- 1Department of Cardiology, Chiba-Nishi General Hospital, Chiba, Japan
- 2Department of Cardiology, Tokyo Medical University Hospital, Tokyo, Japan
Background: Right ventricular pseudoaneurysm (RVP) encased by adjacent pericardial or scar tissue is rare but can be a fatal sequela of cardiac rupture. Differentiating between pseudoaneurysms and true aneurysms is important because they have different natural histories and require distinct treatments. Radiofrequency catheter ablation (RFCA) is a potential cause of RVP; however, RFCA-associated RVP incidence and management remain unclear.
Case presentation: An 88-year-old woman with refractory paroxysmal supraventricular tachycardia was admitted to our hospital. We performed an electrophysiological study, which led to a final diagnosis of atrioventricular nodal reentrant tachycardia, for which successful RFCA was performed. On post-procedural day 2, echocardiography revealed a small right ventricular apical outpouching. Cardiac computed tomography angiography led to the correct diagnosis of RVP, which was successfully treated with surgical repair. The postoperative course was uneventful.
Conclusions: We describe a unique case of RFCA-associated apical RVP. This case highlights the importance of the potential risk of iatrogenic RVP and the value of cardiac computed tomography angiography in diagnosing RVP in patients with right ventricular outpouching.
Highlights
• Right ventricular pseudoaneurysm (RVP) is potentially fatal if left untreated.
• Silent iatrogenic RVP may develop following radiofrequency catheter ablation.
• Cardiac computed tomography angiography is valuable for diagnosis of RVP.
1 Introduction
Ventricular pseudoaneurysms may occur in association with myocardial infarction, trauma, infection, catheter-related procedures, cardiac surgery, or idiopathic (1). Cardiac pseudoaneurysms often occur in the left ventricle, whereas right ventricular pseudoaneurysms (RVP) are rare (2). We describe a unique case of silent iatrogenic RVP secondary to radiofrequency catheter ablation (RFCA).
2 Case description
An 88-year-old woman was admitted with symptomatic paroxysmal supraventricular tachycardia that had persisted for three years. The patient had a history of severe aortic stenosis for which transapical transcatheter aortic valve replacement was performed seven years ago. Her initial vital signs were blood pressure of 79/60 mmHg and heart rate of 164 beats/min. The physical examination findings were unremarkable. Laboratory test revealed elevated serum level of brain natriuretic peptide (171.6 pg/ml, reference: <18.4 pg/ml). Electrocardiography revealed narrow QRS tachycardia with a short RP (Supplementary Figure S1A). Tachycardia was terminated with rapid intravenous administration of adenosine triphosphate (Supplementary Figure S1B). However, the patient experienced frequent paraoxymal supraventricular tachycardia episodes. Echocardiography revealed no structural or functional heart abnormalities (Figure 1A; Supplementary Video S1). Unenhanced computed tomography scans showed no biventricular abnormalities suggestive of myocardial infarction or aneurysm. An electrophysiological study was performed four days after admission. Catheters were placed in the high right atrium, the His bundle region, coronary sinus, and right ventricular (RV) apex. Premature ventricular contractions frequently occurred during RFCA, and, hence, the RV pacing catheter was held tightly to avoid the unintended catheter movement. Based on the electrophysiological study, slow/fast atrioventricular nodal reentrant tachycardia was diagnosed. Subsequent successful anatomical slow-pathway ablation was performed according to standard techniques (Figures 1B,C). We excluded any complications, including cardiac tamponade, on postprocedural echocardiography. Follow-up echocardiography revealed RV apical outpouching on postprocedural day 2 (Figures 1D–F; Supplementary Video S2). The patient was asymptomatic, and her vital signs were stable. Physical examination and electrocardiographic findings were unremarkable (Supplementary Figures S1C,D). Follow-up laboratory tests were close to normal. Differential diagnoses of ventricular outpouching include true aneurysms and pseudoaneurysms. Cardiac computed tomography angiography (CCTA) further characterized the morphology and features of the RV apical outpouching (Figures 2A–D). Note the presence of contrast-filled RV outpouching at the apex that protruded during systole, with a maximum diameter of 12.1 mm and a narrow orifice of 1.5 mm with an orifice-to-maximum diameter ratio of 12.4%, suggestive of RVP. CCTA revealed normal coronary arteries (Figures 2E,F). Pericardial effusion was not observed. A detailed review of the computed tomography images confirmed the absence of RVP before the RFCA procedure and its presence after the procedure (Figure 3). Given the temporal relationship between RFCA and the occurrence of RVP without any other identifiable cause, a final diagnosis of iatrogenic RVP was made. After multidisciplinary discussion, taking into consideration that a ventricular pseudoaneurysm is susceptible to cardiac rupture, the patient underwent urgent surgical repair of the RVP. No bleeding was observed in the pericardial sacs. There was no evidence of pericarditis, intrapericardial bleeding, or cardiac rupture except for a slight bulge at the RV apex. Epicardial echocardiography was used to identify the pseudoaneurysm, as such pseudoaneurysm was difficult to identify by visual examination. Subsequent vertical mattress suture repair with Teflon-felt reinforcement was performed for the RVP. The patient's postoperative course was uneventful, and she remained asymptomatic at the one-year follow-up (Supplementary Figure S2).
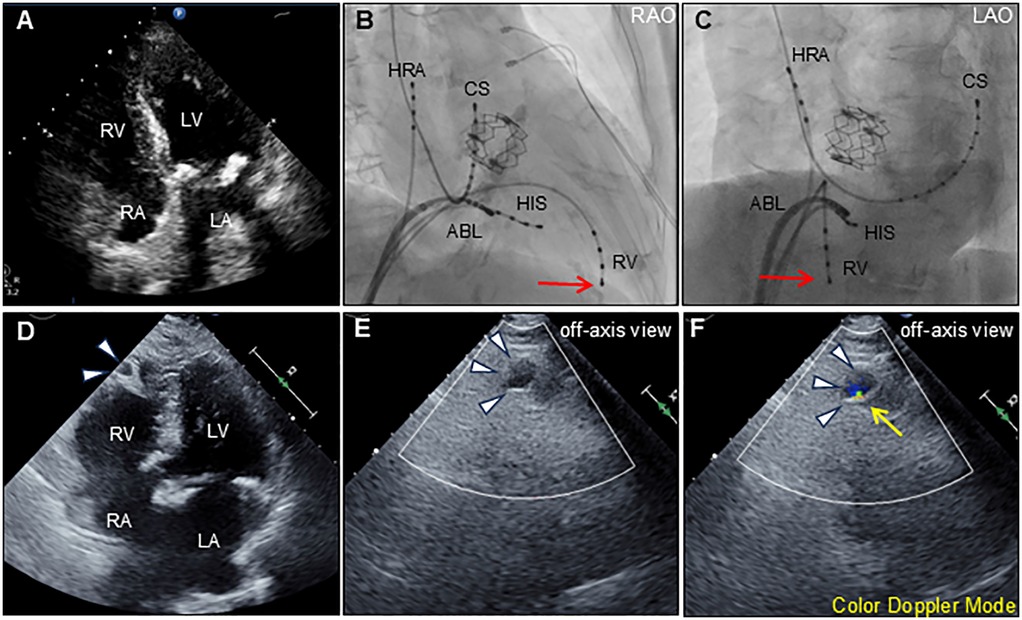
Figure 1. Transthoracic echocardiography and ablation images. Preprocedural transthoracic echocardiography (apical 4-chamber view) shows normal biventricular function with normal anatomy (A) Radiographs of the right anterior oblique [RAO; (B)] and left anterior oblique [LAO; (C)] views show the radiofrequency ablation catheter (ABL) positioned at the posterior right atrial septum. Other catheters were positioned at the high right atrium (HRA), His-bundle (HIS), coronary sinus (CS), and right ventricle (RV). Red arrows indicate the tip of the indwelling RV catheter. Postprocedural transthoracic echocardiography (apical 4-chamber view) reveals an outpouching of the right ventricular apex (arrowheads) (D) Color Doppler echocardiography using an off-axis view reveals outpouching of the right ventricular apex (arrowheads) during diastole (E) and systole (F) The expansion with the antegrade flow at systole, suggests communication between the outpouching and right ventricle (yellow arrow). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
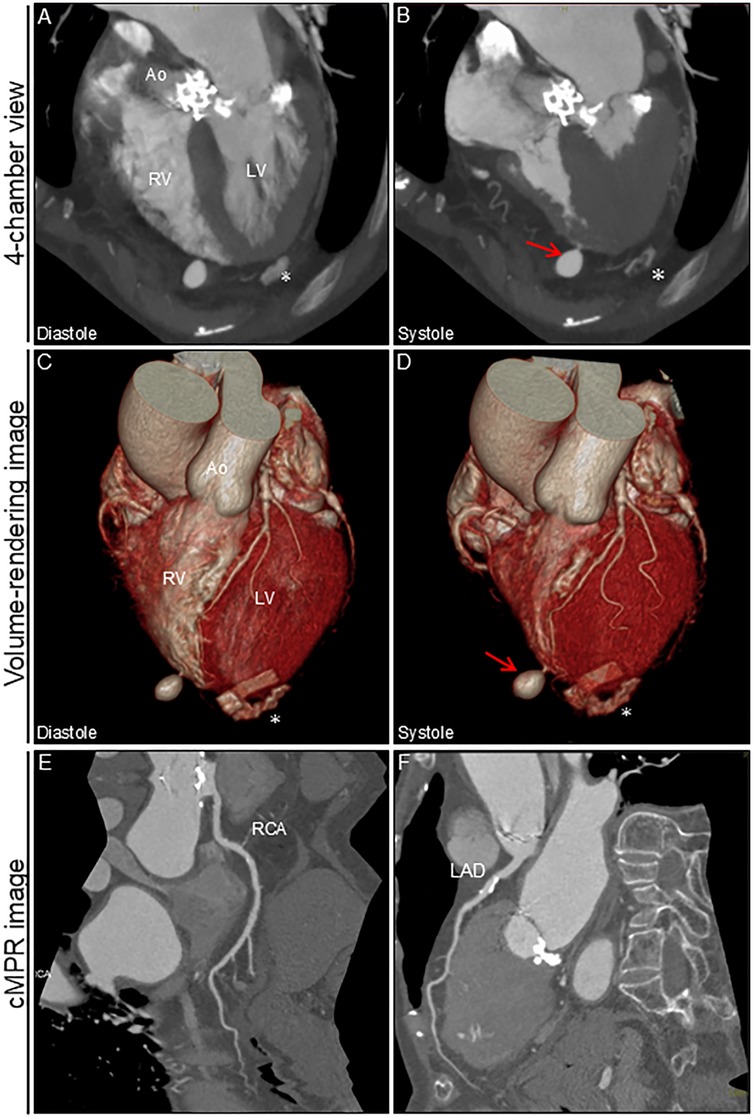
Figure 2. Cardiac computed tomography angiography image after ablation. 4-chamber view and volume-rendering images during diastole (A,C) and systole (B,D). Outpouching with a narrow neck at the apex of the right ventricle is markedly prominent during systole (arrows). No significant pericardial effusion was observed. cMPR images of RCA (E) and LAD (F) * Denotes the remnants of felt in the left ventricular apex after transapical aortic valve replacement. Ao, aorta; cMPR, curved multi-planar reformation; LAD, left anterior descending artery; LV, left ventricle; RCA, right coronary artery; RV, right ventricle.
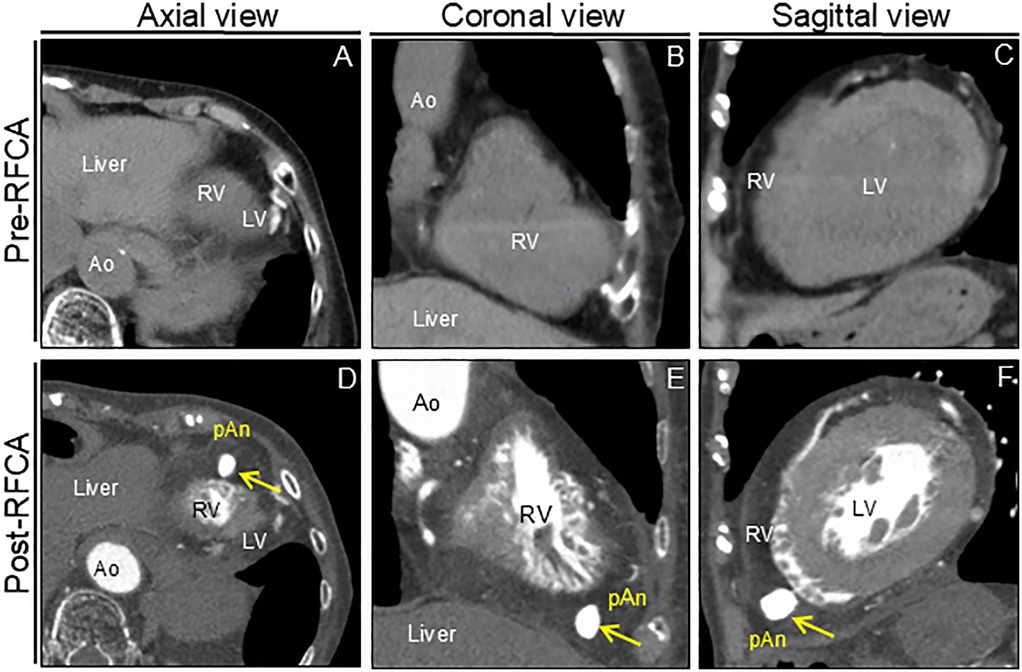
Figure 3. Computed tomography images before and after RFCA. Preprocedural unenhanced computed tomography images confirm normal right ventricular anatomy (A–C). Postprocedural cardiac computed tomography angiography images reveal apical outpouching of the right ventricle (arrows) (D–F). Ao, aorta; LV, left ventricle; pAn; pseudoaneurysm; RFCA; radiofrequency catheter ablation; RV, right ventricle.
3 Discussion
Here, we describe a unique case of silent iatrogenic RVP after RFCA. Our case provides the following clinical lessons: We reviewed the previously reported cases of RFCA-associated cardiac aneurysms (3–25), which were published in English in PubMed between 1985 and 2024 (Table 1). The left ventricle was the most common site of origin of the cardiac aneurysm, followed by the right ventricle, mitral-aortic intervalvular fibrosa, and right atrium. As in previous studies (26), nearly 30% of the patients had no clinical symptoms. The literature documents the triggers, characteristics, management, and outcomes of RVP after RFCA in two cases (6, 11). Notably, RVP developed at a site far from the ablation target site in the present case. Given the histological nature of the RV, which is composed of fragile pectinate muscles with wall thinning, age-related RV myocardial degeneration, physical stress on the RV myocardium due to prior temporary pacing wire placement and improper manipulation of the RV catheter tip might have triggered the RVP. Thus, for medical specialists in RFCA, recognizing these rare RFCA-related complications may aid in early detection and proper management. CCTA with good temporal and spatial resolutions can clearly differentiate ventricular outpouchings (27). Ventricular pseudoaneurysms are characterized by a narrow opening that connects to the cardiac chamber. Pseudoaneurysms expand outward in response to the increased intraventricular pressure during systole. Indeed, CCTA was valuable for the accurate diagnosis of silent iatrogenic RVP in the present case. There are currently no guidelines for the appropriate management of ventricular pseudoaneurysms. Given the high risk of cardiac rupture, surgical repair has been considered the first-line treatment of choice for ventricular pseudoaneurysms (1). Although surgical repair was performed, there was no evidence of impending cardiac rupture in the present case. Considering the high surgical mortality rate of 7%–23% (28), conservative management to reduce the risk of cardiac rupture might be another treatment option (26, 29). Percutaneous embolization with coils offers another promising therapeutic alternative, limited to midterm outcomes (29, 30). Owing to the nature of our hospital, which did not have an experienced interventionalist but had many experienced cardiac surgeons on staff, we decided to opt for surgical treatment in this case. Moreover, in our review, nearly half the patients received conservative management with a favorable prognosis. Noninvasive treatment strategies may be feasible in elderly patients with stable hemodynamics and no risk of cardiac rupture. Data on the long-term outcomes of the above treatments is lacking, and future evidence is awaited to be built up through data accumulation.
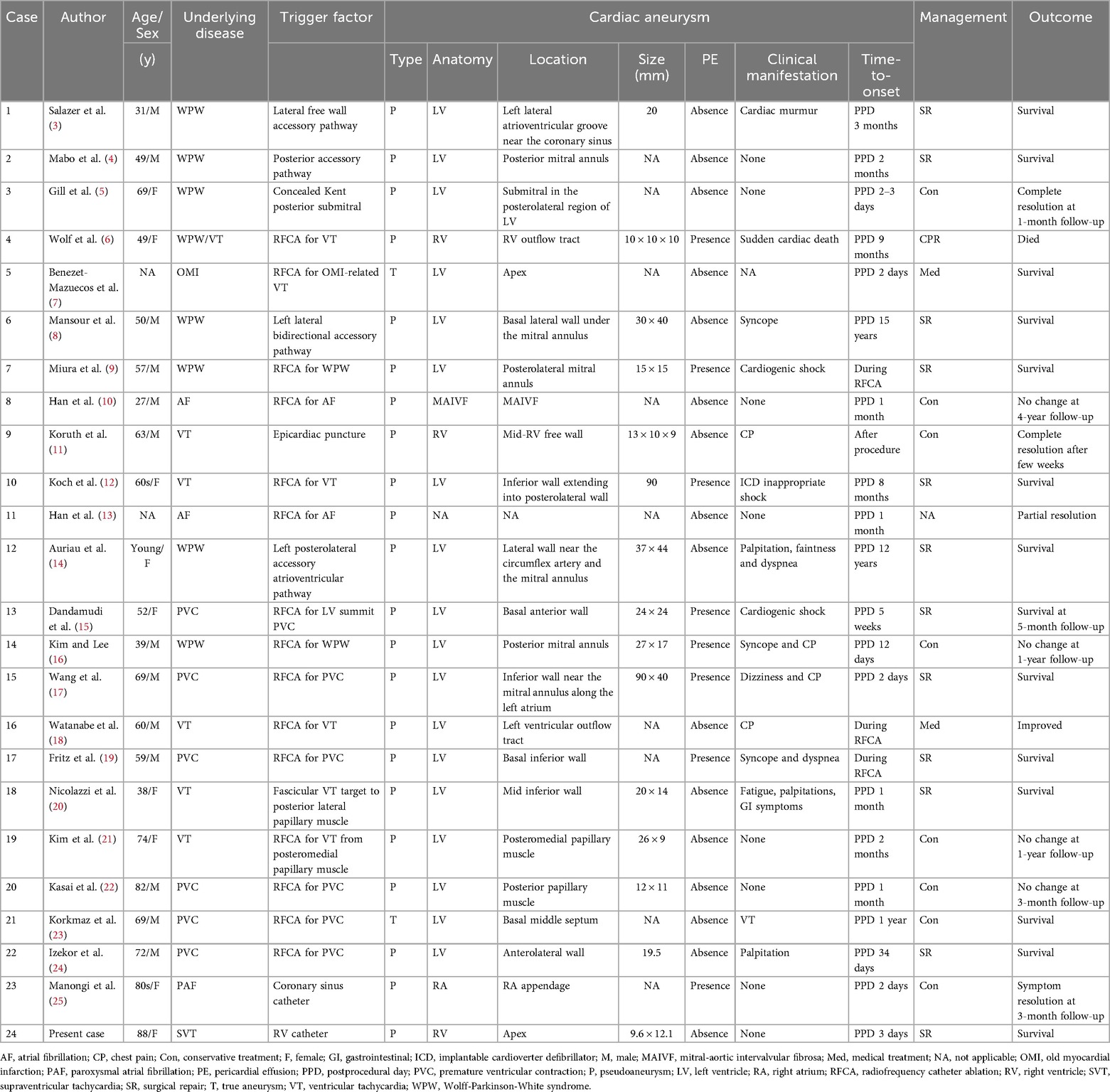
Table 1. Literature review of cases of cardiac aneurysm associated with radiofrequency catheter ablation.
Iatrogenic cardiac pseudoaneurysm have been reported in various clinical reports. Left ventricular pseudoaneurysms (LVPs) are the most frequent type of iatrogenic cardiac pseudoaneurysm, with approximately one-third arising from surgical procedures involving mitral valve replacement (1). Percutaneous device interventions predominantly including transapical transcatheter aortic valve replacement can often cause iatrogenic LVP (31, 32). Cases of transcatheter mitral valve implantation and ventricular septal defect closure device-related LVP (33, 34) have also been reported. Although iatrogenic RVPs are very rare, various case reports describe them in connection with endomyocardial biopsy (35), lead extraction (36), pericardiocentesis (30), Swan-Ganz catheter (37), valve in valve treatment of tricuspid valves (38), placement of a hemodialysis catheter (39), surgery for atrio-ventricular septal defect with tetralogy of Fallot (40) and insertion of central venous line (41). Considering the above diverse case reports, our case highlights that RVP can complicate even a routine procedure.
4 Limitation
Our case was finally diagnosed as iatrogenic RVP based on the temporal relationship between RFCA and evidence of de novo RVP, but the direct causal relationship and detailed mechanism remain unclear. In addition, histological analysis could not be performed in this case. Early onset and the absence of reactive pericardial effusion observed in our case suggests that it may have been a specific subtype of RVP with residual cardiomyocytes that are vulnerable but have not yet ruptured. Future systematic and comprehensive pathological analyses of RFCA-associated ventricular aneurysms are warranted.
5 Conclusions
This is a rare case of silent iatrogenic RVP after RFCA. This case report highlights the importance of recognizing iatrogenic RVP and the clinical significance of CCTA for diagnosis of RVP. Therefore, clinicians should be aware of RVP as a possible complication after RFCA and understand the imaging techniques useful for its early diagnosis and determine appropriate treatment options.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Chiba-Nishi General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SH: Writing – original draft, Writing – review & editing. HY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1631315/full#supplementary-material
Supplementary Figure S1 | Time-course of electrocardiographic change. Electrocardiography (ECG) on emergency arrival (A), after intravenous adenosine triphosphate (ATP) administration (B), before the ablation (ABL) (C), and after the ABL (D). The episode was a narrow QRS with short RP tachycardia. No ST-T changes were observed before or after the ABL. The ECG paper was set to a standard speed of 25 mm/s and voltage (amplitude) of 10 mm/mV.
Supplementary Figure S2 | Timeline of the diagnostics, therapeutic interventions, and disease status of the present case. AS, aortic valve stenosis; ATP, adenosine triphosphate; AVNRT, atrioventricular nodal reentrant tachycardia; BP, blood pressure; CCTA, coronary computer tomography angiography; CT, computer tomography; ECG, electrocardiography; EF, ejection fraction; EPS, electrophysiological study; HR, heart rate; PVC, premature ventricular contraction; PSVT, paroxysmal supraventricular tachycardia; RFCA, radiofrequency catheter ablation; RV, right ventricular; RVP, right ventricular pseudoaneurysm; TA-TAVR, transapical transcatheter aortic valve replacement; and TTE, transthoracic echocardiography.
Supplementary Video S1 | Transthoracic echocardiography before ablation. Preprocedural transthoracic echocardiography (apical 4-chamber view) shows normal biventricular function with normal anatomy.
Supplementary Video S2 | Transthoracic echocardiography after ablation. Postprocedural transthoracic echocardiography (an off-axis view) shows right ventricular pseudoaneurysm.
Abbreviations
CCTA, cardiac computed tomography angiography; LVP, left ventricular pseudoaneurysm; RFCA, radiofrequency catheter ablation; RV, right ventricular; RVP, right ventricular pseudoaneurysm.
References
1. Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. (1998) 32:557–61. doi: 10.1016/s0735-1097(98)00290-3
2. Csapo K, Voith L, Szuk T, Edes I, Kereiakes DJ. Postinfarction left ventricular pseudoaneurysm. Clin Cardiol. (1997) 20:898–903. doi: 10.1002/clc.4960201021
3. Salazer TL, Bacharach JM, Bresnahan JF, Seward JB, Danielson GK. Fistulous pseudoaneurysm complicating surgical accessory pathway interruption for wolff-Parkinson-white syndrome. Mayo Clin Proc. (1992) 67:663–6. doi: 10.1016/s0025-6196(12)60722-8
4. Mabo P, Le Breton H, De Place C, Daubert C. Asymptomatic pseudoaneurysm of the left ventricle and coronary artery fistula after closed-chest ablation of an accessory pathway. Am Heart J. (1992) 124:1637–9. doi: 10.1016/0002-8703(92)90089-e
5. Gill KS, Bansal RC, Pai S, Timothy P. Left ventricular pseudoaneurysm as a complication of electrophysiologic study. J Am Soc Echocardiogr. (2001) 14:228–30. doi: 10.1067/mje.2001.108540
6. Wolf DA, Burke AP, Patterson KV, Virmani R. Sudden death following rupture of a right ventricular aneurysm 9 months after ablation therapy of the right ventricular outflow tract. Pacing Clin Electrophysiol. (2002) 25:1135–7. doi: 10.1046/j.1460-9592.2002.01135.x
7. Benezet-Mazuecos J, Marcos-Alberca P, Farre J. Images in cardiology: giant left ventricular thrombus after radiofrequency ablation of post-infarction ventricular tachycardia: what to do? Heart. (2005) 91:1532. doi: 10.1136/hrt.2004.059600
8. Mansour F, Basmadjian AJ, Bouchard D, Ibrahim R, Guerra PG, Khairy P. Images in cardiovascular medicine. Left ventricular pseudoaneurysm: a late complication of low-energy DC ablation. Circulation. (2006) 113:e780–1. doi: 10.1161/CIRCULATIONAHA.105.591008
9. Miura T, Yamazaki K, Kihara S, Saito S, Miyagishima M, Aomi S, et al. Transatrial repair of submitral left ventricular pseudoaneurysm. Ann Thorac Surg. (2008) 85:643–5. doi: 10.1016/j.athoracsur.2007.08.070
10. Han J, He Y, Gu X, Sun L, Zhao Y, Liu W, et al. Echocardiographic diagnosis and outcome of pseudoaneurysm of the mitral-aortic intervalvular fibrosa: results of a single-center experience in Beijing. Medicine (Baltimore). (2016) 95:e3116. doi: 10.1097/MD.0000000000003116
11. Koruth JS, Aryana A, Dukkipati SR, Pak HN, Kim YH, Sosa EA, et al. Unusual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. (2011) 4:882–8. doi: 10.1161/CIRCEP.111.965731
12. Koch KE, Raiszadeh F, Godelman A, Palma E, Forman R. Giant left ventricular pseudoaneurysm and myocardial dissection as a complication of multiple ventricular tachycardia ablations in a patient with cardiac sarcoidosis. Clin Med Insights Cardiol. (2015) 9:105–7. doi: 10.4137/CMC.S23863
13. Han J, He Y, Li Z, Chen J, Gu X, Pei J, et al. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa in a patient after radio frequency catheter ablation of atrial fibrillation. J Ultrasound Med. (2009) 28:249–51. doi: 10.7863/jum.2009.28.2.249
14. Auriau J, Jankowski A, Defaye P. Large spherical left ventricular pseudoaneurysm: a very rare long-term complication of ablation of an accessory pathway. Europace. (2017) 19:1680. doi: 10.1093/europace/euw259
15. Dandamudi S, Kim SS, Verma N, Malaisrie SC, Tung R, Knight BP. Left ventricular pseudoaneurysm as a complication of left ventricular summit premature ventricular contraction ablation. HeartRhythm Case Rep. (2017) 3:268–71. doi: 10.1016/j.hrcr.2017.01.006
16. Kim D, Lee MY. Spontaneous regression of submitral pseudoaneurysm after radiofrequency catheter ablation in a patient with wolff-Parkinson-white syndrome. HeartRhythm Case Rep. (2018) 4:580–3. doi: 10.1016/j.hrcr.2018.09.002
17. Wang H, Zheng Z, Yao L, Mou Y, Wang X. Giant left ventricular pseudoaneurysm: a rare acute complication of radiofrequency catheter ablation for premature ventricular contraction. J Cardiothorac Surg. (2019) 14:131. doi: 10.1186/s13019-019-0946-3
18. Watanabe T, Imai Y, Morita E, Okuyama T, Watanabe H, Yokota A, et al. Time course of left ventricular pseudoaneurysm after catheter ablation of LVOT tachycardia. JACC Clin Electrophysiol. (2020) 6:248–9. doi: 10.1016/j.jacep.2019.11.011
19. Fritz AV, Luis SA, Bagameri G, Bird J. A case of mistaken identity: pseudoaneurysm masquerading as pericardial effusion following premature ventricular contraction ablation. Eur Heart J Cardiovasc Imaging. (2021) 22:e140. doi: 10.1093/ehjci/jeab047
20. Nicolazzi J, Grizzard J, Kasirajan V, Ellenbogen K, Markley R. Left ventricular pseudoaneurysm caused by radiofrequency catheter ablation: from diagnosis to treatment with multimodality imaging. Circ Cardiovasc Imaging. (2021) 14:e012820. doi: 10.1161/CIRCIMAGING.121.012820
21. Kim M, Park YJ, Yu HT, Kim TH, Uhm JS, Joung B, et al. Case report: delayed ventricular pseudoaneurysm after radiofrequency ablation of left posteromedial papillary muscle ventricular tachycardia. Front Cardiovasc Med. (2022) 9:887190. doi: 10.3389/fcvm.2022.887190
22. Kasai Y, Kasai J, Kitai T, Morita J, Fujita T. Iatrogenic left ventricular pseudoaneurysm after successful radiofrequency catheter ablation for premature ventricular contraction originating from the posterior papillary muscles. Circ J. (2023) 87:464. doi: 10.1253/circj.CJ-22-0553
23. Korkmaz K, Yamanturk YY, Baskovski E, Candemir B, Akyurek O, Altin AT. A rare source of iatrogenic ventricular tachycardia: septal aneurysm due to premature ventricular complex ablation. Anatol J Cardiol. (2023) 27:494–6. doi: 10.14744/AnatolJCardiol.2023.2979
24. Izekor BE, Lovelace J, Giang TK, Ebert E, Olsovsky G. Idioventricular rhythm: a rare presentation of left ventricular pseudoaneurysm following radiofrequency ablation. Cureus. (2024) 16:e55272. doi: 10.7759/cureus.55272
25. Manongi N, Gulkarov I, Kim S, Moustakakis E, Goldbarg S. Nonoperative management of iatrogenic right atrial appendage pseudoaneurysm during catheter ablation. JACC Case Rep. (2024) 29:102147. doi: 10.1016/j.jaccas.2023.102147
26. Yeo TC, Malouf JF, Oh JK, Seward JB. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med. (1998) 128:299–305. doi: 10.7326/0003-4819-128-4-199802150-00010
27. Cresti A, Cannarile P, Aldi E, Solari M, Sposato B, Franci L, et al. Multimodality imaging and clinical significance of congenital ventricular outpouchings: recesses, diverticula, aneurysms, clefts, and crypts. J Cardiovasc Echogr. (2018) 28:9–17. doi: 10.4103/jcecho.jcecho_72_17
28. Hulten EA, Blankstein R. Pseudoaneurysms of the heart. Circulation. (2012) 125:1920–5. doi: 10.1161/circulationaha.111.043984
29. Dudiy Y, Jelnin V, Einhorn BN, Kronzon I, Cohen HA, Ruiz CE. Percutaneous closure of left ventricular pseudoaneurysm. Circ Cardiovasc Interv. (2011) 4:322–6. doi: 10.1161/circinterventions.111.962464
30. Alkhouli M, Waits B, Chaturvedi A, Ling FS. Percutaneous closure of right ventricular pseudoaneurysm. JACC Cardiovasc Interv. (2015) 8:e147–e8. doi: 10.1016/j.jcin.2015.04.019
31. Merchan S, Li CH, Martinez FJ, Kliger C, Jelnin V, Perk G, et al. Novel percutaneous apical exclusion of a left ventricular pseudoaneurysm after complicated transapical transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2015) 8(14):e227–8. doi: 10.1016/j.jcin.2015.06.032
32. Al Ayouby A, Gilard M, Hebert T, Didier R. Case report: iatrogenic left ventricular outflow tract to right atrium fistula after trans-femoral transcatheter aortic valve implantation associated with asymmetric septal hypertrophy. Eur Heart J Case Rep. (2021) 5(3):ytab020. doi: 10.1093/ehjcr/ytab020
33. Vidoni C, Besutti M, Guillon B, Kozmek L, Meneveau N, Chopard R. Left ventricular pseudoaneurysm: an unexpected complication after transcatheter mitral valve implantation. JACC Case Rep. (2025) 30(11):103324. doi: 10.1016/j.jaccas.2025.103324
34. Haddad RN, Bou Houssein H, Adel Hassan A, Kasem M. Closure of a late-onset iatrogenic left ventricular pseudoaneurysm caused by erosion of an apical muscular VSD device. Catheter Cardiovasc Interv. (2025) 106(1):23–7. doi: 10.1002/ccd.31383
35. Rodrigues AC, de Vylder A, Wellens F, Bartunek J, De Bruyne B. Right ventricular pseudoaneurysm as a complication of endomyocardial biopsy after heart transplantation. Chest. (1995) 107(2):566–7. doi: 10.1378/chest.107.2.566
36. Singleton MJ, Brunetti R, Schoenfeld MH, Bhave PD, Zhao DX, Whalen SP. Lead extraction complicated by right ventricular pseudoaneurysm: percutaneous closure with septal occluder device. HeartRhythm Case Rep. (2019) 5(11):542–4. doi: 10.1016/j.hrcr.2019.08.007
37. Esmat HA, Ceylan N, Demir E, Çinkooğlu A. Right ventricular pseudoaneurysm in a young adult following right heart catheterization: a rare case report and review of the literature. Egypt J Radiol Nucl Med. (2021) 52(30):1–5. doi: 10.1186/s43055-021-00413-4
38. Fernandez-Lopez L, Bouvier E, Lefèvre T. Right ventricular pseudoaneurysm after percutaneous tricuspid valve-in-valve implantation. Rev Esp Cardiol. (2018) 71(12):1070. doi: 10.1016/j.rec.2017.11.018
39. Uribe J, Leocachin D, Amaro E, Alvarez B, Jaimes E, Campuzano D, et al. Coil embolization for right ventricular pseudoaneurysm. JACC Case Rep. (2024) 30(1):102734. doi: 10.1016/j.jaccas.2024.102734
40. Calabrò R, Santoro G, Pisacane C, Pacileo G, Russo MG, Vosa C. Repeat syncopal attacks due to postsurgical right ventricular pseudoaneurysm. Ann Thorac Surg. (1999) 68(1):252–4. doi: 10.1016/s0003-4975(99)00497-x
Keywords: right ventricular pseudoaneurysm, radiofrequency catheter ablation, ventricular outpouching, cardiac computed tomography angiography, iatrogenic
Citation: Haruki S and Yamamoto H (2025) Radiofrequency catheter ablation-associated silent iatrogenic right ventricular pseudoaneurysm: a case report and literature review. Front. Cardiovasc. Med. 12:1631315. doi: 10.3389/fcvm.2025.1631315
Received: 19 May 2025; Accepted: 22 September 2025;
Published: 7 October 2025.
Edited by:
Vincenzo Santinelli, IRCCS San Donato Polyclinic, ItalyReviewed by:
Raymond N. Haddad, Assistance Publique-Hôpitaux de Paris (AP-HP), FranceChunChang Qin, First Affiliated Hospital of Chongqing Medical University, China
Copyright: © 2025 Haruki and Yamamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroyuki Yamamoto, eWFtYW1vdG8uaGlyb3l1a2kuNXJAdG9reW8tbWVkLmFjLmpw
 Shogo Haruki
Shogo Haruki Hiroyuki Yamamoto
Hiroyuki Yamamoto