Abstract
Objective:
To investigate the impact of the ventricular pacing modalities and ventricular pacing percentage (VP) on the incidence of new-onset atrial fibrillation (AF) following dual-chamber pacemaker implantation. We aim to explore how these factors, along with left atrial diameter (LAD), contribute to AF development, providing insights into optimizing pacemaker settings to potentially reduce AF risk in pacemaker patients.
Methods:
Between January 1, 2020, and December 31, 2022, a total of 371 patients who underwent initial dual-chamber pacemaker implantation at the Second Affiliated Hospital of Harbin Medical University were enrolled. These patients were categorized into two groups: the non-AF group and the AF group, based on the presence of new-onset AF during one-year follow-up. Regular remote follow-up visits were conducted postoperatively, with endpoint events defined as the detection of AF. A comparative analysis was performed to evaluate the differences in basic characteristics, implantation site of the ventricular lead, and pacemaker programming settings between the two groups.
Results:
The AF group exhibited a significantly higher prevalence of hypertension and a greater proportion of left bundle branch pacing (LBBP) compared to the non-AF group. Additionally, the AF group had a lower proportion of patients with ventricular pacing (VP) in the 0%–39% range, but a higher proportion of patients in the 40%–79% range. Notably, the LBBP cohort demonstrated a significantly lower incidence of AF. The AF group also presented with a larger left atrial diameter (LAD). Multivariate analysis revealed that LBBP and VP in the 0%–39% range were independently associated with a reduced risk of new-onset atrial fibrillation AF. Among the predictive indicators for new-onset AF, both LBBP and LAD demonstrated notable sensitivity and specificity.
Conclusions:
Ventricular pacing modalities and percentage significantly impact new-onset AF after dual-chamber pacemaker implantation. LBBP and lower VP percentages are associated with a lower risk of AF development, whereas a larger LAD may be linked to an increased likelihood of its onset. Optimizing these factors could potentially reduce the risk of AF in pacemaker patients.
Introduction
Sick sinus syndrome (SSS) and atrioventricular block (AVB) are leading indications for pacemaker implantation globally (1). For patients with SSS and AVB requiring permanent pacing, dual-chamber pacing is generally preferred over single-chamber ventricular pacing (2). A comprehensive Cochrane review evaluating pacing modes and patient outcomes concluded that dual-chamber pacing is favored due to its lower incidence of atrial fibrillation (AF) and the reduced occurrence of pacemaker syndrome compared to single-chamber ventricular pacing (2).
Despite this, AF remains the most common cardiac arrhythmia in clinical practice. AF not only leads to a high volume of healthcare consultations but also significantly increases patient morbidity and mortality. The widespread prevalence and serious consequences of AF highlight its critical role as a major public health challenge in modern cardiology (3). The mechanisms by which a dual-chamber pacemaker may induce AF are complex and not yet fully understood. These mechanisms include mechanical stimulation by the pacing electrode such as the ventricular pacing percentage (VP) (4), pacing in the right atrium leading to delayed activation of the left atrium (5), pacemaker-mediated tachycardia that may vary with different ventricular pacing modalities (6), and long-term atrial pacing potentially causing local inflammation or fibrosis (7). The overall pathophysiological processes involved are intricate and still require further clarification.
This study aims to investigate the impact of the ventricular pacing modalities and VP on the incidence of new-onset AF following dual-chamber pacemaker implantation.
Methods
Ethics statement
This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. was approved by the Research Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. Informed consent was obtained from each patient.
Study population
A retrospective analysis was conducted on 870 patients who underwent initial dual-chamber pacemaker implantation at Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University from January 1, 2020, to December 31, 2022. Of these, 371 patients met the inclusion criteria and were enrolled in the study. Participants were categorized into two groups: the AF group and the non-AF group. In this study, AF is defined as clinical AF. The diagnosis of clinical AF requires rhythm documentation via an electrocardiogram (ECG) showing characteristic AF patterns. According to established guidelines, an episode lasting at least 30 s is considered diagnostic of clinical AF. A standard 12-lead ECG or a single-lead ECG tracing of more than 30 s, demonstrating an irregular heart rhythm with no discernible, repetitive P waves and irregular RR intervals (provided atrioventricular conduction is not impaired), is diagnostic for clinical AF (8).
Inclusion Criteria: (i) patients receiving dual-chamber pacemaker implantation; (ii) patients meeting Class I or IIa indications for pacemaker implantation due to conditions such as SSS or AVB (2); (iii) age 18 years or older; (iv) willingness and ability to participate in the study. Exclusion Criteria: (i) preoperative history of atrial fibrillation (AF) or atrial flutter; (ii) prior cardiac surgery for congenital heart disease, cardiomyopathy, valvular disease, or severe myocardial infarction; (iii) post-pacemaker implantation complications, including lead dislocation, pacemaker pocket infection, myocardial perforation, or pacemaker syndrome; (iv) severe liver or kidney disease, hyperthyroidism, malignancy, or other serious illnesses; (v) incomplete clinical data.
Dual-chamber pacemaker implantation
The patient is positioned supine. After routine skin disinfection and draping of the operative field, 1% lidocaine is administered locally 2 cm below the left clavicle along the clavicular line, followed by a 5 cm incision. The subcutaneous tissue is sequentially dissected down to the pectoralis major fascia, creating a 5 × 5 cm pocket. The axillary vein is punctured, a guide wire is inserted, and its entry into the subclavian vein is confirmed by x-ray.
For the left bundle branch pacing (LBBP) group, a C315 HIS delivery sheath is introduced along the guide wire. Right ventriculography is performed to display the tricuspid valve. The electrode is then introduced through the sheath, mapping the His bundle. The electrode is advanced and rotated to locate the proximal LBB. Multiple lead physiological recordings are taken to verify the detection of the LBB potential, the pacing electrode tip, and confirm the pacing pattern indicative of right bundle branch block. Additionally, the recordings are used to ensure that the pacing area is specific to LBBP only and not left ventricular septal pacing. Peak time is measured, and parameters such as ventricular sensing, impedance, and bundle branch threshold are tested. If necessary, a dual lead configuration is employed to find the optimal pacing modalities. After successful implantation, the second electrode is retracted to the atrium and positioned at the top of the atrial septum (Figure 1A). In most cases, the right atrial electrode is placed in the right atrial appendage, with only a few cases requiring placement at the atrial septum due to complications.
Figure 1
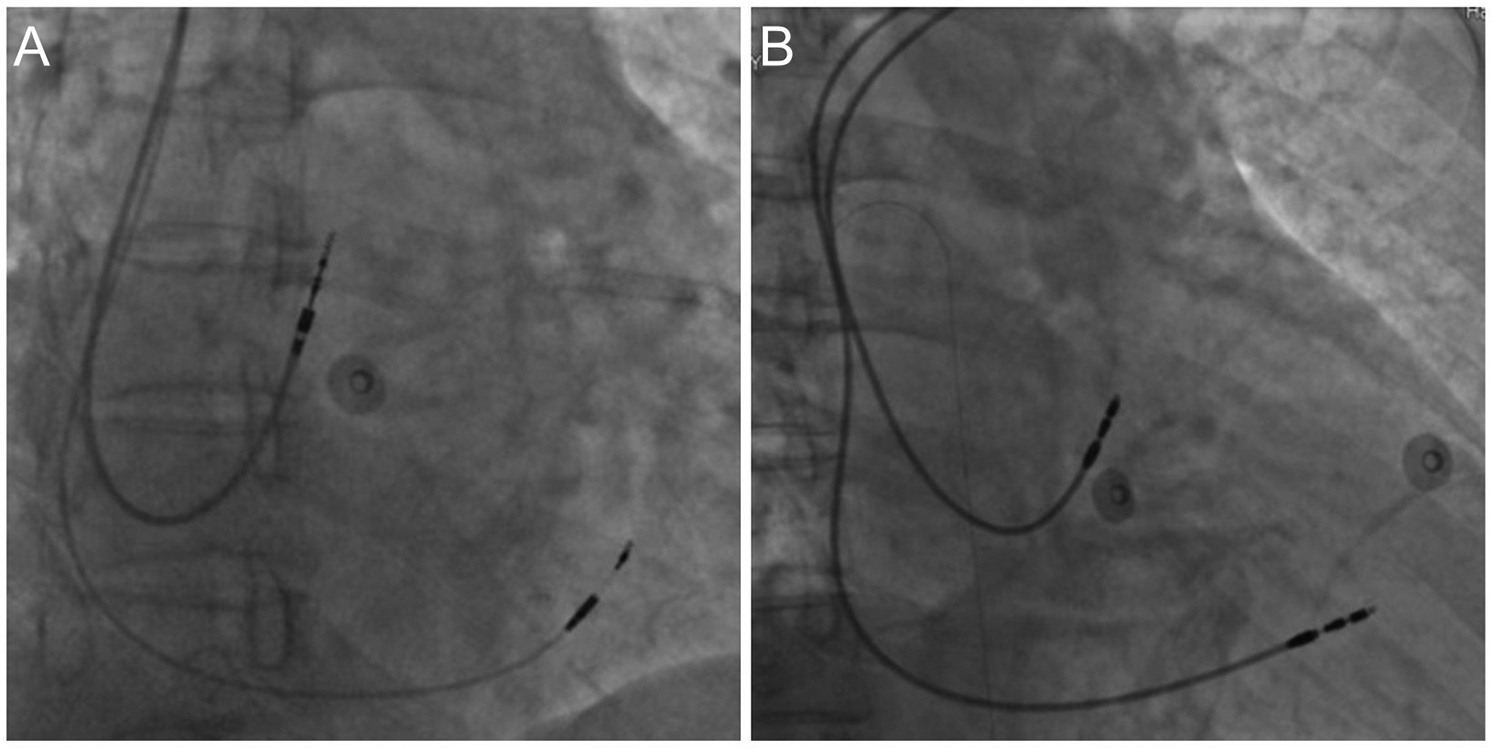
Dual-Chamber pacemaker implantation. (A) Left bundle branch pacing; (B) right ventricular septal pacing.
For the right ventricular septal pacing (RVSP) group, an 8F tear-open sheath is introduced along the guide wire. The right atrium and right ventricular electrodes are introduced through the sheath. The right atrial electrode is secured to the top of the atrial septum, and the right ventricular electrode is fixed to the mid-septum. After electrode placement, testing includes atrial sensing, impedance, and threshold. Pacing the atrium ensures no diaphragmatic contractions. The pulse generator is then connected and placed in the pocket. The electrocardiogram confirms the pacemaker's normal operation (Figure 1B).
The operative area is irrigated with gentamicin and saline, followed by layer-by-layer closure of the pectoralis major and subcutaneous tissue. Tissue glue is used to bond the skin, and the operative site is covered with gauze and dressed for hemostasis.
Clinical data collection
The collected baseline data include patient demographics such as age and gender, as well as medical histories, including hypertension, coronary heart disease, diabetes, and myocardial infarction. Preoperative laboratory indicators are measured, including serum creatinine, brain natriuretic peptide, cardiac troponin I, total cholesterol, low-density lipoprotein, high-density lipoprotein and triglyceride. Echocardiography parameters such as left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and left atrial diameter (LAD) are also recorded, and the echocardiography is also performed preoperatively. In addition, medication history is documented, as well as the pacemaker lead position.
Follow up
All pacemaker patients are followed up for at least one year after the procedure. Remote monitoring of pacemaker function is conducted by professional pacemaker engineers using a pacemaker programmer. This includes the pacemaker's working modes, atrial pacing percentage (AP), VP, and documentation of any newly developed diseases. Data regarding new-onset AF are recorded based on electrocardiogram (ECG) results. For patients without AF, data are primarily derived from their examination at the one-year follow-up.
This follow-up approach allows for a comprehensive assessment of patient outcomes, with a particular focus on the development of new-onset AF, which is a key endpoint of the study. The inclusion of both laboratory and echocardiographic parameters helps in understanding the overall cardiac function and the effects of pacemaker therapy in patients with various underlying cardiovascular conditions.
Statistical analysis
Quantitative data are presented as mean ± standard deviation, while qualitative data are shown as frequency (per centage). Comparisons between groups were performed using the independent two-sample t-test. The chi-square test or Fisher's exact test, as appropriate, was used for categorical variables. For the regression analysis, we categorized patients into two groups: none-AF and AF group. This binary classification was used in both univariate and multivariate logistic regression analyses to identify predictors of new-onset AF. A receiver operating characteristic (ROC) curve was generated to determine the optimal cutoffs for indicators with the best diagnostic sensitivity and specificity. Two-sided P-values <0.05 were considered statistically significant. All statistical analyses were performed using the IBM SPSS statistical software, version 26.0 (IBM SPSS Inc., Armonk, NY, USA).
Results
The baseline characteristics of patients in the non-AF and AF groups are shown in Table 1. The prevalence of hypertension was significantly higher in the AF group compared to the non-AF group (54.17% vs. 37.46%, P = 0.010). Regarding the implantation site of the ventricular lead, the proportion of left bundle branch pacing (LBBP) was significantly lower in the AF group compared to the non-AF group (31.94% vs. 58.86%, P < 0.001). In terms of ventricular pacing percentage (VP), the proportion of patients with VP 0%–39% was significantly lower in the AF group compared to the non-AF group (23.61% vs. 44.48%, P = 0.001). Conversely, the proportion of patients with VP 40%–79% was significantly higher in the AF group compared to the non-AF group (26.39% vs. 11.04%, P = 0.002). Figure 2 demonstrates that the incidence of AF was significantly lower in the LBBP cohort compared to the RVSP cohort (11.56% vs. 28.49%, P < 0.001).
Table 1
| Characteristics | Non-AF group | AF group | P-value |
|---|---|---|---|
| Age, years | 68.00 ± 3.83 | 68.50 ± 3.83 | 0.606 |
| Sex, n (%) | |||
| Male | 143 (47.83) | 37 (51.39) | 0.587 |
| Female | 156 (52.17) | 35 (48.61) | 0.587 |
| Primary disease, n (%) | 0.130 | ||
| SSS | 96 (32.11) | 30 (41.67) | |
| AVB | 203 (67.89) | 42 (58.33) | |
| Medical history, n (%) | |||
| Hypertension | 112 (37.46) | 39 (54.17) | 0.010 |
| DM | 46 (15.38) | 11 (15.28) | 0.982 |
| CHD | 117 (39.13) | 34 (47.22) | 0.210 |
| Implantation site of the ventricular lead, n (%) | <0.001 | ||
| LBBP | 176 (58.86) | 23 (31.94) | |
| RVSP | 123 (41.14) | 49 (68.06) | |
| AP | 0.155 | ||
| <50% | 197 (65.89) | 41 (56.94) | |
| ≥50% | 102 (34.11) | 31 (43.06) | |
| VP | |||
| 0–39% | 133 (44.48) | 17 (23.61) | 0.001 |
| 40–79% | 33 (11.04) | 19 (26.39) | 0.002 |
| ≥80% | 133 (44.48) | 36 (50.00) | 0.430 |
| Medications, n (%) | |||
| Beta-blockers | 25 (8.36) | 7 (9.72) | 0.712 |
| Calcium channel blockers | 85 (28.43) | 19 (26.39) | 0.729 |
| Lipid-lowering drugs | 119 (39.80) | 28 (38.89) | 0.887 |
| ACEI/ARB | 44 (14.72) | 8 (11.11) | 0.429 |
Baseline characteristics of patients between non-AF (n = 299) and AF group (n = 72).
Bold type indicates P value < 0.005.
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively. In the table, the percentages are calculated based on columns. AF, atrial fibrillation; SSS, sick sinus syndrome; AVB, atrioventricular block; DM, diabetes mellitus; CHD, coronary heart disease; LBBP, left bundle branch pacing; RVSP, right ventricular septal pacing; AP, atrial pacing percentage; VP, ventricular pacing percentage; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 2
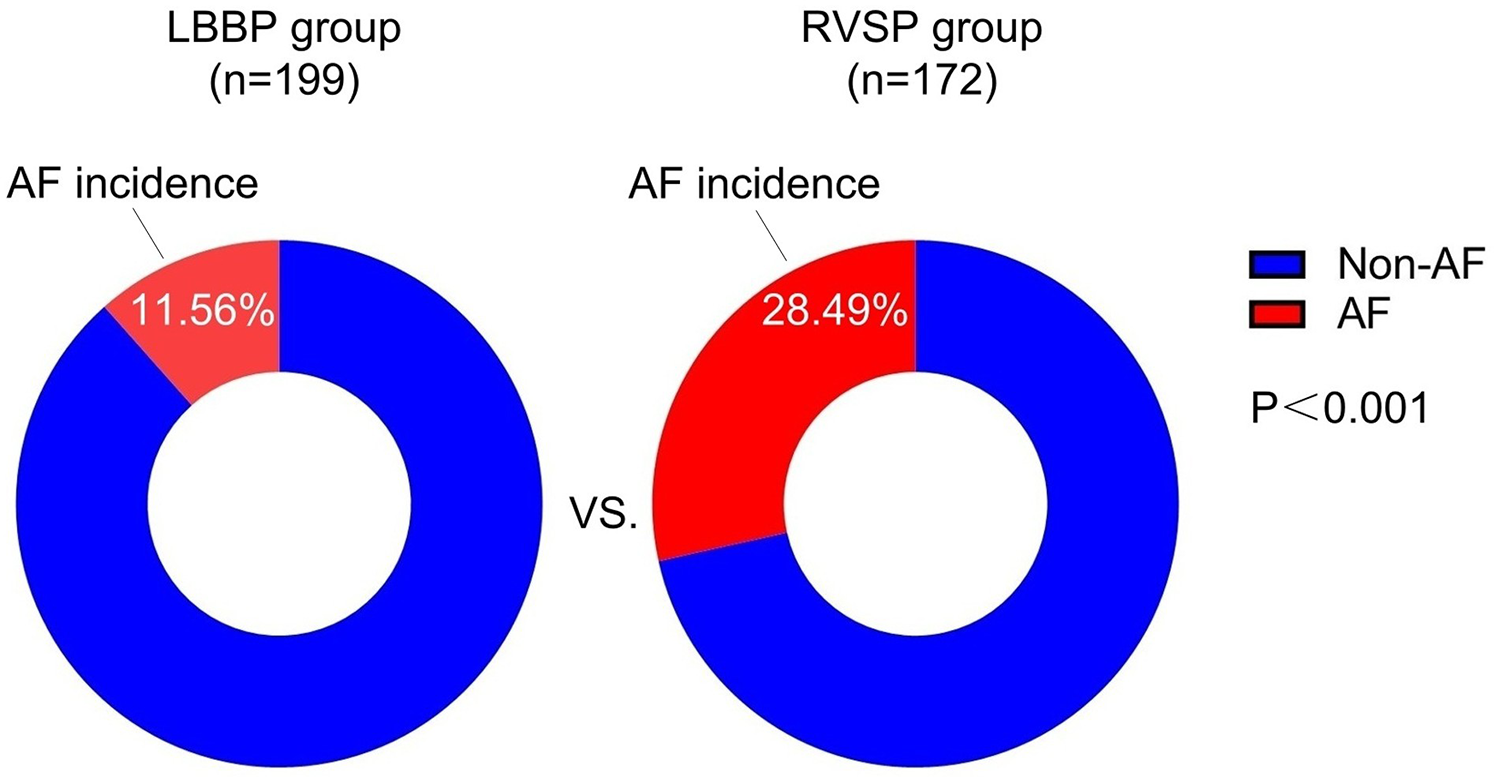
Comparison of the incidence of new-onset AF in relation to different implantation sites of the ventricular lead. AF, atrial fibrillation; LBBP, left bundle branch pacing; RSVP, right ventricular septal pacing.
The characteristics of laboratory testing and echocardiography between the non-AF and AF groups are shown in Table 2. The left atrial diameter (LAD) was significantly larger in the AF group compared to the non-AF group (38.10 ± 1.54 mm vs. 35.90 ± 1.79 mm, P = 0.004).
Table 2
| Characteristics | Non-AF group | AF group | P-value |
|---|---|---|---|
| cTnI, ug/L | 0.842 | ||
| <0.017 | 203 (67.89%) | 48 (66.67%) | |
| ≥0.017 | 96 (32.11%) | 24 (33.33%) | |
| Creatinine, μmol/L | 79.00 ± 6.88 | 77.50 ± 5.42 | 0.632 |
| BNP, pg/ml | 269.00 ± 113.60 | 291.50 ± 100.66 | 0.880 |
| Total cholesterol, mol/L | 4.34 ± 0.94 | 4.33 ± 0.88 | 0.977 |
| LDL, mol/L | 2.54 ± 0.86 | 2.55 ± 0.73 | 0.903 |
| HDL, mol/L | 1.15 ± 0.30 | 1.13 ± 0.31 | 0.682 |
| Triglyceride, mol/L | 1.64 ± 0.75 | 1.73 ± 0.77 | 0.453 |
| LAD, mm | 35.90 ± 1.79 | 38.10 ± 1.54 | 0.004 |
| LVEDD, mm | 46.30 ± 1.58 | 46.35 ± 1.35 | 0.504 |
| LVEF, % | 0.191 | ||
| <50% | 144 (48.16) | 41 (56.94) | |
| ≥50% | 155 (51.84) | 31 (43.06) | |
| Mitral Regurgitation | |||
| Mild | 79 (26.42) | 22 (30.56) | 0.465 |
| Moderate | 179 (59.87) | 41 (56.94) | 0.689 |
| Severe | 41 (13.71) | 9 (12.50) | 1.000 |
Characteristics of laboratory testing and echocardiography between non-AF (n = 299) and AF group (n = 72).
Bold type indicates P value < 0.005.
Mean values (standard deviation) and % (n) were reported for continuous and categorical variables, respectively. In the table, the percentages are calculated based on columns. AF, atrial fibrillation; cTNI, cardiac troponin I; BNP, brain natriuretic peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction.
LBBP [Odds Ratio [OR], 0.315; 95% confidence interval [CI], 0.147–0.600; P = 0.011], VP 0%–39% range (OR, 0.613; 95% CI, 0.208–0.913; P = 0.024) were independently associated with a reduced risk of new-onset AF, while a larger LAD (OR, 1.017; 95% CI, 1.086–1.228; P = 0.025) was independently associated with an increased risk of AF (Table 3). Subgroup analysis for impact of LBBP on new-onset AF in 0%–39% and 40%–79% VP population were shown in Table 4.
Table 3
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 1.019 | 0.994–1.183 | 0.273 | – | – | – |
| Male sex | 1.129 | 1.024–3.034 | 0.132 | – | – | – |
| Hypertension | 4.798 | 2.162–10.645 | 0.021 | 4.646 | 2.132–10.609 | 0.121 |
| LBBP | 0.264 | 0.143–0.506 | 0.001 | 0.315 | 0.147–0.600 | 0.011 |
| VP 0–39% | 0.646 | 0.214–0.950 | 0.001 | 0.613 | 0.208–0.913 | 0.024 |
| VP 40–79% | 1.383 | 1.074–1.839 | 0.006 | 1.304 | 1.067–1.819 | 0.076 |
| LAD | 1.162 | 1.065–1.266 | 0.005 | 1.017 | 1.086–1.228 | 0.025 |
Univariate and multivariate regression analysis for New-onset AF.
Bold type indicates P value < 0.005.
CI, confidence interval; OR, odds ratio; AF, atrial fibrillation; LBBP, left bundle branch pacing; VP, ventricular pacing percentage; LAD, left atrial diameter.
Table 4
| Characteristics | VP 0–39% | VP 40–79% | ||
|---|---|---|---|---|
| HR (95% CI) | P interaction | HR (95% CI) | P interaction | |
| Age, years | 0.167 | 0.092 | ||
| <65 | 2.542 (1.199–5.388) | 2.206 (1.180–4.126) | ||
| ≥65 | 1.749 (0.630–4.857) | 2.055 (1.097–3.850) | ||
| Sex | 0.263 | 0.373 | ||
| Male | 2.051 (0.918–4.587) | 1.944 (0.920–4.105) | ||
| Female | 2.425 (0.968–6.074) | 3.528 (1.047–11.882) | ||
| Hypertension | 0.549 | 0.204 | ||
| Yes | 1.810 (0.837–3.912) | 2.212 (1.044–4.684) | ||
| No | 2.937 (1.096–7.868) | 3.248 (0.978–10.787) | ||
| DM | 0.587 | 0.194 | ||
| Yes | 2.113 (0.918–4.861) | 1.751 (0.588–5.211) | ||
| No | 2.339 (0.956–5.722) | 2.276 (0.955–5.423) | ||
| CHD | 0.351 | 0.265 | ||
| Yes | 1.818 (0.833–3.970) | 1.809 (0.937–3.493) | ||
| No | 2.812 (1.060 −7.461) | 1.043 (0.569–1.909) | ||
| AP | 0.637 | 0.533 | ||
| <50% | 2.261 (1.167–4.378) | 1.417 (0.815–2.465) | ||
| ≥50% | 2.364 (0.511–10.945) | 1.506 (0.887–2.558) | ||
| LAD, mm | 0.197 | 0.737 | ||
| <40 | 2.237 (1.198–4.175) | 1.863 (1.084–3.200) | ||
| ≥40 | 1.500 (0.094–23.981) | 2.555 (0.622–10.485) | ||
Subgroup analysis for impact of LBBP on new-onset AF in 0–39% and 40–79% VP population.
CI, confidence interval; HR, hazard ratio; VP, ventricular pacing percentage; AF, atrial fibrillation; LBBP, left bundle branch pacing; LAD, left atrial diameter; DM, diabetes mellitus; CHD, coronary heart disease; AP, atrial pacing percentage.
Table 5 and Figure 3 displays the sensitivity and specificity of various indicators in predicting new-onset AF. For LBBP, sensitivity was 65%, specificity was 75%, and AUC was 0.738. For the LAD, sensitivity was 60%, specificity was 75%, and the AUC was 0.718 with the cut-off value of 38.10 mm.
Table 5
| Characteristics | LBBP | LAD |
|---|---|---|
| AUC | 0.738 (0.575–0.863) | 0.718 (0.553–0.848) |
| Cut-off value | – | 38.10 |
| Sensitivity | 0.650 (0.622–0.678) | 0.600 (0.590–0.610) |
| Specificity | 0.750 (0.740–0.760) | 0.750 (0.740–0.760) |
| P-value | 0.003 | 0.008 |
Diagnostic performance in predicting new-onset AF with different indicators.
Bold type indicates P value < 0.005.
AF, atrial fibrillation; LBBP, left bundle branch pacing; LAD, left atrial diameter; AUC, area under the curve.
Figure 3
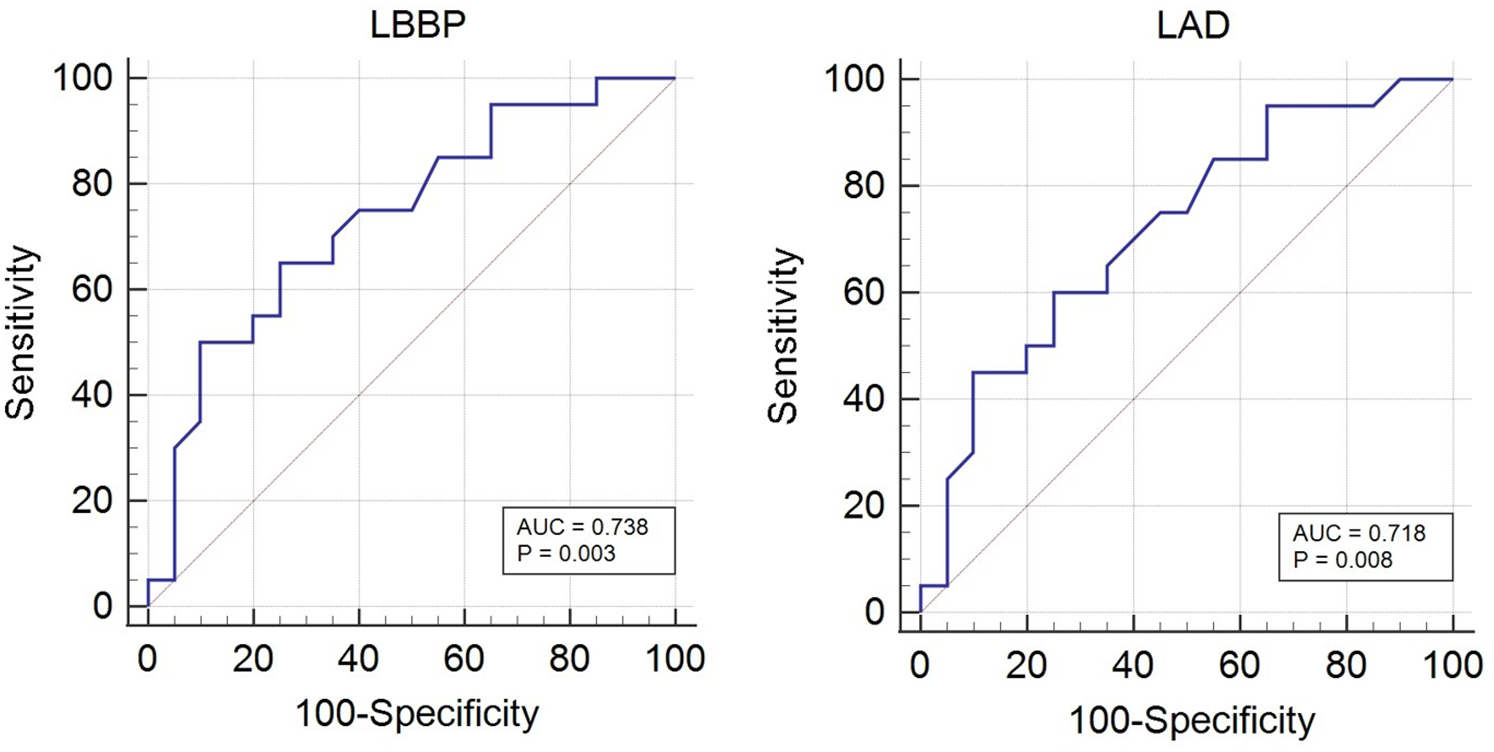
Diagnostic efficiency of LBBP and LAD for new-onset AF using standard ROC curve analysis. AF, atrial fibrillation; LBBP, left bundle branch pacing; LAD, left atrial diameter.
Discussion
The principal findings revealed that the AF group had a lower proportion of patients with ventricular pacing (VP) in the 0%–39% range, but a higher proportion of patients in the 40%–79% range. Notably, the incidence of AF was significantly lower in the LBBP group compared to the RVSP group. LBBP, VP in the 0%–39% range, and LAD were independently associated with new-onset AF. These findings underscore the significant influence of both pacing modalities and percentage of ventricular pacing on the risk of new-onset AF following dual-chamber pacemaker implantation. The results suggest that optimizing these factors, particularly by favoring LBBP and minimizing the VP percentage, may play a crucial role in mitigating the risk of AF in patients with pacemakers. This insight provides valuable guidance for clinicians in tailoring pacemaker implantation strategies to potentially reduce the incidence of post-implantation AF.
Pacemaker implantation, while therapeutic, can potentially impact cardiac function through various mechanisms (9). The implantation process itself causes localized myocardial damage due to electrode lead placement. Moreover, the altered cardiac conduction sequence post-implantation can disrupt normal atrial and ventricular contraction patterns, potentially leading to increased atrial pressure (10). These factors, collectively, may contribute to an increased risk of AF (11). Given these considerations, the selection of an optimal pacing modalities is crucial. An ideal pacing location should minimize myocardial damage, preserve physiological conduction patterns as much as possible, and maintain efficient atrial and ventricular function (12). By optimizing the pacing modalities, it may be possible to mitigate the risk of AF associated with pacemaker implantation while ensuring the device's therapeutic efficacy (13). This underscores the importance of personalized approaches in pacemaker therapy, taking into account individual patient characteristics and the potential long-term consequences of pacing on cardiac electrophysiology and mechanics.
LBBP is an advanced technique derived from His bundle pacing (HBP) (14). It involves advancing the pacing electrode through the right ventricular septum and securing it into the endocardial surface of the left ventricle, where it can capture the left bundle branch or its proximal branches. This results in a narrow QRS complex that resembles right bundle branch block. LBBP typically targets myocardial tissue within the interventricular septum under low-output conditions. Compared to right ventricular pacing (RVP), LBBP is associated with a lower incidence of new-onset AF. Research has demonstrated that in patients with a ventricular pacing burden of ≥20%, LBBP significantly reduces the risk of new-onset AF, with the most notable reductions observed for episodes lasting ≥30 s and ≥6 min. Specifically, LBBP has been shown to reduce the relative risk of AF lasting ≥30 s by 67%, yielding an absolute risk reduction of 13% (15). After adjusting for other predisposing factors for AF, some studies suggest that LBBP may act as an independent protective factor against the development of new-onset AF. Patients with a high ventricular pacing burden, particularly those with a VP ≥20%, may experience more pronounced benefits from LBBP, with greater reductions in the risk of AF onset.
Apart from electrode position, the VP is also a critical factor in the development of new-onset AF following pacemaker implantation. Previous studies have suggested an association between minimal ventricular pacing (MVP) and a reduced risk of AF (16). In the SAVE-PACE randomized trial, the MVP group demonstrated significantly lower ventricular pacing rates (9.1% vs. 99.0%) and a reduced incidence of AF (7.9% vs. 12.7%) compared to the dual chamber pacing group (17). The exact mechanisms by which ventricular pacing contributes to AF remain not fully understood, but it may be linked to asynchronous ventricular pacing and interventricular desynchronization, which could lead to abnormal ventricular excitations (18). Electromechanical desynchrony may have long-term detrimental effects on cardiac structure and function (19). Over time, ventricular remodeling can impair both contraction and relaxation, contribute to mitral regurgitation, and increase LAD (20). These changes collectively disrupt left ventricular hemodynamics, thereby promoting the development of AF.
In our future clinical research, we will place particular emphasis on the monitoring of atrial high rate episodes (AHREs), given their increasing significance in pacemaker recipients. AHREs, although not synonymous with clinical AF, have been recognized as important precursors or predictors for the development of AF, especially when these episodes last longer than 5–6 min (21). Studies have shown that AHREs are closely linked to the onset of AF (22) and associated thromboembolic events (23), highlighting the importance of their detection in identifying patients at higher risk. The detection and monitoring of AHREs using implanted devices, such as pacemakers and defibrillators, is critical in providing valuable insight into the risk of AF and its associated complications (23). Optimizing factors such as atrial pacing percentage and minimizing ventricular pacing in pacemaker patients can reduce the risk of new-onset AF, as a higher ventricular pacing burden is associated with an increased risk of AF development.
Limitation
First, this study was conducted at a single center, which may limit the external validity of the findings. Results may not be applicable to other populations or healthcare settings with different patient demographics or treatment protocols. Second, the retrospective study design carries the risk of inherent biases and limits the ability to establish causality, which could lead to over-correction and unreliable estimates. Third, data on new-onset AF were primarily based on ECG results, which were performed for patients who exhibited symptoms related to AF. However, this approach might not capture all cases, especially in patients with intermittent or asymptomatic AF. Alternative methods such as Holter monitoring or continuous ECG recordings could provide a more accurate assessment. Finally, the outcomes related to different pacing modalities (LBBP vs. RVSP) may be influenced by the experience and technique of the operators performing the implantation, which could introduce variability in the results.
Conclusions
Ventricular pacing modalities and percentage significantly impact new-onset AF after dual-chamber pacemaker implantation. LBBP and lower VP percentages are associated with a lower risk of AF development, whereas a larger LAD may be linked to an increased likelihood of its onset. Optimizing these factors could potentially reduce the risk of AF in pacemaker patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MZ: Writing – original draft. JX: Investigation, Writing – original draft. JW: Writing – original draft, Data curation. YL: Methodology, Writing – original draft. YJ: Writing – original draft, Formal analysis. SL: Writing – review & editing, Supervision. WC: Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the members of the medical staff of the Second Affiliated Hospital of Harbin Medical University for their assistance in the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Dretzke J Toff WD Lip GY Raftery J Fry-Smith A Taylor R . Dual chamber versus single chamber ventricular pacemakers for sick sinus syndrome and atrioventricular block. Cochrane Database Syst Rev. (2004) 2004(2):CD003710. 10.1002/14651858.CD003710.pub2
2.
Kusumoto FM Schoenfeld MH Barrett C Edgerton JR Ellenbogen KA Gold MR et al 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2019) 140(8):e382–482. 10.1161/CIR.0000000000000628
3.
Martignani C Massaro G Biffi M Ziacchi M Diemberger I . Atrial fibrillation: an arrhythmia that makes healthcare systems tremble. J Med Econ. (2020) 23(7):667–9. 10.1080/13696998.2020.1752220
4.
Wilkoff BL Cook JR Epstein AE Greene HL Hallstrom AP Hsia H et al Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA. (2002) 288(24):3115–23. 10.1001/jama.288.24.3115
5.
Bayés de Luna A Platonov P Cosio FG Cygankiewicz I Pastore C Baranowski R et al Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. (2012) 45(5):445–51. 10.1016/j.jelectrocard.2012.06.029
6.
Glikson M Nielsen JC Kronborg MB Michowitz Y Auricchio A Barbash IM et al 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. (2021) 42(35):3427–520. 10.1093/eurheartj/ehab364
7.
Schotten U Verheule S Kirchhof P Goette A . Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. (2011) 91(1):265–325. 10.1152/physrev.00031.2009
8.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomström-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
9.
Somma V Ha FJ Palmer S Mohamed U Agarwal S . Pacing-induced cardiomyopathy: a systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. (2023) 20(2):282–90. 10.1016/j.hrthm.2022.09.019
10.
Wouters PC van Everdingen WM Vernooy K Geelhoed B Allaart CP Rienstra M et al Does mechanical dyssynchrony in addition to QRS area ensure sustained response to cardiac resynchronization therapy? Eur Heart J Cardiovasc Imaging. (2022) 23(12):1628–35. 10.1093/ehjci/jeab264
11.
Sweeney MO Hellkamp AS Ellenbogen KA Greenspon AJ Freedman RA Lee KL et al Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. (2003) 107(23):2932–7. 10.1161/01.CIR.0000072769.17295.B1
12.
Vijayaraman P Chung MK Dandamudi G Upadhyay GA Krishnan K Crossley G et al His bundle pacing. J Am Coll Cardiol. (2018) 72(8):927–47. 10.1016/j.jacc.2018.06.017
13.
Abdelrahman M Subzposh FA Beer D Durr B Naperkowski A Sun H et al Clinical outcomes of his bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. (2018) 71(20):2319–30. 10.1016/j.jacc.2018.02.048
14.
Zhang S Zhou X Gold MR . Left bundle branch pacing. JACC Review Topic of the Week. J Am Coll Cardiol. (2019) 74(24):3039–49. 10.1016/j.jacc.2019.10.039
15.
Ravi V Sharma PS Patel NR Dommaraju S Zalavadia DV Garg V et al New-onset atrial fibrillation in left bundle branch area pacing compared with right ventricular pacing. Circ Arrhythm Electrophysiol. (2022) 15(4):e010710. 10.1161/CIRCEP.121.010710
16.
Sweeney MO Bank AJ Nsah E Koullick M Zeng QC Hettrick D et al Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. (2007) 357(10):1000–8. 10.1056/NEJMoa071880
17.
Cleland JG Coletta AP Abdellah AT Witte KK Hobson N Clark AL . Clinical trials update from heart rhythm 2007 and heart failure 2007: CARISMA, PREPARE, DAVID II, SAVE-PACE, PROTECT and AREA-IN-CHF. Eur J Heart Fail. (2007) 9(8):850–3. 10.1016/j.ejheart.2007.07.003
18.
Occhetta E Bortnik M Magnani A Francalacci G Piccinino C Plebani L et al Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol. (2006) 47(10):1938–45. 10.1016/j.jacc.2006.01.056
19.
Frank BS Schäfer M Douwes JM Ivy DD Abman SH Davidson JA et al Novel measures of left ventricular electromechanical discoordination predict clinical outcomes in children with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. (2020) 318(2):H401–H12. 10.1152/ajpheart.00355.2019
20.
Stanley AW Jr Athanasuleas CL Buckberg GD , RESTORE Group. Left ventricular remodeling and functional mitral regurgitation: mechanisms and therapy. Semin Thorac Cardiovasc Surg. (2001) 13(4):486–95. 10.1053/stcs.2001.30135
21.
Khan AA Boriani G Lip GYH . Are atrial high rate episodes (AHREs) a precursor to atrial fibrillation?Clin Res Cardiol. (2020) 109(4):409–16. 10.1007/s00392-019-01545-4
22.
Vitolo M Imberti JF Maisano A Albini A Bonini N Valenti AC et al Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: a systematic review and meta-analysis. Eur J Intern Med. (2021) 92:100–6. 10.1016/j.ejim.2021.05.038
23.
Meng Y Zhang Y Zhu C Nie C Liu P Chang S et al Atrial high-rate episode burden and stroke risks for patients with device-detected subclinical atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. (2023) 371:211–20. 10.1016/j.ijcard.2022.09.046
Summary
Keywords
atrial fibrillation, left bundle branch pacing, right ventricular septal pacing, ventricular pacing percentage, dual-chamber pacemaker implantation
Citation
Zhang M, Xu J, Wang J, Liu Y, Jin Y, Li S and Cao W (2025) Impact of ventricular pacing modalities and pacing percentage on new-onset atrial fibrillation after dual-chamber pacemaker implantation. Front. Cardiovasc. Med. 12:1615420. doi: 10.3389/fcvm.2025.1615420
Received
21 April 2025
Accepted
06 June 2025
Published
19 June 2025
Volume
12 - 2025
Edited by
Vassil Traykov, Acibadem City Clinic Tokuda Hospital, Bulgaria
Reviewed by
Mohammad Alasti, Monash Health, Australia
Nikolay Poroyliev, University Hospital "Tsaritsa Yoanna", Bulgaria
Updates
Copyright
© 2025 Zhang, Xu, Wang, Liu, Jin, Li and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Wei Cao h04776@hrbmu.edu.cn Shufeng Li h03903@hrbmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.