Abstract
Background:
Cardiovascular diseases (CVDs) are the leading cause of death globally, and chronic inflammation is pivotal in CVDs development. Pro-inflammatory diets may exacerbate inflammation and thus increase CVDs risk. The Dietary Inflammatory Index (DII) is a validated measure of the inflammatory potential of diet. This updated systematic review and meta-analysis was conducted to clarify the association between DII and CVDs incidence and mortality.
Methods:
A comprehensive search was conducted in Pub Med, Web of Science, Embase, Cochrane Library, and China National Knowledge Infrastructure (CNKI) until February 2025. Study quality was assessed using the Newcastle-Ottawa Scale (NOS). Risk ratios (HR) and 95% confidence intervals (CI) were pooled using Review Manager 5.4, with subgroup analyses performed. Sensitivity and publication bias analyses were conducted using Stata 18.0.
Results:
Thirty cohort studies (NOS ≥7) from nine countries, involving 669,205 participants, were included. Compared with the lowest DII category, the highest category was associated with increased risks of CVD incidence [HR = 1.23, 95% CI (1.14–1.33); I2 = 54%] and mortality [HR = 1.29, 95% CI (1.24–1.35); I2 = 16%]. Stratified analyses indicated higher incidence risk among men (HR = 1.51) and higher mortality risk among women (HR = 1.25). Subgroup analyses further revealed a significant positive association between elevated DII and myocardial infarction (HR = 1.41). In models stratified by diabetes history, unadjusted associations were stronger (HR = 1.40), while adjusted associations were attenuated but remained significant, with a significant interaction (P = 0.002). Sensitivity and trim-and-fill analyses confirmed the robustness of these associations (all P < 0.001).
Conclusion:
Higher DII scores, reflecting pro-inflammatory dietary patterns, are significantly associated with increased risks of CVD incidence and mortality. These findings underscore the clinical and public health importance of promoting anti-inflammatory dietary strategies to mitigate the global CVD burden.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD420250654615, PROSPERO, CRD420250654615.
1 Introduction
With the continuous intensification of societal aging and the significant increase in the consumption of ultra-processed foods, the incidence and mortality rates of cardiovascular diseases (CVDs) have been showing a rising trend (1). The Global Burden of Disease Study 2021 (GBD 2021) reported that between 1990 and 2021, the number of new CVD cases rose from 34.74 million to 66.81 million, while deaths increased from 12.33 million to 19.42 million. Although age-standardized incidence and mortality rates declined overall, absolute numbers grew substantially, with marked regional disparities (2) 2021, dietary risk factors were linked to 6.58 million CVD deaths, highlighting the considerable potential of dietary prevention and intervention to reduce the global burden (1). Moreover, evidence from multiple countries indicates that exposure to ultra-processed foods is independently associated with elevated CVD risk, further emphasizing the interplay among diet quality, inflammation, and cardiovascular health, and their public health implications (3, 4).
Based on previous research findings, the pathogenesis of cardiovascular diseases is extremely complex, involving a process of multi-factor interaction and multi-mechanism synergistic effects. In this complex pathological process, chronic inflammatory responses play a key role (5). Inflammatory biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) not only promote the formation of atherosclerotic plaques but also promote plaque instability and thrombosis, thereby triggering acute cardiovascular events (6) a modifiable factor of inflammation, diet's role mechanism has been increasingly focused on. Studies have shown that daily diet can directly influence the body's inflammatory state and can also establish a close link with cardiovascular diseases through the gut microbiota (7). As the “second genome” of humans, the composition and function of the gut microbiota are significantly influenced by daily dietary patterns (8). Long-term consumption of a diet rich in pro-inflammatory nutrients alters the gut microbiota structure, leading to impaired gut barrier function, which in turn triggers chronic inflammatory responses in the body, increasing the risk of cardiovascular disease incidence and mortality, In contrast, diets rich in ω-3 polyunsaturated fatty acids and polyphenols, which are anti-inflammatory nutrients, provide metabolic substrates for beneficial gut bacteria, promoting their proliferation and the production of short-chain fatty acids and other substances. Previous studies have shown that these substances not only regulate immune function, alleviate inflammation, but also improve endothelial cell function and reduce cardiovascular disease risk (9).
With the continuous deepening and expansion of nutritional science, the Dietary Inflammatory Index (DII), a tool designed to reflect the pro- or anti-inflammatory properties of diet, emerged. Constructed by researchers at the University of South Carolina through analyzing and integrating human, animal, and cell experiments, it includes 45 dietary nutrients and 6 inflammatory markers. To reflect specific nutrients' impacts on body inflammatory markers, the authors weighted related studies, assigned each dietary nutrient an inflammatory effect score via different weights, and calculated the impact of an individual's overall diet on body inflammation (10).
Currently, the DII has been widely used to explore links between diet and various inflammation—related diseases (especially cancer, digestive tract diseases, and cardiovascular diseases), becoming a research hotspot in recent years (11). Although existing evidence supports the DII-CVDs association (12), cohort studies published in the past five years have not been systematically evaluated. Thus, conducting a comprehensive and timely Meta-analysis is essential to provide a medical evidence—based basis for constructing precise dietary intervention strategies.
2 Methods
The reporting of this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13), with the protocol registered on PROSPERO (ID: CRD420250654615).
2.1 Search strategy
We systematically searched PubMed, Web of Science, Embase, Cochrane Library, CNKI, Wanfang Data, and VIP databases for studies investigating the association between the DII and the risk of CVDs incidence or mortality. The search timeframe spanned from database inception to February 2025. To minimize omissions, we manually reviewed the reference lists of relevant articles. The detailed search strategy is provided in Supplementary Material 1.
2.2 Study selection
Table 1 outlines the PICOS criteria for study inclusion. Studies meeting the following criteria were eligible: (1) Study type: Cohort studies (retrospective or prospective); (2) Population: Adults aged ≥18 years; (3) Exposure: DII as the primary exposure variable; (4) Outcome: Incident CVDs or CVDs-related mortality; (5) Statistical reporting: Full effect estimates, including hazard ratios (HRs) with 95% confidence intervals (CIs). Exclusion criteria included: (1) Non-original research (e.g., reviews, conference abstracts, book chapters) or secondary evidence (e.g., systematic reviews); (2) Unavailable full-text articles; (3) Duplicate publications; (4) Insufficient data for extraction or conversion; (5) Low methodological quality. Two investigators (YN and QY) independently performed study selection, with discrepancies resolved through team discussions.
Table 1
| PICOS element | Description |
|---|---|
| P | Adults |
| I | Dietary Inflammatory Index |
| C | Highest vs. lowest DII quantiles |
| O | CVDs incidence or mortality |
| S | cohort studies (retrospective or prospective) |
Picos criteria of this study.
PICOS, participant, intervention, comparison, outcome, and study design.
2.3 Data extraction
To ensure comprehensive data extraction, two investigators (YQ and XT) independently performed data extraction using a predefined standardized template. Any discrepancies encountered during the process were resolved through team discussions. Extracted data included: Study characteristics (first author, publication year, study location, study design, follow-up duration); Participant information (age, sex ratio, health status); Assessment methods (dietary survey methods, dietary evaluation tools, criteria for ascertaining CVDs incidence and mortality); Statistical analyses: Hazard ratios (HR) with 95% confidence intervals (CI) comparing the highest vs. lowest DII quantiles.
2.4 Quality assessment
Two investigators (YN and TX) independently evaluated study quality using the Newcastle-Ottawa Scale (NOS), a validated tool developed by the Ottawa Hospital Research Institute for assessing observational studies. The NOS comprises three domains: participant selection (4 items), comparability of groups (1 item), and outcome assessment (3 items), with a maximum score of 9. Studies scoring 7–9 were classified as high quality with low overall bias and included in the systematic review and meta-analysis. Studies scoring ≤6 were excluded due to elevated risk of bias. Discrepancies in scoring were resolved through team consensus.
2.5 Statistical analysis
Data were analyzed using Stata 18.0 and Review Manager 5.4. Pooled HRs with 95% CIs were calculated to evaluate associations between DII and CVDs incidence/mortality. Heterogeneity was assessed using Cochran's Q-test and the I2 statistic (significance level α = 0.1). A fixed-effect model was applied if I2 < 50% and P > 0.1; otherwise, a random-effect model was used. Descriptive analyses were conducted when insufficient data precluded meta-analysis. Subgroup analyses explored heterogeneity sources, sensitivity analyses assessed result robustness, and funnel plots with trim-and-fill adjustments evaluated publication bias.
3 Results
Figure 1 outlines the literature screening process. Initial database searches yielded 3,419 records. After excluding 1,386 duplicates and 1,938 irrelevant studies through title/abstract screening, 95 full-text articles were reviewed. Ultimately, 30 English-language cohort studies met the inclusion criteria.
Figure 1
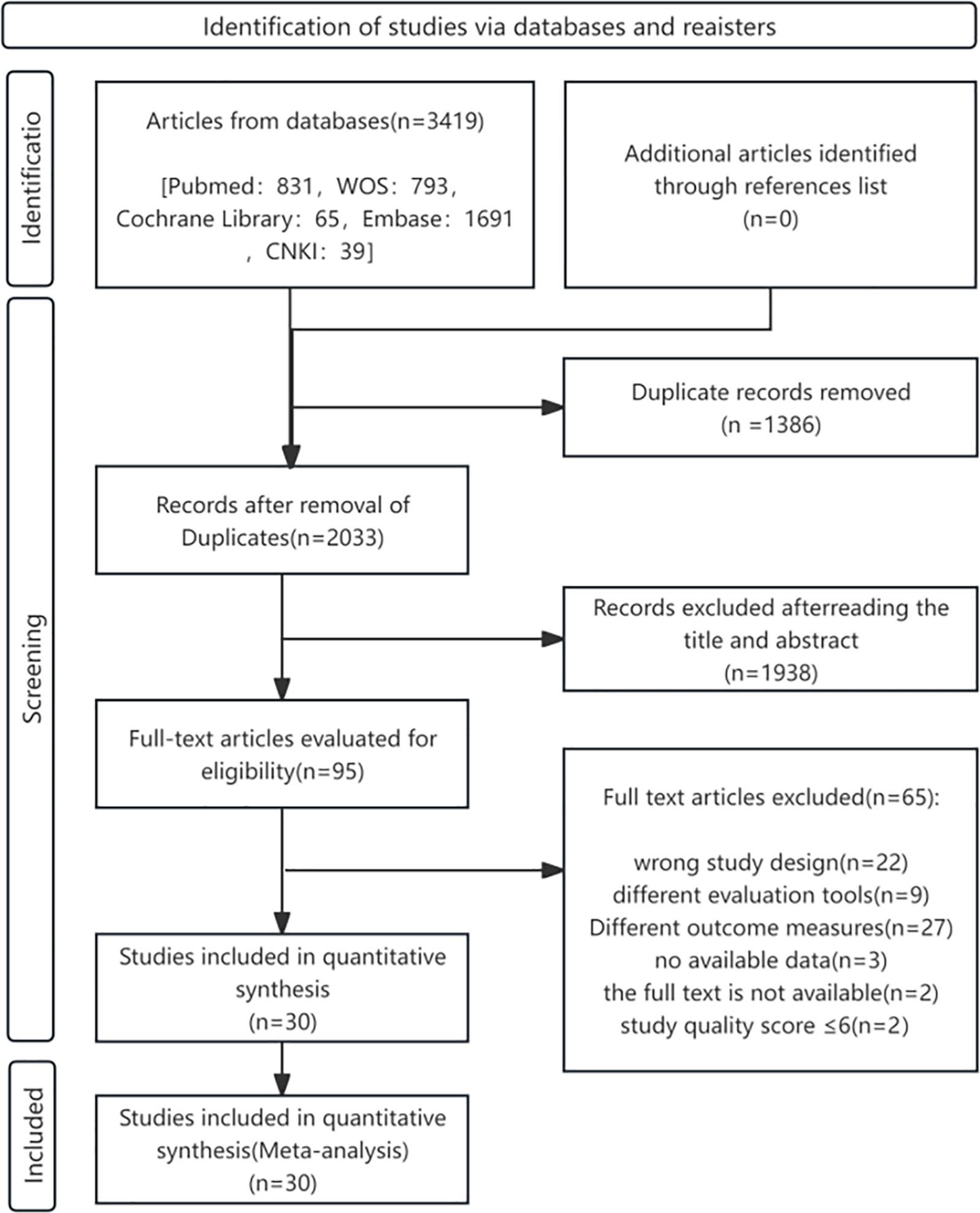
Flowchart of study selection process for the meta-analysis of DII and CVDs risk/mortality.
All the studies mentioned above were evaluated using the Newcastle-Ottawa Scale (NOS), with all scores ≥7, encompassing 669,205 participants across nine countries (14–43). The basic characteristics and quality assessments of studies investigating DII—CVDs incidence and mortality are presented in Tables 2, 3, respectively.
Table 2
| First author | Year of publication | Country | Study type | Follow-up duration (years) | Population | Age (years) | Sample size | Number of incident cases (n) | Dietary assessment method | Outcome measures | Covariates | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asadi et al. (14) | 2019 | Iran | Cohort study | 6 | Normal adults | 35–65 | 4,672 | ①124/②24 | FFQ | ①② | Adjusted for age, energy, BMI, physical activity level, education level, marital status, and smoking status. | 7 |
| Ramallal et al. (19) | 2015 | Spain | Cohort study | 8.9 | College graduates | 38 ± 12 | 18,794 | ①117 | FFQ | ① | Age, sex, cardiovascular risk factors, total energy intake, physical activity, BMI (BMI), educational level, other cardiovascular diseases, baseline special diet, snacking, average sedentary time, average television viewing time | 7 |
| Khan et al. (16) | 2020 | Korea | Cohort study | 7.4 | Normal adults | 40–79 | 1,62,773 | ①1,111/②824/⑤288 | SQ-FFQ | ①②⑤ | Age, smoking, alcohol consumption, physical activity, BMI, and energy intake | 9 |
| Liu et al. (17) | 2024 | Chain | Cohort study | 18 | Normal adults | 40.8 ± 12.0 | 4,822 | ①234/②114/⑤136 | 24HR | ①②⑤ | Age, sex, residential area, smoking status, educational level, physical activity, alcohol intake, hypertension, diabetes, and BMI | 7 |
| Vissers et al. (21) | 2016 | Australia | Cohort study | 11 | Healthy adult women | 52.0 ± 1 | 6,972 | ①335/②69/④191/⑤40 | FFQ | ①②④⑤ | Age, energy level, diabetes, hypertension, smoking status, educational level, menopausal status, physical activity, and alcohol consumption | 7 |
| Francisc et al. (15) | 2020 | Mexico | Cohort study | 11.1 | HCW | 45.0 ± 12.3 | 339 | ③382 | FFQ | ③ | Age, sex, smoking status, educational level, physical activity, sleep duration, and energy intake | 7 |
| Vissers et al. (22) | 2017 | Australia | Cohort study | 12 | Healthy adult women | 52 ± 1 | 7,169 | ③1,681 | FFQ | ③ | Age, energy level, diabetes, smoking status, educational level, menopausal status, physical activity, and BMI | 7 |
| Villaverde et al. (20) | 2024 | Mexico | Cohort study | 5 | HCW | 45.6 ± 7.3 | 1,540 | ③341 | FFQ | ③ | Age, sex, energy intake, physical activity, smoking, sleep, and educational level | 8 |
| MacDonald et al. (18) | 2020 | France | Cohort study | 21 | Healthy adult women | 50.1 ± 6.3 | 46,652 | ③13,183 | FFQ | ③ | Physical activity, smoking, family history of CVDs, educational level, diabetes, dyslipidemia, and BMI | 7 |
| Ze et al. (24) | 2023 | Chain | Cohort study | 7 | Normal adults | 41.85 ± 13.94 | 10,694 | ③3,687 | 24HR | ③ | ge, sex, residential location, education, physical activity level, smoking, alcohol consumption, self-reported diabetes, BMI, waist circumference, baseline systolic and diastolic blood pressure, sodium-to-potassium intake ratio, and intakes of energy, fat, protein, and carbohydrates | 8 |
| Zuercher et al. (25) | 2023 | Spain | Cohort study | 12.9 | PMW | 50–79 | 3,469 | ④118/⑤97 | FFQ | ④⑤ | Age, sex, lifestyle-related risk factors, smoking, alcohol consumption, physical exercise, educational level, diabetes, hypertension, hypercholesterolemia, BMI, and socioeconomic status | 7 |
| Wu et al. (23) | 2023 | Chain | Cohort study | 18 | Normal adults | 45 ± 15 | 14,652 | ②280/⑤404 | 24HR + WFR | ②⑤ | Age, sex, BMI, educational level, region, urbanization index, physical activity, baseline history of hypertension, smoking status, alcohol consumption, total energy intake | 8 |
| Garcia-Arellano et al. (41) | 2015 | Spain | Cohort study | 4.8 | Normal adults | 67.0 ± 6.2 | 7,169 | ①227 | 24HR | ① | Age and sex, overweight/obesity, waist-to-height ratio, total energy intake, smoking status, diabetes mellitus, hypertension, dyslipidemia, family history of premature cardiovascular disease, physical activity, and educational level. | 7 |
| Neufcourt et al. (42) | 2016 | France | Cohort study | 11.4 | Normal adults | 49.1 ± 6.3 | 7,743 | ①292/②93/⑤58 | 24HR | ①②⑤ | sex and energy intake without alcohol, supplementation group, number of 24-h records, education level, marital status, smoking status, and physical activity, BMI | 7 |
Basic characteristics and quality assessment of studies on the risk of CVDs associated with DII.
HCW, healthcare workers; PMW, postmenopausal women; ① = The occurrence of cardiovascular disease; ② = The occurrence of myocardial infarction; ③ = The occurrence of hypertension; ④ = The occurrence of coronary heart disease; ⑤ = The occurrence of stroke; FFQ, Food Frequency Questionnaire; SQ-FFQ, Semi-Quantitative Food Frequency Questionnaire; 24HR, 24-hour dietary recall; WFR, weighed food record; BMI, body mass index.
Table 3
| First author | Year of publication | Country | Study type | Follow-up duration (years) | Population | Age (years) | Sample size (n) | Number of death cases (n) | Dietary assessment method | Outcome measures | Covariates | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. (27) | 2024 | USA | Cohort Study | 18.7 | Normal Adults/NWCO | 46.4 | 1,777/1,744 | ①42/①166 | 24HR | ① | Age, sex, race, ethnicity, income, educational level, physical activity level, and smoking status | 8 |
| Huang et al. (29) | 2023 | USA | Cohort Study | 9 | Hyperuricemic Patients | ≥20 | 3,039 | ①254 | 24HR | ① | Sex, age, race, educational level, PIR, smoking history, alcohol history, hypertension, diabetes, eGFR, serum cholesterol, and serum triglycerides | 7 |
| Ma et al. (30) | 2025 | USA | Cohort Study | 9.5 | MetS | 53.74 ± 0.22 | 13,751 | ①639 | 24HR | ① | Age, sex, race, educational level, BMI, PIR | 7 |
| Majidi et al. (31) | 2024 | USA | Cohort Study | 30 | Normal Adults | 46.90 ± 13.1 | 1,440 | ①118 | FFQ | ① | Smoking status and medical conditions (self-reported diabetes, dyslipidemia, hypertension, angina, heart attack, stroke, cancer, and medication use for heart disease or diabetes), education, BMI, physical activity, and use of dietary supplements | 7 |
| Okada et al. (32) | 2019 | Japan | Cohort Study | 19.3 | Normal Adults | 40–79 | 1,10,585 | ①1,524/②4,248 | FFQ | ①② | Age, geographic region, BMI, educational level, smoking status, exercise habits, sleep duration, history of hypertension and diabetes, and total energy intake | 7 |
| Park et al. (33) | 2018 | USA | Cohort Study | 18.2 | Normal Adults | 45–75 | 1,50,405 | ①3,292 | Q-FFQ | ① | Age, race/ethnicity, body mass index (BMI), history of diabetes, educational level, marital status, physical activity, alcohol intake, smoking status, energy intake, and use of menopausal hormone therapy (women only) | 8 |
| Nitin et al. (34) | 2017 | USA | Cohort Study | 13.5 | Normal Adults | >19 | 12,366 | ①1,233 | 24HR | ① | Age, sex, race, diabetes status, hypertension, physical activity, BMI, PIR, and smoking | 8 |
| Sun et al. (35) | 2023 | USA | Cohort Study | 6.7 | Healthy Older Adults | 73.29 ± 0.10 | 10,827 | ①1,230 | FFQ | ① | Age, sex, race, BMI level, educational level, PIR, smoking status, alcohol status, physical activity, total energy intake, and history of hypertension, diabetes, dyslipidemia, and cardiovascular disease | 7 |
| Veronese et al. (36) | 2020 | Italy | Cohort Study | 12 | Normal Adults | 65.50 ± 9.5 | 1,565 | ①102 | FFQ | ① | Age, sex, smoking status, diabetes, gastric ulcer, gallstone, liver cirrhosis, cancer, acute myocardial infarction, BMI, systolic and diastolic blood pressure, presence of depressive symptoms, presence of hepatic steatosis, energy and alcohol intake, and adherence to the Mediterranean diet | 7 |
| Yang et al. (38) | 2024 | USA | Cohort Study | 18 | Normal Adults | 40.8 ± 12.0 | 4,822 | ①755 | 24HR | ① | Age, sex, educational level, race, BMI, smoking status, alcohol consumption, history of dyslipidemia, diabetes, hypertension, and levels of HbA1c, alanine aminotransferase (ALT), aspartate aminotransferase (AST), sodium, potassium, creatinine, total cholesterol, and high-density lipoprotein cholesterol (HDL-C) | 7 |
| Yuan et al. (39) | 2022 | USA | Cohort Study | 12.9 | Normal Adultsna | 50–79 | 20,762 | ①243 | FFQ | ① | Age, sex, BMI, smoking, hypertension, educational level, dyslipidemia, recreational activity, and moderate or heavy alcohol consumption | 8 |
| Zhou et al. (40) | 2024 | USA | Cohort Study | 6.6 | Normal Adults | 64.66 ± 0.31 | 5,006 | ①532 | 24HR | ① | Marital status, survey cycle, educational level, PIR, BMI, smoking status, alcohol status, CPR, moderate recreational activity, hypertension, diabetes, coronary heart disease, and history of stroke | 8 |
| Deng et al. (28) | 2016 | USA | Cohort Study | 11.25–14 | Normal Adults/Prediabetes/T2MD | 20–90 | 9,631/2,681/968 | ①676/①412/①240 | 24HR | ① | Age, sex, race, HbA1c, current smoking, physical activity, BMI, systolic blood pressure (SBP) | 7 |
| Bondonno et al. (26) | 2017 | Australia | Cohort Study | 15 | PMW | 75.1 ± 2.7 | 1,304 | ②269/③150 | FFQ | ②③ | Age, BMI, energy intake, energy expenditure from physical activity, socioeconomic status, low-dose aspirin use, antihypertensive medication use, statin use, prevalent ASVD, and treatment | 7 |
| Xie et al. (37) | 2024 | USA | Cohort Study | 17 | Normal Adults | 44.93 | 9,788 | ①668 | 24HR | ① | Age, sex, race, waist circumference, physical activity, alcohol status, eGFR, high-sensitivity cardiac troponin T, high-sensitivity cardiac troponin I, HbA1c, Urine albumin-to-creatinine ratio, Low-density lipoprotein cholesterol, CRP, Triglycerides | 7 |
| Ganbaatar et al. (43) | 2023 | Japan | Cohort Study | 29 | Normal Adults | ≥30 | 9,284 | ①1,149/②539/④234/⑤509 | WFR | ①②④⑤ | Age, Sex, BMI, Smoking status, Alcohol consumption status, Work intensity, Energy-adjusted salt intake, Serum total cholesterol, Hypertension status, and Diabetes mellitus history. | 8 |
Basic characteristics and quality assessment of studies on the risk of CVDs mortality associated with DII.
NWCO, normal-weight central obesity; MetS, metabolic syndrome; T2MD, type 2 diabetes mellitus; PMW, postmenopausal women; ① = Mortality due to cardiovascular disease; ② = Mortality due to atherosclerotic cardiovascular disease; ③ = Mortality due to ischemic heart disease; ④ = The occurrence of coronary heart disease; ④ = The occurrence of stroke; FFQ, Food Frequency Questionnaire; 24HR, 24-hour dietary recall; Q-FFQ, Quantitative Food Frequency Questionnaire; ASVD, atherosclerotic vascular disease; WFR, weighed food record; BMI, body mass index; PIR, poverty income ratio; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; CRP, C-reactive protein.
3.1 Meta-Analysis results
3.1.1 Association between DII and CVDs risk
Fourteen studies examined the relationship between DII and CVDs incidence (14–25, 41, 42). Significant heterogeneity was observed across studies (I2 = 54%, P < 0.01), necessitating a random-effects model. The highest DII quantile was associated with a 23% increased risk of CVDs incidence compared to the lowest quantile [HR = 1.23, 95% CI (1.14–1.33)] (Figure 2).
Figure 2
![Forest plot showing the association between the Dietary Inflammatory Index (DII) and cardiovascular diseases (CVDs) incidence. The plot displays the log hazard ratio (log HR) and 95% confidence intervals (CIs) for fourteen studies, represented as squares with horizontal lines indicating the CI. Studies are listed along the left with their respective hazard ratios, weights, and standard errors. The overall effect is shown by the diamond at the bottom, with a pooled hazard ratio of 1.23 [95% CI 1.14-1.33], indicating a 23% increased risk of CVDs incidence for the highest DII quantile compared to the lowest. Significant heterogeneity was observed across studies (I2 = 54%, P < 0.01), requiring a random-effects model](https://www.frontiersin.org/files/Articles/1626523/xml-images/fcvm-12-1626523-g002.webp)
Forest plot of the association between DII and CVDs risk.
Subgroup analyses stratified by outcome type, region, sex, dietary assessment method, BMI adjustment, energy adjustment, and diabetes history are summarized in Table 4. Between-group comparisons indicated significant effect modification by sex, dietary method, energy adjustment, and diabetes history. The strongest and most consistent association was observed for myocardial infarction [HR = 1.41, 95% CI (1.16–1.72)]. Associations were particularly evident among men [HR = 1.51, 95% CI (1.26–1.80)], studies using 24-hour dietary recall [HR = 1.53, 95% CI (1.19–1.94)], studies with energy adjustment [HR = 1.44, 95% CI (1.23–1.69)], and studies without diabetes history adjustment [HR = 1.40, 95% CI (1.25–1.56)].
Table 4
| Subgroup analysis | Number of studies included | Heterogeneity test | Effect model | Pooled effect size HR (95%CI) | Pooled effect size HR test | Intergroup heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| I 2 (%) | P | Z | P | P | ||||
| Specific diseases | 0.050 | |||||||
| CVDs | 7 | 44 | 0.110 | Fixed | 1.33 (1.15–1.53) | 3.92 | 0.001 | |
| HTN | 6 | 57 | 0.040 | Random | 1.14 (1.03–1.26) | 2.47 | 0.010 | |
| MI | 6 | 0 | 0.670 | Fixed | 1.41 (1.16–1.72) | 3.42 | <0.001 | |
| IHD | 2 | 0 | 0.340 | Fixed | 1.08 (0.99–1.18) | 1.73 | 0.080 | |
| Stroke | 6 | 73 | 0.003 | Random | 1.31 (0.94–1.84) | 1.60 | 0.110 | |
| Study region | 0.250 | |||||||
| Europe | 5 | 57 | 0.020 | Random | 1.26 (1.08–1.47) | 2.68 | 0.007 | |
| North America | 2 | 47 | 0.170 | Fixed | 1.40 (0.94–2.08) | 1.65 | 0.100 | |
| Asia | 5 | 45 | 0.080 | Fixed | 1.12 (1.04–1.21) | 3.03 | 0.002 | |
| Oceania | 2 | 0 | 0.61 | Fixed | 1.21 (1.06–1.38) | 2.90 | 0.004 | |
| Gender | 0.006 | |||||||
| Male | 3 | 0 | 0.610 | Fixed | 1.51 (1.26–1.80) | 4.52 | <0.001 | |
| Female | 7 | 32 | 0.130 | Fixed | 1.10 (1.05–1.15) | 4.07 | <0.001 | |
| Dietary assessment method | 0.020 | |||||||
| 24HR | 4 | 74 | <0.001 | Random | 1.53 (1.19–1.94) | 3.29 | 0.01 | |
| FFQ | 8 | 35 | 0.090 | Fixed | 1.09 (1.04–1.13) | 3.96 | <0.001 | |
| SQ-FFQ | 1 | N/A | N/A | N/A | N/A | N/A | N/A | |
| 24HR + WFR | 1 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Adjustment for BMI | 0.670 | |||||||
| Yes | 10 | 55 | 0.001 | Random | 1.22 (1.12–1.31) | 4.91 | <0.001 | |
| No | 4 | 46 | 0.080 | Fixed | 1.25 (1.05–1.51) | 2.44 | 0.010 | |
| Adjustment for physical activity | N/A | |||||||
| Yes | 13 | 55 | <0.001 | Random | 1.24 (1.14–1.34) | 5.33 | <0.001 | |
| No | 1 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Adjustment for energy intake | 0.005 | |||||||
| Yes | 8 | 60 | 0.002 | Random | 1.44 (1.23–1.69) | 4.52 | <0.001 | |
| No | 6 | 39 | 0.070 | Fixed | 1.11 (1.07–1.15) | 4.02 | <0.001 | |
| Adjustment for diabetes history | 0.002 | |||||||
| Yes | 7 | 54 | 0.010 | Random | 1.13 (1.05–1.22) | 3.16 | 0.002 | |
| No | 7 | 0 | 0.490 | Fixed | 1.40 (1.25–1.56) | 5.79 | <0.001 | |
Subgroup analysis of the association between DII and CVDs risk.
N/A, not applicable.
3.1.2 Association between DII and CVDs mortality
Besides the above-mentioned studies, the remaining 16 studies assessed CVDs-related death events (26–40, 43). As shown in Figure 3, the meta-analysis revealed a positive association between the dietary inflammatory index and the risk of CVDs death [I2 = 16%, HR = 1.29, 95%CI (1.24–1.35)].
Figure 3
![Forest plot showing the association between the Dietary Inflammatory Index (DII) and cardiovascular diseases (CVDs) mortality. The plot displays the log hazard ratio (log HR) and 95% confidence intervals (CIs) for 16 studies, with each study represented by a square and horizontal lines indicating the CIs. The overall effect is shown by the diamond at the bottom, with a pooled hazard ratio of 1.29 [95% CI 1.24-1.35], indicating a statistically significant positive association between DII and CVDs mortality. The heterogeneity statistic is I2 = 16%, suggesting low variability across studies. The fixed-effects model was used.](https://www.frontiersin.org/files/Articles/1626523/xml-images/fcvm-12-1626523-g003.webp)
Forest plot of the association between DII and CVDs mortality.
Similarly, subgroup analyses of the included studies were conducted based on factors such as disease status of the subjects, study region, gender, BMI adjustment, physical activity adjustment, dietary assessment method, energy intake adjustment, and diabetes history adjustment to explore the impact of each factor on the study results (Table 5). The subgroup—analysis results indicated that gender significantly contributed to heterogeneity (P < 0.05), with stronger associations observed in women [HR = 1.25, 95% CI (1.12–1.39)]. Other factors (disease status, region, dietary method, BMI adjustment, physical activity adjustment, energy adjustment, diabetes history) did not significantly explain heterogeneity.
Table 5
| Subgroup analysis | Number of studies included | Heterogeneity test | Effect model | Pooled effect size HRHR (95%CI) | Pooled effect size HR test | Intergroup heterogeneity | ||
|---|---|---|---|---|---|---|---|---|
| I 2 (%) | P | Z | P | P | ||||
| Disease status of study participants | 0.200 | |||||||
| Yes | 5 | 0 | 0.530 | Fixed | 1.43 (1.22–1.68) | 4.33 | <0.001 | |
| No | 14 | 25 | 0.150 | Fixed | 1.28 (1.23–1.34) | 10.97 | <0.001 | |
| Study region | 0.740 | |||||||
| USA | 12 | 33 | 0.080 | Fixed | 1.26 (1.20–1.33) | 8.62 | <0.001 | |
| Japan | 2 | 0 | 0.760 | Fixed | 1.36 (1.25–1.48) | 6.97 | <0.001 | |
| Other | 2 | 0 | 0.990 | Fixed | 1.35 (1.19–1.53) | 4.65 | <0.001 | |
| Gender | 0.020 | |||||||
| Male | 6 | 0 | 0.900 | Fixed | 1.09 (1.05–1.14) | 4.16 | <0.001 | |
| Female | 8 | 74 | <0.001 | Random | 1.25 (1.12–1.39) | 4.09 | <0.001 | |
| Dietary assessment method | 0.380 | |||||||
| 24HR | 9 | 0 | 0.870 | Fixed | 1.39 (1.27–1.53) | 7.15 | <0.001 | |
| FFQ | 6 | 60 | 0.020 | Random | 1.18 (1.11–1.25) | 3.50 | <0.001 | |
| Q-FFQ | 1 | N/A | N/A | N/A | N/A | N/A | N/A | |
| WFR | 1 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Adjustment for BMI | 0.830 | |||||||
| Yes | 13 | 12 | 0.300 | Fixed | 1.29 (1.24–1.35) | 11.68 | <0.001 | |
| No | 3 | 43 | 0.150 | Fixed | 1.45 (1.08–1.95) | 2.84 | 0.004 | |
| Adjustment for physical activity | 0.150 | |||||||
| Yes | 11 | 34 | 0.110 | Fixed | 1.26 (1.20–1.32) | 8.91 | <0.001 | |
| No | 5 | 0 | 0.970 | Fixed | 1.41 (1.28–1.56) | 6.82 | <0.001 | |
| Adjustment for energy intake | 0.270 | |||||||
| Yes | 6 | 31 | 0.140 | Fixed | 1.27 (1.21–1.33) | 9.79 | <0.001 | |
| No | 10 | 0 | 0.500 | Fixed | 1.38 (1.26–1.52) | 6.76 | <0.001 | |
| Adjustment for diabetes history | 0.230 | |||||||
| Yes | 10 | 23 | 0.190 | Fixed | 1.27 (1.21–1.33) | 9.94 | <0.001 | |
| No | 6 | 0 | 0.530 | Fixed | 1.39 (1.26–1.52) | 6.75 | <0.001 | |
Subgroup analysis of the association between DII and CVDs mortality.
N/A, not applicable.
3.2 Sensitivity analysis and publication bias
Sensitivity analyses were performed using Stata 18.0. The pooled effect estimates for both CVDs incidence and mortality demonstrated minimal changes upon sequential exclusion of individual studies, indicating robust meta-analysis results (Figures 4, 5). Funnel plots revealed asymmetric scatter distributions, suggesting potential publication bias. Trim-and-fill adjustments were subsequently applied. For CVDs incidence analyses, imputation of 11 hypothetical missing studies under a random-effects model yielded a persistent statistically significant association [HR = 1.106, 95% CI (1.018–1.201), P < 0.001]. Similarly, imputation of 6 hypothetical missing studies in CVDs mortality analyses under a fixed-effects model maintained significance [HR = 1.268, 95% CI (1.218–1.321), P < 0.001], with no reversal in the direction of conclusions. Collectively, these findings confirm the robustness of the meta-analysis results (Figures 6, 7).
Figure 4
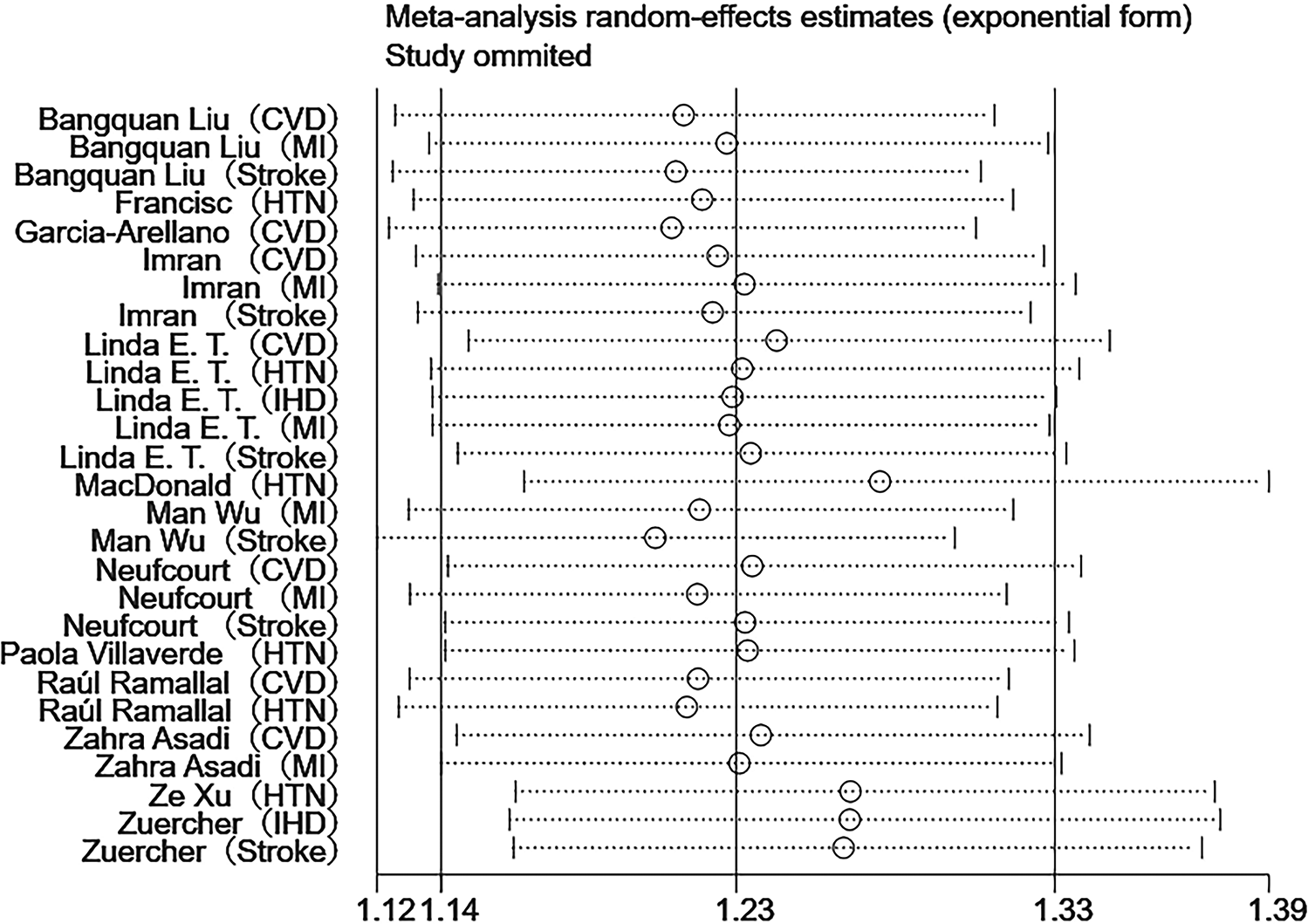
Sensitivity analysis of DII and CVDs risk.
Figure 5
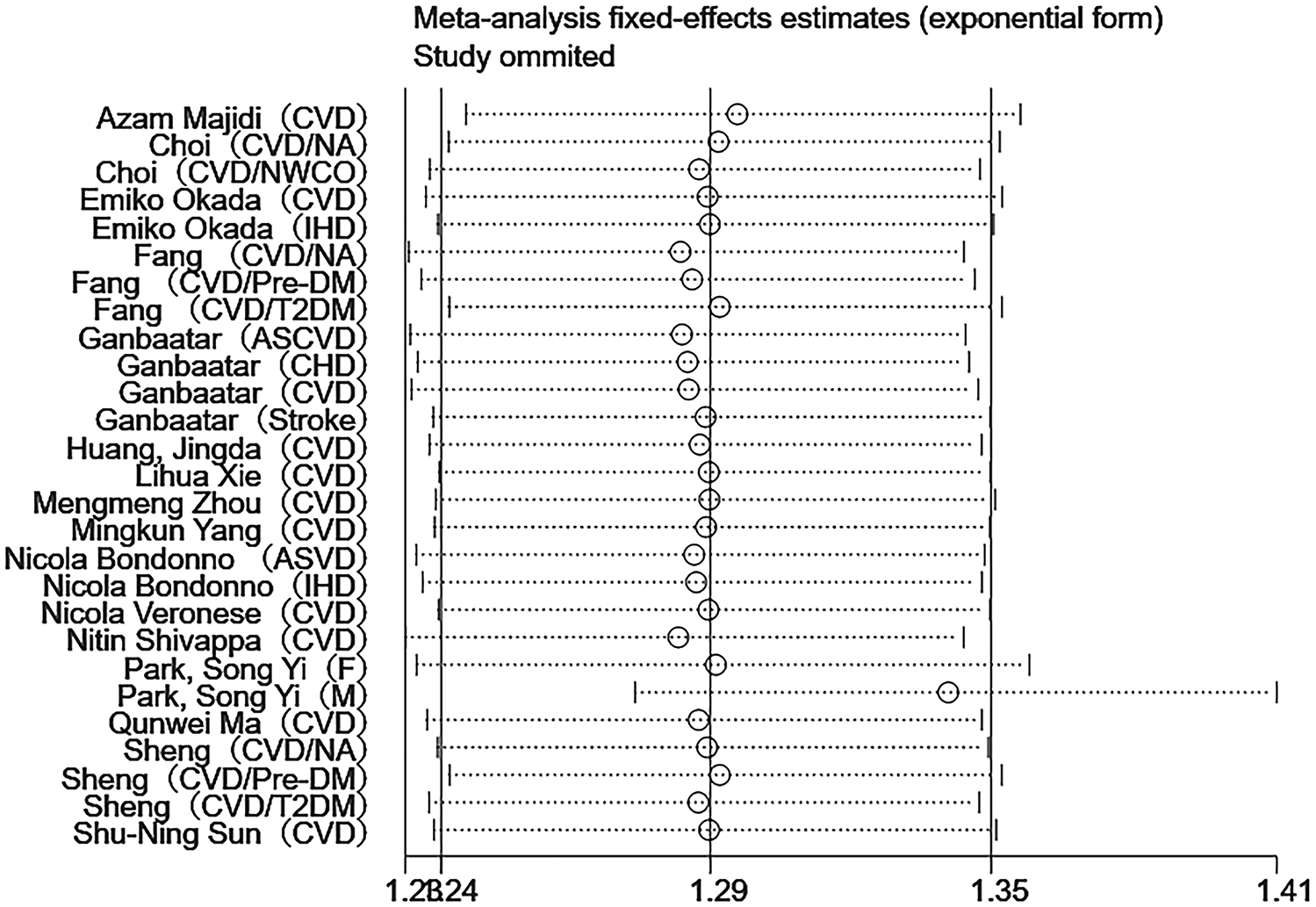
Sensitivity analysis of DII and CVDs mortality.
Figure 6
![Filled funnel plot assessing publication bias for the association between the Dietary Inflammatory Index (DII) and cardiovascular diseases (CVDs) risk using the trim-and-fill method. The plot shows effect sizes (log HR) on the vertical axis and standard errors on the horizontal axis. Circles represent observed studies, while squares indicate imputed (“filled”) studies added under a random-effects model to correct for bias. Imputation of 11 missing studies maintained a statistically significant association [HR = 1.106, 95% CI (1.018-1.201), P < 0.001], suggesting persistent validity despite potential publication bias.](https://www.frontiersin.org/files/Articles/1626523/xml-images/fcvm-12-1626523-g006.webp)
Trim-and-fill funnel plot for the association between DII and CVDs risk.
Figure 7
![Filled funnel plot assessing publication bias for the association between the Dietary Inflammatory Index (DII) and cardiovascular diseases (CVDs) mortality using the trim-and-fill method. The plot displays effect sizes (log HR) on the vertical axis and standard errors on the horizontal axis. Six hypothetical missing studies were imputed under a fixed-effects model, maintaining statistical significance [HR = 1.268, 95% CI 1.218-1.321, P < 0.001] without a change in direction. Circles represent observed studies, and squares denote imputed (“filled”) ones within the pseudo 95% confidence limits, illustrating adjusted symmetry after bias correction.](https://www.frontiersin.org/files/Articles/1626523/xml-images/fcvm-12-1626523-g007.webp)
Trim-and-fill funnel plot for the association between DII and CVDs mortality.
4 Discussion
This updated systematic review and meta-analysis further substantiates the consistent association between pro-inflammatory dietary patterns, as quantified by the Dietary Inflammatory Index (DII), and elevated risks of cardiovascular disease (CVDs) incidence and mortality. Individuals consuming diets with higher inflammatory potential exhibit significantly greater risks of experiencing CVDs events and related deaths compared to those adhering to diets with lower inflammatory potential. These findings align with a growing body of evidence indicating that unhealthy dietary habits—characterized by excessive intake of processed meats, sugar-sweetened beverages, and refined carbohydrates—adversely affect cardiovascular health (44). Consequently, adopting dietary patterns rich in anti-inflammatory components such as fruits, vegetables, and whole grains may serve as an effective strategy to mitigate the population burden of CVDs (45). In this context, the DII emerges as a practical tool that translates complex nutritional data into actionable indicators of dietary inflammatory potential, enabling clinicians to identify high-risk dietary patterns and provide tailored recommendations to patients.
From a mechanistic perspective, pro-inflammatory diets may promote CVD development through chronic low-grade inflammation and oxidative stress (46). Diets with high DII scores are typically rich in saturated fats, trans fatty acids, and added sugars, which can activate the IKKβ/NF-κB signaling pathway, stimulating the release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) (47). This activation initiates pathogenic cascades leading to atherosclerotic plaque formation, vascular remodeling, and increased arterial stiffness (48). Furthermore, diet-induced inflammatory burden destabilizes the fibrous cap of plaques (49). Evidence suggests that inflammation within the fibrous cap promotes degradation of collagen and extracellular matrix, as well as smooth muscle cell apoptosis, resulting in cap thinning and heightened risk of rupture and thrombosis (50).
In addition, high DII diets are often deficient in antioxidants and phytochemicals, which impairs endogenous defense mechanisms and aggravates oxidative stress–induced vascular injury (51). Insufficient dietary fiber intake also reduces the abundance of butyrate-producing bacteria, thereby lowering short-chain fatty acid (particularly butyrate) production and compromising intestinal barrier integrity. Barrier dysfunction combined with dysbiosis facilitates translocation of gut-derived metabolites such as lipopolysaccharide and trimethylamine (TMA) into the circulation, activating Toll-like receptor 4 (TLR4) and triggering inflammatory cascades that lead to cytokine overproduction and exacerbation of vascular and myocardial injury (52, 53).
Moreover, excessive choline and carnitine in high DII diets are metabolized by the gut microbiota into TMA, which is subsequently oxidized in the liver to trimethylamine-N-oxide (TMAO) (53). This metabolite exerts multiple deleterious effects, including enhancing platelet activation to promote thrombosis, activating the NLRP3 inflammasome to aggravate plaque inflammation, facilitating foam cell formation, and contributing to vulnerable plaque characteristics such as thin fibrous caps and increased microvascularization (54–57). Clinically, TMAO levels independently predict major adverse cardiovascular events (MACE) and adverse prognosis in patients with acute coronary syndrome (58). For example, a meta-analysis by Li et al. demonstrated that each 1 μmol/L increase in TMAO was associated with an ∼11% higher risk of MACE (95% CI: 1.07–1.14; P = 0.0000104) (59). Animal studies further revealed that reducing gut-derived metabolites via antibiotic administration significantly attenuated monocyte infiltration and ventricular rupture in myocardial infarction models, supporting a causal role of gut microbiota–mediated inflammation in acute MI (60). Importantly, identical dietary exposures (or equivalent DII scores) do not necessarily result in equal TMAO loads. For instance, microbiota dominated by Firmicutes can substantially enhance TMA-to-TMAO conversion, thereby amplifying pro-inflammatory and pro-thrombotic effects under high DII conditions (61). This provides a biological explanation for interindividual variability in risk responses. Thus, future research on DII–CVD associations should incorporate gut microbiota phenotypes or metabolic capacity into models to better clarify mediating mechanisms and support precision prevention strategies.
Beyond these mechanisms, subgroup analyses in this study indicated potential sex differences in the relationship between dietary inflammation and CVD risk. Elevated DII scores were more strongly associated with CVD incidence among men, whereas the association with CVD mortality was more pronounced among women. This heterogeneity may arise from multiple interacting biological mechanisms. First, estrogen exerts anti-inflammatory and vasoprotective effects. In premenopausal women, estrogen upregulates eNOS expression, enhances nitric oxide production, and suppresses oxidative stress and inflammatory signaling, thereby mitigating vascular injury induced by chronic inflammation (62). However, after menopause, declining estrogen levels weaken this protection, potentially predisposing women to more severe outcomes under high-inflammatory dietary exposure (63). Second, the sex–microbiota–inflammation axis may modulate the strength of DII-related signaling (64). Sex hormones influence microbial composition and metabolic activity, which in turn affect short-chain fatty acid production, intestinal permeability, and endotoxin leakage (65, 66). Under such mechanisms, women may partially buffer pro-inflammatory signaling due to stronger microbial regulatory capacity, whereas men may more readily translate these signals into systemic inflammatory burden. Nonetheless, current evidence is insufficient to conclude that “men are more susceptible to incidence while women are more susceptible to mortality” at equivalent DII levels. Heterogeneity in population composition, follow-up duration, endpoint definitions (incidence vs. mortality), and covariate adjustments across studies complicates interpretation, and most studies lack concurrent assessments of sex hormones, microbiota profiles, inflammatory biomarkers, and sex interactions. Future research should incorporate these mechanistic variables and test sex interactions in larger samples to verify and quantify this heterogeneity.
In addition, methodological subgroup analyses revealed stronger associations in studies using 24HR, studies with energy adjustment, and those without diabetes history adjustment. We interpret these findings as follows: first, compared with FFQ, repeated 24HR captures within-person variation more accurately, thereby reducing non-differential exposure misclassification and biasing effect estimates toward the null (67). Second, energy adjustment “fixes” total energy intake, diminishing confounding from factors such as physical activity and metabolic efficiency, and allowing the analysis to focus on dietary composition–driven inflammatory signals (68). In contrast, adjustment for diabetes history produced weaker associations. This likely reflects the dual role of diabetes history as both a confounder and mediator. Including diabetes as a covariate may block mediating pathways and cause over-adjustment, systematically underestimating the true association (69). However, failure to adjust entirely may leave residual confounding, as diabetes diagnosis and dietary management can alter subsequent DII, while diabetes itself increases CVD risk. Thus, not adjusting could exaggerate or distort the associations. Nevertheless, even in studies adjusting for diabetes, high DII remained positively associated with CVD risk, indicating that DII influences cardiovascular outcomes through mechanisms beyond glycemic pathways, particularly chronic systemic inflammation (70).
Overall, this study demonstrates that pro-inflammatory dietary patterns significantly elevate CVD incidence and mortality, reinforcing the central role of dietary modulation in cardiovascular progression and providing evidence for personalized dietary interventions based on DII. From a practical standpoint, we recommend DII as a supplementary tool for risk stratification and dietary management in addition to traditional CVD risk assessments. Suitable applications include: primary prevention, where baseline nutritional assessments and annual follow-ups are conducted for individuals with multiple cardiometabolic risk factors or family history of early-onset CVD; secondary prevention, where dietary inflammatory load is monitored after hospital discharge and during cardiac rehabilitation follow-up; and clinical decision points such as initiation or intensification of lipid-lowering, glucose-lowering, or weight management interventions, smoking cessation, or exercise prescription. In patients with inflammatory phenotypes or metabolic comorbidities, DII can serve as additional evidence to reinforce lifestyle management. Regarding dietary assessment, repeated 24HR (≥3 non-consecutive recalls, including ≥2 weekdays and ≥1 weekend day) with energy adjustment is recommended for calculating DII (71). In resource-limited settings, simplified FFQs calibrated to local diets may serve as alternatives. For interpretation, DII should be treated as a continuous metric, where negative values indicate relatively anti-inflammatory and positive values relatively pro-inflammatory diets. Given variations in food items, assessment tools, and population characteristics, no universal clinical cutoffs exist. Thus, reporting individuals' percentile rank within a sample, alongside blood pressure, lipids, glucose/HbA1c, anthropometry, and hs-CRP, is a more prudent strategy than using fixed thresholds. Finally, DII interpretation should be linked to heart-healthy dietary patterns and translated into actionable advice. For CVD patients or high-risk individuals, clinicians should encourage increased intake of fruits, dark green leafy vegetables, whole grains, nuts, and ω-3–rich fish, preferential use of liquid plant oils, and restriction of red meat, refined sugars, and fried foods (44, 72). Given the positive correlation between DII and CRP, clinicians may consider presenting improvements in DII alongside reductions in inflammatory biomarkers, thereby helping patients recognize the modifiable “diet–inflammation–CVD event” pathway and motivating them to adopt anti-inflammatory dietary habits in daily life.
4.1 Strengths and limitations
Compared with previous meta-analyses, this study has several strengths: a larger sample size, broader geographic coverage, and stricter methods for covariate control, publication bias assessment, and robustness testing, all of which enhance the reliability and generalizability of the effect estimates. Nevertheless, some limitations should be acknowledged. First, the lack of age-stratified analyses represents a key limitation. As inflammation and dietary habits vary with age, age may act as an important effect modifier. However, incomplete reporting of mean age and standard deviation in some studies prevented subgroup analyses, potentially underestimating or masking age-related effects. Second, although energy adjustment and multiple covariate controls were applied, residual confounding remains inevitable. Potential unmeasured factors—such as medication use, genetic predisposition, and gut microbiota characteristics—may influence inflammation and cardiovascular outcomes. Third, most included studies were conducted in high-income countries, with limited representation from low- and middle-income countries, restricting the generalizability of findings to diverse socioeconomic and nutritional transition contexts.
Future research should integrate multidimensional information including inflammatory biomarkers, microbiota profiles, and genetic susceptibility to clarify biological mechanisms of dietary inflammatory load and explore differential responses across metabolic states and population subgroups.
5 Conclusion
In conclusion, higher Dietary Inflammatory Index (DII) scores are closely associated with increased risks of cardiovascular disease (CVDs) incidence and mortality. Pro-inflammatory diets may accelerate the development of CVDs through systemic inflammation and gut microbiota-mediated pathways. This association may vary by sex and metabolic status, highlighting the importance of nuanced approaches in research and dietary recommendations. Our findings support the promotion of anti-inflammatory dietary patterns as public health measures and clinical interventions to reduce overall cardiovascular risk, advocating for a shift towards more personalized dietary guidance to improve heart health outcomes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YN: Conceptualization, Data curation, Formal analysis, Validation, Visualization, Writing – original draft. QY: Data curation, Formal analysis, Validation, Writing – original draft. TX: Data curation, Validation, Writing – original draft. XL: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1626523/full#supplementary-material
References
1.
Vaduganathan M Mensah GA Turco JV Fuster V Roth GA . The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. (2022) 80(25):2361–71. 10.1016/j.jacc.2022.11.005
2.
GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2100–32. 10.1016/S0140-6736(24)00367-2
3.
Srour B Fezeu LK Kesse-Guyot E Allès B Méjean C Andrianasolo RM et al Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-santé). Bmj. (2019) 365:l1451. 10.1136/bmj.l1451
4.
Juul F Vaidean G Lin Y Deierlein AL Parekh N . Ultra-Processed foods and incident cardiovascular disease in the framingham offspring study. J Am Coll Cardiol. (2021) 77(12):1520–31. 10.1016/j.jacc.2021.01.047
5.
Kheirouri S Alizadeh M . Dietary inflammatory potential and the risk of neurodegenerative diseases in adults. Epidemiol Rev. (2019) 41(1):109–20. 10.1093/epirev/mxz005
6.
Stark K Massberg S . Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. (2021) 18(9):666–82. 10.1038/s41569-021-00552-1
7.
Perler BK Friedman ES Wu GD . The role of the gut Microbiota in the relationship between diet and human health. Annu Rev Physiol. (2023) 85:449–68. 10.1146/annurev-physiol-031522-092054
8.
Campaniello D Corbo MR Sinigaglia M Speranza B Racioppo A Altieri C et al How diet and physical activity modulate gut microbiota, evidence, and perspectives. Nutrients. (2022) 14(12):2456. 10.3390/nu14122456
9.
Damigou E Anastasiou C Chrysohoou C Barkas F Tsioufis C Pitsavos C et al Prevented fractions of cardiovascular disease cases, by long-term adherence to the Mediterranean diet; the ATTICA study (2002–2022). Nutr Metab Cardiovasc Dis. (2024) 35:103777. 10.1016/j.numecd.2024.10.015
10.
Shivappa N Steck SE Hurley TG Hussey JR Hébert JR . Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17(8):1689–96. 10.1017/S1368980013002115
11.
Geng Lan YL Mei Z . Visualization analysis of dietary inflammatory Index research based on web of science. Food Nutr China. (2024) 30(7):5–10; 5. 10.19870/j.cnki.11-3716/ts.20230807.001
12.
Ji M Hong X Chen M Chen T Wang J Zhang N . Dietary inflammatory index and cardiovascular risk and mortality: a meta-analysis of cohort studies. Medicine (Baltimore). (2020) 99(20):e20303. 10.1097/MD.0000000000020303
13.
Boutron I Hoffmann TC Mulrow CD Shamseer L Tetzlaff JM Akl EA et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. 10.1136/bmj.n71
14.
Asadi Z Yaghooti-Khorasani M Ghazizadeh H Sadabadi F Mosa-Farkhany E Darroudi S et al Association between dietary inflammatory index and risk of cardiovascular disease in the mashhad stroke and heart atherosclerotic disorder study population. IUBMB Life. (2020) 72(4):706–15. 10.1002/iub.2172
15.
Canto-Osorio F Denova-Gutierrez E Sánchez-Romero LM Salmerón J Barrientos-Gutierrez T . Dietary inflammatory Index and metabolic syndrome in Mexican adult population. Am J Clin Nutr. (2020) 112(2):373–80. 10.1093/ajcn/nqaa135
16.
Khan I Kwon M Shivappa N Hébert JR Kim MK . Positive association of dietary inflammatory Index with incidence of cardiovascular disease: findings from a Korean population-based prospective study. Nutrients. (2020) 12(2):588. 10.3390/nu12020588
17.
Liu B Ren X Tian W . Dietary inflammatory potential and the risk of nonfatal cardiovascular diseases in the China health and nutrition survey. Nutrition. (2024) 124:112469. 10.1016/j.nut.2024.112469
18.
MacDonald C-J Laouali N Madika A-L Mancini FR Boutron-Ruault M-C . Dietary inflammatory index, risk of incident hypertension, and effect modification from BMI. Nutr J. (2020) 19(1):62. 10.1186/s12937-020-00577-1
19.
Ramallal R Toledo E Martínez-González MA Hernández-Hernández A García-Arellano A Shivappa N et al Dietary inflammatory Index and incidence of cardiovascular disease in the SUN cohort. PLoS One. (2015) 10(9):e0135221. 10.1371/journal.pone.0135221
20.
Villaverde P Rivera-Paredez B Argoty-Pantoja AD Velázquez Cruz R Salmerón J . Dietary inflammatory Index and blood pressure levels in Mexican adults. Nutrients. (2024) 16(18):3052. 10.3390/nu16183052
21.
Vissers LET Waller MA van der Schouw YT Hebert JR Shivappa N Schoenaker DAJM et al The relationship between the dietary inflammatory index and risk of total cardiovascular disease, ischemic heart disease and cerebrovascular disease: findings from an Australian population-based prospective cohort study of women. Atherosclerosis. (2016) 253:164–70. 10.1016/j.atherosclerosis.2016.07.929
22.
Vissers LET Waller M van der Schouw YT Hébert JR Shivappa N Schoenaker DAJM et al A pro-inflammatory diet is associated with increased risk of developing hypertension among middle-aged women. Nutr Metab Cardiovasc Dis. (2017) 27(6):564–70. 10.1016/j.numecd.2017.03.005
23.
Wu M Li S Lv Y Liu K Wang Y Cui Z et al Associations between the inflammatory potential of diets with adherence to plant-based dietary patterns and the risk of new-onset cardiometabolic diseases in Chinese adults: findings from a nation-wide prospective cohort study. Food Funct. (2023) 14(19):9018–34. 10.1039/D3FO02579A
24.
Xu Z Li X Ding L Zhang Z Sun Y . The dietary inflammatory index and new-onset hypertension in Chinese adults: a nationwide cohort study. Food Funct. (2023) 14(24):10759–69. 10.1039/D3FO03767C
25.
Zuercher MD Harvey DJ Santiago-Torres M Au LE Shivappa N Shadyab AH et al Dietary inflammatory index and cardiovascular disease risk in hispanic women from the women’s health initiative. Nutr J. (2023) 22(1):5. 10.1186/s12937-023-00838-9
26.
Bondonno NP Lewis JR Blekkenhorst LC Shivappa N Woodman RJ Bondonno CP et al Dietary inflammatory index in relation to sub-clinical atherosclerosis and atherosclerotic vascular disease mortality in older women. Br J Nutr. (2017) 117(11):1577–86. 10.1017/S0007114517001520
27.
Choi MK Park Y-MM Shivappa N Hong O-K Han K Steck SE et al Inflammatory potential of diet and risk of mortality in normal-weight adults with central obesity. Clin Nutr. (2023) 42(2):208–15. 10.1016/j.clnu.2022.11.019
28.
Deng FE Shivappa N Tang Y Mann JR Hebert JR . Association between diet-related inflammation, all-cause, all-cancer, and cardiovascular disease mortality, with special focus on prediabetics: findings from NHANES III. Eur J Nutr. (2017) 56(3):1085–93. 10.1007/s00394-016-1158-4
29.
Huang J Zhang Y Li J Li H Wei Y Sun M . Association of dietary inflammatory index with all-cause and cardiovascular disease mortality in hyperuricemia population: a cohort study from NHANES 2001 to 2010. Medicine (Baltimore). (2023) 102(51):e36300. 10.1097/MD.0000000000036300
30.
Ma Q Zhang Y Zhang D Liu C Zhu W Wang G et al The relationship between dietary inflammatory index and all-cause and cardiovascular disease-related mortality in adults with metabolic syndrome: a cohort study of NHANES. Front Endocrinol (Lausanne). (2024) 15:1417840. 10.3389/fendo.2024.1417840
31.
Majidi A Hughes MCB Webb IK Miura K van der Pols JC . Inflammatory potential of diet and mortality in Australian adults. Public Health Nutr. (2024) 27(1):e129. 10.1017/S1368980024000909
32.
Okada E Shirakawa T Shivappa N Wakai K Suzuki K Date C et al Dietary inflammatory Index is associated with risk of all-cause and cardiovascular disease mortality but not with cancer mortality in middle-aged and older Japanese adults. J Nutr. (2019) 149(8):1451–9. 10.1093/jn/nxz085
33.
Park S-Y Kang M Wilkens LR Shvetsov YB Harmon BE Shivappa N et al The dietary inflammatory Index and all-cause, cardiovascular disease, and cancer mortality in the multiethnic cohort study. Nutrients. (2018) 10(12):1844. 10.3390/nu10121844
34.
Shivappa N Steck SE Hussey JR Ma Y Hebert JR . Inflammatory potential of diet and all-cause, cardiovascular, and cancer mortality in national health and nutrition examination survey III. Study. Eur J Nutr. (2017) 56(2):683–92. 10.1007/s00394-015-1112-x
35.
Sun S-N Ni S-H Li Y Liu X Deng J-P Ouyang X-L et al Association between dietary inflammatory index with all-cause and cardiovascular disease mortality among older US adults: a longitudinal cohort study among a nationally representative sample. Arch Gerontol Geriatr. (2024) 118:105279. 10.1016/j.archger.2023.105279
36.
Veronese N Cisternino AM Shivappa N Hebert JR Notarnicola M Reddavide R et al Dietary inflammatory index and mortality: a cohort longitudinal study in a Mediterranean area. J Hum Nutr Diet. (2020) 33(1):138–46. 10.1111/jhn.12701
37.
Xie L Liu J Wang X Liu B Li J Li J et al Role of dietary inflammatory index in the association of NT-proBNP with all-cause and cardiovascular mortality in NHANES 1999–2004. Sci Rep. (2024) 14(1):19978. 10.1038/s41598-024-70506-3
38.
Yang M Miao S Hu W Yan J . Association between the dietary inflammatory index and all-cause and cardiovascular mortality in patients with atherosclerotic cardiovascular disease. Nutr Metab Cardiovasc Dis. (2024) 34(4):1046–53. 10.1016/j.numecd.2023.11.015
39.
Yuan S Song C Zhang R He J Dou K . Dietary inflammation Index and its association with long-term all-cause and cardiovascular mortality in the general US population by baseline glycemic Status. Nutrients. (2022) 14(13):2556. 10.3390/nu14132556
40.
Zhou M Cai B Xiao Q Zou H Zeng X Zhao J et al Higher dietary inflammatory Index and increased mortality rate of adults with hyperuricemia: findings from the national health and nutritional examination survey (2001–2018). Arthritis Care Res (Hoboken). (2024) 76(8):1179–86. 10.1002/acr.25336
41.
Garcia-Arellano A Ramallal R Ruiz-Canela M Salas-Salvadó J Corella D Shivappa N et al Dietary inflammatory Index and incidence of cardiovascular disease in the PREDIMED study. Nutrients. (2015) 7(6):4124–38. 10.3390/nu7064124
42.
Neufcourt L Assmann KE Fezeu LK Touvier M Graffouillere L Shivappa N et al Prospective association between the dietary inflammatory Index and cardiovascular diseases in the SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX). Cohort. J Am Heart Assoc. (2016) 5(3):e002735. 10.1161/JAHA.115.002735
43.
Ganbaatar G Okami Y Kadota A Ganbaatar N Yano Y Kondo K et al Association of pro-inflammatory diet with long-term risk of all-cause and cardiovascular disease mortality: NIPPON DATA80. J Atheroscler Thromb. (2024) 31(3):326–43. 10.5551/jat.64330
44.
da Silva A Felício MB Caldas APS Miranda Hermsdorff HH Bersch-Ferreira ÂC Torreglosa CR et al Pro-inflammatory diet is associated with a high number of cardiovascular events and ultra-processed foods consumption in patients in secondary care. Public Health Nutr. (2021) 24(11):3331–40. 10.1017/S136898002000378X
45.
Georgousopoulou EN Kouli G-M Panagiotakos DB Kalogeropoulou A Zana A Chrysohoou C et al Anti-inflammatory diet and 10-year (2002–2012) cardiovascular disease incidence: the ATTICA study. Int J Cardiol. (2016) 222:473–8. 10.1016/j.ijcard.2016.08.007
46.
Steven S Frenis K Oelze M Kalinovic S Kuntic M Bayo Jimenez MT et al Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. (2019) 2019:7092151. 10.1155/2019/7092151
47.
Chai W Morimoto Y Cooney RV Franke AA Shvetsov YB Le Marchand L et al Dietary red and processed meat intake and markers of adiposity and inflammation: the multiethnic cohort study. J Am Coll Nutr. (2017) 36(5):378–85. 10.1080/07315724.2017.1318317
48.
Madan M Bishayi B Hoge M Amar S . Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis. (2008) 197(2):504–14. 10.1016/j.atherosclerosis.2007.02.023
49.
Zhao Z Li L Gao X Hu G Liu G Tao H et al High dietary inflammatory index is associated with decreased plaque stability in patients with coronary heart disease. Nutr Res. (2023) 119:56–64. 10.1016/j.nutres.2023.08.007
50.
Bentzon JF Otsuka F Virmani R Falk E . Mechanisms of plaque formation and rupture. Circ Res. (2014) 114(12):1852–66. 10.1161/CIRCRESAHA.114.302721
51.
Muscolo A Mariateresa O Giulio T Mariateresa R . Oxidative stress: the role of antioxidant phytochemicals in the prevention and treatment of diseases. Int J Mol Sci. (2024) 25(6):3264. 10.3390/ijms25063264
52.
Barillà F Cammisotto V Bartimoccia S Loffredo L Nocella C Bruno N et al Toll-like receptor 4 activation in platelets from myocardial infarction patients. Thromb Res. (2022) 209:33–40. 10.1016/j.thromres.2021.11.019
53.
Gatarek P Kaluzna-Czaplinska J . Trimethylamine N-oxide (TMAO) in human health. Excli J. (2021) 20:301–19. 10.17179/excli2020-3239
54.
Witkowski M Witkowski M Friebel J Buffa JA Li XS Wang Z et al Vascular endothelial tissue factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc Res. (2022) 118(10):2367–84. 10.1093/cvr/cvab263
55.
Zhang X Li Y Yang P Liu X Lu L Chen Y et al Trimethylamine-N-Oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κB) signals. Arterioscler Thromb Vasc Biol. (2020) 40(3):751–65. 10.1161/ATVBAHA.119.313414
56.
Wang Z Klipfell E Bennett BJ Koeth R Levison BS DuGar B et al Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. (2011) 472(7341):57–63. 10.1038/nature09922
57.
Liu X Xie Z Sun M Wang X Li J Cui J et al Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. Int J Cardiol. (2018) 265:18–23. 10.1016/j.ijcard.2018.04.126
58.
Tang TWH Chen H-C Chen C-Y Yen CYT Lin C-J Prajnamitra RP et al Loss of gut Microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. (2019) 139(5):647–59. 10.1161/CIRCULATIONAHA.118.035235
59.
Li D Lu Y Yuan S Cai X He Y Chen J et al Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: an umbrella review and updated meta-analysis. Am J Clin Nutr. (2022) 116(1):230–43. 10.1093/ajcn/nqac074
60.
Rivera K Gonzalez L Bravo L Manjarres L Andia ME . The gut-heart axis: molecular perspectives and implications for myocardial infarction. Int J Mol Sci. (2024) 25(22):12465. 10.3390/ijms252212465
61.
Cho CE Taesuwan S Malysheva OV Bender E Tulchinsky NF Yan J et al Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. (2017) 61(1):1600324. 10.1002/mnfr.201600324
62.
Xing D Nozell S Chen Y-F Hage F Oparil S . Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. (2009) 29(3):289–95. 10.1161/ATVBAHA.108.182279
63.
Tabung FK Steck SE Zhang J Ma Y Liese AD Agalliu I et al Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. (2015) 25(6):398–405. 10.1016/j.annepidem.2015.03.009
64.
Li S Kararigas G . Role of biological sex in the cardiovascular-gut microbiome axis. Front Cardiovasc Med. (2021) 8:759735. 10.3389/fcvm.2021.759735
65.
Peters BA Lin J Qi Q Usyk M Isasi CR Mossavar-Rahmani Y et al Menopause is associated with an altered gut microbiome and estrobolome, with implications for adverse cardiometabolic risk in the hispanic community health study/study of latinos. mSystems. (2022) 7(3):e0027322. 10.1128/msystems.00273-22
66.
Mayneris-Perxachs J Arnoriaga-Rodríguez M Luque-Córdoba D Priego-Capote F Pérez-Brocal V Moya A et al Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status. Microbiome. (2020) 8(1):136. 10.1186/s40168-020-00913-x
67.
Dodd KW Guenther PM Freedman LS Subar AF Kipnis V Midthune D et al Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. (2006) 106(10):1640–50. 10.1016/j.jada.2006.07.011
68.
McCullough LE Byrd DA . Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. (2023) 192(11):1801–5. 10.1093/aje/kwac071
69.
Schisterman EF Cole SR Platt RW . Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. (2009) 20(4):488–95. 10.1097/EDE.0b013e3181a819a1
70.
Hua R Liang G Yang F . Meta-analysis of the association between dietary inflammation index and C-reactive protein level. Medicine (Baltimore). (2024) 103(19):e38196. 10.1097/MD.0000000000038196
71.
Shamah-Levy T Rodríguez-Ramírez S Gaona-Pineda EB Cuevas-Nasu L Carriquiry AL Rivera JA . Three 24-hour recalls in comparison with one improve the estimates of energy and nutrient intakes in an urban mexican population. J Nutr. (2016) 146(5):1043–50. 10.3945/jn.115.219683
72.
Bagheri S Zolghadri S Stanek A . Beneficial effects of anti-inflammatory diet in modulating gut Microbiota and controlling obesity. Nutrients. (2022) 14(19):3985. 10.3390/nu14193985
Summary
Keywords
cardiovascular diseases, dietary inflammatory index, risk, mortality, meta-analysis, updated systematic review
Citation
Ni Y, Yao Q, Xu T and Li X (2025) Dietary inflammatory index and cardiovascular risk and mortality: an updated systematic review and meta-analysis. Front. Cardiovasc. Med. 12:1626523. doi: 10.3389/fcvm.2025.1626523
Received
11 May 2025
Revised
31 October 2025
Accepted
04 November 2025
Published
20 November 2025
Volume
12 - 2025
Edited by
Alexander Akhmedov, University of Zurich, Switzerland
Reviewed by
George Grant, Independent Researcher, Aberdeen, United Kingdom
Stamatia Angeliki Kleftaki, Harokopio University, Greece
Updates
Copyright
© 2025 Ni, Yao, Xu and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xiuchuan Li 18180595453@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.