- 1School of Wushu, Shandong Sport University, Jinan, China
- 2Traditional Sport Institute, Harbin Sport University, Harbin, China
- 3Zaoyang Children’s Sports School, Zaoyang, China
Objective: This study aimed to compare the efficacy of different exercise modalities on cardiac function in patients with myocardial infarction (MI), providing evidence-based recommendations for optimal cardiac rehabilitation programming.
Methods: We conducted a systematic search of seven Chinese and English databases, including CNKI and Web of Science, to identify eligible studies. A network meta-analysis based on the frequency framework was performed using STATA 14.0.
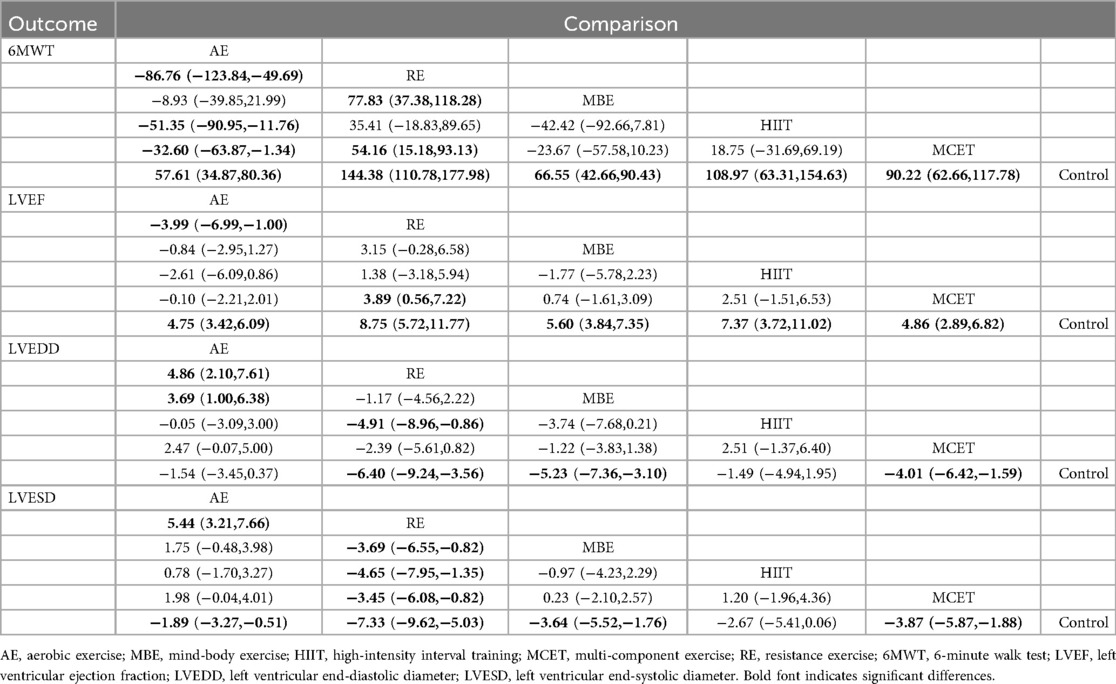
Results: A total of 69 studies involving 5,044 participants were included. Compared to the control group, all exercise interventions significantly improved 6-minute walk test (6MWT) scores in MI patients, with mean differences (MDs) and 95% confidence intervals (CIs) ranging from 57.61 (34.87, 80.36) for aerobic exercise (AE) to 144.38 (110.78, 177.98) for resistance exercise (RE). All modalities enhanced left ventricular ejection fraction (LVEF), with MDs (95% CI) from 4.75 (3.42, 6.09) for AE to 8.75 (5.72, 11.77) for RE. Except for AE, all interventions reduced left ventricular end-diastolic diameter (LVEDD), with MDs (95% CI) from −4.01 (−6.42, −1.59) for multi-component exercise training (MCET) to −6.40 (−9.24, −3.56) for RE. All exercises improved left ventricular end-systolic diameter (LVESD), with MDs (95% CI) from −1.89 (−3.27, −0.51) for AE to −7.33 (−9.62, −5.03) for RE. RE consistently showed a high probability of relatively high efficacy rankings across outcomes (SUCRA: 93.2–99.8).
Conclusion: RE appeared to have a high probability of being a highly effective single modality for improving post-MI cardiac function and remodeling. MCET and mind-body training also offer notable advantages, particularly in reducing ventricular size. Ultimately, rehabilitation programs should be tailored by considering the modality-specific benefits, patient's clinical profile, and functional capacity to optimize outcomes.
Systematic Review Registration: https://inplasy.com/inplasy-2024-11-0016/, identifier INPLASY2024110016.
1 Introduction
Myocardial infarction (MI) is a myocardial necrosis event caused by unstable ischemic syndromes (1), and it remains the leading cause of mortality among cardiovascular diseases (2). This high mortality rate is primarily due to coronary artery stenosis and occlusion, which lead to acute or sustained myocardial ischemia and hypoxia, ultimately resulting in myocardial infarction (3, 4). As one of the major causes of death from coronary heart disease (CHD), MI accounts for over 4 million deaths in Europe and Northeast Asia and is responsible for more than a third of all annual deaths in developed countries (5). In China alone, approximately 2.5 million individuals currently live with MI, with projections estimating an additional 7.5 million cases in the next 15 years (6), and a concerning trend toward younger onset ages (7, 8). Studies show that adverse left ventricular remodeling and heart failure following MI significantly impair patients' quality of life (9). Standard treatments for MI typically include percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG), which improve clinical outcomes, increase survival rates, and reduce mortality (10). However, long-term prognosis—such as effectively managing risk factors, enhancing quality of life, and reducing the recurrence of acute cardiac events—relies heavily on exercise-based cardiac rehabilitation (CR) (11). Therefore, improving the long-term health outcomes and prognosis for MI survivors represents a critical global public health challenge and a focal point in cardiovascular medicine.
Recent studies have demonstrated that regular physical activity is an effective behavioral intervention for improving heart health (12), serving as a key protective factor for MI patients in achieving favorable recovery, low incidence, and reduced mortality risk. Regular exercise exerts an anti-atherosclerotic effect on the vascular system, improves autonomic balance (which lowers the likelihood of dangerous arrhythmias), and promotes myocardial safeguarding from ischemia-reperfusion damage (13). Several studies have shown that exercise-based CR can delay the progression of coronary atherosclerosis, improve long-term mortality in cardiovascular patients, enhance aerobic capacity, and increase quality of life (14, 15). While the benefits of exercise-based CR for CVD patients are widely recognized, the optimal exercise modalities and intensities remain a subject of debate. Some research suggests that aerobic exercise (AE) is an effective form of rehabilitation for enhancing cardiovascular and cardiopulmonary health, with most clinical studies favoring low-intensity, long-duration exercise as the standard for cardiac rehabilitation (16). However, the growing attention given to high-intensity interval training (HIIT) has led to substantial evidence indicating that HIIT is particularly effective in the cardiac rehabilitation of cardiovascular patients, offering advantages over moderate-intensity continuous training (MICT) (17, 18). Furthermore, recent meta-analyses suggest that mind-body exercises (MBE), such as Tai Chi, Baduanjin, and Qigong, are effective in improving cardiac rehabilitation and enhancing cardiopulmonary health in MI patients (19–21). Additionally, recent research has confirmed that resistance exercise (RE) is safe for patients with stable heart failure and has beneficial effects in preventing muscle atrophy and increasing muscle strength and endurance (22).
To date, existing meta-analyses have primarily focused on the effects of single interventions, such as AE (11, 19, 23) or HIIT (24), without conducting a systematic review of how various exercise modalities influence cardiac function in MI patients. More importantly, it is still uncertain which exercise type is the most effective at enhancing cardiac function in these patients. Network meta-analysis (NMA), often referred to as multiple treatment comparison meta-analysis, facilitates a simultaneous comparison of three or more interventions, expanding the scope beyond traditional pairwise analysis. Even in the absence of direct comparisons between two interventions, NMA enables the estimation of the relative effectiveness of all interventions and ranks them accordingly, significantly enhancing the precision of the results (25). Therefore, this study aims to perform an NMA of relevant randomized controlled trials (RCTs) to provide a comprehensive assessment of the effects of mind-body exercise, AE, RE, HIIT, and combined exercise on cardiac function in MI patients, offering stronger evidence to guide the selection of effective cardiac rehabilitation strategies for this population.
2 Methods
This study adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Extension Statement (Supplementary Appendix 1) and has been registered with INPLASY (International Platform of Registered Systematic Review and Meta-analysis Protocols) (Registration Number: INPLASY2024110016).
2.1 Search strategy
We conducted a comprehensive search of eight major Chinese and English databases: China National Knowledge Infrastructure (CNKI), Wanfang Database, Chinese Science and Technology Periodical Database (CSTJ), China Biomedical Database, PubMed, Web of Science, Embase, and The Cochrane Library. The search period spanned from the inception of each database to January 10, 2025. A combination of subject headings and free-text terms was used, with primary search terms including “myocardial infarction”, “cardiovascular strokes”, “exercise”, and “cardiac function”. Detailed search strategies for each database are presented in Supplementary Appendix 2.
2.2 Inclusion criteria
Eligible studies were those that fulfilled these criteria: (1) participants: Adults over the age of 18 who have been diagnosed with myocardial infarction based on clinical examinations such as PCI, dynamic electrocardiogram monitoring, serum or enzymatic tests, x-rays, echocardiography, or coronary angiography (26). No restrictions were placed on participants' race, nationality, or region. (2) Interventions: The control group was administered conventional treatments, including regular medication, typical care, and verbal instruction. The experimental group received exercise interventions in addition to the control treatments, including AE, RE, MBE, HIIT, and multi-component exercise training (MCET). Studies comparing different exercise modalities were also considered. Specific definitions and examples of the various exercise forms are provided in Supplementary Appendix 3. (3) Outcome Measures: Cardiac function was assessed using the six-minute walk test (6WMT), left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and left ventricular end-systolic diameter (LVESD). (4) Study Design: RCTs.
Exclusion criteria included: non-randomized controlled trials; duplicate publications; animal studies; mechanistic pharmacology or drug synthesis research; reviews; studies without clear descriptions of exercise interventions; studies involving participants who were not MI patients; and studies with incomplete data.
2.3 Study selection and data collection
Two researchers conducted screenings of the literature independently to assess eligibility. Duplicate records were removed using reference management software. Afterward, titles and abstracts were reviewed for an initial selection, and the full texts of the remaining articles were downloaded to confirm eligibility for inclusion in the analysis. Discrepancies were resolved through discussion, or a third researcher acted as an arbitrator to determine whether a study should be included.
A pre-designed data extraction form was utilized to systematically collect and organize pertinent information from the studies included in this analysis. The collected data encompassed the author(s), year of publication, average age of participants, gender distribution, specific interventions implemented in both the experimental and control groups, as well as the means and standard deviations recorded prior to and following the interventions, in addition to the sample size. In instances where data were found to be incomplete, the authors of the respective studies were contacted to obtain the necessary information.
2.4 Risk of bias and quality of evidence assessment
Two researchers assessed the risk of bias in the included studies using the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (ROB2) (27). The risk of bias was evaluated across five domains: bias arising from the randomization process; bias due to deviations from the intended interventions; bias from missing outcome data; bias in outcome measurement; and bias due to selective reporting. The overall risk of bias for each study was determined by synthesizing the results from these five domains. Each domain was classified as having high, low, or some risk of bias.
The quality of the evidence was assessed using the CINeMA online tool (28). CINeMA evaluated the risk of bias across six domains: within-study bias, between-study bias, indirectness, imprecision, heterogeneity, and inconsistency. Based on these assessments, the quality of evidence was classified as high, moderate, low, or very low (29). Detailed assessment methods for each domain are provided in Supplementary Appendix 7.
2.5 Statistical analysis
Given that all outcome measures were continuous variables employing identical measurement techniques and units, mean differences (MD) along with their associated 95% confidence intervals (CIs) were utilized to evaluate effect sizes. The NMA was conducted using a frequentist framework in Stata 14.0 (30). A random-effects model was applied to account for heterogeneity across studies due to various factors, providing more conservative confidence intervals (31). This model has been widely utilized in previous studies, and its effectiveness has been verified (32, 33). Network plots were used to visualize the comparisons between interventions, and both the design-by-treatment interaction model and side-splitting methods were employed to assess global and local inconsistency (34, 35). When the global inconsistency test showed no significant results, a consistency model was used for analysis. The Surface Under the Cumulative Ranking (SUCRA) was calculated to determine the relative effectiveness of different interventions, with higher SUCRA values indicating better treatment efficacy (36). It is important to note that the ranking probabilities estimated by SUCRA values should not be interpreted in isolation but rather as one component of a comprehensive assessment that gives greater weight to the magnitude of the MDs, the precision of these estimates (95% CIs), and the overall quality of evidence as assessed by CINeMA. Additionally, network meta-regression was conducted to explore the potential impact of participant age, exercise intervention duration, and baseline severity on the study outcomes. Sensitivity analysis was conducted by excluding studies with a high risk of bias to assess their impact on the results of the NMA. Publication bias was assessed by visual inspection of the comparison-adjusted funnel plot for each outcome network.
3 Results
3.1 Characteristics of included studies
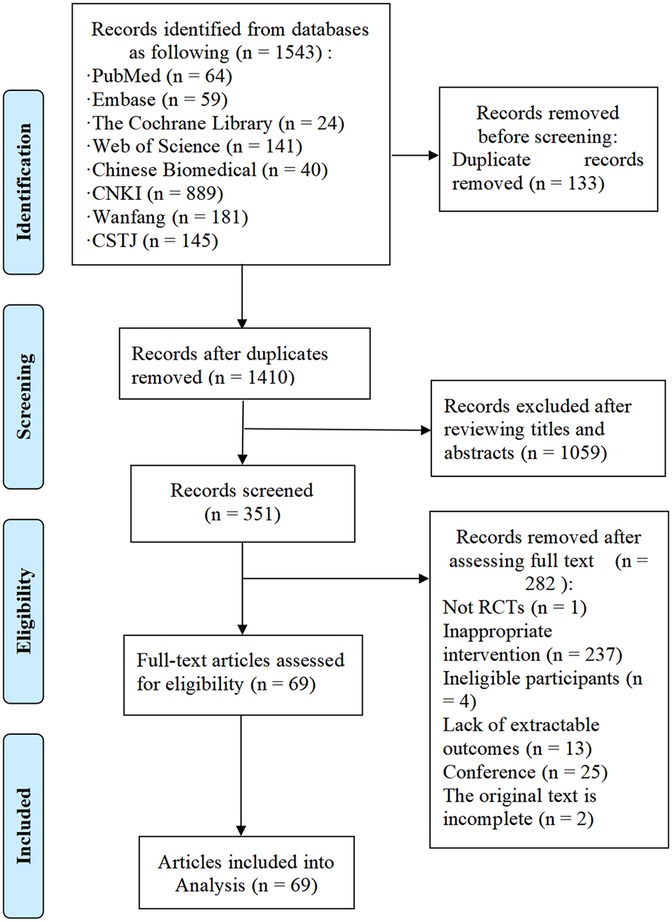
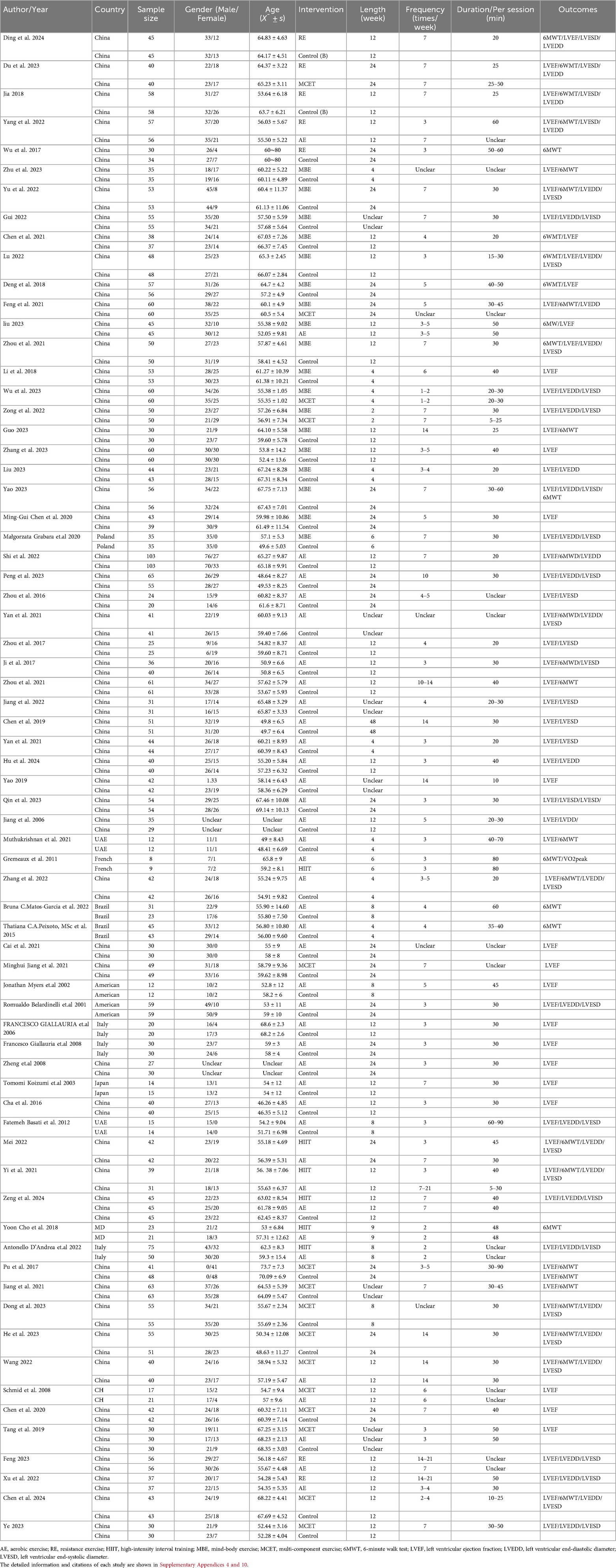
A total of 1,543 studies were initially retrieved from the databases, with 133 duplicates removed. After screening titles and abstracts, 351 studies were considered potentially eligible. Following full-text review, 69 studies were deemed to meet the inclusion criteria. Figure 1 illustrates the detailed literature selection process and Table 1 presents the brief characteristics of the included studies. The majority of the included studies were two-arm trials, comparing different interventions, with only two studies being three-arm trials (37, 38). Among the interventions, AE and mind-body exercise were the most commonly used. Additionally, most studies involving MCET focused on the combined effects of AE and RE. The mean intervention durations were approximately 15.4 weeks for RE, 16.5 weeks for AE, 13.5 weeks for mind-body exercise, 11.8 weeks for HIIT, and 15.2 weeks for MCET. Regarding the assessment of cardiac function outcomes, only two studies, Jonathan Myers et al. (39) and Schmid et al. (40), utilized cardiac magnetic resonance (cardiac MRI); all other studies used echocardiography for these measurements. Detailed characteristics of the included studies are provided in Supplementary Appendix 4.
3.2 Risk of bias
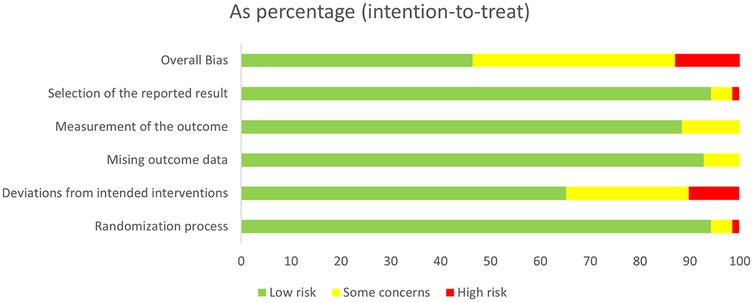
Figure 2 shows the overall risk of bias for the included studies. 33 studies were assessed as having low risk, 28 studies as having some risk, and 8 studies as having high risk. Among all the domains assessed, deviation from the intended interventions was the primary factor influencing study quality. Detailed information on each study's risk of bias in the various domains is presented in Supplementary Appendix 5.
3.3 Network meta-analysis
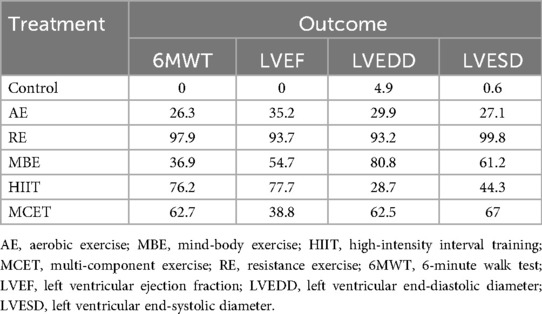
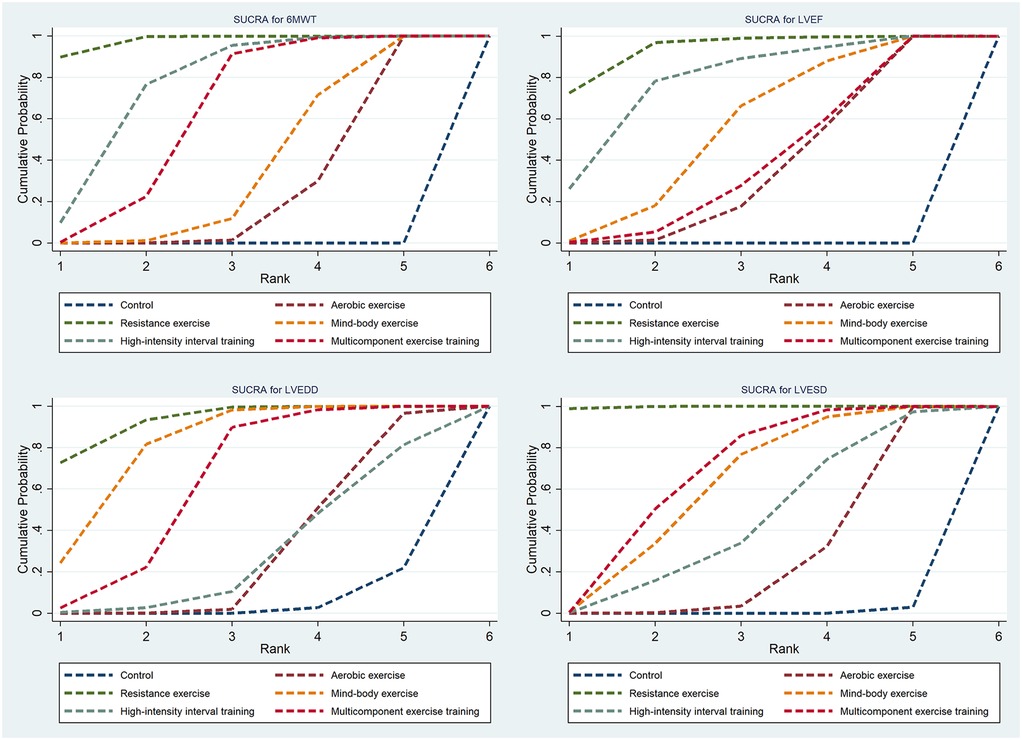
Thirty-three studies reported the 6MWT outcomes, involving 2,613 participants. Figure 3 presents the comparisons between different interventions, with global inconsistency tests indicating no significant inconsistency (P > 0.05). The NMA showed that, compared to the control group, all exercise interventions significantly improved the 6MWT scores in MI patients. The MDs (95% CI) ranged from 57.61 (34.87, 80.36) for AE to 144.38 (110.78, 177.98) for RE (low to moderate evidence quality). Additionally, RE demonstrated significantly greater efficacy than mind-body exercise (MD: 77.83, 95% CI: 37.38, 118.28, low evidence quality) and MCET (MD: 54.16, 95% CI: 15.18, 93.13, low evidence quality). The efficacy of AE was significantly worse than that of RE (MD: −86.76, 95% CI: −123.84, −49.69, very low evidence quality), HIIT (MD: −51.35, 95% CI: −90.95, −11.76, very low evidence quality), and MCET (MD: −32.60, 95% CI: −63.87, −1.34, low evidence quality) (Table 2, Supplementary Appendix 9.4). The probability-based ranking provided by the SUCRA analysis positioned RE as the modality most likely to be the most effective (SUCRA: 97.9), followed by HIIT (SUCRA: 76.2) and MCET (SUCRA: 62.7) (Table 3; Figure 4). Network meta-regression analysis indicated that the average age of participants and intervention duration may influence the efficacy of RE and HIIT, which lowered the quality of evidence for these comparisons (Supplementary Appendix 9).
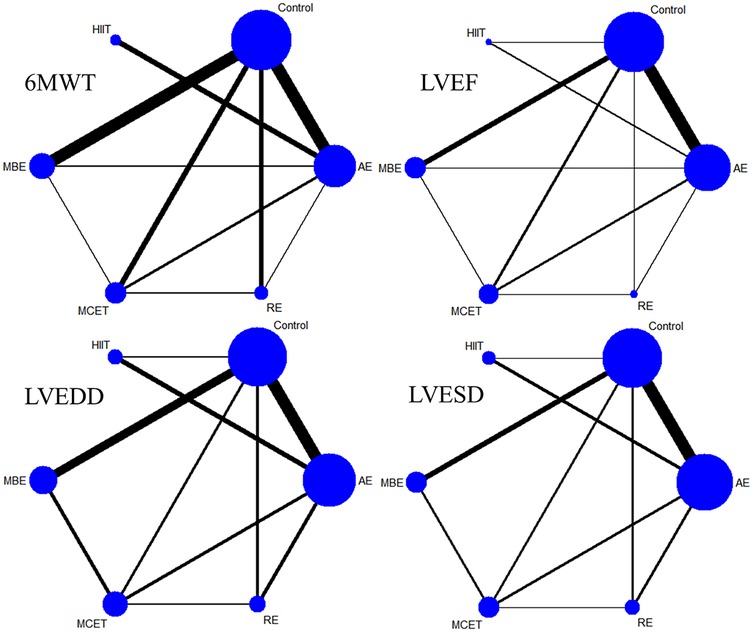
Figure 3. Network plot. AE, aerobic exercise; MBE, mind-body exercise; HIIT, high-intensity interval training; MCET, multi-component exercise; RE, resistance exercise; 6MWT, 6-minute walk test; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter.
Sixty-four studies reported LVEF, involving 4,919 participants. The network plots of comparisons between different interventions are shown in Figure 3, with no significant global inconsistency (P > 0.05). Compared to the control group, all exercise interventions improved LVEF in MI patients, with MDs (95% CI) ranging from 4.75 (3.42, 6.09) for AE to 8.75 (5.72, 11.77) for RE (very low to moderate evidence quality). Furthermore, RE showed significantly greater efficacy than MCET (MD: 3.89, 95% CI: 0.56, 7.22, moderate evidence quality) and AE (MD: −3.99, 95% CI: −6.99, −1.00, low evidence quality), while no significant differences were found between other intervention pairs (Table 2, Supplementary Appendix 9.4). The probabilistic SUCRA analysis was consistent with these findings, indicating that RE had the highest probability of being the most effective intervention (SUCRA: 93.7), followed closely by HIIT (SUCRA: 77.7) and mind-body exercise (SUCRA: 54.7) (Table 3; Figure 4). Regression analysis suggested that baseline severity might be a potential factor influencing RE efficacy (Supplementary Appendix 9).
Thirty-three studies reported LVEDD, involving 2,818 participants. The network plot is shown in Figure 3. Compared to the control group, all interventions, except AE and HIIT, significantly reduced LVEDD in MI patients. Specifically, RE (MD: −6.40, 95% CI: −9.24, −3.56, moderate evidence quality), mind-body exercise (MD: −5.23, 95% CI: −7.36, −3.10, very low evidence quality), and MCET (MD: −4.01, 95% CI: −6.42, −1.59, very low evidence quality) were significantly effective in reducing LVEDD. AE was less effective than RE (MD: 4.86, 95% CI: 2.10, 7.61, low evidence quality) and mind-body exercise (MD: 3.69, 95% CI: 1.00, 6.38, low evidence quality), while RE was significantly more effective than MCET (MD: 3.89, 95% CI: 0.56, 7.22, moderate evidence quality) (Table 2, Supplementary Appendix 9.4). The SUCRA rankings reflected this, with RE (SUCRA: 93.2), mind-body exercise (SUCRA: 80.8) and MCET (SUCRA: 62.5) having relatively high probabilities of being effective interventions for promoting favorable diastolic remodeling (Table 3; Figure 4). Regression analysis suggested that participant age and intervention duration may influence the efficacy of mind-body exercise and MCET (Supplementary Appendix 9).
Thirty-four studies reported LVESD, involving 2,683 participants. The network plot in Figure 3 depicts the comparisons between interventions. The results show that all exercise interventions, except HIIT, were significantly more effective than the control group for improving LVESD in MI patients, with MDs (95% CI) ranging from −1.89 (−3.27, −0.51) for AE to −7.33 (−9.62, −5.03) for RE (very low to moderate evidence quality). Additionally, RE demonstrated greater efficacy compared to AE (MD: 5.44, 95% CI: 3.21, 7.66, low evidence quality), mind-body exercise (MD: −3.69, 95% CI: −6.55, −0.82, moderate evidence quality), HIIT (MD: −4.65, 95% CI: −7.95, −1.35, low evidence quality), and MCET (MD: −3.45, 95% CI: −6.08, −0.82, moderate evidence quality) (Table 2, Supplementary Appendix 9.4). The SUCRA value for RE (99.8) further corroborated its high probability of being a highly effective intervention. MCET (SUCRA: 67.0) and mind-body exercise (SUCRA: 61.2) were ranked as the next potentially effective interventions, although their effect sizes were considerably smaller than that of RE (Table 3; Figure 4). Regression analysis suggested that baseline severity and participant age might be potential factors influencing the efficacy of RE and mind-body exercise (Supplementary Appendix 9).
3.4 Sensitivity analysis
To assess the robustness of our findings, we performed a sensitivity analysis by excluding studies with a high risk of bias, which was primarily due to deviations from intended interventions. The results of this analysis were largely consistent with the primary analysis, although the effect estimates for some between-intervention comparisons were slightly attenuated. This suggests that our main conclusions are robust, even with the inclusion of these higher-risk studies (full results are available in Supplementary Appendix 7).
3.5 Publication bias
Visual inspection of the comparison-adjusted funnel plots for 6MWT, LVEF, LVEDD, and LVESD revealed a relatively symmetrical distribution of study points, indicating no strong evidence of significant publication bias across the network (funnel plots are shown in Supplementary Appendix 8).
4 Discussion
Exercise-based interventions for improving cardiac function in MI patients are widely recognized as beneficial for cardiac function (16). This study is the first to use network meta-analysis to systematically review eligible studies and determine the effects of mind-body exercise, AE, RE, HIIT, and MCET on cardiac function in MI patients. Additionally, regression analysis was employed to explore potential factors influencing exercise efficacy. The aim is to provide a reference for decision-making in exercise interventions within CR for this population. The findings suggest that all types of exercise interventions are likely to effectively improve cardiac function in MI patients, with RE appearing to have a high probability of being a particularly effective intervention for enhancing 6MWT, LVEF, LVEDD, and LVESD. Additionally, HIIT showed notable improvements in 6MWT and LVEF. For LVEF and LVEDD, mind-body exercise was found to be the next most effective after RE.
4.1 The effect of different exercise modalities on cardiac function
A noteworthy finding of this study is the potential superiority of RE over traditional aerobic training in improving the 6MWT distance. While this phenomenon may not be universally applicable, it underscores the necessity of personalized rehabilitation, the core mechanism of which lies in precisely targeting the “weak links” that limit functional capacity in specific patient subgroups. Specifically, for many post-MI patients who are elderly, frail, or have significant sarcopenia, the bottleneck for their functional capacity has shifted from the central cardiopulmonary system to the peripheral skeletal muscle system (41). In these individuals, exercise cessation is often due to lower limb muscle fatigue, insufficient strength, or poor balance, rather than reaching the limits of their cardiopulmonary endurance. RE directly addresses this fundamental peripheral limiting factor by enhancing muscle mass, strength, and neuromuscular coordination (42). This perspective is supported by the findings of a recent network meta-analysis, which not only observed an overall advantage of RE in improving the 6MWT but also revealed through its meta-regression analysis that this advantage was particularly pronounced in older patients or those with poorer baseline functional status (43). The benefits of RE on LVEF and LVESD may be more apparent in study populations with higher baseline disease severity, whereas its impact on the 6MWT is influenced by participant age (44). This finding resonates strongly with the evolving clinical paradigm for frail individuals in post-MI rehabilitation, a population often characterized by advanced age, sarcopenia, and more severe cardiac dysfunction. For these patients, a “resistance-first” approach is increasingly advocated (45). Therefore, it is crucial to emphasize that the “superiority” of RE is conditional; its value does not negate the cornerstone status of aerobic exercise in cardiac rehabilitation but rather positions it as a vital synergistic or preparatory strategy. By strengthening the peripheral musculature first, it not only directly enhances patients' functional performance and sense of security but also lays a solid foundation for them to subsequently engage in more effective and beneficial aerobic training (42). Furthermore, this result reflects the comprehensive nature of the 6MWT as a functional assessment tool. Performance in the 6MWT is not solely dependent on cardiovascular endurance but is also significantly influenced by peripheral factors such as lower limb muscle strength, walking economy, and patient self-efficacy (46). For untrained or frail patients, peripheral muscle fatigue often becomes a limiting factor earlier than For untrained or frail patients, peripheral muscle fatigue often becomes a limiting factor in submaximal exercise earlier than cardiac output (47). By precisely ameliorating this limitation, RE leads to a significant increase in walking distance, which is sensitively captured by the functional endpoint of the 6MWT (48). In terms of assessment methods, although Cardiopulmonary Exercise Testing (CPET) and its parameters (e.g., peak oxygen uptake, VO₂peak; first ventilatory threshold, VT1) represent the “gold standard” for evaluating physiological adaptations, their limited availability in primary care settings restricts their widespread clinical application (49). While the 6MWT may be less sensitive, its simplicity and ease of implementation render it more valuable in real-world clinical practice. Due to the lack of consistent CPET data reporting in the included literature, our study was unable to analyze these more precise physiological indicators, which highlights a direction for future research.
Beyond its direct effects on peripheral muscles, RE also exerts positive influences on the heart itself, consistent with previous research. Studies have demonstrated that RE can enhance cardiac function by improving myocardial contractility, autonomic nervous function, and neuro-cardiovascular stress responses (50). Mechanistically, RE helps improve diastolic function, reduce left ventricular stiffness and filling pressure (51), and positively influences post-MI cardiac remodeling without inducing adverse left ventricular dilation (52). Moreover, by increasing cardiac pressure load, RE can improve subendocardial blood perfusion and decrease myocardial oxygen consumption, thereby alleviating myocardial ischemia (53).These multifaceted benefits collectively support the view that RE should be a core component of comprehensive management in post-MI cardiac rehabilitation.
HIIT has garnered significant attention for its effectiveness in improving patients' LVEF and exercise tolerance. Previous studies have shown that HIIT can mitigate adverse cardiac remodeling and enhance myocardial contractile function by improving glucose and lipid metabolism, reducing oxidative stress, and inhibiting myocardial fibrosis and apoptosis (18, 54, 55). Recent research has further revealed that HIIT can activate the mechano-growth factor (MGF)-related signaling pathway, thereby reducing infarct size and improving cardiac function (56). However, the choice of exercise intensity and modality is critical. Exhausting exercise may impair myocardial contractile function (57, 58), whereas moderate-intensity interval exercise can improve both systolic and diastolic function by optimizing the kinetics of cardiomyocyte calcium transients (59, 60).
Our study found that compared to AE, HIIT, and multicomponent exercise, mind-body exercise demonstrated a superior effect in improving LVEDD and LVESD. This suggests that mind-body exercise, as an effective rehabilitation therapy, may improve cardiac structure and function in MI patients through its unique mechanisms. Mind-body exercise emphasizes the integration of mind and body, promoting physical and mental relaxation and improving myocardial blood supply and oxygenation through coordinated physical movements, rhythmic breathing control, and mental focus (11). Its gentle, rhythmic motions may help optimize diastolic filling efficiency, thereby improving cardiac function and exercise tolerance. Notably, AE did not significantly reduce LVEDD in our analysis. One possible explanation is that the AE protocols in the included studies failed to reach the stimulatory threshold in intensity or duration required to induce beneficial cardiac remodeling (61). Cardiac reverse remodeling is a long-term process that requires a sufficient and sustained stimulus. Another explanation involves the distinct hemodynamic effects of different exercise modalities (45). AE primarily imposes a sustained volume load, whereas other effective interventions (such as resistance or multicomponent training) may confer benefits through different mechanisms (62). For instance, these modalities, by increasing skeletal muscle mass and improving peripheral vascular function, may lead to a long-term reduction in systemic vascular resistance, i.e., a decrease in cardiac afterload. A reduction in afterload is a potent stimulus for decreasing left ventricular dimensions, an effect that may have been less pronounced with the AE protocols analyzed in our study, possibly explaining its non-significant impact on LVEDD. Our results highlight the potential of mind-body exercise and AE as adjunct therapies in cardiac rehabilitation for MI patients, particularly given their safety and convenience. However, definitive conclusions cannot be drawn at this stage due to limitations in the intervention protocols, heterogeneity, and sample sizes of existing studies. Future large-scale, rigorously designed randomized controlled trials are urgently needed to further validate the clinical benefits of mind-body exercise and AE.
4.2 Analysis of sources of heterogeneity
To investigate the potential sources of heterogeneity in our findings, we conducted a network meta-regression analysis (see Supplementary Appendix 7.3). The results revealed that baseline disease severity was a key moderator of the efficacy of RE in improving LVEF and LVESD. Furthermore, the mean age of participants moderated the effect of mind-body exercise on LVEDD and LVESD, as well as the impact of RE on the 6MWT. These findings underscore the importance of comprehensive baseline assessments (including medical history, physical examination, and electrocardiogram) in future studies. This is not only crucial for ensuring the homogeneity of the study population but is also a prerequisite for ensuring the safety of exercise interventions (63). The meta-regression analysis also indicated that the “duration” of the intervention was another significant factor influencing the efficacy of HIIT and MCET on the 6MWT and LVEDD outcomes. The data showed that the intervention period for HIIT was relatively short (mean 11.8 weeks), suggesting that improving cardiac function and exercise tolerance in MI patients through exercise may be a long-term process requiring a longer duration.
The heterogeneity in this study may also stem from the variability in control group interventions. We found that the control groups in the vast majority of studies employed a mixture of various interventions, making a clear, non-overlapping classification extremely difficult. Although we attempted a more granular stratification of the control groups, for example, by creating a separate subgroup for studies using only pharmacological treatment (64, 65), the sample size of such subgroups was too small, leading to insufficient statistical power and potentially misleading results. This inherent variability in control conditions inevitably affects the precise estimation of the relative efficacy of each active intervention, thereby reducing the certainty of our study's conclusions. Additionally, the vast majority of studies (n = 60) explicitly stated that interventions were supervised. Although the remaining nine studies did not specify supervision status, their hospital or rehabilitation center settings suggest that supervised implementation was highly probable (Supplementary Appendix 4). These methodological ambiguities collectively point to an urgent need: future clinical trials in cardiac rehabilitation must precisely define and report the components of control group interventions to facilitate more robust and meaningful evidence synthesis.
The primary source of bias in the included studies was the risk of bias related to deviations from intended interventions, along with the failure to transparently report the random sequence generation process. We conducted a sensitivity analysis by excluding studies with a high risk of bias. The results indicated that the significance of comparisons between different exercise interventions for all outcomes remained unchanged, and their relative ranking of superiority remained stable. This finding from the sensitivity analysis suggests that the core conclusions of our study were not unduly influenced by a few studies of questionable methodological quality. It reflects a degree of consistency and internal homogeneity within the existing body of evidence, where studies from different settings and with varying designs converge towards a clear and robust conclusion. Future research should still aim to optimize current methodological weaknesses, such as reporting of random sequences, standardization of intervention delivery, and blinding of subjective outcomes, as well as increase sample sizes and geographical coverage to further enhance evidence quality and generalizability.
4.3 Limitations
This study has several limitations: (1) although the diagnosis of myocardial infarction in each study was clinically valid, we were unable to assess the differential effects of various exercise modalities on patients with different types of myocardial infarction (e.g., STEMI and NSTEMI) due to insufficient reporting in the original studies. This clinical heterogeneity could affect patient prognosis and response to exercise. (2) The risk of bias, particularly that related to deviations from intended interventions, was a concern in many of the included studies. Although the sensitivity analysis indicated the robustness of our pooled results, the cumulative effect of these biases may have slightly inflated the comparative advantages of some interventions. This potential influence should be considered when interpreting the results and highlights the need for more standardized implementation and reporting in future research. (3) There was considerable heterogeneity in the implementation of exercise interventions across the included studies. For instance, many studies used only qualitative descriptions such as “moderate intensity” or “conventional rehabilitation training” without providing quantifiable parameters like target heart rate zones, percentage of maximal oxygen uptake, or ratings of perceived exertion. This precluded subgroup analyses and network meta-regression based on high- vs. low-to-moderate-intensity interventions. While this issue appears to be common in the field, as observed in similar high-quality studies (32, 66), the unmeasured variability in intensity should be considered with caution when interpreting the results. Future studies could consider using MET intensities to quantify the dose of different exercise interventions for more precise comparisons (67). (4) Although we employed a global search strategy, the included RCTs were predominantly from studies published in China. While this objectively reflects the current distribution of research in this field, it may limit the external validity and global generalizability of our conclusions. Readers should interpret the findings in the context of local clinical practices and population characteristics when extrapolating them to other regions or populations.These limitations somewhat diminish the confidence in the quality of the evidence. Therefore, future research should target these qaps by refining subtype-specific analyses,standardizing quantitative reporting of exercise interventions, and expanding data from diverse regions toenhance the reliability and clinical applicability of evidence in this field.
5 Conclusion
This network meta-analysis evaluated the effects of five different exercise interventions on cardiac function in patients after myocardial infarction. Our findings suggest that RE may be the most effective single modality for improving cardiac function and promoting favorable remodeling post-MI. Its benefits may be particularly pronounced in older or more severely deconditioned patients, supporting a “resistance-first” rehabilitation strategy for this vulnerable population. While MCET and mind-body exercise also offer significant advantages, particularly in reducing ventricular size, claims of any single modality's superiority should be interpreted with caution. The choice of exercise must be individualized, considering the variable quality of evidence for certain comparisons, the patient's specific clinical status, functional capacity, and personal preferences. Ultimately, healthcare professionals should use these findings to guide the design of more tailored and effective rehabilitation programs, moving towards a more personalized exercise prescription approach for post-MI patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
BY: Data curation, Visualization, Conceptualization, Resources, Writing – original draft. LZ: Writing – review & editing, Methodology, Software, Data curation, Formal analysis. LH: Methodology, Data curation, Writing – review & editing, Software, Formal analysis. MZ: Writing – review & editing, Software, Data curation, Formal analysis, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Social Science Planning Project of Shandong Province (Project Approval Number: 24CTYJ13).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1623727/full#supplementary-material
References
1. Anderson JL, Morrow DA. Acute myocardial infarction. New Engl J Med. (2017) 376(21):2053–64. doi: 10.1056/NEJMra1606915
2. Masuda A, Nemoto A, Yamaki T, Oriuchi N, Takenoshita S, Takeishi Y. Assessment of myocardial viability of a patient with old myocardial infarction by (18)F-fluorodeoxyglucose PET/MRI. J Nucl Cardiol. (2018) 25(4):1423–6. doi: 10.1007/s12350-017-0941-9
3. Mangion K, Carrick D, Clerfond G, Rush C, McComb C, Oldroyd KG, et al. Predictors of segmental myocardial functional recovery in patients after an acute ST-elevation myocardial infarction. Eur J Radiol. (2019) 112:121–9. doi: 10.1016/j.ejrad.2019.01.010
4. Mazur R, Buksińska-Lisik M, Mamcarz A. ST-segment elevation myocardial infarction with non-obstructive coronary arteries in a patient with severe diabetic acidosis. Pol Merkur Lekarski. (2018) 45(270):248–50. Available online at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=30693911&query_hl=130693911
5. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8
6. National Center for Cardiovascular Quality Control. 2021 Medical quality report of cardiovascular diseases in China: an executive summary. Chinese Circul J. (2021) 36(11):1041–64. doi: 10.3969/j.issn.1000-3614.2021.11.001
7. Wagner A, Arveiler D, Ruidavets JB, Bingham A, Montaye M, Ferrières J, et al. Gender- and age-specific trends in coronary heart disease mortality in France from 2000 to 2007: results from the MONICA registers. Eur J Prev Cardiol. (2014) 21(1):117–22. doi: 10.1177/2047487312452967
8. Poulter N. Global risk of cardiovascular disease. Heart. (2003) 89(Suppl 2):ii2–5; discussion ii35–7. doi: 10.1136/heart.89.suppl_2.ii2
9. Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. (2017) 19(8):71. doi: 10.1007/s11886-017-0876-4
10. Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. (2009) 11(3):199–205. doi: 10.1007/s11906-009-0035-4
11. Han W, Wenjiao L, Jingbo Z, Lu C, Yan L, Changde J. Meta-Analysis of the effects of tai chi exercise therapy on improving cardiac function in patients with myocardial infarction. Chin J Evid Based Cardiovasc Med. (2020) 12(11):1296–301. doi: 10.3969/j.issn.1674-4055.2020.11.05
12. Vecchiato M, Quinto G, Palermi S, Foccardi G, Mazzucato B, Battista F, et al. Are gyms a feasible setting for exercise training interventions in patients with cardiovascular risk factors? An Italian 10-years cross-sectional survey comparison. Int J Env Res Pub He. (2022) 19(4):2407. doi: 10.3390/ijerph19042407
13. Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. (2018) 15(12):731–43. doi: 10.1038/s41569-018-0065-1
14. Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: the SAINTEX-CAD study. Int J Cardiol. (2015) 179:203–10. doi: 10.1016/j.ijcard.2014.10.155
15. Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Databse Syst Rev. (2016) 2016(1):CD1800. doi: 10.1002/14651858.CD001800.pub3
16. Hambrecht R, Niebauer J, Fiehn E, Kälberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. (1995) 25(6):1239–49. doi: 10.1016/0735-1097(94)00568-B
17. JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J. (2014) 78(8):2022–93. doi: 10.1253/circj.cj-66-0094
18. Elliott AD, Rajopadhyaya K, Bentley DJ, Beltrame JF, Aromataris EC. Interval training versus continuous exercise in patients with coronary artery disease: a meta-analysis. Heart Lung Circ. (2015) 24(2):149–57. doi: 10.1016/j.hlc.2014.09.001
19. Zhang J, Weng J, Yuan M, Shen X, Weng Y, Shen X. Effects of traditional Chinese exercises on cardiac rehabilitation in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2023) 10:1223677. doi: 10.3389/fcvm.2023.1223677
20. Yang YL, Wang YH, Wang SR, Shi PS, Wang C. The effect of tai chi on cardiorespiratory fitness for coronary disease rehabilitation: a systematic review and meta-analysis. Front Physiol. (2017) 8:1091. doi: 10.3389/fphys.2017.01091
21. Gu Q, Wu SJ, Zheng Y, Zhang Y, Liu C, Hou JC, et al. Tai chi exercise for patients with chronic heart failure: a meta-analysis of randomized controlled trials. Am J Phys Med Rehab. (2017) 96(10):706–16. doi: 10.1097/PHM.0000000000000723
22. Fisher S, Smart NA, Pearson MJ. Resistance training in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. (2022) 27(5):1665–82. doi: 10.1007/s10741-021-10169-8
23. Ying X, Quanyu Z, Zhenyang L, Meili L. Meta-Analysis of the effect of baduanjin on left ventricular ejection fraction in patients with acute myocardial infarction. J Clin Mil Med. (2024) 52(06):589–91. doi: 10.16680/j.1671-3826.2024.06.10
24. Yuan Q. Meta-Analysis of the effects of high-intensity interval training on cardiopulmonary adaptation and ventricular remodeling in patients with myocardial infarction (Master thesis). Guangxi Medical University, Nanning (2022).
25. Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. (2017) 12(1):103–11. doi: 10.1007/s11739-016-1583-7
26. Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on coronary angiography) developed in collaboration with the society for cardiac angiography and interventions. Circulation. (1999) 99(17):2345–57. doi: 10.1161/01.cir.99.17.2345
27. Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ Brit Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
28. Nikolakopoulou A, Higgins J, Papakonstantinou T, Chaimani A, Del GC, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. Plos Med. (2020) 17(4):e1003082. doi: 10.1371/journal.pmed.1003082
29. Papakonstantinou T, Nikolakopoulou A, Higgins J, Egger M, Salanti G. CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. (2020) 16(1):e1080. doi: 10.1002/cl2.1080
30. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using stata. Epidemiol Health. (2017) 39:e2017047. doi: 10.4178/epih.e2017047
31. Law M, Jackson D, Turner R, Rhodes K, Viechtbauer W. Two new methods to fit models for network meta-analysis with random inconsistency effects. BMC Med Res Methodol. (2016) 16:87. doi: 10.1186/s12874-016-0184-5
32. Huang X, Zhao X, Li B, Cai Y, Zhang S, Wan Q, et al. Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: a systematic review and network meta-analysis. J Sport Health Sci. (2022) 11(2):212–23. doi: 10.1016/j.jshs.2021.05.003
33. Wu S, Wang L, He Y, Shi F, Zhuang H, Mei L, et al. Effects of different mind-body exercises on glucose and lipid metabolism in patients with type 2 diabetes: a network meta-analysis. Complement Ther Clin. (2023) 53:101802. doi: 10.1016/j.ctcp.2023.101802
34. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3(2):98–110. doi: 10.1002/jrsm.1044
35. Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. (2013) 42(1):332–45. doi: 10.1093/ije/dys222
36. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016
37. Yuhua Z, Di T, Yi L, Lilan L, Yugang R. Effects of different intensity aerobic exercise on cardiopulmonary function,vascular endothelial function and quality of life in patients with cardiac dyfunction after acute myocardial infarction. Prog Mod Biomed. (2024) 24(18):3549–53; 3579. doi: 10.13241/j.cnki.pmb.2024.18.028
38. Yao T, Ping W, Ping Y. The effect of aerobic and resistance exercise guidance on elderly acute myocardial infarction patients after PCI. Contemp Med Symp. (2019) 17(24):10–1. Available online at: https://next.cnki.net/middle/abstract?v=Dm4VI7mKrXNd2HI6_xX-9G96z40jyXPRBJRvzxJ3g-lxKCfu8viRDXMbIqGtmTAjJM6MMFKtiMYqxdVL4rR1XFtGEpK6qCcX7KpON0KrvN7Frb2AAf1Sazhh5B5UnLbfgMhbZKbzCyw2VUJtRphJnksKqTILrOabi-DoaqIYQYcwRt9EpPU00DJhOxYf7r8bje2Z-KWr630=&uniplatform=NZKPT&language=CHS&scence=null
39. Myers J, Wagner D, Schertler T, Beer M, Luchinger R, Klein M, et al. Effects of exercise training on left ventricular volumes and function in patients with nonischemic cardiomyopathy: application of magnetic resonance myocardial tagging. Am Heart J. (2002) 144(4):719–25. doi: 10.1067/mhj.2002.124401
40. Schmid J, Anderegg M, Romanens M, Morger C, Noveanu M, Hellige G, et al. Combined endurance/resistance training early on, after a first myocardial infarction, does not induce negative left ventricular remodelling. Eur J Cardiovasc Prev Rehabil. (2008) 15(3):341–6. doi: 10.1097/HJR.0b013e3282f5dbf5
41. Vigorito C, Abreu A, Ambrosetti M, Belardinelli R, Corrà U, Cupples M, et al. Frailty and cardiac rehabilitation: a call to action from the EAPC cardiac rehabilitation section. Eur J Prev Cardiol. (2017) 24(6):577–90. doi: 10.1177/2047487316682579
42. Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association council on clinical cardiology and council on nutrition, physical activity, and metabolism. Circulation. (2007) 116(5):572–84. doi: 10.1161/CIRCULATIONAHA.107.185214
43. Li JY, Chen L, Wang QC, Zhu J, Ren ZQ, Wang LC. Effects of exercise modalities on physical function and quality of life in patients with heart failure: a systematic review and network meta-analysis. Esc Heart Fail. (2025) 12(4):2427–40. doi: 10.1002/ehf2.15256
44. Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. (2007) 49(24):2329–36. doi: 10.1016/j.jacc.2007.02.055
45. Eleyan L, Gonnah AR, Farhad I, Labib A, Varia A, Eleyan A, et al. Exercise training in heart failure: current evidence and future directions. J Clin Med. (2025) 14(2):359. doi: 10.3390/jcm14020359
46. Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Resp Crit Care. (2013) 188(8):e13–64. doi: 10.1164/rccm.201309-1634ST
47. Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. (2013) 111(10):1466–9. doi: 10.1016/j.amjcard.2013.01.303
48. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, et al. Exercise-Based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol. (2016) 67(1):1–12. doi: 10.1016/j.jacc.2015.10.044
49. Christou GA, Christou MA, Davos CH, Markozannes G, Christou KA, Mantzoukas S, et al. Ergophysiological evaluation of heart failure patients with reduced ejection fraction undergoing exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Hell J Cardiol. (2024) 77:106–19. doi: 10.1016/j.hjc.2024.01.004
50. Badrov MB, Wood KN, Lalande S, Sawicki CP, Borrell LJ, Barron CC, et al. Effects of 6 months of exercise-based cardiac rehabilitation on autonomic function and neuro-cardiovascular stress reactivity in coronary artery disease patients. J Am Heart Assoc. (2019) 8(17):e12257. doi: 10.1161/JAHA.119.012257
51. Qiqi Y, Yang S. Research progress on the effects of resistance exercise in cardiac rehabilitation for the elderly. J Heart. (2021) 33(04):452–5. doi: 10.12125/j.chj.202102015
52. Garza MA, Wason EA, Cruger JR, Chung E, Zhang JQ. Strength training attenuates post-infarct cardiac dysfunction and remodeling. J Physiol Sci. (2019) 69(3):523–30. doi: 10.1007/s12576-019-00672-x
53. CMA Prevention Group of the Cardiovascular Disease Branch, CAOR Cardiovascular Disease Professional Committee. Chinese expert consensus on exercise therapy for patients with coronary heart disease. Chin J Cardiovas Dis. (2015) 43(7):575–88. doi: 10.3760/cma.j.issn.0253-3758.2015.07.004
54. Lu K, Wang L, Wang C, Yang Y, Hu D, Ding R. Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol Med Rep. (2015) 12(2):2374–82. doi: 10.3892/mmr.2015.3669
55. Abad C, Do NA, de Souza LE, Figueroa D, Ramona P, Sartori M, et al. Interval and continuous aerobic exercise training similarly increase cardiac function and autonomic modulation in infarcted mice. J Exerc Rehabil. (2017) 13(3):257–65. doi: 10.12965/jer.1734914.457
56. Bowen L, Lei T, Lili F, Shou P, Zhenjun T. Whole-Body vibration training and high-intensity interval exercise upregulate MGF/MEK/ERK pathway to protect cardiac function and skeletal muscle in myocardial infarction rats. China Sports Sci Technol. (2023) 59(03):58–66. doi: 10.16470/j.csst.2021079
57. Liping G, Ying L, Hong S. Effects of exhaustive exercise on β-adrenergic receptor-mediated myocardial cell contraction in rats. Chin J Appl Physiol. (2013) 29(05):437–40. doi: 10.13459/j.cnki.cjap.2013.05.005
58. Zeppilli P, Biffi A, Cammarano M, Castelletti S, Cavarretta E, Cecchi F, et al. Italian Cardiological guidelines (COCIS) for competitive sport eligibility in athletes with heart disease: update 2024. Minerva Med. (2024) 115(5):533–64. doi: 10.23736/S0026-4806.24.09519-3
59. Wisløff U, Loennechen JP, Currie S, Smith GL, Ellingsen Ø. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. (2002) 54(1):162–74. doi: 10.1016/s0008-6363(01)00565-x
60. Kemi OJ, Haram PM, Wisløff U, Ellingsen Ø. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation. (2004) 109(23):2897–904. doi: 10.1161/01.CIR.0000129308.04757.72
61. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. (2012) 54(5):380–6. doi: 10.1016/j.pcad.2012.01.006
62. Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. (2001) 38(3):860–6. doi: 10.1016/s0735-1097(01)01439-5
63. Donati F, Guicciardi C, Lodi E, Fernando F, Palermi S, Modena MG, et al. Echocardiography in the preparticipation screening: an old topic revisited. J Cardiovasc Med. (2023) 24(5):297–301. doi: 10.2459/JCM.0000000000001460
64. Jian Y, Xiaojing L, Ling O, Niansang L, Chao M, Feng K, et al. Clinical study on the rehabilitation effect of treadmill exercise combined with conventional western medicine therapy on patients with acute myocardial infarction after PCI surgery. Med Innov China. (2021) 18(24):59–62. doi: 10.3969/j.issn.1674-4985.2021.24.015
65. Longyan Y. The effect of simplified ba duan jin on cardiac function and quality of life in patients after stent implantation for acute myocardial infarction. Yishoubaodian. (2020) 17(8):196. Available online at: https://www.cqvip.com/doc/journal/987980728
66. Hou L, Wang Q, Pan B, Li R, Li Y, He J, et al. Exercise modalities for type 2 diabetes: a systematic review and network meta-analysis of randomized trials. Diabetes Metab Res. (2023) 39(1):e3591. doi: 10.1002/dmrr.3591
Keywords: myocardial infarction, exercise training, cardiac function, cardiac rehabilitation, network meta-analysis
Citation: Yu B, Zhao L, Huang L and Zhang M (2025) Which exercise modality is most effective for improving cardiac function in patients with myocardial infarction? A network meta-analysis. Front. Cardiovasc. Med. 12:1623727. doi: 10.3389/fcvm.2025.1623727
Received: 6 May 2025; Accepted: 13 October 2025;
Published: 30 October 2025.
Edited by:
Xiaosong Gu, The Second Affiliated Hospital of Soochow University, ChinaReviewed by:
Georgios A. Christou, University of Ioannina, GreeceSamir José Bolívar González, University of Atlántico, Colombia
Copyright: © 2025 Yu, Zhao, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maolin Zhang, emhhbmdtYW9saW5Ac2RwZWkuZWR1LmNu
 Bo Yu1
Bo Yu1 Maolin Zhang
Maolin Zhang