- 1Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Technology, Boea Wisdom (Hangzhou) Network Technology Co., Ltd., Hangzhou, China
- 3Department of Ultrasound Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China
- 5Department of Vascular Surgery, Traditional Chinese and Western Medicine Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Research Collaboration Group, Cardiovascular Innovation and Research, Cleveland, OH, United States
Objectives: Traditionally, the arterial blood perfusion tube for an extracorporeal left ventricular assist device (LVAD) is placed in the femoral artery, which limits patients' mobility and increases the possibility of lower limb ischemia. We have also developed a new approach for percutaneous placement using the right jugular vein and axillary artery. However, there hasn't been a direct hemodynamic comparison between axillary and femoral perfusion. Therefore, this study estimated the hemodynamic differences between axillary artery and traditional femoral artery perfusion for LVAD.
Methods: Five patients underwent LVAD implantation through the right axillary artery and jugular vein approach at Wuhan Union Hospital. Based on one of their computed tomographic angiography (CTA) data, we established a computational fluid dynamics model. Key hemodynamic parameters, including time-averaged wall shear stress (TAWSS) and oscillatory shear index (OSI), were estimated to be compared.
Results: The axillary perfusion improved perfusion to critical arteries and maintained more stable blood flow distribution, regardless of whether the LVAD supplied 40% or 60% of the total cardiac output. Femoral perfusion caused a substantial reduction in TAWSS and an increase in OSI, suggesting greater blood flow disturbances. In contrast, axillary perfusion maintained TAWSS values closer to normal and exhibited lower OSI, particularly in the thoracic and upper abdominal aorta.
Conclusions: The axillary approach may offer superior hemodynamic performance compared with the conventional femoral approach for percutaneous LVAD, including improved perfusion of abdominal aortic branches and steadier blood flow changes. The jugular-axillary approach could be a promising procedure for future percutaneous LVAD.
Introduction
Extracorporeal left ventricular assist device (Extra-LVAD) is a standard temporary circulatory support device for patients with severe heart failure, whether transitioning to heart transplantation or transitioning to heart repair surgery (1–3). We have developed a new approach using the right jugular vein and the axillary artery (AA) for percutaneous placement to avoid the disadvantages caused by traditional femoral artery (FA) perfusion, as we previously described in a case report (4). Except for our case, some centers used axillary artery perfusion (5–7). However, a direct hemodynamic comparison of LVAD perfusion between the AA and FA approaches has not previously been reported. In this study, based on the computed-tomographic angiography (CTA) data of a male patient with severe heart failure, we established a computational fluid dynamics (CFD) model. We compared hemodynamics during LVAD perfusion using the AA and FA approaches.
The wall shear stress (WSS), a fluid dynamics concept, describes the frictional force a fluid exerts on a solid wall it contacts. Pathologically, changes in WSS can affect vascular endothelial gene expression or solute transport, such as oxygen and low-density lipoprotein (LDL), near the wall, possibly leading to vascular diseases (8–10). Two parameters, time-averaged WSS (TAWSS) and oscillatory shear index (OSI), help characterize WSS. TAWSS averages WSS over a cardiac cycle. Meanwhile, OSI, a dimensionless parameter ranging from 0 to 0.5, measures the direction variability of WSS and indicates steady to highly fluctuating flow. Our study mainly focused on the characteristics of each aorta part.
Materials and methods
Study population
This study was conducted under a protocol approved by the Institutional Review Board of Union Hospital, Affiliated with Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (number: 2023-0429, approval date: June 6, 2023).
The study protocol complies with the ethical principles of the 1975 Declaration of Helsinki. From May 2022 to January 2023, five patients in our center underwent Extra-LVAD placement via the right jugular vein and AA. We used aorta CTA data from one of these patients.
3D Model reconstruction
The contrast-enhanced CTA images were obtained from a heart failure patient with an LVAD. CTA imaging was performed using a dual-source CT scanner (SOMATOM Force, Siemens Medical, Germany, Software Version VA50). Volumetric datasets were retrospectively reconstructed using a multi-segment algorithm, generating thin-section images with a slice thickness of 0.3 mm.
The segmentation region encompassed the aortic lumen and its major branches, extending to the right axillary and femoral arteries as potential LVAD implantation sites. Anatomical model reconstruction was performed through manual segmentation and subsequent smoothing using the open-source software 3D Slicer (Version 5.3.0, National Institutes of Health, USA). The resulting vessel wall model was clipped, and inlet and outlet boundaries were generated to cap the ends, thereby defining a continuous fluid domain for computational simulation.
Computational method
Blood was modeled as an incompressible Newtonian fluid with a density of 1,050 kg/m3 and a dynamic viscosity of 0.0035 Pa·s. The Reynolds-averaged Navier-Stokes (RANS) equations were solved using the Shear-Stress Transport (SST) k-ω turbulence model. This hybrid model was selected for its demonstrated efficacy in predicting flow separation under adverse pressure gradients (11), which is particularly relevant for capturing the jet flow dynamics induced by LVAD insertion, and its ability to accurately resolve near-wall flow, which is critical for reliable Wall Shear Stress (WSS) calculations (12). The model integrates the robustness of the k-ε model in the far-field with the precision of the k-ω model in near-wall regions.
The governing continuity (1) and momentum (2) equations are:
Where ρ is the fluid density, is the velocity vector field, p is the pressure, is the kinematic viscosity.
The spatial discretization employed a second-order upwind scheme, and the pressure-velocity coupling was handled using the Semi-Implicit Method for Pressure-Linked Equations (SIMPLE) algorithm. Solutions were considered converged at each time step when the maximum normalized pressure residual fell below 10−4. The fluid domain was discretized using a hybrid mesh, comprising tetrahedral elements in the core and three layers of prismatic elements adjacent to the wall to capture the boundary layer (Supplementary Figure S1) accurately.
A grid independence study was conducted to ensure robust results (Supplementary Figure S2). Simulations were performed on coarse (∼1 million cells), medium (∼2.2 million cells), and fine (∼5 million cells) meshes for the baseline model (without LVAD). The difference in computed WSS between the medium and fine meshes was less than 1%. Consequently, the medium-resolution mesh was adopted for all subsequent simulations (approximately 2.2 million cells for the baseline, and 2.4 million for LVAD-supported models). The near-wall resolution was controlled to achieve a y+ value of approximately 1, consistent with the SST k-ω model's requirements.
The time step was fixed at 1 ms to ensure temporal accuracy. Simulations were run for three cardiac cycles to achieve a periodic solution, with data from the final cycle used for analysis. The aortic wall was modeled as rigid with a no-slip boundary condition. The simulations were performed using OpenFOAM (version 11).
A physiologically representative flow waveform for the ascending aorta, corresponding to a cardiac output (CO) of 5 L/min for a healthy individual (13), was prescribed at the inlet (Figure 1A). To model the impedance of the peripheral vasculature, a tightly coupled 0D-3D scheme was implemented within OpenFOAM. This scheme solves the three-element Windkessel (0D) model equations in a tightly coupled manner with the 3D Navier-Stokes equations at each time step. A three-element Windkessel (0D) model, characterized by proximal resistance (Rp), compliance (C), and distal resistance (Rd) (14), was applied at each outlet (Figure 2, Supplementary Figure S3). The Windkessel parameters were calibrated to match reference systolic/diastolic pressures and presumed flow splits under normal conditions (without LVAD support).
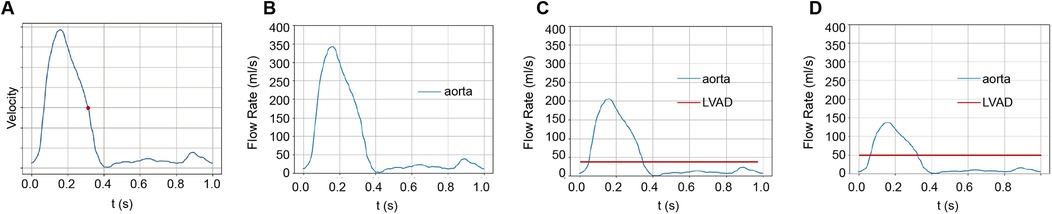
Figure 1. The origins of blood flow during a cardiac cycle. (A) We studied blood flow velocities at the end-systolic phase, marked by the red dot during the cardiac cycle. (B) In the standard group, the heart propels all blood. In this study, we assumed a cardiac output of 5 LPM. (C) With LVAD supplying 40% of total flow, 2 LPM of blood is perfused from the LVAD and 3 LPM from the heart. (D) With LVAD supplying 60% of total flow, 3 LPM perfused from LVAD and 2 LPM from the heart.
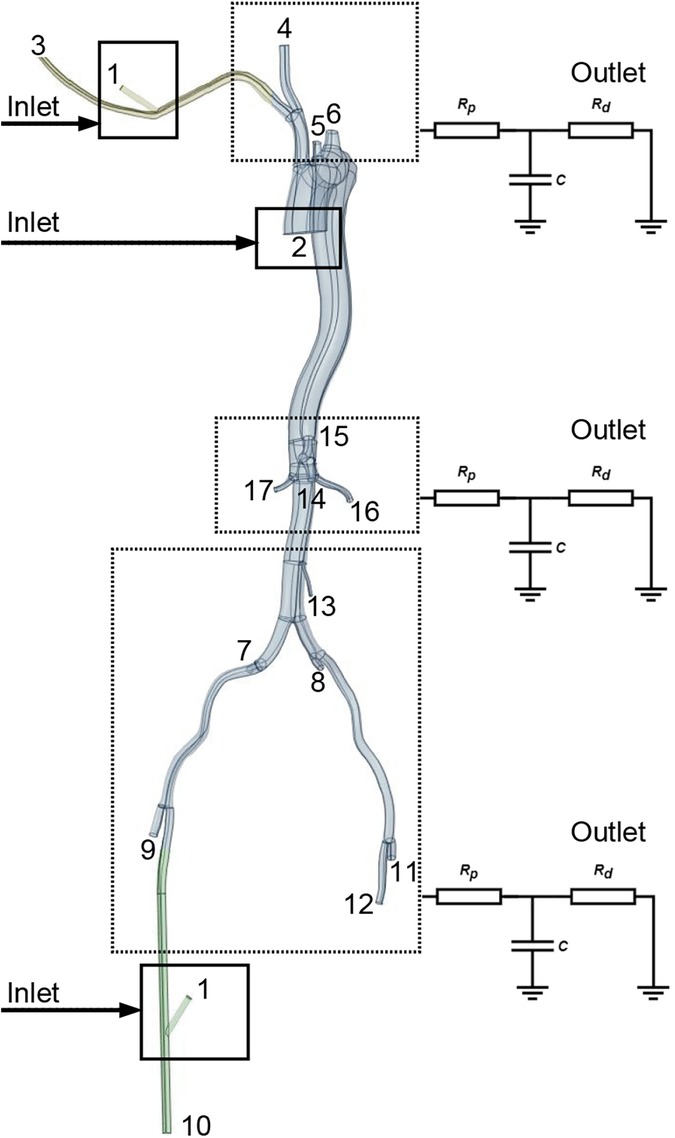
Figure 2. Identification of specific aortic branch locations referenced in Tables 1, 2. Points 1 and 2 signify the LVAD and left ventricle inflow locations. The square denotes the location of the graft for perfusion. Points 3-12 indicate the perfusion outlet sites for each aortic branch, calculated using Windkessel models.

Table 1. Flow divisions in aorta branches with and without LVAD, considering femoral and axillary perfusion at 40% of the total flow.
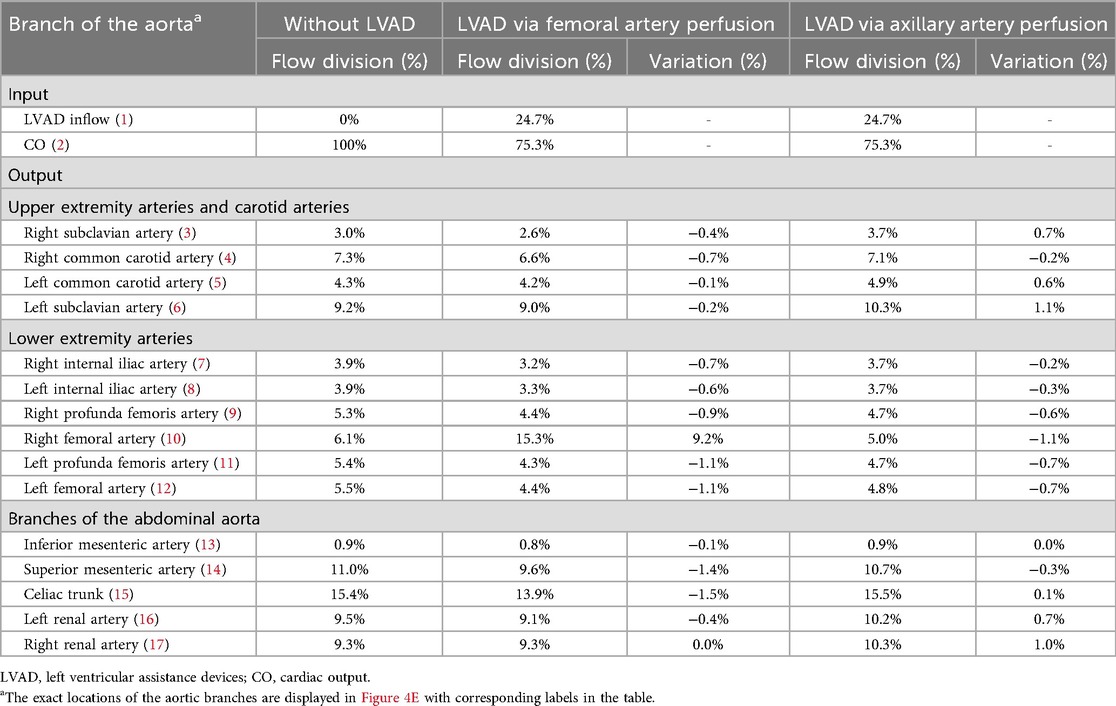
Table 2. Flow divisions in aorta branches with and without LVAD, considering femoral and axillary perfusion at 60% of the total flow.
Boundary conditions
A typical ascending aorta blood flow waveform corresponding to the normal time-average CO, which is assumed to be 5 L/min in this work, was imposed at the inlet, as shown in Figure 1A. The Windkessel models were applied to outlets to represent the impedance of the distal vascular bed to blood flow, as shown in Figure 2 and Supplementary Figure S3.
The LVAD pump function was simplified as a graft inlet with a constant flow rate. Two LVAD support configurations were modeled by introducing a graft at either the FA or the right AA. For each configuration, two constant LVAD flow rates were simulated: 2 L/min and 3 L/min. These were chosen to represent scenarios where the native cardiac output is, respectively, higher or lower than the LVAD output. A summary of the boundary conditions for the standard and LVAD-supported configurations is provided in Supplementary Table S1.
The Windkessel model parameters, calibrated for the baseline condition (without LVAD), were held constant for all simulations with LVAD support. This assumption is based on the established practice in hemodynamic simulations (15–18) and the hypothesis that the characteristics of the distal arterial system remain essentially unchanged immediately post-implantation when total perfusion is restored to a near-normal level (approximately 5 L/min) (19–21). This approach, necessitated by the lack of patient-specific postoperative data, ensures that the observed hemodynamic differences are attributable primarily to the LVAD cannulation site (FA vs. AA).
Results
Aortic branch perfusion estimation via CFD model
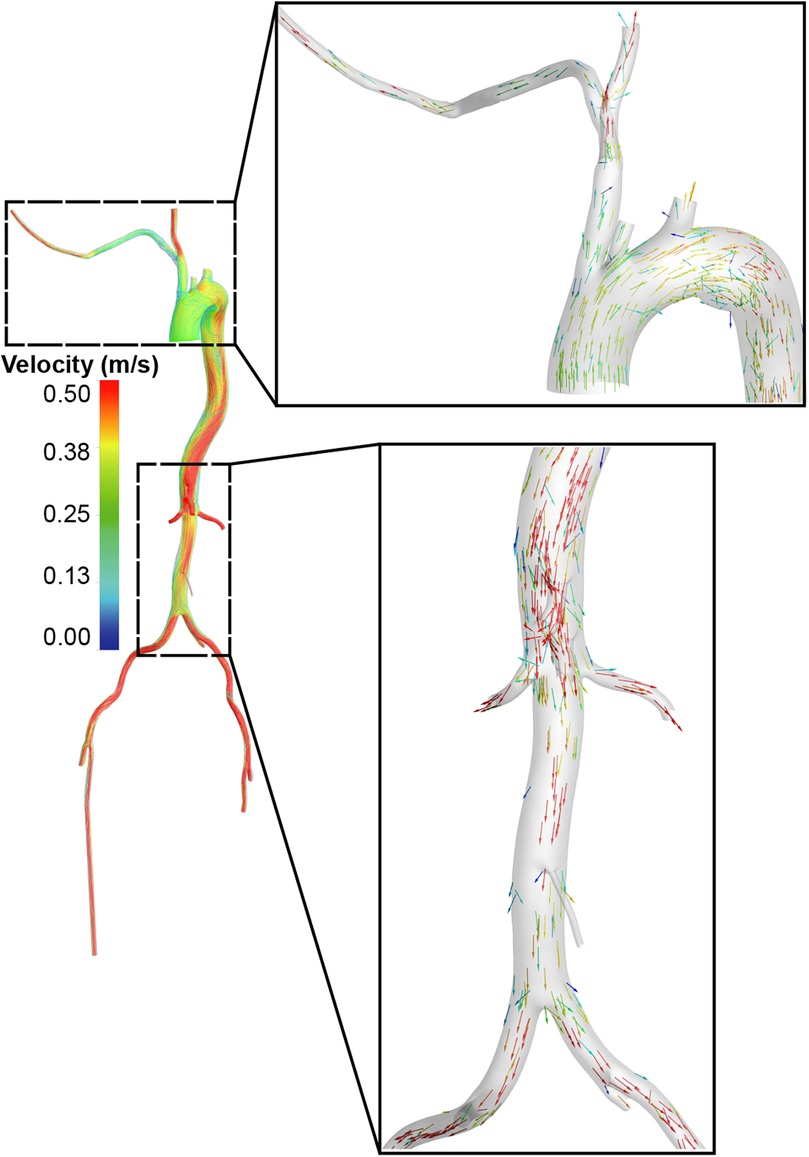
The Extra-LVAD maintained a flow of 1.82-3.25 LPM for all patients, which accounted for 36%–65% of the total flow, with a CO of 5 LPM (Figure 1B). Hence, we studied the hemodynamic changes caused by the Extra-LVAD in our models under two conditions: the LVAD supplying 40% (Figure 1C) and 60% (Figure 1D) of the total flow. Cardiac ejection accounts for 75.3% of total perfusion at the end-systolic phase, with LVAD covering the remaining 24.7%. Tables 1, 2 detail the distribution of blood flow in the aortic branches, with LVAD accounting for 40% or 60% of total blood flow. Figure 2 shows the location of the main aortic branches studied by our research, including the subclavian artery, common carotid artery, internal iliac artery, profunda femoris artery, femoral artery, renal artery, inferior mesenteric artery (IMA), superior mesenteric artery (SMA), and celiac trunk. Figures 3, 4 display the flow patterns. Regardless of the amount of blood flow the LVAD provides, axillary perfusion consistently outperformed femoral perfusion in most of these arteries, except at the placement site and the inferior mesenteric artery.
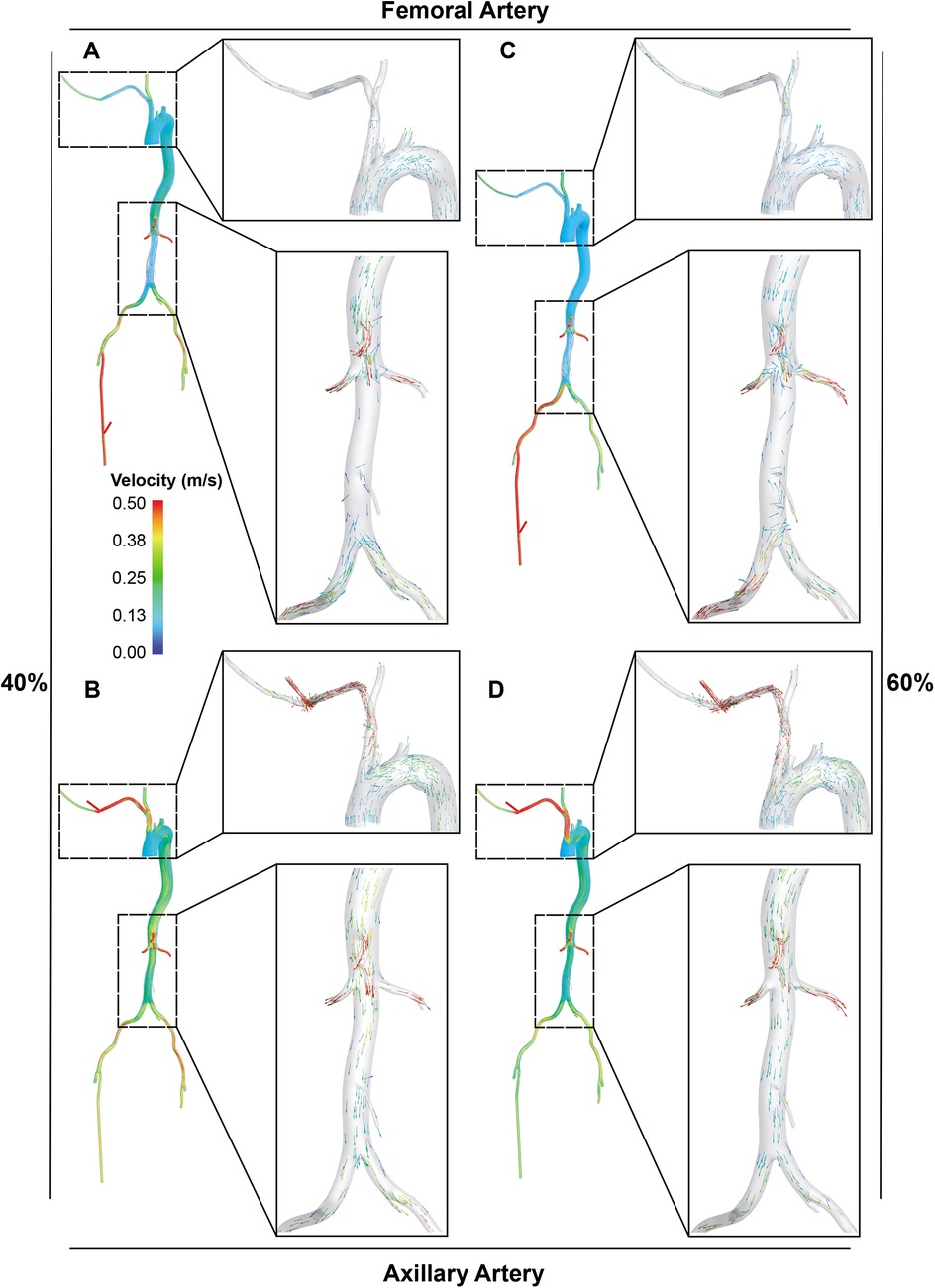
Figure 4. Visualization of blood flow distribution in the aorta and its main branches. A and B. With LVAD supplying 40% of total flow, the blood flow distribution in the femoral (A) and axillary perfusion group (B) is shown. C and D. With LVAD supplying 60% of total flow, blood flow distribution in the femoral (C) and axillary perfusion group (D).
For the abdominal aorta branches, axillary perfusion resulted in a significant decrease in blood supply to the IMA by 44.4% at 40% LVAD flow, but this reduction disappeared at 60% LVAD flow. At 60% LVAD flow, all arteries supplying the intestines and kidneys showed increased blood flow with axillary perfusion compared to femoral perfusion. Specifically, the IMA showed no decrease as opposed to an 11.1% reduction with femoral perfusion, the SMA decreased by 2.8% compared to a 12.7% decrease, the celiac trunk increased by 0.6% instead of decreasing by 9.7% with femoral perfusion, the left renal artery (LRA) increased by 7.4% instead of a 4.2% decrease, and the right renal artery (RRA) increased by 10.8% compared to no change. In the lower extremities, femoral perfusion also led to greater reductions in blood supply. Except for the right femoral artery as the perfusion site, at 60% LVAD flow, blood supply to all lower extremity arteries decreased by more than 15% with femoral perfusion. In comparison, they experienced less than a 13% reduction with axillary perfusion. The upper extremity and carotid artery blood flow remained stable at 40% LVAD, regardless of the perfusion approach. At 60% LVAD flow, axillary perfusion significantly increased blood flow to three of the arteries, including the right subclavian artery (23.3%), the left common carotid artery (14.0%), and the left subclavian artery (12.0%). Still, femoral perfusion decreased the blood flow in these arteries. Blood flow increased notably at the catheter insertion sites, especially the right femoral, with a 151.5% increase at 60% LVAD flow with femoral perfusion.
In terms of blood flow disorders, axillary perfusion may cause turbulent flow in the brachiocephalic trunk (Figures 4B, D), while femoral perfusion may interfere with cardiac ejection in the abdominal aorta, significantly reducing blood flow to it (Figures 4A, C).
WSS assessment in aortic branches via CFD model
The distribution of TAWSS (Figures 5, 6) showed values exceeding 5 Pa at all points where the perfusion graft is attached. With femoral perfusion, TAWSS in the thoracic and upper abdominal aorta above the kidneys decreased significantly to less than 1 Pa, particularly when LVAD contributed 60% of total perfusion. However, in the axillary perfusion group, the distribution of TAWSS values was more similar to that of the standard group, regardless of total perfusion.
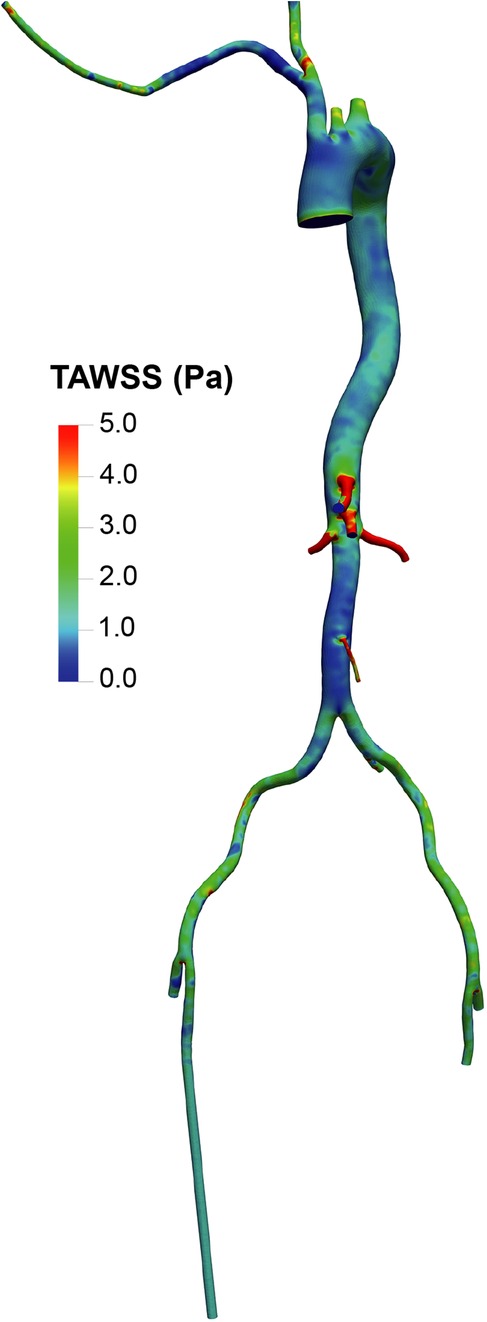
Figure 5. Overview of time-averaged wall shear stress (TAWSS) in the aorta and its branches in the standard group.
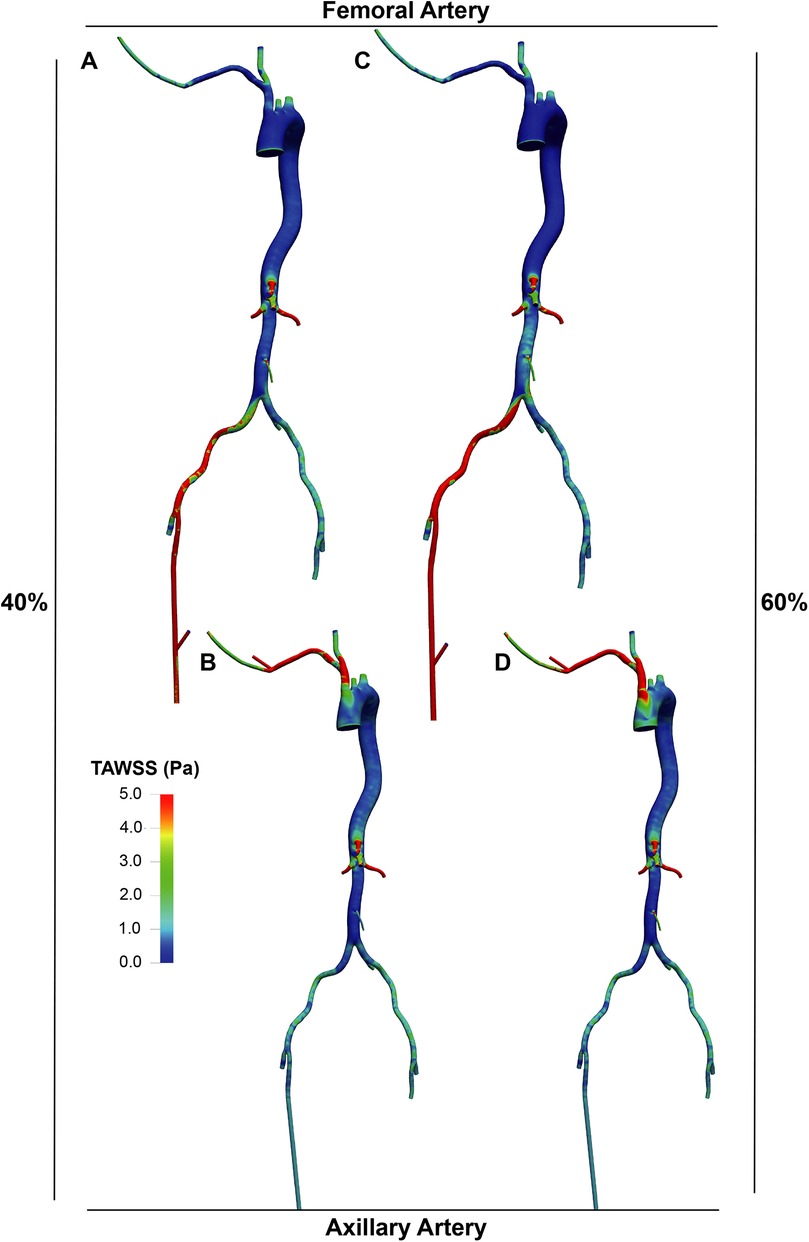
Figure 6. TAWSS distribution in the LVAD group. A and B. With LVAD supplying 40% of total flow, TAWSS distributions in the femoral (A) and axillary (B) perfusion groups. C and D. With LVAD supplying 60% of total flow, TAWSS distributions in the femoral (C) and axillary (D) perfusion groups.
The OSI distribution (Figures 7, 8) was generally lower in axillary perfusion (less than 0.2 in the most regions of the aorta) compared to femoral perfusion (more than 0.2 in the most regions), especially in the thoracic aorta and the upper abdominal aorta. This indicates less pronounced changes in WSS direction with axillary perfusion as LVAD flow increased.
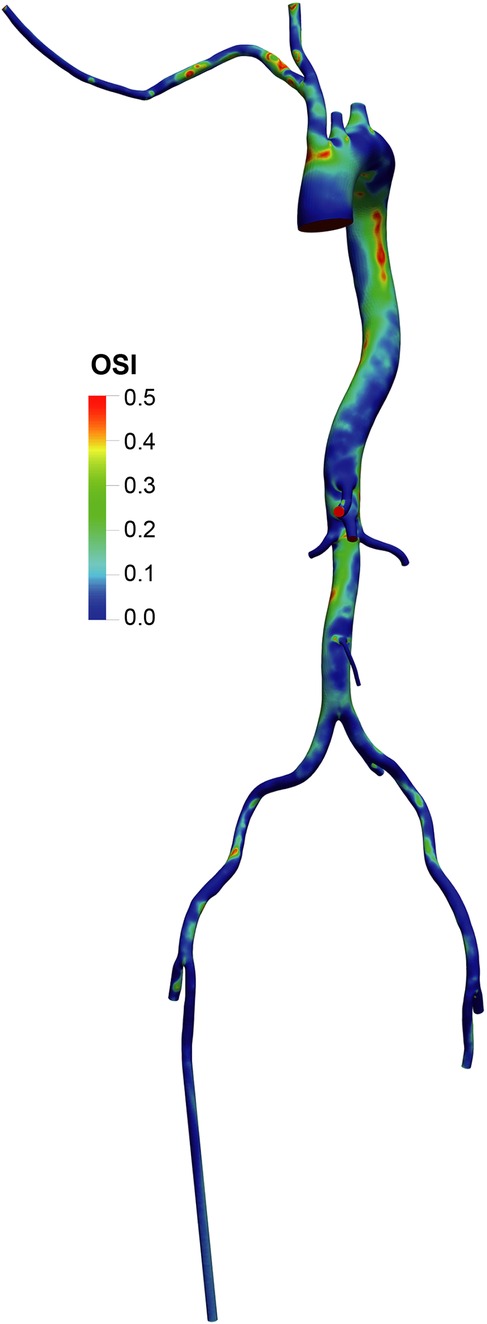
Figure 7. Overview of the oscillatory shear index (OSI) in the aorta and its branches in the standard group.
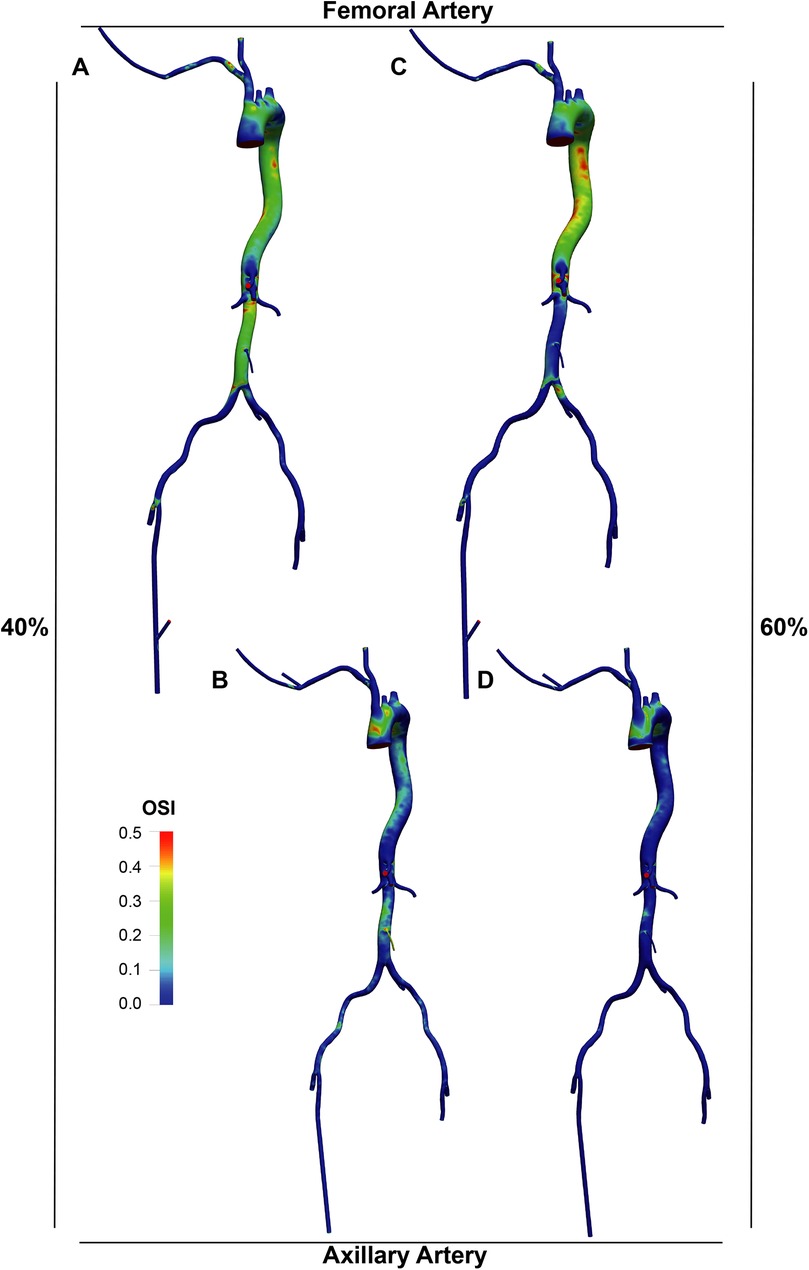
Figure 8. OSI distribution in the LVAD group. A and B. With LVAD supplying 40% of total flow, OSI distributions in the femoral (A) and axillary (B) perfusion groups. C and D. With LVAD supplying 60% of total flow, OSI distributions in the femoral (C) and axillary (D) perfusion groups.
Discussion
This study has several limitations. First, the LVAD was modeled as a constant-flow inlet at 2 L/min and 3 L/min, without dynamic pump-arterial coupling. This simplification may underestimate the competitive interplay between the LVAD and the native heart, potentially leading to inaccuracies in estimated flow rates through the aortic branches. Second, as the primary objective was to compare the two perfusion sites rather than obtain precise hemodynamic values for every small branch, normalized hemodynamic waveforms were employed. Critical misrepresentation would be more likely in a pulsatile environment or with a pulsatile LVAD model, which was not the focus of this study. Furthermore, the model relies on assumptions of rigid, non-remodeling vessel walls and Newtonian blood rheology. Finally, the sample size was limited. These factors collectively differentiate our computational approach from real-world clinical studies, underscoring the need for further investigation.
From a hemodynamic perspective, we explored the pros and cons in two aspects- perfusion and WSS. Our CFD model showed that axillary perfusion resulted in less reduced perfusion in major aortic branches than femoral perfusion. This was consistent regardless of whether the LVAD provided 40% or 60% of total perfusion, except in the intubation arteries and the IMA. Perfusion to the IMA decreased significantly only at the 40% LVAD flow rate, but improved at 60% with axillary perfusion. Therefore, axillary perfusion may not critically compromise IMA perfusion, particularly given that our patients' average initial pump flow was 2.68 (±0.60) L/min (approximately 54% of a 5 L/min CO). Regarding hemodynamic complications, specifically hyperperfusion syndrome (22), our model suggests that the axillary approach may not increase its incidence. Hyperperfusion syndrome is typically associated with over a 100% increase in carotid perfusion (23, 24). The axillary approach resulted in only a 13% increase in carotid artery flow, which may be insufficient to precipitate the syndrome. Conversely, the 24% increase in flow observed in the distal right axillary artery may be clinically relevant, potentially explaining patient reports of arm swelling and numbness.
For the WSS aspect, with femoral perfusion, the TAWSS significantly decreased, with its OSI increased, indicating prevalent aortic blood flow disturbances. However, in the axillary perfusion group, because it provides perfusion in the same direction as the heart, the OSI was generally lower, except at the ascending aorta, and the reduction in TAWSS was less marked. High WSS is usually beneficial, whereas low WSS might be associated with high OSI due to flow reversal (8, 25) and could lead to conditions such as atherosclerosis (8, 26) and aortic or arterial aneurysms (27, 28), possibly due to drastic fluctuations. Based on a CDF model established by our patient's CTA, the axillary approach may offer improved hemodynamics compared to the conventional femoral approach.
Conclusions
We developed a CFD model using patient CTA data to analyze key fluid parameters. Our findings suggest that, within the constraints of a model assuming rigid vessel walls and Newtonian blood flow, the axillary approach may improve overall circulation. It is associated with a more favorable hemodynamic profile, including enhanced perfusion to abdominal aortic branches and more stable flow patterns, compared to the traditional femoral approach. The jugular-axillary approach thus emerges as a promising configuration for future percutaneous extra-LVAD procedures. Further validation through real-world clinical studies is warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Union Hospital, Affiliated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SW: Writing – review & editing, Conceptualization, Writing – original draft. QL: Writing – original draft, Software, Formal analysis, Methodology. PL: Writing – original draft, Methodology, Data curation. CZ: Writing – original draft, Methodology, Data curation. WW: Data curation, Methodology, Writing – original draft. HL: Funding acquisition, Writing – original draft, Writing – review & editing. PY: Methodology, Writing – original draft. LF: Data curation, Investigation, Writing – review & editing. WS: Writing – review & editing. JK: Writing – review & editing. ND: Supervision, Writing – review & editing. SC: Writing – original draft, Funding acquisition, Data curation, Supervision, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Hubei Province (grant 2023AFB1042 to SC and 2025AFB349 to HL) and the National Natural Science Foundation of China (grant 82500579 to HL).
Acknowledgments
We want to thank Professor Houman Khalili, MD, from Memorial Regional Hospital, Department of Cardiology, Hollywood, FL, and Professor I-wen Wang, MD, PhD, from Memorial Regional Hospital, Department of Cardiac Surgery, Hollywood, FL, for their advice on the revision of the manuscript.
Conflict of interest
QL was employed by Boea Wisdom (Hangzhou) Network Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors did not use AI and AI-assisted technologies.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1631144/full#supplementary-material
Supplementary Figure S1 | Detailed mesh with boundary layers for the baseline model (without LVAD).
Supplementary Figure S2 | Grid (Mesh) independence in the standard group (without LVAD). A. The graph (A) plots the computed TAWSS against the total number of grid elements for three systematically refined meshes. TAWSS values are presented for two different surface domains: full wall (B) and clip wall (C). B and C. The orange-shaded regions on the 3D aorta schematics (insets) illustrate the respective surface domains from which the TAWSS values were extracted: Full wall (B), the entire luminal surface of the aortic model, including all major branches. Clip Wall (C), a truncated aortic wall, created by clipping the inlet, outlets, and branch vessels, retaining only the primary aortic trunk to minimize the influence of local flow effects at the boundaries.
Supplementary Figure S3 | The schematic of inlet/outlet boundary conditions and LVAD graft locations.
Abbreviations
AA, axillary artery; CFD, computational fluid dynamics; CO, cardiac output; CTA, computed-tomographic angiography; Extra-LVAD, extracorporeal left ventricular assist device; FA, femoral artery; IMA, inferior mesenteric artery; LPM, liter per minute; LRA, left renal artery; OSI, oscillatory shear index; RRA, right renal artery; SMA, superior mesenteric artery; TAWSS, time-averaged wall shear stress; WSS, wall shear stress.
References
1. Moonsamy P, Axtell AL, Ibrahim NE, Funamoto M, Tolis G, Lewis GD, et al. Survival after heart transplantation in patients bridged with mechanical circulatory support. J Am Coll Cardiol. (2020) 75:2892–905. doi: 10.1016/j.jacc.2020.04.037
2. Trivedi JR, Cheng A, Singh R, Williams ML, Slaughter MS. Survival on the heart transplant waiting list: impact of continuous flow left ventricular assist device as bridge to transplant. Ann Thorac Surg. (2014) 98:830–4. doi: 10.1016/j.athoracsur.2014.05.019
3. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. (2015) 65:e7–e26. doi: 10.1016/j.jacc.2015.03.036
4. Li P, Zhang X, Chen S, Hsu P, Wu T, Qian S, et al. Case report: successful percutaneous extracorporeal magnetic levitation ventricular assist device support in a patient with left heart failure due to dilated cardiomyopathy. Front Cardiovasc Med. (2023) 10:1093794. doi: 10.3389/fcvm.2023.1093794
5. Lewin D, Nersesian G, Lanmüller P, Schoenrath F, Falk V, Potapov EV, et al. Complications related to the access site after transaxillary implantation of a microaxial left ventricular assist device. J Heart Lung Transplant. (2023) 42:679–87. doi: 10.1016/j.healun.2022.12.018
6. El-Sayed Ahmed MM, Aftab M, Singh SK, Mallidi HR, Frazier OH. Left ventricular assist device outflow graft: alternative sites. Ann Cardiothorac Surg. (2014) 3:541–5. doi: 10.3978/j.issn.2225-319X.2014.09.03
7. Schmack L, Ali-Hasan-Al-Saegh S, Weymann A, Pizanis N, Akhyari P, Zubarevich A, et al. Inflammatory and hemolytic responses of microaxial flow pump temporary ventricular assist devices via axillary access in cardiogenic shock. Medicina (Kaunas). (2024) 60(12):1960. doi: 10.3390/medicina60121960
8. Peiffer V, Sherwin SJ, Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res. (2013) 99:242–50. doi: 10.1093/cvr/cvt044
9. Shaaban AM, Duerinckx AJ. Wall shear stress and early atherosclerosis: a review. AJR Am J Roentgenol. (2000) 174:1657–65. doi: 10.2214/ajr.174.6.1741657
10. Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. (2007) 49:2379–93. doi: 10.1016/j.jacc.2007.02.059
11. Menter FR, Kuntz M, Langtry R. Ten years of industrial experience with the SST turbulence model. Turbul Heat Mass Transfer. (2003) 4:625–32.
12. Fraser KH, Taskin ME, Griffith BP, Wu ZJ. The use of computational fluid dynamics in the development of ventricular assist devices. Med Eng Phys. (2011) 33:263–80. doi: 10.1016/j.medengphy.2010.10.014
13. Alastruey J, Xiao N, Fok H, Schaeffter T, Figueroa CA. On the impact of modelling assumptions in multi-scale, subject-specific models of aortic haemodynamics. J R Soc Interface. (2016) 13(119):20160073. doi: 10.1098/rsif.2016.0073
14. Westerhof N, Bosman F, De Vries CJ, Noordergraaf A. Analog studies of the human systemic arterial tree. J Biomech. (1969) 2(2):121–43. doi: 10.1016/0021-9290(69)90024-4
15. Karmonik C, Partovi S, Loebe M, Schmack B, Weymann A, Lumsden AB, et al. Computational fluid dynamics in patients with continuous-flow left ventricular assist device support show hemodynamic alterations in the ascending aorta. J Thorac Cardiovasc Surg. (2014) 147:1326–33.e1321. doi: 10.1016/j.jtcvs.2013.09.069
16. Li L, Shi L, Tan X, Zhao Y. Influence of LVAD cannula outflow graft flow rate and location on fluid-particle interactions and thrombi distribution: a primary numerical study. J Cardiovasc Transl Res. (2024) 17:1316–27. doi: 10.1007/s12265-024-10547-1
17. Black SM. Patient-specific multi-dimensional CFD simulations based on 4D Flow-MRI for the haemodynamic assessment of aortic dissections and perfusion optimisation of vascular grafts. (2024).
18. Black MS, Maclean C, Barrientos PH, Ritos K, McQueen A, Kazakidi A. Calibration of patient-specific boundary conditions for coupled CFD models of the aorta derived from 4D flow-MRI. Front Bioeng Biotechnol. (2023) 11:1178483. doi: 10.3389/fbioe.2023.1178483
19. Salvi L, Alfonsi J, Grillo A, Pini A, Soranna D, Zambon A, et al. Postoperative and mid-term hemodynamic changes after replacement of the ascending aorta. J Thorac Cardiovasc Surg. (2022) 163:1283–92. doi: 10.1016/j.jtcvs.2020.05.031
20. Imamura T, Jeevanandam V, Kim G, Raikhelkar J, Sarswat N, Kalantari S, et al. Optimal hemodynamics during left ventricular assist device support are associated with reduced readmission rates. Circ Heart Fail. (2019) 12:e005094. doi: 10.1161/circheartfailure.118.005094
21. Symons JD, Deeter L, Deeter N, Bonn T, Cho JM, Ferrin P, et al. Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Circ Heart Fail. (2019) 12:e006085. doi: 10.1161/circheartfailure.119.006085
22. Lietz K, Brown K, Ali SS, Colvin-Adams M, Boyle AJ., Anderson D, et al. The role of cerebral hyperperfusion in postoperative neurologic dysfunction after left ventricular assist device implantation for end-stage heart failure. J Thorac Cardiovasc Surg. (2009) 137:1012–9. doi: 10.1016/j.jtcvs.2008.11.034
23. van Mook WNKA, Rennenberg RJMW, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PAM, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. (2005) 4:877–88. doi: 10.1016/s1474-4422(05)70251-9
24. Ascher E, Markevich N, Schutzer RW, Kallakuri S, Jacob T, Hingorani AP. Cerebral hyperperfusion syndrome after carotid endarterectomy: predictive factors and hemodynamic changes. J Vasc Surg. (2003) 37:769–77. doi: 10.1067/mva.2003.231
25. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. (1985) 5:293–302. doi: 10.1161/01.atv.5.3.293
26. Stone PH, Coskun AU, Kinlay S, Popma JJ, Sonka M, Wahle A, et al. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J. (2007) 28:705–10. doi: 10.1093/eurheartj/ehl575
27. Trenti C, Ziegler M, Bjarnegà N, Ebbers T, Lindenberger M, Dyverfeldt P, et al. Wall shear stress and relative residence time as potential risk factors for abdominal aortic aneurysms in males: a 4D flow cardiovascular magnetic resonance case-control study. J Cardiovasc Magn Reson. (2022) 24:18. doi: 10.1186/s12968-022-00848-2
Keywords: heart failure, temporary circulatory support, extracorporeal, left ventricular assist device, percutaneous, right axillary artery, computational fluid dynamics, hemodynamics
Citation: Wang S, Liu Q, Li P, Zhang C, Wu W, Liu H, Yang P, Fang L, Su W, Karimov JH, Dong N and Chen S (2025) Computational hemodynamic assessment of axillary and femoral artery perfusion for extracorporeal left ventricular assist device. Front. Cardiovasc. Med. 12:1631144. doi: 10.3389/fcvm.2025.1631144
Received: 19 May 2025; Revised: 29 October 2025;
Accepted: 4 November 2025;
Published: 2 December 2025.
Edited by:
Tomasz Zieliński, National Institute of Cardiology, PolandReviewed by:
Arka Das, Embry Riddle Aeronautical University, United StatesPravin Kumar Shukla, Motilal Nehru National Institute of Technology Allahabad, India
Copyright: © 2025 Wang, Liu, Li, Zhang, Wu, Liu, Yang, Fang, Su, Karimov, Dong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Chen, c2h1X2NoZW5AaHVzdC5lZHUuY24=
†These authors share first authorship
‡These authors share last authorship
 Shilin Wang
Shilin Wang Qianwen Liu
Qianwen Liu Ping Li
Ping Li Changdong Zhang
Changdong Zhang Wenqian Wu
Wenqian Wu Hao Liu5
Hao Liu5 Jamshid H. Karimov
Jamshid H. Karimov Nianguo Dong
Nianguo Dong