Abstract
Background:
Cryoballoon (CB) ablation is a well-established treatment for atrial fibrillation (AF). The Arctic Front Advance Pro™ (AFA-Pro) system, in use for over a decade, has demonstrated consistent efficacy and safety. Recently, the POLARx™, a novel CB system, has gained attention due to its advanced design and comparable clinical outcomes.
Methods:
We conducted a systematic review and meta-analysis of studies comparing AFA-Pro and POLARx in patients undergoing CB ablation for AF. Outcomes included procedural parameters (procedure time, ablation time, fluoroscopy time, balloon nadir temperature) and safety endpoints such as phrenic nerve palsy (PNP) and Stroke rates.
Results:
There were no significant differences between POLARx and AFA in acute PVI success (OR = 0.49, P = 0.24), procedure time (MD = 4.40, P = 0.14), ablation time (SMD = 0.12, P = 0.10), fluoroscopy time (MD = 0.34, P = 0.67), freezing time and stroke rates. POLARx showed a significant advantage in balloon nadir temperature (P < 0.00001) and demonstrated longer time to isolation (TTI) in the RSPV (MD = 5.49, P = 0.05) and RIPV (MD = 4.54, P = 0.04), while TTI was similar for the LSPV and LIPV. However, POLARx was associated with a higher risk of PNP (OR = 1.87, P = 0.007).
Conclusion:
POLARx demonstrates lower balloon nadir temperature but is associated with a higher risk of phrenic nerve palsy compared to AFA-Pro. These findings provide important insights into the procedural efficiency and safety profiles of these cryoablation systems.
Systematic Review Registration:
identifier CRD42024588371.
1 Introduction
Atrial Fibrillation is a prevalent cardiac arrhythmia marked by rapid and irregular electrical activity in the atria, leading to an increased risk of thromboembolic events (1). According to the 2023 ACC/AHA/ACCP/HRS guideline for diagnosing and managing atrial fibrillation, catheter ablation of AF is classified as a Class 1 indication for first-line therapy in selected patients. Recent studies have shown that catheter ablation is more effective than drug therapy for rhythm control. Cryoballoon (CB) ablation has become increasingly popular due to its ease of use, safety and effectiveness in achieving pulmonary vein isolation (2). The Arctic Front Advance system by Medtronic (USA) is a widely recognized technology, with the Arctic Front Advance Pro being its latest fourth-generation version (3). However, the field of cryoballoon ablation is advancing with the introduction of the POLARx system by Boston Scientific (USA) (4). Preliminary studies suggest that POLARx may offer lower minimal temperatures and shorter procedure time compared to earlier Arctic Front systems (5).
Several centers have reported their clinical experiences comparing the two balloon systems, focusing on acute procedural efficacy, balloon nadir temperature, incidence of phrenic nerve palsy, and acute recurrence of atrial fibrillation (4, 6). A previous meta-analysis by Assaf et al. (7) indicated that the acute outcomes of the POLARx system are comparable to those of the AFA-Pro, despite POLARx demonstrating lower balloon nadir temperatures. Notably, the POLARx system showed a higher rate of time to isolation recordings in the inferior pulmonary veins.
Recently, additional studies have emerged that compare both balloon systems since the publication of that meta-analysis (8, 9). Therefore, we aim to conduct an updated meta-analysis to compare the efficacy, safety, and procedural outcomes of the AFA-Pro and POLARx cryoballoon systems. Our evaluation will focus on procedural efficacy, ablation time, fluoroscopy time, balloon nadir temperature, incidence of phrenic nerve palsy, acute pulmonary vein isolation success, and stroke. Furthermore, we will assess novel outcome measures, including freezing characteristics and TTI recordings in all four pulmonary veins (LIPV, LSPV, RIPV, and RSPV). As the POLARx cryoablation system continues to gain widespread adoption, this updated meta-analysis aims to provide critical insights into its comparative performance, efficacy and safety with the AFA-Pro system.
2 Methodology
This systematic review and meta-analysis were conducted in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines and followed the methodology framework established by the Cochrane Collaboration. The protocol for this review was prospectively registered with PROSPERO (ID: CRD42024588371).
2.1 Data sources and search strategy
A comprehensive search was conducted across several electronic databases, including PubMed, Google Scholar, ScienceDirect, and Clinicaltrials.gov from December 1, 2019 till August 31, 2024. The search strategy aimed to identify studies comparing the AFA-Pro and POLARx cryoballoon systems in patients undergoing catheter ablation for paroxysmal or persistent atrial fibrillation. Keywords used in the search included “AFA-Pro,” “POLARx,” “cryoballoon,” “atrial fibrillation,” “pulmonary vein isolation,” and related terms. No filters were applied based on language, year of publication, or study design. A full search strategy is provided in Supplementary Table 1.
2.2 Study selection and eligibility criteria
Duplicates were removed using EndNote Reference Manager, and two independent reviewers (M.H.S. and F.T.) screened titles and abstracts against predefined eligibility criteria. Full-text articles that met the inclusion criteria were reviewed in detail, with disagreements resolved by a third reviewer (F.S.). In accordance with the Cochrane Collaboration methodology, the study question was structured using the PICO format:
- •
Population: adults aged 18 years or older with paroxysmal or persistent atrial fibrillation (AF) undergoing pulmonary vein isolation.
- •
Intervention: cryoablation using the POLARx cryoballoon system.
- •
Comparator: cryoablation using the AFA-PRO cryoballoon system; and
- •
Outcomes: procedural efficacy (defined as successful pulmonary vein isolation during the index procedure), acute complications (including phrenic nerve palsy and stroke), and intra-procedural metrics (such as ablation time, fluoroscopy time, and balloon nadir temperature).
Eligible studies had to report procedural efficacy as the acute outcome of the cryoballoon ablation procedure, defined by the achievement of pulmonary vein isolation during the index procedure, along with acute complications, including the incidence of phrenic nerve palsy and stroke occurrence. Most of the included studies in our meta-analysis included stroke as a periprocedural complication. Other key intra-procedural parameters, including ablation and fluoroscopy times, as well as balloon nadir temperature, were also reported. Key exclusion criteria included prior left atrial ablation or surgery, reversible causes of atrial fibrillation, severe mitral regurgitation, reduced left ventricular ejection fraction (<35%), or NYHA class III/IV heart failure. Studies were excluded if they did not compare cryoballoon systems or were not published in English.
2.3 Data extraction and quality assessment
Baseline patient characteristics extracted from each included study comprised age, sex, type of atrial fibrillation (AF), presence of hypertension, diabetes, coronary artery disease, and left atrial size. Outcome data collected at the patient level included acute pulmonary vein isolation (PVI) success, operation time, fluoroscopy duration, ablation time, occurrence of phrenic nerve palsy (PNP), and incidence of stroke or transient ischemic attack (TIA). When reported, the following data was obtained for each pulmonary vein (PV): minimum esophageal temperature, balloon nadir temperature, isolation achieved with the first freeze, time-to-isolation (TTI) recording, and TTI values.
The quality of the included studies in this meta-analysis was assessed using two established tools to evaluate the risk of bias: the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (ROB-2) and the Newcastle-Ottawa Scale (NOS). These tools were applied systematically to assess the methodological quality of both randomized controlled trials (RCTs) and non-randomized studies. Two independent reviewers (A.K. and M.H.S.) conducted the risk-of-bias evaluations, with any discrepancies resolved through discussion. The ROB-2 tool is explicitly used for RCTs, assessing five key domains: bias from the randomization process (e.g., sequence generation and allocation concealment), performance bias (deviations from the intended interventions), attrition bias (missing data), detection bias (measurement of outcomes), and reporting bias (selective outcome reporting), with each domain rated as low, high, or unclear risk (10). In contrast, the NOS was used to evaluate cohort and case-control studies in non-randomized designs, focusing on three areas: selection of study groups (e.g., representativeness and exposure ascertainment), comparability of groups (e.g., controlling for confounders), and outcome assessment (e.g., adequacy of follow-up), with studies awarded points based on these criteria; a score of 7 or higher typically suggests a low risk of bias (11).
2.4 Statistical analysis
For statistical analysis, we used RevMan (Version 5.4.1) to pool data from the selected studies. Using the meta-accelerator tool (12), medians and interquartile ranges were used to estimate means and standard deviations (13). Meta-analyses were performed using the random-effects model according to the DerSimonian and Laird method to account for potential heterogeneity across studies (14). Continuous outcomes were expressed as mean differences (MD) or standardized mean differences (SMD), depending on measurement units. For dichotomous outcomes, we calculated odds ratios (ORs) with 95% confidence intervals (CIs). A p-value of <0.05 was considered statistically significant. The I2 statistical test was used to assess heterogeneity between studies. Values of 0%–25% were considered low heterogeneity, 25%–50% moderate, 50%–75% substantial, and >75% high heterogeneity. In cases of significant heterogeneity, we performed sensitivity analyses to test the robustness of the results (15). Sensitivity analysis was performed using a leave-one-out approach, whereby each study was sequentially excluded to evaluate its impact on the overall results. Publication bias was evaluated through both visual and statistical approaches. We generated funnel plots for each pooled analysis and inspected them for asymmetry suggestive of small-study effects. In addition, we applied Begg's rank-correlation test and Egger's weighted regression test to quantify funnel-plot asymmetry; a two-tailed p-value <0.05 on either test was considered indicative of potential publication bias. All publication-bias analyses were conducted in R (meta and metafor packages, version 4.3.3).
3 Results
A total of 2,408 studies were identified after a step-by-step screening process. Following duplicate study removal, assessment of abstracts based on inclusion and exclusion criteria, and a thorough review of the full texts, 16 studies were selected to be included in this analysis (4–6, 8, 9, 16–26). The PRISMA flowchart illustrates the details in Figure 1.
Figure 1
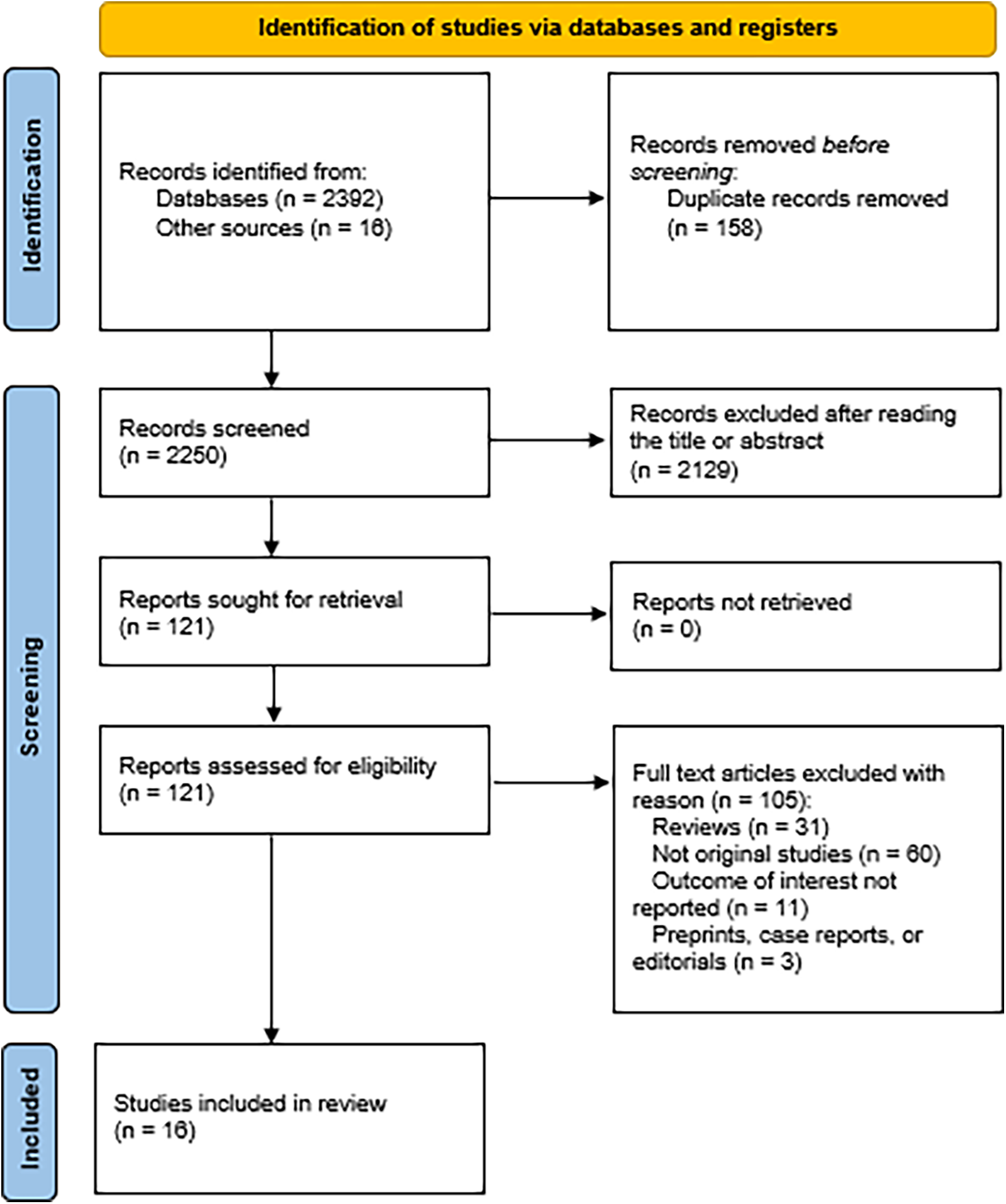
PRISMA flowchart illustrating the screening process.
3.1 Study characteristics
A total of 6,583 individuals participated in the 1 RCT, and 15 cohorts were included in this meta-analysis (4–6, 8, 9, 16–26). Of these, 2,943 belonged to the POLARx group, and 3,640 were in the AFA group. The year of publication ranged from December 1, 2019 to August 1, 2024. The baseline characteristics across studies reveal a predominantly middle-aged to elderly population with a male predominance in both POLARx and AFA Pro groups. Sample sizes ranged widely, from 50 to 2,555 participants, ensuring diverse study designs. The prevalence of paroxysmal atrial fibrillation (PAF) varied between groups, but gender and age were generally well-balanced within individual studies. Baseline and study characteristics are further detailed in Tables 1, 2.
Table 1
| Study name | Sample size (n) | AGE (Mean ± SD) | Percentage of males | Percentage of PAF | Percentage of HTN | Percentage of CAD | Left atrial volume index (Mean ± SD) Or Median (Q1–Q3) | BMI/Obesity | Structural Heart Disease | CHA2DS2VASc | Anticoagulation Regimen (pre-) | Heart Failure (HF) | Left Ventricular Ejection Fraction (LVEF) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Polarx/AFA pro | POLARx | AFA Pro | POLARx | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | POLAR x | AFA Pro | |
| Creta et al. (6) | 80 | 40/40 | 62.8 ± 11.7 | 65.0 ± 12.1 | 57.5 | 55 | 68 | 50 | 42.5 | 35 | N/A | N/A | 38.3 ± 4.1 Mm | 40.2 ± 6.0 mm | N/A | N/A | — | — | N/A | N/A | On uninterrupted (VKA) or (DOAC) | On uninterrupted (VKA) or (DOAC) | N/A | N/A | 80% | 77.5% |
| Knecht et al. (2021) | 80 | 40/40 | 65 ± 11 | 66 ± 9 | 65 | 65 | 58 | 70 | 50 | 50 | 28 | 18 | 36 ± 12 ml/m2 | 41 ± 13 ml/m2 | 28 ± 7 kg/m2 | 27 ± 4 kg/m2 | — | — | N/A | N/A | OAC post-procedure | OAC post-procedure | 13% | 3% | 57 ± 12% | 58 ± 7% |
| Kochi et al. (4) | 70 | 20/50 | 63 ± 59.6 | 61 (53–70.2)a | 60 | 84 | 95 | 94 | 60 | 30 | 5 | 18 | 36 (30–41) ml/m2 | 33 (31–43) ml/m2 | N/A | N/A | — | — | 1.5 (0–2.0)a | 1.0 (0–3.0)a | — | — | N/A | N/A | 60% | 62% |
| Mojica et al. (16) | 60 | 30/30 | 57.47 ± 15.24 | 53.53 ± 16.24 | 66 | 60 | 100 | 100 | 33 | 30 | 3 | 10 | 31.5 ± 8.23 ml/m2 | 31.87 ± 7.31 ml/m2 | N/A | N/A | — | — | 1.27 ± 1.33 | 1.13 ± 1.40 | OAC | OAC | 10% | 3% | N/A | N/A |
| Moser et al. (17) | 100 | 50/50 | 64.5 (57,74)a | 67 (55,75)a | 82 | 62 | 56 | 40 | 60 | 74 | 22 | 28 | N/A | N/A | 27 (24,32)a | 28 (24,31)a | — | — | 2 (1.25,3)a | 3 (2,4)a | oral VKA) or NOACs interrupted 12 h prior procedureru | oral VKA () or NOACs interrupted 12 h prior to procedure | 34% | 28% | 57% | 55% |
| Tilz et al. (5) | 50 | 25/25 | 68 (62,73)a | 69 (59,75)a | 52 | 68 | 48 | 36 | 80 | 72 | 16 | 24 | 25 (25,30)a ml/m2 | 29 (25,35)a ml/m2 | N/A | N/A | NA | NA | LMWH was administered in patients with and INR <2.0. New Oral anticoagulants were re-initiated 6 h post ablation. | LMWH was administered in patients with and INR <2.0. New Oral anticoagulants were re-initiated 6 h post ablation. | Congestive heart failure: 2 (8%) | Congestive heart failure: 4 (16%) | N/A | N/A | ||
| Yap et al. (25) | 110 | 57/53 | 61 (57, 66) | 64 (57, 70) | 57.9 | 67.9 | 75.4 | 75.5 | 31.6 | 58.5 | 14.0 | 9.4 | 41 (36, 44)a LEFT ATRIAL DIAMETER | 41 (37,43)a LEFT ATRIAL DIAMETER | N/A | N/A | CABG: 2 (3.5%) | CABG: 1 (1.9%) | 1 (1,2)a | 2 (0,3)a | DOAC: 57 (100.0%). All patients received oral anticoagulation for at least 4 weeks prior to ablation | DOAC: 48 (90.6%). Vitamin K Antagonist: 3 (5.7%). All patients received oral anticoagulation for at least 4 weeks prior to ablation | N/A | N/A | 63 (60–65)a% | 60 (60–65)a% |
| Bisignani et al. (22) | 80 | 40/40 | 66.6 ± 12.6 | 62.8 ± 11.9 | 55 | 65 | 0 | 0 | 77.5 | 70 | N/A | N/A | 48.1 ± 9.7 | 49 ± 8.3 | N/A | N/A | N/A | N/A | 2.6 ± 1.4 | 2.2 ± 1.5 | N/A | N/A | N/A | N/A | 52.5 ± 6.7% | 55 ± 7.5% |
| Guckel et al. (23) | 687 | 86/601 | 61.3 ± 11.1 | 59.2 ± 20.8 | 69 | 72 | 58 | 58 | 57 | 55 | N/A | N/A | 39.1 ± 6.7 ml/m2 | 39.2 ± 7.3 ml/m2 | 29.6 ± 8.0 kg/m2 | 27.9 ± 4.6 kg/m2 | 13% | 12% | — | — | Phenprocoumon was continued aiming an INR between 2.0 and 3.0. DOAC stopped one hour before ablation | Phenprocoumon was continued aiming an INR between 2.0 and 3.0. DOAC stopped one hour before ablation | N/A | N/A | 52.9 ± 3.7% | 53.5 ± 4.8% |
| Heeger et al. (21) | 205 | 103/102 | 68.7 ± 10.2 | 65.7 ± 12 | 54 | 62 | 51 | 41 | 74 | 70 | 28 | 26 | 32.9 ± 11.4 ml/m2 | 31.7 ± 9.8 ml/m2 | N/A | N/A | N/A | N/A | — | — | Procedure conducted under therapeutic INR values between 2 and 3 for VKAs, morning dose omitted for NOACs | Procedure conducted under therapeutic INR values between 2 and 3 for VKAs, morning dose omitted for NOACs | 11% | 15% | N/A | N/A |
| Honarbakhsh et al. (24) | 1,688 | 844/844 | 61.4 ± 12.8 | 62.3 ± 11.1 | 65.0 | 67.5 | 65.5 | 71.7 | 30.9 | 33.9 | 7.3 | 8.1 | 42.6 ± 4.7 mm | 42.1 ± 6.7 mm | N/A | N/A | 10 | 10.9 | N/A | N/A | Warfarin and DOAC | Warfarin and DOAC | LVEF > 55% 74.8% | LVEF > 55% 77.0% | ||
| Menger et al. (18) | 122 | 61/61 | 63.3 ± 11.8 | 64.8 ± 12.0 | 62.3 | 55.7 | 63.9 | 73.8 | N/A | N/A | N/A | N/A | 3.9 ± 0.7 cm | 3.8 ± 0.6 cm | 29.5 ± 5.0 kg/m2 | 29.2 ± 6.3 kg/m2 | — | — | 2.3 ± 1.6 | 2.5 ± 1.6 | 52 (85.3%) | 58 (95.1%) | N/A | N/A | 53.3 ± 7.8% | 53.4 ± 5.0% |
| Tanese et al. (20) | 267 | 137/130 | 63.3 ± 10.7 | 63.2 ± 11.1 | 59 | 62 | 100 | 100 | 42 | 49 | 7 | 11 | N/A | N/A | N/A | N/A | DCM1% HCM2% VALVULAR 0% | DCM2% HCM1% VALVULAR 1% | — | — | Uninterrupted anticoagulation | Uninterrupted anticoagulation | 3% | 3% | N/A | N/A |
| Knappe et al. (9) | 228 | 114/114 | 67.2 ± 10.1 | 68.0 ± 11.1 | 62.3 | 62.3 | 62.3 | 48.2 | 80.7 | 77.2 | 25.4 | 24.6 | N/A | N/A | 27.2 ± 3.8 kg/m2 | 27.7 ± 4.5 kg/m2 | — | — | 2.7 ± 1.7 | 2.9 ± 1.5 | For patients receiving new oral anticoagulants the morning dose of the procedure was withheld.for patients takingvit k antagonist, procedure was conducted only when inr was between2 and 3 | For patients receiving new oral anticoagulants the morning dose of the procedure was withheld.for patients takingvit k antagonist, procedure was conducted only when inr was between2 and 3 | 9.6% | 10.5% | 58.1 (55.7,63.5)a% | 57.8 (55.1,63.6)a% |
| Reichlin et al. (8) | 201 | 99/102 | 62.2 ± 10.1 | 62.2 ± 9.3 | 65 | 76 | 100 | 100 | 48 | 55 | N/A | N/A | 34 ± 12 ml/m2 | 34 ± 10 ml/m2 | 26.9 ± 4.8 kg/m2 | 26.9 ± 4.8 kg/m2 | — | — | 1.8 ± 1.6 | 1.6 ± 1.4 | Continuous vit k antagonist or DOAC in 78%patients for > _4weeks | Continuous vit k antagonist or DOAC in 84%patients for > _4weeks | N/A | N/A | 60 ± 5% | 61 ± 6% |
| Tachibana et al. (19) | 2,555 | 1,197/1,358 | 68.2 ± 10.8 | 69.4 ± 10.9 | 67.7 | 68.5 | 64.6 | 66.1 | N/A | N/A | N/A | N/A | 38.6 ± 6.9 mm | 39.1 ± 6.3 mm | 23.9 ± 3.9 kg/m2 | 24.3 ± 4.0 kg/m2 | 154 (12.9%) | 168 (12.4%) | 2.2 ± 1.5 | 2.3 ± 1.5 | According to HRS guidelines | According to HRS guidelines | — | — | 60.9 ± 10.7% | 62.6 ± 10.9% |
Baseline clinical and study characteristics of the included study population.
Data is given in median (Q1–Q3).
PAF, paroxysmal atrial firillation; HTN, hypertension; CAD, coronary artery disease.
Table 2
| Study (year) | Country | Study type | Design | Freezing protocol | Bonus freeze | Number of patients in POLARx Group | Number of patients in AFA-Pro group |
|---|---|---|---|---|---|---|---|
| Creta et al. (6) | UK | Prospective | Single center | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s TTI-Guided Strategy: Operator discretion based on TTI, temperature lowering speed and nadir Bonus Freeze or Not: Not | No | 40 | 40 |
| Knecht et al. (2021) | Switzerland | Prospective | Multi-center | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180–240 s TTI-Guided Strategy: TTI-guided Target TTI <60 s Bonus Freeze or Not: No | No | 40 | 40 |
| Kochi et al. (4) | Italy | Prospective | Single-center | Cryoballon Size: 28 mm Duration of Each Freeze: 180 to 300 s Bonus Freeze or Not: No | No | 20 | 50 |
| Mojica et al. (16) | Belgium | Retrospective | Single-center | Cryoballon Size: 28 mm Duration of Each Freeze: Single 180 s TTI-Guided Strategy: TTI-guided (or temperature <−40 °C within 1 min) Bonus Freeze or Not: Bonus freeze delivered if TTI or temperature <−40 °C not met within 1 min | Yes | 30 | 30 |
| Moser et al. (17) | Germany | Prospective | Single-center | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s. If TTI is achieved, continued for 120 s TTI-Guided Strategy: TTI-based ablation protocol Bonus Freeze or Not: No | No | 50 | 50 |
| Tilz et al. (5) | Germany | Prospective | Single-center | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s. If TTI ≥60 s, 180 s plus 180 s bonus TTI-Guided Strategy: TTI-based ablation protocol Bonus Freeze or Not: 180 s bonus freeze if TTI ≥60 s | Yes | 25 | 25 |
| Yap et al. (25) | Croatia, Germany, Netherlands | Prospective | Multi-center | Cryoballon Size: 28 mm Duration of Each Freeze: 180 s if TTI <60 s, otherwise 240 s TTI-Guided Strategy: TTI-guided Bonus Freeze or Not: No bonus freeze | No | 57 | 53 |
| Bisignani et al. (22) | Belgium | Prospective | Single centred | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s. For LAPWI: 120 s. Target Temperature: ≤−40 °C within 60 s TTI-Guided Strategy: Based on temperature attainment (if not ≤−40 °C in 60 s) Bonus Freeze or Not: Extra freeze delivered if the target temperature not attained | NO | 40 | 40 |
| Denise Guckel et al. (2022) | germany | Retrospective | Single centred | Cryoballon Size: 28 mm Duration of Each Freeze: 2 × 180 s per vein Bonus Freeze or Not: No bonus freeze | No | 86 | 601 |
| Heeger et al. (21) | germany | Prospective | Single centred | Cryoballon Size: 28 mm Duration of Each Freeze: 180 s if TTI <60 s. 180 s plus 180 s bonus if TTI ≥60 s TTI-Guided Strategy: TTI-based approach Bonus Freeze or Not: Bonus freeze applied if TTI ≥60 s | Yes | 103 | 102 |
| Honarbakhsh et al. (24) | UK | Prospective | Multi-center | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s. Extended to 240 s at the operator's discretion TTI-Guided Strategy: — Bonus Freeze or Not: Additional consolidating cryoablation at the operator's discretion | Yes | 844 | 844 |
| Menger et al. (18) | Germany | Single centred | Cryoballon Size: 28 mm Duration of Each Freeze: Standard: 180 s. Up to 240 s for late TTI or unsatisfactory temps TTI-Guided Strategy: TTI assessed rigorously Bonus Freeze or Not: No | No | 61 | 61 | |
| Tanese et al. (20) | Belgium, France | Prospective | multi-centred | Cryoballon Size: 28 mm Duration of Each Freeze: Total freezing time set to 180–240 s. Freezing cycle aborted if TTI >60 s TTI-Guided Strategy: TTI-guided. Freeze aborted if TTI >60 s Bonus Freeze or Not: Bonus freeze systematically delivered if no stable PV potential assessed during cryo-application | Yes | 137 | 130 |
| Knappe et al. (9) | Germany | Retrospective | Single center | Duration of Each Freeze:240 s TTI-Guided Strategy: yes Bonus Freeze or Not:No | No | 114 | 114 |
| Reichlin et al. (8) | Switzerland | RCT | Multicentred RCT | Cryoballon Size: 28 mm Duration of Each Freeze: Time-to-effect plus 2 min strategy (if before 60 s temperature was reached then cryoablation was continued for 2 additional minutes) Bonus Freeze or Not: Not | No | 99 | 102 |
| Tachibana et al. (19) | Japan | Prospective | multi-centred | Cryoballon Size: 28 mm Duration of Each Freeze: 180–240 s Bonus Freeze or Not: Not | No | 1,197 | 1,358 |
Study characteristics of the included study population.
PV, pulmonary vein; RCT, randomized controlled trial.
3.2 Meta-analysis of the outcomes
3.2.1 Acute PVI success
Eleven of the Sixteen reported on acute PVI, with a total of 1,640 participants (548 in the POLARx group and 1,092 in the AFA group). Our analysis showed that both techniques were comparable in terms of acute PVI (OR = 0.49, 95% CI: 0.15–1.60, P = 0.24, I2 = 0%. Z = 1.17; Figure 2).
Figure 2
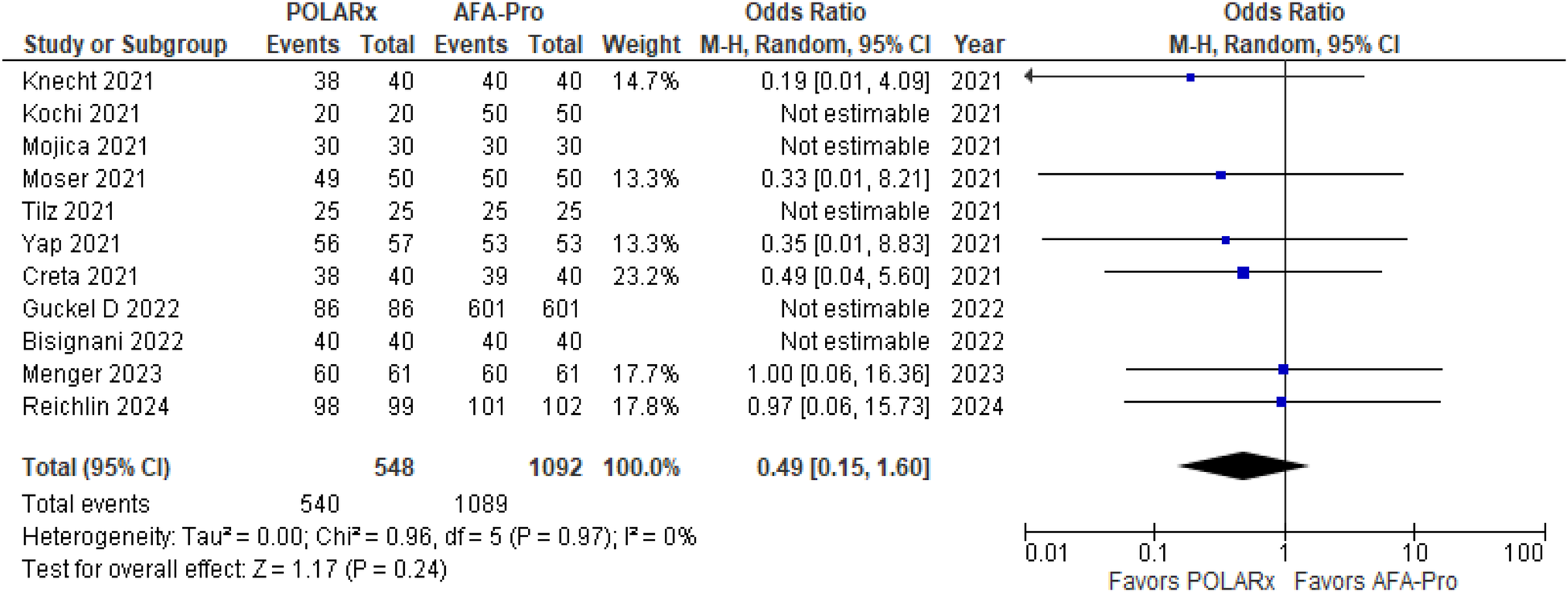
Forest plot illustrating the acute PVI success.
3.2.2 Procedure time
Thirteen studies reported procedure time, with 3,678 participants (1,571 in the POLARx group and 2,107 in the AFA group). Our analysis indicated that POLARx was comparable to ArcticFront in terms of procedure time (MD = 4.40, 95% CI: −1.48 to 10.27, P = 0.14, I2 = 93%, Z = 1.47; Figure 3).
Figure 3
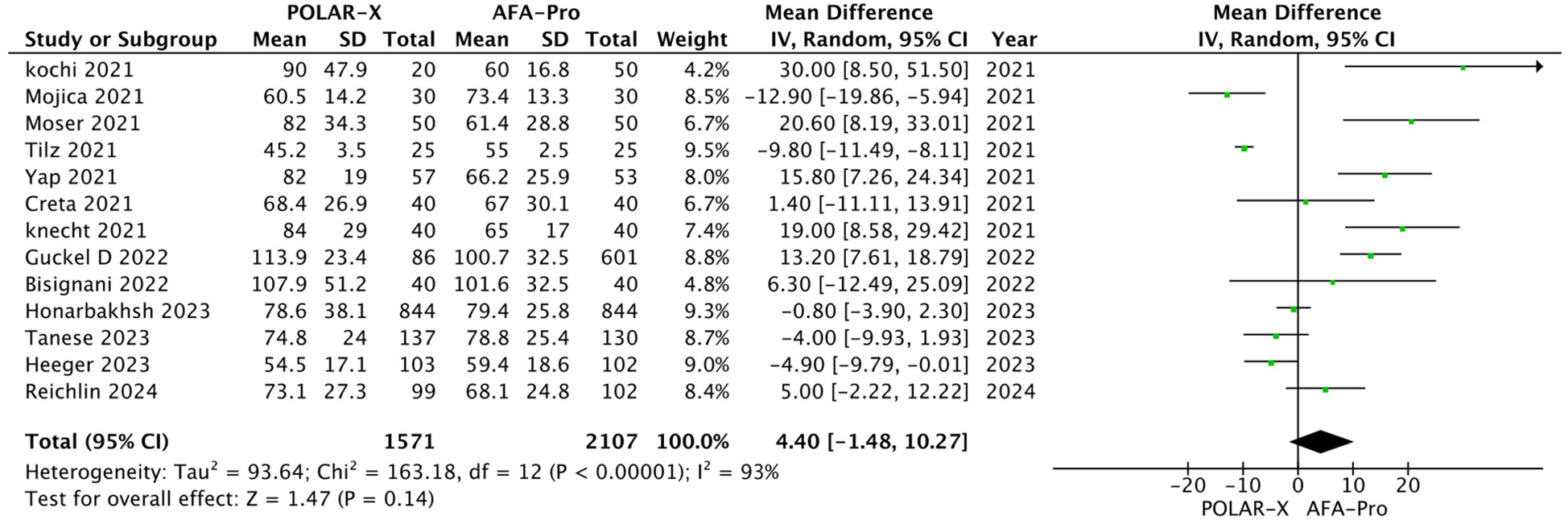
Forest plot illustrating the procedure time.
3.2.3 Ablation time
Six studies evaluated ablation time, and the results showed that there was no notable difference between the two techniques (SMD = 0.12, 95% CI: −0.02 to 0.26, P = 0.10, Z = 1.64; Figure 4), with negligible heterogeneity (I2 = 0%).
Figure 4
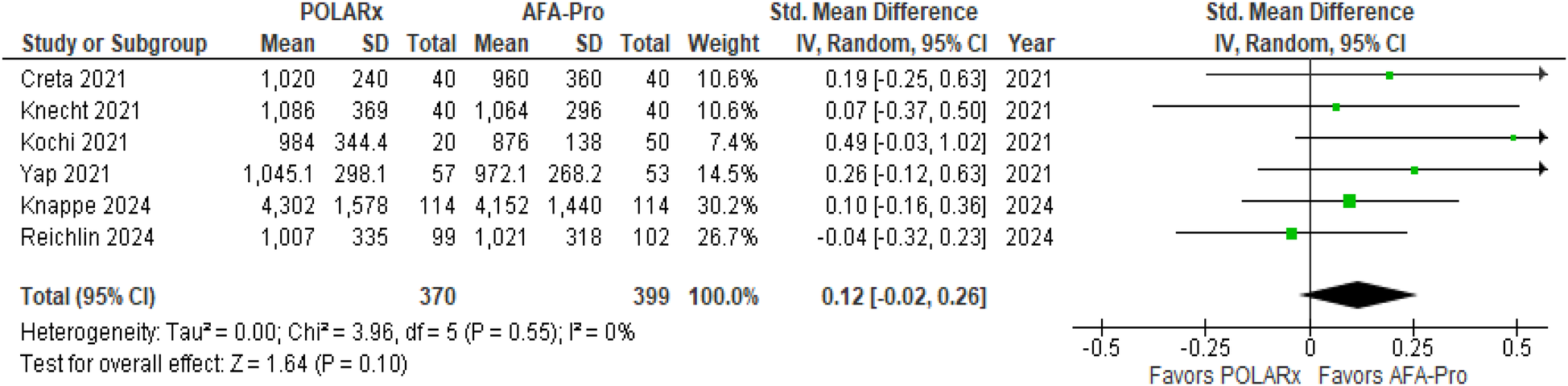
Forest plot illustrating the ablation time.
3.2.4 Fluoroscopy time
Fluoroscopy time was reported in 15 of the 16 studies, involving a total of 4,028 participants (1,746 in the POLARx group and 2,282 in the AFA group). Our analysis revealed that both techniques were comparable in terms of fluoroscopy time (MD = 0.34, 95% CI: −1.22 to 1.91, P = 0.67, I2 = 90%, Z = 0.43; Figure 5).
Figure 5
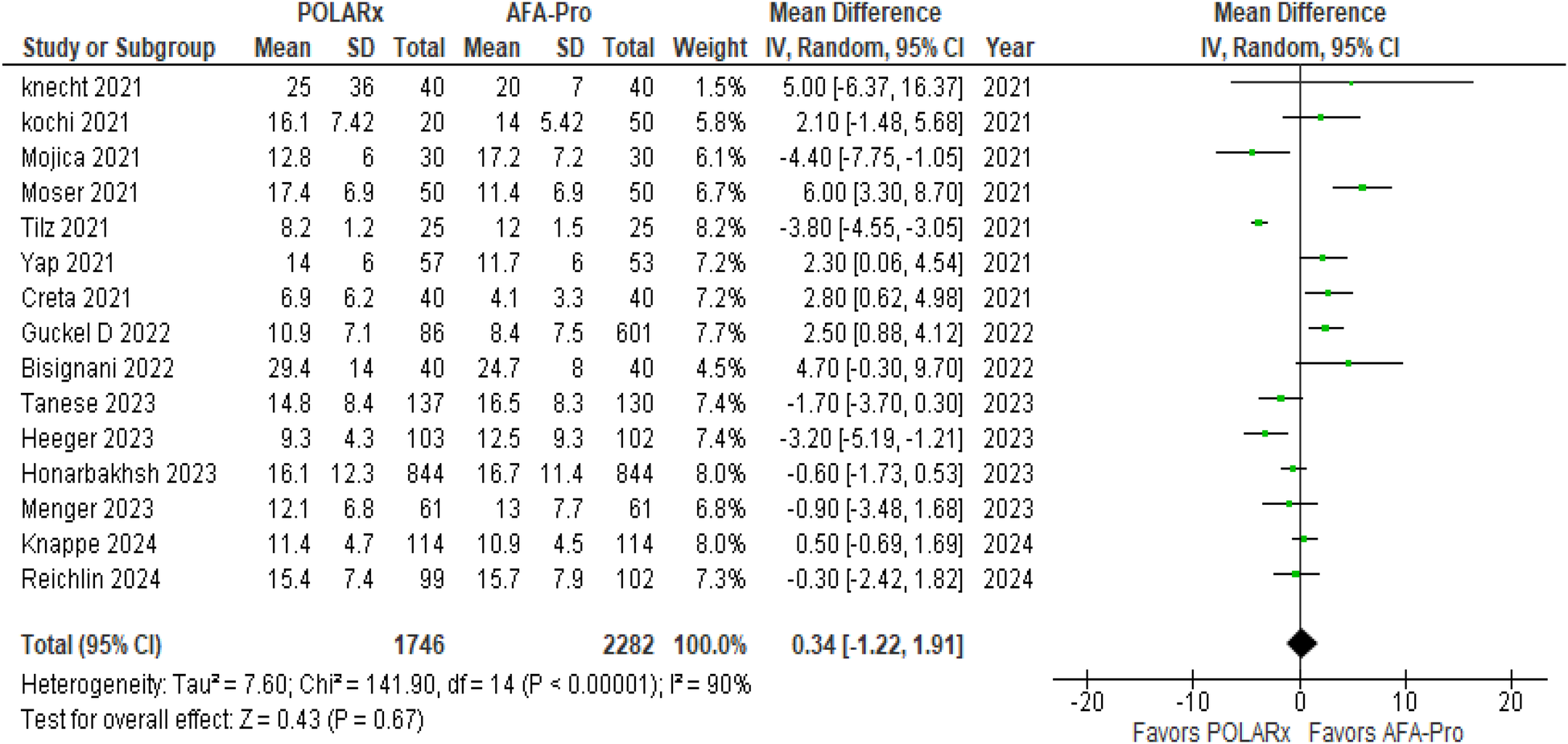
Forest plot illustrating the fluoroscopy time.
3.2.5 Balloon nadir temperature
Balloon nadir Temperature was reported in 12 of the 16 studies. Our analysis showed a significant difference favoring POLARx, with marked improvements in balloon nadir temperature (P < 0.00001). Specifically, for the left superior pulmonary vein (LSPV), the MD was −10.00 (95% CI: −11.33 to −8.66, P < 0.00001, I2 = 89%, Z = 14.68; Figure 6). For the left inferior pulmonary vein (LIPV), the MD was −9.78 (95% CI: −11.42 to −8.15, P < 0.00001, I2 = 93%, Z = 11.75; Figure 6). For the right superior pulmonary vein (RSPV), the MD was −7.72 (95% CI: −9.33 to −6.10, P < 0.00001, I2 = 89%; Z = 9.35 Figure 6), and for the right inferior pulmonary vein (RIPV), the MD was −9.26 (95% CI: −10.89 to −7.63, P < 0. 00001, I2 = 90%, Z = 11.14; Figure 6).
Figure 6
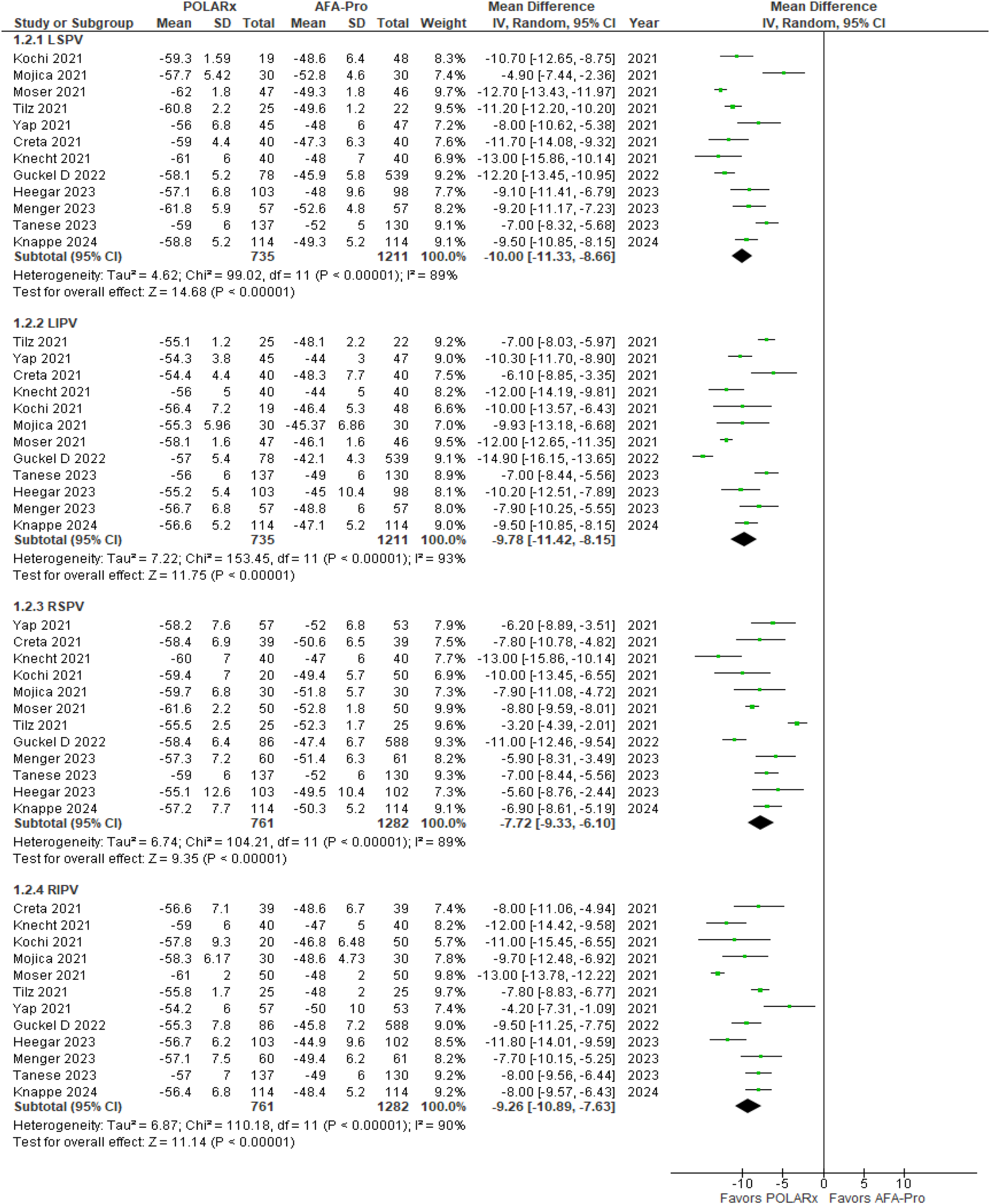
Forest plot illustrating the balloon nadir temperature.
3.2.6 Freezing time
Freezing time data were reported in 5 of the 16 studies. Our analysis showed that both techniques were comparable in terms of freezing time. Specifically, for the LSPV, the freezing time was similar (MD = 7.90, 95% CI: −4.36 to 20.16, P = 0.21, I2 = 22%, Z = 1.26). For the LIPV, there was no notable difference (P = 0.73), indicating similar efficacy (MD = −3.97, 95% CI: −26.66 to 18.72, I2 = 59%, Z = 0.34). For the RSPV, the analysis showed no clear advantage for POLARx (MD = −5.63, 95% CI: −44.96 to 33.70, P = 0.78, I2 = 91%, Z = 0.28), and for the RIPV, there was no marked difference (MD = 1.26, 95% CI: −25.42 to 27.93, P = 0.93, I2 = 73%, Z = 0.09; Supplementary Figure 2). Initial Cochran's Q test indicated heterogeneity (I2 = 59%) for LIPV freezing time, but sensitivity analysis reduced it to 21% as shown in Table 3.
Table 3
| Outcome | Study excluded | I 2 value before S.A/% | I 2 value after S.A/% | Z value (P-Value) before S.A/% | Z value (P-Value) after S.A/% | Mean difference (MD), (95% CI) after SA |
|---|---|---|---|---|---|---|
| Ballon Nadir Temperature LSPV | Tanese et al. (20) | 89 | 84 | 14.68 (<0.00001) | 16.85 (<0.00001) | −10.34 [−11.54, −9.14] |
| Ballon Nadir Temperature LIPV | Tilz et al. (5) | 93 | 90 | 11.75 (<0.00001) | 12.67 (<0.00001) | −10.08 [−11.64, −8.52] |
| Ballon Nadir temperature RSPV | Tilz et al. (5) | 89 | 75 | 9.35 (<0.00001) | 13.27 (<0.00001) | −8.21 [−9.42, −7.00] |
| Ballon Nadir Temperature RIPV | Moser et al. (17) | 90 | 67 | 11.14 (<0.00001) | 15.74 (<0.00001) | −8.82 [−9.92, −7.72] |
| Freezing Time LSPV | Moser et al. (17) | 22 | 0 | 1.26 (0.21) | 0.01 (0.99) | −0.08 [−15.09, 14.94] |
| Freezing Time RSPV | Menger et al. (18) | 91 | 71 | 0.28 (0.78) | 1.17 (0.24) | 14.48 [−9.73, 38.69] |
| Freezing Time RIPV | Tanese et al. (20) | 73 | 0 | 0.09 (0.93) | 1.61 (0.11) | 15.14 [−3.30, 33.58] |
| Freezing Time LIPV | Knappe et al. (9) | 59 | 21 | 0.34 (0.73) | 0.4 (0.69) | 3.61 [−13.89, 21.10] |
| TTI recording LSPV | Tilz et al. (5) | 87 | 50 | 0.11 (0.91) | 0.64 (0.52) | 1.41 [−2.90, 5.73] |
| TTI recording LIPV | Knappe et al. (9) | 70 | 38 | 1.77 (0.08) | 4.02 (<0.0001) | 6.80 [3.48, 10.12] |
| TTI recording RSPV | Tilz et al. (5) | 78 | 62 | 2 (0.05) | 1.49 (0.14) | 4.00 [−1.25, 9.25] |
| TTI recording RIPV | Tilz et al. (5) | 65 | 0 | 2.05 (0.04) | 2.28 (0.02) | 3.49 [0.49, 6.50] |
| Procedure Time | Tilz et al. (5) | 93 | 87 | 1.47 (0.14) | 1.93 (0.05) | 5.76 [−0.08, 11.59] |
| Fluoroscopy Time | Tilz et al. (5) | 90 | 80 | 0.43 (0.67) | 0.98 (0.33) | 0.65 [−0.65, 1.96] |
Tabulated results of sensitivity analyses of each heterogeneous outcome.
LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; TTI, time to isolation; S.A, sensitivity analysis; I2, I-squared (heterogeneity measure); Z, Z-score; P-value, probability value; MD, mean difference; CI, confidence interval.
3.2.7 Time to isolation (TTI)
Nine studies compared TTI, with a total of 1,036 participants (509 in the POLARx group and 527 in the AFA group). The analysis showed that both techniques were comparable in terms of TTI. For the LSPV, the MD was −0.37 (95% CI: −6.67 to 5.93, P = 0.91, I2 = 87%, Z = 0.11; Supplementary Figure 3). For the LIPV, TTI was similar between the techniques (MD = 4.21, 95% CI: −0.46 to 8.88, P = 0.08, I2 = 70%, Z = 1.77; Supplementary Figure 3). For the RSPV, there was a clear advantage for AFA-PRO (MD = 5.49, 95% CI: 0.11–10.88, P = 0.05, I2 = 78%, Z = 2.00; Supplementary Figure 3), with a similar benefit seen for the RIPV (MD = 4.54, 95% CI: 0.21–8.87, P = 0.04, I2 = 65%, Z = 2.05; Supplementary Figure 3).
3.2.8 Phrenic nerve palsy
Phrenic nerve palsy was reported in all the studies, involving 6,583 participants (2,943 in the POLARx group and 3,640 in the AFA group). Our analysis showed that POLARx was associated with a higher risk of phrenic nerve palsy (OR = 1.87, 95% CI: 1.18–2.94, P = 0.007, I2 = 0%, Z = 2.69; Figure 7).
Figure 7
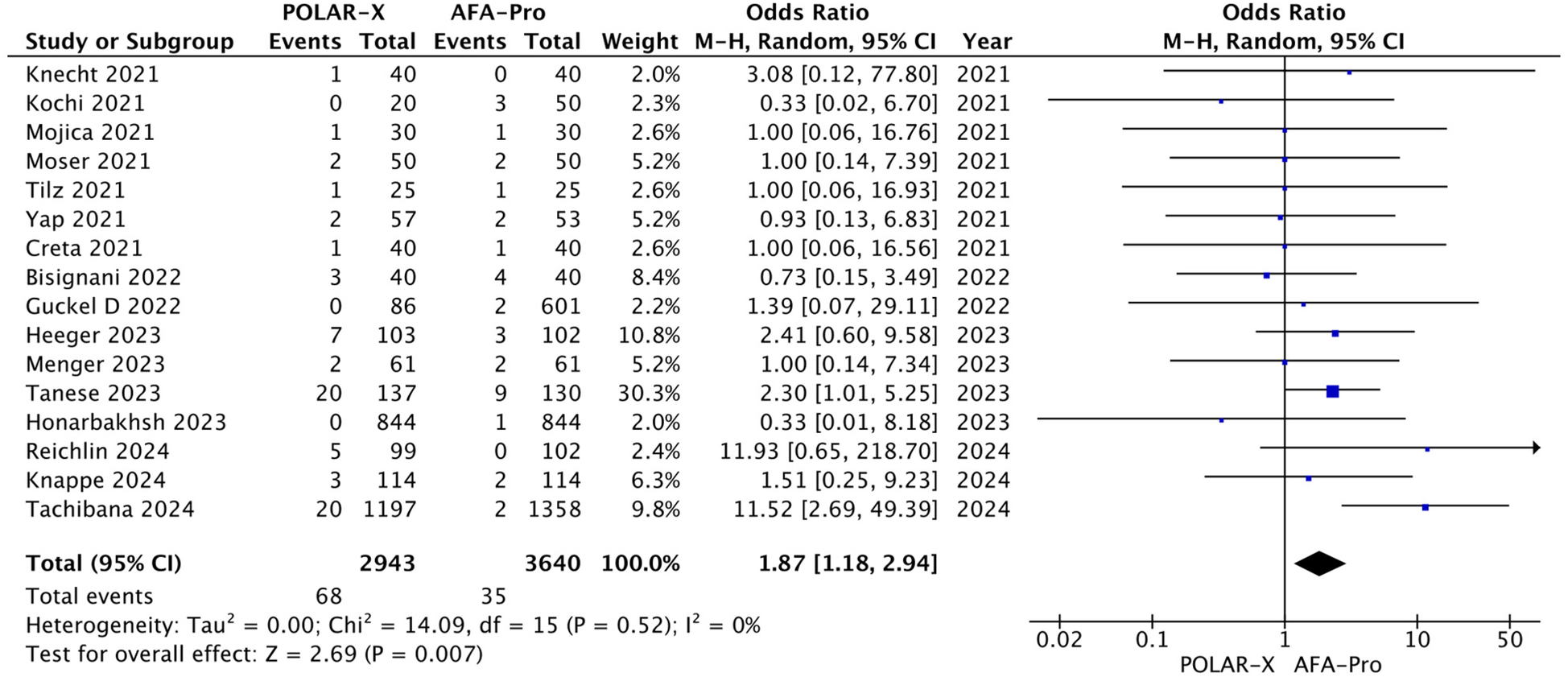
Forest plot illustrating the incidence of phrenic nerve palsy.
3.2.9 Stroke
Nine studies reported on stroke outcomes, and our analysis found that both techniques were comparable in terms of stroke rates (OR = 3.58, 95% CI: 0.58–22.26; P = 0.17, Z = 1.49; Supplementary Figure 4). The heterogeneity was minimal (I2 = 0%).
3.3 Risk of bias assessment of included studies
Specifically, three studies (Mojica et al., Guckel et al., Honarbakhsh et al., and Tachibana et al.) achieved the highest score of 8, reflecting well-defined cohorts, sufficient follow-up duration, and robust outcome assessment as evaluated by NOS. A total of 10 studies were rated as high quality (score 7–8), while 4 studies received scores of 5–6, indicating moderate quality. Areas commonly lacking across studies included documentation of adequate follow-up duration and loss to follow-up information. The COMPARE CRYO Randomized trial showed an overall low risk of bias. Randomization and outcome assessment were appropriately blinded, with objective data collection via implantable cardiac monitors. Minor concerns related to lack of clinician blinding were unlikely to impact outcomes (8). Elaborate findings of each study are further presented in Supplementary Table 2, Figures 1A, 1B.
3.4 Sensitivity analysis
The sensitivity analysis showed a significant reduction in heterogeneity for certain outcomes after excluding specific studies. For instance, Freezing Time for RIPV exclusion of Tanese et al., 2023 decreased from 73% to 0%, and TTI Recording for RIPV removal of Tilz et al., 2021 dropped from 65% to 0%. Freezing Time for LIPV (exclusion of Knappe et al., 2024) also reduced from 59% to 21%, as illustrated in Supplementary Figures 5 and 6. However, several outcomes showed no significant reduction in heterogeneity after excluding a single study, including balloon nadir temperature for LSPV, balloon nadir temperature for LIPV, procedure time, fluoroscopy time, balloon nadir temperature for RSPV, and TTI recording for LSPV. Importantly, many outcomes also showed an increase in Z-values after exclusion, reflecting a stronger overall effect. For instance, the Z-value for Balloon Nadir Temperature (LSPV) rose from 14.68 to 16.85 after excluding Tanese et al., and Procedure Time showed improved effect strength (Z = 1.93 vs. 1.47) after removing Tilz et al. These findings suggest that some heterogeneity was likely due to differences in freezing protocols, balloon systems, or study-level variability rather than random error alone. The summary of the sensitivity analysis results is detailed in Table 3.
3.5 Publication bias
We evaluated potential publication bias across multiple outcomes using both Egger's regression test and Begg's rank correlation test. No significant funnel plot asymmetry was detected for most outcomes. Phrenic Nerve Palsy (Egger's p = 0.1477; Begg's p = 0.2496), Acute PVI Success (Egger's p = 0.5002; Begg's p = 0.4951), and all balloon nadir temperature outcomes including LIPV (Egger's p = 0.4734), RSPV (p = 0.9920), and RIPV (p = 0.2241) showed no evidence of asymmetry. However, Procedure Time demonstrated significant funnel plot asymmetry (Egger's t = 3.2415, df = 10, p = 0.0088), with a large negative limit estimate (b = −12.0260; 95% CI: −18.3466 to −5.7054), suggesting potential small-study effects. Fluoroscopy Time also showed a significant result (Egger's p = 0.0322), with a corresponding limit estimate of b = −3.8988 (95% CI: −6.8080 to −0.9896). Although Begg's tests for these outcomes were not statistically significant (Procedure Time p = 0.4590; Fluoroscopy p = 0.3880), the Egger's results may indicate possible publication bias or small-study effects, particularly for Procedure Time. Balloon Temp in LSPV showed marginal asymmetry (Egger's p = 0.0552), though it did not reach conventional significance. A summary of all results is presented in Supplementary Table 3 and Figures S7A–S7E.
4 Discussion
This meta-analysis examined 16 studies with 6,583 participants (4–6, 8, 9, 16–26) to assess the efficacy and safety of two cryoballoon ablation systems. Overall, the groups did not differ significantly regarding acute PVI, procedure time, ablation time, fluoroscopy time, or freezing time. Notably, POLARx demonstrated much lower balloon nadir temperature in all pulmonary veins, with the LSPV showing the most significant difference. Safety profiles appeared similar with the exception of higher PNP with PolarX.
Earlier meta-analyses in 2021 and 2022 gave helpful information about the relative efficacy of these systems. The 2021 meta-analysis, which included four trials and 310 patients, found no significant differences in primary procedural outcomes, indicating procedural equivalency (28). The 2022 meta-analysis, spanning eight investigations and 1,146 patients, corroborated these findings, adding that POLARx regularly had lower nadir temperatures, notably in RIPV, and had a prolonged TTI in LIPV, indicating potentially better isolation capabilities (7). However, those investigations were constrained in sample sizes and focused on select pulmonary veins, overlooking comprehensive temperature and TTI comparisons. They also lacked detailed analysis of phrenic nerve palsy and device-specific design factors. This updated meta-analysis addresses these gaps by including a larger dataset, evaluating all pulmonary veins, and offering deeper insight into safety and procedural nuances.
The AFA-Pro cryoballoon is frequently used to obtain PVI because it produces effective and homogenous lesions with low arrhythmogenic potential. POLARx has similar qualities, such as a double-layer balloon design and nitrous oxide cooling. Still, it also has a unique design that maintains inner balloon pressure while freezing, making it more compliant (4). This design may allow for more precise lesion development, but also adds a higher learning curve due to its innovative cryoconsole and steerable sheath. This intricacy may explain why certain studies, such as Reichlein et al.'s, reported higher procedure times with POLARx (8). However, we did not find a significant difference in procedure or ablation times between the two systems, aligning with previous meta-analyses.
A notable finding in this study was that POLARx resulted in much lower balloon nadir temperatures, consistent with previous research (5, 18, 24, 29). It is crucial to note that the inner balloon temperature does not always correspond to the surface temperature, as thermocouple position, energy transfer efficiency, balloon depth within the pulmonary vein, and tissue contact area can all influence temperature readings (6, 27). For POLARx, nadir temperatures as low as −53.5 °C (24) or even −56 °C (30) have been linked to acute and sustained PVI success. These findings suggest that the predictors of lesion durability may need to be adjusted for POLARx's lower nadir temperatures and faster cooling profiles.
TTI serves as an essential marker of procedural efficiency in PVI. Previous studies suggested that POLARx had prolonged TTI for the LIPV due to catheter positioning and balloon stability. Our analysis comprehensively evaluated TTI across all pulmonary veins, finding no significant differences in TTI for the LSPV or LIPV. However, there was a notable increase in TTI for the RSPV and RIPV with POLARx. Variations in TTI may be attributed to anatomical differences, as left-sided pulmonary veins tend to have more uniform structures, facilitating energy delivery. In contrast, right-sided veins have variable orientations that could challenge energy delivery and prolong TTI (31). The POLARx system's design, balloon compliance, and cooling mechanisms might improve lesion formation in specific veins. However, operator-dependent factors like optimal contact force and positioning and the proximity of the phrenic nerve to the right-sided veins could further complicate the procedure and affect TTI outcomes. Despite these factors, freezing times were similar between both systems, suggesting comparable procedural cooling efficacy.
Our meta-analysis revealed a increased risk of PNP linked with the POLARx system. This could be explained by POLARx's lower temperatures, which could pose a greater risk to surrounding structures. The POLARx system's DMS™ sensor detects partial PNP with more sensitivity than the AFA-Pro's tactile feedback, perhaps leading to a higher detection rate of transitory PNP (20). The POLARx balloon's compliant design may also allow for deeper advancement into the pulmonary veins, potentially bringing it closer to the phrenic nerve especially in the right-sided veins, where the nerve's anatomical course is more variable (32). Although the majority of phrenic nerve palsy cases are temporary and resolve within weeks to months, persistent dysfunction though relatively uncommon, can result in unilateral diaphragmatic paralysis. This condition may lead to symptoms such as shortness of breath, orthopnea, and decreased exercise tolerance, particularly in individuals with pre-existing pulmonary or cardiac conditions (33). Techniques such as continuous diaphragmatic pacing and compound motor action potential (CMAP) monitoring have been recommended to allow early detection of phrenic nerve impairment and prevent permanent injury (34). To mitigate this risk, clinicians should utilize real-time imaging to ensure optimal balloon positioning, apply electrophysiological mapping to assess phrenic nerve proximity, and consider next-generation devices such as the POLARx Fit, which offers more precise balloon sizing. Moreover, refining freezing techniques and enhancing operator familiarity with the POLARx system may further reduce the likelihood of phrenic nerve injury.
In addition to safety outcomes, long-term efficacy remains a critical aspect of cryoballoon ablation performance. In a study by Tanese et al., atrial fibrillation (AF) recurrence rates during the 3-month blanking period were similar between the AFA-Pro and POLARx systems. Over a mean follow-up of approximately 15–16 months, a slightly higher number of patients in the POLARx group experienced AF recurrence beyond the blanking period; however, by one year post-procedure, the proportion of patients without recurrent AF episodes was comparable across both groups. Interestingly, a greater number of patients treated with POLARx underwent repeat ablation procedures during follow-up, though this did not appear to reflect a higher overall recurrence burden. All repeat procedures were performed using radiofrequency energy rather than cryoballoon technology (20). These findings suggest that both systems provide similar long-term rhythm control, while the increased rate of reintervention in the POLARx group may reflect factors such as the learning curve, patient anatomy, or operator preferences, rather than inherent differences in device efficacy.
4.1 Clinical implications
Our meta-analysis findings provide clinicians with essential recommendations. Because POLARx has lower balloon nadir temperatures, it may be particularly helpful for people who have repeated AF following previous ablation. This could lead to more permanent lesions and a decreased risk of arrhythmia recurrence. The higher risk of phrenic nerve palsy associated with POLARx may call for more cautious treatment in patients with anatomical issues, such as fluctuating right-sided pulmonary vein placements, particularly when treating veins close to the phrenic nerve. POLARx system's lower nadir temperatures and greater balloon compliance may offer advantages in patients with complex or variable pulmonary vein anatomy, potentially improving lesion formation and procedural success. Conversely, the AFA-Pro system may be more suitable for patients with elevated risk of PNP, such as those with prior thoracic surgeries, baseline diaphragmatic weakness, or significant pulmonary disease, given its lower observed PNP incidence. Using AFA-Pro or changing the ablation technique can help lower the chance of issues in certain situations. POLARx may reduce the need for follow-up treatments by improving lesion lifespan in younger individuals or those with a higher arrhythmia load.
Furthermore, for patients with left atrial enlargement or fibrosis, where long-lasting PVI is more problematic, POLARx's superior tissue cooling may aid in forming deeper and more consistent lesions, enhancing long-term outcomes. POLARx may improve procedural results in facilities with experienced operators, particularly for high-risk arrhythmia patients, as long as phrenic nerve injury is adequately controlled. Finally, the cryoballoon system should be tailored to each patient's anatomical characteristics, arrhythmia burden, and clinical experience to provide the highest level of safety and efficacy.
4.2 Limitations
Even though our meta-analysis provides insightful information, there are a few significant limitations to be aware of. The pooled results may have been limited in generalizability due to heterogeneity in study design, sample size, operator experience, and procedural protocols. The variability in freezing techniques (e.g., freeze duration, use of bonus applications), balloon generation used, and procedural workflows (e.g., time-to-isolation strategy, balloon positioning) may have contributed to statistical heterogeneity observed in certain outcomes. Because no single study had a substantial impact on effect sizes, a leave-one-out sensitivity analysis validated the reliability of the results. Moreover, in our assessment of Publication bias assessment, Egger's test revealed possible publication bias for procedure time and fluoroscopy time. Although Begg's test did not confirm this, the small number of included studies limits the power of these assessments. Therefore, findings related to these parameters should be interpreted with caution. Detailed comparisons were further hampered by inconsistent reporting of essential characteristics, such as freezing time and nadir temperature. Future relevance may also be impacted by the POLARx system's challenging learning curve and quick technical developments, such as the POLARx Fit.
5 Conclusion
This meta-analysis concludes that the POLARx and AFA-Pro cryoballoon systems show comparable efficacy and safety profiles in pulmonary vein isolation procedures. Nonetheless, POLARx is linked to lower balloon nadir temperatures and a slightly increased incidence of phrenic nerve palsy, especially in veins on the right side. Although both systems work well, doctors should choose the right one based on patient characteristics, operator experience, and anatomical considerations. Future research should concentrate on large-scale, multicenter randomized studies to validate these findings, evaluate long-term outcomes, and investigate newer device iterations for enhanced procedural efficiency and safety.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MT: Writing – original draft, Investigation, Methodology. MS: Writing – original draft, Conceptualization. RH: Writing – original draft, Methodology, Formal analysis. FS: Conceptualization, Investigation, Methodology, Writing – original draft. AmS: Formal analysis, Investigation, Writing – original draft. AyS: Supervision, Validation, Writing – review & editing. FR: Formal analysis, Funding acquisition, Writing – review & editing. MZ: Formal analysis; Methodology; Data curation. KH: Methodology, Writing – original draft. AK: Methodology, Project administration, Supervision, Writing – original draft. AA: Investigation, Methodology, Project administration, Writing – original draft. SW: Formal analysis, Investigation, Resources, Writing – original draft. HN: Formal analysis, Investigation, Methodology, Writing – original draft. BS: Conceptualization, Methodology, Writing – original draft. ML: Formal analysis, Investigation, Methodology, Writing – original draft. RA: Investigation, Writing – original draft. MS: Supervision, Validation, Writing – review & editing. MC: Supervision, Writing – original draft, Writing – review & editing. MH: Methodology, Writing – review & editing. TA: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. OpenAI's ChatGPT was used solely to improve the readability and language of the manuscript, including grammar correction, sentence clarity, and structure. All scientific input, conclusions, and interpretations are the original work of the authors. The author(s) verify and take full responsibility of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1625399/full#supplementary-material
References
1.
Zulkifly H Lip GYH Lane DA . Epidemiology of atrial fibrillation. Int J Clin Pract. (2018) 72:e13070. 10.1111/IJCP.13070
2.
Anic A Lever N Martin A Breskovic T Sulkin MS Duffy E et al Acute safety, efficacy, and advantages of a novel cryoballoon ablation system for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: initial clinical experience. Europace. (2021) 23:1237–43. 10.1093/EUROPACE/EUAB018
3.
Moltrasio M Sicuso R Fassini GM Riva SI Tundo F Dello Russo A et al Acute outcome after a single cryoballoon ablation: comparison between Arctic front advance and Arctic front advance PRO. Pacing Clin Electrophysiol. (2019) 42:890–6. 10.1111/PACE.13718
4.
Kochi AN Moltrasio M Tundo F Riva S Ascione C Dessanai MA et al Cryoballoon atrial fibrillation ablation: single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform. J Cardiovasc Electrophysiol. (2021) 32:588–94. 10.1111/JCE.14930
5.
Tilz RR Meyer-Saraei R Eitel C Fink T Sciacca V Lopez LD et al Novel cryoballoon ablation system for single shot pulmonary vein isolation—the prospective ICE-AGE-X study. Circ J. (2021) 85:1296–304. 10.1253/CIRCJ.CJ-21-0094
6.
Creta A Kanthasamy V Schilling RJ Rosengarten J Khan F Honarbakhsh S et al First experience of POLARxTM versus Arctic front AdvanceTM: an early technology comparison. J Cardiovasc Electrophysiol. (2021) 32:925–30. 10.1111/JCE.14951
7.
Assaf A Bhagwandien RE Szili-Torok T Yap SC . Comparison of the acute outcome of two cryoballoon technologies for pulmonary vein isolation: an updated systematic review and meta-analysis. IJC Heart Vasculature. (2022) 42:101115. 10.1016/j.ijcha.2022.101115
8.
Reichlin T Kueffer T Knecht S Madaffari A Badertscher P Maurhofer J et al Polarx vs Arctic front for cryoballoon ablation of paroxysmal AF: the randomized COMPARE CRYO study. JACC Clin Electrophysiol. (2024) 10:1367–76. 10.1016/j.jacep.2024.03.021
9.
Knappe V Lahrmann C Funken M Zietzer A Gestrich C Nickenig G et al Comparison of Arctic front advance pro and POLARx cryoballoons for ablation therapy of atrial fibrillation: an intraprocedural analysis. Clin Res Cardiol. (2024) 114:83–92. 10.1007/S00392-024-02398-2
10.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. 10.1136/BMJ.L4898
11.
Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (n.d.). Available online at:https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp(Accessed June 22, 2024).
12.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. 10.1186/1471-2288-14-135
13.
Abbas A Hefnawy MT Negida A . Meta-analysis accelerator: a comprehensive tool for statistical data conversion in systematic reviews with meta-analysis. BMC Med Res Methodol. (2024) 24:243. 10.1186/S12874-024-02356-6
14.
Jackson D Bowden J Baker R . How does the DerSimonian and laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts?J Stat Plan Inference. (2010) 140:961–70. 10.1016/J.JSPI.2009.09.017
15.
Higgins JPT Thompson SG Deeks JJ Altman DG . Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. 10.1136/BMJ.327.7414.557
16.
Mojica J Lipartiti F Al Housari M Bala G Kazawa S Miraglia V et al Procedural safety and efficacy for pulmonary vein isolation with the novel PolarxTM cryoablation system: a propensity score matched comparison with the Arctic FrontTM cryoballoon in the setting of paroxysmal atrial fibrillation. J Atr Fibrillation. (2021) 14:20200455. 10.4022/JAFIB.20200455
17.
Moser F Rottner L Moser J Schleberger R Lemoine M Münkler P et al The established and the challenger: a direct comparison of current cryoballoon technologies for pulmonary vein isolation. J Cardiovasc Electrophysiol. (2022) 33:48–54. 10.1111/JCE.15288
18.
Menger V Frick M Sharif-Yakan A Emrani M Zink MD Napp A et al Procedural performance between two cryoballoon systems for ablation of atrial fibrillation depends on pulmonary vein anatomy. J Arrhythm. (2023) 39:341–51. 10.1002/JOA3.12842
19.
Tachibana S Miyazaki S Nitta J Shirai Y Nagata Y Sagawa Y et al Incidence of phrenic nerve injury during pulmonary vein isolation using different cryoballoons: data from a large prospective ablation registry. Europace. (2024) 26:euae092. 10.1093/EUROPACE/EUAE092
20.
Tanese N Almorad A Pannone L Defaye P Jacob S Ben KM et al Outcomes after cryoballoon ablation of paroxysmal atrial fibrillation with the PolarX or the Arctic front advance pro: a prospective multicentre experience. Europace. (2023) 25:873–9. 10.1093/EUROPACE/EUAD005
21.
Heeger CH Popescu SS Inderhees T Nussbickel N Eitel C Kirstein B et al Novel or established cryoballoon ablation system for pulmonary vein isolation: the prospective ICE-AGE-1 study. Europace. (2023) 25:euad248. 10.1093/EUROPACE/EUAD248
22.
Bisignani A Pannone L Miraglia V Sieira J Iacopino S Bala G et al Feasibility and safety of left atrial posterior wall isolation with a new cryoballoon technology in patients with persistent atrial fibrillation. Pacing Clin Electrophysiol. (2022) 45:605–11. 10.1111/PACE.14495
23.
Guckel D Lucas P Isgandarova K El HM Bergau L Fink T et al Impact of pulmonary vein variant anatomy and cross-sectional orifice area on freedom from atrial fibrillation recurrence after cryothermal single-shot guided pulmonary vein isolation. J Interv Card Electrophysiol. (2022) 65:251–60. 10.1007/S10840-022-01279-W
24.
Honarbakhsh S Martin CA Mesquita J Herlekar R Till R Srinivasan NT et al Atrial fibrillation cryoablation is an effective day case treatment: the UK PolarX vs. Arctic front advance experience. Europace. (2023) 25:euad286. 10.1093/EUROPACE/EUAD286
25.
Yap SC Anic A Breskovic T Haas A Bhagwandien RE Jurisic Z et al Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: insights from an initial multicenter experience. J Cardiovasc Electrophysiol. (2021) 32:580–7. 10.1111/JCE.14915
26.
Knecht S Sticherling C Roten L Badertscher P Chollet L Küffer T et al Technical and procedural comparison of two different cryoballoon ablation systems in patients with atrial fibrillation. J Interv Card Electrophysiol. (2022) 64:409–16. 10.1007/S10840-021-01035-6
27.
Knecht S Sticherling C Roten L Badertscher P Krisai P Chollet L et al Efficacy and safety of a novel cryoballoon ablation system: multicentre comparison of 1-year outcome. Europace. (2022) 24:1926–32. 10.1093/EUROPACE/EUAC094
28.
Assaf A Bhagwandien R Szili-Torok T Yap SC . Comparison of procedural efficacy, balloon nadir temperature, and incidence of phrenic nerve palsy between two cryoballoon technologies for pulmonary vein isolation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. (2021) 32:2424–31. 10.1111/JCE.15182
29.
Martin CA Tilz RRR Anic A Defaye P Luik A de Asmundis C et al Acute procedural efficacy and safety of a novel cryoballoon for the treatment of paroxysmal atrial fibrillation: results from the POLAR ICE study. J Cardiovasc Electrophysiol. (2023) 34:833–40. 10.1111/JCE.15861
30.
Iacopino S Stabile G Fassini G De Simone A Petretta A Moltrasio M et al Key characteristics for effective acute pulmonary vein isolation when using a novel cryoballoon technology: insights from the CHARISMA registry. J Interv Card Electrophysiol. (2022) 64:641–8. 10.1007/S10840-021-01063-2
31.
Pandey NN Taxak A Kumar S . Normal pulmonary venous anatomy and non-anomalous variations demonstrated on CT angiography: what the radiologist needs to know?Br J Radiol. (2020) 93:20200595. 10.1259/BJR.20200595
32.
Pott A Wirth H Teumer Y Weinmann K Baumhardt M Schweizer C et al Predicting phrenic nerve palsy in patients undergoing atrial fibrillation ablation with the cryoballoon—does sex matter? Front Cardiovasc Med. (2021) 8:746820. 10.3389/FCVM.2021.746820/PDF
33.
O’Toole SM Kramer J . Unilateral diaphragmatic paralysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2023). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK557388/(Accessed January 26, 2023).
34.
Gilge JL Prystowsky EN Padanilam BJ Clark BA Shah A Steinberg LA et al Use of diaphragmatic compound motor action potential monitoring to prevent right phrenic nerve palsy during atrial tachycardia ablation. HeartRhythm Case Rep. (2021) 7:739–42. 10.1016/j.hrcr.2021.08.001
Summary
Keywords
cryoballoon ablation, atrial fibrillation, pulmonary vein isolation, POLARx, Arctic front advance
Citation
Tahir MF, Shuja MH, Hannat R, Shaheen F, Sayeed A, Shaukat A, Rajput F, Zahoor MA, Hamid K, Khan A, Ahmed AA, Wasiq SA, Naeem H, Shaikh B, Larik MO, Ahmed R, Shehzad M, Changez MIK, Haider MU and Ayyalu T (2025) Comparative efficacy and safety of POLARx versus Arctic front advance pro cryoballoon systems for pulmonary vein isolation in atrial fibrillation: an updated systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1625399. doi: 10.3389/fcvm.2025.1625399
Received
08 May 2025
Accepted
28 July 2025
Published
18 August 2025
Volume
12 - 2025
Edited by
Vassil Traykov, Acibadem City Clinic Tokuda Hospital, Bulgaria
Reviewed by
Tchavdar Shalganov, National Heart Hospital, Bulgaria
Li-Da Wu, Nanjing Medical University, China
Updates
Copyright
© 2025 Tahir, Shuja, Hannat, Shaheen, Sayeed, Shaukat, Rajput, Zahoor, Hamid, Khan, Ahmed, Wasiq, Naeem, Shaikh, Larik, Ahmed, Shehzad, Changez, Haider and Ayyalu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Raheel Ahmed r.ahmed21@imperial.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.