Abstract
Cardiomyopathy (CM) is a heterogeneous group of diseases characterized by structural and functional changes in the heart, with the exact cause often remaining unknown. CM can arise from both inherited and acquired metabolic disturbances. Alterations in energy production and substrate utilization impair the heart's contractile function and limit its ability to respond to stress. Given the complexity and dynamic nature of CM, as well as the multiple etiologies involved, we reviewed metabolomic studies employing high-throughput platforms to understand how metabolic pathways shift across CM subtypes and how these perturbations may inform clinical translation. Several recurring disruptions emerge across CM with alterations in amino acid metabolism (valine, leucine, methionine, tryptophan, tyrosine); mitochondrial redox imbalance (NAD/NADH shifts, niacinamide, acylcarnitines); and oxidative stress as central hallmarks. Each subtype, however, displays a different emphasis. For instance, hypertrophic CM is characterized by nucleotide remodeling, particularly in cases involving MYBPC3 mutations; dilated CM shows accumulation of Krebs cycle intermediates and trimethylamine-N-oxide; restrictive CM is associated with amino acid stress related to amyloidosis; tachycardia-induced CM involves fatty acid remodeling and elevated uric acid, while Takotsubo CM is linked to ketone utilization and glutamate excitotoxicity. Overall, a single metabolomic profile cannot capture CM. What emerges from this review is that subtype-specific shifts, and the way they interact, provide meaningful insight into disease mechanisms and highlight pathways with diagnostic, prognostic, and therapeutic relevance. This broader perspective shifts the focus beyond narrow comparisons, making the translational relevance of metabolomics in CM more apparent.
Introduction
The World Heart Report 2023 identifies cardiovascular diseases (CVD) as the leading global cause of mortality, accounting for over 20.5 million deaths in 2021 and representing a significant burden of premature non-communicable disease deaths (1, 2). Cardiomyopathies (CM), a subset of CVD, are defined by structural and functional myocardial changes such as altered size, shape, or wall thickness arising from either ischemic (coronary artery disease-related) or non-ischemic origins (3, 4). The Centers for Disease Control and Prevention (CDC) notes that CM etiology often remains idiopathic. However, it may be inherited or acquired, with risk factors including hypertension, arrhythmias, valvular diseases, endocarditis, and metabolic disorders such as diabetes mellitus, Gaucher disease, and mitochondrial dysfunction (5, 6).
CM subtypes, hypertrophic (HCM), dilated (DCM), arrhythmogenic (ACM), restrictive (RCM), left ventricular noncompaction, and Takotsubo, exhibit distinct morphological, genetic, and metabolic profiles (7). In healthy adult hearts, fatty acid oxidation generates approximately 60% of adenosine triphosphate (ATP), with carbohydrates as a secondary substrate. In CM, heightened energy demands drive substrate flexibility, utilizing glucose, fatty acids, and ketone bodies. Disruptions in ATP production rates and substrate preference impair contractility and stress responses, hallmarks observed across CM subtypes (8–11).
Metabolomics, the systematic study of metabolites (amino acids, fatty acids, carbohydrates, nucleotides), provides a window into these biochemical shifts. Key metabolites linked to CM, including branched-chain amino acids (BCAAs), acylcarnitines, glucose, and ketone bodies, reflect altered pathways such as glycolysis and lipid metabolism, offering potential diagnostic and therapeutic targets (Figure 1) (12, 13). These metabolites demonstrate the metabolic pathway changes that occur in CM (14–16), highlighting the disease mechanisms and acting as potential biomarkers for diagnosis and treatment. Although the genetic and structural features of CMs are well established, the metabolic dimension has not been systematically addressed. Previous studies have often restricted their focus to narrow pairwise comparisons, which has left a fragmented view of the field. In this review, we seek to close that gap by bringing together evidence on metabolic remodeling across the full spectrum of CM phenotypes. We aim to move beyond earlier fragmented investigations and to highlight the translational promise of metabolomics for enhancing diagnosis, refining risk stratification, and informing therapeutic strategies in CM.
Figure 1
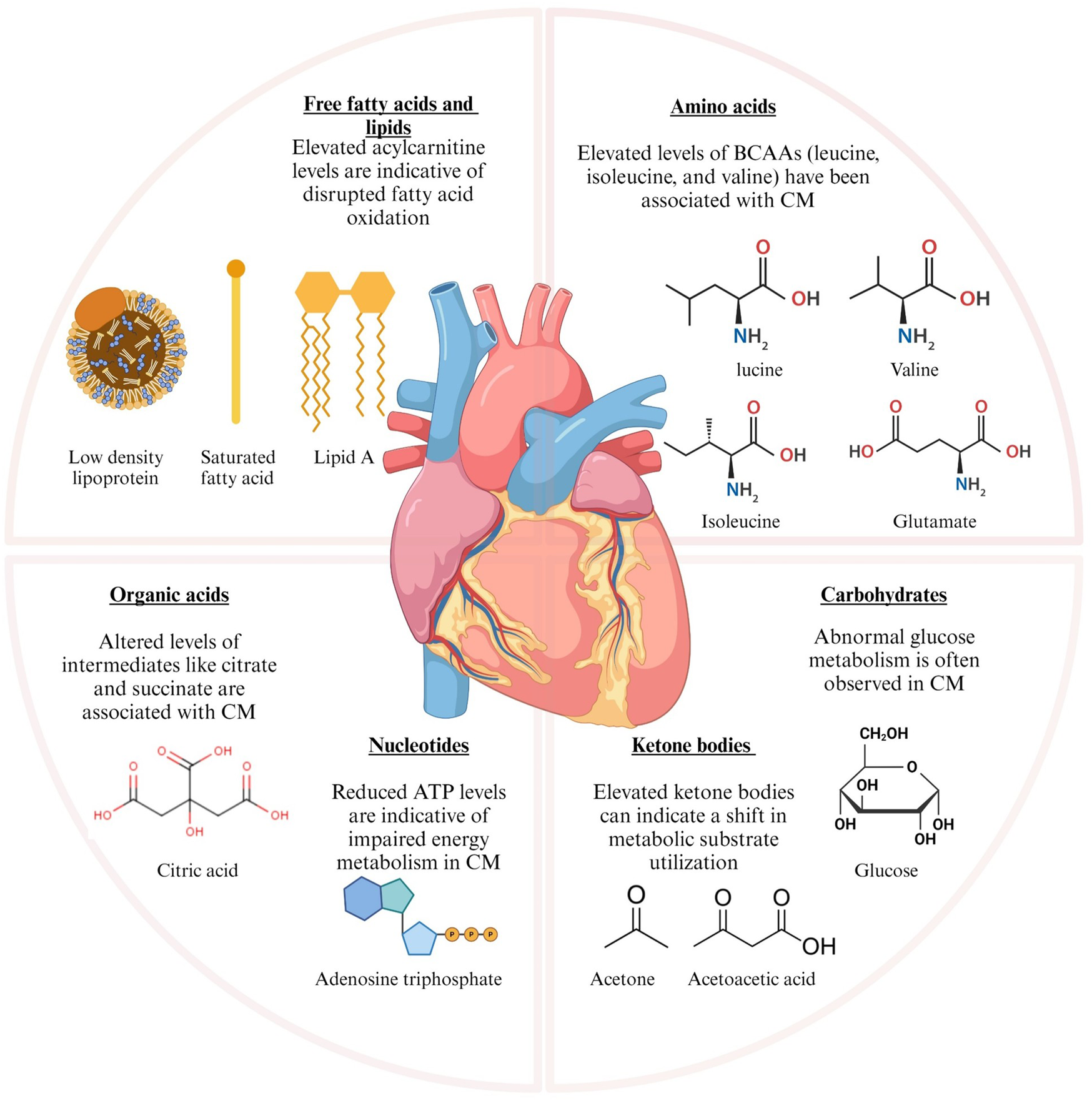
Metabolic alterations in cardiomyopathy and cardiovascular diseases leading to metabolic shifts. The graphic illustrates the principal metabolic alterations identified in cardiomyopathy (CM) and ischemic heart failure. The metabolic changes are classified into various categories based on their biological activities. BCAAs, branched-chain amino acids; CM, cardiomyopathy; ATP, adenosine triphosphate; LDL, low-density lipoprotein. Figure created with BioRender.com.
Cardiomyopathy classifications
The American Heart Association classifies CM into primary (genetic, acquired, or mixed) and secondary (systemic/non-cardiac) forms (Figure 2) (17).
Figure 2
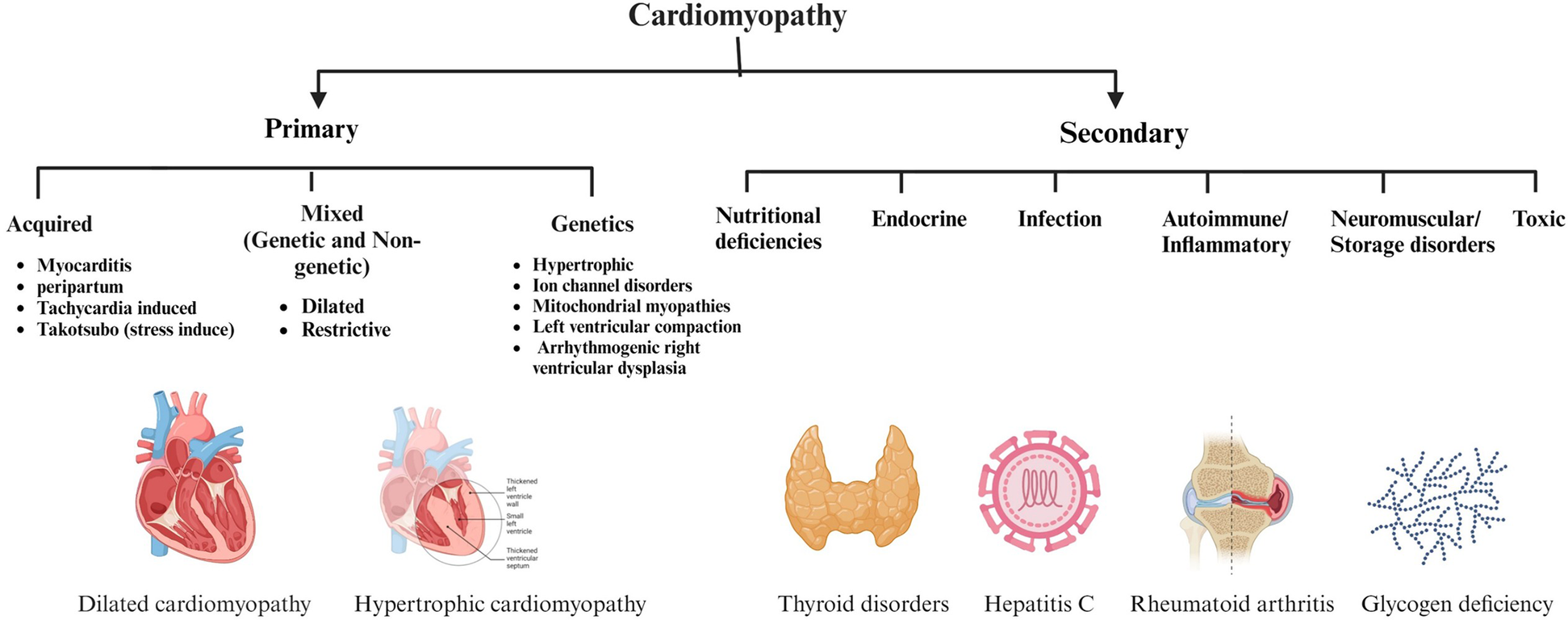
Classification and causes of cardiomyopathy (CM) based on the American Heart Association. This figure categorizes CM into primary and secondary forms, emphasizes the variety of etiologies that can cause CM, including acquired and genetic manifestations, as well as a range of systemic and metabolic conditions. Figure created with BioRender.com.
Primary cardiomyopathy
HCM, the most common genetic CM, involves left ventricular wall thickening, often due to autosomal dominant mutations in genes such as MYH7, MYBPC3, or TNNT2 (18). Arrhythmogenic right ventricular cardiomyopathy (ARVCM), another genetic form, features fibrofatty tissue infiltration in the right ventricle, causing arrhythmias. About 66% of patients test positive for causative genetic mutations (19–21). This tissue replacement impairs contraction, reduces oxygen utilization, and lowers ATP production, thereby driving metabolic shifts and the accumulation of diacylglycerols and ceramides (22, 23).
Acquired CM result from external factors, such as infections in viral myocarditis, pregnancy in peripartum CM, or stress in Takotsubo CM (20). In Takotsubo, stress-induced catecholamine release causes myocyte injury, impairs perfusion, dysregulates fatty acid metabolism, increases inflammatory metabolites (chemokines, cytokines), and elevates oxidative stress via reactive oxygen species (ROS) (24, 25). Mixed CM is a combination of genetic and acquired types associated with both dilated and restrictive forms. DCM involves left ventricle dilation and impaired contraction (17), driven by acquired factors such as myocarditis, cardiotoxins such as alcohol, antineoplastic agents, and metabolic disorders, including insulin resistance, obesity, dyslipidemia, and diabetes. These conditions alter energy utilization, increase oxidative stress, and promote inflammation and fibrosis, often progressing to heart failure (26–28).
RCM features nondilated ventricles with impaired diastolic function, causing wall stiffening and restricted filling (29). Amyloidosis, a primary cause of RCM, involves amyloid protein deposits in the myocardium, leading to cardiomyocyte separation, toxicity, and stiffness (30). These deposits disrupt lipid metabolism, increase lipid peroxidation, reduce mitochondrial respiration, and contribute to severe heart failure (29, 30).
Secondary cardiomyopathy
Endocrine disorders, such as diabetes, thyroid disorders, and obesity, significantly alter carbohydrate and lipid metabolism, frequently increasing ketone body levels (17, 31–33). Viral infections, on the other hand, increase anaerobic glycolysis and hypoxia, resulting in high levels of ROS and lactate (34). Glycogen storage disorders lead to muscular dystrophies and further dysregulate ATP and creatine phosphate levels (35). Nutritional deficiencies, including vitamin C, selenium, and thiamine, disrupt carbohydrate metabolism, leading to elevated pyruvate and lactate levels (36). Toxic causes such as alcohol, anabolic steroids, and radiation disrupt various metabolic pathways through the production of acetaldehyde (37). Collectively, these metabolites play significant roles in the pathogenesis of secondary CM (6).
Metabolomic profiling of cardiovascular diseases
The Human Metabolome Project (HMDB), launched two decades ago, has enabled the quantification of metabolites in body fluids, aiding biomarker discovery, early diagnosis, disease progression, therapeutic response, and drug development (38). Key techniques include NMR and MS platforms, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) or gas chromatography-tandem mass spectrometry (GC-MS) (39). NMR facilitates metabolite identification with minimal sample preparation, while MS offers high sensitivity for ionized molecules (40). High-resolution ¹H NMR provides reproducible results and fast data acquisition (9, 39, 40). Carbon NMR (13C-NMR) reveals structural details, identifies functional groups, and provides more comprehensive positional information compared to 1H-NMR (39). GC-MS requires derivatization for volatile metabolites (41). Whereas LC-MS enables analysis of both polar/non-polar metabolites in complex samples such as blood or urine with minimal derivatization (42).
A single metabolomic approach cannot fully capture the metabolome. Psychogios et al. integrated 1H-NMR, GC-MS, and LC-MS in heart transplant patients, identifying key metabolites like glucose and lactic acid (indicating anaerobic glycolysis in ischemic CM) and arachidonic acid in atherosclerosis (43). High acylcarnitine ratios (C4/C18:2) signal mitochondrial dysfunction and increased CAD risk in diabetic patients (44). Anlar et al. found altered carbohydrate, steroid, and fatty acid metabolism in CAD using UPLC-MS, with elevated mannitol, glucose, and oleoylcarnitine linked to CAD risk (45). Fu et al. noted phospholipid and fatty acid changes such as lysoPC (18:2), lysoPE (20:3), and MG (20:0) aiding CAD diagnosis (46). Harshfield et al. identified creatine and sphingomyelin (d18:2/24:1) associated with cerebral small vessel disease severity using UPLC-MS and NMR from different ethnicities (Caucasians, Caribbean, and African) (47). In peripheral artery disease (PAD), altered lipoprotein and phospholipid metabolism were shown to correlate with mortality (48), while phenylalanine, tyrosine, and oxidized low-density lipoprotein (oxLDL) were associated with arterial stiffness (49). Ismaeel et al. used LC-MS to show phenylalanine/tyrosine ratios and ceramides indicating PAD severity (50).
Rheumatic heart disease (RHD) is characterized by cumulative valvular lesions resulting from acute rheumatic fever and often remains undiagnosed until reaching an advanced stage (51). Das et al. revealed significantly altered pathways, particularly in D-glutamine, D-glutamate, and linoleic acid metabolism via untargeted LC-MS analysis (52). Furthermore, N-acetylneuraminate and arachidonic acid were identified as the primary metabolites, considering them promising therapeutic biomarkers. Congenital heart disease depends on fetal echocardiography. Despite this examination, some cases might be missed. Untargeted metabolomics can detect low-concentration metabolites and predict different subtypes (53). Dong et al. detected changes in nicotinamide adenine dinucleotide (NAD), glutamine, and arginine levels, indicating hypoxia-driven metabolic remodeling (54).
In deep venous thrombosis, overdiagnosis or underdiagnosis due to the poor specificity and moderate sensitivity of D-dimer testing and imaging can lead to fatal pulmonary embolism or increased bleeding risk from excessive anticoagulant use (55). Altered carnitine, glucose, and tryptophan metabolism dysregulate glycolysis and lipid pathways, aiding early diagnosis (56). Chen et al. noted tryptophan derivative DT-26 as an antithrombotic (57). Culler et al. linked myoinositol and carnitine to subclinical cardiac dysfunction in heart failure with preserved ejection fraction (HFpEF) using 1H-NMR (58).
However, the reviewed studies exhibit critical limitations that significantly impair their clinical impact. Small and potentially non-representative sample sizes, a lack of mechanistic depth, reliance on non-specific markers, the absence of diagnostic metrics, limited focus on clinical translation, and the use of cross-sectional designs collectively restrict their generalizability and applicability. Addressing these shortcomings through larger, more diverse cohorts, in-depth mechanistic studies, quantitative validation, longitudinal designs, and practical implementation strategies will be essential to enhance their utility in advancing precision medicine for cardiovascular disease.
Metabolic signature in primary cardiomyopathy
Hypertrophic cardiomyopathy
HCM, a genetic disorder caused by mutations in various genes, poses diagnostic challenges due to variable gene expression within families and phenotypic overlap with conditions like amyloidosis and hypertensive heart disease, risking misdiagnosis (59, 60). Often undetected until complications like sudden cardiac death or heart failure arise, HCM is typically diagnosed using cardiovascular magnetic resonance imaging and electrocardiograms (60). Emerging metabolomic studies offer more profound insights (Table 1). Wang et al. used targeted LC-MS/MS and lipidomics to identify dysregulated metabolites in glycolysis, the Krebs cycle, purine/pyrimidine metabolism, and carnitine synthesis in HCM tissues and plasma, and noted significant alterations in the pentose phosphate pathway and oxidative stress (61). They identified three metabolic subgroups: Subtype 1, characterized by elevated Krebs cycle metabolites such as isocitrate, was associated with better survival, whereas Subtype 2, marked by high purine/pyrimidine metabolites and short-chain carnitines, was linked to worse outcomes. Together, these profiles suggest that such metabolites may serve as both diagnostic and prognostic tools.
Table 1
| Metabolites | Clinical outcomes | Translational implications |
|---|---|---|
| Dimethylglycine, N-acetyl-L-glutamine, β-aminobutyric acid, XMP, GMP, PC38:6p (16:0/22:6), TAG52:2 (C18:0) | Associated with adverse prognosis; predictive of overall survival | Serve as biomarkers of disease severity and mortality risk |
| PG38:6 (18:2/20:4), UDP-galactose, branched-chain amino acids, NADP, acetyl-CoA, NADH | Reflect increased oxidative stress, inflammatory activation, and impaired mitochondrial respiration | Highlight potential druggable pathways for therapeutic modulation in HCM |
| PE32:0 (16:0/16:0), phosphocreatine, 1,3-dimethyluracil, uric acid | Linked to impaired myocardial contractility; metabolic signature associated with MYBPC3 mutations | Useful as diagnostic/prognostic biomarkers and as genotype-specific metabolic readouts |
Representative metabolite alterations in HCM with clinical relevance.
Key metabolites identified in the hypertrophic cardiomyopathy subtype. These metabolites have been linked to diagnostic accuracy, treatment monitoring, risk stratification, and potential therapeutic targeting, underscoring their value for advancing clinical practice.
Previs et al., using LC-MS-based proteomics and targeted metabolomics, reported reduced long-chain acylcarnitine, a shift to ketone metabolism (elevated 3-hydroxybutyrate), and increased branched-chain amino acids in HCM, reflecting reduced ATP availability and impaired contractility (62). In HCM patients with MYBPC3 variants, alterations in acylcarnitine, histidine, lysine, purine, and steroid hormones were associated with disease progression (63). Comparing Dutch MYBPC3 carriers with severe vs. mild/no symptoms, the study identified potential biomarkers for disease severity, offering insights into mechanisms and targeted therapies. Current metabolomic studies in HCM face significant hurdles that limit their translational potential. Uncontrolled external factors like diet, medication (e.g., beta-blockers), and comorbidities introduce confounding variables, while variability in control samples (age, sex differences) undermines biomarker specificity. The predominant focus on tissue-level changes, rather than systemic biomarkers, restricts non-invasive clinical use, and the emphasis on MYBPC3 variant carriers or end-stage HCM patients limits generalizability across genotypes and disease stages. There is growing evidence that links impaired mitochondrial metabolism arising from mutations in either mitochondrial or nuclear DNA to the development of CM, with recent investigations emphasizing their role in disease mechanisms and as potential therapeutic targets (64). Nollet and colleagues, for instance, demonstrated that mitochondrial dysfunction in HCM disrupts cardiomyocyte structure. Yet, these defects could be reversed with the cardiolipin-stabilizing compound elamipretide, which enhances NAD+ availability and supports respiration. Their findings highlight the potential of mitochondria-targeted therapies, particularly in genotype-negative HCM patients (65). In a complementary line of work, Franco and collaborators identified a rare MFN2 mutation (R400Q) that selectively impairs mitophagy, leading to a newly recognized entity termed “mitophagy CM” (66). This mutation, identified in both HCM and DCM and found more frequently among individuals of African ancestry, points to ancestry-specific risks and underscores the value of genetic screening for mitophagy-related defects in otherwise unexplained cases (66).
To enhance translation, future research should control for external factors, match control groups, prioritize blood/urine biomarkers, and include diverse HCM genotypes and stages for broader applicability in early diagnosis and personalized treatment.
Arrhythmogenic cardiomyopathy
Arrhythmogenic CM is a genetically complex disease with variable severity and clinical presentation, which complicates diagnosis even with traditional methods. Metabolomic profiling offers a comprehensive approach to screening and characterization in ACM (67, 68). Volani et al. identified key metabolites in ACM patients’ plasma, revealing dysregulated pathways (68). Notably, α-Aminoadipic acid was found to be downregulated, indicating disrupted lysine degradation. At the same time, propionyl carnitine and asymmetric dimethylarginine were upregulated, pointing to endothelial dysfunction via altered beta-oxidation and nitric oxide synthesis pathways. These metabolites highlight potential biomarkers and therapeutic targets for ACM management.
Dilated cardiomyopathy
DCM, a leading cause of heart failure in adults, presents challenges due to its complex pathophysiology, including systolic dysfunction, ventricular dilation, connective tissue thickening, heart enlargement, and metabolic alterations (28). Standard diagnostic biomarkers like B-type Natriuretic Peptide (BNP), NT-proBNP, troponins, and galectin-3, alongside clinical evaluation and echocardiogram imaging, often lack sensitivity and accuracy to fully capture DCM's complexity and prognosis, especially in comorbid patients (69). Targeted metabolomics offers broader molecular coverage, making it a valuable tool for studying complex diseases (70).
Ampong reviewed metabolomic profiles of DCM in serum, plasma, and heart tissues using GC-MS, LC-MS, UHPLC, and NMR (Table 2) (71). Upregulated metabolites included methylhistidine (protein degradation), prolylhydroxyproline (cardiac remodeling), and isoleucine, linoleic acid, BCAA, and phenylalanine (dysregulated amino acid metabolism, oxidative stress). Other elevated metabolites were glucose, serine, acetate, dimethylsulfone, hypoxanthine, creatinine, creatine, and trimethylamine-N-oxide (TMAO) compared to ischemic CM. Glutamine, piperine, and citrulline were also increased, reflecting metabolic adaptation to stress. Downregulated metabolites included threonine (impaired protein synthesis), histidine, tryptophan, lactate, methionine, formate, arginine, and hydroxytetradecanoylcarnitine. Dysregulation of amino acid and lipid metabolism offers potential biomarkers for differentiating DCM from ischemic CM and for monitoring disease progression.
Table 2
| Metabolites | Clinical outcomes | Translational implications |
|---|---|---|
| Acylcarnitines, succinic acid, malate, methylhistidine, aspartate, methionine, phenylalanine | Associated with the presence of DCM | Support early diagnosis |
| Acylcarnitines, isoleucine, linoleic acid, tryptophan | Reflect changes with treatment | Useful for monitoring therapy response |
| 1-pyrroline-2-carboxylate, lysophosphatidylinositol (16:0/0:0), phosphatidylglycerol, norvaline,phosphatidylcholine | Show distinct metabolic patterns in DCM vs. ICM | Aid in non-invasive differential diagnosis |
| Trimethylamine-N-oxide, creatine, creatinine, lactate | Linked with prognosis in HF secondary to DCM | Predict risk of mortality and guide risk stratification |
Representative metabolite alterations in DCM with clinical relevance.
Key metabolites identified in the dilated cardiomyopathy subtype. These metabolites have been linked to diagnostic accuracy, treatment monitoring, risk stratification, and potential therapeutic targeting, underscoring their value for advancing clinical practice.
Verdonschot et al. performed targeted metabolomics on plasma and urine from DCM patients, using NT-proBNP as a reference (27). The High levels of C16-acylcarnitine, glutamic acid, sialic acid, and cystathionine in severe DCM indicated dysregulated beta-oxidation, mitochondrial dysfunction, systemic inflammation, and oxidative stress, all strongly correlated with NT-proBNP. Conversely, low levels of 3-methylhistidine, urine carnosine, 3-hydroxyisovaleric acid, and citric acid showed an inverse correlation, suggesting reduced protective metabolic responses. These findings support the use of acylcarnitine and amino acid metabolites as adjuncts to NT-proBNP for improved severity stratification in DCM.
Vignoli et al., using untargeted 1H-NMR on serum, confirmed similar disruption in energy and lipid metabolism, inflammation, oxidative stress, and gut microbiome interactions in DCM-induced heart failure (72). High creatinine, succinic acid, lactate, LDL-triglycerides, and TMAO were linked to higher mortality risk, while lower creatine, ApoA2, and HDL phospholipids indicated better outcomes. Combining metabolomics with left ventricular ejection fraction improved prognostic accuracy (hazard ratio: 7.47 to 8.09) compared to NT-proBNP alone. The clinical implications demonstrate the predictive value of serum metabolic markers in predicting mortality and enhancing risk stratification beyond NT-proBNP.
Zhao et al. analyzed plasma metabolomics in DCM and Ischemic CM heart failure patients compared with healthy controls using untargeted LC-MS/MS (73). In DCM, reduced 1-pyrroline-2-carboxylate and altered norvaline levels suggested impaired oxidative stress response and anti-inflammatory capacity, with disruptions in alpha-linolenic acid metabolism. In Ischemic CM, decreased lysophosphatidylinositol (16:0/0:0) reflected altered adrenergic signaling, while elevated phosphatidylglycerol (6:0/8:0) and fatty acid esters pointed to mitochondrial and lipid metabolism dysregulation. Pathways like linoleic acid metabolism, arginine biosynthesis, and D-glutamine/D-glutamate metabolism were dysregulated in Ischemic CM. Both conditions showed altered phosphatidylcholine (18:0/18:3) and glycerophospholipid metabolism, highlighting shared metabolic disturbances. The reviewed studies shed light on the metabolic complexity of DCM, highlighting the potential of plasma-based metabolomics in enhancing diagnostic accuracy and patient stratification.
Overall, these studies highlight the metabolic complexity of DCM and the utility of plasma and tissue-based metabolomics for diagnosis, prognosis, and patient stratification. Vignoli et al. identified a metabolic signature predictive of mortality, Zhao et al. highlighted clear metabolic distinctions between DCM and ischemic CM, and Ampong further revealed disrupted lipid and amino acid pathways central to disease progression. However, all studies remain limited by small sample sizes, methodological variability, and cross-sectional designs, underscoring the need for larger, longitudinal studies with standardized protocols to harness the clinical potential of metabolomics in DCM fully.
Restrictive cardiomyopathy
Cardiac Amyloidosis (CA) is a well-established cause of RCM, yet few studies have characterized its metabolic signature (74). In CA, deposition of immunoglobulin light chain (AL) and transthyretin (ATTR) amyloid leads to ventricular thickening and impaired filling. Diagnosis is challenging and often requires advanced modalities, such as cardiac magnetic resonance, positron emission tomography, or endomyocardial biopsy (74). Proteomic analysis using Nano LC-MS/MS has successfully differentiated CA subtypes (AL, ATTR, serum amyloid A) by detecting amyloid signature proteins like ApoE, ApoA-IV, and SAP, which were upregulated in affected tissues. At the same time, conventional cardiac markers such as troponins I and T were decreased (75).
These shifts indicate misfolded protein accumulation and associated cardiac dysfunction in RCM and suggest that proteomic and metabolomic profiling can differentiate CA subtypes and may complement or reduce reliance on invasive biopsy.
Additionally, Olsson et al. applied untargeted metabolomics (GC-MS and LC-MS) to plasma from ATTRV30M patients, identifying reduced tryptophan, phenylalanine, and tyrosine, along with altered malic acid and acylcarnitines (Table 3). Alterations in plasma amino acids and acylcarnitines may serve as non-invasive biomarkers for monitoring disease progression in ATTR amyloidosis (76).
Table 3
| Metabolites | Clinical outcomes | Translational implications |
|---|---|---|
| Tryptophan, phenylalanine, tyrosine, maltose | Indicators of oxidative stress, endothelial dysfunction, and adrenergic stress | Early biomarkers for diagnosis and stratification, especially in amyloidosis-related RCM |
| Leucine, ketoleucine, malic acid, valine | Reflect mitochondrial dysfunction and impaired contractile performance | Prognostic markers for mitochondrial impairment; aid in detecting restrictive changes before overt heart failure |
| Leucine, niacinamide | Capture metabolic adaptation to energy stress | Candidates for therapeutic monitoring, particularly in NAD⁺ augmentation strategies |
Representative metabolite alterations in RCM with clinical relevance.
Key metabolites identified in the restrictive cardiomyopathy subtype. These metabolites have been linked to diagnostic accuracy, treatment monitoring, risk stratification, and potential therapeutic targeting, underscoring their value for advancing clinical practice.
Inflammatory cardiomyopathy
Myocarditis, an inflammatory CM, is often caused by viruses such as Coxsackie A/B, leading to cardiac dysfunction and remodeling (77, 78). Diagnosis remains difficult due to the non-specificity of biomarkers like troponins and C-reactive protein, and the limited sensitivity of imaging and invasive endomyocardial biopsy (EMB) (79). Although emerging biomarkers such as microRNAs show potential, they are not yet clinically established. Metabolomic profiling offers rapid detection of metabolic shifts, with notable dysregulation in lipid metabolism and amino acid biosynthesis. The PGC-1α/PPARγ axis, central to energy metabolism, appears to be particularly affected (80).
Kong et al. used NMR-based metabolomics in CVB3-induced myocarditis mouse models, revealing elevated leucine, isoleucine, valine, lysine, and 3-hydroxybutyrate in chronic myocarditis, and reduced alanine, glutamate, lactate, and succinate in both acute and DCM stages (81). Disrupted pathways included nicotinamide, alanine-glutamate, and taurine metabolism. The present results may help differentiate between acute, chronic, and dilated cardiomyopathy–transition stages of myocarditis.
A complementary UPLC-MS/MS study by Kong et al. found elevated metabolites such as betaine, shikimic acid, and estrone 3-sulfate, which correlated with gut microbes such as Streptococcus and Enterococcus, linking gut dysbiosis to myocarditis-induced metabolic disturbances (82). Integration of metabolomics with microbiome profiling may provide novel approaches for early diagnosis and therapeutic targeting in viral myocarditis.
Tachycardia-induced cardiomyopathy
Tachycardia-induced CM (TICM) is a reversible form of CM linked to persistent arrhythmias, particularly atrial fibrillation (AF) and atrial flutter. Still, in severe cases, it can progress to cardiogenic shock or sudden death (83, 84). Standard diagnostics include ECG, echocardiography, cardiac MRI, and plasma BNP/NT-proBNP levels, yet these markers lack specificity, making early differentiation from other cardiomyopathies challenging (85).
Lu et al. developed a metabolomics-based model using non-targeted GC-MS and LC-MS to analyze serum from various AF subtypes, identifying increased D-glutamic acid, uric acid, and 2-ketoglutaric acid, and decreased oleic acid and glycerol-2-phosphate across all AF types (86). Unique metabolite changes were observed in AF-related stroke, indicating disruptions in glutamate, glycerophospholipid, and fatty acid metabolism, which may contribute to arrhythmogenesis by promoting membrane instability and energy dysregulation. The current observations could improve early AF/TICM diagnosis and identify high-risk patients predisposed to stroke.
Tu et al. further explored metabolic rewiring in TICM using hiPSC-derived cardiac tissues and a canine tachypacing model (87). They observed increased glucose metabolites (pyruvate, sorbitol, succinic acid) and reduced expression of genes involved in oxidative phosphorylation and fatty acid oxidation (Table 4). This metabolic shift toward glycolysis impaired NAD+ redox balance and increased protein acetylation, including that of SERCA2a, leading to contractile dysfunction. These findings underscore the role of altered energy metabolism in TICM pathogenesis and its potential as a therapeutic target.
Table 4
| Metabolites | Clinical outcomes | Translational implications |
|---|---|---|
| Oleic acid, D-glutamic acid, uric acid, L-acetylcarnitine, decanoylcarnitine, stearic acid, creatinine | Linked with recurrence, oxidative stress, and structural remodeling | Can function as diagnostic markers for recurrence and patient stratification |
| L-lysine, valine, tyrosine, methionine, alanine; evidence of glycolytic shift, protein acetylation, and NAD redox imbalance | Associated with impaired contraction and mitochondrial dysfunction | Useful for therapeutic assessment, particularly when evaluating metabolic modulators |
Representative metabolite alterations in TICM with clinical relevance.
Key metabolites identified in the tachycardia induced cardiomyopathy subtype. These metabolites have been linked to diagnostic accuracy, treatment monitoring, risk stratification, and potential therapeutic targeting, underscoring their value for advancing clinical practice.
Takotsubo/stress cardiomyopathy
Takotsubo Syndrome (TTS) or stress CM is often misdiagnosed as myocardial infarction (MI) due to overlapping features like ECG abnormalities and elevated BNP/NT-proBNP levels. However, these biomarkers lack specificity for TTS and can also be elevated in cardiac and systemic conditions (88, 89). Despite evidence of myocardial inflammation and oxidative stress in TTS, systemic biochemical validation remains limited.
Vanni et al. used ¹H-NMR metabolomics to analyze serum from TTS patients, revealing increased ketone bodies (2-hydroxybutyrate, acetyl-L-carnitine, glutamate) and decreased amino acid metabolites (Table 5) (88). These findings indicate systemic oxidative stress and metabolic dysregulation, which correlated with reduced left ventricular ejection fraction (LVEF), suggesting potential diagnostic and prognostic value.
Table 5
| Metabolites | Clinical outcomes | Translational implications |
|---|---|---|
| Ketone bodies, 2-hydroxybutyrate, glutamate | Higher ketone levels track with disease severity (LVEF decline, arrhythmia risk, ventricular remodeling), redox imbalance reflects stress response; glutamate excess linked to excitotoxicity and poor contractile reserve | Prognostic markers of severity, point to oxidative stress as a treatment target, help in patient risk stratification |
| Acetyl-L-carnitine | Signals mitochondrial dysfunction | Can be followed as a therapeutic monitoring marker, indicating recovery of mitochondrial function |
| Reduced amino acids | Indicate catabolic stress and are associated with myocardial injury and remodeling | Provide rationale for supportive metabolic strategies, including amino acid supplementation |
Representative metabolite alterations in Takotsubo CM with clinical relevance.
Key metabolites identified in the Takotsubo cardiomyopathy subtype. These metabolites have been linked to diagnostic accuracy, treatment monitoring, risk stratification, and potential therapeutic targeting, underscoring their value for advancing clinical practice.
Scally et al. observed that TTS patients, even with preserved LVEF, exhibited reduced peak VO₂ and elevated VE/VCO2 slope, consistent with a heart failure-like phenotype and raising concerns about long-term effects on cardiac function (90). Functional capacity testing may therefore be valuable for detecting persistent cardiac impairment in TTS patients, even after apparent recovery.
Further, a study by Chou et al. using a mouse model of isoprenaline-induced stress demonstrated sustained cardiac damage due to a metabolic shift toward anabolic glucose pathways (glycolysis, hexosamine biosynthesis), persistent oxidative stress, and progressive structural remodeling (91). These findings underscore the importance of early intervention targeting metabolic alterations to prevent long-term cardiac consequences in TTS.
Metabolic signatures in secondary cardiomyopathy
Secondary CM are heart conditions caused by a mix of factors such as tropical living environments, poverty, poor nutrition, and limited access to healthcare. Genetic predisposition also influences how individuals respond to infections. This review highlights how metabolic disturbances such as deficiencies in thiamine, selenium, and calcium, as well as immune-mediated alterations or toxin exposure, contribute to the development of these conditions (Table 6, Figure 3). It also discusses how these insights may inform targeted treatment strategies (92).
Table 6
| CM subtype | Sample type | Cohort | Ethnicity | Metabolic high throughput technique/methods | Upregulated metabolites | Downregulated metabolites | Dysregulated pathways | Ref |
|---|---|---|---|---|---|---|---|---|
| Hypertrophic Cardiomyopathy (HCM) | Cardiac tissue Plasma | Cardiac tissues: 349 patients (with HCM) and 16 controls (without HCM) Plasma: 143 patients (with HCM), 60 controls (without HCM) and 46 patients (with DCM) | Asian (Chinese) | Targeted metabolomics, lipidomics and proteomics analysis. | Purine and pyrimidine nucleotides, Short-chain carnitines | Long-chain carnitines, S-adenosylmethionine | Vit B6 metabolism, Pentose phosphate pathway, Purine and Pyrimidine Metabolism, Glutathione Metabolism | (61) |
| Cardiac tissue | 29 HCM patients carrying variants in MYBPC3 and MYH7, 10 HCM patients with no sarcomere variants, 8 individuals with no significant heart disease as a control group | Caucasian | Proteomics and targeted metabolomics. | Ketone bodies, lactate | Long-chain acylcarnitine's ATP Phosphocreatine NADH NADPH Acetyl-CoA |
Fatty Acid Oxidation Ketone Body Metabolism Amino Acid Metabolism Nucleotide Metabolism |
(62) | |
| Plasma sample | 30 HCM patients carriers of MYBPC3 variants with sever phenotype 30 HCM patients carriers of MYBPC3 with no/mild phenotype 10 HCM patients with no variants in MYBPC3 |
European | Untargeted and targeted Metabolomics using direct-infusion high-resolution mass spectrometry | Acylcarnitine Histidine Lysine |
Di- and oligopeptides Steroid hormone metabolites |
Acylcarnitine Metabolism Histidine Metabolism Lysine Metabolism Purine Metabolism Proteolysis Metabolism |
(63) | |
| Arrhythmogenic Cardiomyopathy (ACM) | Plasma | 36 ACM patients, 27 healthy controls | European | Targeted metabolomics with (UHPLC-MS/MS) | Asymmetric dimethylarginine Propionyl C3 carnitine |
Alpha-aminoadipic acid, Phosphatidylcholines (PCs and lysoPCs), Tryptophan | Lysine Degradation Beta Oxidation of Fatty Acids Tryptophan Metabolism Arginine and Proline Metabolism |
(68) |
| Dilated Cardiomyopathy (DCM) |
Plasma and Urine | 273 patients with DCM at varying disease stages (with LVRR, asymptomatic, or symptomatic) | European | Targeted quantitative metabolomics (using internal isotopic standards) | Long chain acylcarnitine's, Sialic acid Glutamic acid, Cystathionine, 3-Methylhistidine, Urine carnosine |
Citric acid, 3-hydroxyisovaleric acid, Short and medium-chain acylcarnitine's | Impaired fatty acid, Ketone body metabolism, Mitochondrial dysfunction, Inflammatory pathway, Oxidative stress | (27) |
| Serum | 106 patients with heart failure due to DCM | European | NMR Spectroscopy | Trimethylamine-N-oxide Creatinine Lactate Succinic acid |
Creatine Apolipoprotein-A1,A2 HDL-2,3,4 |
Energy production pathways Lipid metabolism TCA cycle dysfunction Gut microbiota involvement |
(72) | |
| Plasma | 38 patients with DCM, 18 patients with Ischemic CM, 20 healthy controls | Asian (Chinese) | LC–MS/MS untargeted metabolomics | O-Acetylcarnitine, Tyramine-O-sulfate and 3-tyramine, Phosphatidylglycerol (6:0/8:0), Fatty acid esters of hydroxy fatty acids, Phosphatidylcholine (18:0/18:3) | 1-Pyrroline-2-carboxylate, Ornithine, Citrulline, Alpha-ketoglutaric acid, Lysophosphatidylinositol (16:0/0:0) | Glycerophospholipid metabolism, Linoleic acid and alpha-linolenic acid metabolism, Arginine and proline metabolism | (73) | |
| Cardiac amyloidosis | Endomyocardial Biopsies | 78 patients with cardiac amyloidosis subgroups are: AL (Immunoglobulin Light Chain) Amyloidosis, ATTR (Transthyretin) Amyloidosis, AA (Inflammatory-Related) Amyloidosis | European | retrospectively analyzed from fresh frozen cardiac amyloidosis patients and from 12 biopsies of unused donor heart explants. Nano LC-MS/MS mass spectrometry-based proteomics |
Branched-chain amino acids (BCAA) Lipid metabolites (e.g., ceramides) Acylcarnitine's Ketone bodies, ApoE, ApoA-IV and SAP |
Glucose metabolism intermediates (e.g., pyruvate and ATP), Taurine, Citrate | Fatty acid oxidation, Glycolysis and gluconeogenesis, TCA cycle, Amino acid metabolism | (75) |
| Plasma | 27 patients with ATTRV30 M amyloidosis, 26 asymptomatic ATTRV30 M carriers, and 26 controls | European | GC-MS and LC-MS | kynurenine, Xanthine, Niacinamide (S1P) | Amino acids, Glucose, L-Carnitine, Acylcarnitines | Tryptophan metabolism, BCAA metabolism, Purine metabolism, Fatty acid oxidation | (76) | |
| Tachycardia-induced cardiomyopathy response to Atrial fibrillation (AF) | Serum | Cohort of 363 patients. Validation phase (134) Discovery phase (229) patient as following: Healthy control =86 participants, Suspected AF N = 30, Cardiogenic ischemic stroke = 32, Other diagnosed AF subtypes =81 |
Chinese | GC-MS and LC-QTOF-MS | Kynurenine, Xanthine, Niacinamide Sphingosine 1-phosphate (S1P) |
Amino acids, Glucose, L-carnitine, Acylcarnitines | Tryptophan metabolism, BCAA metabolism, Purine metabolism, Fatty acid oxidation | (86) |
| Tachycardia-induced cardiomyopathy response to Heart failure (HF) | Human cardiac tissue | Human cardiac tissue data of 16 HF patients, 19 HF with tachycardia patients and 14 Control Canine model of tachycardia-induced HF, Engineered heart tissue derived from human induced pluripotent stem cells | Caucasian | RNA-seq using canine model of tachycardia-induced HF, Engineered heart tissue derived from human induced pluripotent stem cells | Glucose metabolites (dihydroxyacetone phosphate, pyruvate) Serine Ribulose 5-phosphate Sorbitol |
NAD+/NADH ratio | Oxidative phosphorylation TCA cycle Fatty acid oxidation Glycolysis HIF1 signalling Acetylation of sarco/endoplasmic reticulum. Ca2+-ATPase (SERCA2a) |
(87) |
| Takotsubo Cardiomyopathy |
Serum | 10 Takotsubo patients and 10 control subjects | European | 1H-NMR | Ketone bodies (e.g., 3-hydroxybutyrate) 2-hydroxybutyrate Acetyl-L-carnitine Glutamate |
Several amino acids including histidine, arginine methionine, Alanine |
oxidative stress response pathways lipolysis metabolism, and amino acid metabolism | (88) |
| Secondary Autoimmune diseases related to Cardiomyopathy | Serum | Meta analysis based-study of patients diagnosed with multiples autoimmune diseases e.g systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD) | GWAS database | Two-sample Mendelian randomization approach integrating genetic data and metabolite profiling data | 1-Myristoylglycerophosphocholine in RA | Arachidonate (20:4 n6) in IBD, Glycerol in RA, 2-Methoxyacetaminophen sulphate in SLE | Galactose metabolism, Glycine, Serine, and threonine metabolism, Biosynthesis of Unsaturated fatty acids, Glycerophospholipid metabolism, Arachidonic acid pathway | (104) |
| Serum | 71 Young female adults diagnosed with SLE | Caucasian | Lipidomic analysis targeting total cholesterol and triglyceride | Triglycerides, VLDL-C | HDL-C, LDL-C, APO-A, APO-B | Lipid and phospholipid metabolism | (105) |
Overview of metabolic signatures in human models of primary and secondary cardiomyopathy.
Overview of metabolic signatures in human models of primary and secondary cardiomyopathy. This table summarizes recent studies that used high-throughput metabolomics techniques to identify the most upregulated and down regulated altered metabolites and dysregulated pathways in cardiomyopathic cohorts across various ethnicities. ATP, adenosine triphosphate; NADH, nicotinamide adenine dinucleotide (reduced form); NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); LVRR, left ventricular reverse remodeling; HDL, high-density lipoprotein; TCA cycle, tricarboxylic acid cycle (also known as the krebs cycle or citric acid cycle); BCAA, branched-chain amino acids; HIF1, hypoxia-inducible factor 1; VLDL-C, very low-density lipoprotein cholesterol; APO-A, apolipoprotein A; APO-B, apolipoprotein B.
Figure 3
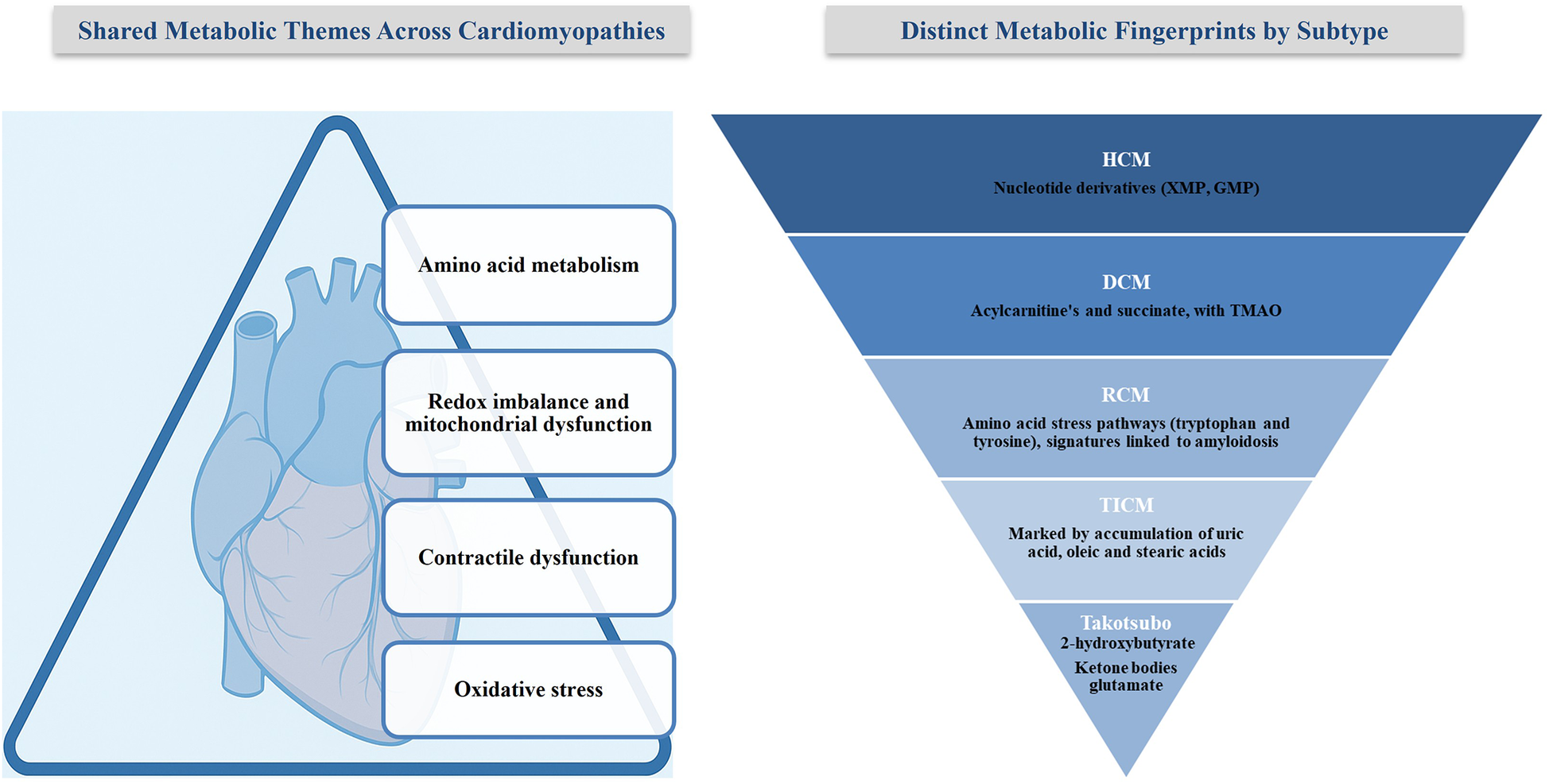
Shared and distinct metabolic alterations in cardiomyopathies. The left panel summarizes shared metabolic themes across CM subtypes. The right panel highlights distinct metabolic fingerprints unique to each subtype.
Nutrition deficiency–related cardiomyopathy
Thiamine (vitamin B1) is essential for carbohydrate, lipid, and branched-chain amino acid metabolism, serving as a cofactor for enzymes including pyruvate dehydrogenase and α-ketoglutarate dehydrogenase. Its deficiency disrupts oxidative metabolism, reduces energy production, and promotes pyruvate accumulation, leading to elevated lactic acid levels. This metabolic imbalance impairs cardiac function and contributes to DCM (93). Thiamine supplementation has been shown to improve left ventricular ejection fraction after several weeks of treatment (94). However, its therapeutic efficacy remains limited by the absence of large randomized controlled trials and variability in patient response, necessitating further investigation to establish standardized clinical protocols.
Keshan disease, an endemic CM associated with selenium deficiency, is characterized by myocardial necrosis and fibrosis (95). Selenium, a critical component of selenoproteins such as glutathione peroxidase, mitigates oxidative stress by neutralizing reactive oxygen species (96). Insufficient selenium levels compromise antioxidant defenses, leaving cardiomyocytes exposed to oxidative damage and upregulating pro-apoptotic pathways. Conversely, excess selenium intake has been associated with adverse outcomes, including reduced cardiac output (97, 98). The mechanisms remain unclear, underscoring the need for precise dosing and longitudinal studies.
Hypocalcemia, most often resulting from hypoparathyroidism or vitamin D deficiency, significantly impairs cardiac function by disrupting calcium-dependent myocardial contraction and rhythm regulation (99, 100).
Calcium homeostasis, modulated by parathyroid hormone and 1,25-dihydroxyvitamin D, is essential for coordinating systole and diastole via interactions with the sarcoplasmic reticulum and ryanodine receptors. Hypocalcemia induces metabolic alterations, including increased serum taurine, glutamine, and lipid dysregulation, which compromise cardiac contractility (101). Clinical reports emphasize hypocalcemia's role in congestive heart failure, advocating its inclusion in differential diagnoses (102, 103). Still, management is complicated by heterogeneous etiologies, requiring individualized therapeutic approaches.
Autoimmune disorders related cardiomyopathy
Notably systemic lupus erythematosus (SLE), rheumatoid arthritis, and dermatomyositis are implicated in secondary CMs through chronic inflammation and immune-driven metabolic dysregulation. In SLE, altered lipid metabolism and xenobiotic pathways enhance oxidative stress and heighten cardiovascular risk (104).
In SLE, reduced levels of 2-methoxy acetaminophen sulfate indicate disrupted xenobiotic metabolism, contributing to oxidative stress and inflammation, both key drivers of CM (104). Zhou et al. further identified dysregulated lipid profiles in young female SLE patients, including reduced Apo A, Apo B, HDL, phosphatidylcholine, and phosphoglyceride, along with elevated triglycerides and VLDL-C (105). These alterations are associated with endothelial dysfunction and increased cardiovascular risk. Persistent inflammation appears to perpetuate these metabolic disturbances, worsening myocardial injury.
In dermatomyositis, elevated betaine levels are associated with impaired amino acid metabolism. While betaine supports methylation and osmoregulation, excess levels can drive the production of TMAO via gut microbiota, an established contributor to atherosclerosis and cardiac dysfunction (106, 107). Monitoring betaine and TMAO levels could help stratify cardiovascular risk in patients with dermatomyositis.
Toxins related cardiomyopathy
Cardiotoxic agents, including anthracyclines (Doxorubicin), psychotropics, and antiretrovirals, precipitate CM primarily through mitochondrial injury and disruption of lipid metabolism (108, 109). Doxorubicin, for instance, upregulates fatty acid transport genes (FABP4), thereby amplifying cardiotoxicity (110). Balancing therapeutic efficacy against cardiotoxic risks remains a clinical challenge, requiring vigilant monitoring and the development of cardioprotective adjuncts.
Although current evidence supports the role of nutritional deficiencies and cardiotoxic agents in secondary CM, significant gaps remain. Thiamine and selenium supplementation have shown potential benefits, but the absence of extensive, well-controlled studies and variability in methodologies limit definitive conclusions. While hypocalcemia's contribution to cardiac dysfunction is established, management is complicated by heterogeneous etiologies. Autoimmune- and toxin-mediated CM underscore the need for precision medicine, yet current therapies broadly address symptoms rather than underlying metabolic pathways. Future research must focus on longitudinal studies that clarify disease mechanisms and refine therapeutic strategies.
Challenges and advances in the metabolomics of cardiomyopathy
While metabolomics has emerged as a powerful tool for exploring the biochemical landscape of CM, its application still faces key challenges. High-throughput platforms are susceptible to low-abundance metabolites but often struggle to detect or quantify metabolites present at higher concentrations reliably. Identification is further complicated by variable biochemical properties, such as polarity, which affect detection and reproducibility. Sample integrity is another limitation; exposure to light or fluctuating temperatures can degrade metabolites, compromising consistency.
Moreover, current metabolomic techniques typically rely on relative signal intensities compared to control samples, which reveal trends but lack the absolute quantification needed for clinical decision-making. The complexity of metabolomic data demands a multidisciplinary approach involving clinicians, biochemists, and data scientists to ensure accurate interpretation.
From a technological perspective, traditional one-dimensional chromatography lacks the resolution to separate closely related analytes in complex biological samples. Advanced platforms, such as two-dimensional gas chromatography (2D-GC) and two-dimensional high-performance liquid chromatography (2D-HPLC), offer greater sensitivity, separation, and sample preservation, but at the expense of high costs and technical complexity, which can limit their application in large-scale or translational studies (111–113).
From a translational standpoint, most current CM studies are cross-sectional, rely on small and homogenous cohorts, and use inconsistent sample processing protocols. These issues limit reproducibility and generalizability. Confounding factors, including age, medication use, dietary habits, and lifestyle, further complicate interpretation.
Conclusion
CM's heterogeneity and lack of specific biomarkers complicate both diagnosis and treatment. Metabolomics offers valuable insights, but its application is limited by technical constraints and insufficient validation. Single-metabolite strategies fail to capture the complexity of metabolic networks, necessitating the integration of multi-omics approaches. This review takes a broader view by comparing metabolic changes across CM subtypes and illustrating their interactions, which provides meaningful insight into disease mechanisms and highlights pathways with diagnostic, prognostic, and therapeutic relevance. This perspective shifts the focus beyond narrow comparisons, making the translational relevance of metabolomics in CM more apparent. We also highlight how advanced techniques, including two-dimensional chromatography and multi-omics integration, can facilitate the development of metabolic biomarker panels with direct clinical applications. Longitudinal studies in diverse cohorts will be crucial for improving diagnostic precision and generalizability. Addressing environmental and comorbid factors is essential, while multidisciplinary collaboration and investment in advanced platforms are vital to advancing metabolomics in precision cardiovascular medicine.
Statements
Author contributions
RA-S: Conceptualization, Writing – review & editing, Investigation, Writing – original draft. GAM: Conceptualization, Resources, Investigation, Visualization, Writing – review & editing. MA-S: Resources, Visualization, Funding acquisition, Writing – review & editing. NA: Writing – review & editing, Resources, Funding acquisition, Visualization, Project administration, Writing – original draft, Conceptualization, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The United Arab Emirates University supported this work. Grant no. 12M213 and 12M154.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT (OpenAI, San Francisco, CA, USA) was used to assist with language refinement and improvements in clarity and readability of this manuscript. The authors are fully responsible for the scientific content.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Lindstrom M DeCleene N Dorsey H Fuster V Johnson CO LeGrand KE et al Global burden of cardiovascular diseases and risks collaboration, 1990–2021. J Am Coll Cardiol. (2022) 80(25):2372–425. 10.1016/j.jacc.2022.11.001
2.
Di Cesare M Perel P Taylor S Kabudula C Bixby H Gaziano TA et al The heart of the world. Glob Heart. (2024) 19(1):11. 10.5334/gh.1288
3.
Ciarambino T Menna G Sansone G Giordano M . Cardiomyopathies: an overview. Int J Mol Sci. (2021) 22(14):7722. 10.3390/ijms22147722
4.
Tymińska A Ozierański K Balsam P Maciejewski C Wancerz A Brociek E et al Ischemic cardiomyopathy versus non-ischemic dilated cardiomyopathy in patients with reduced ejection fraction- clinical characteristics and prognosis depending on heart failure etiology (data from European society of cardiology heart failure registries). Biology. (2022) 11(2):341. 10.3390/biology11020341
5.
(CDC). CfDCaP. About Cardiomyopathy | Heart Disease. (2024). Available online at:https://www.cdc.gov/heart-disease/about/cardiomyopathy.html (Accessed June 01, 2024).
6.
Guertl B Noehammer C Hoefler G . Metabolic cardiomyopathies. Int J Exp Pathol. (2000) 81(6):349–72. 10.1046/j.1365-2613.2000.00186.x
7.
National Heart L, and Blood Institute. (n.d.). Types of Cardiomyopathy 2022. Available online at:https://www.nhlbi.nih.gov/health/cardiomyopathy/types(Accessed May 24, 2024).
8.
Ritterhoff J Tian R . Metabolism in cardiomyopathy: every substrate matters. Cardiovasc Res. (2017) 113(4):411–21. 10.1093/cvr/cvx017
9.
Müller J Bertsch T Volke J Schmid A Klingbeil R Metodiev Y et al Narrative review of metabolomics in cardiovascular disease. J Thorac Dis. (2021) 13(4):2532–50. 10.21037/jtd-21-22
10.
Stanley WC Chandler MP . Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. (2002) 7(2):115–30. 10.1023/A:1015320423577
11.
Taha M Lopaschuk GD . Alterations in energy metabolism in cardiomyopathies. Ann Med. (2007) 39(8):594–607. 10.1080/07853890701618305
12.
Nicholson JK Lindon JC . Systems biology: metabonomics. Nature. (2008) 455(7216):1054–6. 10.1038/4551054a
13.
Dunn WB Ellis DI . Metabolomics: current analytical platforms and methodologies. TrAC Trends Anal Chem. (2005) 24(4):285–94. 10.1016/j.trac.2004.11.021
14.
Wang TJ Larson MG Vasan RS Cheng S Rhee EP McCabe E et al Metabolite profiles and the risk of developing diabetes. Nat Med. (2011) 17(4):448–53. 10.1038/nm.2307
15.
Mazumder PK O’Neill BT Roberts MW Buchanan J Yun UJ Cooksey RC et al Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. (2004) 53(9):2366–74. 10.2337/diabetes.53.9.2366
16.
Neubauer SJ . The failing heart—an engine out of fuel. N Engl J Med. (2007) 356(11):1140–51. 10.1056/NEJMra063052
17.
Arbustini E Narula N Tavazzi L Serio A Grasso M Favalli V et al The MOGE(S) classification of cardiomyopathy for clinicians. J Am Coll Cardiol. (2014) 64(3):304–18. 10.1016/j.jacc.2014.05.027
18.
Alcalai R Seidman JG Seidman CE . Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. (2008) 19(1):104–10. 10.1111/j.1540-8167.2007.00965.x
19.
Maron BJ Towbin JA Thiene G Antzelevitch C Corrado D Arnett D et al Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. (2006) 113(14):1807–16. 10.1161/CIRCULATIONAHA.106.174287
20.
Brieler J Breeden MA Tucker J . Cardiomyopathy: an overview. Am Fam Physician. (2017) 96(10):640–6.
21.
Shah SN Umapathi KK Horenstein MS Oliver TI . Arrhythmogenic right ventricular cardiomyopathy. In: StatPearls publishing editorial team. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at:https://www.ncbi.nlm.nih.gov/books/NBK470378/(Accessed March 20, 2024).
22.
Wenzl FA Ambrosini S Mohammed SA Kraler S Lüscher TF Costantino S et al Inflammation in metabolic cardiomyopathy. Front Cardiovasc Med. (2021) 8:742178. 10.3389/fcvm.2021.742178
23.
Kale D Fatangare A Phapale P Sickmann A . Blood-derived lipid and metabolite biomarkers in cardiovascular research from clinical studies: a recent update. Cells. (2023) 12(24):2796. 10.3390/cells12242796
24.
Ahmad SA Khalid N Ibrahim MA . Takotsubo cardiomyopathy. In: StatPearls publishing editorial team. StatPearls. Treasure Island (FL): StatPearls Publishing (2024). Available online at:https://www.ncbi.nlm.nih.gov/books/NBK430798/(Accessed May 22, 2023).
25.
Prasad A Lerman A Rihal CS . Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. (2008) 155(3):408–17. 10.1016/j.ahj.2007.11.008
26.
Semsarian C MacDonald P Weintraub RG . Dilated cardiomyopathy. (2017).
27.
Verdonschot JAJ Wang P Van Bilsen M Hazebroek MR Merken JJ Vanhoutte EK et al Metabolic profiling associates with disease severity in nonischemic dilated cardiomyopathy. J Card Fail. (2020) 26(3):212–22. 10.1016/j.cardfail.2019.09.004
28.
Schultheiss H-P Fairweather D Caforio ALP Escher F Hershberger RE Lipshultz SE et al Dilated cardiomyopathy. Nat Rev Dis Primers. (2019) 5(1):32. 10.1038/s41572-019-0084-1
29.
Muchtar E Blauwet LA Gertz MA . Restrictive cardiomyopathy. Circ Res. (2017) 121(7):819–37. 10.1161/CIRCRESAHA.117.310982
30.
Jang S Chorna N Rodríguez-Graciani KM Inyushin M Fossati S Javadov S . The effects of amyloid-β on metabolomic profiles of cardiomyocytes and coronary endothelial cells. J Alzheimer’s Dis. (2023) 93(1):307–19. 10.3233/JAD-221199
31.
Wexler RK Elton T Pleister A Feldman D . Cardiomyopathy: an overview. Am Fam Physician. (2009) 79(9):778–84.
32.
Gawałko M Balsam P Lodziński P Grabowski M Krzowski B Opolski G et al Cardiac arrhythmias in autoimmune diseases. Circ J. (2020) 84(5):685–94. 10.1253/circj.CJ-19-0705
33.
Grundy SM . Metabolic syndrome update. Trends Cardiovasc Med. (2016) 26(4):364–73. 10.1016/j.tcm.2015.10.004
34.
Sorouri M Chang T Hancks Dustin C . Mitochondria and viral infection: advances and emerging battlefronts. mBio. (2022) 13(1):e02096–21. 10.1128/mbio.02096-21
35.
Gümüş E Özen H . Glycogen storage diseases: an update. World J Gastroenterol. (2023) 29(25):3932–63. 10.3748/wjg.v29.i25.3932
36.
Spoelstra-de Man AME Oudemans-van Straaten HM Elbers PWG . Vitamin C and thiamine in critical illness. BJA Educ. (2019) 19(9):290–6. 10.1016/j.bjae.2019.05.005
37.
Zakhari S . Overview: how is alcohol metabolized by the body?Alcohol Res Health J National Inst Alcohol Abuse Alcoholism. (2006) 29(4):245–54.
38.
Wishart DS Tzur D Knox C Eisner R Guo AC Young N et al HMDB: the human metabolome database. Nucleic Acids Res. (2007) 35(suppl_1):D521–D6. 10.1093/nar/gkl923
39.
Cheng J Lan W Zheng G Gao X . Metabolomics: a high-throughput platform for metabolite profile exploration. Methods Molec Biol. (2018) 1754:265–92. 10.1007/978-1-4939-7717-8_16
40.
Emwas AH . The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Molec Biol. (2015) 1277:161–93. 10.1007/978-1-4939-2377-9_13
41.
Kiseleva O Kurbatov I Ilgisonis E Poverennaya E . Defining blood plasma and serum metabolome by GC-MS. Metabolites. (2021) 12(1):15. 10.3390/metabo12010015
42.
Zhou B Xiao JF Tuli L Ressom HW . LC-MS-based metabolomics. Mol BioSyst. (2012) 8(2):470–81. 10.1039/C1MB05350G
43.
Psychogios N Hau DD Peng J Guo AC Mandal R Bouatra S et al The human serum metabolome. PLoS One. (2011) 6(2):e16957. 10.1371/journal.pone.0016957
44.
Karagiannidis E Moysidis DV Papazoglou AS Panteris E Deda O Stalikas N et al Prognostic significance of metabolomic biomarkers in patients with diabetes mellitus and coronary artery disease. Cardiovasc Diabetol. (2022) 21(1):70. 10.1186/s12933-022-01494-9
45.
Anlar GG Anwardeen N Al Ashmar S Pedersen S Elrayess MA Zeidan A . Metabolomics profiling of stages of coronary artery disease progression. Metabolites. (2024) 14(6):292. 10.3390/metabo14060292
46.
Fu H Zhu K Zhou D Guan Y Li W Xu S . Identification and validation of plasma metabolomics reveal potential biomarkers for coronary heart disease. Int Heart J. (2019) 60(6):1387–97. 10.1536/ihj.19-059
47.
Harshfield EL Sands CJ Tuladhar AM de Leeuw FE Lewis MR Markus HS . Metabolomic profiling in small vessel disease identifies multiple associations with disease severity. Brain J Neurol. (2022) 145(7):2461–71. 10.1093/brain/awac041
48.
Huang CC McDermott MM Liu K Kuo CH Wang SY Tao H et al Plasma metabolomic profiles predict near-term death among individuals with lower extremity peripheral arterial disease. J Vasc Surg. (2013) 58(4):989–96.e1. 10.1016/j.jvs.2013.04.022
49.
Zagura M Kals J Kilk K Serg M Kampus P Eha J et al Metabolomic signature of arterial stiffness in male patients with peripheral arterial disease. Hypertens Res. (2015) 38(12):840–6. 10.1038/hr.2015.71
50.
Ismaeel A Franco ME Lavado R Papoutsi E Casale GP Fuglestad M et al Altered metabolomic profile in patients with peripheral artery disease. J Clin Med. (2019) 8(9):1463. 10.3390/jcm8091463
51.
Dougherty S Khorsandi M Herbst P . Rheumatic heart disease screening: current concepts and challenges. Ann Pediatr Cardiol. (2017) 10(1):39–49. 10.4103/0974-2069.197051
52.
Das S Kumar Y Sharma S Ray R Arava S Seth S et al An untargeted LC–MS based approach for identification of altered metabolites in blood plasma of rheumatic heart disease patients. Sci Rep. (2022) 12(1):5238. 10.1038/s41598-022-09191-z
53.
Bassareo PP McMahon CJ . Metabolomics: a new tool in our understanding of congenital heart disease. Children. (2022) 9(12):1803. 10.3390/children9121803
54.
Dong S Wu L Duan Y Cui H Chen K Chen X et al Metabolic profile of heart tissue in cyanotic congenital heart disease. Am J Transl Res. (2021) 13(5):4224–32.
55.
Liederman Z Chan N Bhagirath V . Current challenges in diagnosis of venous thromboembolism. J Clin Med. (2020) 9(11):3509. 10.3390/jcm9113509
56.
Franczyk B Gluba-Brzózka A Ławiński J Rysz-Górzyńska M Rysz J . Metabolomic profile in venous thromboembolism (VTE). Metabolites. (2021) 11(8):495. 10.3390/metabo11080495
57.
Chen Y Wang Y Xie Z Ming X Li Z Kong Y . A tryptophan derivative TD-26 attenuates thrombus formation by inhibiting both PI3K/Akt signaling and binding of fibrinogen to integrin αIIbβ3. Biochem Biophys Res Commun. (2015) 465(3):516–22. 10.1016/j.bbrc.2015.08.051
58.
Culler KL Sinha A Filipp M Giro P Allen NB Taylor KD et al Metabolomic profiling identifies novel metabolites associated with cardiac dysfunction. Sci Rep. (2024) 14(1):20694. 10.1038/s41598-024-71329-y
59.
Monda E Limongelli G Pelliccia F . Hypertrophic cardiomyopathy-current challenges and future perspectives. J Clin Med. (2023) 12(18):6093. 10.3390/jcm12186093
60.
Calderon Martinez E Ortiz-Garcia NY Herrera Hernandez DA Arriaga Escamilla D Diaz Mendoza DL Othon Martinez D et al Hypertrophic cardiomyopathy diagnosis and treatment in high- and low-income countries: a narrative review. Cureus. (2023) 15(10):e46330. 10.7759/cureus.46330
61.
Wang W Wang J Yao K Wang S Nie M Zhao Y et al Metabolic characterization of hypertrophic cardiomyopathy in human heart. Nat Cardiovasc Res. (2022) 1(5):445–61. 10.1038/s44161-022-00057-1
62.
Previs MJ O'Leary TS Morley MP Palmer BM LeWinter M Yob JM et al Defects in the proteome and metabolome in human hypertrophic cardiomyopathy. Circ Heart Fail. (2022) 15(6):e009521. 10.1161/CIRCHEARTFAILURE.121.009521
63.
Jansen M Schuldt M van Driel BO Schmidt AF Christiaans I van der Crabben SN et al Untargeted metabolomics identifies potential hypertrophic cardiomyopathy biomarkers in carriers of MYBPC3 founder variants. Int J Mol Sci. (2023) 24(4):4031. 10.3390/ijms24044031
64.
Maack C Dudek J Bertero E Tampakakis E Vernon HJ . Mitochondrial cardiomyopathies: pathogenesis, diagnosis, and treatment. Eur Heart J. (2025):ehaf491. 10.1093/eurheartj/ehaf491
65.
Nollet EE Duursma I Rozenbaum A Eggelbusch M Wüst RCI Schoonvelde SAC et al Mitochondrial dysfunction in human hypertrophic cardiomyopathy is linked to cardiomyocyte architecture disruption and corrected by improving NADH-driven mitochondrial respiration. Eur Heart J. (2023) 44(13):1170–85. 10.1093/eurheartj/ehad028
66.
Franco A Li J Kelly DP Hershberger RE Marian AJ Lewis RM et al A human mitofusin 2 mutation can cause mitophagic cardiomyopathy. eLife. (2023) 12:e84235. 10.7554/eLife.84235
67.
Corrado D Basso C Judge DP . Arrhythmogenic cardiomyopathy. Circ Res. (2017) 121(7):784–802. 10.1161/CIRCRESAHA.117.309345
68.
Volani C Rainer J Hernandes VV Meraviglia V Pramstaller PP Smárason SV et al Metabolic signature of arrhythmogenic cardiomyopathy. Metabolites. (2021) 11(4):195. 10.3390/metabo11040195
69.
Yancy CW Jessup M Bozkurt B Butler J Casey DE Jr. Drazner MH et al 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. (2013) 62(16):e147–239. 10.1016/j.jacc.2013.05.019
70.
Ussher JR Elmariah S Gerszten RE Dyck JR . The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol. (2016) 68(25):2850–70. 10.1016/j.jacc.2016.09.972
71.
Ampong I . Metabolic and metabolomics insights into dilated cardiomyopathy. Ann Nutr Metab. (2022) 78(3):147–55. 10.1159/000524722
72.
Vignoli A Fornaro A Tenori L Castelli G Cecconi E Olivotto I et al Metabolomics fingerprint predicts risk of death in dilated cardiomyopathy and heart failure. Front Cardiovasc Med. (2022) 9:851905. 10.3389/fcvm.2022.851905
73.
Zhao J Yang S Jing R Jin H Hu Y Wang J et al Plasma metabolomic profiles differentiate patients with dilated cardiomyopathy and ischemic cardiomyopathy. Front Cardiovasc Med. (2020) 7:597546. 10.3389/fcvm.2020.597546
74.
Rapezzi C Aimo A Barison A Emdin M Porcari A Linhart A et al Restrictive cardiomyopathy: definition and diagnosis. Eur Heart J. (2022) 43(45):4679–93. 10.1093/eurheartj/ehac543
75.
Noborn F Thomsen C Vorontsov E Bobbio E Sihlbom C Nilsson J et al Subtyping of cardiac amyloidosis by mass spectrometry-based proteomics of endomyocardial biopsies. Amyloid. (2023) 30(1):96–108. 10.1080/13506129.2022.2127088
76.
Olsson M Hellman U Wixner J Anan I . Metabolomics analysis for diagnosis and biomarker discovery of transthyretin amyloidosis. Amyloid. (2021) 28(4):234–42. 10.1080/13506129.2021.1958775
77.
Tymińska A Ozierański K Skwarek A Kapłon-Cieślicka A Baritussio A Grabowski M et al Personalized management of myocarditis and inflammatory cardiomyopathy in clinical practice. J Pers Med. (2022) 12(2):183. 10.3390/jpm12020183
78.
Tschöpe C Ammirati E Bozkurt B Caforio ALP Cooper LT Felix SB et al Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. (2021) 18(3):169–93. 10.1038/s41569-020-00435-x
79.
Basso C . Myocarditis. N Engl J Med. (2022) 387(16):1488–500. 10.1056/NEJMra2114478
80.
Wang L Sun T Liu X Wang Y Qiao X Chen N et al Myocarditis: a multi-omics approach. Clin Chim Acta. (2024) 554:117752. 10.1016/j.cca.2023.117752
81.
Kong Q Gu J Lu R Huang C Chen L Wu W et al NMR-based metabolomic analysis of cardiac tissues clarifies molecular mechanisms of CVB3-induced viral myocarditis and dilated cardiomyopathy. Molecules. (2022) 27(18):6115. 10.3390/molecules27186115
82.
Kong Q Chen L Zeng X Lu F Huang Y Wu W . Alterations of the gut microbiome and metabolic profile in CVB3-induced mice acute viral myocarditis. BMC Microbiol. (2023) 23(1):139. 10.1186/s12866-023-02863-4
83.
Mueller KAL Heinzmann D Klingel K Fallier-Becker P Kandolf R Kilias A et al Histopathological and immunological characteristics of tachycardia-induced cardiomyopathy. J Am Coll Cardiol. (2017) 69(17):2160–72. 10.1016/j.jacc.2017.02.049
84.
Katz M Meitus A Arad M Aizer A Nof E Beinart R . Long-term outcomes of tachycardia-induced cardiomyopathy compared with idiopathic dilated cardiomyopathy. J Clin Med. (2023) 12(4):1412. 10.3390/jcm12041412
85.
Kim DY Kim SH Ryu KH . Tachycardia induced cardiomyopathy. Korean Circ J. (2019) 49(9):808–17. 10.4070/kcj.2019.0199
86.
Lu C Liu C Mei D Yu M Bai J Bao X et al Comprehensive metabolomic characterization of atrial fibrillation. Front Cardiovasc Med. (2022) 9:911845. 10.3389/fcvm.2022.911845
87.
Tu C Caudal A Liu Y Gorgodze N Zhang H Lam CK et al Tachycardia-induced metabolic rewiring as a driver of contractile dysfunction. Nat Biomed Eng. (2024) 8(4):479–94. 10.1038/s41551-023-01134-x
88.
Vanni D Viceconte N Petrella G Biccirè FG Pelliccia F Tanzilli G et al A pilot study on the (1)H-NMR Serum metabolic profile of takotsubo patients reveals systemic response to oxidative stress. Antioxidants. (2021) 10(12):1982. 10.3390/antiox10121982
89.
Pelliccia F Kaski JC Crea F Camici PG . Pathophysiology of takotsubo syndrome. Circulation. (2017) 135(24):2426–41. 10.1161/CIRCULATIONAHA.116.027121
90.
Scally C Rudd A Mezincescu A Wilson H Srivanasan J Horgan G et al Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) cardiomyopathy. Circulation. (2018) 137(10):1039–48. 10.1161/CIRCULATIONAHA.117.031841
91.
Yoganathan T Perez-Liva M Balvay D Le Gall M Lallemand A Certain A et al Acute stress induces long-term metabolic, functional, and structural remodeling of the heart. Nat Commun. (2023) 14(1):3835. 10.1038/s41467-023-39590-3
92.
Sliwa K Viljoen CA Hasan B Ntusi NAB . Nutritional heart disease and cardiomyopathies. J Am Coll Cardiol. (2023) 81(2):187–202. 10.1016/j.jacc.2022.08.812
93.
DiNicolantonio JJ Liu J O'Keefe JH . Thiamine and cardiovascular disease: a literature review. Prog Cardiovasc Dis. (2018) 61(1):27–32. 10.1016/j.pcad.2018.01.009
94.
Piquereau J Boitard SE Ventura-Clapier R Mericskay M . Metabolic therapy of heart failure: is there a future for B vitamins?Int J Mol Sci. (2022) 23(1):30. 10.3390/ijms23010030
95.
Kang D Lee J Wu C Guo X Lee BJ Chun J-S et al The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med. (2020) 52(8):1198–208. 10.1038/s12276-020-0408-y
96.
Cvetinovic N Loncar G Isakovic AM von Haehling S Doehner W Lainscak M et al Micronutrient depletion in heart failure: common, clinically relevant and treatable. Int J Mol Sci. (2019) 20(22):5627. 10.3390/ijms20225627
97.
Shalihat A Hasanah AN Mutakin Lesmana R Budiman A Gozali D . The role of selenium in cell survival and its correlation with protective effects against cardiovascular disease: a literature review. Biomed Pharmacother. (2021) 134:111125. 10.1016/j.biopha.2020.111125
98.
Shimada BK Alfulaij N Seale LA . The impact of selenium deficiency on cardiovascular function. Int J Mol Sci. (2021) 22(19):10713. 10.3390/ijms221910713
99.
Válek M Roblová L Raška I Jr. Schaffelhoferová D Paleček T . Hypocalcaemic cardiomyopathy: a description of two cases and a literature review. ESC Heart Fail. (2020) 7(3):1291–301. 10.1002/ehf2.12693
100.
Eisner D . Calcium in the heart: from physiology to disease. Exp Physiol. (2014) 99(10):1273–82. 10.1113/expphysiol.2013.077305
101.
Podgórska B Wielogórska-Partyka M Godzień J Siemińska J Ciborowski M Szelachowska M et al Applications of metabolomics in calcium metabolism disorders in humans. Int J Mol Sci. (2022) 23(18):10407. 10.3390/ijms231810407
102.
Baqi DH Ahmed SF Baba HO Fattah FH Salih AM Ali RM et al Hypocalcemia as a cause of reversible heart failure: a case report and review of the literature. Ann Med Surg. (2022) 77:103572. 10.1016/j.amsu.2022.103572
103.
Rupasinghe N Ranasinghe P Wanninayake L . Dilated cardiomyopathy due to hypocalcaemia: a case report. J Med Case Rep. (2024) 18(1):204. 10.1186/s13256-024-04505-3
104.
Wang W Huang M Ge W Feng J Zhang X Li C et al Identifying serum metabolite biomarkers for autoimmune diseases: a two-sample mendelian randomization and meta-analysis. Front Immunol. (2024) 15:1300457. 10.3389/fimmu.2024.1300457
105.
Zhou B Xia Y She J . Dysregulated serum lipid profile and its correlation to disease activity in young female adults diagnosed with systemic lupus erythematosus: a cross-sectional study. Lipids Health Dis. (2020) 19(1):40. 10.1186/s12944-020-01232-8
106.
Ilyas A Wijayasinghe YS Khan I El Samaloty NM Adnan M Dar TA et al Implications of trimethylamine N-oxide (TMAO) and betaine in human health: beyond being osmoprotective compounds. Front Mol Biosci. (2022) 9:964624. 10.3389/fmolb.2022.964624
107.
Zhao G He F Wu C Li P Li N Deng J et al Betaine in inflammation: mechanistic aspects and applications. Front Immunol. (2018) 9:1070. 10.3389/fimmu.2018.01070
108.
Albakri A . Drugs-related cardiomyopathy: a systematic review and pooled analysis of pathophysiology, diagnosis and clinical management. Int Med Care. (2019) 3(1):1–19. 10.15761/IMC.1000129
109.
Montastruc G Favreliere S Sommet A Pathak A Lapeyre-Mestre M Perault-Pochat MC et al Drugs and dilated cardiomyopathies: a case/noncase study in the French PharmacoVigilance database. Br J Clin Pharmacol. (2010) 69(3):287–94. 10.1111/j.1365-2125.2009.03596.x
110.
da Cunha Menezes Souza L Fernandes FH Presti PT Anjos Ferreira AL Fávero Salvadori DM . Effect of doxorubicin on cardiac lipid metabolism-related transcriptome and the protective activity of Alda-1. Eur J Pharmacol. (2021) 898:173955. 10.1016/j.ejphar.2021.173955
111.
Pinu FR Goldansaz SA Jaine J . Translational metabolomics: current challenges and future opportunities. Metabolites. (2019) 9(6):108. 10.3390/metabo9060108
112.
Papatheocharidou C Samanidou V . Two-dimensional high-performance liquid chromatography as a powerful tool for bioanalysis: the paradigm of antibiotics. Molecules. (2023) 28(13):5056. 10.3390/molecules28135056
113.
Milani NBL van Gilst E Pirok BWJ Schoenmakers PJ . Comprehensive two-dimensional gas chromatography— a discussion on recent innovations. J Sep Sci. (2023) 46(21):2300304. 10.1002/jssc.202300304
Summary
Keywords
metabolomics, cardiomyopathy, LC-MS, cardiovascular disease, heart failure
Citation
Al-Shahrabi R, Al Mansoori G, Al-Saffar M and Akawi N (2025) Metabolic perturbations in cardiomyopathies: implications for early diagnosis and targeted interventions. Front. Cardiovasc. Med. 12:1616677. doi: 10.3389/fcvm.2025.1616677
Received
23 April 2025
Accepted
30 September 2025
Published
17 October 2025
Volume
12 - 2025
Edited by
Lei Wang, Guangdong Provincial Hospital of Chinese Medicine, China
Reviewed by
Antonietta Franco, Humanitas University, Italy
Chiara Zocchi, Careggi University Hospital, Italy
Updates
Copyright
© 2025 Al-Shahrabi, Al Mansoori, Al-Saffar and Akawi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Nadia Akawi nadia.akawi@uaeu.ac.ae
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.