- Department of Laboratory Medicine, Medical University of Vienna, Vienna, Austria
The human gut microbiota influences host metabolism, immune responses, and inflammation, with microbial dysbiosis linked to metabolic disorders and increased cardiovascular disease risk. Notably, metabolites such as short-chain fatty acids, trimethylamine N-oxide, and bile acids, which are influenced by the microbiome and its functional composition, have been implicated in vascular health, immune modulation, and atherosclerosis. This review summarizes recent findings on the gut-heart axis, demonstrating the intricate interplay between microbial communities, dietary influences and cardiovascular health. Recognizing the microbiome's impact on CVD could yield novel therapeutic targets, including prebiotics, probiotics, and precision medicine approaches that modulate microbial diversity and activities to reduce residual CVD risk.
Introduction
Cardiovascular diseases (CVD), which include coronary artery disease, hypertension, atherosclerosis and stroke, have been designated as the worldwide major cause of death over the last few decades. According to the WHO, CVD accounted for 17.9 million deaths worldwide in 2019, roughly 30% of all deaths that year (1). One of the biggest contributors to the development of cardiovascular complications is atherosclerosis, which involves up to 86% of CVD cases (2). Other prevalent pre-conditions of CVD are hypertension, diabetes mellitus (Type II) and metabolic syndrome. Some key risk factors are physical inactivity, smoking, excessive consumption of a high-calorie diet, sugar and saturated fats, which lead to systemic dyslipidaemia characterised by high amounts of cholesterol and triglycerides in circulation (3, 4). Specifically, low-density lipoproteins (LDL) and its oxidised form, OxLDL, have been shown to be a major driver in the development of CVD, including atherosclerosis. While the onset of CVD is usually characterised by the presence of more than one of the abovementioned risk factors, lipid retention plays a crucial role in disease progression, partly due to its immune-modulatory effects.
Under homeostatic conditions, the immune system plays a critical role in maintaining balance of pro- and anti-inflammatory responses to promote vascular health (5). During atherosclerosis development, this balance is disrupted by non-laminar shear stress at bifurcations of arteries, which also causes endothelial cell dysfunction and accumulation of apolipoprotein B lipoproteins in the subendothelial layer (6, 7). This prompts cytokine release from endothelial cells to recruit innate and adaptive immune cells, most prominently monocytes, which then enter the vascular wall and take up lipids, turning them into so-called lipid-laden foam cells (8). In addition to the continuous immune cell recruitment to the plaque area, vascular smooth muscle cells and fibroblasts are activated, contributing to plaque size and fibrous cap formation (5). The latter leads to stabilisation of the plaque, but may also cause plaque rupture which can cause life-threatening events such as a heart attack or stroke (9). As such, atherosclerotic plaque formation is a lipid- and inflammation-driven process. More recently, studies have focused on the influence of the gut microbiome and its metabolites on these processes, thereby suggesting intestinal dysbiosis as another potential risk factor for CVD, which we will describe in more detail in the following sections.
The microbiome and dysbiosis
The human body encompasses many bacterial, fungal, and viral species, referred to as the microflora or microbiota, that are located at various surfaces on and within the body including the genitourinary tract, skin, respiratory system and gastrointestinal tract (10). Specifically in the small and large intestines, a myriad of bacteria, fungi and protozoa are located that aid in bodily processes (11, 12). So far, the gut microbiome is described to contain trillions of microbes (∼1014 bacterial cells, and up to ∼2,000 identified species), classed in several major families, which support digestion, influence host immunity, mediate cell proliferation and produce essential metabolites (13). As such, they can influence processes including energy uptake, regulation of catabolic processes, metabolite balance, amino acid metabolism, carbohydrate metabolism and lipid metabolism (12, 14). The most prominent bacteria are Bacteroidetes and Firmicutes, and the ratio of these two has been shown to be an indicator of gut health in adults (15).
Recently, the importance of the gut microbiome in various metabolic dysfunction-associated entities has been shown, including obesity, steatotic liver disease, inflammatory bowel syndrome, diabetes type 1 and 2, CVD, multiple sclerosis, autistic spectrum disorders, as well as cancer and brain diseases including Parkinson's disease and Alzheimer's (13, 16). Homeostasis of the gut microbiota is tightly regulated by genetic factors, environmental influences such as the diet, and a specialized mucosal host immune system (17, 18). An imbalance in this regulation results in dysbiosis which can manifest in form of pathological bacterial overgrowth and changes in bacterial diversity characterized by removal of beneficial bacteria (19, 20). Furthermore, dysbiosis promotes an impaired barrier function in the gut, resulting in bacterial translocation into the periphery (21). Consequently, the blood and arterial wall are increasingly exposed to microbial products which can enhance disease progression and systemic inflammation due to the activation of various immune cells (21). Relevantly, increasing evidence suggests that dysbiosis also plays a causative role in atherosclerosis, which will be further discussed below.
Differential gut microbiome composition in CVD
It has previously been demonstrated that individuals with CVD have a differential microbiome composition compared to healthy individuals (22–24). Specifically for atherosclerosis, it was shown that microbial diversity is different between patients with stable and unstable plaques, while dysbiosis also affects lipid metabolism and systemic lipid levels (25, 26). As such, it was found that lower amounts of beneficial bacteria including Bifidobacteria and less short-chain fatty acids (SCFA)-producing bacteria are linked to a higher risk of CVD (24, 27, 28). Further, studies have observed that atherosclerosis associated with less Bacteroides, such as Roseburia intestinalis and Prevotella copri, and an increase in Firmicutes, including Ruminococcus gnavus (29, 30). Additionally, the microbial species Streptococcus and Veillonella have been shown to be present in atherosclerotic plaques, indicating systemic bacterial translocation (31).
One important factor that might contribute to intestinal dysbiosis in CVD is caloric intake and diet composition (32, 33). For example, it was described that diets containing low-fibre are considered to worsen the risks for development of CVD, including through remodelling of the microbiome (34, 35). In contrast, high-fibre diets lowered blood pressure and CVD severity in humans (36). Additionally, heart failure patients showed altered microbial richness, with increased pathogenic bacterial growth - such as Salmonella, Campylobacter and Candida species –, lower SCFA-producing bacteria (e.g., Blautia and Ruminococcus) and reduced alpha- and beta-diversity (37, 38). These changes are all linked to the heightened release of pro-inflammatory signals which can be modulated by the presence of certain pathogenic microflora, or their metabolites. Indeed, several microbiome-dependent metabolites are directly linked to the development of CVD. The most important ones will be discussed in the next section (see Figure 1).
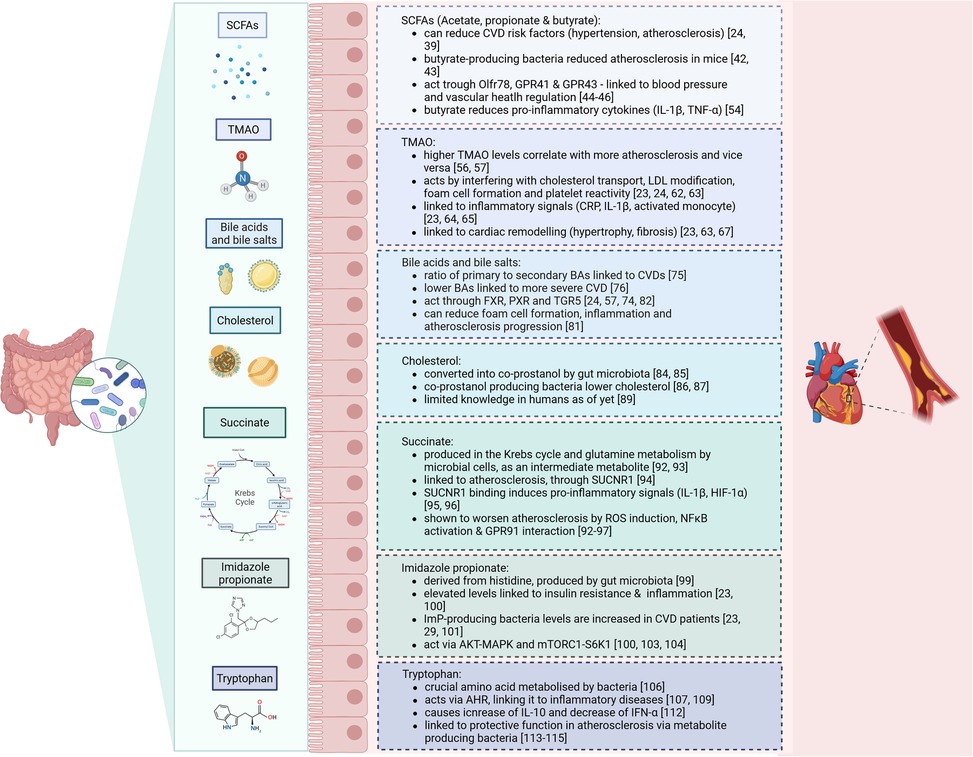
Figure 1. Summary of the microbiome-derived metabolites and their effects on cardiovascular disease.
Microbiome-derived metabolites that can influence CVD
Short-chain fatty acids
SCFA such as acetate, propionate, and butyrate are primary microbial metabolites produced from the fermentation of dietary fibres in the gut (24). Previous research described that SCFA supplements can reduce cardiovascular disease risk factors, including hypertension and atherosclerosis, by fostering beneficial gut bacteria and improving gut health (24, 39). Moreover, studies recognised that hypertensive individuals have aberrant acetyl-CoA production, which is important for synthesising SCFA (40). Specific gut bacteria such as Bacteroides acidifaciens have been linked to reduced blood pressure and improved heart function in animal models, where acetate and propionate supplements alleviate cardiac hypertrophy (41). Butyrate-producing bacteria like Roseburia intestinalis have also been shown to reduce atherosclerosis in mice by strengthening gut barrier function, which decreases translocation of inflammatory molecules into circulation (42, 43).
SCFA primarily act through receptors such as OLFR78, GPR41, and GPR43, which are involved in blood pressure regulation and vascular health (44–46). For example, propionate triggers a hypotensive effect by modulating Gpr41 and Olfr78 expression (47). Butyrate and acetate also enhance endothelial function by increasing nitric oxide bioavailability, contributing to improved vascular health (48). In human studies, high dietary fibre intake has been associated with lower blood pressure, and soluble fibres specifically have shown similar protective effects (49, 50). Mechanistically, SCFAs are also implicated in anti-inflammatory processes. Butyrate is thought to reduce inflammation by modulating gut barrier integrity and inhibiting histone deacetylases, leading to beneficial epigenetic changes in gene expression related to inflammation (51, 52). SCFA have been shown to reduce the production of pro-inflammatory cytokines such as IL-1β and TNF-α in animal models (53). Yet, the benefits of SCFA are complex and can vary. For instance, elevated circulating SCFA levels as a result of a diet high in both fibre and protein correlated with higher LDL cholesterol and blood pressure, and lower HDL cholesterol, potentially raising CVD risk (54). Therefore, while SCFA generally exhibit protective cardiovascular effects, some variations in their impact are noted depending on the fibre source, SCFA type, and the diet's overall composition. Hence, further research, especially in human clinical settings, is needed to better define how specific SCFA and fibre types influence cardiovascular risk, as well as to understand any potential pitfalls associated with high SCFA levels in certain diets.
Trimethylamine N-oxide
Trimethylamine N-oxide (TMAO) is one of the most studied microbiota–host co-metabolite in relation to CVD (23). It is derived from phosphatidylcholine, L-carnitine and choline, and produced by microbial enzymes containing high amounts of trimethylamine (TMA) (24, 55). TMAO and its precursors, particularly L-carnitine, choline, and betaine, have been linked with an increased atherosclerotic burden, showing that higher TMAO levels correlate with increased risk for adverse cardiovascular outcomes (56, 57). Studies have consistently shown a dose-dependent increase in CVD risk with elevated levels of TMAO precursors across diverse populations and meta-analyses involving over 26,000 individuals (23, 58). Also, preclinical studies employing animal models like ApoE−/− mice receiving microbiota transplants from TMAO-producing mice exhibit heightened atherosclerosis, an effect that decreases when plasma TMAO is reduced (59, 60). TMAO production also varies by diet; omnivores generally show greater TMAO synthesis from L-carnitine than vegetarians or vegans, emphasising the influence of dietary habits on gut microbial metabolism (56). Despite the robust evidence connecting TMAO with CVD risk, some inconsistencies remain. Notably, studies investigating the effect of TMAO-rich diets have reported neutral or even beneficial cardiovascular effects of TMAO and its precursors. Observational studies in asymptomatic individuals have found no association between TMAO levels and atherosclerosis progression, suggesting that TMAO may not be an early CVD predictor (24, 61).
Mechanistically, TMAO appears to promote atherosclerosis through several pathways, including effects on cholesterol transport, LDL modification, foam cell formation, and increased platelet reactivity, which enhance clot formation (23, 24, 62, 63). Additionally, TMAO has been linked to increased inflammation markers such as C-reactive protein, IL-1β, and activated monocytes, with higher TMAO levels corresponding to greater inflammatory activity (23, 64, 65). In human studies, TMAO has been associated with atherosclerotic plaque instability and rupture, indicating a role in local vascular remodelling (66). Furthermore, in Western diet-induced obesity models, TMAO has been tied to cardiac remodelling, including hypertrophy and fibrosis, which can impair heart function, and these effects have been diminished by antibiotic use to reduce TMAO levels (23, 63, 67). In Ldlr−/− mice on a high-choline diet, Icam-1, Il-6 and Cox-2 expression was elevated in the aorta, all of which are promoting atherosclerosis through stimulation along the MAPK and NFκB pathways (68). Furthermore, specific inhibitors targeting TMA lyase, the enzyme responsible for TMAO production, have shown promise in reducing TMAO-related atherosclerosis risk in preclinical studies in mice (69, 70). This research exemplifies how microbial metabolism influences CVD risk and suggests that targeting TMAO pathways could be a potential strategy to mitigate cardiovascular risk in susceptible individuals.
Bile acids
The microbiome is also involved in the production and composition of bile acids (BAs), which are saturated or hydroxylated steroids that aid absorption of dietary fats, lipophilic vitamin uptake, and metabolic regulation of lipids, glucose, and energy (24, 57, 71). Initially, primary BAs are derived from cholesterol that is metabolized in the liver. Then, BAs are transported to the gallbladder, from where they enter the duodenum via the bile (57, 72). The gut microbiota deconjugates these primary BAs to produce secondary BAs. Secondary BAs are produced by several bacteria, such as Lactobacillus, Bacteroides (gram-negative), Enterococcus and Clostridium (24, 73). When BAs enter the bloodstream, their associated receptors can influence signalling pathways for metabolism, which has been shown to be involved in CVD risk (74). A higher ratio of secondary to primary BAs has been linked to hypercholesterolemia and cardiovascular diseases, where it may correlate with worse survival in heart failure patients (75). In human studies, circulating levels of BAs, especially lower primary and secondary BA concentrations, are associated with higher severity of coronary artery disease (CAD) (76).
The main pathway by which BAs can influence CVD is through their interactions with nuclear and membrane receptors such as the Farnesoid X-activated receptor (FXR) (24, 57). FXR activation has shown both protective and detrimental effects on atherosclerosis, depending on the experimental model, which suggests that FXR's effects may depend on complex factors such as receptor location and specific BA interactions. For instance, it was shown that FXR activation in certain atherosclerosis-prone mice reduces plaque formation, while in other murine models worsened disease was reported (77–79). Furthermore, FXR also plays a role in the TMAO pathway by regulating FMO3, an enzyme involved in lipid metabolism and inflammation (80). Another membrane receptor, TGR5, has been found to exert anti-inflammatory effects relevant to CVD (74). Activated by secondary BAs, TGR5 can inhibit NFκB signalling, which in turn reduces foam cell formation and inflammation in atherosclerotic lesions (81). In contrast, BAs-mediated signalling via the PXR appears to aggravate atherosclerosis by increasing levels of lipoproteins and upregulating CD36 expression in macrophages (82). Inhibition of PXR has been shown to reduce lipid uptake and alleviate plaque formation, underscoring its complex role in lipid metabolism and plaque development (83).
Taken together, these findings suggest that BAs and their receptors are integral to cardiometabolic regulation, connecting gut, liver, and cardiovascular health. Given these complexities, further investigation is needed to confirm the therapeutic viability of targeting BA and their receptors in human CVD, particularly considering the variable impacts of receptor activation across different tissues and metabolic contexts.
Cholesterol
Besides BA formation from cholesterol in the liver, the gut microbiota directly uses cholesterol to form coprostanol, a non-absorbable sterol eliminated in faeces (84, 85). Currently, the main bacterial genera identified with this cholesterol-reducing ability are Eubacterium (e.g., E. coprostanoligenes) and Bacteroides (e.g., Bacteroides strain D8), though there are likely more undiscovered strains (84). Importantly, animal models support the cholesterol-lowering potential of coprostanol-producing bacteria (86, 87). For instance, hypercholesterolemic rabbits administered these bacteria showed a significant drop in plasma cholesterol that continued beyond 34 days after the last treatment (88). However, human studies on cholesterol conversion to coprostanol have encountered limitations due to small sample sizes, narrow demographic diversity, and unsuccessful strain isolation, which hinder broader conclusions and mechanistic studies (89). Additionally, the specific genes or enzymes involved in intestinal cholesterol conversion remain ill-identified, underscoring the need for studies to better understand microbial contributions to cholesterol metabolism and CVD prevention (89–91).
Succinate
Succinate is a C4-dicarboxylic acid that is produced by human and gut microbial cells as an intermediate metabolite during the Krebs cycle and glutamine metabolism (92). Additionally, succinate can be produced via fermentation of oligosaccharide and polysaccharides, where it acts an intermediate product of propionate synthesis. The main bacterial strain producing succinate are Bacteroidetes (93).
Succinate has been linked to atherosclerosis, as it can function as an inflammatory signal ligand via its receptor SUCNR1, that becomes activated under certain cellular circumstances, such as tissue damage or hypoxia (94). The binding of succinate to its receptor leads to the expression of HIF-1α, IL-1β and other pro-inflammatory cytokines (95, 96). Moreover, succinate induces high levels of ROS in the mitochondria, which supports the conversion of pro-inflammatory macrophages (92, 95, 96). One study showed that the increase in serum IL-1β correlated with succinate in coronary heart disease (97). Additionally, extracellular succinate can interact with GPR91, which is expressed on naïve DCs and macrophages, where it could activate HIF-1α and in turn IL-1β (93, 98). Succinate is also implicated in the NFκB pathway, as it was shown that HUVECS increase NLRP3 and Caspase-1 after stimulation with succinate combined with LPS compared to LPS alone (97). In summary, elevated succinate might amplify the inflammatory response, thereby worsen atherosclerosis.
Imidazole propionate
Imidazole propionate (ImP) is a microbial metabolite derived from the amino acid histidine, undergoing bacterial transformation from urocanate, a compound human cells produce from histidine. While humans do not convert urocanate further, gut bacteria in some individuals metabolize it into ImP (99). This ability to produce ImP has been linked to insulin resistance and T2DM, as studies show that individuals with elevated ImP also often have markers of poor glycaemic control and inflammation, independent of body mass index, chronic kidney disease, or insulin resistance (23, 100). Longitudinal research suggests that individuals who later develop T2DM frequently have elevated plasma urocanate levels prior to diagnosis, indicating ImP production as an early metabolic disruption that may be targetable for intervention (101). ImP production is also associated with a gut bacterial profile enriched in species linked to coronary artery disease, including Clostridium bolteae, Clostridium symbiosum, and Ruminococcus gnavus (23, 29). Dissimilar, the presence of Bacteroides and butyrate-producing bacteria is negatively associated with ImP levels, suggesting a protective effect of these bacteria against ImP production (23, 102).
Mechanistically, ImP has been shown to disrupt insulin signalling by activating the p62-mTORC1-S6K1 and AKT-AMPK pathways, processes involved in insulin resistance (23, 100, 103). Markedly, these mechanisms may extend to CVD risk, since p38γ/δ and mTORC1 signalling are also involved in CVD development (104). Experimental studies found that ImP may even interfere with the effects of metformin, a commonly prescribed diabetes medication, thus compounding metabolic dysregulation (103). In a recent study, ImP levels were notably elevated in individuals with CVD, independent of other traditional risk factors, suggesting a possible link between microbial histidine metabolism and cardiovascular health (105).
Tryptophan
Tryptophan (Trp) is a crucial amino acid that is processed into several metabolites by microbial species in the gastrointestinal tract (106). Mostly, this is done by Clostridium sporogenes, Ruminococcus gnavus, Lactobacillus reuteri, and members of the Bacteroides, Bifidobacteria and Escherichia coli families (107). Many Trp metabolites can interact with aryl hydrocarbon receptor (AHR) and thus regulate epithelial cell function in the intestine (106, 108). This receptor is expressed on the cell surface of DCs, innate lymphoid cells, macrophages, neutrophils and Th17 cells, which also links Trp to several inflammation-driven diseases (107, 109). For example, in HIV patients, Trp has been implicated in promoting atherosclerotic lesions progression, where it was correlated with immune and T cell activation (110, 111). Additionally, Trp has been implicated in macrophage polarisation and effectiveness through AHR (106). In relation to this, a previous study examined that indole metabolites, and related Trp reactions, cause accumulation of IL-10 and downregulation of IFN-α, in macrophages stimulated by LPS (112).
In the context of atherosclerosis, a study demonstrated the athero-protective potential of Trp catabolism. The authors found that the absence of indoleamine 2,3-dioxygenase 1 (IDO) – the rate-limiting enzyme in the kynurenine pathway of tryptophan catabolism – was marked by increased intestinal expression of IFN-y and TNF-α and pro-inflammatory atherosclerotic plaques, characterised by large necrotic cores and high amounts of CD3+ T cells (113, 114). Additionally, the study described that Parabacteroides distasonis, an indole-producing bacterium, was less abundant in IDO deficiency and inversely correlated with atherosclerotic plaque size (114). Overall, this study presents a vital connection between gut Trp, Trp-dependent inflammation and atherosclerosis.
The Trp metabolite Indole-3-propionic acid (IPA) was also investigated in human atherosclerosis. It was found that Peptostreptococcus and Clostridium species, responsible for converting Trp to IPA, were diminished in atherosclerosis patients and associated with lower IPA serum levels (115). Additionally, they demonstrated in mice that IPA was crucial for reducing atherosclerosis burden and linked to higher ABCA1 and reduced miR-142-5p levels – important players for reverse cholesterol transport (RCT) in macrophages (115). Lastly, the lowered serum IPA in CAD patients was found to correlate with the results shown in mice, where the ABCA-1/miR-142-5p signalling pathway was impaired. To summarise, these data suggest that lowered IPA in serum takes part in RCT in macrophages and increased foam cell formation in atherosclerosis patients.
Emerging microbiome-modulating therapeutic applications
Current treatment for CVD is mostly prevention-based with lipid-lowering medication such as statins. However, with these treatments, a large residual risk still remains, which is thought to be due to immunological interactions. With considering the microbiome and their metabolites as a critical player in CVD, targeting these could yield novel effective treatment options. In line, several clinical trials are being initiated to identify the effects of microbiome-targeting approaches for CVD (see Table 1).
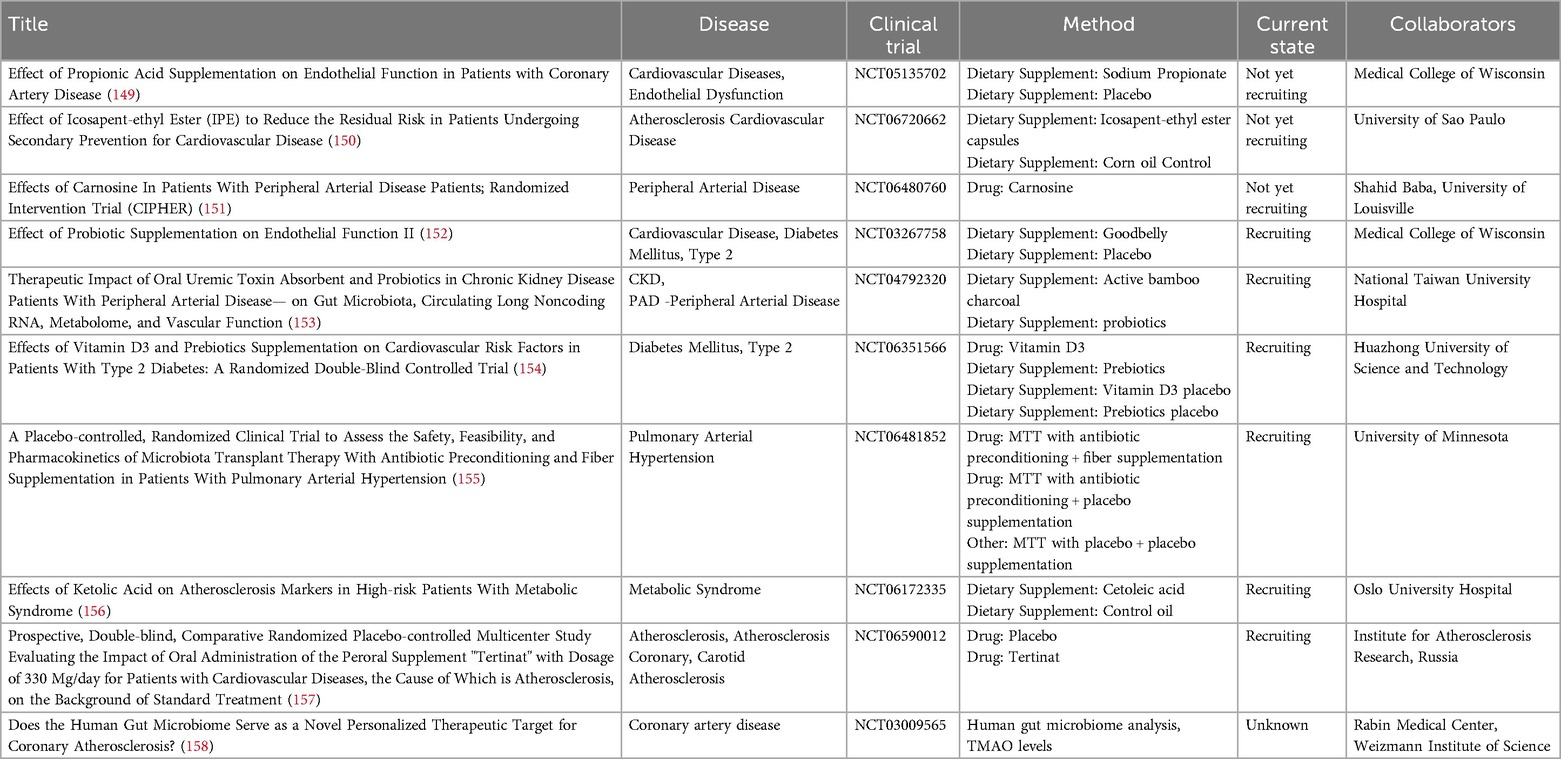
Table 1. Currently not yet recruiting, recruiting and unknown status interventional clinical trials involving microbiome-mediating supplements for treatment of CVDs and related disease.
Probiotics
Probiotics are defined as ingestible living microorganisms that mediate the intestinal microbiota balance to result in health benefits for the host (116). As a viable substance, probiotics need to be able to withstand gastric juice and BAs to keep their viability intact and exert the desired effect when attached to the intestinal lumen (117). The most commonly used strains for probiotics comprise Lactobacillus, Saccharomyces, Enterococcus, Bifidobacterium, Bacillus, Streptococcus and Escherichia (23, 39, 117). The mode of action for probiotics is towards metabolic pathways, mainly the replenishment of beneficial bacteria and thus changing the microbial composition towards a healthier state (21). Furthermore, probiotics are capable of aiding digestion and lactose hydrolysis, facilitating mineral absorption of calcium, iron, manganese and zinc, and upregulating various vitamin biosynthesis, including Vitamin K and riboflavin (118, 119). Probiotics also exert pro-apoptotic, anti-oxidative and anti-proliferative influences on the gut microbiome. Many Lactobacillus- and Bifidobacterium-containing probiotics have also been observed to produce SCFA, thereby potentially improve gut health and its metabolic function (120, 121). Recent studies demonstrated several probiotics to have antihypertensive effects, prominently Lactobacillus plantarum, and that Lactobacillus rhamnosus was capable of lowering cholesterol to improve MASLD (122–124).
Most studies using probiotics in the context of atherosclerosis focus on targeting the traditional risk factors, such as reducing dyslipidaemia, restoring endothelial function, regulating secretion of inflammatory markers and macrophage polarisation (125). A few studies found that atherosclerosis was reduced in ApoE−/− mice when treated with L. acidophilus (ATCC 4356 and 4962), L. rhamnosus GR-1 and A. muciniphila (126–128). Additionally, the total cholesterol and non-HDL cholesterol levels were lowered. In line, A. muciniphila was shown to diminish intestinal permeability and systemic inflammation in ApoE−/− mice (128). These studies demonstrate that probiotics can be considered as a treatment option for CVD.
Prebiotics
Prebiotics are fermented agents that are taken up and processed by the resident microorganisms within the consumer, resulting in distinct microbial profile changes and health benefits (116). Most prebiotics are dietary fibres, which are mainly undigested nor absorbed in the small intestine, but rather are fermented in the distal and large intestines (117). The fermentation releases the prebiotic substances that are then taken up as nutrients for the beneficial Lactobacilli and Bifidobacteria located there (117). Some of the commonly employed carbohydrates include oligofructose, galacto-oligosaccharides and inulin (129). Also, polyphenols and polyunsaturated fatty acids are considered prebiotics (117, 130). These chemicals are converted to conjugated fatty acids to enhance proliferation of beneficial bacteria within the gut. Because prebiotics are differentially degraded by all bacteria, they can be used to selectively change the microbial composition (117).
Importantly, in relation to CVD, treatment with inulin-type fructans in ApoE−/− mice was able to improve the arterial endothelial function (131). Moreover, beta-glucans were capable of enhancing endothelial vascular reactivity and lower total and LDL cholesterol (132). Another study demonstrated that atherosclerosis development could be reduced by alteration of caecal bacteria using a cyclic polymer of glucose, while mannose oligosaccharides were able to reduce serum cholesterol levels and thus halt lesion progression (133, 134). Taken together, these studies demonstrate the potential for arresting atherosclerosis development using prebiotics.
Antibiotics
Antibiotics treatment is one of the most wide-spread techniques to control the gut microbial flora, which has been found to exert various effects on cardiometabolic diseases. An antibiotics mixture consisting of ampicillin plus sulbactam (1 g/L), vancomycin (500 mg/L), ciproflaxin (200 mg/L), imipenem (250 mg/L) and metronidazole (1 g/L) was shown to mediate cholesterol metabolism in ApoE−/− mice and humans by modulation of propionate levels and cholesterol transporter Niemann-Pick C1-like 1 (135). Additionally, the reduction of phytosterol levels by a similar antibiotic treatment (ampicillin 1 g/L, metronidazole 1 g/L, neomycin 1 g/L and vancomycin 0.5 g/L) has also been linked to altered cholesterol metabolism in mice (136). Another study demonstrated that orally administered vancomycin correlated with a reduction in infarct volume and aided in post-infarct cardiac function in rats (137). Other antibiotic applications resulted in lessened inflammation, bacterial translocation, vascular dysfunction and myocardial injury in mice (138, 139).
While these data indicated beneficial outcomes, antibiotics treatment did not affect disease progression in a number of clinical trials (140). In contrast, a longitudinal study in ∼36,000 adult women described that long-time use of various antibiotics associated with heightened risk for CVD via chronic alterations of the microbiome, including depletion of probiotic bacteria (141). The same risk indication of prolonged antibiotics use was also demonstrated in a study in MASLD patients (142). Moreover, in a recent cross-omics analysis employing a human cohort of atherosclerosis patients and ApoE−/− mouse models, it was found that broad-spectrum antibiotics (ampicillin 1 g/L, metronizadole 1 g/L, neomycin 1 g/L and vancomycin 0.5 g/L) worsen atherosclerosis, independent of the type of diet given to the mice (143). Despite antibiotics-mediated loss of bacterial diversity, enhanced atherosclerosis associated with the presence of Lachnospiraceae, Ruminococcaceae, Porphyromonadaceae and Prevotellaceae, which could be better targets for more specific treatments (143). Thus, the impact of antibiotics on CVD may be type- and combination-dependent. One study in metabolic syndrome patients showed that vancomycin has severe effects on microbiota composition, bile acid metabolism and insulin sensitivity compared to amoxicillin which had no effect (144). Also, macrolides such as azithromycin have been correlated with increased risk of cardiovascular death and myocardial infarction, while others like erythromycin or roxithromycin were not (145). Taken together, this indicates some antibiotics are more efficient for intervention than others and that the type, dosage, and combination of antibiotics used appears to be a crucial factor for potential therapeutic applications in CVD.
Faecal microbiota transplantation
While faecal microbiota transplantation (FMT) is widely used to investigate the role of the gut microbiome in health and disease, it also has been tested as an intervention approach in several studies (13, 21). Due to the positive results in regards of Clostridium difficile infections, FMT is currently being studied for other conditions, among them atherosclerosis. One study created an CTRP9-knockout atherosclerotic-prone mouse model, where they performed intragastrical FMT with faecal matter from WT donor mice (146). This study showed that atherosclerosis severity was reduced in mice after FMT and that the transfer of harmful microbiota provoked atherosclerosis development (146). Another study investigating the gut-immune axis in CVD found that germfree mice with FMT from hypertension patients led to increments in blood pressure and inflammation, when compared to germfree mice that received FMT from “healthy” individuals (147). The germfree mice which received the hypertensive FMT displayed an increase in markers for LPS production, which is often triggered by gram-negative bacteria including Klebsiella and Prevotella (10, 147). Both species were previously shown to be enriched in the microbial flora of hypertensive patients, linking dysbiosis and the microbiome to inflammation in CVDs (10, 147). Taken together, these studies demonstrate that FMT could be a potential strategy for atherosclerosis management, via altering or restoring the microbiome.
Other
Certain small molecules have also been developed to specifically target the bacterial growth to limit disease. One study identified two such molecules, specifically cyclic D- and L-α-peptides, which can transfer the bacterial membrane and inhibit bacterial growth (148). These cyclic molecules demonstrated a reduction in atherosclerotic lesion size by 37% and 48% and also lessened cholesterol levels by 37% and 36% in Ldlr−/− mice (148). Furthermore, the peptides led to downregulated expression of pro-inflammatory chemo- and cytokines. These data indicate that cyclic peptides targeting microbial strains could be a promising therapeutic option for atherosclerosis.
Further, the influence of inhibition of TMAO production by commensal bacteria using 3,3-dimethyl-1-butanol (DMB) on atherosclerosis was studied (69). The administration of DMB via the drinking water resulted in lower foam cell formation from macrophages and reduced atherosclerotic plaque sizes in ApoE−/−mice, suggesting that targeting of microbial enzymes could reduce atherosclerosis burden.
Conclusion
The complex relationship between the gut microbiome and CVD underscores the importance of the heart-gut axis in CVD pathology. Microbial dysbiosis disrupts lipid metabolism, promotes inflammation, and facilitates immune dysregulation, all of which contribute to atherosclerosis and other cardiovascular complications. Specific microbial metabolites, particularly SCFA, TMAO, and BAs, modulate immune responses and vascular function, illustrating how dietary and microbial interactions influence CVD risk. Emerging therapeutic strategies, such as microbiota-targeted treatments, prebiotic and probiotic supplementation, show promise in mitigating CVD progression by restoring microbial balance. As our understanding of the functional role of the microbiome in homeostasis and pathology advances, development of novel targeting strategies hold potential to transform CVD management and reduce global cardiovascular mortality. Further research is essential to validate these approaches and translate them into effective clinical interventions.
Author contributions
DH: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. TH: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by a Stand-Alone grant (FWF; P36774-B), and a Zukunftskollegs grant (FWF; ZK81B) to TH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ApoE, apolipoprotein E; AHR, aryl hydrocarbon receptor; Bas, bile acids; CVD, cardiovascular disease; DC, dendritic cell; DMB, 3,3-dimethyl-1-butanol; FMT, faecal microbiota transplantation; FXR, farnesoid X-activated receptor; HDL, high-density lipoprotein; HUVECs, human umbilical vein endothelial cells; IDO, indoleamine 2,3-dioxygenase 1; IL, interleukin; ImP, imidazole propionate; IPA, Indole-3-propionic acid; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPS, lipopolysaccharide; MASLD, metabolic dysfunction-associated steatotic liver disease; OxLDL, oxidized LDL; PXR, pregnane X-activated receptor; RCT, reverse cholesterol transport; ROS, reactive oxygen species; SCFA, short-chain fatty acids; T2DM, type-II diabetes mellitus; TMAO, trimethylamine N-oxide; TNF-α, tumour necrosis factor alpha; Trp, tryptophan.
References
1. Organisation WH. Cardiovascular diseases (CDVs) (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (Accessed February 28, 2025).
2. Di Cesare M. World Heart Report 2023: Confronting the World’s Number One Killer. World Heart Federation (2023). https://world-heart-federation.org/
3. Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592(7855):524–33. doi: 10.1038/s41586-021-03392-8
4. Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. (2006) 47(8, Supplement):C7–C12. doi: 10.1016/j.jacc.2005.09.068
5. Wang X, Chen L, Wei J, Zheng H, Zhou N, Xu X, et al. The immune system in cardiovascular diseases: from basic mechanisms to therapeutic implications. Signal Transduct Target Ther. (2025) 10(1):166. doi: 10.1038/s41392-025-02220-z
6. Souilhol C, Tardajos Ayllon B, Li X, Diagbouga MR, Zhou Z, Canham L, et al. JAG1-NOTCH4 mechanosensing drives atherosclerosis. Sci Adv. (2022) 8(35):eabo7958. doi: 10.1126/sciadv.abo7958
7. Souilhol C, Serbanovic-Canic J, Fragiadaki M, Chico TJ, Ridger V, Roddie H, et al. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat Rev Cardiol. (2020) 17(1):52–63. doi: 10.1038/s41569-019-0239-5
8. Porsch F, Binder CJ. Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2024) 21(11):780–807. doi: 10.1038/s41569-024-01045-7
9. Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. (2013) 11(1):117. doi: 10.1186/1741-7015-11-117
10. Dinakis E, O'Donnell JA, Marques FZ. The gut–immune axis during hypertension and cardiovascular diseases. Acta Physiol. (2024) 240(8):e14193. doi: 10.1111/apha.14193
11. Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease: unveiling the relationship. Front Microbiol. (2022) 13:1–14. doi: 10.3389/fmicb.2022.999001
12. Belizário JE, Faintuch J, Garay-Malpartida M. Gut microbiome dysbiosis and immunometabolism: new frontiers for treatment of metabolic diseases. Mediat Inflamm. (2018) 2018(1):2037838. doi: 10.1155/2018/2037838
13. Bui TVA, Hwangbo H, Lai Y, Hong SB, Choi Y-J, Park H-J, et al. The gut-heart axis: updated review for the roles of microbiome in cardiovascular health. Korean Circ J. (2023) 53(8):499. doi: 10.4070/kcj.2023.0048
14. Cox TO, Lundgren P, Nath K, Thaiss CA. Metabolic control by the microbiome. Genome Med. (2022) 14(1):80. doi: 10.1186/s13073-022-01092-0
15. Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. (2009) 9(1):123. doi: 10.1186/1471-2180-9-123
16. Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. (2018) 56(3):154–62. doi: 10.1007/s12275-018-7548-y
17. Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. (2017) 4:14. doi: 10.1186/s40779-017-0122-9
18. Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. (2016) 535(7610):65–74. doi: 10.1038/nature18847
19. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. (2017) 17(4):219–32. doi: 10.1038/nri.2017.7
20. DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. (2016) 22(5):1137–50. doi: 10.1097/MIB.0000000000000750
21. Benedé-Ubieto R, Cubero FJ, Nevzorova YA. Breaking the barriers: the role of gut homeostasis in metabolic-associated steatotic liver disease (MASLD). Gut Microbes. (2024) 16(1):2331460. doi: 10.1080/19490976.2024.2331460
22. Liu H, Chen X, Hu X, Niu H, Tian R, Wang H, et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. (2019) 7(1):68. doi: 10.1186/s40168-019-0683-9
23. Chakaroun RM, Olsson LM, Bäckhed F. The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol. (2023) 20(4):217–35. doi: 10.1038/s41569-022-00771-0
24. Zhang X, Gérard P. Diet-gut microbiota interactions on cardiovascular disease. Comput Struct Biotechnol J. (2022) 20:1528–40. doi: 10.1016/j.csbj.2022.03.028
25. Mitra S, Drautz-Moses DI, Alhede M, Maw MT, Liu Y, Purbojati RW, et al. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome. (2015) 3(1):38. doi: 10.1186/s40168-015-0100-y
26. Shen X, Li L, Sun Z, Zang G, Zhang L, Shao C, et al. Gut microbiota and atherosclerosis—focusing on the plaque stability. Front Cardiovasc Med. (2021) 8:1–15. doi: 10.3389/fcvm.2021.668532
27. Zhou W, Cheng Y, Zhu P, Nasser MI, Zhang X, Zhao M. Implication of gut microbiota in cardiovascular diseases. Oxid Med Cell Longevity. (2020) 2020(1):5394096. doi: 10.1155/2020/5394096
28. Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, et al. Differential analysis of hypertension-associated intestinal Microbiota. Int J Med Sci. (2019) 16(6):872–81. doi: 10.7150/ijms.29322
29. Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8(1):845. doi: 10.1038/s41467-017-00900-1
30. Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb. (2016) 23(8):908–21. doi: 10.5551/jat.32672
31. Kiouptsi K, Reinhardt C. Contribution of the commensal microbiota to atherosclerosis and arterial thrombosis. Br J Pharmacol. (2018) 175(24):4439–49. doi: 10.1111/bph.14483
32. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. (2016) 375(24):2369–79. doi: 10.1056/NEJMra1600266
33. Lv J-j, Zhang L-j, Yixi Z, Zhang Y-c, Li X-y, Yang C-h, et al. The global burden of cardiovascular disease attributable to diet low in fiber among people aged 60 years and older, 1990–2019: an age–period–cohort analysis of the global burden of disease study. BMC Public Health. (2024) 24(1):2639. doi: 10.1186/s12889-024-19897-6
34. Threapleton DE, Greenwood DC, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Br Med J. (2013) 347(dec19 2):f6879. doi: 10.1136/bmj.f6879
35. Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. (2020) 141(17):1393–403. doi: 10.1161/CIRCULATIONAHA.119.043081
36. Reynolds AN, Akerman A, Kumar S, Diep Pham HT, Coffey S, Mann J. Dietary fibre in hypertension and cardiovascular disease management: systematic review and meta-analyses. BMC Med. (2022) 20(1):139. doi: 10.1186/s12916-022-02328-x
37. Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. (2016) 4(3):220–7. doi: 10.1016/j.jchf.2015.10.009
38. Luedde M, Winkler T, Heinsen F-A, Rühlemann MC, Spehlmann ME, Bajrovic A, et al. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Failure. (2017) 4(3):282–90. doi: 10.1002/ehf2.12155
39. Mao Y, Kong C, Zang T, You L, Wang L-S, Shen L, et al. Impact of the gut microbiome on atherosclerosis. mLife. (2024) 3(2):167–75. doi: 10.1002/mlf2.12110
40. Zaric BL, Radovanovic JN, Gluvic Z, Stewart AJ, Essack M, Motwalli O, et al. Atherosclerosis linked to aberrant amino acid metabolism and immunosuppressive amino acid catabolizing enzymes. Front Immunol. (2020) 11:551758. doi: 10.3389/fimmu.2020.551758
41. Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut Microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. (2017) 135(10):964–77. doi: 10.1161/CIRCULATIONAHA.116.024545
42. Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. (2018) 3(12):1461–71. doi: 10.1038/s41564-018-0272-x
43. Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. (2019) 139(11):1407–21. doi: 10.1161/CIRCULATIONAHA.118.036652
44. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54(9):2325–40. doi: 10.1194/jlr.R036012
45. Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. (2017) 24(7):660–72. doi: 10.5551/jat.RV17006
46. Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. (2016) 48(11):826–34. doi: 10.1152/physiolgenomics.00089.2016
47. Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. (2013) 110(11):4410–5. doi: 10.1073/pnas.1215927110
48. Robles-Vera I, Toral M, de la Visitación N, Aguilera-Sánchez N, Redondo JM, Duarte J. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front Physiol. (2020) 11:277. doi: 10.3389/fphys.2020.00277
49. Khan K, Jovanovski E, Ho HVT, Marques ACR, Zurbau A, Mejia SB, et al. The effect of viscous soluble fiber on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2018) 28(1):3–13. doi: 10.1016/j.numecd.2017.09.007
50. Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. (2005) 23(3):475–81. doi: 10.1097/01.hjh.0000160199.51158.cf
51. Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. (2011) 3(10):858–76. doi: 10.3390/nu3100858
52. Vinolo MAR, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. (2011) 22(9):849–55. doi: 10.1016/j.jnutbio.2010.07.009
53. Aguilar EC, Leonel AJ, Teixeira LG, Silva AR, Silva JF, Pelaez JMN, et al. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr Metab Cardiovasc Dis. (2014) 24(6):606–13. doi: 10.1016/j.numecd.2014.01.002
54. Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. (2020) 111(3):545–54. doi: 10.1093/ajcn/nqz322
55. Kałużna-Czaplińska J, Gątarek P. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. (2021) 20:301–19. doi: 10.17179/excli2020-3239
56. Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. (2019) 129(1):373–87. doi: 10.1172/JCI94601
57. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. (2020) 127(4):553–70. doi: 10.1161/CIRCRESAHA.120.316242
58. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. (2017) 38(39):2948–56. doi: 10.1093/eurheartj/ehx342
59. Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. (2015) 290(9):5647–60. doi: 10.1074/jbc.M114.618249
60. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19(5):576–85. doi: 10.1038/nm.3145
61. Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc. (2015) 4(11):e002699. doi: 10.1161/JAHA.115.002699
62. Ma G, Pan B, Chen Y, Guo C, Zhao M, Zheng L, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. (2017) 37(2):1–12. doi: 10.1042/BSR20160244
63. Chen K, Zheng X, Feng M, Li D, Zhang H. Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol. (2017) 8:139. doi: 10.3389/fphys.2017.00139
64. Chou RH, Chen CY, Chen IC, Huang HL, Lu YW, Kuo CS, et al. Trimethylamine N-oxide, circulating endothelial progenitor cells, and endothelial function in patients with stable angina. Sci Rep. (2019) 9(1):4249. doi: 10.1038/s41598-019-40638-y
65. Farhangi MA, Vajdi M. Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. (2020) 60(16):2801–23. doi: 10.1080/10408398.2020.1770199
66. Tan Y, Sheng Z, Zhou P, Liu C, Zhao H, Song L, et al. Plasma trimethylamine N-oxide as a novel biomarker for plaque rupture in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. (2019) 12(1):e007281. doi: 10.1161/CIRCINTERVENTIONS.118.007281
67. Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. (2019) 99(3):346–57. doi: 10.1038/s41374-018-0091-y
68. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. (2016) 5(2):e002767. doi: 10.1161/jaha.115.002767
69. Wang Z, Roberts Adam B, Buffa Jennifer A, Levison Bruce S, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. (2015) 163(7):1585–95. doi: 10.1016/j.cell.2015.11.055
70. Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, et al. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. (2018) 24(9):1407–17. doi: 10.1038/s41591-018-0128-1
71. Herrema H, Nieuwdorp M, Groen AK. Microbiome and cardiovascular disease. In: von Eckardstein A, Binder CJ, editors. Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets. Cham: Springer International Publishing (2022). p. 311–34.
72. Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. (2006) 47(2):241–59. doi: 10.1194/jlr.R500013-JLR200
73. Ridlon JM, Harris SC, Bhowmik S, Kang D-J, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. (2016) 7(1):22–39. doi: 10.1080/19490976.2015.1127483
74. Kuipers F, Bloks VW, Groen AK. Beyond intestinal soap—bile acids in metabolic control. Nat Rev Endocrinol. (2014) 10(8):488–98. doi: 10.1038/nrendo.2014.60
75. Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. (2017) 23(9):666–71. doi: 10.1016/j.cardfail.2017.06.007
76. Charach G, Argov O, Geiger K, Charach L, Rogowski O, Grosskopf I. Diminished bile acids excretion is a risk factor for coronary artery disease: 20-year follow up and long-term outcome. Therap Adv Gastroenterol. (2018) 11:1756283X17743420. doi: 10.1177/1756283X17743420
77. Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. (2005) 46(12):2595–604. doi: 10.1194/jlr.M500390-JLR200
78. Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, et al. FXR deficiency causes reduced atherosclerosis in ldlr−/− mice. Arterioscler Thromb Vasc Biol. (2006) 26(10):2316–21. doi: 10.1161/01.ATV.0000235697.35431.05
79. Hartman HB, Gardell SJ, Petucci CJ, Wang S, Krueger JA, Evans MJ. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR−/− and apoE−/− mice. J Lipid Res. (2009) 50(6):1090–100. doi: 10.1194/jlr.M800619-JLR200
80. Bennett Brian J, Vallim Thomas QA, Wang Z, Shih Diana M, Meng Y, Gregory J, et al. Trimethylamine-N-Oxide, a metabolite associated with atherosclerosis, exhibits Complex genetic and dietary regulation. Cell Metab. (2013) 17(1):49–60. doi: 10.1016/j.cmet.2012.12.011
81. Pols Thijs WH, Nomura M, Harach T, Lo Sasso G, Oosterveer Maaike H, Thomas C, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. (2011) 14(6):747–57. doi: 10.1016/j.cmet.2011.11.006
82. Zhou C, King N, Chen KY, Breslow JL. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice[S]. J Lipid Res. (2009) 50(10):2004–13. doi: 10.1194/jlr.M800608-JLR200
83. Sui Y, Xu J, Rios-Pilier J, Zhou C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J Lipid Res. (2011) 52(9):1652–9. doi: 10.1194/jlr.M017376
84. Kriaa A, Bourgin M, Potiron A, Mkaouar H, Jablaoui A, Gérard P, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. (2019) 60(2):323–32. doi: 10.1194/jlr.R088989
85. Botham KM, Mayes PA. Cholesterol synthesis, transport, & excretion. In: Rodwell VW, Bender DA, Botham KM, Kennelly PJ, Weil PA, editors. Harper’s Illustrated Biochemistry, 31e. New York, NY: McGraw-Hill Education (2018). p. 266–81.
86. Overturf ML, Smith SA, Gotto AM Jr, Morrisett JD, Tewson T, Poorman J, et al. Dietary cholesterol absorption, and sterol and bile acid excretion in hypercholesterolemia-resistant white rabbits. J Lipid Res. (1990) 31(11):2019–27. doi: 10.1016/S0022-2275(20)42266-7
87. Xu G, Salen G, Shefer S, Tint GS, Nguyen LB, Batta AK, et al. Plant stanol fatty acid esters inhibit cholesterol absorption and hepatic hydroxymethyl glutaryl coenzyme a reductase activity to reduce plasma levels in rabbits. Metab Clin Exp. (2001) 50(9):1106–12. doi: 10.1053/meta.2001.25664
88. Li L, Buhman KK, Hartman PA, Beitz DC. Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Lett Appl Microbiol. (1995) 20(3):137–40. doi: 10.1111/j.1472-765X.1995.tb00410.x
89. Kenny DJ, Plichta DR, Shungin D, Koppel N, Hall AB, Fu B, et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe. (2020) 28(2):245–57.e6. doi: 10.1016/j.chom.2020.05.013
90. Altmann SW, Davis HR, Zhu L-j, Yao X, Hoos LM, Tetzloff G, et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. (2004) 303(5661):1201–4. doi: 10.1126/science.1093131
91. Lu K, Lee M-H, Hazard S, Brooks-Wilson A, Hidaka H, Kojima H, et al. Two genes that map to the STSL locus cause sitosterolemia: genomic structure and spectrum of mutations involving sterolin-1 and sterolin-2, encoded by ABCG5 and ABCG8, respectively. Am J Hum Genet. (2001) 69(2):278–90. doi: 10.1086/321294
92. Xu J, Yang Y, Li X, Ding S, Zheng L, Xiong C, et al. Pleiotropic activities of succinate: the interplay between gut microbiota and cardiovascular diseases. iMeta. (2023) 2(3):1–17. doi: 10.1002/imt2.124
93. Wei YH, Ma X, Zhao JC, Wang XQ, Gao CQ. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes. (2023) 15(1):2190300. doi: 10.1080/19490976.2023.2190300
94. Yuan C, Yu B, Li L, Chen J, Qin W, Zhou Z, et al. SUCNR 1 promotes atherosclerosis by inducing endoplasmic reticulum stress mediated ER-mito crosstalk. Int Immunopharmacol. (2024) 143(Pt 3):113510. doi: 10.1016/j.intimp.2024.113510
95. Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O’Neill LA, et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. (2019) 1(1):16–33. doi: 10.1038/s42255-018-0014-7
96. Mills E, O’Neill LAJ. Succinate: a metabolic signal in inflammation. Trends Cell Biol. (2014) 24(5):313–20. doi: 10.1016/j.tcb.2013.11.008
97. Xu J, Zheng Y, Zhao Y, Zhang Y, Li H, Zhang A, et al. Succinate/IL-1β signaling axis promotes the inflammatory progression of endothelial and exacerbates atherosclerosis. Front Immunol. (2022) 13:1–16. doi: 10.3389/fimmu.2022.817572
98. Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. (2008) 9(11):1261–9. doi: 10.1038/ni.1657
99. Xu Q, Wang W, Li Y, Liu Y, Liu Y. Imidazole propionate in type 2 diabetes mellitus and cardiovascular diseases: a mini review. Front Immunol. (2024) 15:1–6. doi: 10.3389/fimmu.2024.1454210
100. Koh A, Molinaro A, Ståhlman M, Khan MT, Schmidt C, Mannerås-Holm L, et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. (2018) 175(4):947–61.e17. doi: 10.1016/j.cell.2018.09.055
101. Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, et al. A longitudinal big data approach for precision health. Nat Med. (2019) 25(5):792–804. doi: 10.1038/s41591-019-0414-6
102. Koh A, Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. (2020) 78(4):584–96. doi: 10.1016/j.molcel.2020.03.005
103. Koh A, Mannerås-Holm L, Yunn NO, Nilsson PM, Ryu SH, Molinaro A, et al. Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation. Cell Metab. (2020) 32(4):643–53.e4. doi: 10.1016/j.cmet.2020.07.012
104. Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. (2005) 67(4):705–13. doi: 10.1016/j.cardiores.2005.04.018
105. Molinaro A, Bel Lassen P, Henricsson M, Wu H, Adriouch S, Belda E, et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. (2020) 11(1):5881. doi: 10.1038/s41467-020-19589-w
106. Seo S-K, Kwon B. Immune regulation through tryptophan metabolism. Exp Mol Med. (2023) 55(7):1371–9. doi: 10.1038/s12276-023-01028-7
107. Gupta SK, Vyavahare S, Duchesne Blanes IL, Berger F, Isales C, Fulzele S. Microbiota-derived tryptophan metabolism: impacts on health, aging, and disease. Exp Gerontol. (2023) 183:112319. doi: 10.1016/j.exger.2023.112319
108. Zelante T, Iannitti Rossana G, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from Microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. (2013) 39(2):372–85. doi: 10.1016/j.immuni.2013.08.003
109. Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. (2018) 11(4):1024–38. doi: 10.1038/s41385-018-0019-2
110. Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. (2010) 2(32):32ra6. doi: 10.1126/scitranslmed.3000632
111. Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, et al. Plasma tryptophan-kynurenine metabolites are altered in human immunodeficiency virus infection and associated with progression of carotid artery atherosclerosis. Clin Infect Dis. (2018) 67(2):235–42. doi: 10.1093/cid/ciy053
112. Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clément M, et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab. (2015) 22(3):460–71. doi: 10.1016/j.cmet.2015.07.004
113. Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, et al. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A. (2015) 112(42):13033–8. doi: 10.1073/pnas.1517820112
114. Chajadine M, Laurans L, Radecke T, Mouttoulingam N, Al-Rifai R, Bacquer E, et al. Harnessing intestinal tryptophan catabolism to relieve atherosclerosis in mice. Nat Commun. (2024) 15(1):6390. doi: 10.1038/s41467-024-50807-x
115. Xue H, Chen X, Yu C, Deng Y, Zhang Y, Chen S, et al. Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ Res. (2022) 131(5):404–20. doi: 10.1161/CIRCRESAHA.122.321253
116. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18(9):649–67. doi: 10.1038/s41575-021-00440-6
117. Ghanbari F, Hasani S, Aghili ZS, Asgary S. The potential preventive effect of probiotics, prebiotics, and synbiotics on cardiovascular risk factors through modulation of gut microbiota: a review. Food Sci Nutr. (2024) 12(7):4569–80. doi: 10.1002/fsn3.4142
118. Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. (2024) 19(2):275–93. doi: 10.1007/s11739-023-03374-w
119. Farias DP, de Araújo FF, Neri-Numa IA, Pastore GM. Prebiotics: trends in food, health and technological applications. Trends Food Sci Technol. (2019) 93:23–35. doi: 10.1016/j.tifs.2019.09.004
120. Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. (2000) 45(8):1617–22. doi: 10.1023/A:1005577330695
121. Kim KT, Kim JW, Kim SI, Kim S, Nguyen TH, Kang CH. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid Bacteria isolated from canine and feline feces. Microorganisms. (2021) 9(9):1–13. doi: 10.3390/microorganisms9091971
122. Park S, Kang J, Choi S, Park H, Hwang E, Kang Y, et al. Cholesterol-lowering effect of Lactobacillus rhamnosus BFE5264 and its influence on the gut microbiome and propionate level in a murine model. PLoS One. (2018) 13(8):e0203150. doi: 10.1371/journal.pone.0208294
123. Lewis-Mikhael A-M, Davoodvandi A, Jafarnejad S. Effect of lactobacillusplantarum containing probiotics on blood pressure: a systematic review and meta-analysis. Pharmacol Res. (2020) 153:104663. doi: 10.1016/j.phrs.2020.104663
124. Zhou T, Wang Z, Lv X, Guo M, Zhang N, Liu L, et al. Targeting gut S. aureofaciens Tü117 serves as a new potential therapeutic intervention for the prevention and treatment of hypertension. Cell Metab. (2025) 37(2):496–513.e11. doi: 10.1016/j.cmet.2025.01.004
125. Zhai T, Wang P, Hu X, Zheng L. Probiotics bring new hope for atherosclerosis prevention and treatment. Oxid Med Cell Longev. (2022) 2022:3900835. doi: 10.1155/2022/3900835
126. Huang Y, Wang J, Quan G, Wang X, Yang L, Zhong L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl Environ Microbiol. (2014) 80(24):7496–504. doi: 10.1128/AEM.02926-14
127. Fang Y, Chen HQ, Zhang X, Zhang H, Xia J, Ding K, et al. Probiotic administration of lactobacillus rhamnosus GR-1 attenuates atherosclerotic plaque formation in ApoE-/- mice fed with a high-fat diet. Eur Rev Med Pharmacol Sci. (2019) 23(8):3533–41. doi: 10.26355/eurrev_201904_17722
128. Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe−/− mice. Circulation. (2016) 133(24):2434–46. doi: 10.1161/CIRCULATIONAHA.115.019645
129. Oniszczuk A, Oniszczuk T, Gancarz M, Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. (2021) 26(4):1172. doi: 10.3390/molecules26041172
130. Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: safe options for next-generation therapeutics. Appl Microbiol Biotechnol. (2022) 106(2):505–21. doi: 10.1007/s00253-021-11646-8
131. Rault-Nania M-H, Gueux E, Demougeot C, Demigné C, Rock E, Mazur A. Inulin attenuates atherosclerosis in apolipoprotein E-deficient mice. Br J Nutr. (2006) 96(5):840–4. doi: 10.1017/BJN20061913
132. Tiwari U, Cummins E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition. (2011) 27(10):1008–16. doi: 10.1016/j.nut.2010.11.006
133. Sakurai T, Sakurai A, Chen Y, Vaisman BL, Amar MJ, Pryor M, et al. Dietary α-cyclodextrin reduces atherosclerosis and modifies gut flora in apolipoprotein E-deficient mice. Mol Nutr Food Res. (2017) 61(8):1–11. doi: 10.1002/mnfr.201600804
134. Hoving LR, Katiraei S, Heijink M, Pronk A, van der Wee-Pals L, Streefland T, et al. Dietary mannan oligosaccharides modulate gut microbiota, increase fecal bile acid excretion, and decrease plasma cholesterol and atherosclerosis development. Mol Nutr Food Res. (2018) 62(10):e1700942. doi: 10.1002/mnfr.201700942
135. Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. (2021) 43(6):518–33. doi: 10.1093/eurheartj/ehab644
136. Kappel BA, De Angelis L, Puetz A, Ballanti M, Menghini R, Marx N, et al. Antibiotic-induced gut microbiota depletion exacerbates host hypercholesterolemia. Pharmacol Res. (2023) 187:106570. doi: 10.1016/j.phrs.2022.106570
137. Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. (2012) 26(4):1727–35. doi: 10.1096/fj.11-197921
138. Zhao J, Zhang Q, Cheng W, Dai Q, Wei Z, Guo M, et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion. Cardiovasc Res. (2023) 119(6):1390–402. doi: 10.1093/cvr/cvad023
139. Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. (2018) 6(1):66. doi: 10.1186/s40168-018-0441-4
140. Sethi NJ, Safi S, Korang SK, Hróbjartsson A, Skoog M, Gluud C, et al. Antibiotics for secondary prevention of coronary heart disease. Cochrane Database Syst Rev. (2021) 2(2):Cd003610. doi: 10.1002/14651858.cd003610.pub4
141. Heianza Y, Zheng Y, Ma W, Rimm EB, Albert CM, Hu FB, et al. Duration and life-stage of antibiotic use and risk of cardiovascular events in women. Eur Heart J. (2019) 40(47):3838–45. doi: 10.1093/eurheartj/ehz231
142. Kang JH, Park SJ, Jeong S, Park YJ, Kim HJ, Song J, et al. Association between antibiotic use and cardiovascular diseases in metabolic dysfunction-associated steatotic liver disease: a nationally representative retrospective cohort study. Hepatol Res. (2025) 55(2):200–10. doi: 10.1111/hepr.14115
143. Kappel BA, De Angelis L, Heiser M, Ballanti M, Stoehr R, Goettsch C, et al. Cross-omics analysis revealed gut microbiome-related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol Metab. (2020) 36:100976. doi: 10.1016/j.molmet.2020.100976
144. Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. (2014) 60(4):824–31. doi: 10.1016/j.jhep.2013.11.034
145. Wu Y, Bi W-T, Qu L-P, Fan J, Kong X-J, Ji C-C, et al. Administration of macrolide antibiotics increases cardiovascular risk. Front Cardiovasc Med. (2023) 10:1–10. doi: 10.3389/fcvm.2023.1117254
146. Kim ES, Yoon BH, Lee SM, Choi M, Kim EH, Lee B-W, et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp Mol Med. (2022) 54(2):103–14. doi: 10.1038/s12276-022-00728-w
147. Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5(1):14. doi: 10.1186/s40168-016-0222-x
148. Chen PB, Black AS, Sobel AL, Zhao Y, Mukherjee P, Molparia B, et al. Directed remodeling of the mouse gut microbiome inhibits the development of atherosclerosis. Nat Biotechnol. (2020) 38(11):1288–97. doi: 10.1038/s41587-020-0549-5
149. Effect of Propionic Acid Supplementation on Endothelial Function in Patients With Coronary Artery Disease (2021). Available online at: https://clinicaltrials.gov/study/NCT05135702 (Accessed March 07, 2025).
150. Effect of Icosapent-ethyl Ester (IPE) to Reduce the Residual Risk in Patients Undergoing Secondary Prevention for Cardiovascular Disease (2024). Available online at: https://clinicaltrials.gov/study/NCT06720662 (Accessed March 07, 2025).
151. Effects of Carnosine In Patients With Peripheral Arterial Disease Patients; Randomized Intervention Trial (CIPHER) (2024). Available online at: https://clinicaltrials.gov/study/NCT06480760 (Accessed March 07, 2025).
152. Effect of Probiotic Supplementation on Endothelial Function II (2017). Available online at: https://clinicaltrials.gov/study/NCT03267758 (Accessed March 07, 2025).
153. Therapeutic Impact of Oral Uremic Toxin Absorbent and Probiotics in Chronic Kidney Disease Patients With Peripheral Arterial Disease— on Gut Microbiota, Circulating Long Noncoding RNA, Metabolome, and Vascular function (2020). Available online at: https://clinicaltrials.gov/study/NCT04792320 (Accessed March 07, 2025).
154. Effects of Vitamin D3 and Prebiotics Supplementation on Cardiovascular Risk Factors in Patients With Type 2 Diabetes: A Randomized Double-Blind Controlled Trial (2024). Available online at: https://clinicaltrials.gov/study/NCT06351566 (Accessed March 07, 2025).
155. A Placebo-controlled, Randomized Clinical Trial to Assess the Safety, Feasibility, and Pharmacokinetics of Microbiota Transplant Therapy With Antibiotic Preconditioning and Fiber Supplementation in Patients With Pulmonary Arterial Hypertension (2024). Available online at: https://clinicaltrials.gov/study/NCT06481852 (Accessed March 07, 2025).
156. Effects of Ketolic Acid on Atherosclerosis Markers in High-risk Patients With Metabolic Syndrome (Effekt av Ketolinsyre på aterosklerosemarkører i høyrisikopasienter Med Metabolsk Syndrom) (2023). Available online at: https://clinicaltrials.gov/study/NCT06172335 (Accessed March 07, 2025).
157. Prospective, Double-blind, Comparative Randomized Placebo-controlled Multicenter Study Evaluating the Impact of Oral Administration of the Peroral Supplement "Tertinat" with Dosage of 330 Mg/day for Patients with Cardiovascular Diseases, the Cause of Which is Atherosclerosis, on the Background of Standard Treatment (2024). Available online at: https://clinicaltrials.gov/study/NCT06590012 (Accessed March 07, 2025).
158. Does the Human Gut Microbiome Serve as a Novel Personalized Therapeutic Target for Coronary Atherosclerosis? (2016). Available online at: https://clinicaltrials.gov/study/NCT03009565 (Accessed March 07, 2025).
Keywords: atherosclerosis, microbiota, metabolites, immuno-metabolism, dysbiosis
Citation: Hoffelner DK and Hendrikx T (2025) Emerging therapy targets to modulate microbiome-mediated effects evident in cardiovascular disease. Front. Cardiovasc. Med. 12:1631841. doi: 10.3389/fcvm.2025.1631841
Received: 20 May 2025; Accepted: 1 July 2025;
Published: 16 July 2025.
Edited by:
Emiel Van Der Vorst, University Hospital RWTH Aachen, GermanyReviewed by:
Aline Dupont, University Hospital RWTH Aachen, GermanyBen Arpad Kappel, University Hospital RWTH Aachen, Germany
Copyright: © 2025 Hoffelner and Hendrikx. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Hendrikx, dGltLmhlbmRyaWt4QG1lZHVuaXdpZW4uYWMuYXQ=
†ORCID:
Dorothea Katharina Hoffelner
orcid.org/0009-0001-1290-8310
 Dorothea Katharina Hoffelner
Dorothea Katharina Hoffelner Tim Hendrikx
Tim Hendrikx