Abstract
Background:
Atrial fibrillation symptoms are diverse and complex, but symptom networks can visually map the relationships between symptoms and influencing factors, identifying key symptoms and offering better targets for symptom management. However, research on establishing symptom networks in Atrial fibrillation patients is limited.
Aim:
We aimed to construct a symptom network for patients with atrial fibrillation, understand its characteristics, and identify core and bridging symptoms.
Methods:
This cross-sectional study enrolled 384 patients with atrial fibrillation from November 2021 to August 2022 at Tianjin Medical University General Hospital of China. Network analysis methods were utilized to construct the symptom network. Centrality metrics were used to identify important symptoms.
Results:
By incorporating covariates into the symptom network, we revealed that the Mental Health Inventory-5 score was most closely related to “fatigue at rest”. Sex influenced all symptoms except “dizziness” and “shortness of breath at rest”. Left ventricular ejection fraction was closely connected to “exercise intolerance” and “shortness of breath at rest”, while the frail score was closely linked to “exercise intolerance” and “dizziness”. Controlling for covariates, “shortness of breath during physical activity” and “shortness of breath at rest” are atrial fibrillation patients' core symptoms. “Shortness of breath at rest”, “palpitations”, and “chest pain” served as bridging symptoms between symptom clusters.
Conclusion:
Symptom networks can help us understand the relationships between symptoms and influencing factors, as well as the interactions between different atrial fibrillation symptoms.
1 Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia (1) affecting about 1.6% of Chinese adults (2). Atrial fibrillation is associated with increased morbidity, mortality, and a significant economic burden (1). The development of AF is influenced by multiple factors. Conditions such as hyperthyroidism (3) and hypertrophic cardiomyopathy (4) are known to predispose individuals to AF. Additionally, modifiable lifestyle factors are significantly associated with AF risk. Research indicates that smoking more than doubles the risk of AF (5), while endurance exercise training may increase the probability of developing AF by 2- to 10-fold (6). Furthermore, cardiac channelopathies are also closely linked to the occurrence of arrhythmias (7). Further, many atrial fibrillation patients experience symptoms like fatigue, shortness of breath, palpitations (8),, leading to emotional distress and poor quality of life (9, 10). Atrial fibrillation symptoms were strongly associated with multiple factors such as depression, sex, coronary artery disease, diabetes mellitus, and sleep disturbances (11, 12). However, it remains unclear how these factors influence individual or multiple symptoms of atrial fibrillation.
Network analysis is a new approach that offers a comprehensive view by visually representing and quantifying the complex connections between variables in a network graph (13). This method can help visualize the relationships between variables and symptoms, as well as the interactions between different symptoms (14). For example, Bard and colleagues used this method to explore the detailed relationship between insomnia and anxiety or depression symptoms (15). Henneghan and colleagues applied it to examine the detailed connections between symptoms in breast cancer survivors and pro-inflammatory and anti-inflammatory cytokines, suggesting interleukin-2 as a potential mechanism for symptom co-occurrence (16). These examples illustrate that this method can help clarify the relationship between factors and symptoms, aiding in the discovery of symptom mechanisms.
In addition, symptoms of atrial fibrillation are interconnected and mutually influence each other. Different symptoms also play distinct roles and functions within the symptom network (14, 17). Network analysis can help identify core symptoms and bridge symptoms linked to symptom clusters, aiding in understanding the key symptoms driving symptom occurrence and impact, and providing targets for tailored interventions (13). Studies on symptom networks in atrial fibrillation patients are limited; we aim to visualize and analyze the relationships between factors like sleep quality, physiological indicators, psychological health status, and atrial fibrillation symptoms using network analysis. We further seek to explore important symptoms to help understand the mechanisms of symptom interaction to pinpoint intervention targets.
2 Design, materials, and methods
2.1 Participants and methods
This cross-sectional study enrolled 384 patients with atrial fibrillation from November 2021 to August 2022 via convenient sampling at Tianjin Medical University General Hospital. Patients diagnosed with non-valvular atrial fibrillation (18) were included. Patients with active tumors and reversible atrial fibrillation associated with hyperthyroidism or electrolyte imbalances were excluded.
2.2 Sample size
When the network structure contains fewer than 20 nodes, sample sizes ranging from 250 to 350 are generally sufficient to observe moderate sensitivity, high specificity, and strong correlations between edge weights (19). Ultimately, we included 360 participants, meeting the required sample size.
2.3 Data collection
2.3.1 Demographics and clinical data
Socio-demographic data such as age, sex, and body mass index; disease details like atrial fibrillation type and atrial fibrillation duration; laboratory indicators including high-sensitivity C-reactive protein and B-type natriuretic peptide; echocardiographic measurements including left atrial anteroposterior diameter, right atrial transverse diameter, left ventricular end-diastolic diameter, right ventricular transverse diameter, and left ventricular ejection fraction. Echocardiographic and laboratory data were collected on the admission day.
2.3.2 Symptom, mental health status, sleep quality, and frailty evaluation tools
Symptoms were evaluated using the University of Toronto Atrial Fibrillation Severity Scale (AFSS), which assesses symptoms such as palpitations, shortness of breath, fatigue, dizziness, and chest pain at rest and during activity. Each item is scored from 0 to 5, with 0 meaning “no symptoms” and 5 meaning “always present.” The total score ranges from 0 to 35. The Cronbach's α coefficient for this scale in our study was 0.74 (20).
Mental health was assessed using the Mental Health Inventory-5 (MHI-5), which measures emotional well-being, including anxiety, depression, vitality, happiness, and tranquility over the past month. Scores are transformed to a scale of 0 to 100, with higher scores reflecting better mental health. The Cronbach's α coefficient ranged from 0.72 to 0.88 (21).
The Pittsburgh Sleep Quality Index (PSQI) was used to evaluate sleep quality over the past month, with scores ranging from 0 to 21. Higher scores indicate poorer sleep quality. The Chinese version of the PSQI had a Cronbach's α coefficient of 0.71 (22).
Frailty was measured using the Chinese version of the FRAIL scale, which assesses fatigue, resistance, ambulation, illness, and weight changes. Each item is scored from 0 to 1, and a total score of 3 or higher indicates frailty. This scale demonstrated good reliability with a Cronbach's α coefficient of 0.826 (23).
2.3.3 Data collection methods
Upon admission, informed consent was obtained, and a thorough assessment was conducted, covering sociodemographic, clinical, symptom, sleep quality, and mental health information. Patients were fully informed about the study's goals and guidelines. To reduce bias, survey questions were worded consistently and impartially. Of the 400 questionnaires distributed, 384 valid responses were returned, after excluding incomplete forms or those completed in under 5 min, resulting in a 96% response rate.
This study received approval from the Ethics Committee for Clinical Research at Tianjin Medical University General Hospital [Approval No. (IRB2023-WZ-111)]. All procedures adhered to ethical standards and complied with the Declaration of Helsinki.
2.4 Statistical analysis
Statistical analysis utilized SPSS 19.0 and R 4.1.3 software. Missing values for physiological and laboratory data (less than 4% of the sample) were addressed by replacing them with the mean or median. Continuous variables were expressed as mean ± standard deviation or median, while categorical variables were presented as frequencies and percentages. For assessing factors influencing symptom burden in atrial fibrillation patients, we utilized bivariate analysis and linear regression.
2.4.1 Symptom network visualization
Two symptom networks were constructed using distinct graphical approaches: a Mixed Graphical Model (MGM) enrolling covariates which incorporated both continuous (clinical symptoms、left ventricular ejection fraction、frail score、MHI-5 score) and categorical variables (sex), while the “Gaussian Graph” model focused only on continuous variables after controlling for covariates. Covariates were controlled by performing regression analysis on the seven symptom variables, with the residuals from this analysis used as the data for further analysis (24).
The “qgraph” package was used for network visualization. The “Fruchterman-Reingold” algorithm positioned highly connected and numerous symptom nodes at the center of the network and less connected and fewer symptom nodes at the periphery. The “pcor” algorithm was used to reduce false positive results in the symptom network visualization. The accuracy and stability of the network were assessed using the “bootnet” package in R. Accuracy was evaluated by calculating 95% confidence intervals for edge weights based on 1,000 nonparametric bootstrap samples. The stability of node centrality was assessed via 1,000 case-dropping bootstrap samples, which were used to compute the correlation stability coefficient (CS).
2.4.2 Core and bridge symptoms identification
We used three centrality measures: “Betweenness,” “Closeness,” and “Strength” to identify key symptoms. “Betweenness” counts how often a node lies on the shortest path between other nodes. “Closeness” calculates the average distance from a node to all others, highlighting influential nodes. “Strength” measures network connectivity, with higher values indicating more frequent co-occurrence. High centrality nodes were identified as core symptoms. Symptom clusters were identified with the “EGAnet” package's walktrap algorithm, and “bridge strength” was used to find symptoms linking clusters. The “walktrap” algorithm is a built-in function of the “EGAnet” package in R software. It works by calculating the distances between nodes in a graph to identify community structures.
3 Results
3.1 Participant characteristics
The average age of participants was 66.19 ± 9.38 years, with 54.7% male. Of the participants, 58.3% had paroxysmal atrial fibrillation, and the median duration was 18 months (IQR: 7–65). Hypertension and coronary heart disease were present in 62% and 36.5% of participants, respectively (Table 1). The average PSQI and MHI-5 scores were 7.85 and 74.64, respectively, with 61.9% of participants experiencing impaired sleep quality (PSQI > 5).
Table 1
| Item | `x ± S/n(%)/M(IQR) |
|---|---|
| Age (year) | 66.19 ± 9.38 |
| Sex (male) | 210 (54.7%) |
| BMI (Kg/m2) | 25.68 ± 3.41 |
| AF Duration (month) | 18 (7,65) |
| paroxysmal AF | 224 (58.3%) |
| Diabetes | 75 (19.5%) |
| Hypertension | 238 (62%) |
| Coronary heart disease | 140 (36.5%) |
| Ischemic stroke | 91 (23.7%) |
| COPD | 7 (1.8%) |
| OSA | 10 (2.6%) |
| Heart failure | 46 (12%) |
| Medication history | |
| None | 227 (59.3%) |
| AADs | 65 (17%) |
| Rate control | 76 (19.6%) |
| AADs + rate control | 16 (4.1%) |
| RFCA history | 75 (19.6%) |
| Hs-CRP (mg/L) | 1.41(0.70, 3.46) |
| BNP (pg/ml) | 138 (65, 276) |
| LA-ap (mm) | 42.07 ± 5.70 |
| LVEDD (mm) | 48.25 ± 4.42 |
| RA-t (mm) | 40.08 ± 5.21 |
| RV-b (mm) | 32.17 ± 3.42 |
| LVEF (%) | 62%(60%, 64%) |
| MHI-5 score | 74.64 ± 16.31 |
| PSQI score | 7.85 ± 4.33 |
| Frail score | 1.47 ± 1.30 |
Demographic and clinical characteristics of patients with atrial fibrillation (N = 384).
M, median; IQR, interquartile range; BMI, body mass index; RFCA, radiofrequency catheter ablation; AF, atrial fibrillation; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; RA-t, right atrial transverse diameter; LA-ap, left atrial anteroposterior diameter; LVEDD, left ventricular end-diastolic diameter; RV-b, right ventricular transverse diameter; Hs-CRP, high-sensitivity C-reactive protein; AADs, anti-arrhythmic drugs; OSA, obstructive sleep apnea; COPD, chronic obstructive pulmonary disease; MHI-5, Mental Health Inventory-5; PSQI, The Pittsburgh Sleep Quality Index.
3.2 Analysis of the current status and influencing factors of symptom burden in patients with atrial fibrillation
The AFSS score was 9.28 ± 5.19, with 24 patients (6.3%) reporting no symptoms. A total of 360 patients were included in the analysis after excluding non-symptom patients. The bivariate analysis revealed statistical differences in sex, coronary heart disease, heart failure, chronic obstructive pulmonary disease, and atrial fibrillation classification. Variables such as a B-type natriuretic peptide, left ventricular ejection fraction(LVEF), sleep quality, frailty, mental health status, high-sensitivity C-reactive protein, and left atrial diameter showed a correlation with symptom burden (p < 0.05), as detailed in Table 2. Multiple linear regression indicated that sex (β = 18.8, p = 0.007), MHI-5 score (β = −0.06, p = 0.001), left ventricular ejection fraction (β = −13.56, p = 0.010), and frail score (β = 0.68, p = 0.005) were identified as independent influencing factors of symptom burden (Table 3).
Table 2
| Item | t/F/r | p |
|---|---|---|
| Age (year) | 0.05 | 0.322 |
| Sex (male) | −3.84 | <0.001* |
| BMI (Kg/m2) | −0.04 | 0.427 |
| AF Duration (month) | 0.07 | 0.209 |
| paroxysmal AF | −1.95 | 0.051 |
| Diabetes | 0.17 | 0.867 |
| Hypertension | −0.53 | 0.595 |
| Coronary heart disease | −2.91 | 0.004* |
| Ischemic stroke | 0.57 | 0.572 |
| COPD | −2.44 | 0.015* |
| OSA | −0.19 | 0.848 |
| Heart failure | −2.89 | 0.004* |
| Medication history | 0.46 | 0.708 |
| None | ||
| AADs | ||
| Rate control | ||
| AADs + rate control | ||
| RFCA history | −1.59 | 0.113 |
| Hs-CRP (mg/L) | 0.16 | 0.002* |
| BNP (pg/ml) | 0.17 | 0.001* |
| LA-ap (mm) | 0.08 | 0.138 |
| LVEDD (mm) | 0.05 | 0.305 |
| RA-t (mm) | 0.01 | 0.882 |
| RV-b (mm) | 0.00 | 0.999 |
| LVEF (%) | −0.19 | <0.001* |
| MHI-5 score | −0.28 | <0.001* |
| PSQI score | 0.20 | <0.001* |
| Frail score | 0.33 | <0.001* |
The bivariate analysis of symptom burden in atrial fibrillation patients (N = 360).
BMI, body mass index; RFCA, radiofrequency catheter ablatsion; AF, atrial fibrillation; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; RA-t, right atrial transverse diameter; LA-ap, left atrial anteroposterior diameter; LVEDD, left ventricular end-diastolic diameter; RV-b, right ventricular transverse diameter; Hs-CRP, high-sensitivity C-reactive protein; AADs, anti-arrhythmic drugs; OSA, obstructive sleep apnea; COPD, chronic obstructive pulmonary disease; MHI-5:Mental Health Inventory-5; PSQI, The Pittsburgh Sleep Quality Index.
*p < 0.05.
Table 3
| Characteristic | β | SE | β’ | t | p |
|---|---|---|---|---|---|
| Common | 18.80 | 4.59 | 4.09 | 0.000 | |
| Sex (female) | 1.51 | 0.56 | 0.14 | 2.70 | 0.007* |
| LVEF (%) | −13.56 | 5.25 | −0.15 | −2.58 | 0.010* |
| MHI-5 score | −0.06 | 0.02 | −0.17 | −3.29 | 0.001* |
| Frail score | 0.68 | 0.24 | 0.17 | 2.83 | 0.005* |
| Heart failure | 0.72 | 0.87 | 0.05 | 0.83 | 0.406 |
| COPD | 2.52 | 1.86 | 0.07 | 1.35 | 0.177 |
| Coronary heart disease | 0.56 | 0.60 | 0.05 | 0.94 | 0.351 |
| AF type | 0.53 | 0.60 | 0.05 | 0.88 | 0.380 |
| BNP (pg/ml) | 0.00 | 0.00 | 0.03 | 0.44 | 0.663 |
| PSQI score | 0.09 | 0.07 | 0.07 | 1.28 | 0.201 |
| Hs-CRP (mg/L) | 0.00 | 0.04 | −0.01 | −0.10 | 0.923 |
| LA-ap (mm) | 0.00 | 0.06 | 0.00 | −0.03 | 0.973 |
Multiple linear regression for symptom burden of atrial fibrillation patients (N = 360).
R2 = 21.3%, adjusted R2 = 18.4%, F = 7.344, P < 0.001.
AF, atrial fibrillation; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; LA-ap, left atrial anteroposterior diameter; Hs-CRP, high-sensitivity C-reactive protein; COPD, chronic obstructive pulmonary disease; PSQI, The Pittsburgh Sleep Quality Index.
*p < 0.05.
3.3 Symptom network of atrial fibrillation patients after incorporating covariates
Symptoms and codes are named in Table 4. After incorporating covariates such as MHI-5 score, sex, left ventricular ejection fraction, and frail score into the symptom network, it was observed that the MHI-5 score was most closely related to S5 (edge weight = 0.2). Sex influenced all symptoms except S7 and S2. left ventricular ejection fraction was closely connected to S4 (edge weight = 0.13) and S2 (edge weight = 0.07). Frail score was closely linked to S4 (edge weight = 0.14) and S6 (edge weight = 0.04). There were also significant correlations between frail score, MHI-5 score, and sex (Figure 1).
Table 4
| Symptoms | Item |
|---|---|
| Palpitation | S1 |
| Shortness of breath at rest | S2 |
| Shortness of breath during physical activity | S3 |
| Exercise intolerance | S4 |
| Fatigue at rest | S5 |
| Dizziness | S6 |
| Chest pain | S7 |
Symptom code.
Figure 1
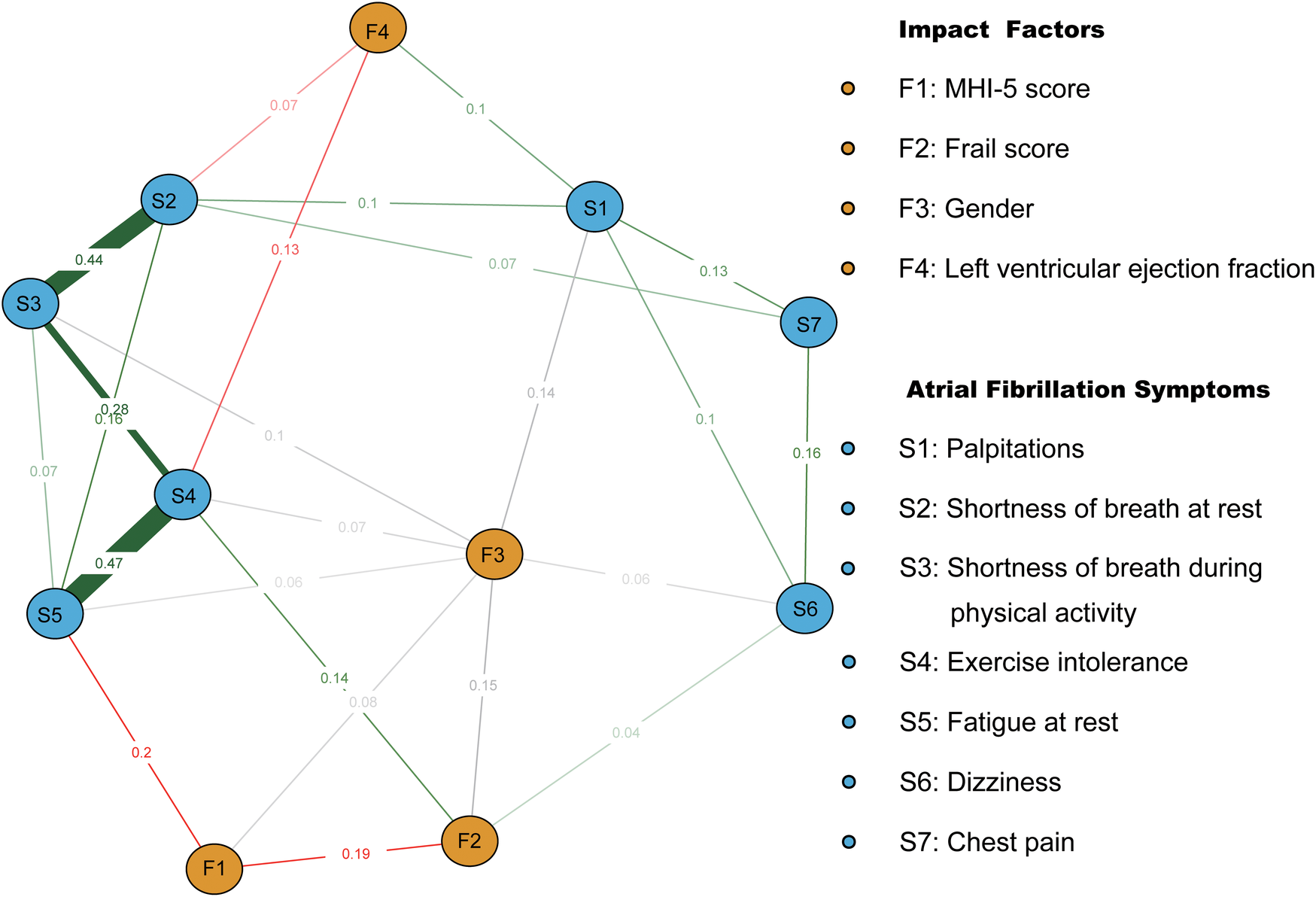
Symptom network of atrial fibrillation patients after incorporating covariates. Red and green line segments represent the association between continuity variables, with red line segments representing a negative correlation between nodes and green representing a positive correlation between nodes. Gray line segments indicate the relationship between categorical covariates and other nodes. Wider segments indicate stronger connectivity between the two.
3.4 The symptom network of atrial fibrillation patients after controlling for the covariates
We identified the top three symptom connections in the network: S4 with S5 (edge weight = 0.48), S2 with S3 (edge weight = 0.46), and S3 with S4 (edge weight = 0.28). Most symptoms showed positive correlations, except for the negative correlations between S2 and S4, S3 and S6, and S1 and S4 (Figure 2). The three strongest “Strength” indicators were S3 (node strength = 0.96), S2 (node strength = 0.88), and S4 (node strength = 0.86). This suggests that the core symptoms of atrial fibrillation patients are shortness of breath during physical activity and at rest (Figure 3A).
Figure 2
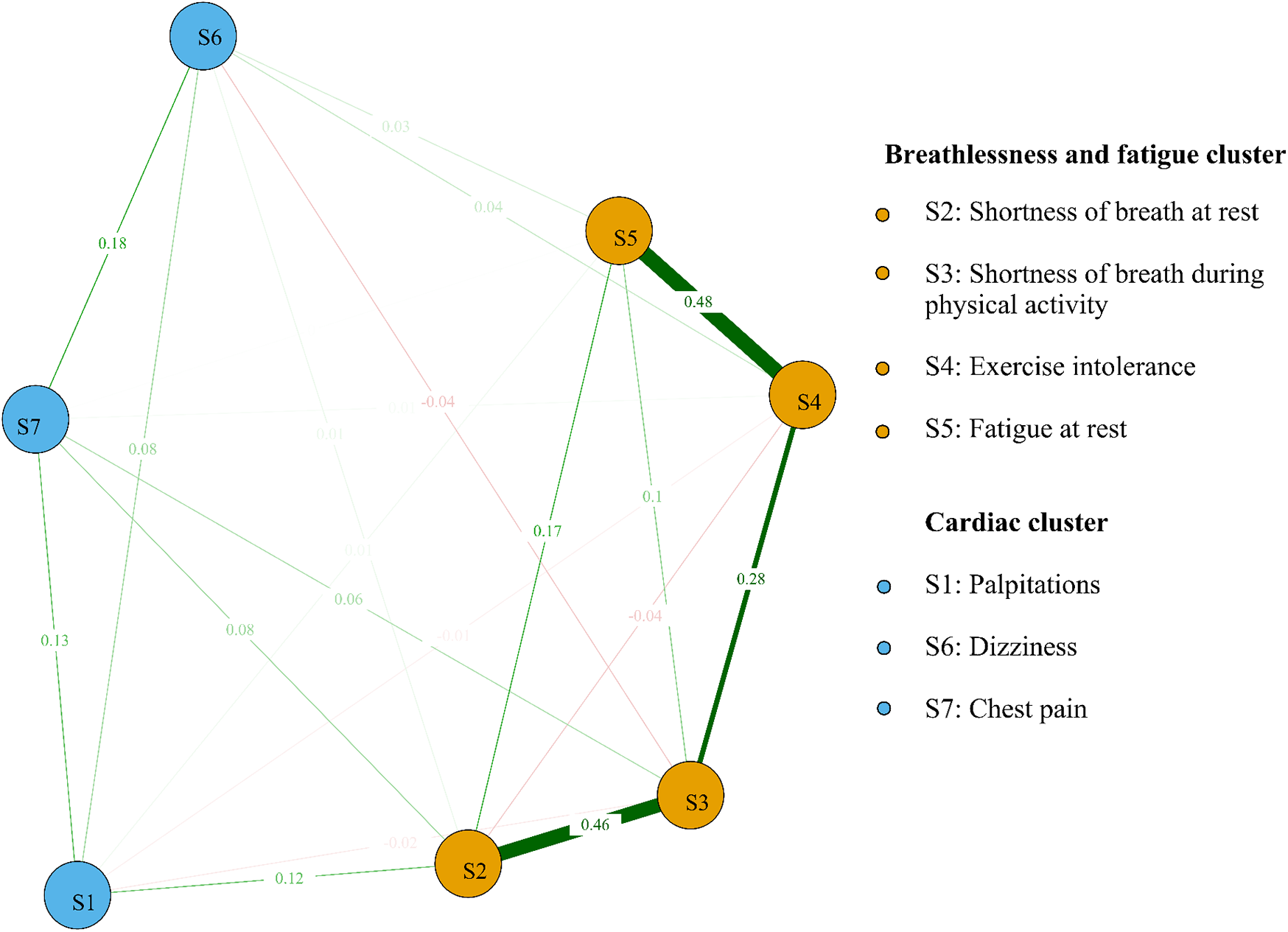
Symptom network and clusters of atrial fibrillation patients. Red line segments represent a negative correlation between nodes, and green represents a positive correlation between nodes. Wider segments indicate stronger connectivity between the two nodes.
Figure 3
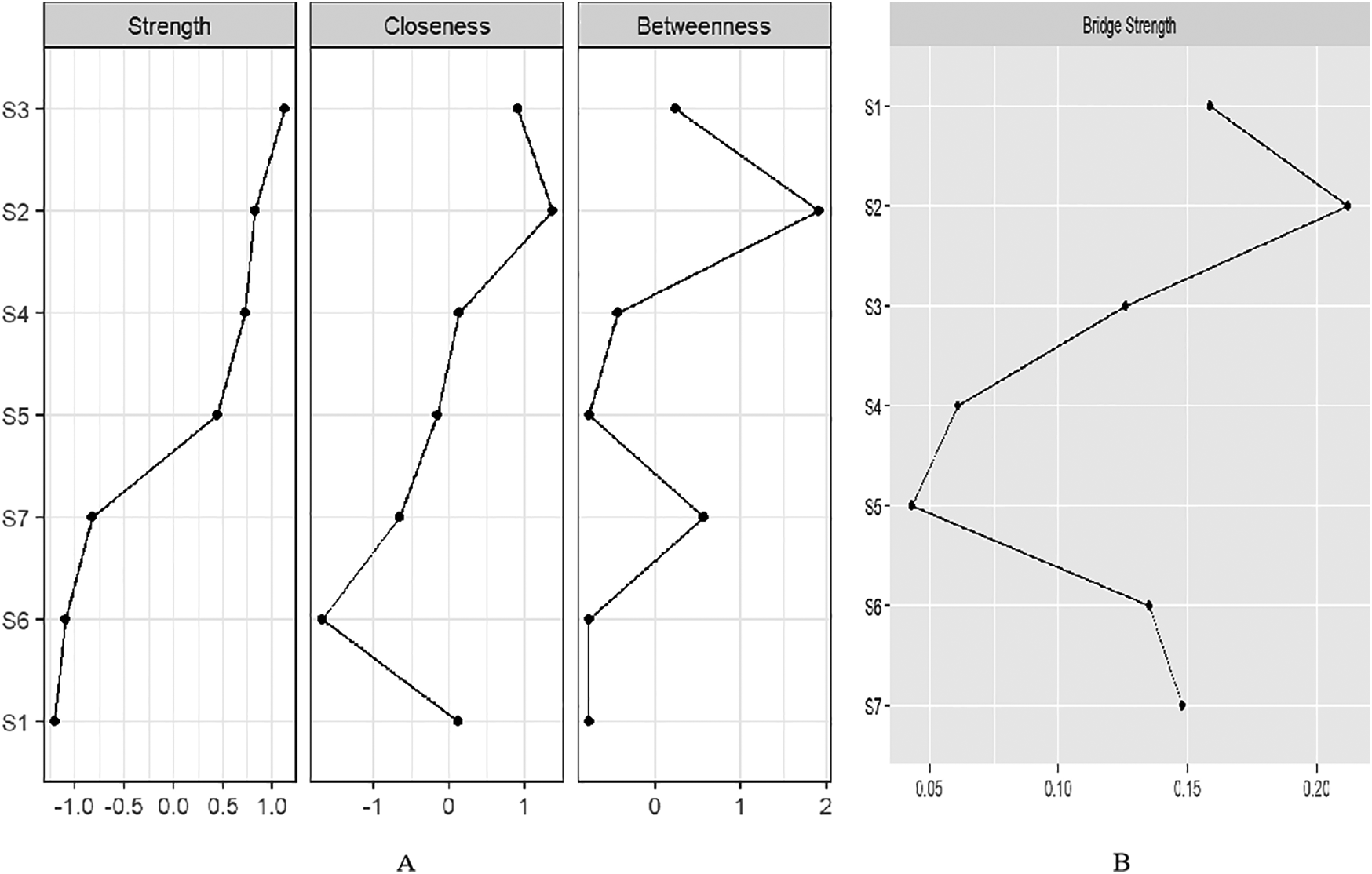
(A) Centrality indices of “Strength”, “Closeness”, and “Betweenness” for 7 symptoms ordered by “Strength”. (B) Bridge strength of symptom nodes.
We identified two symptom clusters: “cardiac cluster” (S1, S6, S7) and “breathlessness and fatigue cluster” (S2, S3, S4, S5) (Figure 2). The top three symptoms with the highest bridge strength were S2 (node bridge strength = 0.21), S1 (node bridge strength = 0.16), and S7 (node bridge strength = 0.15), indicating their role in linking the clusters (Figure 3B).
3.5 Accuracy and stability of the symptom network
Bootstrap confidence intervals for edge weights were relatively narrow, indicating good accuracy of the symptom network (Figure 4). In this study, the CS values for symptom network “strength” centrality were 0.75. These values indicate good stability for symptom node centrality (Figure 5).
Figure 4
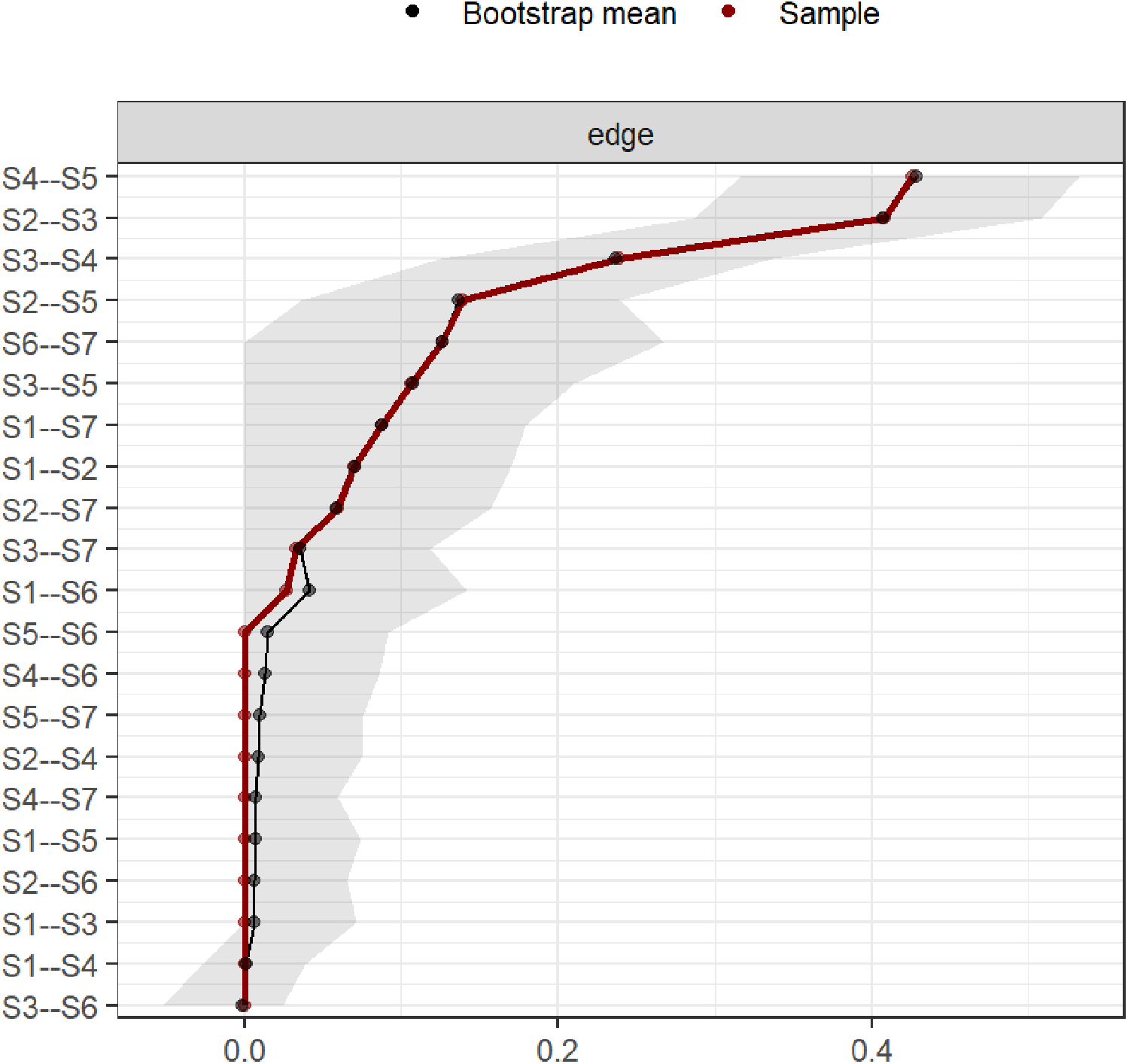
Bootstrap analysis results of edge weights.
Figure 5
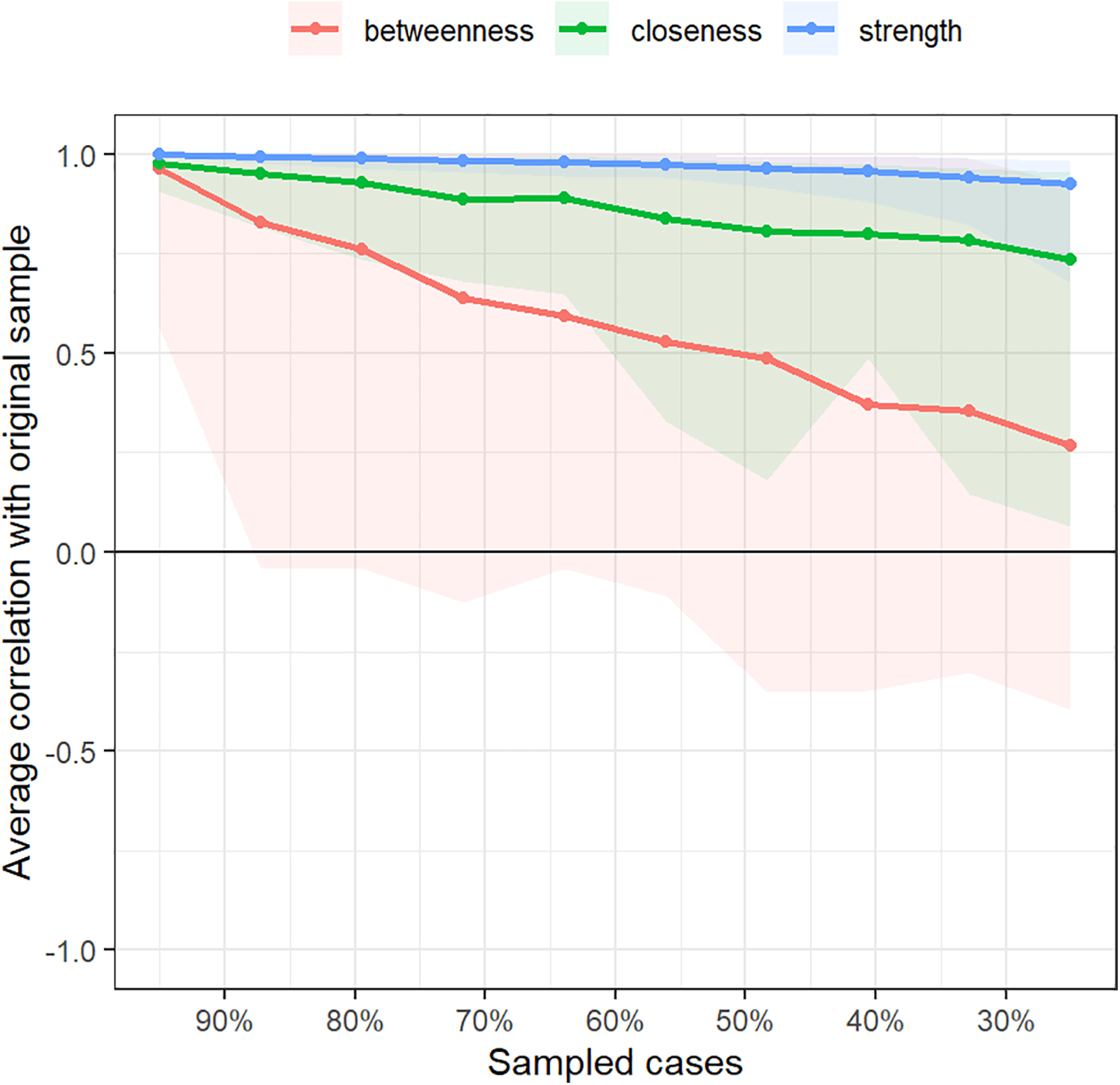
Correlation stability coefficient for strength, betweenness, and closeness of symptom network.
4 Discussion
We observed that worsening mental health status was linked to a higher symptom burden, which aligns with previous research (25). Negative emotions were found to be strong predictors of symptom severity in atrial fibrillation patients (26), affecting symptom perception through attentional bias (27) and psychological rumination (28), often leading to an overestimation of symptom frequency and severity. The study also found a strong association between mental health status and Shortness of breath at rest, similar to Yu (29). This may be related to the chronic low-grade inflammation in atrial fibrillation, which sensitizes the amygdala circuit, activates the neuroimmune network, and induces autonomic hyperreactivity, resulting in difficulty breathing and intolerance to exercise (30). Therefore, healthcare professionals should recognize the impact of emotional distress on symptomatology in atrial fibrillation patients and provide proactive psychological support.
Female patients experience a more severe symptom burden compared to males, a phenomenon supported by numerous studies (31). This may be due to them less likely to be treated with rhythm control strategies (32), more extensive low-voltage areas in the left atrium, higher rates of complex fractionated atrial electrograms (33), and more severe atrial fibrosis in females (34), leading to more pronounced atrial remodeling and increased symptom burden. Furthermore, the study finds that sex has a broad impact on atrial fibrillation symptoms, although the mechanisms remain unclear and require further exploration.
Left ventricular ejection fraction is closely associated with symptoms of exercise intolerance and shortness of breath at rest. The left ventricular ejection fraction reflects the heart's ability to pump blood and its overall function. During atrial fibrillation episodes, hemodynamic changes lead to inadequate blood ejection, causing symptoms like dyspnea and exercise intolerance (35–39). In addition, lower left ventricular ejection fraction is closely linked to persistent atrial fibrillation which experiences more pronounced symptoms of fatigue and exercise intolerance (40).
The more severe the frailty, the heavier the symptom burden, similar to findings in the Slawuta study (41). Frailty, caused by factors such as metabolic and neuroimmune dysfunction, may contribute to increased symptom burden by promoting atrial remodeling through chronic inflammatory responses (42). Covariate-controlled symptom networks show a closer association between frailty and exercise intolerance, as well as chest pain. One possible reason is the similarity between symptoms of atrial fibrillation and frailty, with impaired physical activity and mobility being key features of frailty. Moreover, both conditions share pathological mechanisms such as inflammation and oxidative stress (43). Frailty is also often characterized by a decline in skeletal muscle quantity and quality, which may contribute to exercise intolerance (42). Additionally, covariate-controlled symptom networks indicate a strong association between frailty and mental health status, as well as sex, highlighting the need for multidisciplinary interventions addressing various aspects such as sex and psychological factors to effectively manage symptoms in atrial fibrillation patients with frailty and improve the symptom network.
After controlling for confounding factors and covariates, the network shows that palpitations are negatively correlated with exercise intolerance, while dizziness and exercise intolerance are negatively correlated with shortness of breath. This may be due to atrial fibrillation reducing cardiac output (35–39), leading to symptoms such as exercise intolerance and dizziness. At the same time, reduced cardiac output may decrease pulmonary circulation congestion, which could, in turn, alleviate shortness of breath at rest.
We found that the core symptoms in atrial fibrillation patients were shortness of breath during physical activity and at rest. This is consistent with our previous study (17), highlighting the stability of core symptoms and their representative role in symptomatology. Core symptoms can trigger a range of connected symptoms that can signal the start or worsening of other issues (14). During atrial fibrillation episodes, irregular atrial contractions and reduced ventricular diastolic time decrease effective cardiac output, causing compensatory shortness of breath (35, 36). Hemodynamic changes may increase left ventricular filling pressure, contributing to exercise intolerance (37). Furthermore, atrial fibrillation patients often have endothelial dysfunction and impaired peripheral muscle oxygen uptake, which, along with hemodynamic changes, can result in fatigue and weakness due to altered muscle sensing (38, 39). We identified two symptom clusters involving “cardiac cluster” and “breathlessness and fatigue cluster” in patients with atrial fibrillation, similar to Streur (44). “Shortness of breath at rest”, “palpitations” and “chest pain” were a bridge of clusters with high “bridge strength”. These symptoms can be targeted to improve overall symptom management (14). Breathing difficulties can worsen chest pain by affecting the sympathetic nervous system (37), triggering a cardiac symptom cluster. Additionally, palpitations and chest pain may decrease the desire for physical activity in atrial fibrillation patients, potentially leading to long-term muscle changes and exercise intolerance.
Our study suggests that focusing solely on controlling heart rate and rhythm in atrial fibrillation patients may not be enough (1). Paying attention to core symptoms like shortness of breath could help improve the entire symptom network. Although evidence is limited, treatments such as an ablation procedure and moderate exercise may help ease core symptoms and improve overall symptom management (35, 45).
4.1 Strengths and limitations
Using network analysis, we identified the mechanisms linking symptoms and influencing factors. By calculating centrality measures and bridge strength, we were able to pinpoint core and bridging symptoms, which helps in understanding the emergence of symptoms. This approach provides a better understanding of atrial fibrillation symptoms. However, this study has several limitations: it was a single-center investigation with a small sample size, limiting generalizability; its cross-sectional design reduces the strength of evidence regarding relationships between atrial fibrillation symptoms and other factors; and the use of convenience sampling may have introduced selection bias. Longitudinal studies would better elucidate symptom progression and causal relationships with influencing factors.
5 Conclusion
Employing symptom networks helps uncover the underlying mechanisms behind symptom occurrence, providing a clearer path for identifying targets for symptom management. Mental health was most closely related to “fatigue at rest”. Sex influenced all symptoms except “dizziness” and “shortness of breath at rest”. Left ventricular ejection fraction was closely connected to “exercise intolerance” and “shortness of breath at rest”, while the frail score was closely linked to “exercise intolerance” and “dizziness”. Shortness of breath during physical activity and at rest are identified as core symptoms, while “shortness of breath at rest”, “palpations” and “chest pain” serve as bridging symptoms.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Tianjin Medical University General Hospital [Approval No. (IRB2023-WZ-111)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XY: Conceptualization, Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. HL: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. GL: Data curation, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received funding from The Incubation Project Fund of Mianyang Central Hospital (2023FH004), China's National Clinical Key Specialty Project (XHZDZK013), and Humanities Research Center of Zigong Key Research Base for Philosophy and Social Sciences(JKRWY24-14).
Acknowledgments
We thank all the participants for their contribution to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomstrom-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
2.
Shi S Tang Y Zhao Q Yan H Yu B Zheng Q et al Prevalence and risk of atrial fibrillation in China: a national cross-sectional epidemiological study. Lancet Reg Health West Pac. (2022) 23:100439. 10.1016/j.lanwpc.2022.100439
3.
Frost L Vestergaard P Mosekilde L . Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. (2004) 164(15):1675–8. 10.1001/archinte.164.15.1675
4.
Palyam V Azam AT Odeyinka O Alhashimi R Thoota S Ashok T et al Hypertrophic cardiomyopathy and atrial fibrillation: a review. Cureus. (2022) 14(1):e21101. 10.7759/cureus.21101
5.
Chamberlain AM Agarwal SK Folsom AR Duval S Soliman EZ Ambrose M et al Smoking and incidence of atrial fibrillation: results from the atherosclerosis risk in communities (ARIC) study. Heart Rhythm. (2011) 8(8):1160–6. 10.1016/j.hrthm.2011.03.038
6.
Mont L Elosua R Brugada J . Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace. (2009) 11(1):11–7. 10.1093/europace/eun289
7.
Mascia G Arbelo E Solimene F Giaccardi M Brugada R Brugada J . The long-QT syndrome and exercise practice: the never-ending debate. J Cardiovasc Electrophysiol. (2018) 29(3):489–96. 10.1111/jce.13410
8.
Streur M . Atrial fibrillation symptom perception. J Nurse Pract. (2019) 15(1):60–4. 10.1016/j.nurpra.2018.08.015
9.
Sadlonova M Senges J Nagel J Celano C Klasen-Max C Borggrefe M et al Symptom severity and health-related quality of life in patients with atrial fibrillation: findings from the observational ARENA study. J Clin Med. (2022) 11(4):1140. 10.3390/jcm11041140
10.
Charitakis E Barmano N Walfridsson U Walfridsson H . Factors predicting arrhythmia-related symptoms and health-related quality of life in patients referred for radiofrequency ablation of atrial fibrillation: an observational study (the SMURF study). JACC Clin Electrophysiol. (2017) 3(5):494–502. 10.1016/j.jacep.2016.12.004
11.
von Eisenhart Rothe A Hutt F Baumert J Breithardt G Goette A Kirchhof P et al Depressed mood amplifies heart-related symptoms in persistent and paroxysmal atrial fibrillation patients: a longitudinal analysis–data from the German competence network on atrial fibrillation. Europace. (2015) 17(9):1354–62. 10.1093/europace/euv018
12.
Schnabel RB Pecen L Rzayeva N Lucerna M Purmah Y Ojeda FM et al Symptom burden of atrial fibrillation and its relation to interventions and outcome in Europe. J Am Heart Assoc. (2018) 7(11):e007559. 10.1161/JAHA.117.007559
13.
Isvoranu AM van Borkulo CD Boyette LL Wigman JT Vinkers CH Borsboom D et al A network approach to psychosis: pathways between childhood trauma and psychotic symptoms. Schizophr Bull. (2017) 43(1):187–96. 10.1093/schbul/sbw055
14.
Zhu Z Sun Y Kuang Y Yuan X Gu H Zhu J et al Contemporaneous symptom networks of multidimensional symptom experiences in cancer survivors: a network analysis. Cancer Med. (2023) 12(1):663–73. 10.1002/cam4.4904
15.
Bard HA O'Driscoll C Miller CB Henry AL Cape J Espie CA . Insomnia, depression, and anxiety symptoms interact and individually impact functioning: a network and relative importance analysis in the context of insomnia. Sleep Med. (2023) 101:505–14. 10.1016/j.sleep.2022.12.005
16.
Henneghan A Wright ML Bourne G Sales AC . A cross-sectional exploration of cytokine-symptom networks in breast cancer survivors using network analysis. Can J Nurs Res. (2021) 53(3):303–15. 10.1177/0844562120927535
17.
Lin H Luo H Lin M Li H Sun D . Symptom network and clusters of the multidimensional symptom experience in patients with atrial fibrillation. J Cardiovasc Nurs. (2025) 40(5):441–51. 10.1097/JCN.0000000000001133
18.
Yang PS Sung JH Jang E Yu HT Kim TH Lip GYH et al Application of the simple atrial fibrillation better care pathway for integrated care management in frail patients with atrial fibrillation: a nationwide cohort study. J Arrhythm. (2020) 36(4):668–77. 10.1002/joa3.12364
19.
Constantin M . Sample Size Recommendations for Estimating Cross-Sectional Network Models. (2018).
20.
Dorian P Burk C Mullin CM Bubien R Godejohn D Reynolds MR et al Interpreting changes in quality of life in atrial fibrillation: how much change is meaningful? Am Heart J. (2013) 166(2):381–7.e8. 10.1016/j.ahj.2013.04.015
21.
Li L Wang HM Shen Y . Chinese SF-36 health survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. (2003) 57(4):259–63. 10.1136/jech.57.4.259
22.
Ho KY Lam KKW Xia W Chung JOK Cheung AT Ho LLK et al Psychometric properties of the Chinese version of the Pittsburgh sleep quality Index (PSQI) among Hong Kong Chinese childhood cancer survivors. Health Qual Life Outcomes. (2021) 19(1):176. 10.1186/s12955-021-01803-y
23.
Li Y Zou Y Wang S Li J Jing X Yang M et al A pilot study of the FRAIL scale on predicting outcomes in Chinese elderly people with type 2 diabetes. J Am Med Dir Assoc. (2015) 16(8):714.e7–.e12. 10.1016/j.jamda.2015.05.019
24.
Li X Tian Y Yang J Ning M Chen Z Yu Q et al Network of job demands-resources and depressive symptoms in critical care nurses: a nationwide cross-sectional study. Critical Care (London, England). (2025) 29(1):39. 10.1186/s13054-025-05282-1
25.
Yu SB Hu W Zhao QY Qin M Huang H Cui HY et al Effect of anxiety and depression on the recurrence of persistent atrial fibrillation after circumferential pulmonary vein ablation. Chin Med J. (2012) 125(24):4368–72.
26.
Wheelock KM Kratz A Lathkar-Pradhan S Najarian K Gryak J Li Z et al Association between symptoms, affect and heart rhythm in patients with persistent or paroxysmal atrial fibrillation: an ambulatory pilot study. Am Heart J. (2021) 241:1–5. 10.1016/j.ahj.2021.06.003
27.
Alexeeva I Martin M . Evidence for mood-dependent attentional processing in asthma: attentional bias towards health-threat in depressive mood and attentional avoidance in neutral mood. J Behav Med. (2018) 41(4):550–67. 10.1007/s10865-018-9919-6
28.
Nolen-Hoeksema S Wisco B Lyubomirsky S . Rethinking rumination. Perspect Psychol Sci. (2008) 3(5):400–24. 10.1111/j.1745-6924.2008.00088.x
29.
Yu DS Lee DT Woo J Thompson DR . Correlates of psychological distress in elderly patients with congestive heart failure. J Psychosom Res. (2004) 57(6):573–81. 10.1016/j.jpsychores.2004.04.368
30.
Nusslock R Miller GE . Early-Life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. (2016) 80(1):23–32. 10.1016/j.biopsych.2015.05.017
31.
Gleason KT Dennison Himmelfarb CR Ford DE Lehmann H Samuel L Han HR et al Association of sex, age and education level with patient reported outcomes in atrial fibrillation. BMC Cardiovasc Disord. (2019) 19(1):85. 10.1186/s12872-019-1059-6
32.
Ikemura N Kohsaka S Kimura T Ueda I Katsumata Y Nishiyama T et al Assessment of sex differences in the initial symptom burden, applied treatment strategy, and quality of life in Japanese patients with atrial fibrillation. JAMA Netw Open. (2019) 2(3):e191145. 10.1001/jamanetworkopen.2019.1145
33.
Wong GR Nalliah CJ Lee G Voskoboinik A Chieng D Prabhu S et al Sex-Related differences in atrial remodeling in patients with atrial fibrillation: relationship to ablation outcomes. Circ Arrhythm Electrophysiol. (2022) 15(1):e009925. 10.1161/CIRCEP.121.009925
34.
Li Z Wang Z Yin Z Zhang Y Xue X Han J et al Gender differences in fibrosis remodeling in patients with long-standing persistent atrial fibrillation. Oncotarget. (2017) 8(32):53714–29. 10.18632/oncotarget.16342
35.
Keteyian SJ Ehrman JK Fuller B Pack QR . Exercise testing and exercise rehabilitation for patients with atrial fibrillation. J Cardiopulm Rehabil Prev. (2019) 39(2):65–72. 10.1097/HCR.0000000000000423
36.
Guazzi M Belletti S Tumminello G Fiorentini C Guazzi MD . Exercise hyperventilation, dyspnea sensation, and ergoreflex activation in lone atrial fibrillation. Am J Physiol Heart Circ Physiol. (2004) 287(6):H2899–905. 10.1152/ajpheart.00455.2004
37.
Lindgren TG Fukuoka Y Rankin SH Cooper BA Carroll D Munn YL . Cluster analysis of elderly cardiac patients’ prehospital symptomatology. Nurs Res. (2008) 57(1):14–23. 10.1097/01.NNR.0000280654.50642.1a
38.
Qin S Boidin M Buckley BJR Lip GYH Thijssen DHJ . Endothelial dysfunction and vascular maladaptation in atrial fibrillation. Eur J Clin Investig. (2021) 51(5):e13477. 10.1111/eci.13477
39.
Dubé BP Agostoni P Laveneziana P . Exertional dyspnoea in chronic heart failure: the role of the lung and respiratory mechanical factors. Eur Respir Rev. (2016) 25(141):317–32. 10.1183/16000617.0048-2016
40.
Inohara T Kim S Pieper K Blanco RG Allen LA Fonarow GC et al B-type natriuretic peptide, disease progression and clinical outcomes in atrial fibrillation. Heart. (2019) 105(5):370–7. 10.1136/heartjnl-2018-313642
41.
Slawuta A Jacek P Mazur G Jankowska-Polanska B . Quality of life and frailty syndrome in patients with atrial fibrillation. Clin Interv Aging. (2020) 15:783–95. 10.2147/CIA.S248170
42.
Kranert M Shchetynska-Marinova T Berghoff T Liebe V Doesch C Papavassiliu T et al Arterial stiffness is associated with increased symptom burden in patients with atrial fibrillation. Can J Cardiol. (2020) 36(12):1949–55. 10.1016/j.cjca.2020.08.022
43.
Koca M Yavuz BB Tuna Doğrul R Çalışkan H Şengül Ayçiçek G Özsürekçi C et al Impact of atrial fibrillation on frailty and functionality in older adults. Ir J Med Sci. (2020) 189(3):917–24. 10.1007/s11845-020-02190-x
44.
Streur M Ratcliffe SJ Ball J Stewart S Riegel B . Symptom clusters in adults with chronic atrial fibrillation. J Cardiovasc Nurs. (2017) 32(3):296–303. 10.1097/JCN.0000000000000344
45.
Walfridsson U Hassel Jönsson A Karlsson LO Liuba I Almroth H Sandgren E et al Symptoms and health-related quality of life 5 years after catheter ablation of atrial fibrillation. Clin Cardiol. (2022) 45(1):42–50. 10.1002/clc.23752
Summary
Keywords
atrial fibrillation, symptom network, symptom cluster, frail, mental health
Citation
Sun D, Yang X, Li H, Li G and Lin H (2025) Core and bridging symptoms in patients with atrial fibrillation: a network analysis. Front. Cardiovasc. Med. 12:1617872. doi: 10.3389/fcvm.2025.1617872
Received
27 April 2025
Accepted
14 October 2025
Published
07 November 2025
Volume
12 - 2025
Edited by
Junjie Xiao, Shanghai University, China
Reviewed by
Giuseppe Mascia, University of Genoa, Italy
Dimitrios Tachmatzidis, Aristotle University of Thessaloniki, Greece
Jheng-Yan Wu, Chi Mei Medical Center, Taiwan
Updates
Copyright
© 2025 Sun, Yang, Li, Li and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Hairong Lin 1273373111@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.