Abstract
Stent thrombosis remains a major complication following percutaneous coronary intervention, with significant morbidity and mortality implications. Despite advancements in drug-eluting stents and optimized pharmacotherapy, real-world registry data indicate that definite or probable stent thrombosis occurs in approximately 0.5% of percutaneous coronary intervention cases, with a 30-day mortality rate approaching 25% and a long-term risk exceeding 30% at 10 years. Stent thrombosis is classified based on timing into acute, subacute, late, and very late thrombosis, with subacute and very late stent thrombosis being the most prevalent. Clinical consequences include myocardial infarction, emergent revascularization, and heightened cardiovascular risk, necessitating timely recognition and intervention. Risk factors include patient characteristics, procedural variables, and lesion complexity, with recurrent stent thrombosis remaining a notable concern. This review explores the definitions, classifications, pathophysiology, and risk factors for stent thrombosis while discussing current strategies for prevention and management. Additionally, advancements in stent technology and pharmacologic interventions are examined, underscoring the need for a multidisciplinary approach to mitigate stent thrombosis incidence and improve patient outcomes.
Introduction
Stent thrombosis (ST) remains one of the most significant complications following percutaneous coronary intervention (PCI), carrying significant implications for patient outcomes. Although its overall incidence has decreased in the era of contemporary drug-eluting stents (DES) and optimized pharmacotherapy, ST continues to be associated with substantial morbidity and mortality. Recent real-world registry data show that definite or probable ST occurs in approximately 0.5% of PCI cases, and when it does occur, nearly one in four patients may die within 30 days (1). Long-term data have demonstrated that mortality risk persists well beyond the initial event, rising to over 30% at 10 years (2). The clinical consequences of ST typically include myocardial infarction, emergent revascularization, and a heightened risk of death, emphasizing the need for timely recognition and appropriate management strategies. These findings underscore why, despite modern advancements, a thorough understanding of ST and its clinical course remains essential for guiding effective patient care. The American College of Cardiology (ACC) and American Heart Association (AHA) define ST as the formation of a thrombus within a stent that leads to occlusion of the stented segment. Definite ST requires angiographic confirmation or autopsy findings, while probable ST is suspected in cases of unexplained death within 30 days of stent placement or myocardial infarction attributed to the stented vessel. Possible ST is a diagnosis of exclusion in patients who die more than 30 days post-implantation without another identifiable cause (3).
Figure 1
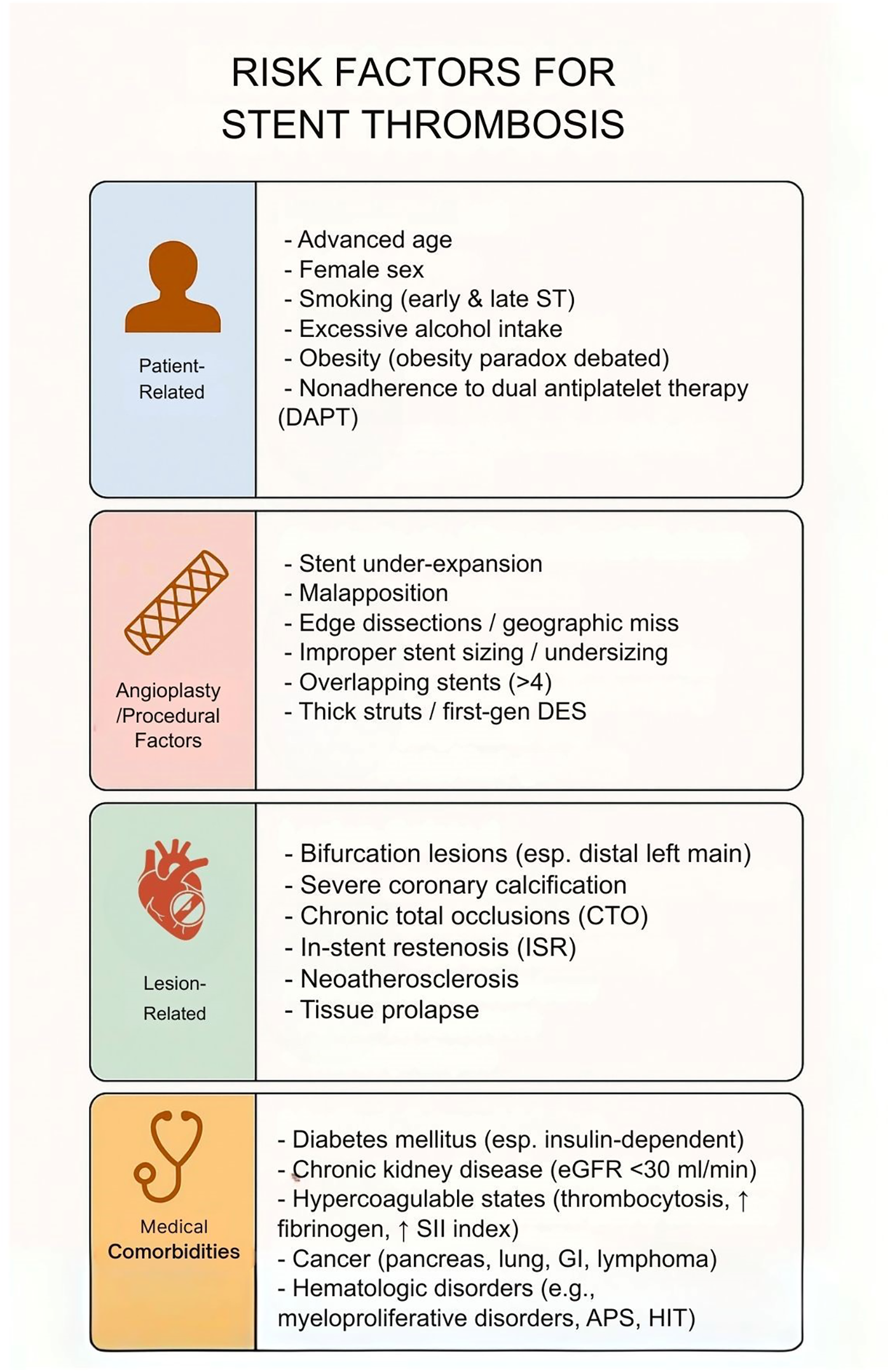
Risk factors for stent thrombosis. This table illustrates that risk factors for stent thrombosis are multifactorial, spanning patient-related, procedural, lesion-related, and comorbid conditions. While nonmodifiable factors such as age and sex contribute, modifiable risks—including smoking, obesity, nonadherence to dual antiplatelet therapy, stent under-expansion, and lesion complexity—play a central role. Recognition of these domains is critical for tailoring procedural strategies, optimizing pharmacotherapy, and reducing early and late stent thrombosis.
ST is classified into four categories based on timing: acute (within 24 h of stent implantation), subacute (24 h to 30 days), late (31 days to one year), and very late (beyond one year) thrombosis (3). Studies have shown variable prevalence rates of ST based on timing. Tariq et al. (4) reported an overall ST rate of 5.8% in a cohort of 569 patients followed for 30 days post-stent implantation, with acute ST accounting for 0.5% and subacute ST for 5.3% (4). The PRESTIGE registry, which utilized optical coherence tomography (OCT), found the prevalence of acute/subacute, late, and very late ST to be 6.1%, 28.6%, and 71.4%, respectively (5). Similarly, the PESTO French Registry identified very late ST as the most common form (75%), followed by subacute (15%), late (6%), and acute ST (4%) (6). Mohamed et al. (7) found that early ST accounted for 52.6% of cases, late ST for 12.0%, and very late ST for 35.4% (7). These studies highlight the variability in ST prevalence based on timing, with subacute and very late ST appearing most frequently. Recognizing these patterns is important for clinical risk stratification and guiding preventive efforts.
The clinical implications of ST are profound. Yang et al. (8) highlighted that early ST is associated with an increased risk of major adverse cardiovascular and cerebrovascular events (MACCE) (8). Tovar Forero et al. (9) reported a 43.7% major adverse cardiovascular event (MACE) rate within 60 days of ST, with 19.5% experiencing cardiac death and 17.9% suffering myocardial infarction (9). Ohno et al. (10) similarly found that ST leads to higher in-hospital mortality and increased cardiovascular complications (10). These findings highlight the importance of close follow-up and proactive treatment during the early phase following ST.
The long-term clinical implications of ST remain concerning, as mortality rates do not decrease over time. Ishihara et al. (2) found that mortality rates increased from 14.6% at 1 year to 33.8% at 10 years post-ST. Additionally, patients with a history of hemodialysis, culprit lesions in the left main trunk or left coronary artery, or elevated creatine kinase levels were at increased risk of mortality (2). Yang et al. (8) meta-analysis further demonstrated that patients with late and very late ST face significantly higher mortality rates at various time points, including in-hospital (P = 0.004), 30-day (P < 0.00001), 1-year (P < 0.00001), and long-term mortality (P = 0.04) (doi:10.1007/s11239-020-02184-7), underscoring the persistent risk even years after the initial event (8). Furthermore, the likelihood of recurrent ST is not negligible; Tovar Forero et al. (9) reported a restenosis rate of 12.1% (9). Given these long-term risks, ongoing surveillance and tailored secondary prevention strategies are essential for optimizing patient outcomes.
While the scope of this review primarily focuses on ST in coronary arteries, it is important to mention that ST in peripheral arteries also results in significant morbidity. The rate of ST in non-coronary vessels varies by vascular territory, stent type, and lesion complexity but is generally significantly higher than in coronary arteries. Large cohort studies reveal a ST rate of 6.1% in th e aortoiliac and femoropopliteal arteries after one year (11) and up to 13.4% in the superficial femoral artery at 5-years (12). Patients who experience stent thrombosis have a markedly increased risk of major adverse limb events, with hazard ratios approaching 5 for events such as repeat revascularization, major amputation, or persistent ischemia within 12 months.
ST remains a serious complication of PCI with significant implications for patient outcomes. This review will explore the definitions, classifications, risk factors, and mechanisms underlying ST, as well as current management and prevention strategies. We will examine how patient characteristics, procedural factors, and lesion complexity contribute to thrombosis risk and discuss the role of pharmacological and interventional therapies. Finally, we will highlight advancements in stent technology and future directions in the field, emphasizing the importance of a multidisciplinary approach to improving patient care and reducing ST incidence.
Risk factors
Risk factors for stent thrombosis are multifactorial, spanning patient characteristics, procedural variables, lesion complexity, and medical comorbidities (Figure 1). Advanced age, female sex, smoking, and obesity have been linked to increased stent thrombosis risk, though an “obesity paradox” is noted in literature with mixed associations. Nonadherence to DAPT is a major risk factor in the early post-PCI period. Procedural factors including stent under-expansion, malapposition, and edge dissections are important risk factors, prompting a use of intravascular imaging for procedural optimization. Lesion characteristics such as bifurcations, calcifications, chronic total occlusions, and in-stent restenosis, increase stent thrombosis risk. Finally, chronic conditions such as diabetes, chronic kidney disease, and hypercoagulable states increase stent thrombosis risk.
Patient factors
Age has been reported as an independent predictor of stent thrombosis, as older patients exhibit more progressive vascular changes and thereby increased coronary artery calcification (13, 14). Coronary artery calcification is a major contributor to stent thrombosis and affects more than 90% of men and 67% of women older than 70 years of age (13). Furthermore, over one-third of all percutaneous coronary interventions occur in patients older than 75 years of age which further heightens the prevalence of stent thrombosis within this cohort. Across genders, female sex is associated with a higher risk of stent thrombosis owing to differences in vessel size, endothelial function, and hormonal predisposition toward thrombosis (15). Smoking is a well-documented independent risk factor for stent thrombosis with reported associations for both early and late events (14, 16–19). In smokers, thrombosis risk is heightened through endothelial dysfunction and platelet reactivity (20). Consequently, smokers exhibit a greater risk of stent thrombosis and generally worse outcomes (19). Risk is compounded in smokers with first-generation DES implants, which may further inhibit endothelial cell proliferation and thereby impair vascular healing in this cohort (20). However, associations are reported between smoking and both early and late stent thrombosis in both first- and second-generation DES (21, 22). In addition to smoking, excessive alcohol consumption has also been reported in association with heightened risk of stent thrombosis (23).
Obesity has been reported as an independent predictor of stent thrombosis, with an estimated 2-fold increase in risk (24). Moreover, overweight (BMI 25-29.9) patients are reported to have an increased 10-year risk of stent thrombosis compared to patients with normal BMI (25). However, conflicting evidence has led to the characterization of an “obesity paradox”, wherein an elevated BMI is often not found to be associated with significantly increased risk or may even be protective of poor ischemic outcomes (26). This phenomenon may be a result of several factors, including more aggressive medical therapy and screening or obese cohorts exhibiting a younger age overall with fewer frailties and better cardiac reserve (27). Obesity may contribute to stent thrombosis risk through platelet hyperactivation and accelerated aggregation, endothelial dysfunction, and chronic inflammation (28). Obese patients may also exhibit antiplatelet resistance, leading to suboptimal platelet inhibition with some clinicians adopting a weight-based dose adjustment strategy (19, 29). Nonadherence to DAPT also is a leading risk factor for stent thrombosis. Termination of DAPT prematurely, either by way of patient self-discontinuation, bleeding diathesis, or periprocedural planning, is one of the strongest and most-reported predictors of stent thrombosis, especially within the 30 day post-PCI period (16, 19, 21, 23, 30). This association is particularly strong with early interruption of clopidogrel, as the median time for thrombosis following clopidogrel discontinuation is approximately 9 days within the first 6 months post-implantation and 104 days beyond 6 months (22, 31). Furthermore, while shorter DAPT durations (3–6 months) are associated with heightened risk of stent thrombosis in ACS patients, evidence for heightened stent thrombosis risk in stable CAD patients is less established (19, 32).
Regional and socioeconomic differences—such as access to contemporary stents, advanced intracoronary imaging, and DAPT significantly impact stent thrombosis rates and outcomes. Multiple large-scale cohort studies and meta-analyses have demonstrated that lower socioeconomic status (SES) measured by income, education, employment, or area deprivation indices—is associated with higher rates of major adverse cardiac events (MACE), including stent thrombosis, recurrent myocardial infarction, and increased long-term mortality after PCI (33, 34). These associations persist even after adjustment for baseline clinical risk factors, though some attenuation occurs, suggesting that both SES and comorbidities contribute independently to adverse outcomes (33). Patients from lower SES backgrounds are less likely to receive contemporary drug-eluting stents, have lower adherence to guideline-recommended dual antiplatelet therapy, and experience higher rates of repeat revascularization and recurrent myocardial infarction, all of which are established risk factors for stent thrombosis (34, 35). Community-level deprivation, as measured by indices such as the Area Deprivation Index, is also linked to higher short-term mortality and readmission rates after PCI, further supporting the role of socioeconomic context in influencing stent-related outcomes (36). These findings highlight the need for targeted interventions to address disparities in access, adherence, and follow-up care to reduce stent thrombosis risk in socioeconomically disadvantaged populations.
Angioplasty-related factors
Stent under-expansion is a strong predictor of stent thrombosis and increases the relative risk by approximately 13 times (37). This phenomenon, wherein a stent does not expand to the intended diameter after deployment, is more frequently observed in DES than BMS and is present in an estimated 26% of early stent thrombosis patients (20, 21). Stent under-expansion may occur due to undersizing of stents, low deployment pressures, or in the setting of heavily calcified lesions (30). This represents a modifiable risk factor, as use of intravascular ultrasound and optical coherence tomography can optimize stent expansion, e.g., with use of minimum stent area (MSA) of >5.0 mm2 (IVUS) or >4.5 mm2 (OCT) as an expansion target or with OCT features such as calcium arc ≥180°, calcium thickness ≥0.5 mm, and lesion length >5 mm as predictors of under-expansion (13, 30). Notably, IVUS-measured MSA following PCA has been reported as a predictor of 2-year stent thrombosis risk (15).Stent geometry also plays a role, as uncovered stent struts disrupt early endothelialization and enable platelet attachment and, by extension, thrombus formation (38). Thicker struts may promote flow recirculation and low ESS, ultimately favoring thrombus formation with higher reported rates of stent thrombosis (39, 40). Thinner struts are reported to be associated with lower rates of stent thrombosis (32). Malapposition is a key mechanism contributing to stent thrombosis that is more commonly seen in VLST in DES compared to BMS (20, 23). This phenomenon, in which part of the implanted stent lacks contact with the surrounding endothelium, may lead to altered local hemodynamic and increased blood viscosity, thereby promoting late stent thrombosis (21, 30). Malapposition is estimated to be present in as much as 18.1% of stent thrombosis patients (15). Edge dissections, wherein stent implantation leads to tearing of the arterial wall at the proximal or distal margin of the stent, has been reported as a procedural risk factor for early stent thrombosis and a major failure mechanism in very late stent thrombosis (20, 22). Expert consensus reports that geographic miss, edge dissections, and intramural hematomas may be strong procedural predictors of early stent thrombosis (30).
Other procedural factors, such as improper stent sizing (i.e., diameter under sizing) leading to incomplete lesion coverage are important risk factors for stent thrombosis (23). Stent under sizing is of particular importance in DES and BRS (40). In an effort to modify this risk, IVUS and OCT have been employed which may lead to a reduction in stent thrombosis risk, particularly in ACS and complex lesions (21, 22). Furthermore, the use of 4+ stents during PCI has been identified as a risk factor for stent thrombosis (17). Overlapping of stents is more frequently observed in stent thrombosis cases compared to non-thrombosis controls (36.0% vs. 24.7%) (41). Finally, first-generation DES generally have a higher risk of early stent thrombosis compared to second-generation DES, owing to polymer coating differences and improved drug release kinetics (31).
Lesion-related factors
Coronary bifurcations are particularly prone to thrombosis due to altered hemodynamics and unbalanced wall shear stress, especially in cases involving stent underexpansion and malapposition (14, 20). Studies involving the REAL-ST and KoST registries reported that bifurcation lesions are significantly more frequent in stent thrombosis patients than in non-thrombosis controls (45.5% vs. 36.5%) (41, 42). Stenting across major side branches is thought to alter flow dynamics and lead to increased platelet activation, leading to increased risk of stent thrombosis, especially within distal left main bifurcation lesions (14, 18, 30). Addressing bifurcations lesions via a two-stent strategy has been reported as a procedural risk factor for early ST (22). Alternative strategies involving overlapping stents are also prone to underexpansion and malapposition (30). Severe coronary calcification leads to stent under-expansion and decreases stent symmetry resulting in malapposition, with suboptimal stent deployment reported in 31%–58% of calcified lesions (30). This ultimately increases risk of stent thrombosis (20, 23, 43). Severe calcification has been estimated to be present in 52.8% of late stent thrombosis cases compared to 14.6% of non-thrombosis controls (41). Calcified lesions generally carry a poorer overall prognosis following PCI, which highlights the importance of intraprocedural lesion preparation and stent deployment in these patients (44). Chronic Total Occlusions (CTOs) also feature higher rates of stent malapposition and under-expansion, leading to increased risk of stent thrombosis (20). CTO's are estimated to be present in 8.3% of stent thrombosis cases vs. 3.3% of non-thrombosis controls (15). Residual plaque burden following intervention in CTO's may contribute to thrombosis formation (18). Tissue prolapse, involving luminal plaque extrusion into the implanted stent, has also been reported as an OCT feature with predictive value of early stent thrombosis in CTO lesions (42). Finally, CTO's are independent predictors of in-stent restenosis, which may further compound risk of stent thrombosis (30). In-Stent Restenosis (ISR), involving vascular smooth muscle cell proliferation into stent struts, also represents an important risk factor of stent thrombosis by way of secondary platelet rupture and thrombus formation (20, 23). ISR is present in an estimated 14.6% of stent thrombosis cases vs. 9.6% of controls (15, 41). ISR is also a known predictor of very late stent thrombosis, especially within second generation DES (42). In cases involving stent under-expansion, this procedural risk factor may contribute to neointimal hyperplasia, accelerating ISR and compounding stent thrombosis risk (44). Finally, neo-atherosclerosis, wherein new atherosclerotic plaques form within the neointima of implanted stents, is a frequent mechanism underlying late and very late stent thrombosis (20). This phenomenon is more common in BMS than in DES, which may contribute to overall heightened stent thrombosis risk in cases involving BMS (20).
Medical comorbidities
Diabetes mellitus is a well-documented predictor for early, late, and very late stent thrombosis following PCI, and insulin dependence is particularly associated with early stent thrombosis (16, 17, 21–23, 31, 39, 41, 42). Diabetes mellitus increases the risk of stent thrombosis via various mechanisms. Firstly, diabetes is associated with increased platelet aggregation, endothelial dysfunction, and a prothrombotic state, all of which contribute directly to stent thrombosis pathophysiology (19, 20). Secondly, diabetes mellitus accelerates atherosclerosis and in-stent neoatherosclerosis, which are key risk factors of very late stent thrombosis (45), Finally, diabetes mellitus leads to reduced arterial healing, increasing stent strut exposure (19). Consequently, diabetes mellitus is associated with higher rates of late stent thrombosis following PCI, with reported rates of 1.7% vs. 0.9% in the E-FIVE and BIOSCIENCE trials (HR = 1.95, P = 0.13), 3% vs. 1.1% in the Western Denmark Heart Registry (RR = 2.56), and an overall OR of 1.95 in a meta-analysis of 18,910 patients (19). In pursuit of avenues to mitigate this risk, polymer-free DES have been identified as a potential solution, with some evidence suggesting a reduction in very late stent thrombosis risk compared to conventional stents (20).
Both chronic kidney disease and end-stage renal disease independently predict stent thrombosis (14, 16, 23). An eGFR < 30 ml/min/m2 has been reported to be associated with both late and very late stent thrombosis (22, 31). CKD may contribute to stent thrombosis risk by promoting arteriosclerosis progression, altered platelet function, systemic inflammation, and a prothrombotic state (19, 20, 45). Furthermore, physicians are more likely to prematurely discontinue DAPT in CKD patients due to an underlying bleeding diathesis which may inadvertently increase stent thrombosis risk (19).
Hypercoagulable states and systemic inflammation, by promoting endothelial damage, are key contributors of stent thrombosis (17). This phenomenon is illustrated through multiple studies evaluating proinflammatory and hypercoagulability biomarkers in stent thrombosis. One retrospective study evaluating the systemic immune-inflammation index found that a value ≥636 is an independent predictor of stent thrombosis (17). Elevated fibrinogen levels have also been observed to a higher degree in patients with very late stent thrombosis relative to controls (45). Thrombocytosis >400 K/ml was estimated to be present in 21.2% of early stent thrombosis patients compared to 7.8% in controls (16, 21). Measures of platelet reactivity are reported to be higher in patients with early stent thrombosis vs. controls (31). Hypercoagulable and proinflammatory states are thought to contribute to thrombosis via the contact activation pathway (46).
Consequently, cancer and thrombophilic hematologic disorders are important risk factors for stent thrombosis. Cancer is reported to be a risk factor for both early and late stent thrombosis, with pancreatic, lung, gastrointestinal cancers, and lymphomas exhibiting particularly increased risks for thrombotic events post-PCI (47, 48). Many chemotherapies used to treat cancer further heighten this risk due to prothrombotic properties (19). Further, myeloproliferative disorders result in aberrant blood counts, leading to a prothrombotic state and increasing risk of stent thrombosis (47). Finally, thrombophilic hematologic disorders such as antiphospholipid syndrome and heparin-induced thrombocytopenia lead to excessive platelet activation and impaired anticoagulation balance, resulting in a heightened risk of stent thrombosis (47). Individualized antithrombotic strategies must therefore be considered in patients with malignancy or other hypercoagulable states (20).
Risk factors and timing
Importantly, the predominant risk factors vary depending on timing. The most common causes of acute stent thrombosis are stent underexpansion and edge dissection along with inadequate antiplatelet therapy initiation (30, 49). Subacute stent thrombosis is most commonly caused by premature discontinuation or inadequate response to dual antiplatelet therapy (DAPT) (30, 50, 51). Late stent thrombosis tends to be related to impaired neointimal healing, which is often due to delayed endothelialization, stent malapposition, or stenting across complex lesions (e.g., bifurcations, overlapping stents) (30). Very late stent thrombosis can be caused by stent malapposition, neoatherosclerosis, and uncovered strut (6, 30). These observations highlight the need for vigilance across all phases after PCI, with tailored strategies to minimize stent thrombosis risk based on patient profile, lesion characteristics, and timing of the event.
Pathophysiology
The pathophysiology of stent-related thrombosis involves complex interactions between stent materials, drug coatings, and the vascular healing response. Stent materials can induce platelet adhesion and activation, leading to thrombus formation. First generation stents such as paclitaxel-eluting stents (PES), contain a polymeric surface that causes a higher degree of platelet activation and deposition compared to bare metal stents (52) Granada et al. observed that the polymeric surface of the PES induce a higher degree of platelet activation and deposition compared to the BMS surface; however, this is not associated with thrombus formation. The mechanism of this is mediated by increased p-selectin expression and platelet-monocyte complex formation. Cobalt-chromium (CoCr), a second-generation DES, significantly induced thrombin generation by the contact and activation of platelets, as demonstrated by Ollivier et al. (53). Drug-eluting stents can further contribute to thrombogenic risk by impairing re-endothelialization, thereby prolonging the exposure of the stent surface to circulating platelets. Sirolimus and paclitaxel, used in first-generation DES, inhibit vascular smooth muscle cell proliferation which delays endothelial healing. This delayed healing is implicated in late and very late stent thrombosis due to prolonged stent exposure (54). CoCr surfaces can trigger leukocyte adhesion, which acts as a scaffold for neutrophil and endothelial cell monolayer formation. This newly formed endothelial phenotype is dysfunctional, impairing effective healing and fostering a pro-inflammatory, pro-thrombotic environment (53).
Role of mechanical and hemodynamic factors
There are three major hemodynamic factors contributing to stent thrombosis: endothelial shear stress (ESS), stent positioning, and stent design. ESS, defined as the tangential force exerted by blood flow on the endothelial surface, is influenced by flow velocity and arterial geometry. When a stent is implanted, it alters this geometry, introducing local flow disturbances and changes in ESS. These regions of low or oscillatory ESS promote platelet adhesion and activation, fostering thrombus formation and local inflammation. Implantation of a stent into an artery imposes geometric changes to flow, introducing shearing stress. This ESS promotes platelet adhesion and activation, which leads to thrombus formation. This induces inflammation, endothelial growth factors such as PDGF, VEGF, and ET-1, which promotes neointimal hyperplasia and endothelialization, creating a pro-thrombotic environment (55). Human studies have revealed an inverse relationship between ESS and extent of ISR after BMS implantation. In DES, ISR occurred more extensively in low-ESS regions after sirolimus-eluting and paclitaxel-eluting stent implantations (56). Together, these factors highlight how mechanical and hemodynamic factors create a pro-thrombotic microenvironment that can persist even with advanced stent technologies.
Stent mispositioning plays a significant role in altering local hemodynamics and increasing the risk of IST. Specifically, SM can lead to regions of ESS, which promote platelet adhesion and thrombus formation. Contributing factors to SM include procedural issues such as stent under sizing or under-expansion, plaque-related mechanisms like positive vessel remodeling, and device-related factors such as delayed endothelialization (57). Protruding struts create localized flow disturbances, acting as a foreign body that disrupts laminar flow and facilitates a procoagulable environment. The extent of stent strut detachment has been shown to correlate strongly with thrombus burden. Qu et al. used computational modeling to demonstrate that shear stress induced by SM is a critical driver of thrombosis, with greater detachment distances associated with increased thrombus formation (58).
Stent design characteristics further influence thrombogenic potential. Strut thickness and geometry play pivotal roles in modulating blood flow and shear stress. Thicker struts and less streamlined designs disturb flow more significantly, increasing the likelihood of thrombus formation. Clinical studies have shown that thick-strutted stents (>162 µm) are approximately 1.5 times more thrombogenic than their otherwise identical thin-strutted counterparts (≈81 µm) (59, 60). In contrast, thin-strutted stents maintain more favorable hemodynamic conditions and are associated with a reduced risk of thrombosis. Together, these findings highlight how mechanical and hemodynamic factors create a pro-thrombotic microenvironment that can persist even with advanced stent technologies.
Evidence from randomized clinical trials
DAPT selection and duration
The DAPT Trial was an international clinical trial to examine the risks and benefits of dual anti-platelet therapy beyond 1 year after placement of a drug-eluting stent as compared with aspirin therapy alone. The DAPT trial had strict inclusion criteria, as only those free from MACE) MACCE), stent thrombosis, repeat revascularization, and moderate or severe bleeding, and who were adherent to DAPT were randomized to either continued thienopyridine or placebo in addition to aspirin for an additional 18 months. This study found that extending DAPT beyond 1 year significantly reduced the risks of ischemic events, such as stent thrombosis, major adverse cardiovascular events, and cerebrovascular events, but was associated with a higher risk of bleeding. The reduction in risk of ischemic events was consistent across stent type. However, this study was limited by selection bias due to exlusion of patients with early events or nonadherence. Furthermore, the trial's run-in period may have led to a healthier, more adherent cohort being randomized, which could underestimate real-world risks and benefit (61). In determining the agent for DAPT, the TRITON-TIMI 38 compared prasugrel with clopidogrel for anticoagulation in adults with moderate to high-risk ACS who were scheduled for PCI. This study found reduction of cardiovascular death, MI, stroke, at a median follow-up of 14.5 months in prasugrel users compared with clopidogrel. This aligned with the information that prasugrel is a greater platelet inhibitor compared with clopidogrel. Although the risk of major and fatal bleeding events was greater with prasugrel, composite clinical benefit appears to favor prasugrel. One exception is noted for patients with a history of cerebrovascular events, for whom the clinical profile of clopidogrel is more favorable. One major downside to this study was that a loading dose of 300 mg of clopidogrel was used, which is now considered suboptimal compared to the 600 mg dose used in contemporary practice. This study cannot be applied to patients who were medicaly managed for ACS or those undergoing elective PCI (62). Meta-analysis performed by Palmerini et al. comparing extended-duration DAPT revealed that shorter DAPT was associated with lower all-cause mortality compared with longer DAPT. Patients in 6-month or 1-year DAPT groups had higher risk of myocardial infarction and stent thrombosis but lower risk of mortality compared with patients treated with DAPT for longer than 1 year. Increased non-cardiovascular mortality (such as higher risk of bleeding) may offset the reduction in cardiac mortality (63).
Procedural techniques
Appropriate stent placement can influence hemodynamic factors and shear stress, increasing the risk of thrombosis. The ADAPT-DES study was a large, prospective, multicenter registry of 9,961 patients that examined whether intravascular ultrasound (IVUS) guidance would improve stent placement compared with angiography guidance. ADAPT-DES included those with successful PCI and only those on clopidogrel and aspirin. Those with failed PCI, nonresponders to clopidogrel (by platelet function testing), and those on other P2Y12 inhibitors were excluded. The study found that IVUS guidance (compared with angiography-guidance) was associated with reduced 1-year rates of definite/probable stent thrombosis, myocardial infarction, and composite adjudicated major adverse cardiac events (ie, cardiac death, myocardial infarction, or stent thrombosis). This indicates that IVUS is a powerful tool in reducing rates of stent thrombosis and MI within 1 year after DES implantation. Limitations of this study include its observational registry design, which introduces potential for unmeasured confounding and selection bias. The results can only be applied to those with successful PCI and on clopidogrel and aspirin, rather than other platelet inhibitors. Lastly, the study population was predominantly from high-volume centers in the US and Europe, which may not reflect outcomes in lower-resource settings. Overall, the study indicates that IVUS may be a powerful tool in reducing rates of stent thrombosis and MI within 1 year after DES implantation (64).
As stent underexpansion is in an important predictor of ST and ISR, techniques such as pre- and post-dilatation have been examined to improve outcomes. Pre-dilatation refers to balloon angioplasty performed before stent deployment to facilitate stent delivery, while post-dilatation is high-pressure balloon inflation after stent placement to optimize stent expansion and apposition. The DISCO trial randomized 416 patients to direct stenting vs. stent implant following balloon pre-dilatation. Patients >75 years old, heavily calcified lesions, bifurcations, total occlusions, left main lesions and very tortuous vessels were excluded. They found that in these non-complex lesions, direct stenting without pre-dilatation is as safe and effective as stenting with pre-dilatation, with similar rates of restenosis and target lesion failure, but with reduced procedure time and radiation exposure (65). Further meta-analyses show that direct stenting modestly reduces periprocedural myocardial infarction and procedural time in simple lesions, but does not consistently lower restenosis or target vessel revascularization rates (66, 67). In complex lesions, however, the data favors using pre-dilatation. A large multicenter registry analysis of 9,525 patients demonstrated that optimal stenting technique including intracoronary imaging-guided pre-dilatation, stent sizing, and post-dilatation was associated with a significantly lower rate of cardiac events, including stent thrombosis, at 3 years in patients with complex lesions (adjusted hazard ratio for composite cardiac events 0.71, 95% CI 0.63–0.81) (68). The ACC,AHA, and SCAI recommend the use of intracoronary imaging and optimal lesion preparation, including pre-dilatation, to minimize stent thrombosis in complex PCI (69).
Although randomized controlled trials are lacking, available studies report mixed findings on the effectiveness of post-dilatation. In a post-hoc analysis of the BASE ACS trial, which originally studied bioactive vs. Everlimus-eluding stents, researchers examined outcomes of patients who underwent stent placement with and without post-dilatation. They found that while the rates of ISR were decreased in those who underwent post-dilatation, the rates of MACE and ST did not differ compared to those without post-dilatation. Post-dilatation was performed at the discretion of the physician rather than randomized, and were done in significantly more complex lesions (70). Furthermore, large registry data from over 90,000 stent implantations found no statistically significant difference in stent thrombosis rates between cases with and without post-dilatation, regardless of lesion complexity (71). A separate large registry of 27,148 patients from Korea examined those who underwent PCI of complex coronary artery stenosis, defined as unprotected left main disease, bifurcate lesion, diffuse-long lesion (>30 mm), or severely calcified lesions on angiography. They found that IVUS-guided post-dilation was significantly associated with a lower risk of the primary outcome (HR: 0.77; 95% CI: 0.63–0.93; P = 0.007), unlike post-dilation without IVUS guidance. Furthermore, compared with implanted stents, IVUS-guided post-dilation used a significantly larger post-dilation balloon (68). This suggests that IVUS-guided post-dilatation may be beneficial in complex coronary artery lesions. Further randomized trials are needed to examine the effectiveness of post-dilatation.
As discussed, bifurcation lesions have been associated with increased rates of ST compared to non-bifurcation lesions. Over the years, several bifurcation techniques have been employed to improve procedural and clinical outcomes (72). The technique descriptions are beyond the scope of this review. Multiple network meta-analyses and randomized trials consistently show that DK crush yields the lowest stent thrombosis rates among bifurcation strategies, with relative risk reductions ranging from 50% to 83% compared to provisional stenting and other two-stent methods such as culotte, T-stenting, and classic crush (73, 74). The randomized trials used in the meta-analyses did not investigate the use of this technique in more severe morbidities, such as in myocardial infarction or left ventricular dysfunction, as well as in more complex lesions such as bifurcation CTO. More studies are needed to examine the effectiveness of DK crush in these populations.
Management of stent thrombosis
IST is a life-threatening complication for patients who have had previous stent placement, requiring urgent intervention to restore coronary perfusion. The cornerstone of management is emergent percutaneous transluminal coronary angioplasty (PTCA), which involves catheterization and balloon angioplasty to reopen the occluded stent. PTCA is often accompanied by additional stenting to help maintain the long-term patency of the lesion (78%). Concurrent antiplatelet therapy is generally recommended, with aspirin in addition to a P2Y12 inhibitor such as prasugrel, ticagrelor, or clopidogrel, to prevent further thrombotic events. Multiple studies have demonstrated >90% rates of successful reperfusion from PTCA in the setting of stent thrombosis. Despite reperfusion, IST is complicated by high rates of myocardial infarction and significant declines in left ventricular ejection fraction. Additionally, patients remain at high risk for death (11%), reinfarction (16%), and recurrent stent thrombosis (12%) in the 6 month period post emergency PTCA (75, 76). Thrombectomy may have a role in cases of higher thrombus burden, as there are lower rates of distal clot embolization and greater epicardial and myocardial reperfusion, however it has not been shown to reduce MACE (77, 78).
Stent selection
Selecting the appropriate stent remains a significant predictor of risk of stent thrombosis. The risk of IST following PCI varies based on the type of stent used, with stent selection being made on a patient-by-patient basis. Polymer-based stents are typically the primary choice of stents used due to their safety and efficacy profiles (69). The main types of stent polymers are durable (permanent) polymers, biodegradable (bioabsorbable) polymers, and polymer-free designs. The polymer type directly influences the risk of stent thrombosis at different time periods after implantation. Durable polymer drug-eluting stents (DP-DES) commonly used include poly(ethylene-co-vinyl acetate) (PEVA), poly(n-butyl methacrylate) (PBMA), and polyvinylidene fluoride-co-hexafluoropropylene (PVDF-HFP). First-generation DP-DES are associated with a higher risk of late and very late stent thrombosis due to chronic vessel wall inflammation and delayed endothelial healing from persistent polymer presence. This risk is less pronounced with second-generation DP-DES, which use more biocompatible polymers and thinner struts, but some risk remains (79–81). Biodegradable polymer drug-eluting stents (BP-DES) are designed so the polymer degrades after drug elution, theoretically reducing chronic inflammation and the risk of very late stent thrombosis. BP-DES include polylactic acid (PLA), polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and poly-D,L-lactide (PDLLA). Meta-analyses show BP-DES have similar rates of acute and subacute stent thrombosis compared to DP-DES, but may offer a modest reduction in very late stent thrombosis (79, 82). Polymer-free stents eliminate polymer-related inflammation entirely, but their clinical performance in terms of stent thrombosis is similar to BP-DES and second-generation DP-DES, with no clear superiority in any time period (83).
The principal alternative to polymer-based stents are the bare metal stent (BMS), which does not use any polymer or drug coating. Bare metal stents experience faster endothelialization and require shorter courses of dual antiplatelet therapy (DAPT), making them the stent of choice for patients with high risk of bleeding or with upcoming procedures that would require interruption of DAPT. BMS are associated with higher rates of restenosis compared to all drug-eluting stents (DES), regardless of polymer type, and have a higher risk of target lesion revascularization. However, BMS have a lower risk of very late stent thrombosis compared to first-generation DES, but not compared to contemporary DES. The ACC, AHA, and SCAI recommend DES over BMS for most patients due to superior efficacy and safety, including lower rates of stent thrombosis and myocardial infarction (69).
Another category of stent includes the BVS, which is designed to be completely absorbed by the body over time, including both the scaffold and any polymer or drug. Older generation BVS, such as the first-generation Absorb everolimus-eluting scaffold, were designed to provide temporary vessel support and drug delivery, then fully resorb, theoretically reducing very late adverse events associated with permanent metallic stents. Compared to second-generation EES, Absorb BVS increased the risk of device thrombosis (hazard ratio 2–4), target lesion failure, and myocardial infarction through 3 years, with the excess risk largely abating after scaffold resorption at 3 year. This pattern is consistent across large pooled analyses and individual randomized trials, including AIDA and ABSORB III/IV (84–87). The most recent clinical trial data on latest-generation bioabsorbable scaffolds with thinner struts indicate that these devices have improved safety and efficacy profiles compared to first-generation bioresorbable vascular scaffolds (BVS), but none have yet demonstrated outcomes superior to or consistently equivalent to second-generation DES such as EES, ZES, or BES in broad patient populations (84, 88–90). In summary, current clinical data do not support the use of either older or newer generation bioabsorbable scaffolds over second-generation sirolimus- or everolimus-eluting DES, which remain the standard of care for safety and efficacy (69).
Role of antiproliferative drugs
DES are coated with antiproliferative agents, which provide both a mechanical and biochemical approach to inhibit lumen re-narrowing. The antiproliferative agents are thought to influence vessel recoil, vessel remodeling, and intimal proliferation, which are the main factors responsible for restenosis. Sirolimus and paclitaxel are the two principal antiproliferative agents used in first-generation DES. Sirolimus (a macrolide immunosuppressant) inhibits the mammalian target of rapamycin (mTOR), blocking smooth muscle cell proliferation in the G1 phase of the cell cycle. Paclitaxel (a taxane) stabilizes microtubules, arresting cell division in the G0–G1 and mitotic phases. Both drugs are delivered via durable polymer coatings on stainless steel stents in first-generation DES (e.g., Cypher for sirolimus, Taxus for paclitaxel) (doi:10.1056/NEJMra1210816, doi:10.1056/NEJMra051091). Head-to-head meta-analyses of sirolimus- and paclitaxel-eluting stents consistently show that sirolimus-eluting stents are superior to paclitaxel-eluting stents in reducing restenosis, target lesion revascularization, and stent thrombosis. Sirolimus-eluting stents (SES) are associated with a lower risk of target lesion revascularization (relative risk reduction ∼30%–40%) and a lower risk of definite or probable stent thrombosis (hazard ratio 0.66, 95% CI 0.46–0.94) compared to paclitaxel-eluting stents (PES), with no significant difference in mortality or myocardial infarction (91–93).
Second-generation DES use sirolimus analogues (everolimus, zotarolimus, biolimus A9) with more biocompatible or biodegradable polymer, further improving safety compared to first-generation SES or PES (91). A meta-analysis of 11 RCTs showed that everolimus-eluting stents (EES) had a reduced the risk of stent thrombosis compared to SES (OR: 0.44, 95% CI: 0.25–0.80) and PES (OR: 0.35, 95% CI: 0.21–0.53) (94). EES also provide lower rates of repeat revascularization and major adverse cardiac events compared to SES and PES, with no significant difference in mortality or cardiac death (95, 96). Zotarolimus-eluting stents (ZES), especially the Resolute platform, have safety profiles similar to EES, with lower stent thrombosis and myocardial infarction rates than SES and PES. Biolimus A9-eluting stents (BES) with biodegradable polymers are non-inferior to EES and ZES for efficacy, but EES and ZES remain the safest overall (97–99).
Intravascular imaging
Intravascular imaging plays a crucial role in identifying the underlying causes of IST and guiding the appropriate interventions. Intravascular Ultrasound (IVUS) and Optical Coherence Tomography (OCT) are the primary modalities used for identifying stent-related complications such as malapposition, underexpansion, neoatherosclerosis, and quantifying thrombus burden. IVUS works through a catheter mounted ultrasound probe, providing real time cross sectional images of the coronary artery. OCT utilizes near-infrared light and contrast dye to offer a higher resolution assessment of stent morphology. IVUS has been used to identify stent underexpansion as a driver of early IST, and malapposition contributing to very late IST (100). Meanwhile, OCT's higher resolution provides superior capabilities for detecting neointimal changes and neoatherosclerosis, a major contributor to late IST (101) Intravascular imaging at the time of PCI is useful in guiding stent sizing, ensuring appropriate expansion, and detecting acute complications such as edge dissections, malapposition, and tissue protrustion (102). The RENOVATE-COMPLEX-PCI demonstrated that intravascular image guided PCI led to lower composite death from cardiac causes, target-vessel–related myocardial infarction, or clinically driven target-vessel revascularization when compared with angiographically guided PCI (103). Given these findings, the American College of Cardiology and American Heart Association recommend the use of intravascular imaging in patients with stent failure to determine the mechanism for stent failure (69).
Duration of dual antiplatelet therapy
The decision on duration of dual antiplatelet therapy (DAPT) after PCI is dependent on the type of stent implanted, complexity of the lesion, and patient specific bleeding and thrombotic risk factorsTraditionally, bare metal stents, with their earlier endothelializationreendothelialization, require at least 30 days of DAPT (104). Previous guidelines recommended the duration of DAPT in patients with drug eluting stents as typically 6–12 months, with longer durations favored in patients presenting with ACS. although this course can be shortened based on the patient's risk factors (69). The latest generation of drug-eluting stents (DES), characterized by enhanced biocompatibility, thinner struts, and improved polymer coatings, has significantly reduced rates of stent thrombosis and myocardial infarction (69). These advancements have allowed for shorter durations of DAPT as brief as 1–3 months, in carefully selected patients, particularly those at high risk for bleeding. After this abbreviated DAPT period, transitioning to P2Y12 inhibitor monotherapy has been shown to lower major bleeding risk without increasing MACCE, compared with the traditional 12-month DAPT regimen. (doi:10.1001/jamacardio.2024.3216). In a systematic review and network meta-analysis in patients with ACS undergoing PCI 24,797 patients received either ticagrelor or prasugrel found that 1 month of DAPT followed by a P2Y12 inhibitors reduced major bleeding (RR, 0.47; 95% CrI, 0.26–0.74), however patients with 3 months of DAPT followed by PGY12 inhibitor was ranked as most optimal for reducing MACCE (RR, 0.85; 95% CrI, 0.56–1.21), despite being statically significant (69). In adition, the STOPDAPT-2 trial found that DAPT as short as one month followed by clopidogrel monotherapy may in fact be superior to the traditional regimen of 12 months of DAPT (105) Similarly, SMART-CHOICE demonstrated that P2Y12 monotherapy after 3 months of DAPT was non-inferior to 12 months of DAPT, with significantly lower bleeding risks (106). In comparison, tThe DAPT trial demonstrated that a 30 month DAPT regimen can significantly reduce the rates of stent thrombosis, however this reduction is accompanied by increased risks of bleeding and all cause mortality (61). More complex lesions are more susceptible to ischemic events such as IST. Involvement of the left main coronary artery, multiple lesions per vessel, lesions longer than 30 mm, and lesions at bifurcations are considered to be complex lesions. Given their increased ischemic risks, the ACC and AHA recommend at least 12 months of DAPT, with consideration of longer regimens based on the patient's bleeding risk assessment (107). The DAPT score is a clinical decision-making tool that can be used to stratify patients based on their bleeding and thrombotic risks and guide the duration of DAPT following coronary stent implantation. This calculator takes into account factors such as age, cigarette smoking, diabetes mellitus, myocardial infarction (MI), early-generation drug-eluting stents, stent diameter <3 mm, left ventricular ejection fraction <30%, presentation with MI, prior PCI, congestive heart failure, and vein graft PCI to identify patients who may benefit from prolonged DAPT. Conversely, a history of prior bleeding, concurrent anticoagulation therapy, female sex, advanced age, low body weight, and chronic kidney disease may place patients at higher risk for bleeding, making them better candidates for shorter DAPT regimens. According to theDAPTstudy, patients with a high DAPT score (≥2) who have tolerated 1 year of therapy without major ischemic or bleeding events are more likely to experience a favorable benefit/risk ratio with prolonged DAPT, whereas those with a low DAPT score (<2) may face increased bleeding without ischemic benefit. Thus, the DAPT score provides a practical and evidence-based approach to individualizing therapy and optimizing outcomes in patients undergoing PCI (104).
Role of cilostazol
Cilostazol, a phosphodiesterase-3 inhibitor, has antiplatelet and vasodilatory properties, making it a potential adjunctive therapy for reducing IST after PCI. While not routinely recommended due to its contraindication in heart failure and potential adverse effects, cilostazol has been studied as part of tiple antiplatelet therapy in high-risk populations. The CILON-T trial demonstrated that cilostazol more effectively reduced platelet activity compared to standard DAPT, however it did not show a reduction in MACE after drug eluting stent implantation (108). Conversely, the CREATIVE trial found that cilostazol reduced MACE, particularly in patients with low responsiveness to clopidogrel as measured by thromboelastography, suggesting a potential role for those with resistance to clopidogrel (109). In clinical practice, cilostazol may be considered in select high risk patients, particularly those with high thrombotic risk, prior IST, or those with clopidogrel resistance (109–111). Additionally, it may be a reasonable option in those with concurrent peripheral artery disease, where its vasodilatory effects can provide additional symptomatic relief (112).
Role of anticoagulation
In certain patient populations, the use of concurrent anticoagulation therapy and DAPT is necessary, with the most common indications being atrial fibrillation, mechanical heart valves, venous thromboembolism, and left ventricular thrombus. However, the combination of DAPT and anticoagulation (triple therapy), significant increases the risk of bleeding, with reported rates rising from 4% to 6% in DAPT to 10%–14% on triple therapy (113). Given this heightened risk, triple therapy should only be used for short durations, after which patients should be continued on anticoagulation plus a single antiplatelet agent. Studies such as the WOEST trial have demonstrated that anticoagulation combined with a single antiplatelet agent confers a lower risk of bleeding without a significant increase in thrombotic events or all-cause mortality (114, 115). Similarly, the ISAR-TRIPLE trial compared six weeks vs. six months of triple therapy, showing no significant difference in ischemic outcomes, but a reduced risk of bleeding with the shorter duration (116). Current recommendations advocate for minimizing the duration of triple therapy and transitioning to dual therapy with an anticoagulant plus single antiplatelet as soon as reasonably possible to balance thrombotic and bleeding risks effectively (117).
Strategies for prevention
Prevention of IST requires a multifaceted approach, incorporating optimal stent selection, precise deployment, and use of intravascular imaging modalities such as IVUS and OCT to ensure proper stent expansion and apposition (102, 103). Advances in stent technology, particularly the development of second and third generation bioresorbable drug eluting stents have significantly reduced the risk of IST by improving endothelialization and reducing thrombogenicity (118, 119). Antiplatelet therapy plays a crucial role in IST prevention, with careful selection of single, dual, or triple antiplatelet therapy to balance ischemic protection with bleeding risk. The duration of antiplatelet therapy must be individualized, ranging from as short as 30 days in high bleeding risk patients, to beyond one year in those at higher risk of ischemic events (69) Adherence to the prescribed therapy is critical, as nonadherence to DAPT is associated with between a 2 and 20 fold increase in IST rates, leading to higher rates of myocardial infarction and mortality (120).
Clinical decision tools such as the DAPT score can aid in risk stratification, helping to personalize therapy duration based on the patient's unique circumstances. By integrating these strategies—careful stent selection, judicious use of intravascular imaging, tailored antiplatelet therapy, and education on medication adherence the risk of IST can be minimized, improving the long-term success of PCI.
Limitations
Despite strong evidence and guideline recommendations to reduce stent thrombosis risk via optimal implantation techniques, imaging guidance, and prolonged dual antiplatelet therapy, adoption in real-world practice remains inconsistent. One major barrier is non-adherence, which is well documented: DAPT use often declines over time, with drop-off by 12 months, driven by bleeding, lack of education, or communication gaps (121). Cost is another recurring obstacle: newer agents (e.g., ticagrelor or prasugrel) are more expensive, and patients with lower income are less likely to adhere to antiplatelet regimens post-PCI (122). Meanwhile, imaging guidance (IVUS, OCT) offers procedural optimization and reduction in thrombosis risk, but uptake remains low. Barriers include high cost, increased procedure time, limited reimbursement, variable operator training, and institutional/regulatory constraints (123).
Beyond these classical barriers, less is known about how provider and patient preferences, and regulatory or system-level constraints, limit uptake of guidelines. Some interventionalists trained principally in angiographic guidance may resist changing habits or feel cognitive dissonance when adopting intravascular imaging (123). On the patient side, preferences regarding bleeding risk, pill burden, cost, or quality-of-life tradeoffs may lead patients to decline or discontinue recommended DAPT therapy, especially when shared decision-making is weak (https://doi.org/10.1007/s40119-024-00372-7) (124). At a higher level, variation in regulatory approval of devices/imaging modalities, institutional policies, reimbursement schemes, and the lack of enforcement of guideline adherence all impose structural friction (125).
Future directions
When developing the next generation of stents, it is crucial to address the current limitations and side effects of current stent. As discussed in the pathophysiology section, stent placement damages the endothelium, triggering platelet activation and aggregation, which significantly increases the risk of restenosis. Therefore, advancements that minimize initial endothelial injury or enhance re-endothelialization could effectively reduce restenosis rates. Regenerative medicine, such as Re-endothelialization stents, which release therapeutic exosomes that accelerate endothelial cell proliferation and migration are one of the handful of innovations created to decrease restenosis. Re-endothelialization stents facilitate early-stage stent coverage but also reduces vascular inflammation and smooth muscle cell overgrowth, mitigating the risk of in-stent restenosis (126). However, need for further long-term studies to evaluate their clinical efficacy and safety compared to traditional stents is still needed.
Cell-captured stents use biomolecule coatings, such as antibodies targeting endothelial progenitor cells (EPCs) (e.g., anti-CD34, VE-cad, or CD133), to attract circulating EPCs and promote natural endothelial regeneration (127, 128). These stents have shown early endothelial coverage and reduced in-stent restenosis in animal models compared to BMS (129). However, clinical outcomes are inconsistent, as studies have shown that captured EPCs do not always contribute significantly to endothelial regeneration, and some cell-capture stents, like those with anti-CD34, may fail to reduce neointimal hyperplasia or even increase restenosis rates (130, 131).
Nitric oxide (NO) stents incorporate NO-producing coatings to enhance vascular healing by preventing thrombosis, reducing inflammation, inhibiting smooth muscle cell (SMC) proliferation, and promoting endothelialization (132). These stents use either NO-releasing coatings, which deliver NO from exogenous donors, or NO-generating coatings, which convert endogenous precursors into NO through catalysts like copper or selenium (133, 134). Despite promising in vitro and in vivo results, challenges include limited NO release duration, potential toxicity of NO donors, and the lack of long-term preclinical studies on NO-generating stents (135, 136).
Lastly, other research has studied VEGF-incorporated stents which utilize vascular endothelial growth factor (VEGF) coatings to promote endothelial regeneration and potentially reduce in-stent restenosis. Recent studies have shown promising results, with VEGF-bound stents selectively capturing EPCs and accelerating endothelialization (137, 138). However, outcomes remain inconsistent, as efficacy depends on co-grafting conditions, the form of VEGF, and the conjugation method (138, 139).
Nanotechnology offers significant advancements in cardiac stents by enhancing drug delivery, biocompatibility, and thrombotic pathway targeting. Liposomes and polymeric micelles facilitate controlled drug release to inhibit SMC proliferation and restenosis, while dendrimers improve gene delivery for rapid endothelial regeneration (140, 141). Lipid-polymer hybrid nanoparticles (LPNs) provide vascular targeting, reducing platelet adhesion and oxidative stress (142, 143). Nonpolymeric nanomaterials, such as TiO₂ nanotubes and diamond-like nanocomposites (DLN), enhance endothelial growth while minimizing platelet adhesion and thrombogenicity (144). These innovations improve stent hemocompatibility and reduce late in-stent restenosis, addressing key limitations of traditional DES. While nanotechnology in cardiac stents holds significant promise, it presents challenges related to toxicity and organ accumulation of nanoparticles, which can lead to adverse effects on macrophage function and increased inflammation (145, 146). Additionally, nanoparticle-induced oxidative stress and membrane disruption from materials like dendrimers and magnetic nanoparticles may contribute to cell death and toxicity, raising concerns for long-term safety in clinical applications (147). The next- generation of stents have been developed with the thought to increase biocompatibility and protect patients from restenosis in the future, but the major limitation in incorporating this new technology is the lack of longitudinal evidence supporting its safety.
Emerging therapies targeting thrombotic pathways
Several novel drug-eluting stent (DES) technologies have been developed to improve cardiovascular outcomes and minimize thrombotic complications in patients undergoing percutaneous coronary intervention (PCI). These include Cyclopentenyl Cytosine (CPEC)-coated stents, surface-degradable DES, and stents modified through various surface engineering techniques.
CPEC-coated stents utilize an anti-proliferative nucleoside analog designed to promote endothelial cell regrowth while minimizing clot formation. By slowly releasing CPEC at the site of implantation, these stents aim to reduce neointimal tissue growth and inhibit platelet activation, ultimately improving vascular healing without the prolonged need for systemic anticoagulation. The gradual release of CPEC from the stent surface reduces neointimal hyperplasia and platelet aggregation, preventing thrombotic complications and enhancing vascular healing, as shown in preclinical models (148). However, there is a lack of human clinical trial data, so the safety, efficacy, and optimal antiplatelet regimen for CPEC-coated stents in real-world populations remain unestablished. Additionally, the long-term effects of CPEC on vascular healing and potential off-target toxicity are unknown, and the translation of findings from animal models to human coronary arteries is uncertain (148). Surface-degradable DES employ a layered coating system that incorporates a biodegradable polymer (such as PTMC) and an antiproliferative agent like rapamycin. These designs work to suppress smooth muscle cell overgrowth and reduce thrombosis while simultaneously encouraging endothelial cell recovery. The addition of an inner titanium oxide (Ti–O) layer further aids in endothelialization, contributing to improved vessel healing and decreased rates of restenosis and thrombotic events. The PTMC/rapamycin combination makes surface-degradable drug-eluting stents a promising solution for preventing restenosis and thrombosis (149). However, meta-analyses and clinical reviews indicate that while biodegradable polymer DES may reduce very late stent thrombosis compared to first-generation DES, they have not demonstrated superiority over second-generation durable polymer everolimus-eluting stents in terms of efficacy or safety. Some bioresorbable scaffolds have shown increased rates of late and very late stent thrombosis, raising concerns about their long-term safety profile (98, 150).
Additionally, advanced surface modification methods, such as plasma oxidation, physical and chemical vapor deposition (PVD/CVD), and electrodeposition—are being applied to stents to enhance their biocompatibility. These approaches alter the stent's surface characteristics, such as texture and charge, to make them less prone to clot formation. For instance, plasma treatments increase surface wettability and reduce platelet adhesion, while coatings like titanium nitride and diamond-like carbon applied via PVD or CVD have been shown to support endothelial cell adhesion and reduce thrombogenicity. Electrodeposition offers precise control over coating thickness and composition, further improving hemocompatibility and reducing thrombogenicity (151). However, there is mixed evidence regarding the impact of advanced surface modification methods on clinically meaningful endpoints such as stent thrombosis and restenosis. Many surface engineering strategies remain at the experimental or early clinical stage, with limited large-scale randomized trial data. The heterogeneity of surface modification methods and lack of standardized evaluation protocols further complicate direct comparison and clinical adoption (151, 152).
Role of artificial intelligence
Artificial intelligence (AI) has the potential to be a valuable tool in medicine, particularly in predicting and preventing stent thrombosis (ST), but current research remains limited. Given our understanding of stent mechanics and patient risk factors, AI, specifically machine learning (ML), could be leveraged to identify individuals at higher risk for ST and tailor prevention strategies accordingly. One study by Gómez et al. (153) developed an ML model using data from the GRACIA-3 trial to stratify patient risk for stent restenosis (SR), demonstrating its potential to reduce unnecessary follow-ups and improve treatment for high-risk individuals (153). However, while ML has shown promise in pattern recognition and predictive analytics, it is only a subset of AI, true AI would likely incorporate broader reasoning, real-time decision-making, and adaptability, which could further enhance risk assessment and personalized treatment strategies.
AI-driven models, such as AI-DAPT, have demonstrated superior predictive accuracy compared to traditional risk scores by dynamically assessing ischemic and bleeding risks in post-stent implantation patients. By using vast EHR data and advanced machine learning algorithms, AI-DAPT can identify complex, nonlinear relationships among clinical variables, offering a more personalized approach to DAPT management. This adaptability is particularly relevant to stent thrombosis prevention, as AI models could continuously update risk predictions based on evolving patient data, ensuring timely intervention. As research advances, integrating AI-driven models with real-time patient data and clinical insights may provide more accurate predictions and ultimately help prevent ST. Moreover, incorporating multimodal data sources and deep learning techniques could further refine risk assessment, improving long-term cardiovascular outcomes (154, 155). However, the clinical utility of AI in this domain is still developing, necessitating further validation through large-scale studies and real-world implementation.
Conclusion
ST remains a complex and multifactorial complication following PCI, influenced by patient-specific characteristics, procedural factors, lesion complexity, and underlying medical co-morbidities. Advanced age, female sex, smoking, and obesity are notable patient-related risk factors that increase thrombosis risk. These factors, alongside nonadherence to DAPT, remain significant modifiable risks, especially in the early post-PCI period. Furthermore, procedural factors like stent under-expansion, malposition, edge dissections, and improper stent sizing necessitate the use of advanced intravascular imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), which can optimize stent placement and reduce complications. Complex lesion characteristics, including bifurcations, calcifications, chronic total occlusions (CTOs), and in-stent restenosis, increase the risk of thrombosis and highlight the need for tailored interventional strategies. In addition, chronic conditions such as diabetes, chronic kidney disease (CKD), and hypercoagulable states exacerbate thrombotic risk through mechanisms like endothelial dysfunction and impaired arterial healing. Studies have shown that diabetes accelerates atherosclerosis and impairs stent healing, leading to higher rates of late and very late ST. Given the interplay of these risk factors, a comprehensive, multidisciplinary approach is essential to minimize the incidence of ST and improve long-term patient outcomes. This approach must include individualized procedural techniques, medical management tailored to the patient's unique risk factors, and rigorous adherence to DAPT.
The pathophysiology of ST is multifaceted, driven by platelet activation, delayed endothelialization, and hemodynamic factors. Studies have shown that first-generation drug-eluting stents (DES), such as paclitaxel-eluting stents, cause heightened platelet activation due to their polymeric surfaces, while second-generation cobalt-chromium DES exacerbate thrombin generation. Delayed endothelialization, particularly with sirolimus- and paclitaxel-eluting stents, prolongs stent exposure to circulating blood elements, sustaining a prothrombotic state. Additionally, hemodynamic alterations, such as endothelial shear stress, stent mispositioning, and strut design, contribute significantly to thrombogenicity by promoting platelet adhesion and thrombus formation. Stent misalignment and thicker struts exacerbate low-shear environments conducive to thrombus development, underscoring the need for precise stent placement. These findings further reinforce the importance of continued advancements in stent technology and implantation techniques to mitigate thrombosis risk. Given these complex interactions, multidisciplinary management, incorporating cardiologists, interventional specialists, and pharmacologists, is crucial to ensure that all aspects of patient care, from procedural optimization to pharmacological intervention and long-term management, are addressed. Multimodal strategies that integrate state-of-the-art imaging, personalized treatment plans, and adherence to evidence-based therapies are critical for improving patient outcomes and reducing ST incidence. Despite advances in stent technology and pharmacotherapy, several challenges remain in minimizing the incidence of ST. There is an urgent need for further research to better understand the role of specific lesion characteristics, stent designs, and patient risk factors in stent thrombosis development. Clinical trials, such as those by Palmerini et al. (63) and studies involving advanced intravascular imaging techniques, should continue to focus on optimizing procedural strategies and stent material innovation (63). Additionally, research into new therapeutic agents, such as those targeting thrombotic pathways and advancements in nanotechnology for stent coatings, holds promise for enhancing endothelial healing and reducing thrombosis risk.
As stent thrombosis remains a critical issue in PCI outcomes, a continued, multidisciplinary effort combining clinical expertise, technological innovation, and patient-centered care is essential to improving the prevention, diagnosis, and management of this complication.
Statements
Author contributions
AF: Writing – original draft, Writing – review & editing. SE: Writing – review & editing. AW: Writing – review & editing. BE: Writing – review & editing. BG: Writing – review & editing. RS: Writing – review & editing. AR: Writing – review & editing. IR: Writing – review & editing. HH: Writing – review & editing. MA: Writing – review & editing. MK: Writing – review & editing. MS: Writing – review & editing, Writing – original draft. CK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Batchelor R Dinh D Brennan A Lefkovits J Reid C Duffy SJ et al Incidence, predictors and clinical outcomes of stent thrombosis following percutaneous coronary intervention in contemporary practice. Heart Lung Circ. (2020) 29(10):1433–9. 10.1016/j.hlc.2019.10.009
2.
Ishihara T Okada K Kida H Tsujimura T Iida O Okuno S et al Long-term outcomes and clinical predictors of mortality following occurrence of stent thrombosis. J Am Heart Assoc. (2022) 11(7):e023276. 10.1161/JAHA.121.023276
3.
Hicks KA Tcheng JE Bozkurt B Cutlip DE Farb A Fonarow GC et al 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American college of cardiology/American heart association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation. (2015) 132(4):302–61. 10.1161/CIR.0000000000000156
4.
Tariq S Kumar R Fatima M Saghir T Masood S Karim M . Acute and sub-acute stent thrombosis: frequency, predictors and features in patients undergoing primary percutaneous intervention at a tertiary care cardiac centre. IJC Heart Vasc. (2020) 26:100427. 10.1016/j.ijcha.2019.100427
5.
Adriaenssens T Joner M Godschalk TC Malik N Alfonso F Xhepa E et al Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE consortium (prevention of late stent thrombosis by an interdisciplinary global European effort). Circulation. (2017) 136(11):1007–21. 10.1161/CIRCULATIONAHA.117.026788
6.
Souteyrand G Amabile N Mangin L Chabin X Meneveau N Cayla G et al Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. (2016) 37(15):1208–16. 10.1093/eurheartj/ehv711
7.
Mohamed MM Sirker A Chieffo A Avanzas P Nolan J Rashid M et al Temporal patterns, characteristics, and predictors of clinical outcomes in patients undergoing percutaneous coronary intervention for stent thrombosis. EuroIntervention. (2022) 18(9):729–39. 10.4244/EIJ-D-22-00049
8.
Yang YX Liu Y Li XW Lu P-J Wang J Li C-P et al Clinical outcomes after percutaneous coronary intervention for early versus late and very late stent thrombosis: a systematic review and meta-analysis. J Thromb Thrombolysis. (2021) 51(3):682–92. 10.1007/s11239-020-02184-7
9.
Tovar Forero MN Zanchin T Masdjedi K van Zandvoort LJC Kardys I Zijlstra F et al Incidence and predictors of outcomes after a first definite coronary stent thrombosis. EuroIntervention. (2020) 16(4):e344–50. 10.4244/EIJ-D-19-00219
10.
Ohno Y Yamaji K Kohsaka S Inohara T Amano T Ishii H et al Incidence and in-hospital outcomes of patients presenting with stent thrombosis (from the Japanese nationwide percutaneous coronary intervention registry). Am J Cardiol. (2020) 125(5):720–6. 10.1016/j.amjcard.2019.12.005
11.
Katsanos K Al-Lamki SAM Parthipun A Spiliopoulos S Patel SD Paraskevopoulos I et al Peripheral stent thrombosis leading to acute limb ischemia and major amputation: incidence and risk factors in the aortoiliac and femoropopliteal arteries. Cardiovasc Intervent Radiol. (2017) 40(3):351–9. 10.1007/s00270-016-1513-0
12.
Bradaric C Koppara T Müller A Haller B Ott I Cassese S et al Incidence and predictors of stent thrombosis after endovascular revascularisation of the superficial femoral artery. EuroIntervention. (2019) 15(12):e1107–14. 10.4244/EIJ-D-19-00187
13.
Shah M Najam O Bhindi R De Silva K . Calcium modification techniques in complex percutaneous coronary intervention. Circ Cardiovasc Interv. (2021) 14(5):e009870. 10.1161/CIRCINTERVENTIONS.120.009870
14.
Franchin L Kang J De Filippo O Gwon H-C Piroli F Kim H-S . Incidence and predictors of stent thrombosis in patients treated with stents for coronary bifurcation narrowing (from the BIFURCAT registry). Am J Cardiol. (2021) 156:24–31. 10.1016/j.amjcard.2021.06.031
15.
Fujimura T Matsumura M Witzenbichler B Metzger DC Rinaldi MJ Duffy PL . Stent expansion indexes to predict clinical outcomes. JACC Cardiovasc Interv. (2021) 14(15):1639–50. 10.1016/j.jcin.2021.05.019
16.
Kumar R Tariq S Fatima M Saghir T Batra MK Karim M et al Validity of the stent thrombosis risk score in predicting early stent thrombosis after primary percutaneous coronary intervention. J Saudi Heart Assoc. (2020) 32(2):256–62. 10.37616/2212-5043.1024
17.
Zheng PG Chen P Wang LJ Zhang N . The association of the systemic immune-inflammation index and stent thrombosis in myocardial infarction patients after coronary stent implantation—a retrospectively study. J Thorac Dis. (2023) 15(4):1726–33. 10.21037/jtd-23-363
18.
Capodanno D Angiolillo DJ . Personalised antiplatelet therapies for coronary artery disease: what the future holds. Eur Heart J. (2023) 44(32):3059–72. 10.1093/eurheartj/ehad362
19.
Anghel L Tudurachi BS Tudurachi A Zăvoi A Clement A Roungos A et al Patient-related factors predicting stent thrombosis in percutaneous coronary interventions. J Clin Med. (2023) 12(23):7367. 10.3390/jcm12237367
20.
Polimeni A Sorrentino S Spaccarotella C Mongiardo A Sabatino J De Rosa S et al Stent thrombosis after percutaneous coronary intervention. Cardiol Clin. (2020) 38(4):639–47. 10.1016/j.ccl.2020.07.008
21.
Ullrich H Münzel T Gori T . Coronary stent thrombosis — predictors and prevention. Dtsch Ärztebl Int. (2020) 117:320. 10.3238/arztebl.2020.0320
22.
Kuramitsu S Sonoda S Ando K Otake H Natsuaki M Anai R et al Drug-eluting stent thrombosis: current and future perspectives. Cardiovasc Interv Ther. (2021) 36(2):158–68. 10.1007/s12928-021-00754-x
23.
Kamenik M . Stent thrombosis during and after acute coronary syndromes: patient-related factors and operator-related factors. Anatol J Cardiol. (2020) 4:274–9. 10.14744/AnatolJCardiol.2020.69679
24.
Nascimento Silva JS Barros D Cantarelli IML L F Alves RC Falcão FJdA et al Predictors of coronary stent thrombosis: a case–control study. J Thromb Thrombolysis. (2018) 46(3):420–6. 10.1007/s11239-018-1699-x
25.
Ndrepepa G Kufner S Cassese S Joner M Xhepa E Sager HB et al Body mass index and 10-year clinical outcomes after percutaneous coronary intervention—interaction with age, sex, diabetic status and clinical presentation. J Clin Med. (2025) 14(5):1413. 10.3390/jcm14051413
26.
Wolny R Maehara A Liu Y Zhang Z Mintz GS Redfors B et al The obesity paradox revisited: body mass index and long-term outcomes after PCI from a large pooled patient-level database. EuroIntervention. (2020) 15(13):1199–208. 10.4244/EIJ-D-19-00467
27.
Jahangiri Y Kerrigan T Li L Prosser D Brar A Righetti J et al Risk factors for stent graft thrombosis after transjugular intrahepatic portosystemic shunt creation. Cardiovasc Diagn Ther. (2017) 7(S3):S150–8. 10.21037/cdt.2017.10.03
28.
Bindlish S Ng J Ghusn W Fitch A Bays HE . Obesity, thrombosis, venous disease, lymphatic disease, and lipedema: an obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obes Pillars. (2023) 8:100092. 10.1016/j.obpill.2023.100092
29.
Ono M Chichareon P Tomaniak M Kawashima H Takahashi K Kogame N et al The association of body mass index with long-term clinical outcomes after ticagrelor monotherapy following abbreviated dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a prespecified sub-analysis of the GLOBAL LEADERS trial. Clin Res Cardiol. (2020) 109(9):1125–39. 10.1007/s00392-020-01604-1
30.
Klein LW Nathan S Maehara A Messenger J Mintz GS Ali ZA et al SCAI expert consensus statement on management of in-stent restenosis and stent thrombosis. J Soc Cardiovasc Angiogr Interv. (2023) 2(4):100971. 10.1016/j.jscai.2023.100971
31.
Lim S Hong SJ Kim JH Cha J-J Joo HJ Park JH et al High platelet reactivity strongly predicts early stent thrombosis in patients with drug-eluting stent implantation. Sci Rep. (2024) 14(1):520. 10.1038/s41598-023-50920-9
32.
Yang Ff Song H Qin Wb Tang W-z Zhan L-j Zhang L-w et al Five-year outcomes of bioresorbable stent therapy for coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Rev Cardiovasc Med. (2024) 25(7):238. 10.31083/j.rcm2507238
33.
Jakobsen L Niemann T Thorsgaard N Thuesen L Lassen JF Jensen LO et al Dimensions of socioeconomic status and clinical outcome after primary percutaneous coronary intervention. Circ Cardiovasc Interv. (2012) 5(5):641–8. 10.1161/CIRCINTERVENTIONS.112.968271
34.
Jones DA Howard JP Rathod KS Gallagher SM Knight CJ Jain AK et al The impact of socio-economic status on all-cause mortality after percutaneous coronary intervention: an observational cohort study of 13,770 patients. EuroIntervention. (2015) 10(11):e1–8. 10.4244/EIJV10I10A196
35.
Shimony A Zahger D Ilia R Shalev A Cafri C . Impact of the community’s socioeconomic status on characteristics and outcomes of patients undergoing percutaneous coronary intervention. Int J Cardiol. (2010) 144(3):379–82. 10.1016/j.ijcard.2009.04.033
36.
Hannan EL Wu Y Cozzens K Friedrich M Walford G Ling FSK et al The association of socioeconomic factors with percutaneous coronary intervention outcomes. Can J Cardiol. (2022) 38(1):13–22. 10.1016/j.cjca.2021.09.029
37.
Avcı İİ Zeren G Sungur MA Akdeniz E Şimşek B Yılmaz MF et al Enhanced stent imaging system guided percutaneous coronary intervention is linked to optimize stent placement. Angiology. (2024) 75(1):54–61. 10.1177/00033197221130203
38.
Jinnouchi H Sato Y Cheng Q Janifer C Kutyna M Cornelissen A et al Thromboresistance and endothelial healing in polymer-coated versus polymer-free drug-eluting stents: implications for short-term dual anti-platelet therapy. Int J Cardiol. (2021) 327:52–7. 10.1016/j.ijcard.2020.11.030
39.
Saito A Dai Z Ono M Kanie T Takaoka Y Mizuno A et al The relationship between coronary stent strut thickness and the incidences of clinical outcomes after drug-eluting stent implantation: a systematic review and meta-regression analysis. Catheter Cardiovasc Interv. (2022) 99(3):575–82. 10.1002/ccd.29922
40.
Leesar MA Feldman MD . Thrombosis and myocardial infarction: the role of bioresorbable scaffolds. J Cardiovasc Aging. (2023) 3(1):7. 10.20517/jca.2022.41
41.
Hamana T Otake H Kuramitsu S Shinozaki T Ohya M Horie K et al Association between cancer history and second-generation drug-eluting stent thrombosis: insights from the REAL-ST registry. Thromb J. (2023) 21(1):60. 10.1186/s12959-023-00503-5
42.
Condello F Spaccarotella C Sorrentino S Indolfi C Stefanini GG Polimeni A . Stent thrombosis and restenosis with contemporary drug-eluting stents: predictors and current evidence. J Clin Med. (2023) 12(3):1238. 10.3390/jcm12031238
43.
Karamasis GV Varlamos C Benetou DR Kalogeropoulos AS Keeble TR Tsigkas G et al The usefulness of intracoronary imaging in patients with ST-segment elevation myocardial infarction. J Clin Med. (2023) 12(18):5892. 10.3390/jcm12185892
44.
Ng P Maehara A Kirtane AJ McEntegart M Jaffer FA Doshi D et al Management of coronary stent underexpansion. J Am Coll Cardiol. (2025) 85(6):625–44. 10.1016/j.jacc.2024.12.009
45.
Wang X Chen X Tian T You H Li Y Wu M et al A scoring system to predict the occurrence of very late stent thrombosis following percutaneous coronary intervention for acute coronary syndrome. Sci Rep. (2020) 10(1):6378. 10.1038/s41598-020-63455-0
46.
Galli L Sator A Schauer S Bräu K Bernhard J Hengstenberg C et al Platelets, biomarkers of coagulation and fibrinolysis, and early coronary stent thrombosis. J Clin Med. (2024) 14(1):56. 10.3390/jcm14010056
47.
Biggart R Davies C Joshi N . A review of systemic hematological manifestations and stent thrombosis. Cardiol Rev. (2023). 10.1097/CRD.0000000000000535. Publish Ahead of Print.
48.
Guo W Fan X Lewis BR Johnson MP Rihal CS Lerman A et al Cancer patients have a higher risk of thrombotic and ischemic events after percutaneous coronary intervention. JACC Cardiovasc Interv. (2021) 14(10):1094–105. 10.1016/j.jcin.2021.03.049
49.
Heestermans AACM Van Werkum JW Zwart B Van der heyden JA Kelder JC Breet NJ et al Acute and subacute stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: incidence, predictors and clinical outcome. J Thromb Haemost. (2010) 8(11):2385–93. 10.1111/j.1538-7836.2010.04046.x
50.
D’Ascenzo F Bollati M Clementi F Castagno D Lagerqvist B de la Torre Hernandez JM et al Incidence and predictors of coronary stent thrombosis: evidence from an international collaborative meta-analysis including 30 studies, 221,066 patients, and 4276 thromboses. Int J Cardiol. (2013) 167(2):575–84. 10.1016/j.ijcard.2012.01.080
51.
Cayla G Hulot JS O’Connor SA Pathak A Scott SA Gruel Y et al Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. (2011) 306(16):1765–74. 10.1001/jama.2011.1529
52.
Granada JF Alviar CL Wallace-Bradley D Osteen M Dave B Tellez A et al Patterns of activation and deposition of platelets exposed to the polymeric surface of the paclitaxel eluting stent. J Thromb Thrombolysis. (2010) 29(1):60–9. 10.1007/s11239-009-0348-9
53.
Ollivier V Roques C Receveur N Gratz M Feldman L Letourneur D et al Bioreactivity of stent material: activation of platelets, coagulation, leukocytes and endothelial cell dysfunction in vitro. Platelets. (2017) 28(6):529–39. 10.1080/09537104.2016.1252836
54.
Lüscher TF Steffel J Eberli FR Joner M Nakazawa G Tanner FC et al Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. (2007) 115(8):1051–8. 10.1161/CIRCULATIONAHA.106.675934
55.
Koskinas KC Chatzizisis YS Antoniadis AP Giannoglou GD . Role of endothelial shear stress in stent restenosis and thrombosis. J Am Coll Cardiol. (2012) 59(15):1337–49. 10.1016/j.jacc.2011.10.903
56.
Gijsen FJH Oortman RM Wentzel JJ Schuurbiers JCH Tanabe K Degertekin M et al Usefulness of shear stress pattern in predicting neointima distribution in sirolimus-eluting stents in coronary arteries. Am J Cardiol. (2003) 92(11):1325–8. 10.1016/j.amjcard.2003.08.017
57.
Taniwaki M Radu MD Zaugg S Amabile N Garcia-Garcia HM Yamaji K . Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. (2016) 133(7):650–60. 10.1161/CIRCULATIONAHA.115.019071
58.
Qu Z Wei H Du T Qiao A . Computational simulation of stent thrombosis induced by various degrees of stent malapposition. Front Bioeng Biotechnol. (2022) 10:1062529. 10.3389/fbioe.2022.1062529
59.
Kolandaivelu K Swaminathan R Gibson WJ Kolachalama VB Nguyen-Ehrenreich K-L Giddings VL et al Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. (2011) 123(13):1400–9. 10.1161/CIRCULATIONAHA.110.003210
60.
Ng J Bourantas CV Torii R Ang HY Tenekecioglu E Serruys PW et al Local hemodynamic forces after stenting: implications on restenosis and thrombosis. Arterioscler Thromb Vasc Biol. (2017) 37(12):2231–42. 10.1161/ATVBAHA.117.309728
61.
Mauri L Kereiakes DJ Yeh RW Driscoll-Shempp P Cutlip DE Steg PG et al Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. (2014) 371(23):2155–66. 10.1056/NEJMoa1409312
62.
Wiviott SD Braunwald E McCabe CH Montalescot G Ruzyllo W Gottlieb S et al Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2007) 357(20):2001–15. 10.1056/NEJMoa0706482
63.
Palmerini T Benedetto U Bacchi-Reggiani L Riva DD Biondi-Zoccai G Feres F et al Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. (2015) 385(9985):2371–82. 10.1016/S0140-6736(15)60263-X
64.
Witzenbichler B Maehara A Weisz G Neumann F-J Rinaldi MJ Metzger DC et al Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. (2014) 129(4):463–70. 10.1161/CIRCULATIONAHA.113.003942
65.
Martínez-Elbal L . Direct coronary stenting versus stenting with balloon pre-dilation: immediate and follow-up results of a multicentre, prospective, randomized study. The DISCO trial. Eur Heart J. (2002) 23(8):633–40. 10.1053/euhj.2001.2893
66.
Piscione F Piccolo R Cassese S Galasso G D'Andrea C De Rosa R et al Is direct stenting superior to stenting with predilation in patients treated with percutaneous coronary intervention? Results from a meta-analysis of 24 randomised controlled trials. Heart. (2010) 96(8):588–94. 10.1136/hrt.2009.183277
67.
Magalhaes MA Minha S Lhermusier T Pendyala L Escarcega RO Baker NC et al Does direct stenting with drug-eluting stents improve outcome? A meta-analysis of 10,900 patients. Catheter Cardiovasc Interv. (2017) 90(2):213–22. 10.1002/ccd.26861
68.
Park H Ahn JM Kang DY Lee J-B Park S Ko E et al Optimal stenting technique for complex coronary lesions. JACC Cardiovasc Interv. (2020) 13(12):1403–13. 10.1016/j.jcin.2020.03.023
69.
Lawton JS Tamis-Holland JE Bangalore S Bates ER Beckie TM Bischoff JM et al 2021 ACC/AHA/SCAI guideline for coronary artery revascularization. J Am Coll Cardiol. (2022) 79(2):e21–e129. 10.1016/j.jacc.2021.09.006
70.
Karjalainen PP Niemelä M Laine M Airaksinen JKE Ylitalo A Nammas W . Usefulness of post-coronary dilation to prevent recurrent myocardial infarction in patients treated with percutaneous coronary intervention for acute coronary syndrome (from the BASE ACS trial). Am J Cardiol. (2017) 119(3):345–50. 10.1016/j.amjcard.2016.09.057
71.
Fröbert O Sarno G James SK Saleh N Lagerqvist B . Effect of stent inflation pressure and post-dilatation on the outcome of coronary artery intervention. A report of more than 90 000 stent implantations. PLoS One. (2013) 8(2):e56348. 10.1371/journal.pone.0056348
72.
Sawaya FJ Lefèvre T Chevalier B Garot P Hovasse T Morice M-C et al Contemporary approach to coronary bifurcation lesion treatment. JACC Cardiovasc Interv. (2016) 9(18):1861–78. 10.1016/j.jcin.2016.06.056
73.
Wang R Ding Y Yang J Wang K Gao W Fang Z et al Stenting techniques for coronary bifurcation disease: a systematic review and network meta-analysis demonstrates superiority of double-kissing crush in complex lesions. Clin Res Cardiol. (2022) 111(7):761–75. 10.1007/s00392-021-01979-9
74.
Park DY An S Jolly N Attanasio S Yadav N Rao S et al Systematic review and network meta-analysis comparing bifurcation techniques for percutaneous coronary intervention. J Am Heart Assoc. (2022) 11(12):e025394. 10.1161/JAHA.122.025394
75.
Wenaweser P Rey C Eberli FR Togni M Tüller D Locher S et al Stent thrombosis following bare-metal stent implantation: success of emergency percutaneous coronary intervention and predictors of adverse outcome. Eur Heart J. (2005) 26(12):1180–7. 10.1093/eurheartj/ehi135
76.
Parodi G Memisha G Bellandi B Valenti R Migliorini A Carrabba N et al Effectiveness of primary percutaneous coronary interventions for stent thrombosis. Am J Cardiol. (2009) 103(7):913–6. 10.1016/j.amjcard.2008.12.006
77.
Waldo SW Armstrong EJ Yeo KK Patel M Reeves R MacGregor JS et al Procedural success and long-term outcomes of aspiration thrombectomy for the treatment of stent thrombosis. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. (2013) 82(7):1048–53. 10.1002/ccd.25007
78.
Mahmoud KD Vlaar PJ van den Heuvel AFM Hillege HL Zijlstra F de Smet BJGL . Usefulness of thrombus aspiration for the treatment of coronary stent thrombosis. Am J Cardiol. (2011) 108(12):1721–7. 10.1016/j.amjcard.2011.07.044
79.
Srdanovic I . Factors influencing 1st and 2nd generation drug-eluting stent performance: understanding the basic pharmaceutical drug-in-polymer formulation factors contributing to stent thrombosis do we really need to eliminate the polymer?J Pharm Pharm Sci. (2021) 24:435–61. 10.18433/jpps32053
80.
Palmerini T Biondi-Zoccai G Della Riva D Mariani A Genereux P Branzi A et al Stent thrombosis with drug-eluting stents. J Am Coll Cardiol. (2013) 62(21):1915–21. 10.1016/j.jacc.2013.08.725
81.
Shah R Rao SV Latham SB Kandzari DE . Efficacy and safety of drug-eluting stents optimized for biocompatibility vs bare-metal stents with a single month of dual antiplatelet therapy: a meta-analysis. JAMA Cardiol. (2018) 3(11):1050. 10.1001/jamacardio.2018.3551
82.
Wang Y Liu S Luo Y Wang F Liu H Li L et al Safety and efficacy of degradable vs. permanent polymer drug-eluting stents: a meta-analysis of 18,395 patients from randomized trials. Int J Cardiol. (2014) 173(1):100–9. 10.1016/j.ijcard.2014.02.023
83.
Yaylak B Polat F Onuk T Akyüz Ş Çalık AN Çetin M et al The relation of polymer structure of stent used in patients with acute coronary syndrome revascularized by stent implantation with long-term cardiovascular events. Catheter Cardiovasc Interv. (2023) 102(7):1186–97. 10.1002/ccd.30881
84.
Stone GW Kereiakes DJ Gori T Metzger DC Stein B Erickson M et al 5-year outcomes after bioresorbable coronary scaffolds implanted with improved technique. J Am Coll Cardiol. (2023) 82(3):183–95. 10.1016/j.jacc.2023.05.003
85.
Power DA Camaj A Kereiakes DJ Ellis SG Gao R Kimura T et al Early and late outcomes with the absorb bioresorbable vascular scaffold. JACC Cardiovasc Interv. (2025) 18(1):1–11. 10.1016/j.jcin.2024.08.050
86.
Kerkmeijer LK Renkens MR Tijssen RT Hofma SH van der Schaaf RvdS Arkenbout EK et al Long-term clinical outcomes of everolimus-eluting bioresorbable scaffolds versus everolimus-eluting stents: final five-year results of the AIDA randomised clinical trial. EuroIntervention. (2022) 17(16):1340–7. 10.4244/EIJ-D-21-00419
87.
Kereiakes DJ Ellis SG Metzger DC Caputo RP Rizik DG Teirstein PS et al Clinical outcomes before and after complete everolimus-eluting bioresorbable scaffold resorption: five-year follow-up from the ABSORB III trial. Circulation. (2019) 140(23):1895–903. 10.1161/CIRCULATIONAHA.119.042584
88.
Liu S Nie S Hou Y Huang G Fu G Zhou H et al A randomized comparison of bioheart sirolimus-eluting bioresorbable scaffold and everolimus-eluting stents. JACC Cardiovasc Interv. (2025) 18(1):15–27. 10.1016/j.jcin.2024.09.043
89.
Abraham WT Kuck KH Goldsmith RL Lindenfeld J Reddy VY Carson PE . A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. (2018) 6(10):874–83. 10.1016/j.jchf.2018.04.010
90.
Jiang J Li C Chen D Song L Cui Z Li P et al Firesorb bioresorbable scaffold for de novo coronary artery disease: 1-year clinical outcomes. BMC Med. (2025) 23(1):419. 10.1186/s12916-025-04254-0
91.
Stefanini GG Holmes DR . Drug-eluting coronary-artery stents. N Engl J Med. (2013) 368(3):254–65. 10.1056/NEJMra1210816
92.
Stettler C Wandel S Allemann S Kastrati A Morice MC Schömig A et al Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. (2007) 370(9591):937–48. 10.1016/S0140-6736(07)61444-5
93.
Kastrati A Dibra A Eberle S Mehilli J Suárez de Lezo J Goy J-J et al Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA. (2005) 294(7):819. 10.1001/jama.294.7.819
94.
Park KW Kang SH Velders MA Shin D-H Hahn S Lim W-H et al Safety and efficacy of everolimus- versus sirolimus-eluting stents: a systematic review and meta-analysis of 11 randomized trials. Am Heart J. (2013) 165(2):241–250.e4. 10.1016/j.ahj.2012.08.007
95.
Toyota T Shiomi H Morimoto T Kimura T . Meta-analysis of long-term clinical outcomes of everolimus-eluting stents. Am J Cardiol. (2015) 116(2):187–94. 10.1016/j.amjcard.2015.03.059
96.
Stone GW Rizvi A Newman W Mastali K Wang JC Caputo R et al Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. (2010) 362(18):1663–74. 10.1056/NEJMoa0910496
97.
Navarese EP Tandjung K Claessen B Andreotti F Kowalewski M Kandzari DE et al Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. Br Med J. (2013) 347:f6530. 10.1136/bmj.f6530
98.
Bangalore S Toklu B Amoroso N Fusaro M Kumar S Hannan EL et al Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. Br Med J. (2013) 347:f6625–f6625. 10.1136/bmj.f6625
99.
Von Birgelen C Kok MM Van Der Heijden LC Danse PW Schotborgh CE Scholte M . Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. (2016) 388(10060):2607–17. 10.1016/S0140-6736(16)31920-1
100.
Armstrong EJ Kwa AT Yeo KK Mahmud E Javed U Patel M et al Angiographically confirmed stent thrombosis in contemporary practice: insights from intravascular ultrasound. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. (2013) 81(5):782–90. 10.1002/ccd.24460
101.
Kawasaki M Bouma BE Bressner J Houser SL Nadkarni SK MacNeill BD et al Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J Am Coll Cardiol. (2006) 48(1):81–8. 10.1016/j.jacc.2006.02.062
102.
Maehara A Matsumura M Ali ZA Mintz GS Stone GW . IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. (2017) 10(12):1487–503. 10.1016/j.jcmg.2017.09.008
103.
Lee JM Choi KH Song YB Lee J-Y Lee S-J Lee SY et al Intravascular imaging–guided or angiography-guided complex PCI. N Engl J Med. (2023) 388(18):1668–79. 10.1056/NEJMoa2216607
104.
Levine GN Bates ER Bittl JA Brindis RG Fihn SD Fleisher LA et al 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134(10):e123–155. 10.1161/CIR.0000000000000404
105.
Watanabe H Domei T Morimoto T Natsuaki M Shiomi H Toyota T et al Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. (2019) 321(24):2414–27. 10.1001/jama.2019.8145
106.
Hahn JY Song YB Oh JH Chun WJ Park YH Jang WJ et al Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. (2019) 321(24):2428–37. 10.1001/jama.2019.8146
107.
Yeh RW Kereiakes DJ Steg PG Cutlip DE Croce KJ Massaro JM et al Lesion complexity and outcomes of extended dual antiplatelet therapy after percutaneous coronary intervention. J Am Coll Cardiol. (2017) 70(18):2213–23. 10.1016/j.jacc.2017.09.011
108.
Suh JW Lee SP Park KW Lee H-Y Kang H-J Koo B-K et al Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. (2011) 57(3):280–9. 10.1016/j.jacc.2010.08.631
109.
Tang YD Wang W Yang M Zhang K Chen J Qiao S et al Randomized comparisons of double-dose clopidogrel or adjunctive cilostazol versus standard dual antiplatelet in patients with high posttreatment platelet reactivity: results of the CREATIVE trial. Circulation. (2018) 137(21):2231–45. 10.1161/CIRCULATIONAHA.117.030190
110.
Jeong YH Lee SW Choi BR Kim I-S Seo M-K Kwak CH et al Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity. J Am Coll Cardiol. (2009) 53(13):1101–9. 10.1016/j.jacc.2008.12.025
111.
Geng Df Liu M Jin Dm Wu W Deng J Wang Jf . Cilostazol-based triple antiplatelet therapy compared to dual antiplatelet therapy in patients with coronary stent implantation: a meta-analysis of 5,821 patients. Cardiology. (2012) 122(3):148–57. 10.1159/000338812
112.
Kalantzi K Tentolouris N Melidonis AJ Papadaki S Peroulis M Amantos KA et al Efficacy and safety of adjunctive cilostazol to clopidogrel-treated diabetic patients with symptomatic lower extremity artery disease in the prevention of ischemic vascular events. J Am Heart Assoc. (2021) 10(1):e018184. 10.1161/JAHA.120.018184
113.
Amsterdam EA Wenger NK Brindis RG Casey DE Ganiats TG Holmes DR et al 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 64(24):e139–228. 10.1016/j.jacc.2014.09.017
114.
Dewilde WJM Oirbans T Verheugt FWA Kelder JC De Smet BJ Herrman J-P et al Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet Lond Engl. (2013) 381(9872):1107–15. 10.1016/S0140-6736(12)62177-1
115.
Agarwal N Jain A Mahmoud AN Bishnoi R Golwala H Karimi A et al Safety and efficacy of dual versus triple antithrombotic therapy in patients undergoing percutaneous coronary intervention. Am J Med. (2017) 130(11):1280–9. 10.1016/j.amjmed.2017.03.057
116.
Fiedler KA Maeng M Mehilli J Schulz-Schüpke S Byrne RA Sibbing D et al Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol. (2015) 65(16):1619–29. 10.1016/j.jacc.2015.02.050
117.
January CT Wann LS Calkins H Chen LY Cigarroa JE Cleveland JC . 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140(2):104–32. 10.1161/CIR.0000000000000665
118.
Tada T Byrne RA Simunovic I King LA Cassese S Joner M et al Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv. (2013) 6(12):1267–74. 10.1016/j.jcin.2013.06.015
119.
Varenhorst C Lindholm M Sarno G Olivecrona G Jensen U Nilsson J et al Stent thrombosis rates the first year and beyond with new- and old-generation drug-eluting stents compared to bare metal stents. Clin Res Cardiol Off J Ger Card Soc. (2018) 107(9):816–23. 10.1007/s00392-018-1252-0
120.
Brodie BR Garg A Stuckey TD Kirtane AJ Witzenbichler B Maehara A et al Fixed and modifiable correlates of drug-eluting stent thrombosis from a large all-comers registry: insights from ADAPT-DES. Circ Cardiovasc Interv. (2015) 8(10):e002568. 10.1161/CIRCINTERVENTIONS.114.002568
121.
Czarny MJ Nathan AS Yeh RW Mauri L . Adherence to dual antiplatelet therapy after coronary stenting: a systematic review. Clin Cardiol. (2014) 37(8):505–13. 10.1002/clc.22289
122.
LaRosa AR Swabe GM Magnani JW . Income and antiplatelet adherence following percutaneous coronary intervention. Int J Cardiol Cardiovasc Risk Prev. (2022) 14:200140. 10.1016/j.ijcrp.2022.200140
123.
Escaned J Lombardi M Götberg M Amabile N Banning A Barbato E et al Factors contributing to low utilization of intracoronary imaging in clinical practice: a white paper. J Soc Cardiovasc Angiogr Interv. (2025) 4(7):103607. 10.1016/j.jscai.2025.103607
124.
Cohen M Jones C . Patient and physician perspectives on the benefits and risks of antiplatelet therapy for acute coronary syndrome. Cardiol Ther. (2024) 13(3):631–43. 10.1007/s40119-024-00372-7
125.
Wang T Tan JY Liu XL Zhao I . Barriers and enablers to implementing clinical practice guidelines in primary care: an overview of systematic reviews. BMJ Open. (2023) 13(1):e062158. 10.1136/bmjopen-2022-062158
126.
Hu S Li Z Shen D Huang K Su T Dinh P-U et al Exosome-eluting stents for vascular healing after ischaemic injury. Nat Biomed Eng. (2021) 5(10):1174–88. 10.1038/s41551-021-00705-0
127.
Lee JM Choe W Kim BK Seo W-W Lim W-H Kang C-K et al Comparison of endothelialization and neointimal formation with stents coated with antibodies against CD34 and vascular endothelial-cadherin. Biomaterials. (2012) 33(35):8917–27. 10.1016/j.biomaterials.2012.08.066
128.
Pang JH Farhatnia Y Godarzi F Tan A Rajadas J Cousins BG et al In situ endothelialization: bioengineering considerations to translation. Small. (2015) 11(47):6248–64. 10.1002/smll.201402579
129.
Wu X Yin T Tian J Tang C Huang J Zhao Y et al Distinctive effects of CD34- and CD133-specific antibody-coated stents on re-endothelialization and in-stent restenosis at the early phase of vascular injury. Regen Biomater. (2015) 2(2):87–96. 10.1093/rb/rbv007
130.
Blessing R Ahoopai M Geyer M Brandt M Zeiher AM Münzel T et al The bioengineered combo dual-therapy CD34 antibody-covered sirolimus-eluting coronary stent in patients with chronic total occlusion evaluated by clinical outcome and optical coherence tomography imaging analysis. J Clin Med. (2020) 10(1):80. 10.3390/jcm10010080
131.
Evans CE Iruela-Arispe ML Zhao YY . Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathol. (2021) 191(1):52–65. 10.1016/j.ajpath.2020.10.001
132.
Rao J Pan Bei H Yang Y Liu Y Lin H Zhao X . Nitric oxide-producing cardiovascular stent coatings for prevention of thrombosis and restenosis. Front Bioeng Biotechnol. (2020) 8:578. 10.3389/fbioe.2020.00578
133.
Fan Y Zhang Y Zhao Q Xie Y Luo R Yang P et al Immobilization of nano Cu-MOFs with polydopamine coating for adaptable gasotransmitter generation and copper ion delivery on cardiovascular stents. Biomaterials. (2019) 204:36–45. 10.1016/j.biomaterials.2019.03.007
134.
Yang Z Zhao X Hao R Tu Q Tian X Xiao Y et al Bioclickable and mussel adhesive peptide mimics for engineering vascular stent surfaces. Proc Natl Acad Sci. (2020) 117(28):16127–37. 10.1073/pnas.2003732117
135.
Enayati M Schneider KH Almeria C Grasl C Kaun C Messner B et al S-nitroso human serum albumin as a nitric oxide donor in drug-eluting vascular grafts: biofunctionality and preclinical evaluation. Acta Biomater. (2021) 134:276–88. 10.1016/j.actbio.2021.07.048
136.
Midgley AC Wei Y Li Z Kong D Zhao Q . Nitric-oxide-releasing biomaterial regulation of the stem cell microenvironment in regenerative medicine. Adv Mater. (2020) 32(3):1805818. 10.1002/adma.201805818
137.
Takabatake S Hayashi K Nakanishi C Hao H Sakata K Kawashiri M et al Vascular endothelial growth factor–bound stents: application of in situ capture technology of circulating endothelial progenitor cells in porcine coronary model. J Intervent Cardiol. (2014) 27(1):63–72. 10.1111/joic.12087
138.
Wang J Xue Y Liu J Hu M Zhang H Ren K et al Hierarchical capillary coating to biofunctionlize drug-eluting stent for improving endothelium regeneration. Research. (2020) 2020:2020/1458090. 10.34133/2020/1458090
139.
Yang J Zeng Y Zhang C Chen Y-X Yang Z Li Y et al The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent. Biomaterials. (2013) 34(6):1635–43. 10.1016/j.biomaterials.2012.11.006
140.
Chan JW Lewis DR Petersen LK Moghe PV Uhrich KE . Amphiphilic macromolecule nanoassemblies suppress smooth muscle cell proliferation and platelet adhesion. Biomaterials. (2016) 84:219–29. 10.1016/j.biomaterials.2015.12.033
141.
Paul A Elias CB Shum-Tim D Prakash S . Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci Rep. (2013) 3(1):2366. 10.1038/srep02366
142.
Déglise S Bechelli C Allagnat F . Vascular smooth muscle cells in intimal hyperplasia, an update. Front Physiol. (2023) 13:1081881. 10.3389/fphys.2022.1081881
143.
Chan JM Rhee JW Drum CL Bronson RT Golomb G Langer R et al In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid–polymeric nanoparticles. Proc Natl Acad Sci. (2011) 108(48):19347–52. 10.1073/pnas.1115945108
144.
Junkar I Kulkarni M Benčina M Kovač J Mrak-Poljšak K Lakota K et al Titanium dioxide nanotube arrays for cardiovascular stent applications. ACS Omega. (2020) 5(13):7280–9. 10.1021/acsomega.9b04118
145.
Inglut CT Sorrin AJ Kuruppu T Vig S Cicalo J Ahmad H et al Immunological and toxicological considerations for the design of liposomes. Nanomater Basel Switz. (2020) 10(2):190. 10.3390/nano10020190
146.
Zhang N Li C Zhou D Ding C Jin Y Tian Q et al Cyclic RGD functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biomater. (2018) 70:227–36. 10.1016/j.actbio.2018.01.038
147.
Janaszewska A Lazniewska J Trzepiński P Marcinkowska M Klajnert-Maculewicz B . Cytotoxicity of dendrimers. Biomolecules. (2019) 9(8):330. 10.3390/biom9080330
148.
Cai D Chen AC Zhou R Murashita T Fay WP Chen SY . Enhanced reendothelialization and thrombosis prevention with a new drug-eluting stent. Cardiovasc Drugs Ther. (2024) 4:765–774. 10.1007/s10557-024-07584-y
149.
Hou R Wu L Wang J Yang Z Tu Q Zhang X et al Surface-degradable drug-eluting stent with anticoagulation, antiproliferation, and endothelialization functions. Biomolecules. (2019) 9(2):69. 10.3390/biom9020069
150.
Kalra A Rehman H Khera S Thyagarajan B Bhatt DL Kleiman NS et al New-generation coronary stents: current data and future directions. Curr Atheroscler Rep. (2017) 19(3):14. 10.1007/s11883-017-0654-1
151.
Raikar AS Priya S Bhilegaonkar SP Somnache SN Kalaskar DM . Surface engineering of bioactive coatings for improved stent hemocompatibility: a comprehensive review. Materials. (2023) 16(21):6940. 10.3390/ma16216940
152.
Udriște AS Burdușel AC Niculescu AG Rădulescu M Grumezescu AM . Coatings for cardiovascular stents—an up-to-date review. Int J Mol Sci. (2024) 25(2):1078. 10.3390/ijms25021078
153.
Sampedro-Gómez J Dorado-Díaz PI Vicente-Palacios V Sánchez-Puente A Jiménez-Navarro M San Roman JA et al Machine learning to predict stent restenosis based on daily demographic, clinical, and angiographic characteristics. Can J Cardiol. (2020) 36(10):1624–32. 10.1016/j.cjca.2020.01.027
154.
Li F Rasmy L Xiang Y Feng J Abdelhameed A Hu X et al Dynamic prognosis prediction for patients on DAPT after drug-eluting stent implantation: model development and validation. J Am Heart Assoc. (2024) 13(3):e029900. 10.1161/JAHA.123.029900
155.
Woudstra P Grundeken MJ Kraak RP Hassell MECJ Arkenbout EK Baan J et al Amsterdam Investigator–initiateD Absorb strategy all-comers trial (AIDA trial): a clinical evaluation comparing the efficacy and performance of ABSORB everolimus-eluting bioresorbable vascular scaffold strategy vs the XIENCE family (XIENCE PRIME or XIENCE Xpedition) everolimus-eluting coronary stent strategy in the treatment of coronary lesions in consecutive all-comers: rationale and study design. Am Heart J. (2014) 167(2):133–40. 10.1016/j.ahj.2013.09.017
Summary
Keywords
stent thrombosis, coronary artery disease, percuataneous coronary intervention, coronary stenting, ischaemic heart disease (IHD)
Citation
Flowers A, Evenhuis B, Gabanic B, Weiss A, Eisenberg S, Siddiqui R, Rizwan A, Riaz I, Hassan Virk HU, Alam M, Khawaja M, Strauss M and Krittanawong C (2025) Stent thrombosis: a contemporary guide to definitions, risk factors, and management. Front. Cardiovasc. Med. 12:1622235. doi: 10.3389/fcvm.2025.1622235
Received
02 May 2025
Accepted
01 October 2025
Published
21 October 2025
Volume
12 - 2025
Edited by
Akhilesh Kumar, Fortis Escorts Heart Institute, India
Reviewed by
Ehsan Moradi Farsani, Tehran University of Medical Sciences, Iran
Nilashish Dey, Fortis Healthcare, India
Updates
Copyright
© 2025 Flowers, Evenhuis, Gabanic, Weiss, Eisenberg, Siddiqui, Rizwan, Riaz, Hassan Virk, Alam, Khawaja, Strauss and Krittanawong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Markus Strauss markus.strauss@ukmuenster.de Chayakrit Krittanawong Chayakrit.Krittanawong@gmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.