- 1Department of Medicine, Henry Ford Hospital, Detroit, MI, United States
- 2Division of Internal Medicine, Rutgers Health Community Medical Center, New Brunswick, NJ, United States
- 3Division of Cardiology, Department of Medicine, Henry Ford Hospital, Detroit, MI, United States
- 4Harrington Heart & Vascular Institute, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 5Department of Neurosurgery, The Loyal and Edith Davis Neurosurgical Research Laboratory, Barrow Neurological Institute, St. Joseph's Hospital and Medical Center, Phoenix, AZ, United States
- 6Division of Neurosurgery, Department of Surgery, Chulabhorn Hospital, Chulabhorn Royal Academy, Bangkok, Thailand
- 7Department of Medicine, Adena Regional Medical Center, Chillicothe, OH, United States
- 8Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, MN, United States
- 9Division of Health Care Policy and Research, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, United States
- 10The Texas Heart Institute, Baylor College of Medicine, Houston, TX, United States
- 11Department of Cardiology I- Coronary and Peripheral Vascular Disease, Heart Failure Medicine, University Hospital Muenster, Muenster, Germany
- 12Department of Cardiology, Faculty of Health, School of Medicine, University Witten/Herdecke, Witten, Germany
- 13HumanX, Delaware City, DE, United States
Background: Patients admitted with acute ischemic stroke (AIS) may experience accompanying acute ST-Segment myocardial infarction after AIS. The cardiovascular risks, incidence, complications, and outcomes of acute STEMI in patients hospitalized with AIS remains underexplored.
Methods: We evaluated 2,804,819 patients that presented with AIS who were listed in the National Inpatient Sample from 2016 to 2021. AIS and STEMI were defined according to the ICD-10 Diagnostic Codes. Patients with Non-STEMI were excluded. The risk of specific complications and outcomes were expressed as percentages. Multivariable logistic regression analysis was used to examine the association of STEMI with a primary outcome of mortality and secondary outcomes. The temporal trend of both the incidence of STEMI after AIS as well as the mortality rate between 2016 and 2021 were expressed as percentages over time.
Results: Of the total (n = 2,804,819) patients with AIS, 6,550 also had STEMI diagnosed during the hospitalization. Of these, 1,635 (24.96%) died in the STEMI group and 86,810 (3.10%) died in the group without STEMI. All of the secondary outcome measures were significantly associated with a diagnosis of STEMI. STEMI was associated with mortality [OR 7.43 (95% CI, 6.44–8.57); P < 0.001], cardiogenic shock [OR 29.64, (95% CI, 22.64–38.81); P < 0.001], cardiac arrest [OR, 7.76 (95% CI, 6.01–10.03); P < 0.001], and AKI [OR 1.96 (95% CI, 1.72–2.23); P < 0.001] among other complications. When assessed yearly, the temporal trend of STEMI among AIS patients showed a decrease in frequency from about 0.3% in 2016 to about 0.2% in 2021. Furthermore, comparing the mortality between AIS patients with and without STEMI showed a significant difference with a higher mortality in the AIS with STEMI population.
Conclusions: Patients admitted with acute ischemic stroke who had STEMI have a significant mortality increase compared to those who did not have STEMI. They also had a significant increase in secondary complications including cardiac arrest, cardiogenic shock, AKI, and need for further medical interventions. Temporally, we have seen a decrease in STEMI after AIS over the interval.
Introduction
Stroke and ST-segment elevation myocardial infarction (STEMI) are two leading causes of death and disability worldwide. Epidemiologically, both conditions are driven by atherosclerosis and share several risk factors, including hypertension, diabetes, smoking, and hyperlipidemia. The global burden of stroke remains high, with an estimated 12.2 million new strokes each year, leading to 6.5 million deaths annually. Similarly, STEMI contributes significantly to the overall burden of ischemic heart disease, with thousands of new cases reported each year. In the United States alone, there are approximately 800,000 strokes and 900,000 STEMI events annually.
Despite sharing common pathophysiological mechanisms, including atherosclerosis, thrombosis, and inflammation, the interplay between these two conditions when they occur concurrently remains poorly understood because although, both stroke and myocardial infarction have been studied in isolation, the intersection of these two critical events presents unique challenges that are not adequately addressed by current clinical practices. Moreover, the absence of robust contemporary data impedes efforts to fully understand the changing epidemiology of this overlap, the associated risk factors, and the optimal therapeutic strategies.
Given the paucity of contemporary data, our objective was to investigate the trends, clinical characteristics, and outcomes of patients hospitalized with AIS, stratified by the presence of STEMI.
Methods
This retrospective cohort study used the National Inpatient Sample (NIS), the largest publicly available all-payer inpatient database in the United States, which is part of the Healthcare Cost and Utilization Project (HCUP). The NIS provides a 20% stratified sample of U.S. hospital discharges and allows for the generation of nationally representative estimates of inpatient outcomes.
Study population
We included adult patients (aged ≥18 years) hospitalized between 2016 and 2021 with a primary diagnosis of AIS identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes I63.x.x From this cohort, patients who had a concurrent diagnosis of STEMI during the same hospitalization were identified using the ICD−10-CM codes including I21.0, I21.1, I21.2, I21.3, I22.0, I22.1, I22.8 and I22.9. We excluded patients with non-ST elevation myocardial infarction (NSTEMI), intracranial hemorrhage, and those with missing data on key demographic and clinical variables.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included the occurrence of acute kidney injury (AKI), cardiogenic shock, cardiac arrest, gastrointestinal (GI) bleeding, pericardial effusion, cardiac tamponade, cost of hospitalization and length of stay. In addition, we evaluated the trends of STEMI among AIS hospitalization as well as the trends of in-hospital mortality among AIS hospitalizations, stratified by presence of STEMI.
Statistical analysis
All analyses accounted for the complex survey design using appropriate discharge-level weights to produce national estimates. Descriptive statistics were computed, with continuous variables presented as means (± standard deviations) and categorical variables as proportions. For comparisons between groups (AIS with and without STEMI), we used the chi-square test for categorical variables and the linear regression for continuous variables. Multivariable logistic regression models were implemented to assess the outcomes, adjusting for patient demographics, comorbidities, and hospital characteristics. Results were presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). All statistical analyses were performed using Stata version 17.0 (StataCorp LLC, College Station, TX).
Results
We evaluated 2,804,819 patients who presented to the hospital with Acute Ischemic Stroke. Of these patients who presented primarily for the AIS, 6,550 had a STEMI diagnosed during the hospitalization while 2,798,269 patients did not have a STEMI.
In the AIS population without concomitant STEMI, the average age was 70 years old and 49.78% of the cohort was female. In addition, the predominant race was white (68.14% of the population), followed by African American and Hispanic. In the AIS stroke that had concurrent STEMI, the average age was 70.48 years old, and 49.31% of the cohort was female. In addition, the predominant race was white (68.17% of the population) followed by African American and Hispanic.
Significant comorbidities that existed among both groups included heart failure, atrial fibrillation, valvular heart disease, hypertension, family history of ischemic heart disease, hyperlipidemia, hypothyroidism, liver disease, coagulopathy, obesity, fluid and electrolyte disorders, alcohol use, depression, prior percutaneous coronary intervention, prior myocardial infarction, prior stroke, cancer, obstructive sleep apnea (Table 1).
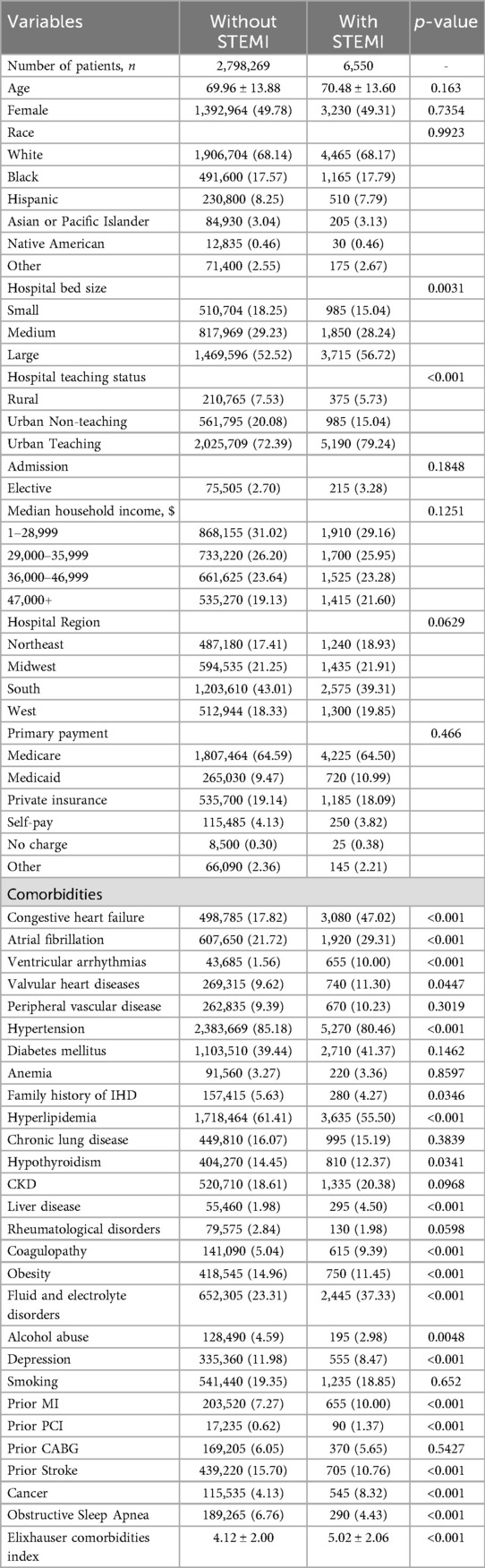
Table 1. Baseline characteristics of acute ischemic stroke patients with and without ST-elevation myocardial infarction (STEMI).
In patients with AIS and concomitant STEMI, compared to those without STEMI, there were statistically significant differences in mortality, AKI, cardiac arrest, cardiogenic shock, GI bleeding, pericardial effusion, cardiac tamponade, septic shock, cost of hospitalization, and length of stay. The adjusted odds ratio for mortality was 7.43 (CI of 6.44–8.57 with p-value < .001), the adjusted odds ratio for AKI was 1.96 (CI of 1.72–2.23 with p-value < .001), the adjusted odds ratio for cardiac arrest was 7.76 (CI of 6.01–10.03 with p-value < .001), the adjusted odds ratio for cardiogenic shock was 29.64 (CI of 22.64–38.81 with p-value < .001). There was also a statistically significant difference in the need for procedures between the two groups in terms of requiring PCI, Fibrinolysis, mechanical intracranial thrombectomy, blood transfusion, use of mechanical circulatory support, ICD or pacemaker insertion, tracheostomy, and intubation (Tables 2, 3). We also sought to evaluate the yearly incidence of STEMI among those hospitalized with acute ischemic stroke between 2016 and 2021. Between 2016 and 2021, the incidence of STEMI among AIS admissions decreased from about 3 cases per 1,000 patients to about 2 cases per 1,000 patients and demonstrated a modest decline over the time. Figure 1 illustrates the annual incidence expressed as a percent of patients who had AIS with STEMI among all patients with AIS for each given year, and provides a more accurate depiction of trends in this population and shows that from 2016 to 2021, the frequency of STEMI in this population has decreased from around 0.30% in 2016 to about 0.20% in 2021. Furthermore, Figure 2 compared mortality of hospitalizations with acute ischemic stroke stratified between those who had concomitant STEMI and those who did not. It showed a significant difference with a higher mortality in the AIS with STEMI population compared to without STEMI.
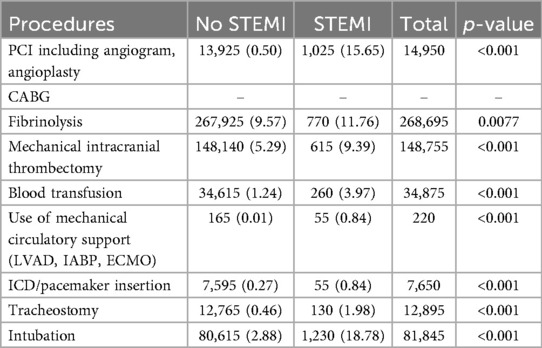
Table 2. Procedures between acute ischemic stroke patients with and without ST-elevation myocardial infarction (STEMI).
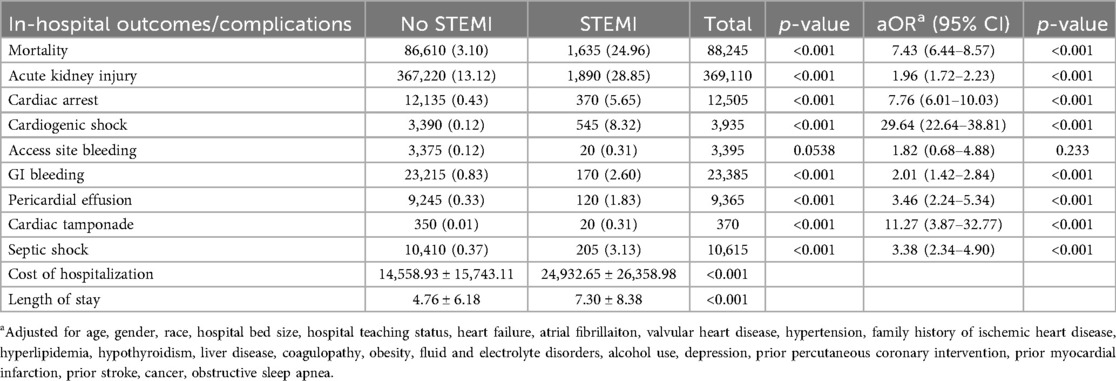
Table 3. Unadjusted and adjusted outcomes between acute ischemic stroke patients with and without ST-elevation myocardial infarction (STEMI).
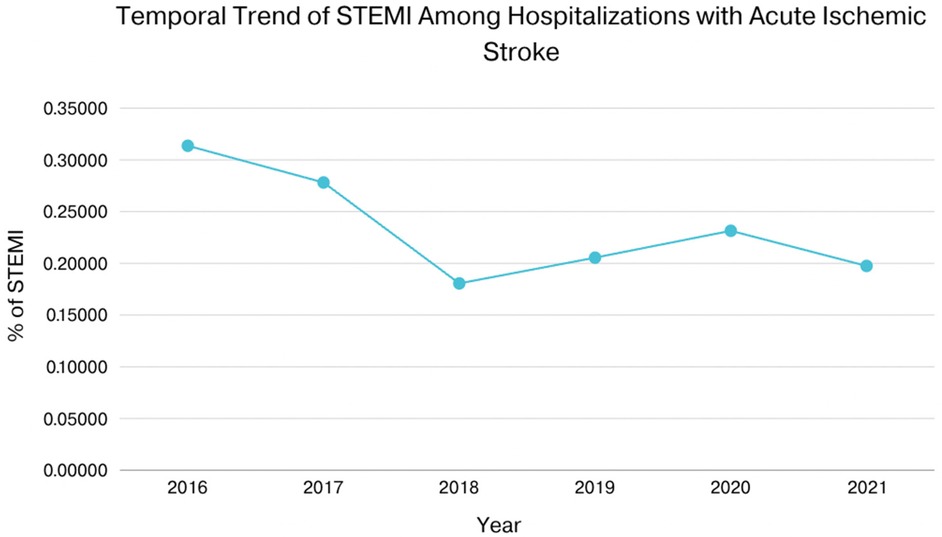
Figure 1. Shows that from 2016 to 2021, the yearly incidence of STEMI in this population has decreased from around 0.3% in 2016 to about 0.2% in 2021.
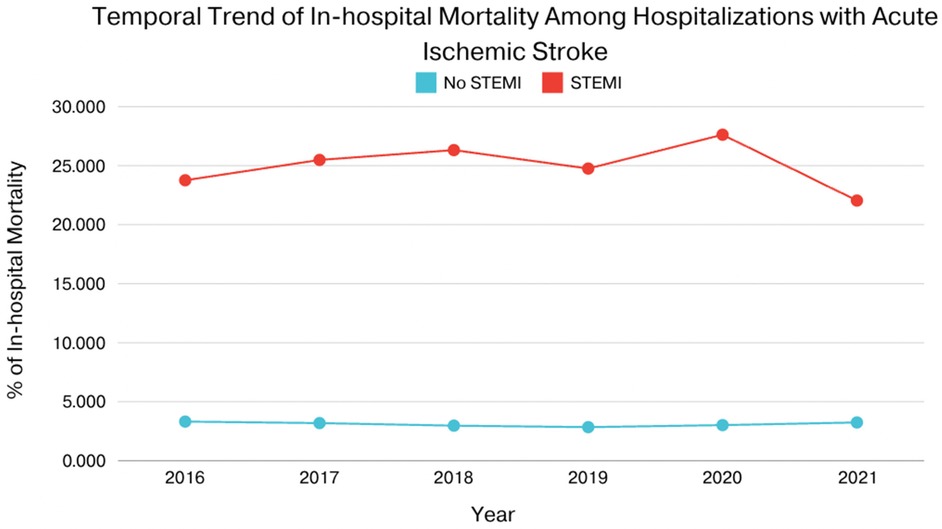
Figure 2. Compared mortality of hospitalizations with acute ischemic stroke stratified between those who had concomitant STEMI and those who did not.
Discussion
The pathogenesis of acute ischemic stroke (AIS) and ST segment elevation myocardial infarction (STEMI) share several similarities, primarily characterized by ischemic etiology and inflammatory processes. Both conditions result from a significant reduction in blood flow due to the obstruction of blood vessels; STEMI typically occurs due to rupture of an atherosclerotic plaque and subsequent thrombus formation in coronary arteries, while AIS may arise from thromboembolic occlusion of cerebral arteries or large vessel disease. In both scenarios, inflammatory processes, platelet activation, and coagulation play critical roles in thrombus formation, contributing to tissue damage. However, key differences exist between the two conditions. Notably, the affected anatomical regions differ, with STEMI impacting the heart and AIS affecting the brain, which has profound implications for tissue response and recovery mechanisms. Additionally, the underlying causes of each condition can vary. While both conditions can arise from atherosclerosis, STEMI is more frequently associated with acute plaque rupture and thrombosis, whereas AIS can also occur due to systemic factors like atrial fibrillation or carotid artery disease, leading to embolic strokes (1) Treatment strategies also differ significantly; STEMI is primarily managed through percutaneous coronary intervention or thrombolytics, AIS often requires thrombolysis or mechanical thrombectomy. Recent multicenter studies have further refined predictors of poor outcomes and hemorrhagic transformation following alteplase in AIS, underscoring the importance of individualized risk assessment in this setting (2, 3). These findings reinforce that while thrombolysis remains a cornerstone of AIS management, its risks, particularly in patients with atrial fibrillation or concomitant cardiac disease, necessitate careful patient selection and multidisciplinary decision-making. The time sensitivity for intervention varies. While both conditions are considered acute emergencies, the brain is less tolerant of ischemia compared to the heart, necessitating faster intervention for AIS to minimize irreversible damage. Although the pathogenesis of both acute ischemic stroke and STEMI are often similar and linked to parallel factors, there is not significant data studying and managing patients admitted for stroke who then experience a STEMI.
There is also a mechanistic link between AIS and STEMI. The occurrence of STEMI in patients hospitalized with AIS may be explained through several interconnected mechanisms collectively described as the stroke-heart syndrome (4) acute brain injury can trigger autonomic dysregulation with excessive sympathetic activation and parasympathetic withdrawal, resulting in a catecholamine surge that predisposed to myocardial ischemia, arrhythmia, and contractile dysfunction. This neurocardiogenic injury is further compounded by systemic inflammatory responses and endothelial dysfunction which amplify pro-thrombotic pathways and microvascular damage (5). In addition, stroke itself promotes a transient hypercoagulable state through platelet activation, increasing circulating clotting factors, and impaired fibrinolysis, therapy increasing the risk of coronary artery thrombosis (6). Finally, AIS patients frequently have concomitant atherosclerotic disease, which, in the setting of heightened hemodynamic stress and metabolic demand, may destabilize vulnerable coronary plaques and precipitate acute coronary occlusion (7–9). Together, these mechanisms provide a pathophysiological framework that explains the markedly higher rates of myocardial infarction, cardiogenic shock, and mortality observed in our cohort of AIS patients with STEMI (10, 11).
In 2003, Adam et al. (12) described coronary risk evaluation in patients with TIA and ischemic stroke. Given that stroke and myocardial infarction share common risk factors and pathological mechanisms, coronary artery disease is an important cause of death in patients with cerebrovascular disease. This Scientific statement was made to highlight issues in management of CAD patients with brain ischemia who do not have previously identified CAD, and provide recommendations for further research to determine the optimal approach to recognize and treat asymptomatic CAD in patients with cerebral ischemia. This was a stepping stone in increasing research in not just CAD but also incidence and treatment of STEMI in cerebrovascular patients.
Amarenco et al. (7) describes coronary artery disease and risk of major vascular events after cerebral infarction. This was the first study to correlate angiographic findings with subsequent cardiovascular events in patients with cerebral infarction. In patients with cerebral infarction, asymptomatic CAD is highly predictive of future major cardiovascular events.
These studies were also frameworks for further investigation that focused not only on CAD, but also on myocardial infarctions. Toueze and colleagues (13) performed a meta-analysis and systematic review to determine the risk of MI and nonstroke vascular death after TIA, utilizing 39 cohort studies with a total of 65,996 patients. Through previous analyses, it was found that compared with the general population, stroke patients have an increased risk of death that notably results from myocardial infarction. But there was no reliable estimation of the absolute risk of MI and vascular death after stroke and high risk populations. This systemic review and meta-analysis aimed to study the absolute risk of MI and vascular death after stroke or TIA. The main result of this meta-analysis is that after a stroke or a TIA, the risks of MI and nonstroke vascular death are each about 2% per year, which is usually considered high absolute risks in different guidelines for assessment of cardiovascular risk. Given that patients with TIA or stroke have a relatively high risk of MI and nonstroke vascular death, it leads us to where further research may be needed such as the need to identify the determinants of coronary artery disease in stroke patients. It also urges us to improve secondary prevention. These can be in the form of preventive measures without CAD screening, systematic screening of asymptomatic patients, selective screening based on risk stratification, while also being conscious of the downfalls of over-screening. Further research to identify and address factors that lead to risk of CAD and address the best possible interventions to decrease both cardiac morbidity and mortality in stroke patients.
In 2017, in Alqahtani et al. (14) studied the incidence and outcomes of myocardial infarction in patients admitted with ischemic stroke. They utilized the National Inpatient Sample to identify patients with AIS between 2003 and 2014 who were then analyzed for incidence of MI and in hospital mortality. They found that AMI after a stroke carried a substantial in-hospital mortality and cost of care and that although coronary angiography was utilized in a minority of these patients, there may be improved survival in those who underwent coronary angiography with or without intervention. They suggested further studies were needed to discern the ideal approach to addressing STEMI in AIS patients.
Our data seems to be congruent with the results of Alqahtani et al. and Amerenco et al. in which we found a significant mortality increase in those admitted with AIS who experienced STEMI in comparison to those who did not have a STEMI. There also was a significant improvement in hospital mortality with those who ended up undergoing cardiac catheterization. The studies differed in that Al Qahtani et al. reported an increase in myocardial infarction over their studied time interval, however, this was mainly driven by an increase in diagnosis of NSTEMI's whereas our study showed a decrease in myocardial infarction, and focused particularly on STEMI's.
However, there were also several limitations of the study. First, the National Inpatient Sample (NIS) is a discharge-level administrative database without patient identifiers; therefore, repeat hospitalizations for the same individual cannot be distinguished. Second, important clinical details that influence outcomes in acute ischemic stroke (AIS) are not available, including baseline modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS) scores when not coded, TOAST classification, vascular territory, blood pressure trajectories, temperature at presentation, and door-to-needle times. Third, while we report rates of fibrinolysis and mechanical thrombectomy, the NIS does not capture the specific thrombolytic agent (e.g., alteplase vs. tenecteplase), the timing of administration, or subsequent hemorrhagic complications. Thus, not separating acute ischemic stroke by severity which is a key confounder. Fourth, as with all administrative datasets, diagnoses and procedures are subject to potential coding misclassification. However, prior validation studies have demonstrated high accuracy for both ischemic stroke and STEMI codes in ICD-10. Despite these limitations, the large, nationally representative sample and robust associations observed strengthen the validity and clinical relevance of our findings.
In conclusion, our study highlights the incidence of STEMI in patients already hospitalized with AIS, risk factors that increase the risk, and the outcomes and complications that tend to arise after experiencing a STEMI after a stroke. This dual pathology requires an integrated approach to management that addresses the complexities of treating concurrent ischemic events.
The question of whether cardiac screening should be intensified in AIS has been increasingly recognized within the broader concept of stroke-heart interactions. Current AHA/ASA stroke guidance recommends obtaining a 12-lead ECG and baseline cardiac troponin at admission, along with continuous in-hospital rhythm monitoring to detect atrial fibrillation and malignant arrhythmias. In patients with symptoms, ischemic-type ECG changes, or dynamic troponin elevation, serial ECG's and repeat troponin testing over the first 24–48 h are advised, ideally with early cardiology involvement. For secondary prevention, particularly in cryptogenic stroke, extended outpatient rhythm monitoring, including long-term patch monitoring or implantable loop recorders, is supported because it significantly improves atrial fibrillation detection and alters management. While stroke-specific ACS algorithms are lacking, contemporary cardiology guidelines provide a framework for management when STEMI is suspected during AIS hospitalization. This includes rapid ECG acquisition, serial high-sensitivity troponin testing, and multidisciplinary decision-making to balance urgent revascularization with the elevated risk of intracranial bleeding after thrombolysis or thrombectomy. Recent guidance now further emphasizes risk-adapted strategies: the 2024 ACC Expert Consensus Pathway recommends tailoring arrhythmia monitoring such as short-term event monitor, patch, or implantable devices according to stroke subtype and embolic risk, while the 2025 ACC/AHA ACS guideline highlights the importance of serial high-sensitivity troponin testing and structured multidisciplinary care pathways when ACS is suspected during AIS hospitalization (15, 16). Collectively, these recommendations support a selective, risk-based intensification of cardiac monitoring rather than a universal escalation for all AIS patients.
Future research should focus on elucidating the underlying mechanisms that predispose stroke patients to STEMI, as understanding these pathways may inform targeted therapies and preventive strategies. Furthermore, establishing clear protocols for the early identification and management of STEMI in hospitalized AIS patients is essential. Medicinal therapeutic approaches, such as the dual antiplatelet therapy or novel anticoagulants warrant exploration in this unique patient population to reduce the risk of recurrent events. Additionally, the development of interdisciplinary care teams that include neurologists, cardiologists, and rehabilitation specialists could facilitate comprehensive treatment plans and improve overall outcomes. As the field of cardiovascular and cerebrovascular medicine continues to evolve, ongoing clinical trials are necessary to evaluate the efficacy and safety of various interventions either medical or surgical, ultimately aiming to enhance patient care and reduce mortality and morbidity in this vulnerable group. Addressing the intersection of AIS and STEMI will be crucial for optimizing the management of these patients and improving long term outcomes in those who unique challenges faced by individuals experiencing concurrent ischemic events.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
AM: Writing – review & editing, Writing – original draft. SA: Writing – review & editing. YQ: Writing – review & editing. HH: Writing – review & editing. JT: Writing – review & editing. IR: Writing – review & editing. ZW: Writing – review & editing. MA: Writing – review & editing. MS: Writing – review & editing. CK: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
CK is the founder of HumanX, a cutting-edge company at the intersection of artificial intelligence, blockchain to facilitate precision medicine precision medicine and innovation in healthcare; he declares that his affiliation with HumanX is not a competing interest. CK discloses the following relationships – Member of the American College of Cardiology Solution Set Oversight Committee, the American Heart Association Committee of the Council on Genomic and Precision Medicine, The Lancet Digital Health (Advisory Board), European Heart Journal Digital Health (Editorial board), JACC: Asia (Section Editor), The Journal of Scientific Innovation in Medicine (Associate Editor).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adams RD, Biller J. Stroke. In: Ropper AH, Brown RH, editors. Principles of Neurology. New York, NY: McGraw-Hill (2005). p. 1193–213.
2. Zeinhom MG, Khalil MFE, Kamel IFM, Kohail AM, Rezk SR, Elbassiouny A, et al. Predictors of the unfavorable outcomes in acute ischemic stroke patients treated with alteplase: a multi-center randomized trial. Sci Rep. (2024) 14:5960. doi: 10.1038/s41598-024-56067-5
3. Ahmed SR, Zeinhom MG, Ebied AAMK, Kamel IFM, Almoataz MA, Daabis AMA, et al. A multi-center study on the predictors of different subtypes of hemorrhagic transformation of brain infarction after thrombolysis in atrial fibrillation patients presented with embolic stroke. Sci Rep. (2025) 15:15655. doi: 10.1038/s41598-025-97968-3
4. Wang L, Xiong X, Zheng H, Chen Z, Wang Y. Stroke—heart syndrome: a review of clinical manifestations, mechanisms, and treatment. Front Cardiovasc Med. (2024) 11:1432102. doi: 10.3389/fcvm.2024.1432102
5. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brain–heart interaction: cardiac complications after stroke. Circ Res. (2017) 121(4):451–68. doi: 10.1161/CIRCRESAHA.117.311170
6. Hankey GJ. Stroke and the risk of myocardial infarction and other vascular events. Stroke. (2020) 51(11):3360–70. doi: 10.1161/STROKEAHA.120.030236
7. Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard J-M, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. (2011) 42:22–9. doi: 10.1161/STROKEAHA.110.584086
8. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S, et al. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. (2007) 38(8):2295–302. doi: 10.1161/STROKEAHA.106.471813
9. Seifi A, Carr K, Maltenfort M, Paczynski RP, Wilterdink JL, Moussouttas M, et al. The incidence and risk factors of associated acute myocardial infarction (AMI) in acute cerebral ischemic (ACI) events in the United States. PLoS One. (2014) 9(8):e105785. doi: 10.1371/journal.pone.0105785
10. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association [published correction appears in circulation. 2016 Apr 12;133(15):e599. doi: 10.1161/CIR.0000000000000409]. Circulation. (2016) 133(4):e38–e360. doi: 10.1161/CIR.0000000000000350
11. Otite FO, Khandelwal P, Malik AM, Chaturvedi S, Sacco RL, Romano JG. Ten-Year temporal trends in medical complications after acute intracerebral hemorrhage in the United States. Stroke. (2017) 48(3):596–603. doi: 10.1161/STROKEAHA.116.015746
12. Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke. Circulation. (2003) 108:1278–90. doi: 10.1161/01.CIR.0000090444.87006.CF
13. Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke. Stroke. (2005) 36:2748–55. doi: 10.1161/01.STR.0000190118.02275.33
14. Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. (2017) 48(11):2931–8. doi: 10.1161/STROKEAHA.117.018408
15. Spooner MT, Messé SR, Chaturvedi S, Do MM, Gluckman TJ, Han JK, et al. 2024 ACC expert consensus decision pathway on practical approaches for arrhythmia monitoring after stroke: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. (2025) 85(6):657–81. doi: 10.1016/j.jacc.2024.10.100
16. Rao SV, O’Donoghue ML, Ruel M, Rab T, Tamis-Holland JE, Alexander JH, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI guideline for the management of patients with acute coronary syndromes: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2025) 151(13):e771–e862. doi: 10.1161/CIR.0000000000001309
Keywords: stroke, acute ischeamic stroke, myocardial infarction, ST-segment-elevation myocardial infarction, stroke patient care
Citation: Mahmood A, Ang SP, Qadeer YK, Hassan Virk HU, Tangsrivimol JA, Riaz I, Wang Z, Alam M, Strauss M and Krittanawong C (2025) Incidence and outcomes of hospitalized acute ischemic stroke patients with subsequent ST-segment-elevation myocardial infarction. Front. Cardiovasc. Med. 12:1630805. doi: 10.3389/fcvm.2025.1630805
Received: 18 May 2025; Accepted: 17 September 2025;
Published: 30 October 2025.
Edited by:
Gaetano Santulli, Albert Einstein College of Medicine, United StatesReviewed by:
Mohamed G. Zeinhom, Kafrelsheikh University, EgyptSwati Chand, Westchester Medical Center, United States
Copyright: © 2025 Mahmood, Ang, Qadeer, Hassan Virk, Tangsrivimol, Riaz, Wang, Alam, Strauss and Krittanawong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chayakrit Krittanawong, Q2hheWFrcml0LktyaXR0YW5hd29uZ0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Arhum Mahmood
Arhum Mahmood Song Peng Ang
Song Peng Ang Yusuf Kamran Qadeer3
Yusuf Kamran Qadeer3 Jonathan Alan Tangsrivimol
Jonathan Alan Tangsrivimol Zhen Wang
Zhen Wang Markus Strauss
Markus Strauss Chayakrit Krittanawong
Chayakrit Krittanawong