Abstract
The mechanistic target of rapamycin (mTOR) signaling pathway is a central regulator of cellular physiology, modulating processes such as metabolism, protein synthesis, growth, and various forms of cell death. Increasing evidence has revealed that dysregulation of mTOR activity, often triggered or exacerbated by aberrant post-translational modifications (PTMs), contributes to the onset and progression of cardiovascular diseases (CVDs), including atherosclerosis, myocardial infarction, heart failure, and ischemia-reperfusion injury. PTMs such as phosphorylation, ubiquitination, SUMOylation, acetylation, and glycosylation alter mTOR's upstream regulators and downstream effectors, influencing the balance between apoptosis, autophagy, pyroptosis, and ferroptosis. These regulatory mechanisms provide a molecular basis for cell fate decisions during cardiovascular stress and injury. In this review, we systematically summarize recent advances in the understanding of PTM-mediated control of mTOR signaling, with a focus on cardiovascular pathophysiology. We also highlight emerging therapeutic strategies that target PTMs or the mTOR axis, including mTOR inhibitors, AMPK activators, proteasome blockers, and SUMOylation modulators, all of which show promise in preclinical or clinical settings. Understanding how PTMs fine-tune mTOR activity and cell death may pave the way for novel, targeted interventions in cardiovascular medicine and offer potential avenues for the development of precision therapies.
1 Introduction
Cardiovascular diseases (CVDs), including atherosclerosis, myocardial infarction, and aortic dissection (AD), remain the leading cause of mortality worldwide, posing a significant global health challenge (1, 2). These conditions arise from complex interactions among inflammation, oxidative stress, and cell death, which encompasses both non-programmed forms, such as necrosis, and various types of programmed cell death (PCD), including apoptosis, ferroptosis, pyroptosis, and autophagy-dependent cell death (3–5). While necrosis primarily contributes to acute injuries, PCD plays a pivotal role in chronic pathological remodeling and tissue degeneration (6). Moreover, recent studies highlight the interplay between cell death modalities in CVDs, mediated by shared mechanisms such as oxidative stress and inflammatory signaling (7, 8). For example, necrosis-induced release of damage-associated molecular patterns (DAMPs) can exacerbate inflammation, triggering ferroptosis or pyroptosis and amplifying cardiovascular damage (9, 10). These interconnected processes underscore the critical need for a comprehensive understanding of the regulatory pathways that govern cell death in cardiovascular pathophysiology.
The mammalian target of rapamycin (mTOR) signaling pathway, a master regulator of cellular growth, metabolism, and survival, has emerged as a pivotal modulator of cell death in CVDs (11, 12). In addition to its canonical roles, mTOR signaling is intricately regulated by post-translational protein modifications such as phosphorylation, ubiquitination, and SUMOylation (13, 14). These modifications serve as molecular switches, bridging upstream stimuli and downstream responses, including oxidative stress regulation and ferroptosis (15). Investigating these regulatory mechanisms not only deepens our understanding of CVD progression but also opens new avenues for therapeutic interventions.
By focusing on the interplay between mTOR signaling, post-translational protein modifications, and cell death, this review provides novel insights into the pathogenesis of CVDs. These findings emphasize the potential for targeting these pathways to mitigate oxidative stress and cell death, ultimately advancing the development of innovative strategies for CVD treatment and prevention.
2 The role of mTOR signaling in cardiovascular diseases
mTOR is a highly conserved serine/threonine kinase that plays a central role in regulating cellular processes such as growth, metabolism, proliferation, and survival (16, 17). It integrates signals from nutrients, energy status, and growth factors to modulate key cellular activities, thereby ensuring proper cellular function in response to environmental changes (18). mTOR operates through two distinct complexes, mTORC1 and mTORC2, each of which plays specific roles in cellular regulation (19).
2.1 mTOR function and regulation
mTORC1 is the more extensively studied of the two complexes and is a key regulator of cell growth and metabolism (Figure 1) (20, 21). It promotes protein synthesis by activating key effectors such as S6K1 (ribosomal protein S6 kinase 1) and 4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1), which control translation initiation and protein synthesis. mTORC1 also regulates lipid biosynthesis and autophagy, coordinating these processes with nutrient availability (21–25). One of the key mechanisms by which mTORC1 exerts its effects is through the regulation of the AMPK (AMP-activated protein kinase) pathway, which responds to cellular energy levels (26, 27). In states of nutrient deficiency or stress, AMPK is activated and inhibits mTORC1, promoting energy conservation through autophagy (28, 29).
Figure 1
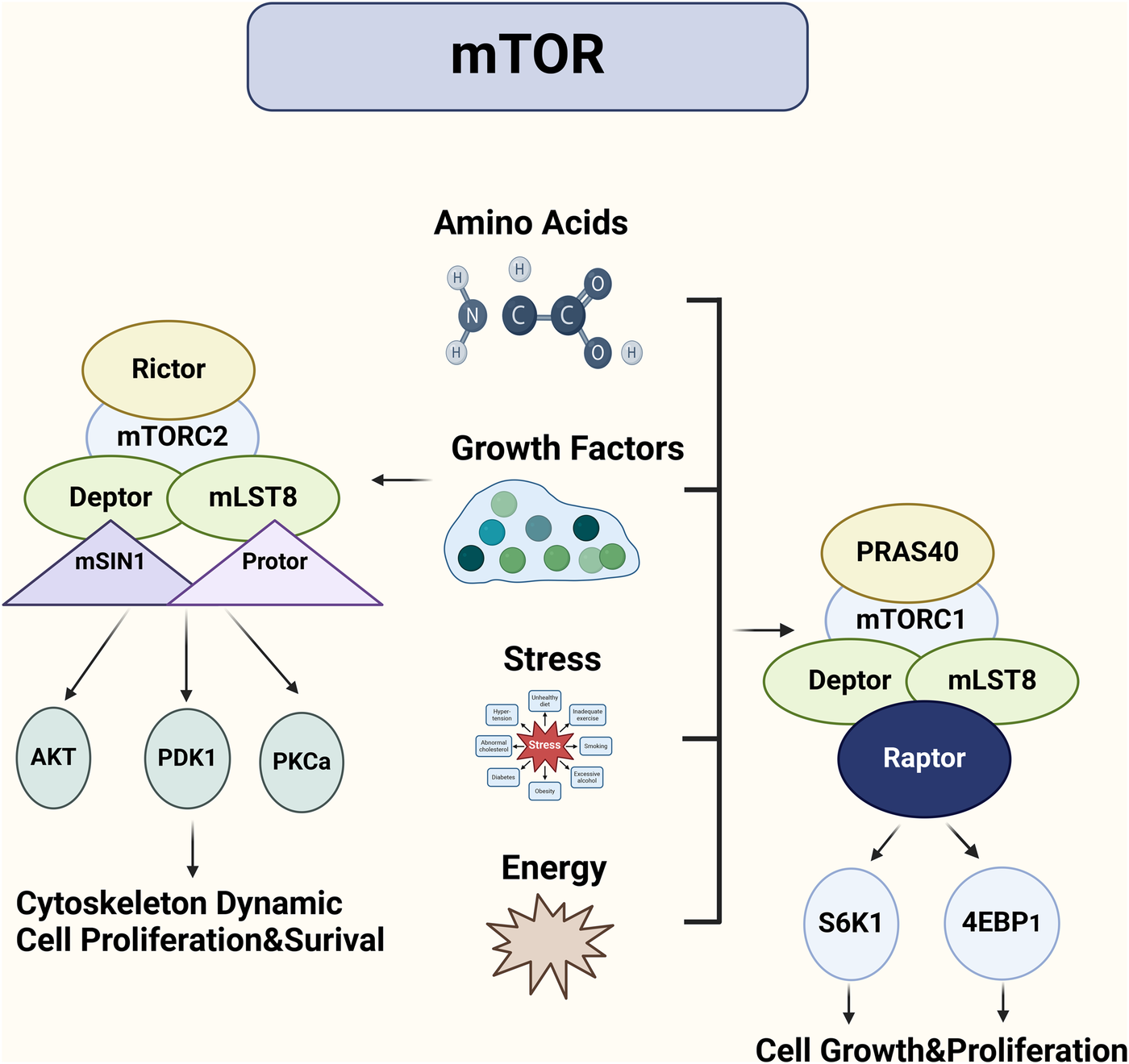
mTORC1 and mTORC2: distinct complexes regulating cell growth and survival in cardiovascular contexts. The mTOR signaling pathway regulates cell growth through two complexes: mTORC1 and mTORC2. mTORC1, acting as a highly sensitive sensor of growth factors and nutrients, is primarily responsible for promoting cell growth and proliferation. It activates protein synthesis by phosphorylating S6K and 4E-BP1. In contrast, mTORC2 is more involved in cell survival, metabolism, and cytoskeletal remodeling. By phosphorylating AKT/PKB, mTORC2 promotes cell survival and influences cell shape and movement.
A study demonstrated the role of mTORC1 in muscle growth and metabolism, showing that mTORC1 activity is a critical determinant of protein synthesis in muscle cells (30). Furthermore, mTORC1 activity has been shown to be regulated by the availability of amino acids, particularly leucine, which acts as a signal for mTORC1 activation to promote cell growth and proliferation (31, 32). This has significant implications in contexts like cancer, where excessive mTORC1 activation contributes to uncontrolled cell proliferation (Figure 1) (33, 34).
mTORC2, in contrast to mTORC1, primarily regulates cell survival, cytoskeletal organization, and metabolism, and its role is less well understood (35, 36). One of the key functions of mTORC2 is the phosphorylation of Akt, a central regulator of cell survival and metabolism (37). Akt activation promotes glucose uptake, inhibits apoptosis, and supports cell proliferation. mTORC2 also regulates the actin cytoskeleton, influencing cell shape and motility, which is particularly important in cancer metastasis and tissue regeneration (38, 39).
A recent study emphasized the role of mTORC2 in endothelial cell function, showing that mTORC2 inhibition leads to a reduction in vascular remodeling, indicating its critical involvement in endothelial cell survival and function in vascular diseases (40).Additionally, mTORC2-mediated phosphorylation of Akt has been implicated in the development of atherosclerosis, where it contributes to endothelial dysfunction and vascular inflammation (41, 42).
2.2 mTOR in CVDs
The mechanistic target of rapamycin (mTOR) plays a multifaceted role in the pathogenesis of CVDs, influencing cellular processes such as growth, metabolism, proliferation, and survival (43). mTOR is involved in regulating oxidative stress, inflammation, cell death, and vascular remodeling, all of which are crucial in the development and progression of CVDs (44, 45). Its activity, however, varies between different cardiovascular conditions, and dysregulation of mTOR signaling is associated with several pathological processes, including atherosclerosis, myocardial infarction, heart failure, and coronary artery disease (46).
In atherosclerosis, mTOR activation drives several processes contributing to plaque formation and instability (47). It promotes the proliferation and migration of vascular smooth muscle cells (VSMCs), facilitating the formation of the fibrous cap and contributing to plaque expansion (48). mTOR also influences endothelial cell function, increasing endothelial permeability and inducing oxidative stress, which exacerbates vascular inflammation and promotes foam cell formation (44, 49). Inflammatory cytokines, such as TNF-α, and factors like advanced glycation end products (AGEs) also contribute to mTOR activation, further enhancing the inflammatory environment within plaques (50, 51). This persistent activation of mTOR accelerates the development of atherosclerosis, increases plaque vulnerability, and contributes to plaque rupture, a key event in the pathogenesis of acute coronary events (52).
mTOR's role in myocardial infarction (MI) and heart failure is similarly complex (53, 54). After MI, mTOR signaling is involved in myocardial hypertrophy, fibrosis, and tissue remodeling (55). It promotes the production of extracellular matrix proteins, such as collagen, and inhibits autophagy, a process critical for clearing damaged cellular components (56). This results in increased fibrosis and scarring, impairing the heart's ability to regenerate and repair itself (56). While mTOR activation can initially promote hypertrophic responses in the myocardium as an adaptive mechanism to stress, prolonged activation contributes to pathological remodeling, including ventricular dilation and heart failure (57, 58). Moreover, excessive mTOR signaling can exacerbate ischemic injury by promoting oxidative stress and inflammation, which leads to further tissue damage (59).
In coronary artery disease (CAD), mTOR signaling is integral to the regulation of endothelial function, vascular tone, and smooth muscle cell behavior (Table 1) (60). mTOR modulates the response to oxidative stress in endothelial cells, contributing to endothelial dysfunction, a key early event in CAD (61). mTOR also plays a role in VSMC proliferation and migration, which are important in the development of neointimal hyperplasia following vascular injury (62). In this regard, mTOR regulates the expression of matrix metalloproteinases (MMPs) and other enzymes involved in extracellular matrix remodeling, leading to changes in vascular wall structure and increased susceptibility to rupture in atherosclerotic lesions (63, 64). Furthermore, the activation of mTOR in the context of CAD is associated with an imbalance in the vascular response to stress and injury, ultimately promoting the progression of the disease (65).
Table 1
| Disease | mTORC1-mediated mechanism | mTORC2-mediated mechanism | References |
|---|---|---|---|
| Atherosclerosis | Promotes inflammation, increases ROS production, facilitates foam cell formation, accelerates plaque growth | Improves endothelial function, inhibits inflammation, reduces vascular damage | (66, 67) |
| Aortic dissection | Activates MMPs, promotes vascular wall degeneration, increases oxidative stress, leads to vascular structural instability | Promotes vascular repair, inhibits endothelial cell damage | (68, 69) |
| Myocardial infarction | Increases oxidative stress and cell death, exacerbates ischemia-reperfusion injury | Protects cardiomyocyte survival, improves heart function, reduces oxidative stress damage | (53) |
| Hypertension | Promotes vasoconstriction, increases vascular tension, induces endothelial dysfunction | Enhances endothelial relaxation function, reduces hypertension-induced vascular damage | (70, 71) |
| Heart failure | Activates fibrotic pathways, causes myocardial remodeling, increases energy metabolism dysregulation | Protects cardiomyocytes, regulates energy metabolism adaptation, alleviates cardiac burden | (72, 73) |
| Diabetes-related cardiovascular disease | Promotes lipid accumulation, exacerbates oxidative stress, increases the risk of arteriosclerosis | Improves glucose metabolism homeostasis, alleviates cardiovascular damage caused by diabetes | (74, 75) |
| Cardiac hypertrophy | Promotes protein synthesis, accelerates cardiomyocyte hypertrophy, increases oxidative stress | Regulates antioxidant mechanisms, reduces ROS accumulation in cells, inhibits cardiac hypertrophy | (76, 77) |
| Arrhythmia | Causes calcium overload, disrupts myocardial electrical activity stability, increases the risk of arrhythmia | Maintains intracellular calcium homeostasis, reduces oxidative stress-induced electrical activity abnormalities | (78, 79) |
Functional differences between mTORC1 and mTORC2 in cardiovascular diseases.
In heart failure, mTOR signaling also plays a pivotal role in the transition from compensatory hypertrophy to decompensated heart failure (Table 1) (80). Dysregulated mTOR activity has been implicated in the pathogenesis of heart failure with both reduced and preserved ejection fraction (HFrEF and HFpEF) (Table 1) (81, 82). In the failing heart, mTOR activation may increase oxidative stress, leading to mitochondrial dysfunction and contractile dysfunction (83, 84). mTOR's role in fibrosis, inflammation, and autophagy inhibition in the heart further accelerates cardiac remodeling, fibrosis, and loss of myocardial function (85).
Given mTOR's central role in the pathogenesis of these cardiovascular conditions, targeting this pathway holds significant therapeutic promise (Table 1) (86). However, mTOR functions as a complex hub that regulates multiple cellular processes, and its effects in CVDs are context-dependent (Table 1). While mTOR inhibition with rapamycin or other mTOR inhibitors has shown promise in preclinical models, clinical translation remains challenging due to the broad effects of mTOR on various cell types and tissues (87). Furthermore, as mTOR signaling has both protective and deleterious roles in different stages of CVDs, a more nuanced approach is required (88). Selective modulation of mTOR complexes (such as mTORC1 or mTORC2) or targeting downstream effectors may provide a more refined therapeutic strategy, potentially improving clinical outcomes by mitigating the adverse effects of excessive mTOR activation without compromising its beneficial roles in tissue repair and regeneration (Table 1).
In conclusion, mTOR is a pivotal regulator in the pathogenesis of CVDs, influencing numerous aspects of cardiovascular function (Table 1). A better understanding of the intricate mechanisms by which mTOR regulates oxidative stress, inflammation, and cell death will be critical for the development of targeted therapies (89). Tailoring mTOR inhibition or modulation to specific disease contexts, timing, and patient populations will be essential to maximize therapeutic benefits and minimize potential risks (90).
In order to better understand the distinct roles that mTORC1 and mTORC2 play in various cardiovascular diseases, the following table summarizes their respective mechanisms and effects across a range of conditions. This comparison highlights how mTOR signaling pathways contribute to disease progression and how differential activation of mTORC1 and mTORC2 could off.
3 Mechanisms underlying cell death in cardiovascular disease
Cell death is a central pathological event in CVDs, contributing to the progression of conditions such as atherosclerosis, myocardial infarction, and heart failure (Figure 2) (91). The intricate interplay between programmed cell death (PCD) and non-programmed cell death (non-PCD) shapes the structural and functional outcomes of cardiovascular tissues (92, 93). Below, we detail the mechanisms underlying these two forms of cell death and their implications in CVD pathophysiology.
Figure 2
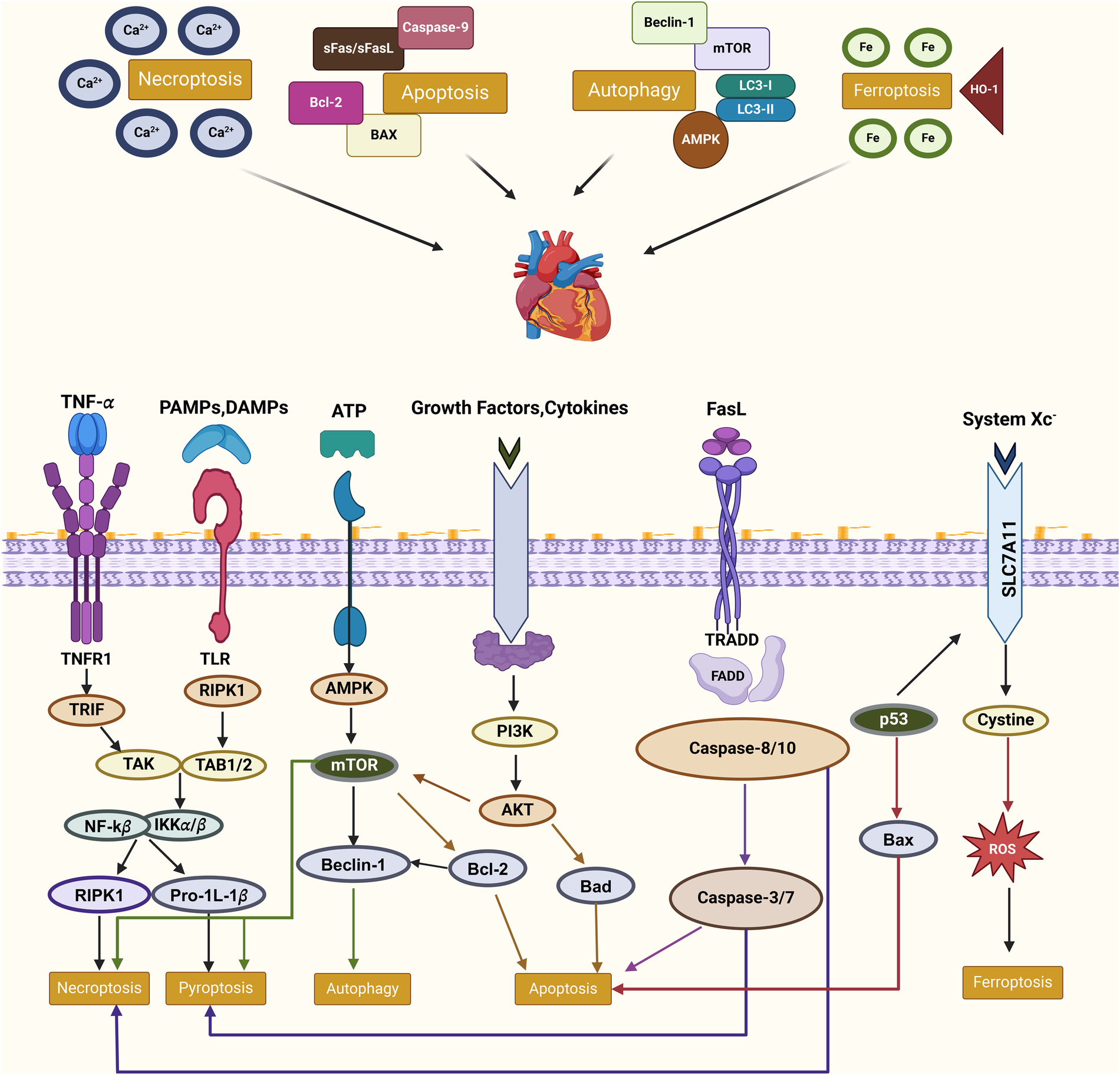
Molecular interplay of PCD pathways in cardiovascular diseases this figure illustrates the interconnected molecular mechanisms of PCD pathways—apoptosis, necroptosis, autophagy, and ferroptosis—in CVDs. Key regulators such as RIPK1, mTOR, and AMPK mediate these processes, influenced by external signals like TNF-α and oxidative stress. Crosstalk between pathways, including shared mediators like RIPK1 and the interplay of autophagy with apoptosis or ferroptosis, highlights their collective roles in inflammation, oxidative stress, and cell fate. These relationships underscore the therapeutic potential of targeting PCD in CVD management.
3.1 Programmed cell death in cardiovascular diseases
PCD refers to tightly regulated cellular processes involving molecular pathways that orchestrate cell demise (94). Its various forms—such as apoptosis, autophagy-dependent cell death, ferroptosis, and pyroptosis—play distinct but interconnected roles in cardiovascular pathology (95).
Apoptosis, a caspase-mediated process, is pivotal in both acute and chronic cardiovascular injury (96). In myocardial infarction, ischemia-reperfusion injury triggers mitochondrial dysfunction and cytochrome c release, activating apoptotic cascades (97).Similarly, in atherosclerosis, the apoptosis of vascular smooth muscle cells weakens plaque stability, predisposing it to rupture and thrombosis (98).
Autophagy is primarily a survival mechanism, but excessive autophagy contributes to cell death under stress conditions, such as hypoxia or oxidative stress (99). In heart failure, dysregulated autophagy exacerbates cardiomyocyte loss and disrupts myocardial contractility, while in vascular diseases, impaired autophagic clearance leads to foam cell accumulation and plaque progression (100, 101).
Iron-dependent lipid peroxidation characterizes ferroptosis, which is distinct from other PCD modalities (102). This form of cell death is implicated in oxidative stress-driven endothelial dysfunction and vascular remodeling (103). For instance, ferroptosis accelerates smooth muscle cell depletion in aortic dissection, compromising vessel integrity and facilitating aneurysm formation (104).
Gasdermin-mediated pyroptosis serves as a pivotal nexus between cell death and inflammation (105). Activation of the NLRP3 inflammasome in macrophages triggers pyroptosis, leading to the release of pro-inflammatory cytokines and subsequent destabilization of atherosclerotic plaques (106). In the context of heart failure, pyroptosis amplifies myocardial inflammation, thereby exacerbating pathological cardiac remodeling (107).
3.2 Non-programmed cell death in cardiovascular diseases
Non-PCD encompasses unregulated, passive forms of cell death, including necrosis and lysosome-mediated cell death, which are frequently observed in acute tissue damage associated with CVDs (Table 2) (108).
Table 2
| Type of cell death | Subtype | Key mechanisms | Role in CVDs | References |
|---|---|---|---|---|
| PCD | Apoptosis | Activation of caspases, release of cytochrome c from mitochondria, DNA fragmentation | Promotes cardiomyocyte apoptosis, endothelial dysfunction, and plaque destabilization | (109) |
| Autophagy-dependent death | Excessive autophagosome activation leading to lysosomal dysfunction | Loss of cardiomyocytes, endothelial dysfunction, and foam cell accumulation | (110) | |
| Ferroptosis | Iron-dependent lipid peroxidation and inactivation of glutathione peroxidase 4 (GPX4) | Loss of smooth muscle cells, vascular wall instability, and aggravated arterial remodeling | (111) | |
| Pyroptosis | Activation of inflammasomes and gasdermin-mediated membrane pore formation | Release of inflammatory cytokines, destabilization of atherosclerotic plaques, and amplification of myocardial inflammation | (112) | |
| Non-PCD | Necrosis | Cell membrane rupture and release of DAMPs, triggering secondary inflammation | Amplifies inflammatory responses, forms necrotic cores, and increases plaque rupture risk | (113) |
| Lysosome-mediated death | Lysosomal membrane permeabilization and release of cathepsins into the cytoplasm | Increases proteotoxic stress, oxidative stress, and cardiomyocyte loss | (114) |
Classification and pathophysiological roles of cell death mechanisms in cardiovascular diseases.
Necrosis is characterized by membrane rupture and the uncontrolled release of intracellular contents, leading to secondary inflammation (115). In myocardial infarction, massive necrosis induced by prolonged ischemia activates inflammatory cascades, aggravating cardiac dysfunction (116). Additionally, necrotic core formation within atherosclerotic plaques promotes plaque instability, increasing the risk of rupture (Table 2) (117).
Lysosomal membrane permeabilization releases cathepsins and other hydrolytic enzymes into the cytosol, initiating cell death (118). In diabetic cardiomyopathy, lysosomal dysfunction exacerbates oxidative stress and lipid accumulation, driving myocardial damage (119). Similarly, in heart failure, impaired lysosomal clearance of damaged organelles contributes to proteotoxic stress and cardiomyocyte loss (120).
3.3 Synergy between programmed and non-programmed cell death
The interactions between PCD and non-PCD amplify cardiovascular damage, creating a feedback loop of inflammation and oxidative stress (121). For example, necrosis-derived damage-associated molecular patterns (DAMPs) not only exacerbate inflammatory responses but also trigger ferroptosis and pyroptosis, amplifying endothelial and myocardial injury (122, 123). Similarly, pyroptosis-induced cytokine release can aggravate necrotic cell death in vascular lesions, perpetuating plaque vulnerability (Table 2) (124).
The dichotomy and interplay between PCD and non-PCD underscore the complexity of cell death mechanisms in CVDs. Understanding these processes at a molecular level offers opportunities for developing therapeutic strategies to mitigate cardiovascular damage by targeting specific forms of cell death. Future research focusing on the crosstalk between these pathways may pave the way for novel, integrative treatments for cardiovascular diseases (Table 2). To better illustrate these mechanisms and their respective roles in CVDs, the following table provides a structured overview of both PCD and NPCD, emphasizing their pathophysiological implications in various cardiovascular conditions.
4 The relationship between mTOR and cell death in cardiovascular diseases
The connection between mTOR and cell death pathways is vital for understanding the mechanisms underlying cardiovascular pathophysiology and for identifying potential therapeutic targets (125). This section explores how mTOR modulates both types of cell death and their implications in CVDs.
4.1 mTOR and programmed cell death: mechanisms and implications
Programmed cell death (PCD) refers to a controlled process by which cells undergo death in response to specific signals (126). It includes various forms, such as apoptosis, autophagy, ferroptosis, and pyroptosis, each of which plays distinct roles in cardiovascular pathophysiology (127). mTOR is a key player in regulating PCD, acting as a switch that can either promote or inhibit these processes depending on the cellular context.
4.1.1 mTOR and apoptosis
mTOR influences apoptosis through several downstream targets, including the pro-survival factors such as Bcl-2 and anti-apoptotic proteins (128). Dysregulation of mTOR can lead to the excessive apoptosis of endothelial cells, VSMCs, and cardiomyocytes, contributing to the progression of diseases such as atherosclerosis, myocardial infarction, and aortic dissection (129). In atherosclerosis, ubiquitination of mTORC1 components by E3 ligases such as FBXW7 has been shown to regulate macrophage apoptosis, influencing plaque stability. This highlights the role of mTORC1 ubiquitination in disease progression. In atherosclerosis, ubiquitination of mTORC1 components by E3 ligases such as FBXW7 has been shown to regulate macrophage apoptosis, influencing plaque stability (130). This highlights the role of mTORC1 ubiquitination in disease progression.
4.1.2 mTOR and autophagy
Autophagy, a cellular process that removes damaged organelles and proteins, is another PCD process regulated by mTOR (131). mTOR inhibition promotes autophagy, which may help clear damaged components in the cardiovascular system (132). However, excessive autophagy can also be detrimental, leading to cell death and contributing to vascular remodeling and heart failure (133). During ischemia-reperfusion injury, acetylation of Raptor modulates mTORC1 activity, thereby regulating cardiomyocyte autophagy and reducing necrotic cell death (134). This PTM acts as a critical switch under oxidative stress.
4.1.3 mTOR and ferroptosis
Ferroptosis, a recently identified form of iron-dependent cell death, is regulated by mTOR in certain cardiovascular diseases (135). mTOR-mediated signaling pathways, including those regulating oxidative stress and iron homeostasis, can influence the initiation of ferroptosis, which has been implicated in diseases like atherosclerosis and ischemic heart disease (136).
4.1.4 mTOR and pyroptosis
Pyroptosis, a form of inflammatory cell death, is another PCD type regulated by mTOR (137). Inflammatory cytokines and ROS production, both modulated by mTOR, can drive pyroptosis in vascular cells, contributing to the inflammation and vascular damage seen in CVDs (138). In heart failure models, SUMOylation of mTORC2-associated proteins influences inflammasome activation and pyroptosis, contributing to myocardial inflammation and dysfunction (139).
4.2 mTOR and non-programmed cell death: role in cardiovascular pathology
Non-programmed cell death, primarily represented by necrosis, is characterized by uncontrolled cell rupture and inflammation (126). While less regulated than PCD, necrosis is a significant driver of acute tissue injury in cardiovascular diseases, and mTOR plays a role in its regulation (140).
4.2.1 mTOR and necrosis
mTOR's role in necrosis is complex and context-dependent. Under conditions of excessive oxidative stress, such as ischemia-reperfusion injury, mTOR activation may contribute to the necrotic death of endothelial cells, VSMCs, and cardiomyocytes (141). This necrosis exacerbates tissue injury and promotes inflammation, creating a cycle of further cell damage.
4.2.2 mTOR and inflammation
mTOR also regulates the inflammatory response, which is a critical mediator of necrosis. In the context of CVDs, mTOR activation in immune cells like macrophages and neutrophils can lead to the release of pro-inflammatory cytokines, amplifying the tissue damage and necrosis seen in conditions like myocardial infarction and aortic dissection (142).
In summary, mTOR plays a dual role in both programmed and non-programmed cell death in cardiovascular diseases. The pathways it regulates can either protect against cell death and promote tissue repair or exacerbate cell loss and inflammation. Understanding the intricate relationship between mTOR and cell death mechanisms is crucial for developing novel therapeutic strategies targeting mTOR signaling in cardiovascular diseases.
5 Protein modifications: the bridge between mTOR and cell death
PMs play a pivotal role in linking mTOR signaling to cell death pathways, serving as a dynamic regulatory bridge in CVDs (Figure 3) (143). These modifications, including phosphorylation, ubiquitination, SUMOylation, acetylation, and glycosylation, finely regulate the activity, stability, and interactions of mTOR components (144, 145). Dysregulation of these modifications can trigger maladaptive responses, such as apoptosis, ferroptosis, and pyroptosis, influencing disease progression (Figure 3) (146, 147). Understanding the interplay between PMs and mTOR signaling offers novel therapeutic strategies to modulate cell fate and improve cardiovascular outcomes.
Figure 3
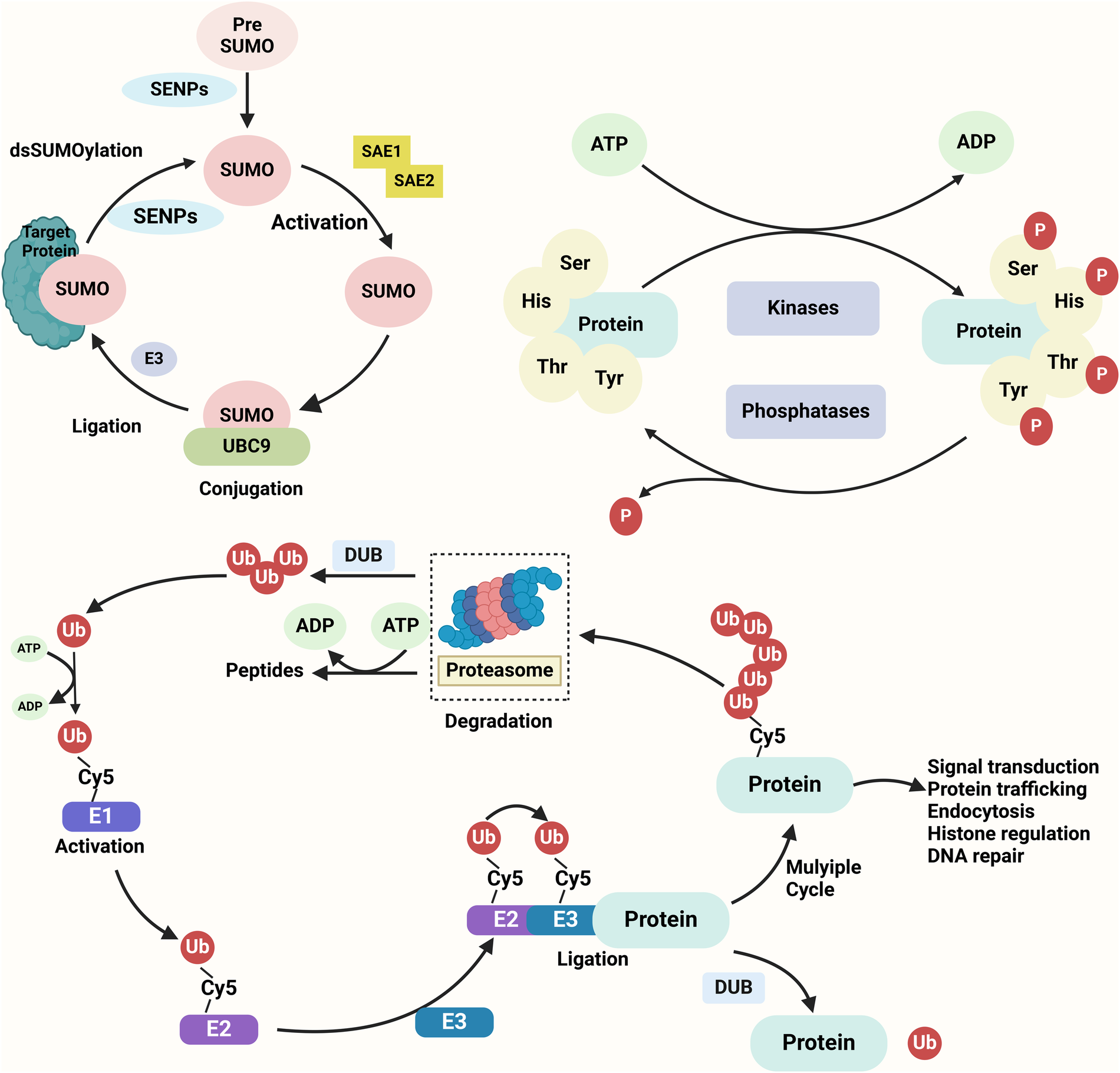
Key protein modifications in cellular regulation SUMOylation, phosphorylation, and ubiquitination are three essential post-translational modifications regulating protein function. SUMOylation involves the covalent attachment of SUMO to lysine residues on target proteins through a cascade of E1 activation, E2 conjugation, and E3 ligation, modulating processes such as nuclear transport, protein stability, and transcription. Phosphorylation, catalyzed by kinases, adds phosphate groups to serine, threonine, or tyrosine residues, influencing signaling pathways and cellular functions. Ubiquitination, mediated by E1, E2, and E3 enzymes, attaches ubiquitin to lysine residues, marking proteins for proteasomal degradation or altering their cellular roles. These modifications are reversible and tightly regulated, playing pivotal roles in cell cycle, apoptosis, and stress responses.
5.1 Protein modifications as critical regulators of mTOR signaling and cell death pathways in cardiovascular diseases
PMs represent a critical mechanism linking mTOR signaling to cell death pathways, serving as a dynamic regulatory bridge in CVDs (148). These modifications, including phosphorylation, ubiquitination, SUMOylation, acetylation, and glycosylation, orchestrate the balance between cell survival and death by modulating the activity, stability, and interactions of mTOR signaling components (149, 150).
Phosphorylation is one of the most extensively studied PMs in mTOR regulation (151). It enhances mTOR activity under physiological conditions but, when dysregulated, can trigger maladaptive responses such as apoptosis, ferroptosis, or pyroptosis (Table 3) (152). For instance, hyper-phosphorylation of mTOR and its downstream targets, such as p70S6 K or 4E-BP1, contributes to oxidative stress and cell death during ischemia-reperfusion injury (153, 154). Conversely, targeted inhibition of aberrant phosphorylation has been shown to mitigate myocardial damage in preclinical models (155).
Table 3
| Protein modification | Protein modification | Regulation of cell death | References |
|---|---|---|---|
| Phosphorylation | Activates mTORC1, inhibits mTORC2 | Apoptosis Autophagy Necroptosis |
(156) |
| Ubiquitination | Modulates mTOR stability and activity | Apoptosis Pyroptosis Ferroptosis |
(157) |
| SUMOylation | Inhibits mTORC1, promotes autophagy | Inhibits apoptosis Autophagy |
(158) |
| Acetylation | Activates mTORC1, inhibits mTORC2 | Apoptosis Ferroptosis Necrosis |
(159) |
| Methylation | Modulates mTORC1 activity | Apoptosis Autophagy |
(160) |
| Glycosylation | Affects mTORC1 localization | Regulates apoptosis necrosis |
(161) |
| Palmitoylation | Regulates mTORC1 and mTORC2 activity | Cell survival and death | (162) |
Regulatory mechanisms of protein modifications linking mTOR signaling and cell death in cardiovascular diseases.
Ubiquitination also plays a pivotal role in regulating mTOR signaling and cell death (Table 3) (163). K48-linked ubiquitination typically mediates proteasomal degradation, thereby suppressing mTOR activity, while K63-linked ubiquitination stabilizes the mTOR complex, promoting survival signals (164). Dysregulation of ubiquitination in conditions such as atherosclerosis exacerbates foam cell formation and apoptosis, destabilizing plaques and worsening disease progression (Table 3) (165, 166).
SUMOylation, a reversible PM involving the attachment of small ubiquitin-like modifiers, fine-tunes the activity of mTOR pathway components such as Raptor and TSC2 (167, 168). This modification can either promote autophagy during early stress adaptation or facilitate apoptosis under sustained oxidative stress (Table 3) (169). In VSMCs, SUMOylation influences phenotypic switching, playing a dual role in vascular remodeling and disease progression, as seen in aortic dissection and hypertension (170, 171).
Acetylation, another critical PM, regulates mTOR-mediated mitochondrial function and cardiomyocyte survival (Table 3) (172). For example, the acetylation status of mTOR targets can alter energy metabolism and redox balance during heart failure, while deacetylation therapies targeting the SIRT1-mTOR axis have shown potential in alleviating diabetic cardiomyopathy (173, 174).
Among the various PTMs involved in cardiovascular regulation of mTOR signaling, ubiquitination and acetylation are particularly well-characterized (175). Ubiquitination, mediated by E1 activating enzymes, E2 conjugating enzymes, and E3 ligases (such as FBXW7 and TRAF6), regulates mTOR complex stability and degradation (176, 177). K48-linked ubiquitination often targets mTORC1 components for proteasomal degradation, especially under stress conditions such as hypoxia or oxidative injury, leading to autophagy activation (178). In contrast, K63-linked ubiquitination may promote mTOR signaling under inflammatory conditions (179). Deubiquitinases (e.g., USP9X, OTUD7B) can stabilize mTOR and suppress cell death pathways (180, 181). Acetylation, catalyzed by enzymes such as p300/CBP or GCN5, modulates mTOR signaling by modifying upstream regulators (e.g., TSC2) or mTORC1 components (e.g., Raptor) (182). For instance, stress-induced acetylation of Raptor has been shown to enhance its interaction with mTOR, thereby suppressing autophagy (183). Conversely, deacetylases like SIRT1 can promote autophagy and inhibit apoptosis by deacetylating Atg proteins or FoxO transcription factors (184). These modifications often occur in a stimulus- and context-dependent manner, serving as rapid switches in the cellular response to cardiovascular injury.
These diverse PMs not only regulate mTOR activity but also mediate its downstream impact on cell fate, highlighting their therapeutic potential (185, 186). Pharmacological modulation of specific PMs has emerged as a promising approach to mitigate CVD progression (187). Drugs such as rapamycin derivatives (targeting phosphorylation), proteasome inhibitors (modulating ubiquitination), and SUMOylation inhibitors (e.g., TAK-981) have shown preclinical efficacy in restoring mTOR homeostasis and reducing pathological cell death (188).
In summary, protein modifications serve as a crucial bridge between mTOR signaling and cell death mechanisms, offering novel insights into the pathophysiology of CVDs. By targeting these modifications, innovative therapies could be developed to finely tune mTOR activity and improve cardiovascular outcomes.
5.2 Cross-talk between protein modifications in mTOR-dependent cell death
The intricate interplay between PMs represents a fundamental mechanism in regulating mTOR-dependent cell death pathways (147). These modifications, including phosphorylation, ubiquitination, and SUMOylation, rarely act in isolation; instead, they form a dynamic network of cross-talk that orchestrates mTOR activity and downstream signaling (189). By influencing the stability, localization, and functional output of mTOR and its associated proteins, this cross-regulation ensures cellular adaptation to environmental stresses (190). Disruptions in this delicate balance, however, can drive pathological cell death processes, such as apoptosis and necrosis, contributing to the progression of CVDs (191, 192). Exploring the cooperative and antagonistic interactions among PMs unveils new therapeutic possibilities for targeting mTOR in CVDs.
Notably, several PTMs regulating mTOR also impact other key signaling pathways involved in cardiovascular pathogenesis (193). For example, K63-linked ubiquitination of TRAF6 can activate both mTORC1 and NF-κB signaling under inflammatory stress (194). Similarly, acetylation of STAT3 and mTOR by p300 under oxidative conditions promotes pro-hypertrophic gene expression and suppresses autophagy (195). These shared modifications reflect a level of multi-pathway integration in which PTMs serve as central regulatory nodes, coordinating diverse cellular outcomes in cardiovascular injury.
5.2.1 Phosphorylation and ubiquitination cross-talk in mTOR-dependent cell death
Phosphorylation and ubiquitination are two of the most common protein modifications in cells, and they often interact with each other to regulate cell growth, division, and death (196, 197). Phosphorylation regulates protein function by activating or inhibiting specific kinases and phosphatases, while ubiquitination controls the stability and activity of proteins by marking them for degradation (198, 199).
The activation and function of mTOR are largely regulated by phosphorylation by upstream kinases such as AMPK and Akt (200, 201). The activity of these kinases is often closely linked to the ubiquitination process (202). For example, some proteins may be marked for ubiquitination and degradation following phosphorylation, or their half-life may be prolonged by the removal of ubiquitin (169). Activation of mTORC1 is associated with the phosphorylation of specific substrates (such as p70S6 K, 4EBP1), and the stability of these substrates may be influenced by ubiquitination (203, 204). Under stress conditions (e.g., oxidative stress or hypoxia), mTORC1 may regulate the ubiquitination of these substrates to control the balance between cell growth and death (205, 206).
Ubiquitination not only regulates the stability of mTOR downstream effectors but also participates in feedback regulation by controlling the activity of mTOR itself (207). By regulating the deubiquitination process of mTOR, the cell can precisely control the activation state of mTOR (208). During cell death, excessive activation of mTOR may lead to metabolic imbalances, while moderate ubiquitination modification can limit mTOR activity by promoting the degradation of mTOR complexes, preventing the cell from entering a state of overgrowth or metabolic imbalance (209, 210).
5.2.2 SUMOylation and phosphorylation cross-talk in mTOR-mediated cell death
SUMOylation and phosphorylation are two important protein modifications in cells, with SUMOylation mainly regulating protein localization, stability, and function, while phosphorylation directly affects protein activity and interactions with other molecules (211–213).
SUMOylation can regulate the assembly and stability of mTOR, affecting its interactions with upstream regulators (such as Raptor) and downstream effectors (such as S6 K, 4EBP1) (214–216). For example, SUMOylation can increase the stability of Raptor, promoting mTORC1 activation, thus supporting cell growth and metabolism (217–219). During stress responses or drug treatment, SUMOylation and phosphorylation together regulate the activity of mTOR and influence cell death decisions (220). SUMOylation can also indirectly affect autophagy by regulating proteins associated with autophagy (such as LC3, Atg5) (221, 222).
In many cases, phosphorylation and SUMOylation occur in alternating modifications on the same protein, mutually regulating each other (223, 224). For example, under certain conditions, key regulatory proteins in the mTOR pathway may be activated by phosphorylation and then modulated by SUMOylation to promote cell growth and metabolism (225). In other cases, the introduction of SUMOylation may alter the output of phosphorylation responses, thereby affecting the cell death mechanisms (226, 227). The cross-regulation between SUMOylation and phosphorylation in mTOR-mediated cell death could determine whether cells enter apoptotic, necrotic, or autophagic death, especially in the context of cardiovascular diseases like myocardial ischemia or atherosclerosis (169, 228).
5.2.3 Ubiquitination and SUMOylation in mTOR-mediated cell death
Ubiquitination and SUMOylation are both critical protein modification mechanisms within the cell, and they have a complex interaction (229, 230). While each modification mechanism acts independently, they often work in concert to regulate the mTOR pathway and cell death.
Ubiquitination and SUMOylation not only independently play roles in mTOR signaling, but they also influence each other, thus regulating protein function (231). For example, certain key regulatory proteins in the mTOR pathway (such as TSC2, Rheb) may be regulated through multiple modifications, including phosphorylation, ubiquitination, and SUMOylation, affecting mTOR activation (232–234). Under stress conditions, SUMOylation may prevent ubiquitination-mediated degradation of certain proteins by altering their localization, while ubiquitination may remove SUMO modifications, thereby changing the function of the protein (235).
In cardiovascular diseases, the cross-talk between ubiquitination and SUMOylation may regulate mTOR's stability and activity, controlling the growth and death of cells such as cardiomyocytes, endothelial cells, and smooth muscle cells (236). For example, in pathological conditions like atherosclerosis and myocardial infarction, these protein modifications may determine whether cells enter apoptosis, necrosis, or autophagy (237, 238).
6 Therapeutic potential of targeting protein modifications in mTOR-dependent cell death
PMs such as phosphorylation, ubiquitination, SUMOylation, and acetylation play pivotal roles in mTOR-dependent cell death pathways, offering potential therapeutic avenues for CVDs (239). This section discusses how targeting PMs can address specific disease mechanisms, with a focus on detailed disease contexts, pharmacological interventions, and emerging therapeutic approaches.
6.1 Targeting protein modifications in cardiovascular diseases
By influencing mTOR activity and its downstream signaling, specific PMs such as phosphorylation, ubiquitination, SUMOylation, and acetylation orchestrate the balance between cell survival and death (240). Dysregulation of these processes contributes to various CVDs, including atherosclerosis, myocardial infarction, heart failure, diabetic cardiomyopathy, and hypertension (241, 242).
In atherosclerosis, hyperactivation of the mTOR pathway exacerbates oxidative stress and foam cell apoptosis, destabilizing plaques and promoting vascular inflammation (243, 244). Phosphorylation of mTOR downstream effectors like p70S6 K and 4E-BP1 is often aberrant, driving endothelial dysfunction and VSMC phenotypic switching (245, 246). Concurrently, ubiquitination dysregulation modulates foam cell formation and inflammatory responses (247). Therapeutically, rapamycin and its derivatives, such as everolimus, mitigate these effects by attenuating mTOR phosphorylation (248).Proteasome inhibitors like bortezomib adjust the ubiquitination machinery, reducing foam cell apoptosis and stabilizing plaques (249, 250). These interventions highlight the potential of targeting mTOR-associated PMs to address the underlying pathology of atherosclerosis.
In ischemia-reperfusion injury (IRI) and myocardial infarction, the role of mTOR in cell death becomes evident through its modulation of apoptosis, necrosis, and ferroptosis (251, 252). SUMOylation of TSC2 during ischemia enhances mTOR activity, worsening oxidative stress and apoptotic signaling (Table 4) (253). Upon reperfusion, excessive phosphorylation of mTOR downstream proteins exacerbates inflammation, promoting cell death and myocardial dysfunction (254). Moreover, acetylation abnormalities impair mitochondrial function and energy metabolism, aggravating injury (Table 4) (255, 256). Interventions such as metformin, which activates AMPK and indirectly suppresses mTOR phosphorylation, have demonstrated cardioprotective effects by reducing apoptosis (257, 258). SUMOylation inhibitors like TAK-981 attenuate oxidative stress and protect mitochondrial integrity, while SIRT1 activators, including resveratrol, restore acetylation balance and metabolic homeostasis, reducing infarct size in preclinical studies (259, 260).
Table 4
| Cardiovascular disease | Protein modification | Mechanism | Therapeutic strategy | References |
|---|---|---|---|---|
| Atherosclerosis | Phosphorylation Ubiquitination |
mTOR hyperactivation → oxidative stress, foam cell apoptosis, plaque instability | Rapamycin derivatives (e.g., everolimus), proteasome inhibitors (e.g., bortezomib) | (261) |
| Ischemia-reperfusion injury | SUMOylation Phosphorylation Acetylation |
SUMOylation of TSC2 → mTOR activation, oxidative stress; acetylation imbalance → mitochondrial dysfunction | Metformin, SUMOylation inhibitors (e.g., TAK-981), SIRT1 activators (e.g., resveratrol) | (262) |
| Myocardial infarction | Phosphorylation Acetylation |
Hyper-phosphorylation of mTOR effectors → apoptosis and inflammation | AMPK activators (e.g., metformin), Acetylation modulators | (263) |
| Heart failure | Phosphorylation Ubiquitination Acetylation |
mTOR hyperactivation → hypertrophy, fibrosis; Ubiquitination dysregulation → inflammation | Rapamycin derivatives, Proteasome inhibitors, Acetylation modulators (e.g., resveratrol) | (264) |
| Diabetic cardiomyopathy | Ubiquitination Acetylation |
Hyperglycemia → ubiquitination dysregulation, oxidative stress; Acetylation imbalance → inflammation | HDAC inhibitors (e.g., vorinostat), AMPK activators (e.g., metformin) | (265) |
| Hypertension | SUMOylation Phosphorylation |
SUMOylation of VSMCs → phenotypic switching; mTOR hyperphosphorylation → endothelial dysfunction | SUMOylation inhibitors, Rapamycin derivatives | (266) |
Key protein modifications in mTOR-driven cardiovascular pathologies.
In chronic heart failure, aberrant PMs exacerbate pathological remodeling and cell death (Table 4) (267). Persistent mTOR overactivation, driven by excessive phosphorylation, leads to maladaptive cardiac hypertrophy and fibrosis (268). Ubiquitination dysregulation amplifies inflammatory cascades, while impaired acetylation compromises mitochondrial function and energy production (269). Rapamycin derivatives alleviate fibrosis by suppressing mTOR phosphorylation, whereas proteasome inhibitors adjust ubiquitination to curb inflammation and cell death (270). Additionally, acetylation modulators such as resveratrol enhance mitochondrial function, mitigating heart failure progression (271, 272).
Diabetic cardiomyopathy (DCM) exemplifies the interplay between metabolic derangements and PM dysregulation (Table 4) (273).In hyperglycemic conditions, disrupted ubiquitination destabilizes mTOR complexes, leading to heightened oxidative stress and apoptosis (274). Simultaneously, acetylation imbalance undermines mitochondrial function, exacerbating inflammation and fibrosis (275). Therapeutic agents like HDAC inhibitors (e.g., vorinostat) restore acetylation homeostasis and mitochondrial function, reducing inflammation in diabetic hearts (276). AMPK activators, including metformin, indirectly suppress mTOR hyperactivation and alleviate metabolic stress, improving cardiac outcomes in DCM models (Table 4) (277, 278).
Hypertension-induced vascular remodeling is another context in which PMs and mTOR dysregulation converge (Table 4) (279).Excessive SUMOylation in VSMCs drives their phenotypic switching, contributing to vascular stiffening and thickening (280). Additionally, hyperphosphorylation of mTOR effectors exacerbates endothelial dysfunction, increasing oxidative stress and apoptosis (281). Therapeutic strategies targeting these modifications, such as SUMOylation inhibitors to prevent VSMC phenotypic changes and rapamycin derivatives to curb mTOR hyperphosphorylation, show promise in mitigating vascular remodeling and improving vascular health (282, 283).
In summary, the intricate interplay between PMs, mTOR signaling, and cell death mechanisms highlights a promising therapeutic avenue for combating CVDs. Targeting specific PMs not only restores mTOR homeostasis but also addresses the underlying pathophysiological processes driving disease progression. Future research focusing on the development of PM-modulating drugs may unlock novel strategies to improve cardiovascular outcomes.
6.2 Targeted pharmacological strategies for mTOR-related protein modifications in cardiovascular diseases
The growing understanding of protein modifications and mTOR signaling in cardiovascular diseases has paved the way for the development of targeted therapies (Table 5). Rapamycin and its analogs have demonstrated efficacy in reducing atherosclerotic plaque burden by modulating mTORC1 ubiquitination pathways (284). Meanwhile, histone deacetylase inhibitors that affect acetylation status of mTOR regulators show promise in limiting ischemia-reperfusion injury (285). These therapies focus on modulating key pathways, including mTOR inhibition, regulation of protein modifications, and attenuation of pathological cell death. The table below summarizes critical drugs, their mechanisms of action, and their clinical relevance in cardiovascular diseases (Table 5).
Table 5
| Drug category | Representative drugs | Mechanism of action | References |
|---|---|---|---|
| mTOR inhibitors | Sirolimus Everolimus Temsirolimus |
Inhibit mTORC1 activity, reduce cardiac hypertrophy, inflammation, and fibrosis, regulate cell proliferation and metabolism | (286) |
| AMPK activators | Metformin AICAR |
Activate AMPK pathway, inhibit mTORC1, restore energy metabolism, reduce phosphorylation imbalance and oxidative stress | (287) |
| Proteasome inhibitors | Bortezomib Carfilzomib |
Inhibit proteasome activity, reduce abnormal protein degradation, stabilize mTOR complex, and inhibit apoptosis and foam cell formation | (288) |
| E3 ligase modulators | MLN4924 Thalidomide derivatives |
Regulate E3 ligase activity, mitigate inflammation-mediated cell death and stress responses | (289) |
| SUMOylation inhibitors | TAK-981 Anacardic acid |
Inhibit SUMOylation, reduce inflammation and phenotypic switching, improve pathological vascular smooth muscle cell behavior | (290) |
| HDAC inhibitors | Vorinostat Panobinostat Trichostatin A |
Regulate acetylation levels, reduce myocardial fibrosis and inflammation, improve metabolic imbalance and cardiac remodeling | (291) |
| SIRT1 activators | Resveratrol Nicotinamide Mononucleotide |
Activate SIRT1 deacetylation, regulate mTOR signaling, enhance antioxidant action and mitochondrial function | (292) |
| PI3K/AKT inhibitors | LY294002 Wortmannin |
Inhibit PI3K/AKT-mTOR pathway, reduce excessive cell proliferation and inflammatory responses | (293) |
| Antioxidants | N-Acetylcysteine Coenzyme Q10 |
Reduce oxidative stress, inhibit apoptosis and inflammation through mTOR and downstream pathways | (294) |
| Iron chelators | Deferoxamine Deferiprone |
Reduce iron-dependent oxidative stress and ferroptosis, improve cell survival via mTOR-related mechanisms | (295) |
| Anti-inflammatory drugs | Colchicine Canakinumab |
Reduce inflammatory mediator release, regulate mTOR-associated cell death processes, stabilize lesions | (296) |
| mTOR activators | MHY1485 | Selectively activate mTORC1 to promote cell survival and reduce cardiomyocyte apoptosis | (297) |
Therapeutic compounds targeting mTOR signaling or protein modifications in cardiovascular disease models.
This table provides a comprehensive overview of the therapeutic landscape targeting protein modifications and mTOR signaling in cardiovascular diseases, emphasizing both established treatments and promising drug candidates.
Notably, several ongoing clinical trials support the translational relevance of targeting mTOR or PTM pathways in cardiovascular diseases (193). For instance, everolimus, an mTORC1 inhibitor, is being evaluated in patients with coronary artery disease via bioresorbable scaffolds (NCT03039751) (298). In addition, the HDAC inhibitor vorinostat, which affects protein acetylation, is under investigation in ischemic heart conditions for its potential to modulate myocardial remodeling (NCT02455034) (299). These studies highlight the clinical momentum toward PTM- and mTOR-based therapies in CVD.
6.3 Current limitations and translational challenges
Despite the promising advances in targeting mTOR signaling and PTM pathways for cardiovascular disease treatment, several translational hurdles remain (300). A key concern is the lack of tissue and cell-type specificity of many mTOR inhibitors and PTM modulators, which may lead to off-target effects such as metabolic imbalance, immune suppression, or unintended cell death in non-target tissues (301). Additionally, the intracellular delivery of these agents is often inefficient, particularly for compounds targeting specific protein modifications that require nuclear or organelle-level access (302). Conventional systemic administration may result in suboptimal bioavailability and dose-limiting toxicity. Furthermore, the temporal dynamics of PTMs pose a challenge, as modulating transient or reversible modifications in vivo requires precise control (303). Overcoming these obstacles will require improved delivery systems (e.g., nanoparticle carriers or tissue-specific vectors), enhanced selectivity of inhibitors, and deeper understanding of context-dependent mTOR–PTM–cell death networks in various CVD settings (193).
7 Conclusion
In conclusion, the mechanistic target of rapamycin (mTOR) signaling pathway and its associated protein modifications are integral to the regulation of various cellular processes such as growth, metabolism, survival, and death (304, 305). These pathways are critically involved in the pathophysiology of cardiovascular diseases (CVDs), including atherosclerosis, heart failure, ischemia-reperfusion injury, and hypertension (306, 307). Dysregulation of mTOR activity, often through aberrant protein modifications such as phosphorylation, ubiquitination, SUMOylation, acetylation, and glycosylation, contributes to maladaptive cellular responses and promotes the progression of these diseases (218). The complex interactions between mTOR signaling and protein modifications play a pivotal role in determining the balance between cell survival and death, influencing processes like apoptosis, autophagy, ferroptosis, and necrosis (308).
Targeting mTOR signaling and its associated protein modifications has emerged as a promising therapeutic strategy in cardiovascular medicine. The development of drugs that modulate mTOR activity, such as mTOR inhibitors, AMPK activators, and proteasome inhibitors, has shown potential in preclinical studies and early clinical trials for restoring homeostasis and reducing pathological cell death. Moreover, pharmacological interventions targeting specific protein modifications, including SUMOylation inhibitors and anti-inflammatory agents, offer new avenues for therapeutic intervention in CVDs. These strategies aim to modulate the intricate cellular mechanisms regulated by mTOR and its modifications, thus offering a refined approach to managing cardiovascular diseases.
However, while significant progress has been made, further research is needed to deepen our understanding of the precise molecular mechanisms through which mTOR signaling and protein modifications contribute to cardiovascular pathology. In particular, the development of more selective and effective drugs that can target specific aspects of mTOR-related pathways and protein modifications is crucial for translating these findings into clinical practice. Additionally, clinical trials evaluating the long-term safety and efficacy of these therapeutic strategies in diverse patient populations will be essential in establishing their therapeutic potential.
Ultimately, the exploration of mTOR signaling and protein modifications represents a promising frontier in cardiovascular research, with the potential to revolutionize the treatment of CVDs. By targeting these molecular pathways, we can offer more personalized and effective therapies, improving patient outcomes and quality of life for individuals suffering from cardiovascular diseases. These disease-specific mechanistic insights emphasize the importance of targeting discrete mTOR-PTM-cell death axes for precision therapy in cardiovascular diseases. Future studies should focus on delineating these pathways in diverse pathologies to optimize therapeutic interventions.
Statements
Author contributions
JG: Funding acquisition, Supervision, Validation, Writing – original draft, Writing – review & editing. YW: Conceptualization, Writing – original draft, Writing – review & editing. ZW: Project administration, Writing – original draft. ZZ: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (grant nos. 82300526).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Garady L Soota A Shouche Y Chandrachari KP Srikanth KV Shankar P et al A narrative review of the role of blood biomarkers in the risk prediction of cardiovascular diseases. Cureus. (2024) 16(12):e74899. 10.7759/cureus.74899
2.
Stiefel M Brito da Silva H Schmied CM Niederseer D . Exercise, sports, and cardiac rehabilitation recommendations in patients with aortic aneurysms and post-aortic repair: a review of the literature. J Cardiovasc Dev Dis. (2024) 11(12):379. 10.3390/jcdd11120379
3.
Elkammash A Zaki A Tawfik O Gouda S . Ferroptosis: a key driver in atherosclerosis progression and arterial disease. Rev Cardiovasc Med. (2024) 25(12):441. 10.31083/j.rcm2512441
4.
Tan R Ge C Yan Y Guo H Han X Zhu Q et al Deciphering ferroptosis in critical care: mechanisms, consequences, and therapeutic opportunities. Front Immunol. (2024) 15:1511015. 10.3389/fimmu.2024.1511015
5.
Wang W Wang XM Zhang HL Zhao R Wang Y Zhang HL et al Molecular and metabolic landscape of adenosine triphosphate-induced cell death in cardiovascular disease. World J Cardiol. (2024) 16(12):689–706. 10.4330/wjc.v16.i12.689
6.
Zhou RP Liang HY Hu WR Ding J Li SF Chen Y et al Modulators of Asic1a and its potential as a therapeutic target for age-related diseases. Ageing Res Rev. (2023) 83:101785. 10.1016/j.arr.2022.101785
7.
Williams PT . Quantile-specific heritability of inflammatory and oxidative stress biomarkers linked to cardiovascular disease. J Inflamm Res. (2022) 15:85–103. 10.2147/jir.S347402
8.
You N Liu G Yu M Chen W Fei X Sun T et al Reconceptualizing endothelial-to-mesenchymal transition in atherosclerosis: signaling pathways and prospective targeting strategies. J Adv Res. (2025). 10.1016/j.jare.2024.12.049
9.
Madreiter-Sokolowski CT Hiden U Krstic J Panzitt K Wagner M Enzinger C et al Targeting organ-specific mitochondrial dysfunction to improve biological aging. Pharmacol Ther. (2024) 262:108710. 10.1016/j.pharmthera.2024.108710
10.
Hilgendorf I Frantz S Frangogiannis NG . Repair of the infarcted heart: cellular effectors, molecular mechanisms and therapeutic opportunities. Circ Res. (2024) 134(12):1718–51. 10.1161/circresaha.124.323658
11.
Liu Y Liu Q Shang H Li J Chai H Wang K et al Potential application of natural compounds in ischaemic stroke: focusing on the mechanisms underlying “lysosomocentric” dysfunction of the autophagy-lysosomal pathway. Pharmacol Ther. (2024) 263:108721. 10.1016/j.pharmthera.2024.108721
12.
Yarmohammadi F Hesari M Shackebaei D . The role of mTOR in doxorubicin-altered cardiac metabolism: a promising therapeutic target of natural compounds. Cardiovasc Toxicol. (2024) 24(2):146–57. 10.1007/s12012-023-09820-7
13.
Nwabo Kamdje AH Dongmo Fogang HP Mimche PN . Role of epigenetic in cancer biology, in hematologic malignancies and in anticancer therapy. Front Mol Med. (2024) 4:1426454. 10.3389/fmmed.2024.1426454
14.
Tessier S Martin-Martin N de Thé H Carracedo A Lallemand-Breitenbach V . Promyelocytic leukemia protein, a protein at the crossroad of oxidative stress and metabolism. Antioxid Redox Signal. (2017) 26(9):432–44. 10.1089/ars.2016.6898
15.
Tang Y Ju W Liu Y Deng Q . The role of Sirt1 in autophagy and drug resistance: unveiling new targets and potential biomarkers in cancer therapy. Front Pharmacol. (2024) 15:1469830. 10.3389/fphar.2024.1469830
16.
Zhou XH Luo YX Yao XQ . Exercise-driven cellular autophagy: a bridge to systematic wellness. J Adv Res. (2025). 10.1016/j.jare.2024.12.036
17.
Chen J Li H Zhuo J Lin Z Hu Z He C et al Impact of immunosuppressants on tumor pulmonary metastasis: new insight into transplantation for hepatocellular carcinoma. Cancer Biol Med. (2024) 21(11):1033–49. 10.20892/j.issn.2095-3941.2024.0267
18.
Cao Z Tian K Ran Y Zhou H Zhou L Ding Y et al Beclin-1: a therapeutic target at the intersection of autophagy, immunotherapy, and cancer treatment. Front Immunol. (2024) 15:1506426. 10.3389/fimmu.2024.1506426
19.
Patel A Nguyen L Shea C Singh S Venketaraman V . The role of mtor in mycobacterium tuberculosis infection. Biomedicines. (2024) 12(10):2238. 10.3390/biomedicines12102238
20.
Chen T Lin X Lu S Li B . V-Atpase in cancer: mechanistic insights and therapeutic potentials. Cell Commun Signal. (2024) 22(1):613. 10.1186/s12964-024-01998-9
21.
Pandrangi SL Chittineedi P Manthari RK Suhruth B . Impact of oxytosis on the cross-talk of mTORC with mitochondrial proteins in drug-resistant cancer stem cells. J Cell Physiol. (2024) 239(12):e31421. 10.1002/jcp.31421
22.
Evans JF McCormack FX Sonenberg N Krymskaya VP . Lost in translation: a neglected mTOR target for lymphangioleiomyomatosis. Eur Respir Rev. (2023) 32(169):230100. 10.1183/16000617.0100-2023
23.
Mir SA Dar A Alshehri SA Wahab S Hamid L Almoyad MAA et al Exploring the mTOR signalling pathway and its inhibitory scope in cancer. Pharmaceuticals (Basel). (2023) 16(7):1004. 10.3390/ph16071004
24.
Zhang S Lin X Hou Q Hu Z Wang Y Wang Z . Regulation of Mtorc1 by amino acids in mammalian cells: a general picture of recent advances. Anim Nutr. (2021) 7(4):1009–23. 10.1016/j.aninu.2021.05.003
25.
Xiang H Zhang J Lin C Zhang L Liu B Ouyang L . Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B. (2020) 10(4):569–81. 10.1016/j.apsb.2019.10.003
26.
Smiles WJ Ovens AJ Oakhill JS Kofler B . The metabolic sensor AMPK: twelve enzymes in one. Mol Metab. (2024) 90:102042. 10.1016/j.molmet.2024.102042
27.
Zeng F Cao J Li W Zhou Y Yuan X . Fnip1: a key regulator of mitochondrial function. Biomed Pharmacother. (2024) 177:117146. 10.1016/j.biopha.2024.117146
28.
Ren Q Sun Q Fu J . Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy. (2024) 20(2):221–41. 10.1080/15548627.2023.2254191
29.
Feng Y Chen Y Wu X Chen J Zhou Q Liu B et al Interplay of energy metabolism and autophagy. Autophagy. (2024) 20(1):4–14. 10.1080/15548627.2023.2247300
30.
Gibbons E Minor BMN Hammes SR . Lymphangioleiomyomatosis: where endocrinology, immunology and tumor biology meet. Endocr Relat Cancer. (2023) 30(9):e230102. 10.1530/erc-23-0102
31.
Roch T Akymenko O Krüger A Jung F Ma N Lendlein A . Expression pattern analysis and activity determination of matrix metalloproteinase derived from human macrophage subsets. Clin Hemorheol Microcirc. (2014) 58(1):147–58. 10.3233/ch-141885
32.
Bednarczyk M Dąbrowska-Szeja N Łętowski D Dzięgielewska-Gęsiak S Waniczek D Muc-Wierzgoń M . Relationship between dietary nutrient intake and autophagy-related genes in obese humans: a narrative review. Nutrients. (2024) 16(23):4003. 10.3390/nu16234003
33.
Jiao L Liu Y Yu XY Pan X Zhang Y Tu J et al Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduct Target Ther. (2023) 8(1):15. 10.1038/s41392-022-01285-4
34.
Cormerais Y Vučetić M Parks SK Pouyssegur J . Amino acid transporters are a vital focal point in the control of Mtorc1 signaling and cancer. Int J Mol Sci. (2020) 22(1):23. 10.3390/ijms22010023
35.
Hou J Nie Y Wen Y Hua S Hou Y He H et al The role and mechanism of AMPK in pulmonary hypertension. Ther Adv Respir Dis. (2024) 18:17534666241271990. 10.1177/17534666241271990
36.
Marafie SK Al-Mulla F Abubaker J . MTOR: its critical role in metabolic diseases, cancer, and the aging process. Int J Mol Sci. (2024) 25(11):6141. 10.3390/ijms25116141
37.
Jhanwar-Uniyal M Zeller SL Spirollari E Das M Hanft SJ Gandhi CD . Discrete mechanistic target of rapamycin signaling pathways, stem cells, and therapeutic targets. Cells. (2024) 13(5):409. 10.3390/cells13050409
38.
Ragupathi A Kim C Jacinto E . The Mtorc2 signaling network: targets and cross-talks. Biochem J. (2024) 481(2):45–91. 10.1042/bcj20220325
39.
Panwar V Singh A Bhatt M Tonk RK Azizov S Raza AS et al Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. (2023) 8(1):375. 10.1038/s41392-023-01608-z
40.
Zieba J Munivez E Castellon A Jiang MM Dawson B Ambrose CG et al Fracture healing in collagen-related preclinical models of osteogenesis imperfecta. J Bone Miner Res. (2020) 35(6):1132–48. 10.1002/jbmr.3979
41.
Linton MF Moslehi JJ Babaev VR . AKT signaling in macrophage polarization, survival, and atherosclerosis. Int J Mol Sci. (2019) 20(11):2703. 10.3390/ijms20112703
42.
Kurdi A De Meyer GR Martinet W. Potential therapeutic effects of mTOR inhibition in atherosclerosis. Br J Clin Pharmacol. (2016) 82(5):1267–79. 10.1111/bcp.12820
43.
Stanciu SM Jinga M Miricescu D Stefani C Nica RI Stanescu S II et al mTOR dysregulation, insulin resistance, and hypertension. Biomedicines. (2024) 12(8):1802. 10.3390/biomedicines12081802
44.
Shi J Liu X Jiao Y Tian J An J Zou G et al mTOR pathway: a key player in diabetic nephropathy progression and therapeutic targets. Genes Dis. (2025) 12(2):101260. 10.1016/j.gendis.2024.101260
45.
Darwish R Alcibahy Y Bucheeri S Albishtawi A Tama M Shetty J et al The role of hypothalamic microglia in the onset of insulin resistance and type 2 diabetes: a neuro-immune perspective. Int J Mol Sci. (2024) 25(23):13169. 10.3390/ijms252313169
46.
Gong J Gao X Ge S Li H Wang R Zhao L . The role of cGAS-STING signalling in metabolic diseases: from signalling networks to targeted intervention. Int J Biol Sci. (2024) 20(1):152–74. 10.7150/ijbs.84890
47.
Chang S Wang Z An T . T-cell metabolic reprogramming in atherosclerosis. Biomedicines. (2024) 12(8):1844. 10.3390/biomedicines12081844
48.
Al Attar AA Fahed GI Hoballah MM Pedersen S El-Yazbi AF Nasser SA et al Mechanisms underlying the effects of caloric restriction on hypertension. Biochem Pharmacol. (2022) 200:115035. 10.1016/j.bcp.2022.115035
49.
Aziz A Ganesan Nathan K Kamarul T Mobasheri A Sharifi A . The interplay between dysregulated metabolites and signaling pathway alterations involved in osteoarthritis: a systematic review. Ther Adv Musculoskelet Dis. (2024) 16:1759720x241299535. 10.1177/1759720×241299535
50.
Sumiyoshi R Koga T Kawakami A . Biomarkers and signaling pathways implicated in the pathogenesis of idiopathic multicentric castleman disease/thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly (TAFRO) syndrome. Biomedicines. (2024) 12(6):1141. 10.3390/biomedicines12061141
51.
Vargas-Soria M García-Alloza M Corraliza-Gómez M . Effects of diabetes on microglial physiology: a systematic review of in vitro, preclinical and clinical studies. J Neuroinflammation. (2023) 20(1):57. 10.1186/s12974-023-02740-x
52.
Chen C Wang J Liu C Hu J Liu L . Pioneering therapies for post-infarction angiogenesis: insight into molecular mechanisms and preclinical studies. Biomed Pharmacother. (2023) 166:115306. 10.1016/j.biopha.2023.115306
53.
Li JP Qiu S Tai GJ Liu YM Wei W Fu MM et al NLRP3 inflammasome-modulated angiogenic function of EPC via PI3K/Akt/mTOR pathway in diabetic myocardial infarction. Cardiovasc Diabetol. (2025) 24(1):6. 10.1186/s12933-024-02541-3
54.
Donadeu L Gomez-Olles S Casanova F Torija A Lopez-Meseguer M Boada-Pérez M et al Role of SARS-CoV-2-specific memory B cells promoting immune protection after booster vaccination in solid organ transplantation. Front Immunol. (2024) 15:1463769. 10.3389/fimmu.2024.1463769
55.
Duan Q Yang W Zhu X Feng Z Song J Xu X et al Deptor protects against myocardial ischemia-reperfusion injury by regulating the mTOR signaling and autophagy. Cell Death Discov. (2024) 10(1):508. 10.1038/s41420-024-02263-1
56.
Li X Wang Z Mouton AJ Omoto ACM da Silva AA do Carmo JM et al Sestrin2 attenuates myocardial endoplasmic reticulum stress and cardiac dysfunction during ischemia/reperfusion injury. J Am Heart Assoc. (2024) 13(21):e035193. 10.1161/jaha.124.035193
57.
Baxan N Zhao L Ashek A Niglas M Wang D Khassafi F et al Deep phenotyping the right ventricle to establish translational MRI biomarkers for characterization of adaptive and maladaptive states in pulmonary hypertension. Sci Rep. (2024) 14(1):29774. 10.1038/s41598-024-79029-3
58.
Sung PH Yue Y Chen YL Chiang JY Cheng BC Yang CC et al Combined dapagliflozin and roxadustat effectively protected heart and kidney against cardiorenal syndrome-induced damage in rodent through activation of cell stress-Nfr2/ARE signalings and stabilizing HIF-1α. Biomed Pharmacother. (2024) 180:117567. 10.1016/j.biopha.2024.117567
59.
Bernis ME Burkard H Bremer AS Grzelak K Zweyer M Maes E et al The neuroprotective effects of caffeine in a neonatal hypoxia-ischemia model are regulated through the AMPK/mTOR pathway. Int J Biol Sci. (2025) 21(1):251–70. 10.7150/ijbs.101087
60.
Stock AT Parsons S Hansen JA D’Silva DB Starkey G Fayed A et al mTOR signalling controls the formation of smooth muscle cell-derived luminal myofibroblasts during vasculitis. EMBO Rep. (2024) 25(10):4570–93. 10.1038/s44319-024-00251-1
61.
Shi W Zhang J Zhao W Yue M Ma J Zeng S et al Intracellular iron deficiency and abnormal metabolism, not ferroptosis, contributes to homocysteine-induced vascular endothelial cell death. Biomedicines. (2024) 12(10):2301. 10.3390/biomedicines12102301
62.
Zhang Q Miao M Cao S Liu D Cao Z Bai X et al PCSK9 promotes vascular neointimal hyperplasia through non-lipid regulation of vascular smooth muscle cell proliferation, migration, and autophagy. Biochem Biophys Res Commun. (2025) 742:151081. 10.1016/j.bbrc.2024.151081
63.
Sun D Du Y . O304 alleviates abdominal aortic aneurysm formation via AMPK/mTOR/MMP pathway activation. Front Pharmacol. (2024) 15:1457817. 10.3389/fphar.2024.1457817
64.
Huang J Zhuang J Wang J Shan Z . Montelukast inhibits abdominal aortic aneurysm formation in mice via activating the AMPK/mTOR signalling pathway. Langenbecks Arch Surg. (2024) 409(1):362. 10.1007/s00423-024-03527-1
65.
Xiang T Sun F Liu T Zhao J Yang J Ouyang D et al EBV-associated epithelial cancers cells promote vasculogenic mimicry formation via a secretory cross-talk with the immune microenvironment. Theranostics. (2024) 14(13):5123–40. 10.7150/thno.100171
66.
De Paoli F Staels B Chinetti-Gbaguidi G . Macrophage phenotypes and their modulation in atherosclerosis. Circ J. (2014) 78(8):1775–81. 10.1253/circj.cj-14-0621
67.
Xia B Lu YL Peng J Liang JW Li FQ Ding JY et al Galactin-8 DNA methylation mediates macrophage autophagy through the MAPK/mTOR pathway to alleviate atherosclerosis. Sci Rep. (2025) 15(1):603. 10.1038/s41598-024-85036-1
68.
Shen X Xie X Wu Q Shi F Chen Y Yuan S et al S-adenosylmethionine attenuates angiotensin II-induced aortic dissection formation by inhibiting vascular smooth muscle cell phenotypic switch and autophagy. Biochem Pharmacol. (2024) 219:115967. 10.1016/j.bcp.2023.115967
69.
Yu L Huang T Zhao J Zhou Z Cao Z Chi Y et al Branched-chain amino acid catabolic defect in vascular smooth muscle cells drives thoracic aortic dissection via mTOR hyperactivation. Free Radic Biol Med. (2024) 210:25–41. 10.1016/j.freeradbiomed.2023.11.002
70.
Xie S Zhao J Zhang F Li X Yu X Shu Z et al Dehydrodiisoeugenol inhibits PDGF-BB-induced proliferation and migration of human pulmonary artery smooth muscle cells via the mTOR/HIF1-α/HK2 signaling pathway. Toxicol Appl Pharmacol. (2024) 495:117212. 10.1016/j.taap.2024.117212
71.
Han X Zhu QQ Li Z He JK Sun Y Zhong QH et al 4-hydroxychalcone attenuates AngII-induced cardiac remodeling and dysfunction via regulating PI3K/AKT pathway. Hypertens Res. (2024) 48. 10.1038/s41440-024-02068-w
72.
Mao S Yang M Liu H Wang S Liu M Hu S et al Serinc2 antagonizes pressure overload-induced cardiac hypertrophy via regulating the amino acid/mTORC1 signaling pathway. Biochim Biophys Acta Mol Basis Dis. (2025) 1871:167650. 10.1016/j.bbadis.2024.167650
73.
Richards D Fujito H Shanbhag A Akhavan B Frame E Hayes S et al Utility of mechanistic target of rapamycin inhibitors in cardiac sarcoidosis. J Card Fail. (2024). 10.1016/j.cardfail.2024.10.444
74.
Saha S Fang X Green CD Das A . mtorc1 and Sglt2 inhibitors-a therapeutic perspective for diabetic cardiomyopathy. Int J Mol Sci. (2023) 24:15078. 10.3390/ijms242015078
75.
Noori T Sureda A Shirooie S . Role of natural mTOR inhibitors in treatment of diabetes mellitus. Fundam Clin Pharmacol. (2023) 37(3):461–79. 10.1111/fcp.12851
76.
Zhao YJ Wu WH Niu KM Zhang WJ Li SR Bao RL et al Xinkeshu formula restrains pathological cardiac hypertrophy through metabolic remodeling via AMPK/mTOR pathway. Phytomedicine. (2024) 136:156309. 10.1016/j.phymed.2024.156309
77.
Ma H Ge Y Di C Wang X Qin B Wang A et al Gq262 attenuates pathological cardiac remodeling by downregulating the AKT/mTOR signaling pathway. Int J Mol Sci. (2024) 25(19):10297. 10.3390/ijms251910297
78.
Ren W Huang Y Meng S Cao Z Qin N Zhao J et al Salidroside treatment decreases the susceptibility of atrial fibrillation in diabetic mice by reducing mtor-Stat3-mcp-1 signaling and atrial inflammation. Int Immunopharmacol. (2024) 142(Pt B):113196. 10.1016/j.intimp.2024.113196
79.
Mustafa HJ Javinani A Morning ML D’Antonio F Pagani G Puranik PM et al Characteristics and outcomes of fetal cardiac rhabdomyoma with or without mTOR inhibitors, a systematic review and meta-analysis. Prenat Diagn. (2024) 44(10):1251–67. 10.1002/pd.6640
80.
Zhao D Xu R Zhou Y Wu J Zhang X Lin H et al ORP5 promotes cardiac hypertrophy by regulating the activation of mTORC1 on lysosome. J Adv Res. (2024). 10.1016/j.jare.2024.12.014
81.
Tan C Zhou H Xiong Q Xian X Liu Q Zhang Z et al Cromolyn sodium reduces LPS-induced pulmonary fibrosis by inhibiting the EMT process enhanced by MC-derived IL-13. Respir Res. (2025) 26(1):3. 10.1186/s12931-024-03045-0
82.
Aytekin A Kadakal H Mihcioglu D Gurer T . Bioinformatics analysis of miR-2861 and miR-5011-5p that function as potential tumor suppressors in colorectal carcinogenesis. BMC Med Genomics. (2025) 18(1):1. 10.1186/s12920-024-02080-6
83.
Li Y Liu Z Yan H Zhou T Zheng L Wen F et al Polygonatum sibiricum polysaccharide ameliorates skeletal muscle aging and mitochondrial dysfunction via PI3K/AKT/mTOR signaling pathway. Phytomedicine. (2024) 136:156316. 10.1016/j.phymed.2024.156316
84.
Kibalnyk Y Afanasiev E Noble RMN Watson AES Poverennaya I Dittmann NL et al The chromatin regulator Ankrd11 controls cardiac neural crest cell-mediated outflow tract remodeling and heart function. Nat Commun. (2024) 15(1):4632. 10.1038/s41467-024-48955-1
85.
Luo J He M Liang C Huang X Zhu Y Hu D et al Canagliflozin reverses doxorubicin-induced cardiotoxicity via restoration of autophagic homeostasis. Toxicol Appl Pharmacol. (2024) 495:117183. 10.1016/j.taap.2024.117183
86.
Yin J Lu Y Liu Y Shi Q Shi M Zhu Z et al Siglec11 promotes M2 macrophage polarization through AKT-mTOR signaling and facilitates the progression of gastric cancer. J Immunother Cancer. (2025) 13(1):e146821. 10.1136/jitc-2024-010162
87.
Remy D Antoine-Bally S de Toqueville S Jolly C Macé AS Champenois G et al TFEB triggers a matrix degradation and invasion program in triple-negative breast cancer cells upon mTORC1 repression. Dev Cell. (2024) 60:1018–35. 10.1016/j.devcel.2024.12.005
88.
Jasińska-Stroschein M Glajzner P . Searching for old and new small-molecule protein kinase inhibitors as effective treatments in pulmonary hypertension-a systematic review. Int J Mol Sci. (2024) 25(23):12858. 10.3390/ijms252312858
89.
Wright B King S Suphioglu C . The importance of phosphoinositide 3-kinase in neuroinflammation. Int J Mol Sci. (2024) 25(21):11638. 10.3390/ijms252111638
90.
Yao Z Chen H . Everolimus in pituitary tumor: a review of preclinical and clinical evidence. Front Endocrinol (Lausanne). (2024) 15:1456922. 10.3389/fendo.2024.1456922
91.
Diao L Wu Y Jiang X Chen B Zhang W Chen L et al Human umbilical cord mesenchymal stem cell-derived exosomes modulate the NLRP3 inflammasome/caspase-1 pathway to repress pyroptosis induced by hypoxia/reoxygenation in cardiac microvascular endothelial cells. Int Heart J. (2024) 65(6):1107–17. 10.1536/ihj.23-500
92.
Roudi HS Safaei R Dabbaghi MM Fadaei MS Saberifar M Sakhaee K et al Mechanistic insights on cardioprotective properties of ursolic acid: regulation of mitochondrial and non-mitochondrial pathways. Curr Pharm Des. (2024) 31. 10.2174/0113816128344497241120025757
93.
Bhat OM Mir RA Nehvi IB Wani NA Dar AH Zargar MA . Emerging role of sphingolipids and extracellular vesicles in development and therapeutics of cardiovascular diseases. Int J Cardiol Heart Vasc. (2024) 53:101469. 10.1016/j.ijcha.2024.101469
94.
Delcheva G Stefanova K Stankova T . Ceramides-emerging biomarkers of lipotoxicity in obesity, diabetes, cardiovascular diseases, and inflammation. Diseases. (2024) 12(9):195. 10.3390/diseases12090195
95.
Zhao X Liang Z Zhao W Tao Y Hao Y Liu Y et al ALKBH5 deletion ameliorates inflammation by regulating IRF3 signaling in an M(6)a-dependent manner after myocardial infarction. Biochem Biophys Res Commun. (2025) 742:151039. 10.1016/j.bbrc.2024.151039
96.
Pang B Dong G Pang T Sun X Liu X Nie Y et al Advances in pathogenesis and treatment of vascular endothelial injury-related diseases mediated by mitochondrial abnormality. Front Pharmacol. (2024) 15:1422686. 10.3389/fphar.2024.1422686
97.
Vilahur G Ben-Aicha S Gutiérrez M Radike M Mendieta G Ramos L et al Cardioprotection exerted by intravenous statin at index myocardial infarction event attenuates cardiac damage upon recurrent infarction. Cardiovasc Res. (2025). 10.1093/cvr/cvae264
98.
Shi Y He T Liu H Li X Li Z Wen Q et al Ganglioside GA2-mediated caspase-11 activation drives macrophage pyroptosis aggravating intimal hyperplasia after arterial injury. Int J Biol Sci. (2025) 21(1):433–53. 10.7150/ijbs.97106
99.
Seale B Slotabec L Nguyen JD Wang H Patterson C Filho F et al Sestrin2 serves as a scaffold protein to maintain cardiac energy and metabolic homeostasis during pathological stress. Faseb J. (2024) 38(20):e70106. 10.1096/fj.202401404R
100.
Zhang H Xu T Mei X Zhao Q Yang Q Zeng X et al PINK1 modulates Prdx2 to reduce lipotoxicity-induced apoptosis and attenuate cardiac dysfunction in heart failure mice with a preserved ejection fraction. Clin Transl Med. (2025) 15(1):e70166. 10.1002/ctm2.70166
101.
Guan J Mo H Virak V Guo R Que D Yu W et al eEF2K alleviates doxorubicin-induced cardiotoxicity by inhibiting GSK3β and improving autophagy dysfunction. Cell Biol Toxicol. (2024) 41(1):15. 10.1007/s10565-024-09966-2
102.
Zhou X Wang H Yan B Nie X Chen Q Yang X et al Ferroptosis in cardiovascular diseases and ferroptosis-related intervention approaches. Cardiovasc Drugs Ther. (2024). 10.1007/s10557-024-07642-5
103.
Saedi S Tan Y Watson SE Wintergerst KA Cai L . Potential pathogenic roles of ferroptosis and cuproptosis in cadmium-induced or exacerbated cardiovascular complications in individuals with diabetes. Front Endocrinol (Lausanne). (2024) 15:1461171. 10.3389/fendo.2024.1461171
104.
Jinson S Zhang Z Lancaster GI Murphy AJ Morgan PK . Iron, lipid peroxidation and ferroptosis play pathogenic roles in atherosclerosis. Cardiovasc Res. (2024) 121:44–61. 10.1093/cvr/cvae270
105.
Miao M Yang Y Dai H . Current research status and future prospects of NLRP3 inflammasome in cardiovascular diseases: a bibliometric and visualization analysis. Front Cardiovasc Med. (2024) 11:1407721. 10.3389/fcvm.2024.1407721
106.
Guo NK Si LN Li PQ Gan GF . Nano acacetin mitigates intestinal mucosal injury in sepsis rats by protecting mitochondrial function and regulating TRX1 to inhibit the NLRP3 pyroptosis pathway. Int J Nanomedicine. (2024) 19:14125–41. 10.2147/ijn.S497081
107.
Zheng YY Shen DN Peng XL San WQ Zhou QY Yang SJ et al TRADD-mediated pyroptosis contributes to diabetic cardiomyopathy. Acta Pharmacol Sin. (2025) 46:940–50. 10.1038/s41401-024-01450-1
108.
Li W Liu Y Xu R Zong Y He L Hu J et al M(6)A modification in cardiovascular disease: with a focus on programmed cell death. Genes Dis. (2024) 11(5):101039. 10.1016/j.gendis.2023.05.023
109.
Contursi A Tacconelli S Di Berardino S De Michele A Patrignani P . Platelets as crucial players in the dynamic interplay of inflammation, immunity, and cancer: unveiling new strategies for cancer prevention. Front Pharmacol. (2024) 15:1520488. 10.3389/fphar.2024.1520488
110.
He W Sun Z Tong G Zeng L He W Chen X et al FUNDC1 alleviates doxorubicin-induced cardiotoxicity by restoring mitochondrial-endoplasmic reticulum contacts and blocked autophagic flux. Theranostics. (2024) 14(9):3719–38. 10.7150/thno.92771
111.
Gao Y Wang B Hu M Ma Y Zheng B . The role of iron in atherosclerosis and its association with related diseases. Curr Atheroscler Rep. (2024) 27(1):1. 10.1007/s11883-024-01251-1
112.
Han G Hu K Luo T Wang W Zhang D Ouyang L et al Research progress of non-coding RNA regulating the role of panoptosis in diabetes mellitus and its complications. Apoptosis. (2025) 30:516–36. 10.1007/s10495-024-02066-w
113.
Ma Z Li M Guo R Tian Y Zheng Y Huang B et al Treating myocardial infarction via a nano-ultrasonic contrast agent-mediated high-efficiency drug delivery system targeting macrophages. Sci Adv. (2025) 11(1):eadp7126. 10.1126/sciadv.adp7126
114.
Hou X Xiao H Zhang Y Zeng X Huang M Chen X et al Transient receptor potential channel 6 knockdown prevents apoptosis of renal tubular epithelial cells upon oxidative stress via autophagy activation. Cell Death Dis. (2018) 9(10):1015. 10.1038/s41419-018-1052-5
115.
Yu L Jin W Deng D Wang Y Chen Q Zhang Y et al Investigation of anti-apoptotic effects and mechanisms of astragaloside iv in a rat model of cerebral ischemia-reperfusion injury. CNS Neurosci Ther. (2025) 31(1):e70209. 10.1111/cns.70209
116.
Lee KCY Williams AL Hara A Khadka VS Hayashi J Shohet RV . Loss of PKM2 dysregulates inflammatory signaling in the infarcted murine heart. Physiol Rep. (2025) 13(1):e70193. 10.14814/phy2.70193
117.
Tian W Cao H Li X Gong X Yu X Li D et al Adjunctive PCSK9 inhibitor evolocumab in the prevention of early neurological deterioration in non-cardiogenic acute ischemic stroke: a multicenter, prospective, randomized, open-label, clinical trial. CNS Drugs. (2025) 39:197–208. 10.1007/s40263-024-01145-5
118.
Yang N Dong Z Xiao W Deng S Li Y Hua L et al Hexamethylene amiloride induces lysosome-mediated cell death in multiple myeloma through transcription factor E3. Cell Death Discov. (2024) 10(1):505. 10.1038/s41420-024-02269-9
119.
Rabinovich-Nikitin I Kirshenbaum LA . YAP/TFEB pathway promotes autophagic cell death and hypertrophic cardiomyopathy in lysosomal storage diseases. J Clin Invest. (2021) 131(5):e010162. 10.1172/jci146821
120.
Yagi M Do Y Hirai H Miki K Toshima T Fukahori Y et al Improving lysosomal ferroptosis with NMN administration protects against heart failure. Life Sci Alliance. (2023) 6(12):e202302116. 10.26508/lsa.202302116
121.
Blomgren K Leist M Groc L . Pathological apoptosis in the developing brain. Apoptosis. (2007) 12(5):993–1010. 10.1007/s10495-007-0754-4
122.
Chuang YT Yen CY Tang JY Chang FR Tsai YH Wu KC et al The modulation of immune cell death in connection to micrornas and natural products. Front Immunol. (2024) 15:1425602. 10.3389/fimmu.2024.1425602
123.
Zhang W Jiang L Tong X He H Zheng Y Xia Z . Sepsis-induced endothelial dysfunction: permeability and regulated cell death. J Inflamm Res. (2024) 17:9953–73. 10.2147/jir.S479926
124.
Dow CT Kidess Z . Proposing bromo-epi-androsterone (BEA) for perioperative neurocognitive disorders with interleukin-6 as a druggable target. J Clin Anesth. (2025) 101:111736. 10.1016/j.jclinane.2024.111736
125.
Guo M Qiu MY Zeng L Nie YX Tang YL Luo Y et al Acidosis induces autophagic cell death through Asic1-mediated Akt/mTOR signaling in HT22 neurons. Toxicology. (2025) 511:154045. 10.1016/j.tox.2025.154045
126.
Wolf G Craigon C Teoh ST Essletzbichler P Onstein S Cassidy D et al The efflux pump ABCC1/MRP1 constitutively restricts protac sensitivity in cancer cells. Cell Chem Biol. (2024) 60:1018–35. 10.1016/j.chembiol.2024.11.009
127.
Liu H Song Y Wang H Zhou Y Xu M Xian J . Deciphering the power of resveratrol in mitophagy: from molecular mechanisms to therapeutic applications. Phytother Res. (2025) 39:1319–43. 10.1002/ptr.8433
128.
Xia TS Xu SY Lai LY Jiang YP Wang NN Xin HL . Bitter acids from Humulus lupulus L. alleviate D-galactose induced osteoblastic senescence and bone loss via regulating AKT/mTOR-mediated autophagy. J Food Drug Anal. (2024) 32(4):506–19. 10.38212/2224-6614.3508
129.
Zhou B Liu Y Ma H Zhang B Lu B Li S et al Zdhhc1 deficiency mitigates foam cell formation and atherosclerosis by inhibiting PI3K-Akt-mTOR signaling pathway through facilitating the nuclear translocation of P110α. Biochim Biophys Acta Mol Basis Dis. (2025) 1871(2):167577. 10.1016/j.bbadis.2024.167577
130.
Xie F Liu B Qiao W He JZ Cheng J Wang ZY et al Smooth muscle NF90 deficiency ameliorates diabetic atherosclerotic calcification in male mice via FBXW7-AGER1-AGEs axis. Nat Commun. (2024) 15(1):4985. 10.1038/s41467-024-49315-9
131.
Du L Xiao Y Wei Q Guo Z Li Y . Preparation, evaluation, and bioinformatics study of hyaluronic acid-modified ginsenoside Rb1 self-assembled nanoparticles for treating cardiovascular diseases. Molecules. (2024) 29(18):4423. 10.3390/molecules29184425
132.
Anilkumar SA Dutta S Aboo S Ismail A . Vitamin D as a modulator of molecular pathways involved in CVDs: evidence from preclinical studies. Life Sci. (2024) 357:123062. 10.1016/j.lfs.2024.123062
133.
Zarzycka W Kobak KA King CJ Peelor FF Miller BF 3rd Chiao YA . Hyperactive mTORC1/4EBP1 signaling dysregulates proteostasis and accelerates cardiac aging. Geroscience (2024) 47:1823–36. 10.1007/s11357-024-01368-w
134.
Qi J Li Q Xin T Lu Q Lin J Zhang Y et al MCOLN1/TRPML1 in the lysosome: a promising target for autophagy modulation in diverse diseases. Autophagy. (2024) 20(8):1712–22. 10.1080/15548627.2024.2333715
135.
Liu X Tuerxun H Zhao Y Li Y Wen S Li X et al Crosstalk between ferroptosis and autophagy: broaden horizons of cancer therapy. J Transl Med. (2025) 23(1):18. 10.1186/s12967-024-06059-w
136.
Wang J Huang Z Li Y Li Q Li X Chen L . Electroacupuncture improves cognitive function in high-fat diet/streptozocin-induced type 2 diabetic mice by inhibiting autophagy-related ferroptosis. Exp Anim. (2024) 74:197–208. 10.1538/expanim.24-0072
137.
Liu C Huang X Kong J Li X Wang Y Zhang F et al Podophyllotoxin mediates hepatic toxicity via the C5a/C5aR/ROS/NLRP3 and cGMP/PKG/mTOR axis in rats based on toxicological evidence chain (TEC) concept by phosphoproteomic analysis. Ecotoxicol Environ Saf. (2024) 289:117441. 10.1016/j.ecoenv.2024.117441
138.
Zhang X Jiang X Deng H Yu G Yang N Al Mamun A et al Engineering exosomes from fibroblast growth factor 1 pre-conditioned adipose-derived stem cells promote ischemic skin flaps survival by activating autophagy. Mater Today Bio. (2024) 29:101314. 10.1016/j.mtbio.2024.101314
139.
Sun X Tang X Qiu H . Cardiac-specific suppression of valosin-containing protein induces progressive heart failure and premature mortality correlating with temporal dysregulations in mTOR complex 2 and protein phosphatase 1. Int J Mol Sci. (2024) 25(12):6445. 10.3390/ijms25126445
140.
Zhang RX Zhai YY Ding RR Huang JH Shi XC Liu H et al FNDC1 is a myokine that promotes myogenesis and muscle regeneration. Embo J. (2025) 44(1):30–53. 10.1038/s44318-024-00285-0
141.
Zein L Dietrich M Balta D Bader V Scheuer C Zellner S et al Linear ubiquitination at damaged lysosomes induces local NFKB activation and controls cell survival. Autophagy. (2025) 21:1–21. 10.1080/15548627.2024.2443945
142.
Barcena ML Aslam M Norman K Ott C Ladilov Y . Role of AMPK and sirtuins in aging heart: basic and translational aspects. Aging Dis. (2024). 10.14336/ad.2024.1216
143.
Wang Q Liu X Yuan J Yang T Ding L Song B et al NEK6 regulates autophagy through the mTOR signaling pathway to alleviate cerebral ischemia-reperfusion injury. Mol Brain. (2024) 17(1):96. 10.1186/s13041-024-01166-7
144.
Waypa GB Smith KA Mungai PT Dudley VJ Helmin KA Singer BD et al Mitochondria regulate proliferation in adult cardiac myocytes. J Clin Invest. (2024) 134(13):e165482. 10.1172/jci165482
145.
Wang B Shi D Yang S Lian Y Li H Cao M et al Mitochondrial trna pseudouridylation governs erythropoiesis. Blood. (2024) 144(6):657–71. 10.1182/blood.2023022004
146.
Sirera J Sarlak S Teisseire M Carminati A Nicolini VJ Savy C et al Disrupting USP39 deubiquitinase function impairs the survival and migration of multiple myeloma cells through ZEB1 degradation. J Exp Clin Cancer Res. (2024) 43(1):335. 10.1186/s13046-024-03241-2
147.
Huang C Zhang X Wu SX Chang Q Zheng ZK Xu J . METTL3, m6A modification, and EGR1: interplay affecting myocardial I/R injury outcomes. Cell Biol Toxicol. (2024) 41(1):7. 10.1007/s10565-024-09937-7
148.
Zahid R Wang J Cai Z Ishtiaq A Liu M Ma D et al Single chain fragment variable, a new theranostic approach for cardiovascular diseases. Front Immunol. (2024) 15:1443290. 10.3389/fimmu.2024.1443290
149.
Guo B Zhang F Yin Y Ning X Zhang Z Meng Q et al Post-translational modifications of pyruvate dehydrogenase complex in cardiovascular disease. iScience. (2024) 27(9):110633. 10.1016/j.isci.2024.110633
150.
Wang Y Hu J Wu S Fleishman JS Li Y Xu Y et al Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases. Signal Transduct Target Ther. (2023) 8(1):449. 10.1038/s41392-023-01720-0
151.
Cheng X Wang K Zhao Y Wang K . Research progress on post-translational modification of proteins and cardiovascular diseases. Cell Death Discov. (2023) 9(1):275. 10.1038/s41420-023-01560-5
152.
Yang J Guo Q Feng X Liu Y Zhou Y . Mitochondrial dysfunction in cardiovascular diseases: potential targets for treatment. Front Cell Dev Biol. (2022) 10:841523. 10.3389/fcell.2022.841523
153.
Laurindo LF Sosin AF Lamas CB de Alvares Goulart R Dos Santos Haber JF Detregiachi CRP et al Exploring the logic and conducting a comprehensive evaluation of adiporon-based adiponectin replacement therapy against hormone-related cancers-a systematic review. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397(4):2067–82. 10.1007/s00210-023-02792-z
154.
Gao R Lu Y Zhang W Zhang Z . The application of berberine in fibrosis and the related diseases. Am J Chin Med. (2024) 52(3):753–73. 10.1142/s0192415×24500307
155.
Artemenko M Zhong SSW To SKY Wong AST . P70 S6 kinase as a therapeutic target in cancers: more than just an mTOR effector. Cancer Lett. (2022) 535:215593. 10.1016/j.canlet.2022.215593
156.
Dilber Y Çeker HT Öztüzün A Çırçırlı B Kırımlıoğlu E Barut Z et al Sparstolonin B reduces estrogen-dependent proliferation in cancer cells: possible role of ceramide and PI3K/AKT/mTOR inhibition. Pharmaceuticals (Basel). (2024) 17(12):1564. 10.3390/ph17121564
157.
Li G Wen Z Xiong S . Microenvironmental β-TrCP negates amino acid transport to trigger CD8(+) T cell exhaustion in human non-small cell lung cancer. Cell Rep. (2025) 44(1):115128. 10.1016/j.celrep.2024.115128
158.
Wen D Xiao H Gao Y Zeng H Deng J. N6-methyladenosine-modified SENP1, identified by IGF2BP3, is a novel molecular marker in acute myeloid leukemia and aggravates progression by activating AKT signal via de-SUMOylating HDAC2. Mol Cancer (2024) 23(1):116. 10.1186/s12943-024-02013-y
159.
Shao Q Wykretowicz J Hu N Bedi K Rizk M Malek IA et al Aberrant BCAT1 expression augments mTOR activity and accelerates disease progression in chronic lymphocytic leukemia. Leukemia. (2024) 39:112–21. 10.1038/s41375-024-02448-8
160.
Li Y Guo C Zhang F Cheng S Li Y Luo S et al Dnmt1 inhibition improves the activity of memory-like natural killer cells by enhancing the level of autophagy. Mol Biol Rep. (2024) 52(1):68. 10.1007/s11033-024-10181-9
161.
Kugiyama N Nagaoka K Yamada R Watanabe T Yamazaki H Ushijima S et al Serum Mac2-binding protein glycosylated isomer (M2BPGi) as a prognostic biomarker in pancreatic ductal adenocarcinoma: iCAFs-derived M2BPGi drives tumor invasion. J Gastroenterol. (2024) 60:479–95. 10.1007/s00535-024-02195-8
162.
Palenca I Basili Franzin S Zilli A Seguella L Troiani A Pepi F et al N-palmitoyl-d-glucosamine limits mucosal damage and VEGF-mediated angiogenesis by PPARα-dependent suppression of pAkt/mTOR/HIF1α pathway and increase in PEA levels in AOM/DSS colorectal carcinoma in mice. Phytother Res. (2024) 38(11):5350–62. 10.1002/ptr.8303
163.
Huang J Zhu Z Schlüter D Lambertsen KL Song W Wang X . Ubiquitous regulation of cerebrovascular diseases by ubiquitin-modifying enzymes. Clin Transl Med. (2024) 14(5):e1719. 10.1002/ctm2.1719
164.
Rahman S Wolberger C . Breaking the K48-chain: linking ubiquitin beyond protein degradation. Nat Struct Mol Biol. (2024) 31(2):216–8. 10.1038/s41594-024-01221-w
165.
Wang F Lerman A Herrmann J . Dysfunction of the ubiquitin-proteasome system in atherosclerotic cardiovascular disease. Am J Cardiovasc Dis. (2015) 5(1):83–100.
166.
Li Y Yu J Zeng Z Lin W . Regulation of ubiquitination in sepsis: from PAMP versus DAMP to peripheral inflammation and cell death. Front Immunol. (2024) 15:1513206. 10.3389/fimmu.2024.1513206
167.
Jin JY Wei XX Zhi XL Wang XH Meng D . Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol Sin. (2021) 42(5):655–64. 10.1038/s41401-020-00518-y
168.
Tsang SV Rainusso N Liu M Nomura M Patel TD Nakahata K et al LncRNA PVT-1 promotes osteosarcoma cancer stem-like properties through direct interaction with TRIM28 and TSC2 ubiquitination. Oncogene. (2022) 41(50):5373–84. 10.1038/s41388-022-02538-w
169.
Zotta A O’Neill LAJ Yin M . Unlocking potential: the role of the electron transport chain in immunometabolism. Trends Immunol. (2024) 45(4):259–73. 10.1016/j.it.2024.02.002
170.
Deng Z Long D Li C Liu H Li W Zhong Y et al IRF1-mediated upregulation of PARP12 promotes cartilage degradation by inhibiting PINK1/Parkin dependent mitophagy through ISG15 attenuating ubiquitylation and SUMOylation of MFN1/2. Bone Res. (2024) 12(1):63. 10.1038/s41413-024-00363-3
171.
Xiong Z Yang L Zhang C Huang W Zhong W Yi J et al MANF facilitates breast cancer cell survival under glucose-starvation conditions via PRKN-mediated mitophagy regulation. Autophagy. (2025) 21(1):80–101. 10.1080/15548627.2024.2392415
172.
Kleimann P Irschfeld LM Grandoch M Flögel U Temme S . Trained innate immunity in animal models of cardiovascular diseases. Int J Mol Sci. (2024) 25(4):2312. 10.3390/ijms25042312
173.
Cheng J Zhang Y Ge Y Li W Cao Y Qu Y et al Sodium butyrate promotes milk fat synthesis in bovine mammary epithelial cells via GPR41 and its downstream signalling pathways. Life Sci. (2020) 259:118375. 10.1016/j.lfs.2020.118375
174.
Quan J Jia Z Liu L Tian J . The effect of long-term administration of green tea catechins on aging-related cardiac diastolic dysfunction and decline of troponin I. Genes Dis. (2025) 12(2):101284. 10.1016/j.gendis.2024.101284
175.
Bogetofte H Ryan BJ Jensen P Schmidt SI Vergoossen DLE Barnkob MB et al Post-translational proteomics platform identifies neurite outgrowth impairments in Parkinson’s disease GBA-N370s dopamine neurons. Cell Rep. (2023) 42(3):112180. 10.1016/j.celrep.2023.112180
176.
Cheng Q Li Z Li Y Chen L Chen D Zhu J . The emerging role and mechanism of E2/E3 hybrid enzyme UBE2O in human diseases. Biomedicines. (2025) 13(5):1082. 10.3390/biomedicines13051082
177.
Sun J Liu C Lang C Wang J Li Q Peng C et al Inhibiting neddylation: a new strategy for tumor therapy. J Pharm Anal. (2025) 15(5):101140. 10.1016/j.jpha.2024.101140
178.
Li M Huang W Zhang Y Du Y Zhao S Wang L et al Glucose deprivation triggers DCAF1-mediated inactivation of Rheb-mTORC1 and promotes cancer cell survival. Cell Death Dis. (2024) 15(6):409. 10.1038/s41419-024-06808-1
179.
Wang W Yang Y Shi Y Xiang T Xie J . E3 ubiquitin ligase STUB1 affects the mTORC1 pathway through p62 and participates in regulating the differentiation of follicular helper T cells in rheumatoid arthritis. Clin Immunol. (2023) 255:109736. 10.1016/j.clim.2023.109736
180.
Zheng S Zhu J Wang C Wu Y Sun S Guo H et al USP9x-mediated deubiquitination of raptor contributes to autophagy impairment and memory deficits in P301s mice. Cell Commun Signal. (2024) 22(1):516. 10.1186/s12964-024-01872-8
181.
Zhang J Zha Y Jiao Y Li Y Zhang S . Protective role of cezanne in doxorubicin-induced cardiotoxicity by inhibiting autophagy, apoptosis and oxidative stress. Toxicology. (2023) 485:153426. 10.1016/j.tox.2023.153426
182.
Cheong MC Mackowiak B Kim HB Hernandez G Nandu T Vale K et al Ethanol induction of FGF21 in the liver is dependent on histone acetylation and ligand activation of ChREBP by glycerol-3-phosphate. Proc Natl Acad Sci U S A. (2025) 122(22):e2505263122. 10.1073/pnas.2505263122
183.
Du C Zhu Y Duan J Yang Y Ren Y Mu L et al A-485 alleviates fibrosis and apoptosis in kidney by disrupting tandem activation of acetylation and phosphorylation on Stat3. Biomed Pharmacother. (2025) 188:118217. 10.1016/j.biopha.2025.118217
184.
Yu F Zhao H Luo L Wu W . Nicotinamide adenine dinucleotide supplementation to alleviate heart failure: a mitochondrial dysfunction perspective. Nutrients. (2025) 17(11):1855. 10.3390/nu17111855
185.
Mao S Liu ZY Liu ZY Liu P Lin LC Zhang Y et al Phase separation of epigenetic landscape in cardiovascular diseases. Biomed Pharmacother. (2024) 181:117654. 10.1016/j.biopha.2024.117654
186.
Wang K Wang Y Li Y Fang B Li B Cheng W et al The potential of RNA methylation in the treatment of cardiovascular diseases. iScience. (2024) 27(8):110524. 10.1016/j.isci.2024.110524
187.
Li B Wang K Cheng W Fang B Li YH Yang SM et al Recent advances of PIWI-interacting RNA in cardiovascular diseases. Clin Transl Med. (2024) 14(8):e1770. 10.1002/ctm2.1770
188.
Du Y Yang L Wang X Jiang N Zhou Y Chen R et al Proteome profiling of experimental autoimmune encephalomyelitis mouse model and the effect of a sumo E1 inhibitor. J Proteome Res. (2024) 23(12):5312–25. 10.1021/acs.jproteome.4c00229
189.
Datta S Rahman MA Koka S Boini KM . High mobility group box 1 (HMGB1): molecular signaling and potential therapeutic strategies. Cells. (2024) 13(23):1946. 10.3390/cells13231946
190.
Kowalczyk P Krych S Kramkowski K Jęczmyk A Hrapkowicz T . Effect of oxidative stress on mitochondrial damage and repair in heart disease and ischemic events. Int J Mol Sci. (2024) 25(22):12467. 10.3390/ijms252212467
191.
Peng J Zhu H Ruan B Duan Z Cao M . miR-155 promotes M6A modification of SOX2 mRNA through targeted regulation of HIF-1α and delays wound healing in diabetic foot ulcer in vitro models. J Diabetes Investig. (2025) 16(1):60–71. 10.1111/jdi.14327
192.
Li L Wang J Zhang D Deng L Zhao X Wang C et al Resveratrol relieves myocardial ischemia-reperfusion injury through inhibiting AKT nitration modification. Redox Rep. (2024) 29(1):2420564. 10.1080/13510002.2024.2420564
193.
Yin S Liu L Gan W . The roles of post-translational modifications on mtor signaling. Int J Mol Sci. (2021) 22(4):1784. 10.3390/ijms22041784
194.
O’Sullivan PA Aidarova A Afonina IS Manils J Thurston TLM Instrell R et al CARD14 signalosome formation is associated with its endosomal relocation and mTORC1-induced keratinocyte proliferation. Biochem J. (2024) 481(18):1143–71. 10.1042/bcj20240058
195.
Alsamri H Alneyadi A Muhammad K Ayoub MA Eid A Iratni R . Carnosol induces P38-mediated ER stress response and autophagy in human breast cancer cells. Front Oncol. (2022) 12:911615. 10.3389/fonc.2022.911615
196.
Hertzog N Duman M Bochud M Brügger-Verdon V Gerhards M Schön F et al Hypoxia-induced conversion of sensory Schwann cells into repair cells is regulated by HDAC8. Nat Commun. (2025) 16(1):515. 10.1038/s41467-025-55835-9
197.
Liu Z Liu J Wei Y Li J Zhang J Yu R et al Ubiquitin-specific protease 25 ameliorates ulcerative colitis by regulating the degradation of phosphor-Stat3. Cell Death Dis. (2025) 16(1):5. 10.1038/s41419-024-07315-z
198.
Nakashima M Pinkaew D Pal U Miyao F Huynh H Tanaka L et al Fortilin binds CTNNA3 and protects it against phosphorylation, ubiquitination, and proteasomal degradation to guard cells against apoptosis. Commun Biol. (2025) 8(1):1. 10.1038/s42003-024-07399-5
199.
Zhu M Zhong W Wong S Luo X Hong Z Lin J et al E3 ubiquitin ligase ITCH-mediated proteasomal degradation of WBP2 sensitizes breast cancer cells to chemotherapy through restraining AMOTL2/C-JUN axis. Biochem Pharmacol. (2024) 232:116720. 10.1016/j.bcp.2024.116720
200.
Longo M Bishnu A Risiglione P Montava-Garriga L Cuenco J Sakamoto K et al Opposing roles for AMPK in regulating distinct mitophagy pathways. Mol Cell. (2024) 84(22):4350–67.e9. 10.1016/j.molcel.2024.10.025
201.
Seager R Ramesh NS Cross S Guo C Wilkinson KA Henley JM . SUMOylation of MFF coordinates fission complexes to promote stress-induced mitochondrial fragmentation. Sci Adv. (2024) 10(40):eadq6223. 10.1126/sciadv.adq6223
202.
Caba C Black M Liu Y DaDalt AA Mallare J Fan L et al Autoinhibition of ubiquitin-specific protease 8: insights into domain interactions and mechanisms of regulation. J Biol Chem. (2024) 300(10):107727. 10.1016/j.jbc.2024.107727
203.
Li M Chen X Qu P Shao Z Shi L Quan H et al FBXO22 inhibits colitis and colorectal carcinogenesis by regulating the degradation of the S2448-phosphorylated form of mTOR. Proc Natl Acad Sci U S A. (2024) 121(45):e2402035121. 10.1073/pnas.2402035121
204.
Jiao D Sun H Zhao X Chen Y Lv Z Shi Q et al mTORC1/S6K1 signaling promotes sustained oncogenic translation through modulating CRL3(IBTK)-mediated ubiquitination of eIF4A1 in cancer cells. Elife. (2024) 12:P92236. 10.7554/eLife.92236
205.
Mukherjee R Bhattacharya A Mello-Vieira J Kuncha SK Hoffmann M Gonzalez A et al Serine ubiquitination of SQSTM1 regulates NFE2L2-dependent redox homeostasis. Autophagy. (2024) 21:1–17. 10.1080/15548627.2024.2404375
206.
Bu WJ Li SS Liu C Wang YH Lu JR Dong CR et al Nepetin limits NLRP3 inflammasome activation and alleviates NLRP3-driven inflammatory diseases via PINK1-dependent mitophagy. Free Radic Biol Med. (2024) 227:420–33. 10.1016/j.freeradbiomed.2024.12.027
207.
Yang P Gao S Shen J Liu T Lu K Han X et al TRIM21-mediated ubiquitination of SQSTM1/p62 abolishes its Ser403 phosphorylation and enhances palmitic acid cytotoxicity. Autophagy. (2025) 21(1):178–90. 10.1080/15548627.2024.2394308
208.
Smirnov EY Silonov SA Shmidt EA Nozdracheva AV Pleskach NM Kuranova ML et al PML nuclear bodies and cellular senescence: a comparative study of healthy and premature aging syndrome donors’ cells. Cells. (2024) 13(24):2075. 10.3390/cells13242075
209.
Patranabis S . Recent advances in the miRNA-mediated regulation of neuronal differentiation and death. Neuromolecular Med. (2024) 26(1):52. 10.1007/s12017-024-08820-2
210.
Meng W Li L . N6-methyladenosine modification of SPOP relieves ferroptosis and diabetic cardiomyopathy by enhancing ubiquitination of VDAC3. Free Radic Biol Med. (2025) 226:216–29. 10.1016/j.freeradbiomed.2024.11.025
211.
Wang P Qiu J Fang Y Li S Liu K Cao Y et al SENP3 inhibition suppresses hepatocellular carcinoma progression and improves the efficacy of anti-PD-1 immunotherapy. Cell Death Differ. (2025) 32:959–72. 10.1038/s41418-024-01437-9
212.
Zang X He XY Xiao CM Lin Q Wang MY Liu CY et al Correction: circular RNA-encoded oncogenic PIAS1 variant blocks immunogenic ferroptosis by modulating the balance between SUMOylation and phosphorylation of STAT1. Mol Cancer. (2024) 23(1):278. 10.1186/s12943-024-02200-x
213.
Cheng Z Cheng Z Zhang Y Zhang S . “Intrinsic disorder-protein modification-LLPS-tumor” regulatory axis: from regulatory mechanisms to precision medicine. Biochim Biophys Acta Rev Cancer. (2024) 1880(1):189242. 10.1016/j.bbcan.2024.189242
214.
Montero-Martin N Girón MD Vílchez JD Salto R . Sodium tungstate promotes neurite outgrowth and confers neuroprotection in Neuro2a and SH-SY5Y cells. Int J Mol Sci. (2024) 25(17):9150. 10.3390/ijms25179150
215.
Martin N Schwamborn K Urlaub H Gan B Guan JL Dejean A . Spatial interplay between PIASy and FIP200 in the regulation of signal transduction and transcriptional activity. Mol Cell Biol. (2008) 28(8):2771–81. 10.1128/mcb.01210-07
216.
Meng L Du CP Lu CY Zhang K Li L Yan JZ et al Neuronal activity-induced SUMOylation of Akt1 by PIAS3 is required for long-term potentiation of synaptic transmission. Faseb Jj. (2021) 35(8):e21769. 10.1096/fj.202002728R
217.
Wang J Zheng X Wang X Zhong D Zhou G . E2 ubiquitin-conjugating enzymes regulates dengue virus-2 replication in aedes albopictus. Microorganisms. (2024) 12(12):2508. 10.3390/microorganisms12122508
218.
Park SLL Ramírez-Jarquín UN Shahani N Rivera O Sharma M Joshi PS et al SUMO modifies GβL and mediates mTOR signaling. J Biol Chem. (2024) 300(4):105778. 10.1016/j.jbc.2024.105778
219.
Sun F Wang FX Zhu H Yue TT Yang CL Luo JH et al SUMOylation of PDPK1 is required to maintain glycolysis-dependent CD4T-cell homeostasis. Cell Death Dis. (2022) 13(2):181. 10.1038/s41419-022-04622-1
220.
Yan Y Ollila S Wong IPL Vallenius T Palvimo JJ Vaahtomeri K et al SUMOylation of AMPKα1 by PIAS4 specifically regulates MTORC1 signalling. Nat Commun. (2015) 6:8979. 10.1038/ncomms9979
221.
Gao A Wang M Tang X Shi G Hou K Fang J et al NDP52 SUMOylation contributes to low-dose x-rays-induced cardiac hypertrophy through PINK1/Parkin-mediated mitophagy via MUL1/SUMO2 signalling. J Cell Physiol. (2024) 239(1):79–96. 10.1002/jcp.31145
222.
Lee B Oh Y Cho E DiAntonio A Cavalli V Shin JE et al FK506-binding protein-like and FK506-binding protein 8 regulate dual leucine zipper kinase degradation and neuronal responses to axon injury. J Biol Chem. (2022) 298(3):101647. 10.1016/j.jbc.2022.101647
223.
Miao C Huang Y Zhang C Wang X Wang B Zhou X et al Post-translational modifications in drug resistance. Drug Resist Updat. (2025) 78:101173. 10.1016/j.drup.2024.101173
224.
Liu H McCollum A Krishnaprakash A Ouyang Y Shi T Ratovitski T et al Roscovitine, a CDK inhibitor, reduced neuronal toxicity of mHTT by targeting HTT phosphorylation at S1181 and S1201 in vitro. Int J Mol Sci. (2024) 25(22):12315. 10.3390/ijms252212315
225.
Ho JSY Jou E Khong PL Foo RSY Sia CH . Epigenetics in heart failure. Int J Mol Sci. (2024) 25(22):12010. 10.3390/ijms252212010
226.
Li K Xie X Gao R Chen Z Yang M Wen Z et al Spatiotemporal protein interactome profiling through condensation-enhanced photocrosslinking. Nat Chem. (2025) 17(1):111–23. 10.1038/s41557-024-01663-1
227.
Shah RB Li Y Yu H Kini E Sidi S . Stepwise phosphorylation and SUMOylation of PIDD1 drive PIDDosome assembly in response to DNA repair failure. Nat Commun. (2024) 15(1):9195. 10.1038/s41467-024-53412-0
228.
Liu X Sha J Wang L Wang Z Fang Z Han X et al Rnf111 has a pivotal role in regulating development of definitive hematopoietic stem and progenitor cells through the Smad2/3-gcsfr/NO axis in zebrafish. Haematologica. (2024) 110:385–96. 10.3324/haematol.2024.285438
229.
Sun L Guo X Yu M Wang XF Ren H Wang X . Human ANP32a/B are SUMOylated and utilized by avian influenza virus NS2 protein to overcome Species-specific restriction. Nat Commun. (2024) 15(1):10805. 10.1038/s41467-024-55034-y
230.
Xia Q Que M Zhan G Zhang L Zhang X Zhao Y et al SENP6-mediated deSUMOylation of Nrf2 exacerbates neuronal oxidative stress following cerebral ischemia and reperfusion injury. Adv Sci (Weinh). (2024) 12:e2410410. 10.1002/advs.202410410
231.
Giaimo BD Ferrante F Borggrefe T . Lysine and arginine methylation of transcription factors. Cell Mol Life Sci. (2024) 82(1):5. 10.1007/s00018-024-05531-6
232.
Liu M Zeng T Zhang X Liu C Wu Z Yao L et al ATR/Chk1 signaling induces autophagy through sumoylated RhoB-mediated lysosomal translocation of TSC2 after DNA damage. Nat Commun. (2018) 9(1):4139. 10.1038/s41467-018-06556-9
233.
Wang Q Zhang X Chen L Weng S Xia Y Ye Y et al Regulation of the expression of DAPK1 by SUMO pathway. Biomolecules. (2019) 9(4):151. 10.3390/biom9040151
234.
Amemiya Y Ioi Y Araki M Kontani K Maki M Shibata H et al Calmodulin enhances mTORC1 signaling by preventing TSC2-Rheb binding. J Biol Chem. (2024) 302:108122. 10.1016/j.jbc.2024.108122
235.
Vuillefroy de Silly R Pericou L Seijo B Crespo I Irving M . Acidity suppresses CD8+T-cell function by perturbing IL-2, mTORC1, and c-Myc signaling. Embo J. (2024) 43(21):4922–53. 10.1038/s44318-024-00235-w
236.
Chen N Wang X Guo Y Zhao M Cao B Zhan B et al IL-37d suppresses Rheb-mTORC1 axis independently of TCS2 to alleviate alcoholic liver disease. Commun Biol. (2024) 7(1):756. 10.1038/s42003-024-06427-8
237.
Guo W Zong S Liu T Chao Y Wang K . The role of NOP58 in prostate cancer progression through SUMOylation regulation and drug response. Front Pharmacol. (2024) 15:1476025. 10.3389/fphar.2024.1476025
238.
Kotani H Yamano T Boucher JC Sato S Sakaguchi H Fukuda K et al Comprehensive antitumor immune response boosted by dual inhibition of SUMOylation and MEK in MYC-expressing KRAS-mutant cancers. Exp Hematol Oncol. (2024) 13(1):94. 10.1186/s40164-024-00563-x
239.
Jian D Li H Wang C Li F Li R Jin S et al METTL3-mediated m6A modification of ISG15 mRNA regulates doxorubicin-induced endothelial cell apoptosis. J Cell Mol Med. (2025) 29(1):e70339. 10.1111/jcmm.70339
240.
O’Connor PJ Haapala JL Dehmer SP Chumba LN Ekstrom HL Asche SE et al Clinical decision support and cardiometabolic medication adherence: a randomized clinical trial. JAMA Netw Open. (2025) 8(1):e2453745. 10.1001/jamanetworkopen.2024.53745
241.
Li F Zhang Y Wang Y Cai X Fan X . Cytokine gene variants as predisposing factors for the development and progression of coronary artery disease: a systematic review. Biomolecules. (2024) 14(12):1631. 10.3390/biom14121631
242.
Wang P Wang L Liu C Hu Y Feng G Lian Z et al YAP K236 acetylation facilitates its nucleic export and deprived the protection against cardiac hypertrophy in mice. Pharmacol Res. (2025) 211:107573. 10.1016/j.phrs.2024.107573
243.
Potì F Scalera E Feuerborn R Fischer J Arndt L Varga G et al Sphingosine 1-phosphate receptor 1signaling in macrophages reduces atherosclerosis in LDL receptor-deficient mice. JCI Insight. (2024) 9(24):e158217. 10.1172/jci.insight.158127
244.
Kang N Kim J Kwon M Son Y Eo SK Baryawno N et al Blockade of mTORC1 via rapamycin suppresses 27-hydroxycholestrol-induced inflammatory responses. Int J Mol Sci. (2024) 25(19):10381. 10.3390/ijms251910381
245.
Kumariya S Grano de Oro A Nestor-Kalinoski AL Joe B Osman I . Gut microbiota-derived metabolite, shikimic acid. Inhibits vascular smooth muscle cell proliferation and migration. Biochem Pharmacol. (2024) 229:116524. 10.1016/j.bcp.2024.116524
246.
Lee S Lee DH Lee JP Han JH . Rosuvastatin activates autophagy via inhibition of the Akt/mTOR axis in vascular smooth muscle cells. Korean J Physiol Pharmacol. (2025) 29(1):117–26. 10.4196/kjpp.24.284
247.
Ma M Song L Yan H Liu M Zhang L Ma Y et al Low dose tunicamycin enhances atherosclerotic plaque stability by inducing autophagy. Biochem Pharmacol. (2016) 100:51–60. 10.1016/j.bcp.2015.11.020
248.
Ugusman A Hisam NSN Othman NS Anuar NNM Hamid AA Kumar J et al Pharmacological interventions for intraplaque neovascularization in atherosclerosis. Pharmacol Ther. (2024) 261:108685. 10.1016/j.pharmthera.2024.108685
249.
Peng X Sun B Tang C Shi C Xie X Wang X et al HMOX1-LDHB interaction promotes ferroptosis by inducing mitochondrial dysfunction in foamy macrophages during advanced atherosclerosis. Dev Cell. (2024) 60:112–21. 10.1016/j.devcel.2024.12.011
250.
Polusani SR Cortez V Esparza J Nguyen HN Fan H Velagaleti GVN et al Oxidatively modified low-density lipoproteins are potential mediators of proteasome inhibitor resistance in multiple myeloma. Int J Cancer. (2021) 148(12):3032–40. 10.1002/ijc.33497
251.
Talabieke S Yang X Yang J Wan Q Zhu D Rao H et al Arachidonic acid synergizes with aspirin preventing myocardial ischemia-reperfusion injury and mitigates bleeding risk. Cardiovasc Res. (2025) 121:775–87. 10.1093/cvr/cvae254
252.
Tsurusaki S Kizana E . Mechanisms and therapeutic potential of multiple forms of cell death in myocardial ischemia-reperfusion injury. Int J Mol Sci. (2024) 25(24):13492. 10.3390/ijms252413492
253.
Qiao W Zang Z Li D Shao S Li Q Liu Z . Liensinine ameliorates ischemia-reperfusion-induced brain injury by inhibiting autophagy via PI3K/AKT signaling. Funct Integr Genomics. (2023) 23(2):140. 10.1007/s10142-023-01063-7
254.
Hu F Hu T He A Yuan Y Wang X Zou C et al Puerarin protects myocardium from ischaemia/reperfusion injury by inhibiting ferroptosis through downregulation of VDAC1. J Cell Mol Med. (2024) 28(24):e70313. 10.1111/jcmm.70313
255.
Zhao A Lei W Tian J Wu X Li M Zhang Y et al Mangiferin attenuates myocardial ischemia reperfusion injury by regulating the Gas6/axl signaling pathway. Phytother Res. (2025) 39:1388–402. 10.1002/ptr.8423
256.
Zhou W Wang S Yang J Shi Q Feng N Gao K et al Snhg16 alleviates pulmonary ischemia-reperfusion injury by promoting the Warburg effect through regulating Mtch2 expression: experimental studies. Int J Surg. (2025) 111:1874–90. 10.1097/js9.0000000000002217
257.
Santos-Báez LS Diaz-Rizzolo DA Borhan R Popp CJ Sordi-Guth A DeBonis D et al Predictive models of post-prandial glucose response in persons with prediabetes and early onset type 2 diabetes: a pilot study. Diabetes Obes Metab. (2025) 27:1515–25. 10.1111/dom.16160
258.
Witek P Bolanowski M Krętowski A Głowińska A . Pasireotide-induced hyperglycemia in cushing’s disease and acromegaly: a clinical perspective and algorithms proposal. Front Endocrinol (Lausanne). (2024) 15:1455465. 10.3389/fendo.2024.1455465
259.
Heynen G Baumgartner F Heider M Patra U Holz M Braune J et al SUMOylation inhibition overcomes proteasome inhibitor resistance in multiple myeloma. Blood Adv. (2023) 7(4):469–81. 10.1182/bloodadvances.2022007875
260.
Oliveira F Sousa Soares E Pillmann Ramos H Lättig-Tünnemann G Harms C Cimarosti H et al Renal protection after hemorrhagic shock in rats: possible involvement of sumoylation. Biochem Pharmacol. (2024) 227:116425. 10.1016/j.bcp.2024.116425
261.
Sennett C Jia W Khalil JS Hindle MS Coupland C Calaminus SDJ et al α-synuclein deletion impairs platelet function: a role for snare complex assembly. Cells. (2024) 13(24):2089. 10.3390/cells13242089
262.
Oeing CU Jun S Mishra S Dunkerly-Eyring BL Chen A Grajeda MI et al MTORC1-regulated metabolism controlled by TSC2 limits cardiac reperfusion injury. Circ Res. (2021) 128(5):639–51. 10.1161/circresaha.120.317710
263.
Zi-Chang N Ran A Hui-Hui S Qi J Jun-Li S Yan-Xu C et al Columbianadin ameliorates myocardial injury by inhibiting autophagy through the PI3K/Akt/mTOR signaling pathway in AMI mice and hypoxic H9c2 cells. Phytother Res. (2024) 39:521–35. 10.1002/ptr.8387
264.
Chen X Ruiz-Velasco A Zou Z Hille SS Ross C Fonseka O et al PAK3 exacerbates cardiac lipotoxicity via Srebp1c in obesity cardiomyopathy. Diabetes. (2024) 73(11):1805–20. 10.2337/db24-0240
265.
Parichatikanond W Pandey S Mangmool S . Exendin-4 exhibits cardioprotective effects against high glucose-induced mitochondrial abnormalities: potential role of glp-1 receptor and mTOR signaling. Biochem Pharmacol. (2024) 229:116552. 10.1016/j.bcp.2024.116552
266.
Al-Othman R Al-Jarallah A Babiker F . High-density lipoprotein protects normotensive and hypertensive rats against ischemia-reperfusion injury through differential regulation of mTORC1 and mTORC2 signaling. Front Pharmacol. (2024) 15:1398630. 10.3389/fphar.2024.1398630
267.
Podyacheva E Snezhkova J Onopchenko A Dyachuk V Toropova Y . The role of micrornas in the pathogenesis of doxorubicin-induced vascular remodeling. Int J Mol Sci. (2024) 25(24):13335. 10.3390/ijms252413335
268.
Rakhe N Bhatt LK . Valosin-containing protein: a potential therapeutic target for cardiovascular diseases. Ageing Res Rev. (2024) 101:102511. 10.1016/j.arr.2024.102511
269.
Vicenzetto C Giordani AS Menghi C Baritussio A Scognamiglio F Pontara E et al Cellular immunology of myocarditis: lights and shades-a literature review. Cells. (2024) 13(24):2082. 10.3390/cells13242082
270.
Sheng K Ran Y Feng X Wang Y Zhou S Guan Y et al Ptn secreted by cardiac fibroblasts promotes myocardial fibrosis and inflammation of pressure overload-induced hypertrophic cardiomyopathy through the ptn-Sdc4 pathway. Life Sci. (2025) 363:123356. 10.1016/j.lfs.2024.123356
271.
Gong W Zhang N Sun X Zhang Y Wang Y Lv D et al Cardioprotective effects of polydatin against myocardial injury in hfd/stz and high glucose-induced diabetes via a caveolin 1-dependent mechanism. Phytomedicine. (2024) 135:156055. 10.1016/j.phymed.2024.156055
272.
Jin S Kang PM . A systematic review on advances in management of oxidative stress-associated cardiovascular diseases. Antioxidants (Basel). (2024) 13(8):923. 10.3390/antiox13080923
273.
Lasheras J Pardo R Velilla M Poncelas M Salvatella N Simó R et al Cardiac-specific overexpression of ERRγ in mice induces severe heart dysfunction and early lethality. Int J Mol Sci. (2021) 22(15):8047. 10.3390/ijms22158047
274.
Kambis TN Shahshahan HR Mishra PK . Metabolites and genes behind cardiac metabolic remodeling in mice with type 1 diabetes mellitus. Int J Mol Sci. (2022) 23(3):1392. 10.3390/ijms23031392
275.
Ahmed LA Shiha NA Attia AS . Escitalopram ameliorates cardiomyopathy in type 2 diabetic rats via modulation of receptor for advanced glycation End products and its downstream signaling cascades. Front Pharmacol. (2020) 11:579206. 10.3389/fphar.2020.579206
276.
Bocchi L Motta BM Savi M Vilella R Meraviglia V Rizzi F et al The histone deacetylase inhibitor suberoylanilide hydroxamic acid (Saha) restores cardiomyocyte contractility in a rat model of early diabetes. Int J Mol Sci. (2019) 20(8):1873. 10.3390/ijms20081873
277.
Kounatidis D Vallianou NG Karampela I Rebelos E Kouveletsou M Dalopoulos V et al Anti-diabetic therapies and cancer: from bench to bedside. Biomolecules. (2024) 14(11):1479. 10.3390/biom14111479
278.
Nawaz L Grieve DJ Muzaffar H Iftikhar A Anwar H . Methanolic extract of phoenix dactylifera confers protection against experimental diabetic cardiomyopathy through modulation of glucolipid metabolism and cardiac remodeling. Cells. (2024) 13(14):1196. 10.3390/cells13141196
279.
Alnsasra H Asleh R Khalil F Akiki E Briasoulis A Dean PG et al Treatment with mTOR inhibitors as primary immunosuppression after combined heart and kidney transplantation. J Card Fail. (2024). 10.1016/j.cardfail.2024.10.451
280.
Xu Y Zhang H Chen Y Pober JS Zhou M Zhou JH et al SRF SUMOylation modulates smooth muscle phenotypic switch and vascular remodeling. Nat Commun. (2024) 15(1):6919. 10.1038/s41467-024-51350-5
281.
Orsini F Bosica M Martucci A De Paola M Comolli D Pascente R et al SARS-Cov-2 nucleocapsid protein induces tau pathological changes that can be counteracted by SUMO2. Int J Mol Sci. (2024) 25(13):7169. 10.3390/ijms25137169
282.
Li Puma DD Colussi C Bandiera B Puliatti G Rinaudo M Cocco S et al Interleukin 1β triggers synaptic and memory deficits in herpes simplex virus type-1-infected mice by downregulating the expression of synaptic plasticity-related genes via the epigenetic Mecp2/Hdac4 complex. Cell Mol Life Sci (2023) 80(6):172. 10.1007/s00018-023-04817-5
283.
Han J Wan L Jiang G Cao L Xia F Tian T et al ATM controls the extent of DNA end resection by eliciting sequential posttranslational modifications of CTIP. Proc Natl Acad Sci U S A. (2021) 118(12):e2022600118. 10.1073/pnas.2022600118
284.
Kuang DD Zhang T Guo XY Pan LH Li QM Luo JP et al Tea polysaccharide ameliorates atherosclerosis by inhibiting insulin resistance-mediated hepatic VLDL overproduction. J Agric Food Chem. (2025) 73(15):8959–77. 10.1021/acs.jafc.4c11144
285.
Mangione MC Wen J Cao DJ . Mechanistic target of rapamycin in regulating macrophage function in inflammatory cardiovascular diseases. J Mol Cell Cardiol. (2024) 186:111–24. 10.1016/j.yjmcc.2023.10.011
286.
Mohammadhosseini M Enright T Duvall A Chitsazan A Lin HY Ors A et al Targeting the CD74 signaling axis suppresses inflammation and rescues defective hematopoiesis in Runx1-familial platelet disorder. Sci Transl Med. (2025) 17(780):eadn9832. 10.1126/scitranslmed.adn9832
287.
Liu J Li X Li Y Gong Q Luo K . Metformin-based nanomedicines for reprogramming tumor immune microenvironment. Theranostics. (2025) 15(3):993–1016. 10.7150/thno.104872
288.
Gasparini VR Rampazzo E Barilà G Buratin A Buson E Calabretto G et al Proteasome inhibitors induce apoptosis in ex vivo cells of T-cell prolymphocytic leukemia. Int J Mol Sci. (2024) 25(24):13573. 10.3390/ijms252413573
289.
Zhou Q Yu H Chen Y Ren J Lu Y Sun Y . The CRL3(KCTD10) ubiquitin ligase-USP18 axis coordinately regulates cystine uptake and ferroptosis by modulating SLC7A11. Proc Natl Acad Sci U S A. (2024) 121(28):e2320655121. 10.1073/pnas.2320655121
290.
Kaito S Aoyama K Oshima M Tsuchiya A Miyota M Yamashita M et al Inhibition of topors ubiquitin ligase augments the efficacy of DNA hypomethylating agents through Dnmt1 stabilization. Nat Commun. (2024) 15(1):7359. 10.1038/s41467-024-50498-4
291.
Khatun S Dasgupta I Sen S Amin SA Qureshi IA Jha T et al Histone deacetylase 8 in focus: decoding structural prerequisites for innovative epigenetic intervention beyond hydroxamates. Int J Biol Macromol. (2025) 284(Pt 2):138119. 10.1016/j.ijbiomac.2024.138119
292.
Siri M Maleki MH Meybodi SM Mazhari SA Saviri FG Dehghanian A et al Enhancing wound healing via modulation of autophagy-induced apoptosis: the role of nicotinamide riboside and resveratrol in streptozotocin-treated diabetic rat. J Nutr Biochem. (2024) 137:109811. 10.1016/j.jnutbio.2024.109811
293.
Wu LY Enkhjargal B Xie ZY Travis ZD Sun CM Zhou KR et al Recombinant OX40 attenuates neuronal apoptosis through OX40-OX4L/PI3K/AKT signaling pathway following subarachnoid hemorrhage in rats. Exp Neurol. (2020) 326:113179. 10.1016/j.expneurol.2020.113179
294.
Li X Zou J Lin A Chi J Hao H Chen H et al Oxidative stress, endothelial dysfunction, and N-acetylcysteine in type 2 diabetes mellitus. Antioxid Redox Signal. (2024) 40(16–18):968–89. 10.1089/ars.2023.0524
295.
Chen Y Li X Wang S Miao R Zhong J . Targeting iron metabolism and ferroptosis as novel therapeutic approaches in cardiovascular diseases. Nutrients. (2023) 15(3):591. 10.3390/nu15030591
296.
d’Aiello A Filomia S Brecciaroli M Sanna T Pedicino D Liuzzo G . Targeting inflammatory pathways in atherosclerosis: exploring new opportunities for treatment. Curr Atheroscler Rep. (2024) 26(12):707–19. 10.1007/s11883-024-01241-3
297.
Wei Y Li W Huang J Braunstein Z Liu X Li X et al Midline-1 regulates effector T cell motility in experimental autoimmune encephalomyelitis via mTOR/microtubule pathway. Theranostics. (2024) 14(3):1168–80. 10.7150/thno.87130
298.
Oliva A Angiolillo DJ Valgimigli M Cao D Sartori S Bangalore S et al One- versus three-month DAPT after everolimus-eluting stent implantation in diabetic patients at high bleeding risk: results from the XIENCE short DAPT programme. EuroIntervention. (2025) 21(12):e668–80. 10.4244/eij-d-24-00897
299.
Rameika N Tsiara I Zhang X Haberek W Rendo V Kundu S et al NAT2 activity increases cytotoxicity of anthracycline antibiotics and HDAC inhibitors. Biochim Biophys Acta Mol Basis Dis. (2025) 1871(5):167755. 10.1016/j.bbadis.2025.167755
300.
Ma K Xian W Liu H Shu R Ge J Luo ZQ et al Bacterial ubiquitin ligases hijack the host deubiquitinase Otub1 to inhibit mTORC1 signaling and promote autophagy. Autophagy. (2024) 20(9):1968–83. 10.1080/15548627.2024.2353492
301.
Xu Y Qian C Wang Q Song L He Z Liu W et al Deacetylation of ATG7 drives the induction of macroautophagy and LC3-associated microautophagy. Autophagy. (2024) 20(5):1134–46. 10.1080/15548627.2023.2287932
302.
Fang L Ford-Roshon D Russo M O’Brien C Xiong X Gurjao C et al RNF43 G659fs is an oncogenic colorectal cancer mutation and sensitizes tumor cells to PI3K/mTOR inhibition. Nat Commun. (2022) 13(1):3181. 10.1038/s41467-022-30794-7
303.
Jiang Y Li Y Liu C Zhang L Lv D Weng Y et al Isonicotinylation is a histone mark induced by the anti-tuberculosis first-line drug isoniazid. Nat Commun. (2021) 12(1):5548. 10.1038/s41467-021-25867-y
304.
Jiang B Wang Y Zhi X Liu A Wang L Wang X et al Elucidating the mechanism of action of astragalus polysaccharide on ionizing radiation-induced myocardial damage based on network pharmacology and experimental research. Int Immunopharmacol. (2025) 145:113758. 10.1016/j.intimp.2024.113758
305.
Li Y Zhang X Jiang G Min X Kong Q Liu L et al Downregulation of HSPA12A protects heart against sepsis through suppressing mTOR-mediated inflammatory response in cardiomyocytes. Int Immunopharmacol. (2025) 145:113721. 10.1016/j.intimp.2024.113721
306.
Fan QQ Zhai BT Qiao JX Zhang D Sun J Zhang XF et al Study on the underlying mechanism of Huachansu capsule induced cardiotoxicity of normal rat by integrating transcriptomics, metabolomics and network toxicology. J Ethnopharmacol. (2025) 336:118751. 10.1016/j.jep.2024.118751
307.
Ferrito N Báez-Flores J Rodríguez-Martín M Sastre-Rodríguez J Coppola A Isidoro-García M et al Biomarker landscape in rasopathies. Int J Mol Sci. (2024) 25(16):8563. 10.3390/ijms25168563
308.
Chen Y Peng W Tao Q Li S Wu Z Zhou Y et al Increased small ubiquitin-like modifier-activating enzyme SAE1 promotes hepatocellular carcinoma by enhancing mTOR sumoylation. Lab Invest. (2023) 103(1):100011. 10.1016/j.labinv.2022.100011
Summary
Keywords
MTOR signaling, protein modifications, cardiovascular diseases, cell death, therapeutic strategies
Citation
Guo J, Wu Y, Wan Z and Zhang Z (2025) Post-translational modifications orchestrate mTOR-driven cell death in cardiovascular disease. Front. Cardiovasc. Med. 12:1620669. doi: 10.3389/fcvm.2025.1620669
Received
30 April 2025
Accepted
30 June 2025
Published
15 July 2025
Volume
12 - 2025
Edited by
Gianfranco Pintus, University of Sharjah, United Arab Emirates
Reviewed by
Zhen-Guo Ma, Renmin Hospital of Wuhan University, China
Song Zihao, People's Hospital of Guangxi Zhuang Autonomous Region, China
Chengyi Li, Southern Medical University, China
Updates
Copyright
© 2025 Guo, Wu, Wan and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jiawei Guo guojw9@mail2.sysu.edu.cn Zhaoshan Zhang zhangzhaoshan.stu@yangtzeu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.