- 1Department of Pulmonary and Critical Care Medicine, Xiangtan Central Hospital, The Affiliated Hospital of Hunan University, Xiangtan, China
- 2Medical Department, Xiangtan Central Hospital, The Affiliated Hospital of Hunan University, Xiangtan, China
- 3Department of Cardiology, Xiangtan Central Hospital, The Affiliated Hospital of Hunan University, Xiangtan, China
Background: Clinical studies on heart failure (HF) with mildly reduced left ventricular ejection fraction (HFmrEF) are gradually increasing. However, relatively few studies have examined patients with HFmrEF after myocardial infarction (MI), and the prognosis of such patients remains unclear. Therefore, we conducted a retrospective evaluation of HFmrEF patients with/without MI using a propensity score matching analysis (PSMA).
Methods: A total of 1,691 patients with HFmrEF were included in this study. Of these patients, 873 had a diagnosis of MI, and 818 did not. After propensity score matching, we used Kaplan–Meier analysis and Cox regression to compare all-cause mortality, cardiovascular death, or HF readmission (CV events).
Results: After the first PSMA, the MI group had a lower risk of all-cause mortality [hazard ratio (HR) 0.6; 95% confidence interval (95% CI) 0.5–0.8] compared with the non-MI group; however, there was no significant difference in the incidence of CV events (HR 0.9; 95% CI 0.7–1.2). After the second PSMA, which additionally matched for PCI performance in the MI group, there were no differences in the risk of all-cause mortality (HR 1.0; 95% CI 0.7–1.5) or CV events (HR 1.1; 95% CI 0.8–1.5) between the MI and non-MI groups.
Conclusions: There was no difference in all-cause mortality and CV events between patients with HFmrEF with and without MI. However, among patients with HFmrEF and MI, those who underwent PCI had a much lower risk of all-cause mortality compared with patients with HFmrEF without MI and those with HFmrEF after MI who did not undergo PCI.
Introduction
Patients with heart failure (HF) and a left ventricular ejection fraction (LVEF) between the ranges for heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) are referred to as “HF with mid-range ejection fraction (EF)” or “HF with mildly reduced EF” (1). Because LVEF is lower than normal, they are classified as having HF with mildly reduced EF(HFmrEF) according to the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America Guideline for the Management of Heart Failure (2). In addition, the 2021 European Society of Cardiology heart failure guidelines define HFmrEF as HF with LVEF 41%–49% (3). In recent years, the global incidence of heart failure seems to have progressively increased each year (4–6). One of the main reasons for the increase in HF is the substantial increase in the survival rate following a diagnosis of MI, which inadvertently affects the survival of more patients with left ventricular dysfunction. Although the number of studies reported on patients with HFmrEF has been increasing, few have focused on patients with HFmrEF after MI. These patients may have a different prognosis than other patients with HF. Thus, we conducted a retrospective evaluation to compare outcomes between HFmrEF patients with and without a diagnosis of MI.
Patients and methodologies
The study protocol was approved by the Ethics Committee of Xiangtan Central Hospital (Xiangtan, China) and conformed to the principles outlined in the Declaration of Helsinki (7). Informed consent was obtained from all patients or their guardians before the inception of the study protocols.
This study included patients admitted to our hospital between 1 January 2015 and 31 August 2020. HFmrEF was defined according to the ESC 2021 guidelines as a LVEF of 41%–49% measured by transthoracic echocardiography during the index hospitalization, combined with symptoms and/or signs of heart failure corresponding to New York Heart Association (NYHA) functional class II–IV. Myocardial infarction (MI) was diagnosed according to the Fourth Universal Definition of MI. In this study, all MI cases occurred prior to or during the index hospitalization in which HFmrEF was diagnosed. Patients with a history of MI after the diagnosis of HFmrEF were not included. The temporal sequence was determined based on hospital admission records, discharge summaries, and prior medical documentation.
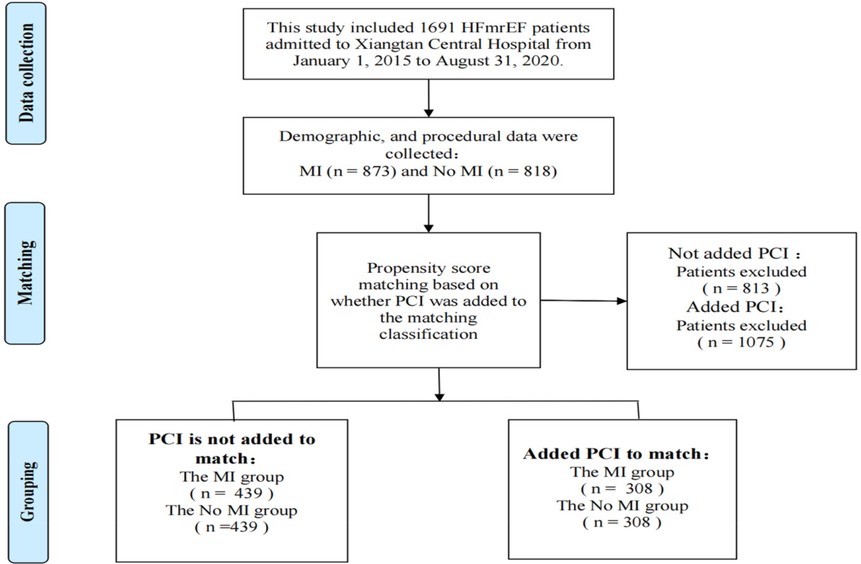
A total of 1,691 patients with HFmrEF were included in the study: 873 were diagnosed with MI, and 818 did not suffer from MI. Malignant tumors or other non-cardiac diseases with an expected survival time of less than 1 year were excluded from both groups. The primary endpoint of this study was all-cause mortality, and the secondary endpoints were cardiovascular (CV) events, defined as a composite of cardiovascular death and readmission for heart failure.
Data collection and follow-up
Demographic and procedural data were collected from hospital charts or databases. Follow-up was conducted on all study participants until 31 August 2021, through clinical telephone interviews and community visits. The median follow-up time was 33 months (interquartile range: 20–50 months).
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation. The first propensity score matching analysis (PSMA) was performed using a multivariate logistic regression model based on the following factors: age, sex, body mass index (BMI), diabetes, hypertension, hyperlipidemia, current smoker, coronary heart disease, atrial fibrillation, previous stroke, chronic obstructive pulmoriary disease, renal insufficiency, creatinine, New York Heart Association functional class, use of respirator, and use of electrocardiogram monitoring. Pairs of patients with or without MI were derived within a quarter of the standard deviation of the estimated propensity using 1:1 greedy nearest-neighbor matching. This strategy resulted in 439 matching pairs per group [percutaneous coronary intervention (PCI) was not included as a matching variable in the first PSMA]. The second propensity score matching analysis was performed, adding the factor of whether PCI had been performed while retaining other factors from the first analysis. This yielded 308 pairs per group.
The propensity score matching analyses were intentionally structured to reflect the study's primary objective—namely, to explore prognostic differences between HFmrEF patients with and without MI and to further assess the effect of PCI within the MI subgroup. Alternative grouping strategies, such as dividing patients according to primary or secondary outcomes, may provide additional perspectives but would shift the analytic framework away from MI status, which was the central hypothesis of this study. Moreover, by definition, patients without MI did not undergo PCI, and this limitation has been acknowledged in the Discussion section.
Clinical characteristics between groups were compared using t-tests for continuous measures and chi-square tests for categorical variables. The Kaplan–Meier method was used to estimate cumulative event incidence, and a Cox proportional hazards model was constructed to assess the hazard ratio (HR) for each event between the two groups. Cox regression was conducted as a univariable analysis because the PSM procedure had already balanced all measured covariates, making further multivariable adjustment unnecessary. The balance of measured variables between groups after propensity score matching was analyzed using paired t-tests for continuous measures and McNemar's test for categorical variables. After propensity score matching, differences in cumulative event rates were analyzed using the stratified Cox procedure.
P-values were obtained using the Kruskal–Wallis rank-sum test for continuous variables and Fisher's exact probability test for count variables. Results were considered significant when P < 0.05. All analyses were performed with R (http://www.R-project.org) and EmpowerStats software (https://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA).
Results
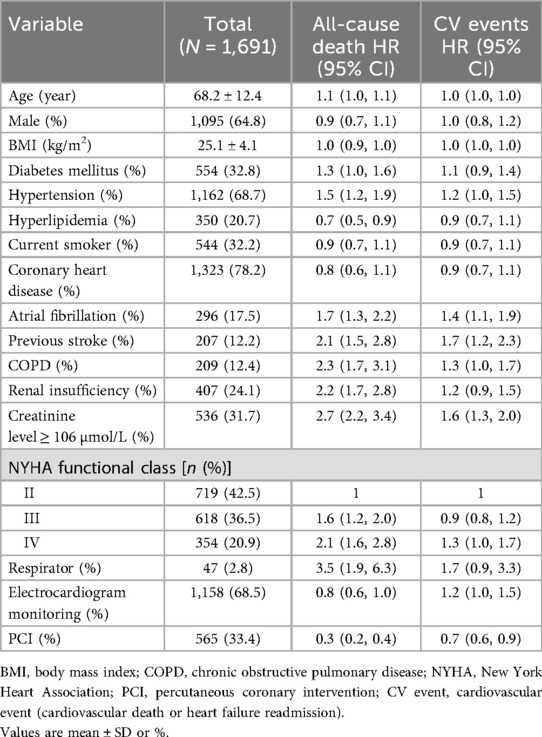
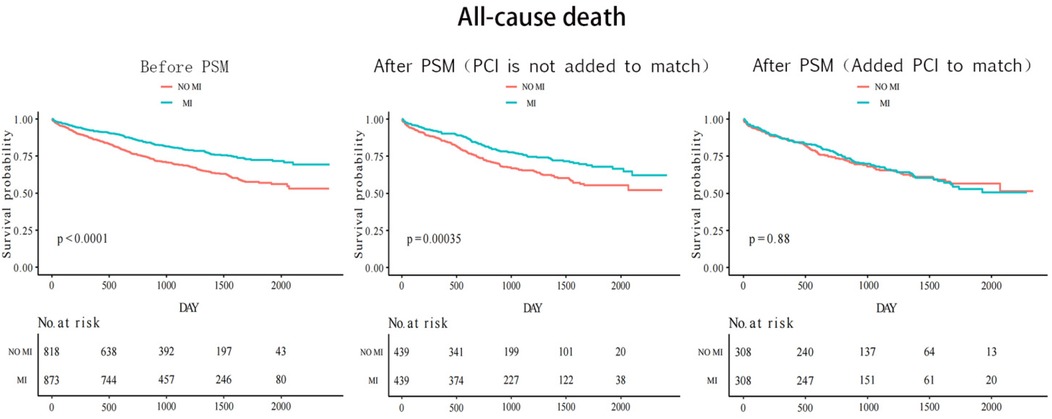
Table 1 shows the baseline characteristics of outcome events before propensity score matching (N = 1,691). Risk factors for all-cause mortality were: age [HR 1.1; 95% confidence interval (95% CI) 1.0–1.1; P < 0.001], hypertension (HR 1.5; 95% CI 1.2–1.9; P < 0.001), atrial fibrillation (HR 1.7; 95% CI 1.3–2.2; P < 0.001), diabetes (HR 1.3; 95% CI 1.0–1.6; P = 0.033), previous stroke (HR 2.1; 95% CI 1.5–2.8), P < 0.001), chronic obstructive pulmonary disease (COPD) (HR 2.3; 95% CI 1.7–3.1; P < 0.001), renal insufficiency (HR 2.2; 95% CI 1.7–2.8; P < 0.001), NYHA class III (HR 1.6; 95% CI 1.2–2.0; P < 0.001), NYHA class IV (HR 2.1; 95% CI 1.6–2.8; P < 0.001), ventilator use (HR 3.5; 95% CI 1.9–6.3; P < 0.001), and creatinine ≥106 µmol/L (HR 2.7; 95% CI 2.2–3.4; P < 0.001). The presence or absence of myocardial infarction was a protective factor for all-cause death (HR 0.5; 95% CI 0.4–0.7; P < 0.001). PCI or not is a protective factor for all-cause death (HR 0.3; 95% CI 0.2–0.4; P < 0.001) and cardiovascular events (HR 0.7; 95% CI 0.6–0.9; P = 0.001). Therefore, these factors that had a significant impact on the outcome events and other common influencing factors were included in the propensity score matching analysis. The purpose of performing two propensity score matching analyses was to make the two matched groups more comparable and determine whether the protective factors of myocardial infarction on outcome events were related to PCI. Among the 1,691 HFmrEF patients enrolled, 873 had been diagnosed with an MI, whereas 818 had reported being free of any episodes of an MI. A total of 439 matching pairs were obtained after the first propensity score matching analysis, and 308 matching pairs were obtained after the second propensity score matching analysis (Figure 1).
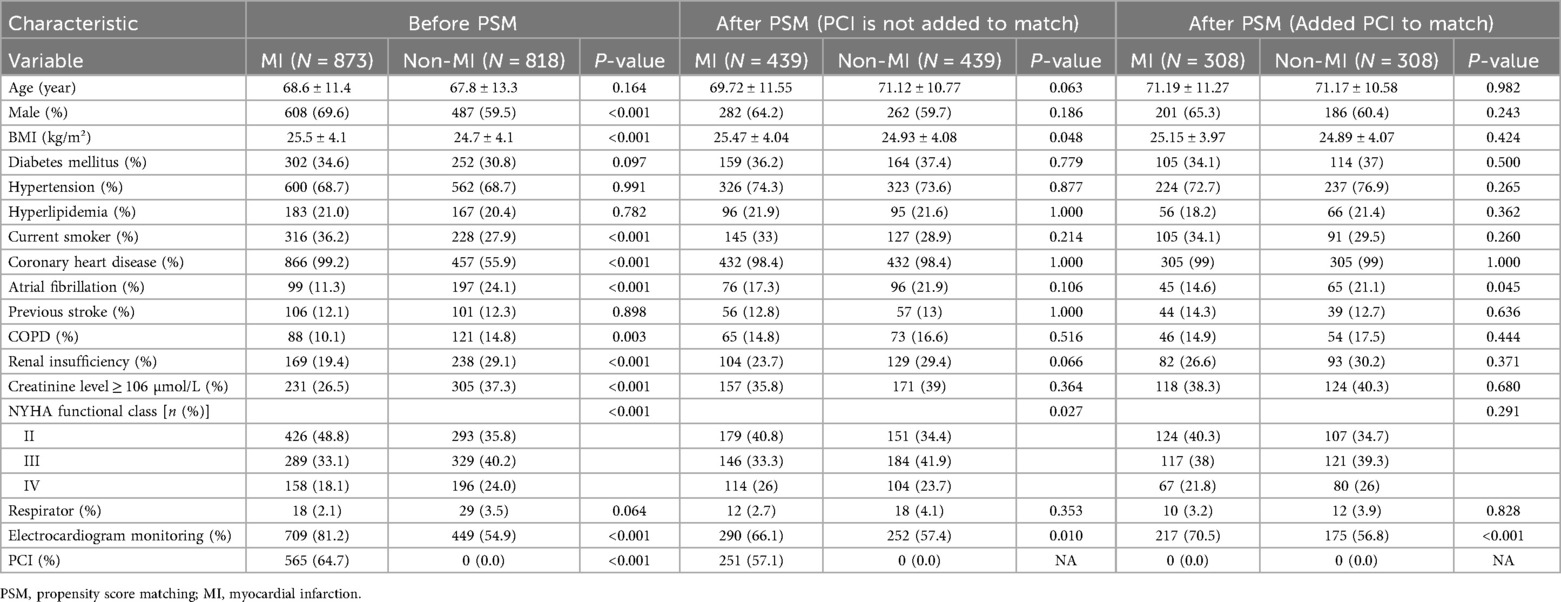
Table 2 shows the patient profiles before and after propensity score matching. Before propensity score matching, the patients in the MI group were more likely to be male (P < 0.001), to be current smokers (P < 0.001), and to have coronary heart disease (P < 0.001), electrocardiogram monitoring (P < 0.001), PCI (P < 0.001), and higher BMI values (P < 0.001). Compared with the MI group, the non-MI group had higher rates of atrial fibrillation (P < 0.001), COPD (P = 0.003), renal insufficiency (P < 0.001), and creatinine ≥106 µmol/L (P < 0.001), NYHA class III (P < 0.001), and NYHA class IV (P < 0.001). The two groups had patients with similar ages (68.6 ± 11.4 and 67.8 ± 13.3 with and without myocardial infarction, respectively, P = 0.164) and comparable rates of diabetes (P = 0.097), hypertension (P = 0.991), and hyperlipidemia (P = 0.997). 0.782), previous stroke (P = 0.898), and ventilator use (P = 0.064). Of the 439 matched pairs obtained after the first match, 290 in the MI group underwent PCI. However, the 308 matched pairs obtained after adding PCI for the second time to the matching group excluded all patients who underwent PCI. After the first and second propensity score matching, the two groups were well matched on parameters.
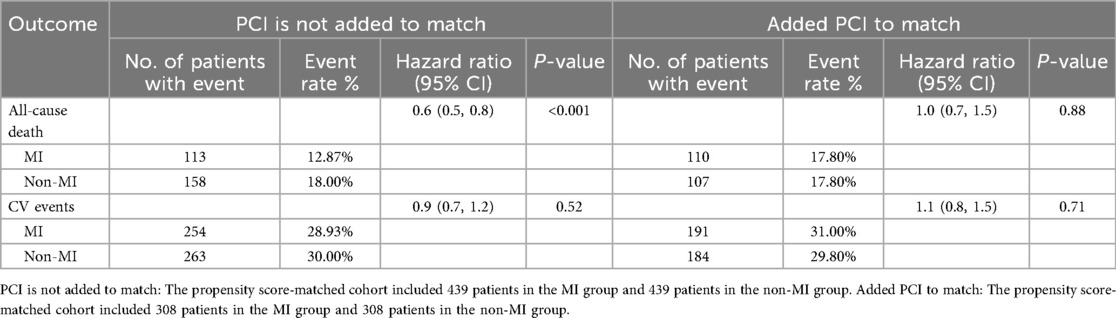
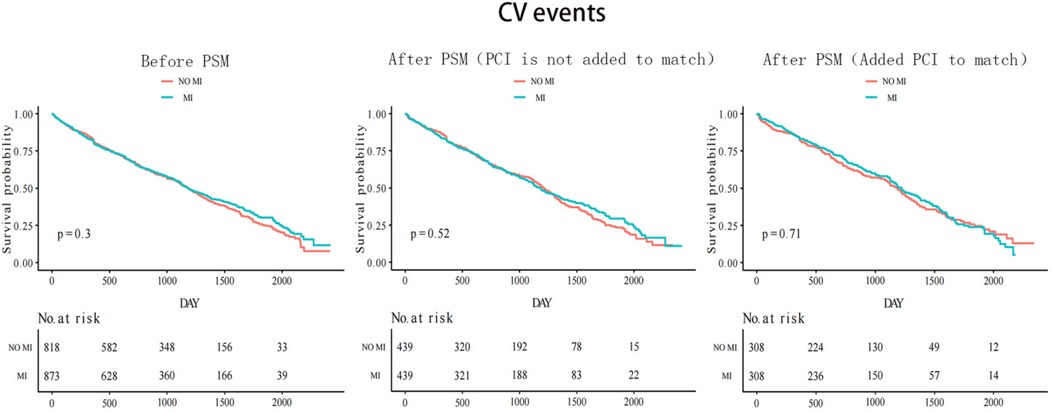
Table 3 presents the risk of primary and secondary outcomes in the propensity score-matched cohort. Without adding PCI to the matched 439 pairs, the MI group had 113 all-cause deaths (12.87%) compared with 158 all-cause deaths (18.00%) in the non-MI group (HR 0.6; 95% CI 0.5–0.8; P < 0.001). After adding PCI to the matched 308 pairs, there were 110 all-cause deaths (17.80%) in the MI group and 107 all-cause deaths (17.80%) in the non-MI group (HR 1.0; 95% CI 0.7–1.5; P = 0.88). In the first PSMA, CV events occurred in 254 patients (28.93%) with MI and 263 patients (30.00%) without MI (HR 0.9; 95% CI 0.7–1.2; P = 0.52). In the second PSMA (with PCI matched), CV events occurred in 191 patients (31.00%) with MI and 184 patients (29.80%) without MI (HR 1.1; 95% CI 0.8–1.5; P = 0.71).
The median follow-up time was 33 months for both groups with and without myocardial infarction. Figure 2 shows that before the propensity score match, the Kaplan–Meier cumulative all-cause mortality was lower in the MI group than that in the non-MI group (P < 0.0001). After the first propensity score matching, the MI group still had lower all-cause mortality than that of the non-MI group (P = 0.00035). However, after the second addition of PCI for matching, all-cause mortality was similar in both groups (P = 0.88). CV events were similar between the two groups before and after matching. Before matching, the results revealed a statistically significant value of P = 0.3, whereas after the first matching, the P-value was 0.52, and after the second matching, it was 0.71 (Figure 3).
Discussion
There were three primary outcomes determined from our study. Firstly, HFmrEF without a diagnosis of MI had higher all-cause mortality than HFmrEF patients with MI after adjusting for the first propensity score. The second finding suggested that after adding PCI to the second propensity score, the rates of all-cause death and CV events were similar in patients with and without MI with HFmrEF. Lastly, patients with HFmrEF post-MI who underwent PCI had a lower risk of all-cause mortality compared with patients with HFmrEF without MI and those with HFmrEF post-MI without PCI.
Although several studies have reported data on post-MI heart failure in recent decades (8, 9), few have directly compared post-MI HF with non-post-MI HF. For example, a study of 1,260 MI patients undergoing PCI showed that although patients with HFmrEF after MI had similar baseline characteristics, their hospitalization rates, long-term mortality, and heart failure rehospitalization differed from those of patients with HFrEF and HFpEF (10). Other studies have shown that after acute MI, the predominant HF subtypes are HFmrEF and HFpEF rather than HFrEF (11). Our cohort specifically compared HFmrEF patients with and without MI, thereby addressing a gap in the existing literature.
In the present study, PCI emerged as a strong protective factor in discharged patients with HFmrEF. Loss of cardiac function after MI remains a leading cause of morbidity in developed countries (12), and early revascularization is the only therapy shown to reduce mortality in post-MI HF with cardiogenic shock (13). PCI enables rapid relief of acute thrombotic occlusion, treatment of underlying atherosclerotic and thrombotic risk, attenuation of adverse ventricular remodeling (14), and reduction of arrhythmias. Evidence from the EPICOR study involving 11,931 ACS patients demonstrated that higher in-hospital coronary revascularization rates were independently associated with lower adjusted 2-year mortality (15). Similarly, Núñez-Gil et al. (16) found that mild HF after MI was associated with poor prognosis and increased short-term mortality, supporting the use of aggressive strategies including early catheterization and revascularization. These observations are consistent with our conclusion that PCI plays a protective role in HFmrEF.
Previous studies have reported variations in LVEF among patients with post-MI HF. Kamon et al. (17) found that HF with non-reduced EF was the predominant subtype after AMI. Alkhalil et al. (18) showed that HFmrEF after STEMI carried higher risks of death, HF hospitalization, and ventricular arrhythmias than preserved EF. Other studies have also documented distinct characteristics and prognosis for HFmrEF after MI compared with HFrEF and HFpEF (19, 20). Most research has examined either MI or HF in isolation, whereas our study directly compared HFmrEF with and without MI, finding that the prognostic differences are largely mediated by PCI use.
This study has several limitations. First, although propensity score matching was applied to reduce selection bias, the retrospective design cannot exclude unmeasured confounding. Second, the study population was derived from a single heart center in China, limiting generalizability. Third, data on the long-term use of statins, renin–angiotensin system blockers, and beta-blockers were unavailable, preventing assessment of their potential effects on morbidity and mortality. Fourth, PCI could only be assessed within the MI subgroup, as patients without MI by definition did not undergo PCI. This limits the interpretation of PCI's protective effect across the entire HFmrEF population. Fifth, although PSM balanced baseline covariates, the reduced sample size and event counts after matching may have decreased statistical power, especially in the secondary PSM analyses. Finally, data collection was based on medical records and follow-up interviews, which may be subject to reporting inaccuracies or incomplete documentation.
In conclusion, there were no differences in all-cause mortality and CV events in patients with HFmrEF with or without MI after accounting for the second propensity score matching analysis. When PCI status was included in the PSMA, patients with HFmrEF after myocardial infarction who underwent PCI had a lower risk of all-cause mortality compared with those with HFmrEF without myocardial infarction and those with HFmrEF after myocardial infarction without PCI. While no significant difference in CV events was observed, most patients with post-MI heart failure are those with preserved and mildly reduced EF. Therefore, early blood reperfusion is recommended to reduce the long-term mortality of heart failure after myocardial infarction.
This work represents an advance in biomedical science because we determined that PCI is a protective factor for all-cause death, while no significant difference in CV events was observed. Most patients with post-MI heart failure are those with preserved and mildly reduced EF.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Xiangtan Central Hospital (No. 20211036). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective observational nature of this study. The research was conducted using de-identified data from previously collected medical records, and no direct patient interaction or intervention was involved.
Author contributions
ZL: Methodology, Writing – original draft, Conceptualization, Visualization, Data curation. LZ: Writing – original draft. JZ: Supervision, Validation, Writing – review & editing. MJ: Writing – review & editing, Supervision, Funding acquisition, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study is supported by the Scientific Bureau of Xiangtan City (SF-ZDJH20231037), Medical Association of Xiangtan City (2023-xtyx-35), and Medical Association of Xiangtan City (2024-xtyx-38), Xiangtan City, Hunan Province, China.
Acknowledgments
We thank TopEdit (https://www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures. J Am Coll Cardiol. (2014) 63(21):2304–22. doi: 10.1016/j.jacc.2014.03.016
2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. (2022) 79(17):e263–421. doi: 10.1016/j.jacc.2021.12.012
3. Bauersachs J, Soltani S. Herzinsuffizienzleitlinien 2021 der ESC. Herz. (2022) 47(1):12–8. doi: 10.1007/s00059-021-05084-5
4. Savarese G, Lund L. Global public health burden of heart failure. Card Fail Rev. (2017) 3(1):7. doi: 10.15420/cfr.2016:25:2
5. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide: addressing heart failure. ESC Heart Fail. (2014) 1(1):4–25. doi: 10.1002/ehf2.12005
6. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, et al. Prevention of heart failure: a scientific statement from the American Heart Association councils on epidemiology and prevention, clinical cardiology, cardiovascular nursing, and high blood pressure research; quality of care and outcomes research interdisciplinary working group; and functional genomics and translational biology interdisciplinary working group. Circulation. (2008) 117(19):2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965
7. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310(20):2191. doi: 10.1001/jama.2013.281053
8. Lin CF, Chang YH, Yu FC, Tsai CT, Chen CC, Liu HY, et al. Risk of heart failure following drug-eluting stent implantation in patients with non–ST–elevation myocardial infarction. Atherosclerosis. (2021) 316:84–9. doi: 10.1016/j.atherosclerosis.2020.10.012
9. Dobre D, Kjekshus J, Rossignol P, Girerd N, Benetos A, Dickstein K, et al. Heart rate, pulse pressure and mortality in patients with myocardial infarction complicated by heart failure. Int J Cardiol. (2018) 271:181–85. doi: 10.1016/j.ijcard.2018.05.017
10. Karabağ Y, Çınar T, Çağdaş M, Rencüzoğulları İ, Tanık VO. In-hospital and long-term prognoses of patients with a mid-range ejection fraction after an ST-segment myocardial infarction. Acta Cardiol. (2019) 74(4):351–58. doi: 10.1080/00015385.2018.1501140
11. Kamon D, Sugawara Y, Soeda T, Okamura A, Nakada Y, Hashimoto Y, et al. Predominant subtype of heart failure after acute myocardial infarction is heart failure with non-reduced ejection fraction. ESC Heart Fail. (2021) 8(1):317–25. doi: 10.1002/ehf2.13070
12. Weir RA, McMurray JJ. Epidemiology of heart failure and left ventricular dysfunction after acute myocardial infarction. Curr Heart Fail Rep. (2006) 3(4):175–80. doi: 10.1007/s11897-006-0019-5
13. Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC Heart Fail. (2018) 6(3):179–86. doi: 10.1016/j.jchf.2017.09.015
14. Martin K, Huang C-L, Caplice N. Regenerative approaches to post-myocardial infarction heart failure. Curr Pharm Des. (2014) 20(12):1930–40. doi: 10.2174/13816128113199990450
15. Bueno H, Rossello X, Pocock SJ, Van de Werf F, Chin CT, Danchin N, et al. In-hospital coronary revascularization rates and post-discharge mortality risk in non–ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. (2019) 74(11):1454–61. doi: 10.1016/j.jacc.2019.06.068
16. Núñez-Gil IJ, García-Rubira JC, Luaces M, Vivas D, De Agustín JA, González-Ferrer JJ, et al. Mild heart failure is a mortality marker after a non-ST-segment acute myocardial infarction. Eur J Intern Med. (2010) 21(5):439–43. doi: 10.1016/j.ejim.2010.06.003
17. Kamon D, Sugawara Y, Soeda T, Okamura A, Nakada Y, Hashimoto Y, et al. Predominant subtype of heart failure after acute myocardial infarction is heart failure with non-reduced ejection fraction. ESC Heart Fail. (2021) 8(1):317–25. doi: 10.1002/ehf2.13070
18. Alkhalil M, Kearney A, MacElhatton D, Fergie R, Dixon L. The prognostic role of mid-range ejection fraction in ST-segment elevation myocardial infarction. Int J Cardiol. (2020) 321:12–7. doi: 10.1016/j.ijcard.2020.07.001
19. Jiang Y, Hu S, Cao M, Li X, Zhou J, Ding B, et al. Evaluation of acute myocardial infarction patients with mid-range ejection fraction after emergency percutaneous coronary intervention. Postgrad Med J. (2019) 95(1125):355–60. doi: 10.1136/postgradmedj-2018-136334
Keywords: heart failure with mildly reduced ejection fraction, myocardial infarction, percutaneous coronary intervention, outcome, PSMA
Citation: Liu Z, Zhang L, Zeng J and Jiang M (2025) The outcome of possible events following the hospital discharge of patients with HFmrEF after MI as compared with those with HFmrEF without MI: a propensity score matching analysis. Front. Cardiovasc. Med. 12:1622220. doi: 10.3389/fcvm.2025.1622220
Received: 2 May 2025; Accepted: 30 October 2025;
Published: 17 November 2025.
Edited by:
Maria Concetta Pastore, University of Siena, ItalyReviewed by:
Pengchao Tian, Capital Medical University, ChinaDaniela Bacich, University Hospital Padua, Italy
Copyright: © 2025 Liu, Zhang, Zeng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Zeng, emVuZ2ppYW5waW5nMTk2MUAxNjMuY29t; Mingyan Jiang, amlhbmdtaW5neWFuMTk3OUAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID:
Zhican Liu
orcid.org/0000-0002-5532-1632
Lingling Zhang
orcid.org/0009-0005-6902-3475
Jianping Zeng
orcid.org/0000-0002-4485-6164
Mingyan Jiang
orcid.org/0000-0001-8558-2609
 Zhican Liu1,†,‡
Zhican Liu1,†,‡ Lingling Zhang
Lingling Zhang Mingyan Jiang
Mingyan Jiang