Abstract
Aims:
This study compared characteristics of patients with heart failure (HF) prescribed vericiguat vs. eligible patients with HF not prescribed vericiguat, and sought to identify factors associated with vericiguat use in real-world settings.
Methods:
We analysed 2022–2023 Adelphi HF cross-sectional survey from United States physicians and their adult patients. Patients prescribed vericiguat were compared with patients eligible for but not prescribed vericiguat. Vericiguat eligibility was defined as ≥1 prior HF hospitalization at any time, ejection fraction (EF) <45%, and no stage 5 chronic kidney disease or need for dialysis. Both cohorts were compared descriptively, and logistic regression used to identify factors associated with vericiguat non-use.
Results:
Overall, 93 physicians reported data on 228 patients with HF (mean age [SD]: 66.8 years [11.8], 65.7% male, 60.1% White), with 98 patients prescribed vericiguat and 130 patients eligible but not prescribed vericiguat. Patients eligible but not prescribed vericiguat had more comorbid hypertension (62.3% vs. 45.9%), hyperlipidemia (52.3% vs. 34.7%), and lower EF (mean [SD]: 34.7% [5.8%] vs. 41.7% [9.6%]), all p < 0.05. For every 1% increase in EF above 38%, odds of being prescribed vericiguat increased by 44% (Odds Ratio [CI]: 1.44 [1.28, 1.63]; p < 0.05).
Conclusion:
Among patients with HF in contemporary US clinical practice, patients prescribed vericiguat have distinct demographic and clinical profiles compared to eligible patients not prescribed vericiguat. Future research should confirm these findings and explore whether subgroups of eligible patients less likely to be prescribed vericiguat may benefit from targeted implementation initiatives.
Introduction
Patients with worsening heart failure events (WHFE), defined as heart failure hospitalization (HFH) or use of outpatient intravenous diuretics, are at increased risk for downstream HFH and cardiovascular (CV) mortality (1). Vericiguat, a first-in-class soluble guanylate cyclase stimulator, received approval in the United States (US) in 2021 for the treatment of HF with reduced ejection fraction (HFrEF) following a WHFE based on findings from the VICTORIA clinical trial (2). Subsequently, the 2022 American Heart Association/American College of Cardiology (AHA/ACC) guidelines recommend vericiguat to reduce the risk of HFH and CV mortality following a WHFE, among patients with HFrEF receiving guideline-directed medical therapy (GDMT) (3).
However, despite proven benefits (2), utilization of vericiguat remains low in the US (4). Understanding factors associated with non-use of vericiguat among eligible patients in real-world clinical settings is important to inform targeted implementation strategies aimed at reducing the impact of HF-related morbidity and mortality in this population. This study compared the demographic and clinical characteristics of patients prescribed vericiguat, to eligible patients not prescribed vericiguat, and identify factors associated with vericiguat use in real-world settings.
Methods
Study design and data source
Data were collected from the Adelphi Real World HF Disease Specific Programme (DSP)™, a cross-sectional survey of physicians and their consulting patients, conducted in the US between August 2022 and February 2023. DSP methodology has been previously described and validated (5, 6).
Data collection adhered to the European Pharmaceutical Marketing Research Association guidelines and therefore ethics committee approval was not required.
Study population and variables
Physicians completed an electronic patient record form (ePRF) for ≤10 consecutively consulting patients with HF and 1 additional ePRF for their next consulting patient with HF prescribed vericiguat. Patients eligible for inclusion in the DSP were ≥18 years of age, had a physician-confirmed diagnosis of HF, and were not participating in a clinical trial.
Patients were grouped into two cohorts: “patients eligible but not being prescribed vericiguat” had a history of ≥1 HFH at any time, a most recent EF <45% (based on the HFrEF definition in the VICTORIA trial) (2), and had no evidence of chronic kidney disease Stage 5 or need for dialysis. “Patients prescribed vericiguat” were prescribed vericiguat at the time of survey. Data collected included patient demographic and clinical characteristics, medical history, laboratory measures, and current HFrEF treatment.
Statistical analysis
Characteristics of patients who were prescribed vericiguat were compared with those of patients eligible but not prescribed vericiguat. Comparisons utilized Mann–Whitney tests for ordered categorical variables, T-test for continuous variables, and Fisher's Exact Test for nominal categorical variables. Additionally, a logistic regression was used to identify factors associated with vericiguat use (Figure 1). Ejection fraction linearity was assessed and there was found to be a non-linear relationship, so a linear spline with knot at the median value of EF (38%) was used within the model. Covariates included in the model are listed in the footnote to Figure 1.
Figure 1
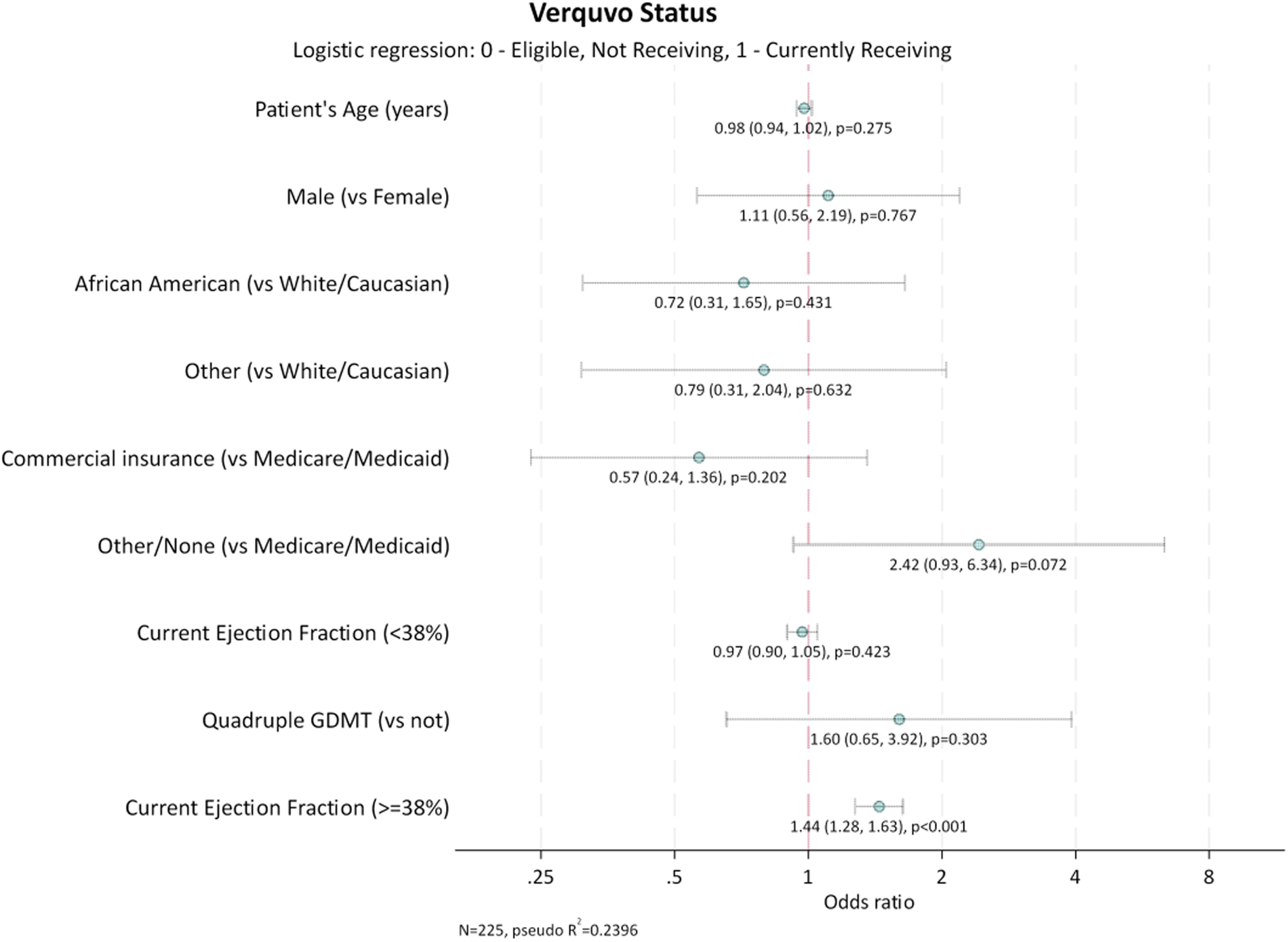
Factors associated with vericiguat use. GDMT, guideline-directed medical therapy. Quadruple GDMT was defined as use of angiotensin receptor neprilysin inhibitors (ARNi) or angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB), and beta-blockers, and mineralocorticoid receptor antagonists (MRA), and sodium-glucose cotransporter-2 inhibitors (SGLT2i). Age and EF are reported as continuous variables. Sex, ethnicity, insurance status and receiving quadruple GDMT are reported as non-continuous variables. Interpretation: Odds ratio per 1% increase of ejection fraction.
All analyses were conducted using Stata 18 (StataCorp 2023. Stata Statistical Software: Release 18. College Station, TX: StataCorp LLC).
Results
A total of 93 physicians (58 [61.7%] cardiologists and 36 [38.3%] primary care physicians) provided information for 228 patients with HF. This study cohort included 98 (43.0%) patients prescribed vericiguat, and 130 (57.0%) patients eligible but not prescribed vericiguat.
Most demographics, clinical characteristics, and treatment patterns were similar in the two groups (Table 1). However, patients prescribed vericiguat were more frequently from the Midwest (39.8%) and 44.6% of the patients eligible but not prescribed vericiguat were likely from the West (44.6%). Health insurance other than Medicare, Commercial or Medicaid insurance was more common among patients prescribed vericiguat than patients eligible but not prescribed vericiguat (14.3% vs. 4.6%, p = 0.016).
Table 1
| Variable | Overall (n = 228) | Eligible, not prescribed vericiguat (n = 130)a | Prescribed vericiguat (n = 98)b | p-value |
|---|---|---|---|---|
| Age | ||||
| Years, mean (SD) | 66.8 (11.76) | 67.4 (12.21) | 66.0 (11.15) | 0.383 |
| Sex | ||||
| Male sex, n (%) | 150 (65.79) | 88 (67.69) | 62 (63.27) | 0.573 |
| Ethnicity | ||||
| White, n (%) | 137 (60.09) | 76 (58.46) | 61 (62.24) | 0.587 |
| Black, n (%) | 44 (19.30) | 28 (21.54) | 16 (16.33) | 0.397 |
| Hispanic, n (%) | 28 (12.28) | 15 (11.54) | 13 (13.27) | 0.690 |
| Otherc, n (%) | 22 (9.65) | 12 (9.23) | 10 (10.20) | 0.824 |
| US region | ||||
| West, n (%) | 79 (34.65) | 58 (44.62) | 21 (21.43) | <0.001 |
| Northeast, n (%) | 67 (29.39) | 39 (30.00) | 28 (28.57) | |
| Midwest, n (%) | 59 (25.88) | 20 (15.38) | 39 (39.80) | |
| South, n (%) | 23 (10.09) | 13 (10.00) | 10 (10.20) | |
| Insurance status | ||||
| Medicare, n (%) | 128 (56.14) | 75 (57.69) | 53 (54.08) | 0.593 |
| Commercial, n (%) | 72 (31.58) | 46 (35.38) | 26 (26.53) | 0.195 |
| Medicaid, n (%) | 11 (4.82) | 4 (3.08) | 7 (7.14) | 0.213 |
| Otherd, n (%) | 20 (8.77) | 6 (4.62) | 14 (14.29) | 0.016 |
| No insurance coverage | 2 (0.88) | 0 (0.00) | 2 (2.04) | 0.184 |
| Top 5 comorbidities | ||||
| Hypertension, n (%) | 126 (55.26) | 81 (62.31) | 45 (45.92) | 0.016 |
| Hyperlipidemia, n (%) | 102 (44.74) | 68 (52.31) | 34 (34.69) | 0.011 |
| Diabetes, n (%) | 73 (32.02) | 48 (36.92) | 25 (25.51) | 0.085 |
| Osteoarthritis, n (%) | 37 (16.23) | 25 (19.23) | 12 (12.24) | 0.204 |
| Depression, n (%) | 35 (15.35) | 19 (14.62) | 16 (16.33) | 0.716 |
| Time since HF diagnosise | ||||
| Mean (SD) years | 2.9 (2.87) | 3.2 (3.19) | 2.4 (2.23) | 0.072 |
| HF hospitalizationf | ||||
| Never, n (%) | 42 (20.00) | 0 (0.00)g | 42 (52.76) | <0.001 |
| Within past 6 months, n (%) | 22 (11.17) | 18 (14.63) | 4 (5.41) | |
| More than 6 months ago, n (%) | 133 (65.51) | 105 (85.37) | 28 (37.84) | |
| Time since most recent hospitalization (days) | ||||
| Median (IQR) | 90.5 (22.0, 220.5) | 84.5 (17.0, 185.0) | 201.0 (62.0, 242.0) | 0.498 |
| EF at time of visit | ||||
| Mean (SD) | 37.71 (8.43) | 34.69 (5.83) | 41.71 (9.62) | <0.001 |
| Current treatment | ||||
| Angiotensin converting enzyme inhibitor (ACEi), n (%) | 66 (28.95) | 53 (40.77) | 13 (13.27) | <0.001 |
| Angiotensin receptor blockers (ARB), n (%) | 32 (14.04) | 26 (20.00) | 6 (6.12) | 0.003 |
| ACEi/ARB, n (%) | 90 (39.47) | 71 (54.62) | 19 (19.39) | <0.001 |
| Angiotensin receptor blocker + neprilysin inhibitor (ARNi), n (%) | 94 (41.23) | 62 (47.69) | 32 (32.65) | 0.030 |
| Beta-blockers, n (%) | 163 (71.49) | 118 (90.77) | 45 (45.92) | <0.001 |
| Mineralocorticoid receptor antagonists (MRA), n (%) | 68 (29.82) | 37 (28.46) | 31 (31.63) | 0.662 |
| Sodium-glucose cotransporter-2 inhibitors (SGLT2i), n (%) | 62 (27.19) | 37 (28.46) | 25 (25.51) | 0.654 |
| Receiving triple therapy | ||||
| Yes, n (%)h | 28 (12.28) | 17 (13.08) | 11 (11.22) | 0.839 |
| No, n (%) | 200 (87.72) | 113 (86.92) | 87 (88.78) | |
| Receiving quadruple GDMT | ||||
| Yes, n (%)i | 32 (14.04) | 20 (15.38) | 12 (12.24) | 0.566 |
| No, n (%) | 196 (86.0) | 110 (84.62) | 86 (87.76) | |
Patient demographics, characteristics, and treatment patterns by prescription of vericiguat.
BMI, body mass index; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDMT, guideline-directed medical therapy; HF, heart failure; IQR, interquartile range; NT-proBNP, N-terminal pro–B-type natriuretic peptide; SD, standard deviation; US, United States. Data points which add to >100% are due to the question being multiple choice and some respondents selecting >1 option.
Eligibility criteria for “Eligible but not currently prescribed vericiguat:” Patients eligible but not being prescribed vericiguat at the time of survey had a history of ≥1 HFH at any time, an EF <45% at their last assessment, were part of the random sample, and had no evidence of chronic kidney disease Stage 5 or need for dialysis.
Eligibility criteria for “Currently prescribed vericiguat:” HF diagnosis and being prescribed vericiguat at the time of survey.
Comprised of Native American, Asian (Indian subcontinent), South-East Asian, Asian (other), Middle Eastern and Other not included in the list.
Comprised of Health Insurance Exchange Plan, Cobra (continuation coverage), Non-Medicare Retired Benefit and Tricare/Veterans Healthcare.
Overall, 175 patients had data stating how long it had been since they were diagnosed with HF, 104 among patients eligible but not prescribed vericiguat, and 71 among patients who were prescribed vericiguat.
Overall, 210 patients had data about previous HFH, 130 among patients eligible but not prescribed vericiguat, and 80 among patients who were prescribed vericiguat.
Inclusion criteria for patients eligible but not prescribed vericiguat included history of HFH. As a result, all patients in this group have ≥1 HFH in their medical record.
Triple therapy was defined as angiotensin receptor neprilysin inhibitors (ARNi) or angiotensin-converting enzyme inhibitors (ACEi) or angiotensin ii receptor blockers (ARB), and beta-blockers, and mineralocorticoid receptor antagonists (MRA). Note that patients receiving quadruple therapy which included the triple treatment defined here were excluded from these analyses.
Quadruple GDMT was defined as use of ARNi or ACEi or ARB, and beta-blockers, and MRA, and sodium-glucose cotransporter-2 inhibitors (SGLT2i).
Compared to patients prescribed vericiguat, those eligible but not prescribed vericiguat had a higher prevalence of hypertension (62.3% vs. 45.9%, p = 0.016), and hyperlipidemia (52.3% vs. 34.7%, p = 0.011), but lower ejection fraction [(EF) 34.7% vs. 41.7%, p < 0.001]. Compared to patients prescribed vericiguat, a higher proportion of patients eligible but not being prescribed vericiguat received at least 1 GDMT drug class, including beta-blockers (90.8% vs. 45.9%, p < 0.001), angiotensin receptor neprilysin inhibitors [(ARNi) 47.7% vs. 32.6%, p = 0.030], angiotensin-converting enzyme inhibitors [(ACEi) 40.8% vs. 13.3%, p < 0.001], and angiotensin II receptor blockers [(ARB) 20.0% vs. 6.1%, p = 0.003]. Only a small proportion of patients in both groups were receiving triple (overall: 12.2%) or quadruple (overall: 14.0%) GDMT.
Factors associated with vericiguat prescription
For every 1% increase in EF at and above the median (≥38%), the odds of being prescribed vericiguat increased by 44% [odds ratio (CI): 1.44 (1.28, 1.63); p < 0.001], although among lower EF <38% there was no significant relationship. All other candidate covariates did not have a significant association with vericiguat use.
Discussion
Although prior studies have described the clinical profile of patients eligible for vericiguat (7), the current study examined how eligible patients compare with patients actually prescribed vericiguat in US clinical practice. Moreover, the current study also identified factors independently associated with vericiguat use in the US, highlighting patient subgroups where targeted implementation efforts may be particularly needed.
Patients prescribed vericiguat were more often from the Midwest while patients eligible but not prescribed vericiguat were more often from the West, highlighting potential geographic differences in clinician practices. Additionally, it should be noted that, by definition, all eligible patients not receiving vericiguat in the current study had a history of HFHs and exhibited a lower mean EF, indicating substantial risk of downstream WHFEs. Non-prescription of vericiguat in this population can be viewed as a missed opportunity for further reducing residual clinical risk, with current HF guidelines recommending consideration of vericiguat following WHFE to reduce subsequent rates of cardiovascular death or HF hospitalization.
Overall, only 14% of patients in the study sample were prescribed quadruple GDMT. This is consistent with prior studies that have found low rates of quadruple GDMT use ranging from 0.8% to 15.3% among patients with HF (8, 9), which suggests potential treatment inertia. Prior data suggest that use of vericiguat may be particularly helpful in 2 key patient profiles with worsening HF: (1) patients already receiving standard GDMTs in order to lower their residual clinical risk, and (2) patients unable to tolerate or with contraindications to other GDMTs. In the current study, use of background ACEI/ARB/ARNI and beta-blocker therapy were significantly lower among patients prescribed vericiguat compared with patients eligible but not prescribed vericiguat. This could suggest preferential use of vericiguat in this second clinical profile of patients unable to tolerate various components of quadruple therapy. From the standpoint of tolerability, vericiguat has minimal to no effect on systolic blood pressure and kidney function. Likewise, vericiguat can be initiated with an eGFR as low as 15 ml/min/1.73 m2 (2, 10, 11). In combination, these features support the strong safety and tolerability profile of vericiguat among patient potentially ineligible or intolerant to other GDMTs. The analysis of factors associated with vericiguat use revealed increased odds of vericiguat use in patients with higher EF across the ranges of EF ≥38% to <45%. There was no relationship between EF and vericiguat prescription among patients with EF < 38%. Whether this relationship among patients with EF ≥38% reflects a true tendency among prescribers vs. a chance finding requires confirmation in future studies with large sample sizes. Likewise, although other candidate variables such as age, sex, race, and insurance status were not significantly associated with likelihood of vericiguat prescription in the current study, these results should be verified in larger samples of patients.
The current study has several limitations inherent to survey research, such as sample and recall bias and unobserved data (e.g., EF at the point of treatment initiation). Firstly, patients in the vericiguat prescription group were chosen based on their prescription status, whereas the comparator group of eligible patients not prescribed vericiguat were randomly chosen based on eligibility criteria. Hence, these data cannot be used to estimate the rate of vericiguat uptake in clinical practice. Further to this, the eligible but not prescribed vericiguat group used history of HF hospitalization at any time as a criterion for inclusion. This limitation is relevant as it must be considered that this group includes patients who may have been hospitalized long before this study and have since remained clinical stable on optimized treatment. Second, the sample size was modest and future studies with larger sample sizes are needed to confirm these findings. The regression analysis was limited by sample size, and we were unable to include all potential covariates that may be associated with the outcome. Non-significant covariates may be due to insufficient power to detect small differences between groups. Thirdly, data on the clinician's reasoning for prescribing vericiguat were not collected, and the role of clinician perception of safety and tolerability in driving vericiguat prescription remains speculative. This limitation is relevant when considering the observed inverse association between use of vericiguat and other GDMTs. For example, it remains unclear if clinicians perceived patients on quadruple medical therapy as already “optimally treated”, potentially prompting less use of vericiguat. Furthermore, the degree to which clinicians might consider the specific patient comorbidities when making prescription decisions is unclear (12). Likewise, the underlying clinical rationale for patients with lower EF being less likely to be prescribed vericiguat remains unknown. Future dedicated studies of physician perceptions and decision-making surrounding prescription of vericiguat and other GDMTs are needed.
Conclusion
Overall, our findings provide real-world insights into the patient profile of patients with HF prescribed vericiguat in routine US clinical practice, and the factors independently associated with vericiguat prescription among eligible patients. Future research efforts should aim to confirm these associations and explore whether subgroups of eligible patients less likely to be prescribed vericiguat may benefit from targeted implementation initiatives.
Statements
Data availability statement
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to lucy.hargreaves@omc.com.
Ethics statement
The DSP is a market research study conducted in accordance with the European Pharmaceutical Market Research Association's (EphMRA) code of conduct. DSPs meet all the requirements outlined by the EphMRA code of conduct and have been confirmed as market research by various regulatory authorities. Therefore, an ethical review is not required. Despite this, Adelphi Real World seek Ethics/IRB US exemption for all DSPs. The Heart Failure DSP, upon which this manuscript is based, successfully gained exemption from Pearl IRB. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin.
Author contributions
SG: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. CC: Supervision, Writing – review & editing. LH: Formal analysis, Methodology, Writing – review & editing. KT: Formal analysis, Methodology, Writing – review & editing. SB: Formal analysis, Methodology, Writing – review & editing. AS: Writing – review & editing. EO: Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Data collection was undertaken by Adelphi Real World (ARW) as part of an independent survey, entitled the ARW Heart Failure Disease Specific Programme (DSP). Merck Sharp & Dohme LLC, a subsidiary of Merck & Co. Inc., Rahway, NJ, USA did not influence the original survey through either contribution to the design of questionnaires or data collection. The analysis described here used data from the ARW Heart Failure DSP. The DSP is a wholly owned ARW product. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA is one of multiple subscribers to the DSP. Publication of survey results was not contingent on the subscriber's approval or censorship of the manuscript. Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA funded the analysis and development of the publication, including medical writing services.
Conflict of interest
SG has received research support from the Duke University Department of Medicine Chair's Research Award, American Heart Association, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Merck (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), Novartis, Otsuka, Pfizer, and Sanofi; has served on advisory boards or as consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Corcept Therapeutics, Corteria Pharmaceuticals, CSL Vifor, Cytokinetics, Idorsia, Lexicon, Lilly, Merck (a subsidiary of Merck & Co., Inc., Rahway, NJ, USA), Novo Nordisk, Otsuka, PharmaIN, Recordati, Roche Diagnostics, Sanofi, scPharmaceuticals, Sumitomo, Tricog Health; and has received speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Lexicon, Novo Nordisk, and Roche Diagnostics.
CC, AS, and EO are full time employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder had the following involvement in this study: study design, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
GreeneSJBauersachsJBrugtsJJEzekowitzJALamCSPLundLHet alWorsening heart failure: nomenclature, epidemiology, and future directions: JACC review topic of the week. J Am Coll Cardiol. (2023) 81(4):413–24. 10.1016/j.jacc.2022.11.023
2.
ArmstrongPWPieskeBAnstromKJEzekowitzJHernandezAFButlerJet alVericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. (2020) 382(20):1883–93. 10.1056/NEJMoa1915928
3.
HeidenreichPABozkurtBAguilarDAllenLAByunJJColvinMMet al2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(18):e876–94. 10.1161/CIR.0000000000001063
4.
VictoresABashLDRuBMcMullanCMelaragnoMStevensonAet alWho? Characterizing vericiguat use in a real-world HFrEF patient population. J Am Coll Cardiol. (2023) 81(8_Supplement):290. 10.1016/S0735-1097(23)00734-9
5.
AndersonPHigginsVCourcyJDoslikovaKDavisVAKaravaliMet alReal-world evidence generation from patients, their caregivers and physicians supporting clinical, regulatory and guideline decisions: an update on disease specific programmes. Curr Med Res Opin. (2023) 39(12):1707–15. 10.1080/03007995.2023.2279679
6.
BabineauxSMCurtisBHolbrookTMilliganGPiercyJ. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific programme. BMJ Open. (2016) 6(8):e010352. 10.1136/bmjopen-2015-010352
7.
KhanMSXuHFonarowGCLautschDHilkertRAllenLAet alApplicability of vericiguat to patients hospitalized for heart failure in the United States. JACC Heart Fail. (2023) 11(2):211–23. 10.1016/j.jchf.2022.11.007
8.
MorrisAACoyleCMinJMarks-AnglinAFonarowGC. Factors associated with adherence to guideline-directed medical therapy (GDMT) among U.S. patients with heart failure with reduced ejection fraction (HFrEF). J Am Coll Cardiol. (2024) 83(13_Supplement):327. 10.1016/S0735-1097(24)02317-9
9.
GreeneSJAyodeleIPierceJBKhanMSLewseySCYancyCWet alEligibility and projected benefits of rapid initiation of quadruple therapy for newly diagnosed heart failure. JACC Heart Fail. (2024) 12(8):1365–77. 10.1016/j.jchf.2024.03.001
10.
LamCSPMulderHLopatinYVazquez-TanusJBSiuDEzekowitzJet alBlood pressure and safety events with vericiguat in the VICTORIA trial. J Am Heart Assoc. (2021) 10(22):e021094. 10.1161/JAHA.121.021094
11.
VoorsAAMulderHReyesECowieMRLassusJHernandezAFet alRenal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (vericiguat global study in subjects with HFrEF) trial. Eur J Heart Fail. (2021) 23(8):1313–21. 10.1002/ejhf.2221
12.
LavalleCMarianiMVSeverinoPPalombiMTrivignoSD'AmatoAet alEfficacy of modern therapies for heart failure with reduced ejection fraction in specific population subgroups: a systematic review and network meta-analysis. Cardiorenal Med. (2024) 14(1):570–80. 10.1159/000541393
Summary
Keywords
vericiguat, heart failure, eligibility, United States, ejection fraction
Citation
Greene SJ, Coyle CR, Hancock LN, Tebbs KW, Barlow SG, Stevenson AS and Obi EN (2025) Eligible patients with heart failure prescribed vs. not prescribed vericiguat in the United States. Front. Cardiovasc. Med. 12:1633435. doi: 10.3389/fcvm.2025.1633435
Received
22 May 2025
Accepted
18 July 2025
Published
26 August 2025
Volume
12 - 2025
Edited by
Andre Rodrigues Duraes, Federal University of Bahia (UFBA), Brazil
Reviewed by
Nicola Pierucci, Sapienza University of Rome, Italy
Matteo Toma, San Martino Hospital (IRCCS), Italy
Germano Souza, Hospital Regional de São José dos Campos, Brazil
Updates
Copyright
© 2025 Greene, Coyle, Hancock, Tebbs, Barlow, Stevenson and Obi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen J. Greene stephen.greene@duke.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.