW values were displayed on a bull's eye plot. The global MW index (GWI) was calculated as the average of all 17 segments. Global constructive work (GCW) was defined as the sum of positive work generated by myocardial shortening during systole and negative work during isovolumic relaxation. Global wasted work (GWW) represented the energy loss from myocardial lengthening in systole and shortening during isovolumic relaxation. Global MW efficiency (GWE) was calculated as GCW divided by the sum of GCW and GWW (9).
Figure 3
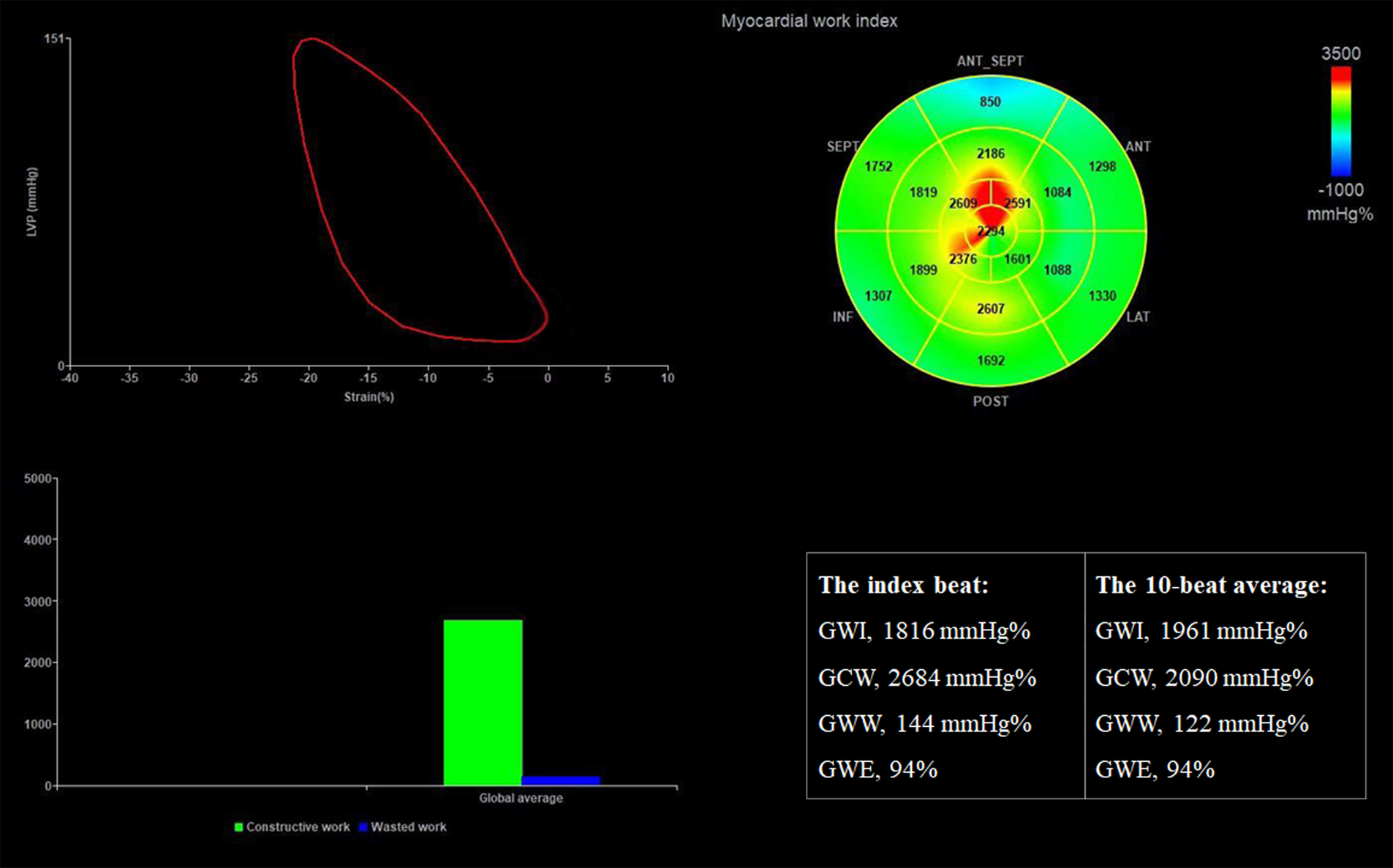
Assessment of LV MW parameters in the same patient. The results show that GWI, GCW, GWW, and GWE derived from the index beat are similar to or the same as the average value over 10 beats. GCW, global constructive work; GWE, global myocardial work efficiency; GWI, global myocardial work index; GWW, global wasted work.
Reproducibility analysis
To assess the intra- and inter-observer reproducibility of the index beat method for assessing MW parameters, 24 individuals were randomly selected for re-analysis. Two independent observers (L-YK and X-JW) measured the same cine loops separately, and the same observer (L-YK) re-evaluated the images at least 4 weeks apart. The results were expressed as intra-class correlation coefficients (ICC) and 95% confidence intervals (CI). Excellent agreement was defined by an ICC > 0.75.
Statistical analysis
Continuous variables are expressed as mean ± SD or median (inter-quartile range [IQR]), as appropriate. Categorical variables are presented as frequency (%). The Kolmogorov–Smirnov test was used to assess the normality of data distribution. Lin's Concordance Correlation Coefficient Power Analysis was used to estimate the sample needed. A minimum sample size of 101 subjects was estimated to achieve a power of 0.90, assuming a concordance correlation coefficient of 0.991. Differences between the values obtained by the index beat method and the average method were compared using paired Student's t-tests or Wilcoxon signed-rank tests, as appropriate. Agreement between the two methods was evaluated using the ICC (95% CI) based on a single-rater, two-way random effect model with absolute agreement. Bland–Altman analysis was not applied because the distribution of the differences between the two measurements was skewed. All clinical and echocardiographic data were analyzed using standard statistical software (SPSS 21.0 and MedCalc 15.0), and statistical significance was defined as p < 0.05.
Results
Patient characteristics and traditional echocardiographic parameters
The baseline demographic and echocardiographic characteristics of the patients are shown in Table 1. A total of 120 patients with AF during the initial echocardiographic examination were enrolled as the study population (63.33% men, mean age 71.30 ± 9.11 years). Of the patients, 102/120 (85.0%) had persistent AF. Most (69/120, 57.5%) patients fell in New York Heart Association functional class I. The median LVEF was 62.0% (14.0) (IQR 20%–76%). In total, 12 (10%) patients had LVEF ≤ 40%.
Table 1
| Variables | Participants (N = 120) |
|---|---|
| Demographics | |
| Age (years) | 71.30 ± 9.11 |
| Male | 76 (63.33) |
| Clinical parameters | |
| BSA (m2) | 1.77 ± 0.19 |
| BMI (kg/m2) | 24.98 ± 3.68 |
| Pulse (bpm) | 74.91 ± 14.24 |
| Systolic blood pressure (mmHg) | 126.72 ± 17.67 |
| Diastolic blood pressure (mmHg) | 79.38 ± 11.30 |
| Type of AF, N (%) | |
| Paroxysmal AF | 15 (12.50) |
| Persistent AF | 102 (85.00) |
| Permanent AF | 3 (2.50) |
| Status of cardiac function | |
| NYHA, N (%) | |
| Class I | 69 (57.50) |
| Class II | 31 (25.83) |
| Class III | 16 (13.33) |
| Class IV | 4 (3.33) |
| History of diseases, N (%) | |
| Hypertension | 76 (63.33) |
| Diabetes | 33 (27.50) |
| Heart failure | 31 (25.83) |
| Mitral stenosis | 16 (13.33) |
| Hypertrophic cardiomyopathy | 4 (3.33) |
| Multiple myeloma | 1 (0.83) |
| Coronary heart disease | 23 (19.17) |
| Cancer | 15 (12.50) |
| Hyperthyroidism | 5 (4.17) |
Characteristics of the study population.
AF, atrial fibrillation; BSA, body surface area; BMI, body mass index; bpm, beats per minutes; NYHA, New York Heart Association.
Myocardial strain and work
STE analysis was successfully performed in all patients. The median frame rate of traditional 2DE images and triplane echocardiography was 56.5 Hz (7.2 Hz) and 49.1 Hz (6.3 Hz), respectively. Baseline echocardiographic characteristics are listed in Table 2. It should be noted that among the 129 patients enrolled for STE analysis (Figure 1), as many as 43 (33.3%) patients did not present an index beat at the initial analysis. However, analysis of other cine loops from triplane datasets stored during the index examination helped find out 34 patients with at least one index beat, thus resulting in 9 (6.9%) patients excluded from further analysis. Among the 120 eligible patients, 46 (38.3%) had more than two index beats (35 patients had two, nine patients had three, and two patients had four index beats).
Table 2
| Variables | Participants (N = 120) |
|---|---|
| Frame rate of 2DE | 56.50 (7.20) |
| Frame rate of 3PE | 49.10 (6.30) |
| Average index beat R-R (RR0) (ms) | 731.71 (305.76) |
| Average pre-index beat R-R (RR1) | 722.89 (195.28) |
| Index beat ratio of RR1/RR2 | 1.00 (0.04) |
| Left ventricle | |
| EDD (mm) | 49.00 (8.50) |
| EDV (mL) | 75.50 (34.50) |
| ESV (mL) | 27.00 (22.00) |
| EF (%) | 62.00 (14.00) |
| Left atrium | |
| Anteroposterior diameter (mm) | 46.00 (10.00) |
| Axial diameter (mm) | 65.50 (12.00) |
| Transverse diameter (mm) | 53.00 (8.00) |
| LAVi-max (mL/m2) | 93.50 (56.50) |
| LAVi-min (mL/m2) | 74.00 (49.50) |
| Doppler echocardiography | |
| e'-Septal (cm/s) | 8.00 (3.85) |
| e'-Lateral (cm/s) | 12.00 (4.25) |
| E/e' | 10.00 (5.80) |
| Moderate and above MR, N (%) | 43 (35.83) |
| Moderate and above TR, N (%) | 60 (50.00) |
Traditional echocardiogram parameters (N = 120).
2DE, 2-dimensional echocardiography; 3PE, triplane echocardiography; EDD, end-diastolic dimension; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; FAC, fraction area change; HR, heart rate; LAVi, left atrial volume indexed; MR, mitral regurgitation; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
The median R-R interval of 10 beats was 753.28 ms (202.07 ms, range 485.9–1,268.5 ms) (Table 2), among whom 4 (3.3%) had a mean R-R interval <500 ms (range 485.9–496.5 ms), corresponding to a mean heart rate of 122–128 beats per min. The median R-R interval of index beat was 731.71 ms (305.76 ms, range 384.6–1,818.2 ms) (P = 0.87 compared with the average R-R of 10 beats). The median RR1/RR2 ratio of the index beat was 1.0 (IQR 0.4).
Table 3 shows the overall GLS and apical four-chamber GLS derived from the index beat closely approximated the corresponding 10-beat average values, with only small inter-method differences. These findings are consistent with those of a previous study on apical four-chamber GLS using the index beat method (15). The ICCs for GLS measurement by the two methods were also excellent (Table 4). Notably, only 1 (0.83%) of the 120 patients showed an index beat-derived GLS that differed from the average by more than 5% (index beat: −5.6% vs. mean: −10.79%). This patient had a wide GLS range, fluctuating from −19.8% to −4.0%, with R-R intervals varying from 377.36 to 1,000.00 ms. Among the 120 index beats analyzed, 7 (5.8%) had R-R intervals <500 ms (range 384.6–491.8 ms), of whom only 1 (14.3%) case had a non-representative GLS value.
Table 3
| Variables | Average value | Index beat value | Inter-method difference | P-value | Maximal difference among the 10 beats |
|---|---|---|---|---|---|
| Myocardial strain | |||||
| GLS-A4C (%) | −14.17 ± 4.07 | −14.40 ± 4.36 | 0.10 (1.70) | 0.190 | 6.1 (3.1) |
| GLS-overall (%) | −13.93 ± 4.18 | −14.14 ± 4.41 | 0.22 (1.13) | 0.062 | 5.6 (2.8) |
| Myocardial work | |||||
| GWI (mmHg%) | 1,157.19 ± 416.83 | 1,188.98 ± 452.96 | −35.7 (173.53) | 0.014* | 575 (301.5) |
| GCW (mmHg%) | 1,721.46 ± 524.69 | 1,732.46 ± 524.24 | 2.8 (134.03) | 0.403 | 467 (207.75) |
| GWW (mmHg%) | 237.95 (183.60) | 207.50 (207.25) | 21.95 (82.15) | 0.001* | 227 (217.25) |
| GWE (%) | 85.80 (11.05) | 86.50 (12.75) | −1.25 (3.95) | 0.000* | 11.5 (8) |
| HR (bpm) | 83.10 (20.03) | 82.00 (32.50) | 0.85 (21.28) | 0.349 | 46.5 (25.5) |
| R-R interval (ms) | 753.28 (202.07) | 731.71 (305.76) | 4.87 (191.29) | 0.869 | 434.81 (274.58) |
Results of and difference in the myocardial strain and work parameters between the two methods.
A4C, apical four-chamber view; GCW, global constructive work; GLS, global longitudinal strain; GWE, global myocardial work efficiency; GWI, global myocardial work index; GWW, global wasted work; HR, heart rate.
P < 0.05.
Table 4
| Variables | ICC (95% CI) | P-value |
|---|---|---|
| GLS-A4C | 0.92 (0.89–0.95) | <0.001 |
| GLS-overall | 0.96 (0.94–0.97) | <0.001 |
| GWI | 0.93 (0.90–0.95) | <0.001 |
| GCW | 0.98 (0.96–0.98) | <0.001 |
| GWW | 0.87 (0.82–0.91) | <0.001 |
| GWE | 0.88 (0.82–0.92) | <0.001 |
| HR | 0.66 (0.55–0.75) | <0.001 |
| R-R | 0.60 (0.47–0.70) | <0.001 |
Agreement analysis between the measurements by the two methods.
A4C, apical four-chamber view; CI, confidence interval; GCW, global constructive work; GLS, global longitudinal strain; GWE, global myocardial work efficiency; GWI, global myocardial work index; GWW, global wasted work; HR, heart rate; ICC, intra-class correlation coefficient.
For MW parameters, no differences were found in GCW measured using the two methods. The index beat method yielded slightly higher GWI and GWE, and slightly lower GWW, compared with the average method; however, these inter-method differences were small, especially when compared with the maximal differences observed among the 10 beats, which reflect random measurement variability (Table 3). Consistency analysis demonstrated excellent agreement between methods for MW parameters, with all ICC values at >0.75 (Table 4).
Reproducibility analysis
For intra-observer repeat assessment of overall GLS, GWI, GCW, GWW, and GWE, ICC was 0.987 (95% CI, 0.971–0.994), 0.876 (95% CI, 0.720–0.945), 0.981 (95% CI, 0.957–0.992), 0.852 (95% CI, 0.660–0.936), and 0.904 (95% CI, 0.778–0.958), respectively (all P = 0.000). For inter-observer repeat analysis of overall GLS, GWI, GCW, GWW, and GWE, ICC was 0.97 (95% CI, 0.947–0.990), 0.892 (95% CI, 0.753–0.953), 0.927 (95% CI, 0.830–0.968), 0.834 (95% CI, 0.618–0.928), and 0.877 (95% CI, 0.708–0.947), respectively (all P = 0.000).
Discussion
To our knowledge, this is the largest prospective study to evaluate the reliability of the index beat method for assessing LV MW in AF patients across the full range of LVEF. We found that LV GWI, GCW, GWW, and GWE derived from the index beat closely matched the corresponding 10-beat average values and showed excellent reproducibility.
AF is characterized by irregular ventricular rhythm and beat-to-beat variation in ventricular contractility (4). Our study is unique in that it overcomes these limitations and extends the use of overall GLS and LV MW evaluation to the AF population, while also demonstrating a less time-consuming approach. Overall GLS derived from 17 LV segments has been validated as a robust measure of LV global systolic function, offering greater sensitivity than either LVEF or apical four-chamber GLS alone (6, 21, 22). However, in AF patients, data on 17-segment GLS have been scarce. Lee et al. (15) assessed LV GLS in AF patients but only analyzed the apical four-chamber view, covering six LV segments. Bunting et al. (23) measured overall GLS in AF using conventional 2DE, acquiring apical two-, three-, and four-chamber views separately, and then averaging of the sectional GLS values. Given the beat-to-beat variability in AF, such summation and averaging may fail to represent the true contractile status of any single beat (24). Our study addresses this challenge by using triplane echocardiography, which enables simultaneous acquisition of apical two-, three-, and four-chamber views with a single probe, allowing precise overall GLS measurement for each heartbeat. As MW is derived from overall GLS, this approach is feasible for extended use in AF. The present study provides robust supporting evidence.
Non-invasive MW indices are novel LV systolic function parameters incorporating LV afterload into GLS analysis (20, 25), with incremental diagnostic and prognostic value in multiple clinical scenarios (9, 25). However, in AF patients confronted with the same irregular ventricular rhythm problem, few studies have reported its use. Liu et al. (11) quantified MW in 51 patients with persistent AF; however, in their study, the apical views were acquired separately. Thus, the accuracy of the global value for each beat remains uncertain. To our knowledge, the present study is the first to assess LV MW with precise heartbeats in AF.
It is generally accepted that, in patients with AF, multiple measurements with subsequent averaging represent the standard approach for quantifying LV systolic function parameters (13). Nonetheless, evidence has shown that, to achieve an estimation of cardiac output with a variability of <2% compared with the mean of four beats in sinus rhythm, the number of beats required in AF is approximately three times greater (26). This is undoubtedly time-consuming for daily practice. Previous studies have validated the reliability and reproducibility of the index beat method for evaluating LV volume change rate (EF), intracardiac pressure change, aortic flow velocity and stroke distance, as well as GLS of the apical four-chamber view (14–17, 19, 27–29). Our study confirmed the reliability and reproducibility of the index beat method compared to the 10-beat average for quantifying overall GLS and global MW in AF. The present study showed higher GWI and GWE, and lower GWW using the index beat approach. These inter-method differences, although statistically significant, were small. Strong consistency was also supported by high ICC values. Further research including larger population remains warranted.
A previous study evaluating the index beat method defined the index beat as the beat with R-R intervals >500 ms (15). In contrast, our results show that measurements derived from the index beat with R-R intervals <500 ms can also be representative. In our cohort, seven patients had index beats with R-R intervals in the range of 384.6–491.8 ms, which produced representative results. This finding is clinically significant, as rapid heart rates and short R-R intervals are common in AF patients.
Limitations
First, nearly one-third of patients did not demonstrate an eligible beat during the initial evaluation. Although re-analysis of additional triplane datasets improved the detection rate, this process was more time-consuming. With future advancements in machine learning and algorithms, real-time calculation of cardiac cycle length and automatic identification of the index beat may become feasible. Second, at present, only GE echocardiographic systems provide software capable of performing 2D STE in triplane datasets or MW analysis (20), which may limit generalizability across different commercial platforms. Nonetheless, as software capabilities evolve, more commercially available analysis tools can be anticipated. Third, this is a single-center study with a relatively small sample size; however, the number of patients was sufficient to achieve 90% power and a 99% concordance correlation coefficient. Besides, our findings in a heterogeneous AF population provide supportive evidence that may enhance the real-world applicability of the index beat method.
Conclusion
In patients with AF undergoing echocardiography, global MW indices derived from the index beat were comparable with those calculated from 10 consecutive beats, demonstrating good reproducibility. Therefore, this approach offers a reliable method for advanced quantification of LV global systolic function in AF. Future multicenter studies with larger populations, broader workstation compatibility, and more advanced software are warranted to further validate and expand its clinical utility.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board of Beijing Tsinghua Changgung Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
L-YK: Conceptualization, Writing – review & editing, Software, Writing – original draft, Funding acquisition, Data curation, Formal analysis, Methodology. X-JW: Software, Writing – review & editing, Data curation, Methodology. L-LC: Data curation, Methodology, Conceptualization, Investigation, Writing – review & editing. WX: Validation, Methodology, Conceptualization, Writing – review & editing. FL: Conceptualization, Investigation, Supervision, Writing – review & editing, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was sponsored by Beijing Hospitals Authority Youth Programme (No. 12022B4009).
Acknowledgments
We thank Professor Getu Zhaori and Ms. Xiao-Yan Wang for their assistance during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Chugh SS Havmoeller R Narayanan K Singh D Rienstra M Benjamin EJ et al Worldwide epidemiology of atrial fibrillation. Circulation. (2014) 129(8):837–47. 10.1161/CIRCULATIONAHA.113.005119
2.
January CT Wann LS Calkins H Chen LY Cigarroa JE Cleveland JC Jr et al 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2019) 140:e126. 10.1161/CIR.0000000000000665
3.
Van Gelder IC Rienstra M Bunting KV Casado-Arroyo R Caso V Crijns H et al 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2024) 45:3314–414. 10.1093/eurheartj/ehae176
4.
Brookes CIO White PA Staples M Oldershaw PJ Redington AN Collins PD et al Myocardial contractility is not constant during spontaneous atrial fibrillation in patients. Circulation. (1998) 98(17):1762–8. 10.1161/01.CIR.98.17.1762
5.
Trivedi SJ Campbell T Stefani L Kumar S Thomas L . Speckle-tracking strain echocardiography in the assessment of myocardial mechanics in patients with idiopathic ventricular arrhythmias: a longitudinal follow-up study. Circ Arrhythm Electrophysiol. (2020) 13(9):e008748. 10.1161/circep.120.008748
6.
Houbois CP Nolan M Somerset E Shalmon T Esmaeilzadeh M Lamacie MM et al Serial cardiovascular magnetic resonance strain measurements to identify cardiotoxicity in breast cancer: comparison with echocardiography. JACC Cardiovasc Imaging. (2021) 14(5):962–74. 10.1016/j.jcmg.2020.09.039
7.
Medvedofsky D Milhorini Pio S Weissman NJ Namazi F Delgado V Grayburn PA et al Left ventricular global longitudinal strain as a predictor of outcomes in patients with heart failure with secondary mitral regurgitation: the COAPT trial. J Am Soc Echocardiogr. (2021) 34(9):955–65. 10.1016/j.echo.2021.04.003
8.
Boe E Skulstad H Smiseth OA . Myocardial work by echocardiography: a novel method ready for clinical testing. Eur Heart J Cardiovasc Imaging. (2019) 20(1):18–20. 10.1093/ehjci/jey156
9.
Edwards NFA Scalia GM Shiino K Sabapathy S Anderson B Chamberlain R et al Global myocardial work is superior to global longitudinal strain to predict significant coronary artery disease in patients with normal left ventricular function and wall motion. J Am Soc Echocardiogr. (2019) 32(8):947–57. 10.1016/j.echo.2019.02.014
10.
Hedwig F Nemchyna O Stein J Knosalla C Merke N Knebel F et al Myocardial work assessment for the prediction of prognosis in advanced heart failure. Front Cardiovasc Med. (2021) 8:691611. 10.3389/fcvm.2021.691611
11.
Liu T Liu H Song Y Huang Y Zhang C . Quantitative assessment of left ventricular myocardial work in patients with different types of atrial fibrillation by non-invasive pressure-strain loop technique. Echocardiography. (2024) 41(3):e15801. 10.1111/echo.15801
12.
Gherbesi E Bergamaschi L Pizzi C Carugo S . Targeting subclinical ventricular dysfunction in atrial fibrillation: what can myocardial work add?Echocardiography. (2024) 41(5):e15830. 10.1111/echo.15830
13.
Lang RM Badano LP Mor-Avi V Afilalo J Armstrong A Ernande L et al Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. 10.1016/j.echo.2014.10.003
14.
Sumida T Tanabe K Yagi T Kawai J Konda T Fujii Y et al Single-beat determination of Doppler-derived aortic flow measurement in patients with atrial fibrillation. J Am Soc Echocardiogr. (2003) 16(7):712–5. 10.1016/S0894-7317(03)00275-X
15.
Lee CS Lin TH Hsu PC Chu CY Lee WH Su HM et al Measuring left ventricular peak longitudinal systolic strain from a single beat in atrial fibrillation: validation of the index beat method. J Am Soc Echocardiogr. (2012) 25(9):945–52. 10.1016/j.echo.2012.06.006
16.
Kusunose K Yamada H Nishio S Tomita N Hotchi J Bando M et al Index-beat assessment of left ventricular systolic and diastolic function during atrial fibrillation using myocardial strain and strain rate. J Am Soc Echocardiogr. (2012) 25(9):953–9. 10.1016/j.echo.2012.06.009
17.
Kong LY Sun LL Chen LL Lv X Liu F . Value of index beat in evaluating left ventricular systolic and diastolic function in patients with atrial fibrillation: a dual pulsed-wave Doppler study. Ultrasound Med Biol. (2020) 46(2):255–62. 10.1016/j.ultrasmedbio.2019.10.028
18.
Mitchell C Rahko PS Blauwet LA Canaday B Finstuen JA Foster MC et al Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. (2019) 32(1):1–64. 10.1016/j.echo.2018.06.004
19.
Muntinga H Gosselink A Blanksma P De Kam PJ Wall E Crijns H . Left ventricular beat to beat performance in atrial fibrillation: dependence on contractility, preload, and afterload. Heart. (1999) 82(5):575–80. 10.1136/hrt.82.5.575
20.
Manganaro R Marchetta S Dulgheru R Ilardi F Sugimoto T Robinet S et al Echocardiographic reference ranges for normal non-invasive myocardial work indices: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. (2019) 20(5):582–90. 10.1093/ehjci/jey188
21.
Plana JC Galderisi M Barac A Ewer MS Ky B Scherrer-Crosbie M et al Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2014) 27(9):911–39. 10.1016/j.echo.2014.07.012
22.
Abou R van der Bijl P Bax JJ Delgado V . Global longitudinal strain: clinical use and prognostic implications in contemporary practice. Heart. (2020) 106(18):1438–44. 10.1136/heartjnl-2019-316215
23.
Bunting KV Gill SK Sitch A Mehta S Connor O Lip K et al Improving the diagnosis of heart failure in patients with atrial fibrillation. Heart. (2021) 107(11):902–8. 10.1136/heartjnl-2020-318557
24.
Su HM Lin TH Hsu PC Lee WH Chu CY Lee CS et al Global left ventricular longitudinal systolic strain as a major predictor of cardiovascular events in patients with atrial fibrillation. Heart. (2013) 99(21):1588–96. 10.1136/heartjnl-2013-304561
25.
Boe E Russell K Eek C Eriksen M Remme EW Smiseth OA et al Non-invasive myocardial work index identifies acute coronary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur Heart J Cardiovasc Imaging. (2015) 16(11):1247–55. 10.1093/ehjci/jev078
26.
Dubrey SW Falk RH . Optimal number of beats for the Doppler measurement of cardiac output in atrial fibrillation. J Am Soc Echocardiogr. (1997) 10(1):67–71. 10.1016/S0894-7317(97)80034-X
27.
Kusunose K Yamada H Nishio S Tomita N Niki T Yamaguchi K et al Clinical utility of single-beat E/e’ obtained by simultaneous recording of flow and tissue Doppler velocities in atrial fibrillation with preserved systolic function. JACC Cardiovasc Imaging. (2009) 2(10):1147–56. 10.1016/j.jcmg.2009.05.013
28.
Yamaguchi K Yoshitomi H Ito S Adachi T Takahashi N Tanabe K . Single-beat determination of global longitudinal speckle strain in patients with atrial fibrillation. J Echocardiogr. (2012) 10(3):90–4. 10.1007/s12574-012-0135-z
29.
Yamanaka-Funabiki K Onishi K Tanabe M Dohi K Ito M Ohte N et al Single beat determination of regional myocardial strain measurements in patients with atrial fibrillation. J Am Soc Echocardiogr. (2006) 19(11):1332–7. 10.1016/j.echo.2006.05.026
Summary
Keywords
atrial fibrillation, left ventricular systolic function, index beat, single beat, myocardial work
Citation
Kong L-Y, Wang X-J, Chen L-L, Xiang W and Liu F (2025) Global myocardial work parameters measured by the index beat method are comparable to the average of 10 beats in patients during atrial fibrillation. Front. Cardiovasc. Med. 12:1612962. doi: 10.3389/fcvm.2025.1612962
Received
16 April 2025
Accepted
04 August 2025
Published
26 August 2025
Volume
12 - 2025
Edited by
Sebastian Kelle, German Heart Center Berlin, Germany
Reviewed by
Diana Ruxandra Hădăreanu, University of Medicine and Pharmacy of Craiova, Romania
Karina Bunting, University of Birmingham, United Kingdom
Updates
Copyright
© 2025 Kong, Wang, Chen, Xiang and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Fang Liu fliu2084@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.