- 1Mehiläinen Airport Health Centre, Vantaa, Finland
- 2Department of Forensic Medicine, University of Helsinki, Helsinki, Finland
- 3Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa
- 4Cardiovascular Research Laboratory, Wihuri Research Institute, Helsinki, Finland
Heterozygous familial hypercholesterolemia (HeFH) is a genetic disorder that is characterized by a lifelong elevation of low-density lipoprotein cholesterol (LDL-C) due to impaired clearance by dysfunctional LDL receptors (1). About 30% of HeFH subjects also have elevated levels of another genetically determined atherogenic lipoprotein, namely lipoprotein(a) [(Lp(a)] (1). With a worldwide prevalence of HeFH of approximately 1 in 300 (2, 3), it can be estimated that there are over 10 million HeFH patients with an elevated Lp(a) level over 50 mg/dl (>125 nmol/L). As both elevated LDL-C and Lp(a) are associated with an increased risk for premature atherosclerotic cardiovascular disease (ASCVD), in those HeFH patients with both conditions, the risk of premature ASCVD is likely to be even greater (4). Importantly, Bhatia and coworkers (2025) have shown that in statin-treated patients, when compared with an Lp(a) level of 5 mg/dl, higher levels of Lp(a) are log-linearly associated with increasing ASCVD risk.
Current guidelines recommend that statin therapy be initiated in childhood in subjects diagnosed with HeFH (5). However, no clear recommendations exist for treating elevated Lp(a) in subjects with HeFH. Currently, an oral small molecule Lp(a) inhibitor and several Lp(a) lowering antisense oligonucleotide and siRNA formulations are in clinical trials (6–11). The current trials involve only adult subjects, and the question has been raised whether Lp(a)-lowering drugs should also be considered and tested in young HeFH patients (12). Below, we will critically review the evidence that elevated Lp(a) may accelerate the development of atherosclerosis in young HeFH individuals, and if it does, then this would support pharmacological primary prevention for elevated Lp(a) in young HeFH patients.
In a recent Norwegian study, Lp(a) levels were measured in 438 children with genotypically confirmed HeFH, and approximately 24% of the girls and 17% of the boys in this cohort had elevated Lp(a) levels above 50 mg/dl (approximately 125 nmol/L), i.e., a level considered to be significantly atherogenic (13). In children with HeFH of either sex, increased Lp(a) concentrations are therefore more prevalent than in the general population. In addition, in a large cross-sectional study of 1,960 patients with HeFH and their 957 non-HeFH relatives, higher Lp(a) levels were found in those with HeFH, particularly in those with pre-existing ASCVD, compared to their non-HeFH relatives (14) suggesting that elevated Lp(a) levels predispose to a heightened risk of ASCVD in patients with HeFH.
Regarding the pathophysiological importance of an elevated Lp(a) level, impaired endothelium-dependent dilation has been observed in HeFH children as young as seven years old (15). Furthermore, in the study by Charakida et al. (16), inflammatory and hemostatic abnormalities that associated with vascular dysfunction were present in HeFH children with elevated Lp(a) levels but not in those HeFH children without. An additional interesting piece of information comes from the study by Hegele and coworkers (17), who studied stress thallium scans in HeFH children with a family history of premature ASCVD. They found that in HeFH children aged 9–23 years, Lp(a) levels tended to be higher in those in whom the stress thallium heart scan revealed a reduced coronary blood flow. Moreover, in a recent 20-year follow-up study of 200 HeFH children aged 8–18 years who participated in a statin trial in which the carotid intima-media thickness was serially measured as an indicator of subclinical atherosclerosis it appeared that a high Lp(a) level contributed significantly to the progression of carotid intima-media thickness [β adjusted 0·0073 mm per 50 nmol/L increase in lipoprotein(a); 95% CI (0·0013–0·0132); p = 0·017] (18). The authors of this study concluded that an elevated level of Lp(a) is an independent risk factor for early atherosclerosis and that, therefore, it is essential to measure Lp(a) in young patients with HeFH. Despite this interesting finding which strongly supports Lp(a) as a risk factor for early atherosclerosis in HeFH patients, a very recent study of 143 young adults with HeFH (age 31.8 ± 3.2 years) failed to find an association with carotid arterial stiffness and Lp(a) levels (12). However, arterial stiffness may not be a good measure of early atherosclerosis in young adults, and other surrogate markers of early signs of atherosclerosis, such as plaque formation, may be more suitable to evaluate the Lp(a)-mediated contribution to atherosclerosis in young FH patients.
Despite lipid-lowering treatment (LLT), exposure to even moderately increased LDL-C levels from birth in HeFH patients results in premature development of ASCVD, particularly coronary atherosclerosis. This was eloquently shown in a study in which 90 HeFH patients (mean age 41 ± 3 years) having LLT initiated before or after age 25 were compared to an age- and sex-matched unaffected control group (19). FH patients had a higher cumulative LDL-C exposure (181 ± 54 vs. 105 ± 33 mmol/L ∗ years) and higher prevalence of coronary plaque compared with controls [46 (51%) vs. 10 (22%), OR 3.66 (95% CI 1.62–8.27)]. Every 75 mmol/L ∗ years, cumulative exposure to LDL-C was associated with a doubling in percent atheroma. Early treated patients had a modestly lower cumulative LDL-C exposure than did late-treated FH patients (167 ± 41 vs. 194 ± 61 mmol/L ∗ years; P = 0.045), without significant difference in coronary atherosclerosis. This study emphasizes the need for early initiation of intensive lipid-lowering treatment in subjects with HeFH.
In a recent study by Shishikura et al. (20) analyzed 439 patients with coronary artery disease (CAD) using near-infrared spectroscopy (NIRS) imaging, which enables the quantitative evaluation of lipidic-plaque materials. NIRS-derived maximum 4 mm lipid-core burden index (MaxLCBI4 mm) ≥ 400 is associated with an increased risk of cardiovascular events (19). In this study the coexistence of LDL-C < 70 mg/dl and Lp(a) < 50 mg/dl showed an approximately 70% lower risk (adjusted odds ratio: 0.30; 95% confidence interval: 0.13–0.68) of MaxLCBI4 mm ≥ 400 when compared with the reference group [LDL-C ≥ 70 mg/dl and Lp(a) ≥ 50 mg/dl] (20). Thus, the findings of this study strongly support the idea of treating Lp(a) to stabilize atherosclerotic plaques.
The findings of a very recent study suggest that the optimal cut-off point for Lp(a) in HeFH patients needs to be below 10 mg/dl (approximately 25 nmol/L), i.e., a level which is significantly lower than that associated with increased ASCVD risk in the general population (above 30 mg/dl or 75 nmol/L) (21). Importantly, since the atherosclerosis-promoting effect of a high Lp(a) level, like that of a high LDL-C level, is present from birth, HeFH patients with elevations in both LDL-C and Lp(a) may present with their first cardiovascular event before the age of 35 years (22).
Worldwide, FH remains underdiagnosed and, even if diagnosed, undertreated or untreated, with most patients only having LLT aimed at lowering LDL-C commenced in adulthood. As discussed above, this is especially deleterious for those FH patients who, in addition to the high LDL-C levels, also have high Lp(a) levels and are, therefore, even at greater risk for an ASCVD event. However, treatment aimed at concurrently lowering Lp(a) is presently not considered. Notably, a recent German Lipoprotein Apheresis Registry follow-up study involving non-FH patients with an isolated elevated Lp(a) level showed that the patients benefited from a reduction of the Lp(a) level in that the number of ASCVD events was reduced (23). So, in FH patients whose coronary atherosclerotic plaques may be stenotic and show characteristics of advanced vulnerability already in the teens, specific Lp(a)-lowering drug therapy should be considered and should accompany an early and effective LDL-C-lowering strategy to prevent or at least delay, the onset of premature ASCVD.
It has already been demonstrated in adult non-HeFH patients that the addition of the PCSK9 inhibitor, alirocumab, reduces major acute cardiovascular events, including coronary heart disease death, non-fatal myocardial infarction, fatal/non-fatal ischemic stroke, unstable angina requiring hospitalization to a greater degree among those patients who had a high baseline Lp(a) level (24). Therefore, the availability of the PCSK-9 inhibitors seems to offer a unique opportunity for primary prevention in HeFH children with a double heritable risk (25). Including a PCSK9 inhibitor in the intensive treatment schedule necessary for these very high-risk HeFH children with both elevated LDL-C and Lp(a) levels should be considered. However, because of their additive lifelong cumulative burden, it remains to be proven whether an optimal early primary prevention of HeFH necessitates targeting both atherogenic lipoproteins (26) (Figure 1).
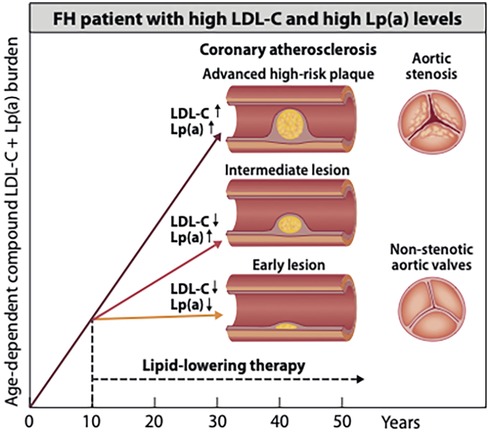
Figure 1. Potential beneficial effects of early initiation of pharmacotherapies to delay the onset of atherosclerosis in an HeFH patient with elevation of both LDL-C and Lp(a) potential beneficial effects of early started pharmacotherapies on the development of coronary atherosclerosis in an HeFH patient with elevation of both LDL-C and Lp(a) level. About one-third of patients with HeFH have since birth a genetically determined elevation of both low-density lipoprotein cholesterol (LDL-C) and lipoprotein (a) [(Lp(a)]. Such joint elevation of two atherogenic lipoproteins strongly accelerates the development of atheroscleroticcardiovascular disease (ASCVD) and the rates of ASCVD events. The figure shows illustratively that in an untreated HeFH patient as young as 40 years old, an advanced high-risk coronary plaque may develop. In contrast, when efficient LDL-C-lowering or combined LDL-C- and Lp(a)-lowering pharmacotherapy is already started in early childhood, only an intermediate or an early lesion may have developed by early middle age. On the right side of the image, severely stenotic and non-stenotic aortic valves are shown. Although aortic stenosis in HeFH without elevated Lp(a) is rare, a joint elevation of Lp(a) is likely to increase the risk for aortic stenosis (27). Accordingly, an early start of lowering LDL-C and, particularly, of Lp(a) is expected to reduce the risk of developing this disease condition.
Summary
• Heterozygous familial hypercholesterolemia (HeFH) is characterized by a lifelong elevation of low-density lipoprotein cholesterol (LDL-C).
• About 30% of HeFH subjects also have elevated levels of another atherogenic lipoprotein, namely lipoprotein(a) [(Lp(a)].
• Currently, an oral small molecule Lp(a) inhibitor and several Lp(a) lowering antisense oligonucleotides and siRNA formulations are in clinical trials for the treatment of elevated Lp(a).
• Elevated Lp(a) impairs endothelium-dependent dilation, and this has been observed in HeFH children as young as seven years old.
• High Lp(a) level has been shown to contribute significantly progression of carotid intima-media thickness in HeFH children aged 9 to 23 years.
• The findings of a very recent study suggest that the optimal cut-off point for Lp(a) in HeFH patients needs to be below 10 mg/dL.
• In optimal early primary prevention of HeFH both elevated LDL-C and Lp(a) levels should be considered, because of their additive lifelong cumulative burdens.
Author contributions
AV: Writing – review & editing. FJR: Writing – review & editing, Writing – original draft, Conceptualization. PTK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
AV has received consultancy fees Amgen and Novartis. FJR has received research grants, honoraria, or consulting fees for professional input and/or delivered lectures from Amgen, AstraZeneca, MSD, Novartis, Sanofi, Regeneron, Ultragenyx, Chiesi, Cipla, Silence Therapeutics, Verve Therapeutics and LIB Therapeutics. PTK has received consultancy fees, lecture honoraria, and/or travel fees from Amgen, Novartis, Raisio Group, and Sanofi.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vuorio A, Watts GF, Schneider WJ, Tsimikas S, Kovanen PT. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J Intern Med. (2020) 287(1):2–18. doi: 10.1111/joim.12981
2. Akioyamen LE, Genest J, Shan SD, Reel RL, Albaum JM, Chu A, et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open. (2017) 7(9):e016461. doi: 10.1136/bmjopen-2017-016461
3. Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide prevalence of familial hypercholesterolemia: meta-analyses of 11 million subjects. J Am Coll Cardiol. (2020) 75(20):2553–66. doi: 10.1016/j.jacc.2020.03.057
4. Jha M, McCarthy IR, Gelfand EV. Lipoprotein(a)—from biomarker to therapy: a review for the clinician. Am J Cardiol. (2025) 245(25):42–53. doi: 10.1016/j.amjcard.2025.02.034
5. Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev. (2019) 2019(11):CD006401. doi: 10.1002/14651858.CD006401.pub5
6. Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, et al. Single ascending dose study of a short interfering RNA targeting lipoprotein(a) production in individuals with elevated plasma lipoprotein(a) levels. JAMA. (2022) 327(17):1679–87. doi: 10.1001/jama.2022.5050
7. O'Donoghue ML, López JA, Knusel B, Gencer B, Wang H, Wu Y, et al. Study design and rationale for the olpasiran trials of cardiovascular events and lipoprotein(a) reduction-DOSE finding study (OCEAN(a)-DOSE). Am Heart J. (2022) 251:61–9. doi: 10.1016/j.ahj.2022.05.004
8. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I, Tardif JC, Baum SJ, Steinhagen-Thiessen E, et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. (2020) 382(3):244–55. doi: 10.1056/NEJMoa1905239
9. Paragh G, Zilahi P, Kolozsvári LR, Lőrincz H, Fülöp P, Harangi M. Novel therapeutic approaches for the management of elevated lipoprotein(a): from traditional agents to future treatment options. Life. (2024) 14:374. doi: 10.3390/life14030374
10. De Los Reyes C, Rikhi RR, Doherty S, Hernandez S, Mirzai S, Shapiro MD, et al. Current clinical trials for treating elevated lipoprotein(a). Curr Cardiovasc Risk Rep. (2025) 19:7. doi: 10.1007/s12170-025-00759-8
11. Nicholls SJ, Ni W, Rhodes GM, Nissen SE, Navar AM, Michael LF, et al. Oral muvalaplin for lowering of lipoprotein(a): a randomized clinical trial. JAMA. (2025) 333(3):222–31. doi: 10.1001/jama.2024.24017
12. van den Bosch SE, Boer LM, Revers A, Schrauben EM, Ooij PV, Nederveen AJ, et al. Association between lipoprotein(a) and arterial stiffness in young adults with familial hypercholesterolemia. J Clin Med. (2025) 14(5):1611. doi: 10.3390/jcm14051611
13. Johansen AK, Bogsrud MP, Thoresen M, Christensen JJ, Narverud I, Langslet G, et al. Lipoprotein(a) in children and adolescents with genetically confirmed familial hypercholesterolemia followed up at a specialized lipid clinic. Atheroscler Plus. (2024) 57:13–8. doi: 10.1016/j.athplu.2024.06.002
14. Alonso R, Andres E, Mata N, Fuentes-Jiménez F, Badimón L, López-Miranda J, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol. (2014) 63(19):1982–9. doi: 10.1016/j.jacc.2014.01.063
15. Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. (1994) 93(1):50–5. doi: 10.1172/JCI116983
16. Charakida M, Tousoulis D, Skoumas I, Pitsavos C, Vasiliadou C, Stefanadi E, et al. Inflammatory and thrombotic processes are associated with vascular dysfunction in children with familial hypercholesterolemia. Atherosclerosis. (2009) 204(2):532–7. doi: 10.1016/j.atherosclerosis.2008.09.025
17. Hegele RA, Connelly PW, Cullen-Dean G, Rose V. Elevated plasma lipoprotein(a) associated with abnormal stress thallium scans in children with familial hypercholesterolemia. Am J Cardiol. (1993) 72(5):402–6. doi: 10.1016/0002-9149(93)91130-a
18. de Boer LM, Wiegman A, Kroon J, Tsimikas S, Yeang C, Peletier MC, et al. Lipoprotein(a) and carotid intima-media thickness in children with familial hypercholesterolaemia in The Netherlands: a 20-year follow-up study. Lancet Diabetes Endocrinol. (2023) 11(9):667–74. doi: 10.1016/S2213-8587(23)00156-0
19. Ibrahim S, Reeskamp LF, de Goeij JN, Hovingh GK, Planken RN, Bax WA, et al. Beyond early LDL cholesterol lowering to prevent coronary atherosclerosis in familial hypercholesterolaemia. Eur J Prev Cardiol. (2024) 31:892–900. doi: 10.1093/eurjpc/zwae028
20. Shishikura D, Kataoka Y, Nicholls SJ, Ray KK, Puri R, Kusumoto H, et al. Characterization of lipidic plaque features in association with LDL-C<70 mg/dl and lipoprotein(a) <50 mg/dl. J Clin Lipidol. (2025):in press. doi: 10.1016/j.jacl.2024.12.019
21. Woźniak E, Broncel M, Woźniak A, Satała J, Pawlos A, Bukowska B, et al. Lipoprotein(a) is associated with DNA damage in patients with heterozygous familial hypercholesterolemia. Sci Rep. (2024) 148(1):2564. doi: 10.1038/s41598-024-52571-w
22. Li S, Zhang HW, Guo YL, Wu NQ, Zhu CG, Zhao X, et al. Familial hypercholesterolemia in very young myocardial infarction. Sci Rep. (2018) 8(1):8861. doi: 10.1038/s41598-018-27248-w
23. Schettler VJ, Selke N, Jenke S, Zimmermann T, Schlieper G, Bernhardt W, et al. The German lipoprotein apheresis registry-summary of the eleventh annual report. Atherosclerosis. (2024) 398:118601. doi: 10.1016/j.atherosclerosis.2024.118601
24. Bittner VA, Schwartz GG, Bhatt DL, Chua T, De Silva HA, Diaz R, et al. Alirocumab and cardiovascular outcomes according to sex and lipoprotein(a) after acute coronary syndrome: a report from the ODYSSEY OUTCOMES study. J Clin Lipidol. (2024) 18(4):e548–61. doi: 10.1016/j.jacl.2024.04.122
25. Hegele RA. PCSK9 inhibition in children with familial hypercholesterolaemia. Lancet Diabetes Endocrinol. (2022) 10(10):686–8. doi: 10.1016/S2213-8587(22)00254-6
26. Vuorio A, Raal FJ, Kovanen PT. Extension of heterozygous familial hypercholesterolemia treatment recommendations by including both low-density lipoprotein and lipoprotein(a) burden—a unique opportunity to improve patient prognosis. Eur J Prev Cardiol. (2024):zwae304. doi: 10.1093/eurjpc/zwae304
Keywords: lipoprotein(a), plaques, atherosclerotic cardiovascular disease, low-density lipoprotein cholesterol, familial hypercholesterolemia
Citation: Vuorio A, Raal FJ and Kovanen PT (2025) Elevated levels of lipoprotein(a) and low-density lipoprotein cholesterol in familial hypercholesterolemia patients: is dual primary prevention already in sight?. Front. Cardiovasc. Med. 12:1624049. doi: 10.3389/fcvm.2025.1624049
Received: 6 May 2025; Accepted: 16 June 2025;
Published: 1 July 2025.
Edited by:
Jose Luis Sanchez-Quesada, Sant Pau Institute for Biomedical Research, SpainReviewed by:
Antonio Pérez Pérez, Universitat Autònoma de Barcelona, SpainGemma Rojo Martínez, Regional University Hospital of Malaga, Spain
Copyright: © 2025 Vuorio, Raal and Kovanen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alpo Vuorio, YWxwby52dW9yaW9AZ21haWwuY29t
 Alpo Vuorio
Alpo Vuorio Frederick J. Raal
Frederick J. Raal Petri T. Kovanen4
Petri T. Kovanen4