Abstract
Introduction:
Aortic stenosis (AS) is a common valvular disease with a complex and incompletely defined genetic architecture. The contribution of inherited factors may be particularly prominent in consanguineous populations, where familial clustering suggests a strong hereditary component. We investigated the genetic basis of AS in a consanguineous Lebanese family.
Methods:
We performed clinical phenotyping and trio whole-exome sequencing (WES) on a 12-year-old female proband with severe valvular AS and her phenotypically normal consanguineous parents. Variants were assessed with standard filtering for rarity, predicted functional impact, and biological plausibility, with particular attention to genes implicated in cardiovascular development and signaling pathways.
Results:
The proband presented with severe aortic stenosis, bicuspid aortic valve, dilated aortic root and ascending aorta, and mild -moderate tricuspid regurgitation, requiring multiple interventions (balloon valvuloplasty, Ross procedure, and right ventricle-pulmonary artery conduit replacement). WES identified three heterozygous variants in genes belonging to the Wnt signaling pathway APCDD1, DVL1, and AXIN2 in the proband, which were inherited from the normal parents.
Discussion:
The co-occurrence of heterozygous variants in Wnt pathway genes in a child with severe AS highlights a potential polygenic or pathway-level contribution to disease susceptibility, even within a consanguineous context. These findings support a role for Wnt signaling in aortic valve development and pathology, motivating further functional studies and broader cohort analyses to clarify pathogenicity, segregation, and clinical relevance.
1 Introduction
Aortic stenosis (AS) is a valvular disease characterized by restricted blood flow through the aortic valve and narrowing of the left ventricular outflow tract often leading to left ventricular systolic dysfunction (1). Early onset AS is associated with gene mutations affecting valvulogenesis (2), which can lead to more severe phenotypes and an earlier onset of AS. In Lebanon, consanguinity rates exceed 35% in some areas (3), increasing the likelihood of autosomal recessive mutations (4) that can cause AS. The genetics of familial AS in consanguineous populations Remains poorly characterized. This study aims to identify novel pathologic variants underlying this disease.
2 Case presentation
2.1 Patient information
We report the case of a 12-year-old female diagnosed with severe aortic stenosis and a bicuspid aortic valve, with the only child of phenotypically normal consanguineous parents. The patient had progressive aortic valve dysfunction and calcification from an early age, requiring multiple interventions (Figure 1). In June 2009, she underwent balloon valvuloplasty, which was initially effective with only mild residual stenosis. However, further valvular dysfunction required a Ross procedure in February 2015, during which the aortic valve was replaced with her native pulmonary valve. A 16 mm Contegra conduit was placed to replace the right ventricular outflow tract, and a 20 mm Dacron tube graft was used to replace the ascending aorta. In March 2016, the patient required an 18 mm RV-PA conduit replacement to restore right ventricular outflow tract continuity due to calcification of the previous one.
Figure 1
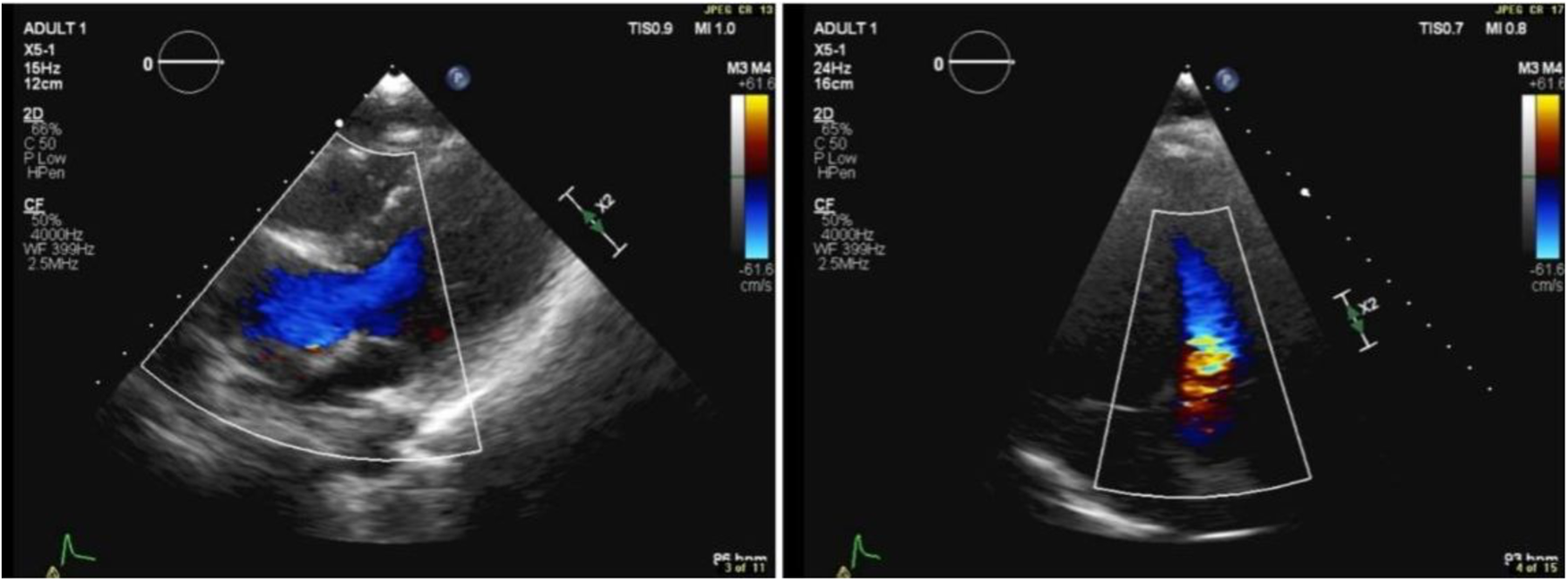
Transthoracic echocardiographic color Doppler showing flow turbulence through the RV-PA conduit. Peak gradient: 25 mmHg; mean gradient: 13 mmHg.
2.2 Diagnostic workup
In April 2018, transthoracic echocardiography was performed as part of the patient's follow-up. The imaging revealed a dilated aortic root, with the sinus of Valsalva measuring 3.1 cm. Some cardiac valve anomalies were present, including mild-to-moderate tricuspid regurgitation (TR), and trace mitral regurgitation (MR). There was no evidence of shunting at the atrial, ventricular, or arterial levels. The other cardiac chambers are grossly normal in size, thickness, and systolic function and hemodynamic assessment showed increased flow velocity at the level of the conduit to the pulmonary artery branches, with a peak systolic gradient of 25 mmHg and a mean gradient of 13 mmHg. Right ventricular (RV) function remained within normal limits, and there were no signs of pericardial effusion or aortic coarctation.
2.3 Genetic findings
Whole-exome sequencing (WES) was performed on genomic DNA extracted from peripheral blood samples of the patient and her parents (the only three individuals analyzed in this study). A stringent variant filtering strategy was implemented, adhering to the American College of Medical Genetics and Genomics (ACMG) guidelines for variant interpretation and classification, and informed by a comprehensive literature review to identify optimal filtering criteria for whole-exome sequencing (WES) analyses in rare congenital heart diseases. Initially, homozygous variants predicted to be pathogenic or likely pathogenic (based on ACMG categories PVS1, PS1-PS4, PM1-PM6, PP1-PP5, and absence of benign criteria BA1-BS4) and with a minor allele frequency (MAF) below 1% were screened to detect potential autosomal recessive inheritance. However, no homozygous deleterious variants meeting these stringent criteria were found in this family. Relevant de novo variants were also excluded from subsequent analyses. Analyses then focused on identifying compound heterozygous mutations within individual candidate genes. Despite a thorough assessment, none of the detected compound heterozygous variants within single genes met the ACMG criteria or were supported by literature evidence as disease-causing for congenital heart defects. Therefore, further analyses examined compound heterozygous variants affecting multiple genes within the same biological pathway. This rigorous filtering approach resulted in the identification of three variants (Table 1) across genes implicated in cardiac development: a maternally inherited variant in APCDD1 (adenomatosis polyposis coli down-regulated), and paternally inherited variants in AXIN2 (axis inhibition protein 2) and DVL1 (Disheveled segment polarity protein 1).
Table 1
| Gene | Variant | Chr | Coordinate | Type | Genotype | Exonic | Inherited From | Read Depth | dbSNP ID | Sift | PolyPhen | CADD Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APCDD1 | C>C/T | 18 | 1,0487,749 | snv | het | Yes | Mother | 92 | rs114154601 | Deleterious (0.04) | Probably damaging (0.993) | 24.2 |
| DVL1 | G>G/A | 1 | 1,275,810 | snv | het | Yes | Father | 76 | rs747373290 | Deleterious (0) | Probably damaging (0.942) | 26.8 |
| AXIN2 | G>G/A | 17 | 63,532,604 | snv | het | Yes | Father | 55 | rs142670753 | Deleterious (0.02) | Possibly damaging (0.885) | 28.1 |
Filtered-Genetic variants.
These three genes have been found to be implicated in the valvular developmental under the WNT signaling pathway, implying that a single or combination of these genes could contribute to the patient's aortic stenosis phenotype.
3 Discussion
This study describes the genetic basis of valvular aortic stenosis in one consanguineous Lebanese family and has identified novel variants in APCDD1, AXIN2, and DVL1, which collectively might explain the phenotype.
Previous studies have linked aortic stenosis to mutations in multiple different genes, most noticeably NOTCH1which was highly correlated with AS given its important role in valvulogenesis and cardiac development (5). Other studies have also identified Lp(a), which is involved in lipid transport, as well as IL6, which is implicated in multiple different inflammatory pathways, as contributors to AS pathogenesis (6, 7), while several others have associated AS to the WNT signaling pathway (8) thus, in addition to valvular calcification and inflammation, alternative pathways involving extracellular matrix remodeling and WNT signaling may contribute to the development AS which emphasizes the genetic heterogeneity of the disease and the need for further research in this field.
In our case, compound heterozygous mutations were detected in APCDD1, AXIN2, and DVL1. Although no variant alone was classified as pathogenic, the presence of heterozygous mutations in APCDD1, AXIN2, and DVL1, all of which are implicated in WNT signaling, suggests a polygenic pathogenesis of AS with a combinatorial inheritance from the parents. In the context of congenital aortic stenosis, the identified missense variants in DVL1, AXIN2, and APCDD1 collectively suggest an intriguing mechanism implicating enhanced WNT signaling activity. Specifically, the missense variant in DVL1 may enhance its affinity toward the Frizzled (Fz) receptor, thereby potentiating WNT pathway activation through stabilized receptor interaction and downstream signaling. Concurrently, the variant identified in AXIN2 potentially reduces its affinity for β-catenin, impairing the effective assembly of the β-catenin degradation complex. This decreased affinity can result in elevated cytoplasmic β-catenin levels, facilitating nuclear translocation and enhanced transcription of WNT-responsive genes involved in valvular interstitial cell proliferation and matrix remodeling. Similarly, the missense variant in APCDD1 may result in reduced affinity toward LRP5/6 co-receptors, diminishing its inhibitory capacity on WNT signaling (Figure 2). Collectively, these subtle molecular alterations—enhanced DVL1-Fz interaction, diminished AXIN2–β-catenin interaction, and attenuated APCDD1–LRP5/6 binding—could synergistically amplify canonical WNT signaling. Such a mechanism aligns well with documented evidence that dysregulated WNT signaling contributes to pathological valve thickening, fibrosis, and calcification—hallmarks of aortic stenosis (9).
Figure 2
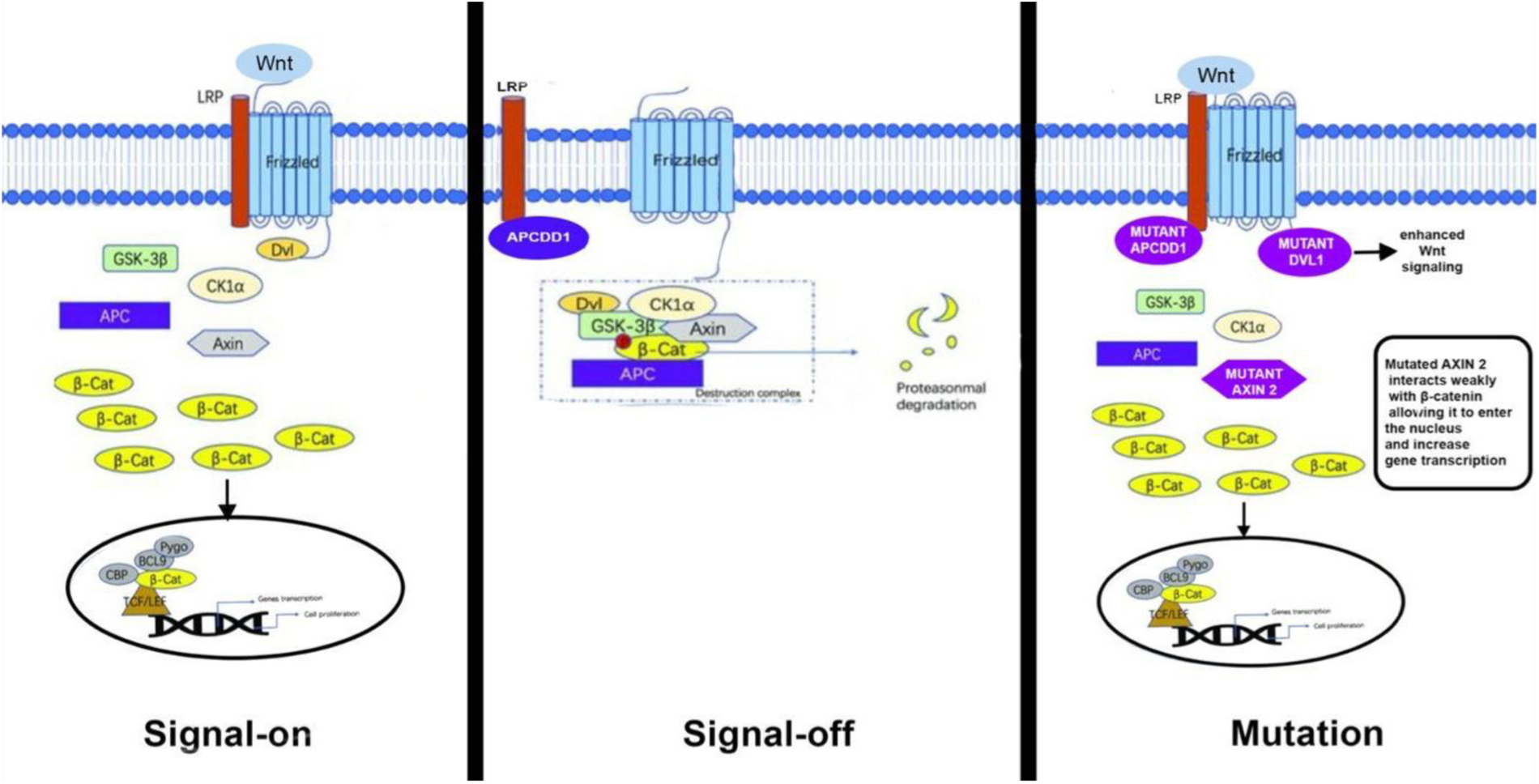
Schematic diagram of the canonical WNT signaling pathway.
These findings emphasize the delicate balance within the WNT signaling pathway critical for normal aortic valve formation and underscore how slight functional changes in pathway components can collectively predispose individuals to congenital valve malformations, including aortic stenosis.
These results support the developing pattern of polygenic inheritance patterns in congenital heart diseases (CHD)where different variants within the same functional pathway act synergistically and increase disease risk (10). This becomes especially relevant in consanguineous populations where deleterious mutations become more apparent and may be at an increased risk of developing CHD.
Uncovering the genetic variants of AS has major potential in the field of precision medicine, especially in high-risk groups, including consanguineous families despite the very limited outcomes for the autosomal recessive pattern postulate. Genetic screening could provide early diagnosis of AS and enable the development of specific personalized therapies.
4 Strengths and limitations
Our study provides insights into the genetic basis of AS through whole exome sequencing allowing for fast and accurate variant identification. Furthermore, this study adds to the limited research and literature on the genetic variants of valvular aortic stenosis. However, the small sample size (a single affected patient analyzed with her parents) limits the generalizability of the results. No other consanguineous families with AS or unrelated controls were included, so the proposed contribution of WNT pathway variants to this patient's phenotype remains speculative. Future studies involving larger cohorts and functional analyses are planned to validate these preliminary findings. Additionally, undetected genetic or epigenetic changes may influence disease severity, as our study did not investigate non-coding regions of the DNA.
5 Conclusion
This study broadens the genetic outlook of AS by confirming the WNT pathway through combinatorial interaction as a major player in congenital heart diseases. These findings point out the complexity of AS genetics, the interplay of multiple different genetic pathways in its pathology, and the need for continued research into its genetic basis. Future studies involving additional patients are warranted to validate these WNT-related genetic findings and confirm their contribution to AS pathogenesis.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1237610.
Ethics statement
The studies involving humans were approved by Institution Review Board (IRB) at the American University of Beirut (AUB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
WA: Methodology, Visualization, Investigation, Writing – review & editing, Writing – original draft, Formal analysis. FM: Data curation, Software, Visualization, Formal analysis, Investigation, Writing – review & editing, Methodology. MA: Data curation, Validation, Visualization, Writing – review & editing, Formal analysis, Investigation. FB: Resources, Methodology, Visualization, Data curation, Investigation, Validation, Writing – review & editing, Supervision, Funding acquisition, Conceptualization. GN: Formal analysis, Resources, Writing – review & editing, Visualization, Investigation, Supervision, Methodology, Conceptualization, Validation, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research project was funded by a Medical Practice Plan (MPP) grant from the Faculty of Medicine at AUB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Nishimura RA Otto CM Bonow RO Carabello BA Erwin JP Gentile F et al 2014 AHA/ACC guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. (2014) 63(22):e57–e185. 10.1016/j.jacc.2014.02.536
2.
Wang Y Fang Y Lu P Wu B Zhou B . NOTCH signaling in aortic valve development and calcific aortic valve disease. Front Cardiovasc Med. (2021) 8:682298. 10.3389/fcvm.2021.682298
3.
Barbour B Salameh P . Consanguinity in Lebanon: prevalence, distribution and determinants. J Biosoc Sci. (2009) 41(4):505–17. 10.1017/S0021932009003290
4.
Bittles AH Black ML . Evolution in health and medicine sackler colloquium: consanguinity, human evolution, and complex diseases. Proc Natl Acad Sci U S A. (2010) 107(Suppl 1):1779–86. 10.1073/pnas.0906079106
5.
Irtyuga O Malashicheva A Zhiduleva E Freylikhman O Rotar O Bäck M et al NOTCH1 mutations in aortic stenosis: association with osteoprotegerin/RANK/RANKL. Biomed Res Int. (2017) 2017:6917907. 10.1155/2017/6917907
6.
Sticchi E Giusti B Cordisco A Gori AM Sereni A Sofi F et al Role of lipoprotein (a) and LPA KIV2 repeat polymorphism in bicuspid aortic valve stenosis and calcification. Intern Emerg Med. (2019) 14(1):45–50. 10.1007/s11739-018-1925-8
7.
Junco-Vicente A Solache-Berrocal G Del Río-García Á Rolle-Sóñora V Areces S Morís C et al IL6 Gene polymorphism association with calcific aortic valve stenosis. Front Cardiovasc Med. (2022) 9:989539. 10.3389/fcvm.2022.989539
8.
Khan K Yu B Kiwan C Shalal Y Filimon S Cipro M et al The role of wnt/β-catenin pathway mediators in aortic valve stenosis. Front Cell Dev Biol. (2020) 8:862. 10.3389/fcell.2020.00862
9.
Pahnke A Conant G Huyer LD Zhao Y Feric N Radisic M . The role of wnt regulation in heart development, cardiac repair and disease. Biochem Biophys Res Commun. (2016) 473(3):698–703. 10.1016/j.bbrc.2015.11.060
10.
Jin SC Homsy J Zaidi S Lu Q Morton S DePalma SR et al Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. (2017) 49(11):1593–601. 10.1038/ng.3970
Summary
Keywords
aortic stenosis, consanguinity, whole-exome sequencing, wnt signaling pathway, polygenic inheritance, congenital heart disease, case report
Citation
Ataya W, Mohammed F, Arabi M, Bitar F and Nemer G (2025) Case Report: Novel combinatorial factors in the WNT pathway in a pediatric case of valvular aortic stenosis from Lebanon: a brief report. Front. Cardiovasc. Med. 12:1614666. doi: 10.3389/fcvm.2025.1614666
Received
19 April 2025
Accepted
23 September 2025
Published
07 October 2025
Volume
12 - 2025
Edited by
Gianluca Lucchese, Guy’s and St Thomas’ NHS Foundation Trust, United Kingdom
Reviewed by
Stiljan Hoxha, University of Verona, Italy
Rajdeep Bilkhu, St Thomas’ Hospital, United Kingdom
Updates
Copyright
© 2025 Ataya, Mohammed, Arabi, Bitar and Nemer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Fadi Bitar fadi.bitar@aub.edu.lb Georges Nemer gnemer@hbku.edu.qa
†These authors have contributed equally to this work
‡These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.