- Department of Ultrasound, The Affiliated Hospital of Yunnan University, The Second People’s Hospital of Yunnan Province, Kunming, China
In China, the healthcare burden of cardiovascular diseases (CVDs) will continue to rise due to the pressure of the aging population, which has posed higher demands for CVDs prevention and treatment. Ultrasound-targeted microbubble destruction (UTMD) is an ultrasound-triggered drug delivery technique based on microbubbles. This technique utilizes the principles of cavitation and sonoporation to enhance the delivery of genes or drugs to target tissue. This review article will provide an overview of studies using UTMD to treat CVDs over the last decade. In light of these studies, we underscore the potential therapeutic targets and delineate the practical substances that can be loaded onto microbubbles. Additionally, a discussion is provided regarding the limitations and prospects of this field.
1 Introduction
Cardiovascular diseases (CVDs) remains the predominant cause of mortality and premature death in China, accounting for 48% and 45.86% of total deaths in rural and urban populations respectively (1, 2). Atherosclerosis, the most prevalent form of CVDs, manifests as lipid accumulation and chronic inflammation in large arteries, leading to critical complications including ischemic heart disease and cerebrovascular accidents (3). Over the past three decades, China has faced escalating challenges in CVDs management due to the dual pressures of rising atherosclerotic cases and demographic aging, which necessitates innovative approaches for prevention and therapeutic intervention (4).
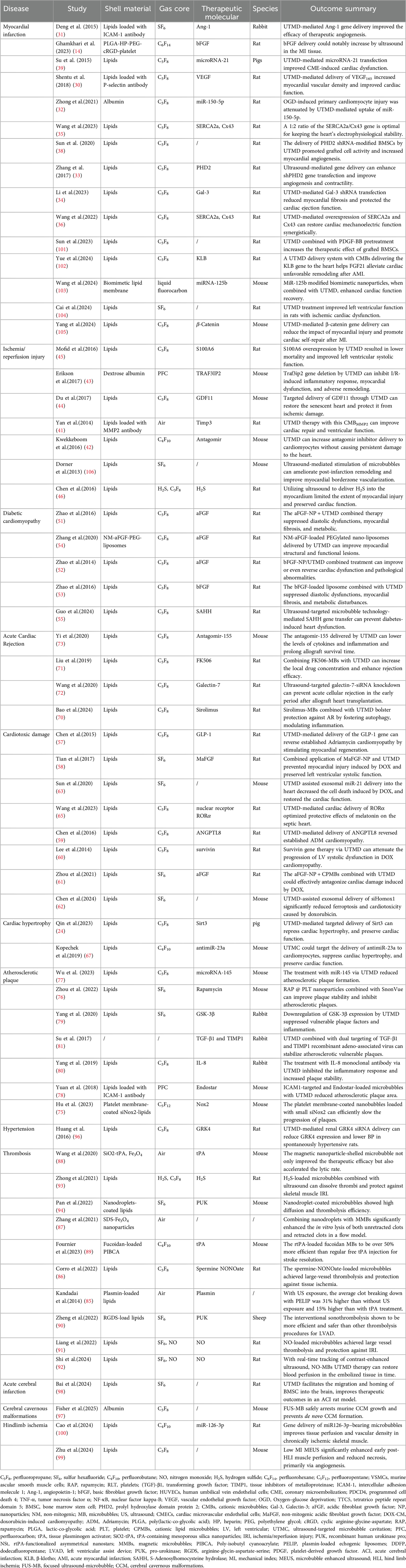
Ultrasound has a long history as a diagnostic imaging technique. Additionally, it has the capacity to modulate the spatial and temporal release of medication. Due to its biocompatibility and low attenuation in tissue, ultrasound demonstrates the potential for remote activation, which has driven the development of smart medicine delivery systems. In recent years, this field has expanded to therapeutic applications, such as ultrasound-targeted microbubble destruction (UTMD) (5, 6). The non-invasive and target-specific nature of UTMD renders it a promising drug and gene delivery strategy (7). Recent studies suggest a promising future for UTMD-based therapies in the management of CVDs (8–10), indicating a potential for advancement in the field of precision medicine (Figure 1).
Figure 1. Preclinical evaluation of UTMD in cardiovascular therapy. Created in BioRender. YANG, S. (2025) https://BioRender.com/pdf8y59, licensed under Academic license.
A systematic literature search was conducted in PubMed using the key term “ultrasound-targeted microbubble disruption” to evaluate the therapeutic advancements of UTMD in CVDs over the past decade. Particular emphasis was placed on innovative approaches for microbubble functionalization. Furthermore, a critical examination of current technological limitations and translational challenges is undertaken to inform future research directions.
2 Overview of UTMD
Ultrasound serves as a critical diagnostic modality providing non-invasive real-time imaging capabilities in clinical practice. Microbubbles, when employed as contrast agents, significantly enhance the assessment of tissue perfusion, hemodynamic parameters, and pathological features such as lesions or vascular abnormalities — capabilities that establish them as indispensable tools for the diagnosis and clinical evaluation of cardiovascular diseases (11).
In 1998, Dr. Price and colleagues advanced the notion of UTMD in a publication in the “Circulation” journal (12). Over recent decades, UTMD has evolved into a prominent therapeutic strategy. Ultrasound-mediated techniques have garnered significant attention for their tripartite capability in precisely controlling drug activation, real-time monitoring, and spatiotemporally regulated release. Functioning as acoustic energy transducers, microbubbles serve as critical vectors for ultrasound-triggered delivery of both chemical agents and engineered nanoparticles. Several methods are employed to prepare microbubbles, such as atomization and reconstitution, cross-linking polymerization, and emulsion solvent evaporation (13). Gas-filled microbubbles (MBs) with diameters of 1–8 μm are conventionally employed as intravascular ultrasound contrast agents. The gaseous core provides the necessary echogenicity, while the surrounding shell prevents rapid dissolution of the core, thereby ensuring MB stability. These shells may consist of albumin, surfactants, phospholipids, proteins, mesoporous silica, or biocompatible and biodegradable polymers (14, 15). The utilization of insoluble, high-density inert gases—such as perfluorocarbons and sulfur hexafluoride—as core materials significantly enhances microbubble stability. Due to their low blood solubility, these gases prolong microbubble circulation within the vascular system. For instance, air-filled contrast agents (e.g., Albunex and Levovist) exhibit circulation times of approximately 5 min. In contrast, microbubbles containing fluorinated gases (e.g., SonoVue and Optison) demonstrate circulation times exceeding 10 min (16).
A significant disparity in their capabilities of drug delivery was observed due to substantial discrepancies in microbubble shell composition, gaseous composition, and size distribution. In addition, through surface functionalization or core-loading strategies, these microcarriers can encapsulate diverse therapeutic payloads including small-molecule drugs, nucleic acids, and immunotherapeutic antigens. Following intravenous administration, the acoustically driven inertial cavitation of microbubbles at target sites generates localized shear stresses and microstreaming effects, enabling site-specific payload release with enhanced bioavailability (Figure 2) (15).
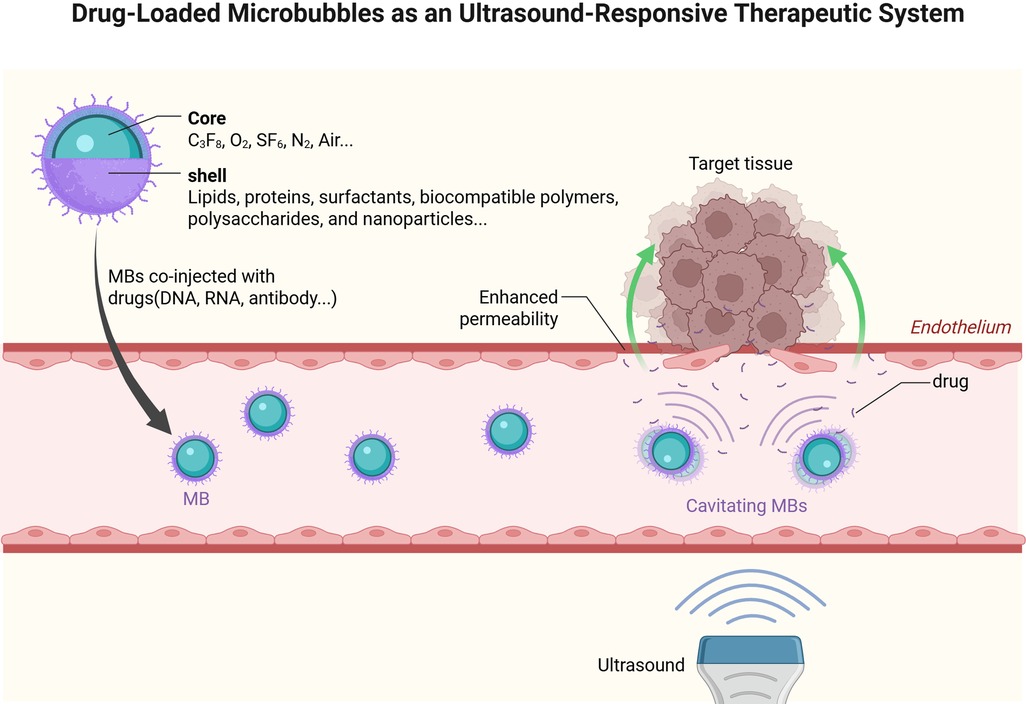
Figure 2. Drug-Loaded microbubbles as an ultrasound-responsive therapeutic system. Created in BioRender. YANG, S. (2025) https://BioRender.com/18kn4mw, licensed under Academic license.
The mechanical effects induced by ultrasonic fields, including acoustic radiation force, microstreaming, shear stress, and related phenomena, stem from the momentum transfer associated with sound waves. The occurrence of more substantial mechanical effects is plausible when the MBs interact with ultrasonic beams (17). In the context of low acoustic pressures, these MBs undergo symmetrically periodic expansion and compression, oscillating synchronously with the incident ultrasonic wave. The process in question is referred to as “stable cavitation.” During this process, the MBs undergo an expansion phase in which they extend to the point of coming into proximity with the blood vessel wall. This expansion causes the adjacent endothelium to separate from its neighbors. During the subsequent compression phase, the MBs undergo a process of shrinkage, causing invaginations in the endothelial cells that line the vessel. This process disrupt the tight junctions between the endothelial cells, resulting in a disruption of the tissue's structural integrity (18, 19). In the presence of elevated acoustic pressures, MBs undergo a violent collapse during the compression phase. This process is referred to as inertial cavitation. The propensity of microbubbles to undergo sustained stable cavitation or inertial cavitation depends on multiple factors, chief among which is the mechanical index (MI)—defined as the peak negative acoustic pressure divided by the square root of the ultrasound frequency. The collapse of MBs has been demonstrated to generate shock waves of increased strength, as well as microstreaming, micro-jetting, and tangential stresses. These phenomena have been observed to result in the perforation of cellular membranes. Impulse forces exerted on the endothelial cell membrane can directly result in the formation of transient, nonselective, and repairable pores, a phenomenon referred to as the sonoporation effect (15, 20).
Experimental evidence confirms that microbubble-mediated vascular permeability enhancement—driven by ultrasound-induced shear stress (e.g., 1 MHz, MI = 0.8)—is typically transient and occurs without vascular rupture when using pulsed ultrasound (0.1–10 ms pulses) with low duty cycles, restoring endothelial barrier function within minutes to hours while triggering Ca2+ signaling. However, excessive acoustic energy [e.g., high mechanical index (MI >1.3), prolonged burst length, or extended exposure] can induce violent microbubble collapse, causing localized endothelial damage through large-amplitude oscillations, endothelial cell death due to delayed pore resealing, and—under extreme conditions (MI 1.3–2.0)—vascular rupture and hemorrhage (21). Critical damage thresholds depend on both acoustic parameters (pulse scheme, microbubble properties/concentration) and biological factors (calcium-dependent repair mechanisms, membrane composition), as well as tissue-specific vessel characteristics (22).
Microbubble suspensions exhibit excellent biocompatibility as delivery platforms, demonstrating minimal systemic toxicity and negligible immunogenicity (23). This favorable safety profile stems from the fate of their components: Perfluorocarbon (PFC) gases (e.g., perfluoropropane, sulfur hexafluoride) are chemically inert, non-metabolized, and primarily eliminated via exhalation through the lungs within minutes to hours after administration. Meanwhile, the encapsulating shell materials (lipids, proteins, polymers) undergo biodegradation and renal clearance. Supporting this safety, Qin et al.'s cardiomyopathy study using repeated ultrasound-mediated delivery (three sessions at 1-day intervals) of Sirt3 plasmid via cationic microbubbles suppressed hypertrophic phenotypes—including cardiac enlargement, fibrosis, and apoptosis—at both 7 days and 2 months post-treatment. Critically, multi-organ assessment (kidneys, liver, lungs) confirmed no treatment-related damage, demonstrating high safety with minimal off-target effects (24).
Clinically administered via peripheral intravenous injection, these contrast agents enable non-invasive ultrasound-targeted microbubble destruction (UTMD) procedures—a key translational advantage for therapeutic applications (25). Microbubbles crucially protect encapsulated bioactive molecules from enzymatic degradation and immune clearance during circulation. Subsequent ultrasound-triggered inertial cavitation then enables site-specific payload release. This mechanism enhances therapeutic precision while minimizing off-target effects. Furthermore, microbubbles with functionalized surfaces achieve molecular-level targeting, significantly improving spatiotemporal control in diagnostics and therapeutics. Preclinical evidence confirms that molecularly targeted microbubbles substantially enhance gene delivery efficiency compared to non-targeted agents. For instance, in cardiovascular models, Xie et al. demonstrated the feasibility of endothelial-targeted (P-selectin/ICAM-1) gene-carrying microbubbles, achieving a 5-fold higher transfection efficiency than non-targeted controls at MI 0.6 in murine hindlimb ischemia (26). Similarly, Zhou et al. used ICAM-1-targeted microbubbles to deliver Ang1 in rabbit myocardial infarction models, yielding approximately 3-fold greater delivery efficiency vs. non-targeted bubbles using a Philips iE33 system (1.7 MHz, MI 1.3) (27).
3 Potential application of UTMD in cardiovascular diseases
3.1 Ischemic heart disease
3.1.1 Myocardial infarction (MI)
As a common cardiovascular disease, myocardial infarction (MI) is characterized by the irreversible necrosis of myocardium due to oxygen deprivation. MI typically progresses to impaired diastolic function, myocardial fibrosis, malignant arrhythmia, weakened ventricular contraction, heart failure (HF), and even sudden death (28). Although revascularization is performed via stenting or bypassing of the infarcted artery, ventricular dysfunction remains inescapable after an extensive MI (29). Ultrasound-targeted microbubble destruction UTMD can facilitate drug, gene, and cell delivery into the infarcted heart. Current research demonstrates feasible targeting ligands, such as P-selectin (30) and ICAM-1 (31), which are expressed in endothelial cells, as well as glycoprotein IIb/IIIa receptors on activated platelets (14). In rats, UTMD-mediated local transfection of VEGF (30), miR-150-5p (32), PHD2 (33), Gal-3 (34), and SERCA2a-Cx43 (35, 36) has been used to protect the heart from complications following acute MI. Despite ongoing debate over the efficacy of cardiac stem cell therapy, a recent study suggests that the underlying biological mechanism involves an acute sterile immune response to improve heart function (37). For example, UTMD-delivered PHD2 shRNA-modified BMSCs were shown to enhance grafted cell homing, activity, and myocardial angiogenesis in infarcted hearts (38). Additionally, studies on Ang-1 delivery in rabbits (31), and microRNA-21 delivery in pigs (39) further demonstrate the therapeutic potential of UTMD in treating post-MI cardiac injury.
3.1.2 Ischemia/reperfusion injury (I/R)
Early and successful revascularization can significantly improve clinical outcomes in patients with acute MI. However, reperfusion may paradoxically exacerbate myocardial damage, a phenomenon termed ischemia-reperfusion (I/R) injury. Ischemia initiates inflammatory cascades, and the subsequent restoration of blood flow further activates additional inflammatory pathways, thereby amplifying this paradoxical injury (40). To enhance tissue-specific targeting, a feasible strategy involves conjugating thiolated MMP2 antibodies to cationic microbubbles (41). Experimental studies have demonstrated direct cardiac delivery of therapeutic agents to mitigate I/R injury: in mice, Atagomir (42), TRAF3IP2 (43), GDF11 (44), and in rats, Timp3 (41), S100A6 (45), and hydrogen sulfide (46) were successfully utilized to protect myocardial tissue.
3.2 Cardiomyopathies
3.2.1 Diabetic cardiomyopathy (DCM)
Diabetes mellitus and its complications represent a significant global health burden, affecting populations across both developed and developing nations. Cardiovascular pathologies constitute the predominant cause of mortality in diabetic populations, with diabetic cardiomyopathy (DCM) emerging as a distinct myocardial disorder independent of hypertension or coronary artery disease (47). This metabolic cardiomyopathy, driven by chronic hyperglycemia, insulin resistance, and compensatory hyperinsulinemia, manifests through characteristic pathological progression: mitochondrial oxidative stress initiates cardiomyocyte apoptosis, followed by extracellular matrix remodeling (myocardial fibrosis), compensatory hypertrophy, impaired ventricular relaxation (diastolic dysfunction), and ultimately progresses to impaired cardiac contraction (systolic failure) (48). Despite significant advancements in glucose-lowering therapy for diabetes in recent years, conventional medications have proven ineffective in halting the progression of diabetic cardiomyopathy (49).
Animal experiments indicate that acidic fibroblast growth factor (aFGF) and basic fibroblast growth factor (bFGF) are involved in regulating cardiac angiogenesis and repair, suggesting their potential as therapeutic agents for the treatment of DCM (50). However, conventional intracardiac administration poses significant limitations due to invasiveness and procedural risks, hindering clinical translation. This therapeutic impasse has driven innovation in targeted delivery systems, with UTMD demonstrating remarkable efficacy in preclinical models. Mechanistic studies reveal that UTMD-mediated aFGF delivery attenuates ventricular remodeling through activation of PI3 K/Akt signaling pathways (51), while bFGF administration via UTMD enhances angiogenesis via VEGF upregulation and improves cardiac function parameters in DCM models (52, 53). Moreover, PEGylated nanoliposomes could serve as suitable carriers to enhance the stability of non-mitogenic aFGF (NM-aFGF) during storage and systemic circulation (54).
Emerging gene therapy approaches utilizing UTMD demonstrate particular promise. Targeted delivery of S-adenosylhomocysteine hydrolase (SAHH) via UTMD technology has shown capacity to restore ventricular function in DCM. The cardioprotective effects appear mediated through activation of the energy-sensing AMP-activated protein kinase (AMPK)/forkhead box O3 (FOXO3)/sirtuin 3 (SIRT3) axis, a critical pathway modulating mitochondrial biogenesis and oxidative stress responses (55). These findings demonstrate UTMD's utility as a precision cardiac therapeutic platform, enabling site-specific treatment delivery with minimized systemic toxicity.
3.2.2 Cardiotoxic damage
Doxorubicin (DOX), also known as Adriamycin (ADM), is a potent chemotherapeutic agent widely used in the treatment of multiple malignancies. However, its clinical utility is significantly limited by dose-dependent cardiotoxicity, often leading to cardiomyopathy and posing critical therapeutic challenges (56). Resolving this therapeutic dilemma necessitates novel cardioprotective approaches. Recent preclinical studies utilizing doxorubicin-induced cardiomyopathy rodent models have validated UTMD-facilitated precision delivery of multiple therapeutics - including GLP-1 (57), MaFGF (58), ANGPTL8 (59), survivin (60), and aFGF (61) - demonstrating capacity to ameliorate or reverse the established ADM cardiomyopathy. Exosome-mediated nucleic acid delivery has shown therapeutic promise, but its clinical application remains constrained by poor heart-targeting efficiency. The UTMD platform addresses this challenge by enabling precise cardiac targeting, having successfully delivered siHomox1 (62) and miR-21 (63), with demonstrated efficacy in mitigating doxorubicin-induced myocardial damage and functional decline.
Sepsis has been identified as the foremost cause of mortality within intensive care units, accounting for approximately one-fourth of all cases. Concomitant heart dysfunction has been demonstrated to increase the risk of mortality in patients with severe sepsis. Despite the evident decline in cardiac performance observed in patients with sepsis, there remains a paucity of consensus or guidelines regarding the management of sepsis-induced cardiomyopathy (64). Notably, in septic cardiomyopathy models, the UTMD-mediated cardiac delivery of RORα significantly enhanced melatonin's cardioprotective effects in sepsis. Given the favorable biocompatibility, safety profile, and delivery efficiency of UTMD technology, the combined therapeutic approach of melatonin with RORα/cationic microbubbles may represent a promising strategy for managing sepsis-induced cardiomyopathy (65).
3.2.3 Cardiac hypertrophy
Pathological cardiac hypertrophy develops through multiple etiological pathways, encompassing ischemic injury (myocardial infarction), structural valvular abnormalities (aortic stenosis, mitral/aortic regurgitation), metabolic dysregulation in storage disorders, chronic pressure overload (hypertension), and heritable mutations affecting sarcomeric protein genes. Distinct from the compensatory mechanisms of physiological hypertrophy, this maladaptive process evolves through aberrant molecular signaling cascades (66), ultimately culminating in heart failure decompensation, fatal arrhythmogenesis, and sudden cardiac death. Emerging therapeutic interventions utilizing UTMD technology show significant preclinical efficacy. In porcine models of myocardial hypertrophy, UTMD-facilitated Sirt3 gene delivery demonstrates therapeutic efficacy by enhancing sustained cardiac functional recovery while attenuating pathological myocardial remodeling (24). Furthermore, UTMD-mediated antimiR-23a delivery achieves targeted myocardial inhibition of pathological hypertrophy and preservation of left ventricular systolic performance at a dosage 200-fold lower than systemic administration (67).
3.3 Acute cardiac rejection
In the absence of contraindications, heart transplantation (HT) remains the standard therapeutic intervention for end-stage heart failure. However, acute rejection (AR) persists as a prevalent complication during the first post-transplant year and is independently associated with accelerated cardiac allograft vasculopathy and irreversible graft dysfunction, significantly impacting long-term outcomes (68). Current AR management relies on high-dose systemic immunosuppression, including calcineurin inhibitors, corticosteroids, and polyclonal antibody therapies. These regimens carry substantial toxicity profiles, manifesting as nephrotoxicity, metabolic derangements (hypertension, hyperlipidemia, glucose metabolism dysregulation), neurocognitive impairment, opportunistic infections, and elevated malignancy risk—collectively diminishing quality of life and threatening post-transplant survival (69). UTMD presents a new, low side - effect approach for AR - targeted therapy. Compared to direct sirolimus administration, ultrasound-targeted microbubbles carrying sirolimus can achieve a local drug concentration 15 times higher (70). In gene therapy, UTMD has been combined with efficient FK506 (71), galectin-7-siRNA (72), and Antagomir-155 (73) delivery methods to treat AR.
3.4 Atherosclerotic plaque
Atherosclerosis poses a significant threat to cardiovascular health, with plaque rupture being a primary trigger for acute complications. These rupture-prone plaques are typically characterized by lipid-rich cores, frequent intraplaque hemorrhage, and thin collagen caps that are infiltrated with inflammatory cells (74). This pathological process is the leading cause of cardiovascular disease morbidity and mortality. However, achieving effective localized therapeutic intervention for atherosclerotic plaque lesions remains a substantial clinical challenge. Current treatment strategies are often hampered by inadequate target-specific, which compromise both treatment precision and biological efficacy (75).
Recent studies have demonstrated the potential of various therapeutic agents in stabilizing vulnerable plaques and even inhibiting atherosclerotic plaque progression. For instance, in ApoE−/− mice, the application of rapamycin (76), microRNA-145 (77), Endostar (78), and Nox2 (75) has shown promise in stabilizing plaques. Similarly, in rabbits, the use of GSK-3β (79), IL-8 monoclonal antibody (80), TGF-β1, and TIMP1 (81) delivered via UTMD, has also yielded favorable outcomes. Collectively, these findings underscore the potential of UTMD as a practical and promising technique for the efficient and safe management of atherosclerosis.
3.5 Thrombolysis
Thrombosis remains a major global cause of morbidity and mortality by triggering vascular occlusion and subsequent cardiovascular events like acute myocardial infarction, ischemic stroke, and pulmonary embolism (82). To address this, endovascular sonothrombolysis—a technique using ultrasound-enhanced clot lysis via acoustic cavitation—has emerged as a promising adjuvant therapy. Clinical validation of this ultrasound-mediated strategy comes from a phase II clinical trial, which demonstrated both feasibility and safety when combining microbubble technology with standard intra-arterial thrombolysis (83). Latest research indicates that the stronger cavitation effect induced by dual-frequency ultrasound enhances the removal of retracted clots by up to 85% compared to single-frequency ultrasound, which further demonstrates its potential for treating deep vein thrombosis (DVT) (84).
Beyond conventional thrombolytics such as tissue plasminogen activator (tPA) and pro-urokinase (PUK), the therapeutic potential of plasmin (85) and spermine-NONOate (86) has been evaluated in combination with UTMD. Building on these advancements, current research frontiers in sonothrombolysis focus on engineering multifunctional microbubbles to overcome limitations of traditional thrombolytic agents. Notably, magnetic nanoparticle-conjugated microbubbles represent a significant advancement, enabling spatial guidance via external magnetic fields while maintaining ultrasound-triggered drug release capabilities (87, 88). In parallel, surface functionalization strategies employing fucoidan (89) and arginine-glycine-aspartate-serine (RGDS) peptides enhance thrombus-specific targeting (90). Furthermore, therapeutic gases like nitric oxide (NO) (91, 92) and hydrogen sulfide (H₂S) (93) have been engineered into the gaseous cores of microbubbles, exhibiting dual functionality: thrombus dissolution and mitigation of tissue ischemia-reperfusion injury (IRI). Most recently, a breakthrough approach involving microbubbles integrated with phase-change nanodroplets has emerged. These hybrid systems generate transient micropores within thrombi through acoustic-triggered phase transition, thereby significantly enhancing therapeutic agent penetration (94).
3.6 Other applications relevant to CVDs
In China, hypertension stands as the principal modifiable risk factor for CVDs, accounting for approximately 43% of cardiovascular morbidity and mortality (95). Recent advancements in UTMD technology demonstrate promising therapeutic applications for hypertension management. Experimental studies in spontaneously hypertensive rats (SHRs) reveal that UTMD-mediated renal delivery of GRK4-specific siRNA effectively suppresses renal GRK4 expression, thereby restoring dopamine D1 receptor (D1R) signaling pathways. This molecular intervention enhances renal sodium excretion through improved D1R-mediated natriuresis and diuresis, ultimately achieving sustained blood pressure reduction (96).
In the treatment of cerebrovascular diseases, a recent study supports FUS-MB (Focused Ultrasound with Microbubbles) as a minimally invasive therapeutic modality. This technique can therapeutically control the growth and de novo formation of cerebral cavernous malformations (CCMs) even without drug delivery (97). Meanwhile, the therapeutic potential of UTMD extends beyond molecular targeting to enhancing cellular therapies. In rodent models of cerebral ischemia induced by middle cerebral artery occlusion (MCAO), UTMD application significantly improves the homing efficiency of intravenously administered bone marrow stromal cells (BMSCs) to ischemic regions. This targeted cellular delivery correlates with reduced infarct volume and improved neurological outcomes. These benefits are potentially mediated through the modulation of matrix metalloproteinase-8 (MMP8) activity—a key regulator of extracellular matrix remodeling in ischemic injury (98).
In an ischemic hindlimb model, low-mechanical-index (MI) microbubble-enhanced ultrasound (MEUS) demonstrated significantly enhanced efficacy in augmenting muscle blood perfusion and reducing necrosis during the early postoperative phase. These effects are primarily attributable to angiogenesis stimulated by low-MI MEUS (99). Additionally, UTMD offers a noninvasive approach to facilitate microRNA delivery, enabling site-specific transfection with minimal systemic or off-target effects. Studies in ischemic hindlimb models show that ultrasound-mediated delivery of miR126-3p–loaded carrier microbubbles to chronically ischemic skeletal muscle promotes enhanced tissue perfusion, increased vascular density, arteriolar formation, and neovessel maturation. Critically, this technique induces no substantial off-target effects in remote organs (100).
4 Discussion
Under ultrasound irradiation, commercially available microbubbles exemplified by SonoVue® (Bracco Imaging) demonstrate significant cardioprotective properties even when not loaded with therapeutics. Mechanistic studies attribute these effects to the upregulation of key signaling molecules including vascular endothelial growth factor-α (VEGF-α), insulin-like growth factor-1 (IGF-1), and caveolin-3 (Cav-3), coupled with the liberation of bioactive mediators such as nitric oxide (NO) and adenosine triphosphate (ATP) (104, 106). Notably, UTMD technology has emerged as a promising strategy for enhancing stem cell homing efficacy. Experimental evidence confirms that UTMD not only promotes targeted migration of mesenchymal stem cells (MSCs) to lesion sites but also preserves their fundamental biological characteristics, including proliferation capacity, apoptotic regulation, and cell cycle dynamics (38, 101). Furthermore, microbubble-assisted exosomal delivery systems have demonstrated therapeutic potential in cardiovascular applications. While therapeutic exosome administration shows cardioprotective benefits against toxic injury, limitations persist in cardiac-specific targeting efficiency. UTMD-mediated cavitation effects offer a technological breakthrough by significantly improving myocardial accumulation of exosomal therapeutics, thereby addressing current delivery challenges (62, 63).
Recent breakthroughs in MB nanotechnology have transformed gas-core contrast agents into multifunctional therapeutic systems through advanced engineering strategies. In cardiovascular applications, ligand-functionalized MBs enable molecular-level targeting via specific ligand-receptor interactions. The utilization of dual-modality theranostic contrast agents, compatible with ultrasound and MRI imaging, can be achieved by loading drugs and magnetic materials into the MBs. Furthermore, the polymeric MBs' thicker shell can accommodate a greater quantity of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles, a property that facilitates the mediation and monitoring of drug delivery (107). It is noteworthy that nanoscale bubbles have the capacity to extend their effects beyond the vascular confinement, thereby facilitating the transport of therapeutic agents across cellular barriers. The utilization of biosynthetic gas vesicles holds considerable promise for facilitating large-scale, non-invasive imaging techniques, thereby enabling the visualization of genetically modified bacteria within living subjects. This development stands to significantly contribute to the advancement of diagnostic and therapeutic cellular agents (108).
Gene delivery systems represent a frontier application with transformative potential in ultrasound-mediated therapy. The process of gene delivery is of paramount importance, as it pertains to the transplantation of foreign DNA to the cells of a host organism. This process is employed within the realm of biomedical research and gene therapy (109). There exist two fundamental gene delivery systems: viral and non-viral. Sonoporation, due to its non-invasiveness, high spatio-temporal resolution and tissue penetration through ultrasound-induced microbubble cavitation, has clear advantages over other modalities. Mechanically, ultrasonic excitation at the appropriate frequency and energy can cause microbubbles to vibrate, expand and collapse, leading to various stable or inertial cavitation effects including microstreams and microjets (110, 111). These physical forces result in the formation of transient and repairable pores in the cellular membrane, thereby facilitating the entry of foreign substances. Pre-clinical investigations have demonstrated that microbubble cavitation-based gene delivery holds considerable promise as a therapeutic modality (112). It has been employed extensively for the delivery of transgenes in the treatment of numerous diseases. Viral vectors are a means of facilitating the transfer of genetic material from one organism to another. This process, known as transduction, involves the use of viruses as vehicles to introduce foreign DNA into the cells of a recipient organism. Despite their capacity to elicit effective gene expression due to their viral configuration, which hinders degradation, numerous studies have demonstrated that the utilization of these carriers is encumbered by several limitations. These limitations encompass immunogenicity (113), off-target delivery (114), and arduous vector production (115). The substantial internal space of ultrasound-responsive microbubbles renders them optimal for use as viral vectors. Consequently, gene transfer strategies that employ acoustically triggered microbubble collapse might effectively circumvent antiviral immune responses while improving targeting. Non-viral gene carriers have gained significant interest due to their comparatively reduced toxicity and immunogenicity in contrast to viral vectors. Nevertheless, the limitations inherent to non-viral carriers include the low levels of protein expression and the inefficient gene transfer efficiency (116). To address this issue, Xie et al. (117) proposed a gene delivery strategy that utilizes ultrasound to directly deliver plasmid DNA into nuclei via gas vesicles (GVs)-based intracellular cavitation. The pDNA-binding GVs are internalized by cells, leading to the formation of intracellular cavitation when exposed to acoustic irradiation, thereby delivering their pDNA payloads into the nuclei.
These innovative targeting approaches - encompassing biochemical specificity, physical guidance, and nanoscale biodistribution - are redefining precision medicine in cardiovascular interventions, particularly for pathologies requiring temporally controlled, site-specific treatment administration. Building upon this paradigm shift in therapeutic delivery, over the past decade, therapeutic research in cardiovascular diseases has predominantly centered on acute myocardial infarction and heart failure, while peripheral arterial disease and cerebrovascular disorders have remained relatively underexplored in preclinical investigations (Table 1). This research gap persists despite growing clinical needs, notably in cerebrovascular therapeutics where conventional drug delivery systems face substantial anatomical barriers. Emerging as a technological breakthrough in this context, the integration of focused ultrasound (FUS) with microbubbles (MBs) has demonstrated significant potential for treating cerebrovascular diseases (118, 119). This noninvasive method enables targeted drug delivery to the brain. At relatively low frequencies, concentrated ultrasound waves can traverse the skull to generate an acoustic field within the brain tissue. When MBs circulate in an ultrasonic field, they undergo oscillation at the same frequency as the ultrasound, a process termed cavitation. In this context, MBs function as cavitation nuclei, moderating the effects of ultrasound while simultaneously inducing transient and reproducible openings of the blood-brain barrier (BBB) (120). The mechanism underpinning FUS-mediated BBB disruption involves the dynamic biomechanical interaction between oscillating MBs and the cerebrovascular structures within the ultrasonic field. This interaction exhibits pronounced parametric sensitivity, being critically dependent on three interrelated factors: 1. acoustic exposure parameters, 2. The physicochemical properties of MBs, and 3. regional vascular density (121). Ultrasound-mediated BBB disruption, supported by well-established mechanisms, preclinical research, and clinical trials, has confirmed its scalability and favorable safety profile. Repeated administrations have not resulted in any clinically detectable tissue damage or neurological complications.
Beyond current clinical trials of focused ultrasound-mediated blood-brain barrier opening for brain tumors, Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis, emerging applications target neuropsychiatric disorders (including major depression and substance addiction) and central pain syndromes. To translate these diverse applications into clinical practice, integrated systems enabling real-time therapy control and safety validation become paramount. The combination of clinically approved MRI-guided focused ultrasound (MRgFUS) with engineered, shell-optimized microbubbles establishes a robust platform for evaluating therapeutic interventions across diverse patient groups. Real-time MRI monitoring, coupled with advanced microbubble formulations, further provides critical evidence regarding safety and feasibility, particularly for cohorts with neurological impairments that require therapies targeting the central nervous system (CNS) (122).
UTMD embodies precision medicine principles through its unique mechanism, demonstrating substantial clinical promise. Preclinical studies validate its safety profile, with microbubbles exhibiting high circulatory stability and biocompatibility in vivo. Surface modification strategies further enhance microbubble localization, enabling UTMD to achieve tissue-specific targeting. Nevertheless, technical challenges persist regarding clinical translation.
The current absence of mass-production manufacturing methodologies for ultrasound-responsive microbubbles remains a critical barrier. Furthermore, refining the physicochemical composition of these contrast agents represents a pivotal challenge for their clinical translation. Specifically, next-generation formulations must simultaneously demonstrate enhanced drug-loading efficiency, consistent acoustic responsiveness, and convenient production-preservation-transportation workflows to meet clinical demands. Beyond these engineering bottlenecks, the transition from preclinical validation to human applications introduces additional biological complexities. While the feasibility of UTMD has been extensively validated in rodent models, the inherent anatomical complexity and physiological heterogeneity of human systems necessitate a phased translational approach. This progression should prioritize rigorous validation in large-animal models before advancing to controlled human trials, ensuring interspecies compatibility in microbubble-mediated therapeutic delivery.
The clinical implementation of UTMD necessitates stringent control over interdependent acoustic parameters - including frequency, mechanical index (MI), pulse duration, and duty cycle (5) - which require systematic calibration to achieve therapeutic bioeffects (mechanical/thermal) while avoiding off-target tissue damage. This precision engineering challenge is particularly evident in focused ultrasound-mediated BBB opening, where safety profiles exhibit significant context-dependency influenced not only by acoustic variables but also by microbubble characteristics (size distribution, shell composition) and vascular anatomical constraints (123). The complex interplay between inertial cavitation thresholds, microbubble oscillation dynamics, and non-linear acoustic interactions mandates the development of multiparametric optimization frameworks. Such protocols must balance BBB permeability enhancement with preservation of neurovascular integrity, requiring real-time feedback systems capable of adjusting sonication parameters in response to dynamic physiological feedback. Emerging evidence indicates that FUS-mediated BBB opening may inadvertently provoke neuroinflammatory cascades (124). Therefore, a critical technological barrier persists: the absence of robust, non-invasive tools for real-time tracking of neuroinflammatory dynamics in vivo. Addressing this unmet need requires prioritized development of targeted molecular imaging probes capable of selectively detecting inflammation-associated biomarkers. Such innovations would not only enable safety profiling during FUS interventions but also facilitate precision modulation of treatment parameters to optimize therapeutic outcomes while mitigating adverse effects.
UTMD has demonstrated unique advantages in cardiovascular disease management, particularly through its capacity for targeted drug/gene delivery mediated by ultrasound-triggered microbubble cavitation. The convergence of these platforms could address critical challenges in cardiovascular therapies, including tissue specificity, delivery efficiency, and safety. However, clinical translation requires systematic validation of their synergistic mechanisms, long-term biocompatibility, and spatiotemporal control precision. By advancing these ultrasound-mediated technologies, we may pioneer a new era in cardiovascular care—from molecular level interventions to organ monitoring—driving a shift towards personalised medicine.
Author contributions
SY: Writing – review & editing, Writing – original draft. RZ: Writing – review & editing. HZ: Software, Supervision, Writing – review & editing. YL: Writing – review & editing, Project administration, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the grant for National Natural Science Funds (82260095) of China, the Cardiovascular Ultrasound Innovation team of Yunnan province (202305AS350021), and Yunnan University Medical Research Fund (YDYXJJ-2024-0041).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. (2016) 387(10015):251–72. doi: 10.1016/S0140-6736(15)00551-6
2. Group C C H a D R W. Summary of China cardiovascular health and disease report 2022. Chin Circ J. (2023) 38(6):583–612.
3. Björkegren JLM, Lusis AJ. Atherosclerosis: recent developments. Cell. (2022) 185(10):1630–45. doi: 10.1016/j.cell.2022.04.004
4. Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. (2019) 16(4):203–12. doi: 10.1038/s41569-018-0119-4
5. Liu S, Zhang Y, Liu Y, Wang W, Gao S, Yuan W, et al. Ultrasound-targeted microbubble destruction remodels tumour microenvironment to improve immunotherapeutic effect. Br J Cancer. (2023) 128(5):715–25. doi: 10.1038/s41416-022-02076-y
6. Tu B, Li Y, Wen W, Liu J. Bibliometric and visualized analysis of ultrasound combined with microbubble therapy technology from 2009 to 2023. Front Pharmacol. (2024) 15:1418142. doi: 10.3389/fphar.2024.1418142
7. Panje CM, Wang DS, Willmann JK. Ultrasound and microbubble-mediated gene delivery in cancer: progress and perspectives. Invest Radiol. (2013) 48(11):755–69. doi: 10.1097/RLI.0b013e3182982cc1
8. Rix A, Curaj A, Liehn E, Kiessling F. Ultrasound microbubbles for diagnosis and treatment of cardiovascular diseases. Semin Thromb Hemost. (2020) 46(5):545–52. doi: 10.1055/s-0039-1688492
9. Qian L, Thapa B, Hong J, Zhang Y, Zhu M, Chu M, et al. The present and future role of ultrasound targeted microbubble destruction in preclinical studies of cardiac gene therapy. J Thorac Dis. (2018) 10(2):1099–111. doi: 10.21037/jtd.2018.01.101
10. Mayer CR, Bekeredjian R. Ultrasonic gene and drug delivery to the cardiovascular system. Adv Drug Delivery Rev. (2008) 60(10):1177–92. doi: 10.1016/j.addr.2008.03.004
11. Bernier M, Abdelmoneim SS, Stuart Moir W, Eifert Rain SSJ, Chandrasekaran K, Ammash NM, et al. CUTE-CV: a prospective study of enhanced left atrial appendage visualization with microbubble contrast agent use during transesophageal echocardiography guided cardioversion. Echocardiography. (2013) 30(9):1091–7. doi: 10.1111/echo.12240
12. Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. (1998) 98(13):1264–7. doi: 10.1161/01.CIR.98.13.1264
13. Kumar M, Kumar D, Chopra S, Mahmood S, Bhatia A. Microbubbles: revolutionizing biomedical applications with tailored therapeutic precision. Curr Pharm Des. (2023) 29(44):3532–45. doi: 10.2174/0113816128282478231219044000
14. Ghamkhari A, Tafti HA, Rabbani S, Ghorbani M, Ghiass MA, Akbarzadeh F, et al. Ultrasound-triggered microbubbles: novel targeted core-shell for the treatment of myocardial infarction disease. ACS Omega. (2023) 8(12):11335–50. doi: 10.1021/acsomega.3c00067
15. Li H, Zhang Y, Shu H, Lv W, Su C, Nie F. Highlights in ultrasound-targeted microbubble destruction-mediated gene/drug delivery strategy for treatment of malignancies. Int J Pharm. (2022) 613:121412. doi: 10.1016/j.ijpharm.2021.121412
16. Chen HH, Matkar PN, Afrasiabi K, Kuliszewski MA, Leong-Poi H. Prospect of ultrasound-mediated gene delivery in cardiovascular applications. Expert Opin Biol Ther. (2016) 16(6):815–26. doi: 10.1517/14712598.2016.1169268
17. Athanassiadis AG, Ma Z, Moreno-Gomez N, Melde K, Choi E, Goyal R, et al. Ultrasound-responsive systems as components for smart materials. Chem Rev. (2022) 122(5):5165–208. doi: 10.1021/acs.chemrev.1c00622
18. Al-Jawadi S, Thakur SS. Ultrasound-responsive lipid microbubbles for drug delivery: a review of preparation techniques to optimise formulation size, stability and drug loading. Int J Pharm. (2020) 585:119559. doi: 10.1016/j.ijpharm.2020.119559
19. Hahmann J, Ishaqat A, Lammers T, Herrmann A. Sonogenetics for monitoring and modulating biomolecular function by ultrasound. Angew Chem Int Ed Engl. (2024) 63(13):e202317112. doi: 10.1002/anie.202317112
20. Yang Y, Li Q, Guo X, Tu J, Zhang D. Mechanisms underlying sonoporation: interaction between microbubbles and cells. Ultrason Sonochem. (2020) 67:105096. doi: 10.1016/j.ultsonch.2020.105096
21. Singh D, Memari E, He S, Yusefi H, Helfield B. Cardiac gene delivery using ultrasound: state of the field. Mol Ther Methods Clin Dev. (2024) 32(3):101277. doi: 10.1016/j.omtm.2024.101277
22. Yemane PT, Åslund AKO, Snipstad S, Bjørkøy A, Grendstad K, Berg S, et al. Effect of ultrasound on the vasculature and extravasation of nanoscale particles imaged in real time. Ultrasound Med Biol. (2019) 45(11):3028–41. doi: 10.1016/j.ultrasmedbio.2019.07.683
23. Unger E, Porter T, Lindner J, Grayburn P. Cardiovascular drug delivery with ultrasound and microbubbles. Adv Drug Delivery Rev. (2014) 72:110–26. doi: 10.1016/j.addr.2014.01.012
24. Qin X, Cai P, Liu C, Chen K, Jiang X, Chen W, et al. Cardioprotective effect of ultrasound-targeted destruction of Sirt3-loaded cationic microbubbles in a large animal model of pathological cardiac hypertrophy. Acta Biomater. (2023) 164:604–25. doi: 10.1016/j.actbio.2023.04.020
25. Cai W, Lv W, Feng Y, Yang H, Zhang Y, Yang G, et al. The therapeutic effect in gliomas of nanobubbles carrying siRNA combined with ultrasound-targeted destruction. Int J Nanomed. (2018) 13:6791–807. doi: 10.2147/IJN.S164760
26. Xie A, Belcik T, Qi Y, Morgan TK, Champaneri SA, Taylor S, et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging. (2012) 5(12):1253–62. doi: 10.1016/j.jcmg.2012.05.017
27. Zhou Q, Deng Q, Hu B, Wang Y-J, Chen J-L, Cui J-J, et al. Ultrasound combined with targeted cationic microbubble-mediated angiogenesis gene transfection improves ischemic heart function. Exp Ther Med. (2017) 13(5):2293–303. doi: 10.3892/etm.2017.4270
28. Xu C, Xu L, Ohorodnyk P, Roth M, Chen B, Li S. Contrast agent-free synthesis and segmentation of ischemic heart disease images using progressive sequential causal GANs. Med Image Anal. (2020) 62:101668. doi: 10.1016/j.media.2020.101668
29. St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the survival and ventricular enlargement (SAVE) trial. Circulation. (1997) 96(10):3294–9. doi: 10.1161/01.CIR.96.10.3294
30. Shentu W-H, Yan C-X, Liu C-M, Qi R-X, Wang Y, Huang Z-X, et al. Use of cationic microbubbles targeted to P-selectin to improve ultrasound-mediated gene transfection of hVEGF165 to the ischemic myocardium. J Zhejiang Univ Sci B. (2018) 19(9):699–707. doi: 10.1631/jzus.B1700298
31. Deng Q, Hu B, Cao S, Song H-N, Chen J-L, Zhou Q. Improving the efficacy of therapeutic angiogenesis by UTMD-mediated ang-1 gene delivery to the infarcted myocardium. Int J Mol Med. (2015) 36(2):335–44. doi: 10.3892/ijmm.2015.2226
32. Zhong X, Chen Y, Long X, Chen H, Zheng Z, Pan H, et al. Ultrasound-targeted microbubble destruction (UTMD)-mediated miR-150-5p attenuates oxygen and glucose deprivation-induced cardiomyocyte injury by inhibiting TTC5 expression. Mol Biol Rep. (2022) 49(7):6041–52. doi: 10.1007/s11033-022-07392-3
33. Zhang L, Sun Z, Ren P, You M, Zhang J, Fang L, et al. Localized delivery of shRNA against PHD2 protects the heart from acute myocardial infarction through ultrasound-targeted cationic microbubble destruction. Theranostics. (2017) 7(1):51–66. doi: 10.7150/thno.16074
34. Li W, Jin Q, Zhang L, He S, Song Y, Xu L, et al. Ultrasonic microbubble cavitation deliver gal-3 shRNA to inhibit myocardial fibrosis after myocardial infarction. Pharmaceutics. (2023) 15(3):729. doi: 10.3390/pharmaceutics15030729
35. Wang W, Tayier B, Guan L, Yan F, Mu Y. Optimization of the cotransfection of SERCA2a and Cx43 genes for myocardial infarction complications. Life Sci. (2023) 331:122067. doi: 10.1016/j.lfs.2023.122067
36. Wang W, Tayier B, Guan L, Yan F, Mu Y. Pre-transplantation of bone marrow mesenchymal stem cells amplifies the therapeutic effect of ultrasound-targeted microbubble destruction-mediated localized combined gene therapy in post-myocardial infarction heart failure rats. Ultrasound Med Biol. (2022) 48(5):830–45. doi: 10.1016/j.ultrasmedbio.2022.01.004
37. Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. (2020) 577(7790):405–9. doi: 10.1038/s41586-019-1802-2
38. Sun Z, Xie Y, Lee RJ, Chen Y, Jin Q, Lv Q, et al. Myocardium-targeted transplantation of PHD2 shRNA-modified bone mesenchymal stem cells through ultrasound-targeted microbubble destruction protects the heart from acute myocardial infarction. Theranostics. (2020) 10(11):4967–82. doi: 10.7150/thno.43233
39. Su Q, Li L, Liu Y, Zhou Y, Wang J, Wen W. Ultrasound-targeted microbubble destruction-mediated microRNA-21 transfection regulated PDCD4/NF-κB/TNF-α pathway to prevent coronary microembolization-induced cardiac dysfunction. Gene Ther. (2015) 22(12):1000–6. doi: 10.1038/gt.2015.59
40. Algoet M, Janssens S, Himmelreich U, Gsell W, Pusovnik M, Van Den Eynde J, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. (2023) 33(6):357–66. doi: 10.1016/j.tcm.2022.02.005
41. Yan P, Chen K-J, Wu J, Sun L, Sung H-W, Weisel RD, et al. The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials. (2014) 35(3):1063–73. doi: 10.1016/j.biomaterials.2013.10.043
42. Kwekkeboom RFJ, Sluijter JPG, Van Middelaar BJ, Metz CH, Brans M, Kamp O, et al. Increased local delivery of antagomir therapeutics to the rodent myocardium using ultrasound and microbubbles. J Controlled Release. (2016) 222:18–31. doi: 10.1016/j.jconrel.2015.11.020
43. Erikson JM, Valente AJ, Mummidi S, Kandikattu HK, Demarco VG, Bender SB, et al. Targeting TRAF3IP2 by genetic and interventional approaches inhibits ischemia/reperfusion-induced myocardial injury and adverse remodeling. J Biol Chem. (2017) 292(6):2345–58. doi: 10.1074/jbc.M116.764522
44. Du G-Q, Shao Z-B, Wu J, Yin W-J, Li S-H, Wu J, et al. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res Cardiol. (2017) 112(1):7. doi: 10.1007/s00395-016-0593-y
45. Mofid A, Newman NS, Lee PJH, Abbasi C, Matkar PN, Rudenko D, et al. Cardiac overexpression of S100A6 attenuates cardiomyocyte apoptosis and reduces infarct size after myocardial ischemia-reperfusion. J Am Heart Assoc. (2017) 6(2):e004738. doi: 10.1161/JAHA.116.004738
46. Chen G, Yang L, Zhong L, Kutty S, Wang Y, Cui K, et al. Delivery of hydrogen sulfide by ultrasound targeted microbubble destruction attenuates myocardial ischemia-reperfusion injury. Sci Rep. (2016) 6:30643. doi: 10.1038/srep30643
47. Dillmann WH. Diabetic cardiomyopathy. Circ Res. (2019) 124(8):1160–2. doi: 10.1161/CIRCRESAHA.118.314665
48. Jia G, Demarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. (2016) 12(3):144–53. doi: 10.1038/nrendo.2015.216
49. Xie S-Y, Liu S-Q, Zhang T, Shi W-K, Xing Y, Fang W-X, et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. (2024) 149(9):684–706. doi: 10.1161/CIRCULATIONAHA.123.065603
50. Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y. Acidic and basic fibroblast growth factors involved in cardiac angiogenesis following infarction. Int J Cardiol. (2011) 152(3):307–13. doi: 10.1016/j.ijcard.2010.07.024
51. Zhao Y-Z, Zhang M, Wong HL, Tian X-Q, Zheng L, Yu X-C, et al. Prevent diabetic cardiomyopathy in diabetic rats by combined therapy of aFGF-loaded nanoparticles and ultrasound-targeted microbubble destruction technique. J Controlled Release. (2016) 223:11–21. doi: 10.1016/j.jconrel.2015.12.030
52. Zhao Y-Z, Tian X-Q, Zhang M, Cai L, Ru A, Shen X-T, et al. Functional and pathological improvements of the hearts in diabetes model by the combined therapy of bFGF-loaded nanoparticles with ultrasound-targeted microbubble destruction. J Controlled Release. (2014) 186:22–31. doi: 10.1016/j.jconrel.2014.04.054
53. Zhao Y-Z, Zhang M, Tian X-Q, Zheng L, Lu C-T. Using basic fibroblast growth factor nanoliposome combined with ultrasound-introduced technology to early intervene the diabetic cardiomyopathy. Int J Nanomed. (2016) 11:675–86. doi: 10.2147/IJN.S99376
54. Zhang M, Zhu N-W, Ma W-C, Chen M-J, Zheng L. Combined treatment with ultrasound-targeted microbubble destruction technique and NM-aFGF-loaded PEG-nanoliposomes protects against diabetic cardiomyopathy-induced oxidative stress by activating the AKT/GSK-3β1/nrf-2 pathway. Drug Delivery. (2020) 27(1):938–52. doi: 10.1080/10717544.2020.1785052
55. Guo X, Chen K, Ji L, Wang S, Ye X, Xu L, et al. Ultrasound-targeted microbubble technology facilitates SAHH gene delivery to treat diabetic cardiomyopathy by activating AMPK pathway. IScience. (2024) 27(2):108852. doi: 10.1016/j.isci.2024.108852
56. Chen Y, Shi S, Dai Y. Research progress of therapeutic drugs for doxorubicin-induced cardiomyopathy. Biomed Pharmacother. (2022) 156:113903. doi: 10.1016/j.biopha.2022.113903
57. Chen S, Chen J, Huang P, Meng X-L, Clayton S, Shen J-S, et al. Myocardial regeneration in Adriamycin cardiomyopathy by nuclear expression of GLP1 using ultrasound targeted microbubble destruction. Biochem Biophys Res Commun. (2015) 458(4):823–9. doi: 10.1016/j.bbrc.2015.02.038
58. Tian X-Q, Ni X-W, Xu H-L, Zheng L, Zhuge D-L, Chen B, et al. Prevention of doxorubicin-induced cardiomyopathy using targeted MaFGF mediated by nanoparticles combined with ultrasound-targeted MB destruction. Int J Nanomed. (2017) 12:7103–19. doi: 10.2147/IJN.S145799
59. Chen S, Chen J, Meng X-L, Shen J-S, Huang J, Huang P, et al. ANGPTL8 reverses established adriamycin cardiomyopathy by stimulating adult cardiac progenitor cells. Oncotarget. (2016) 7(49):80391–403. doi: 10.18632/oncotarget.13061
60. Lee PJH, Rudenko D, Kuliszewski MA, Liao C, Kabir MG, Connelly KA, et al. Survivin gene therapy attenuates left ventricular systolic dysfunction in doxorubicin cardiomyopathy by reducing apoptosis and fibrosis. Cardiovasc Res. (2014) 101(3):423–33. doi: 10.1093/cvr/cvu001
61. Zhou N-Q, Fang Z-X, Huang N, Zuo Y, Qiu Y, Guo L-J, et al. aFGF targeted mediated by novel nanoparticles-microbubble Complex combined with ultrasound-targeted microbubble destruction attenuates doxorubicin-induced heart failure via anti-apoptosis and promoting cardiac angiogenesis. Front Pharmacol. (2021) 12:607785. doi: 10.3389/fphar.2021.607785
62. Chen J, Qiu S, Liu Y, Sun W, Zhou T, Zhao L, et al. Ultrasound targeted microbubble destruction assisted exosomal delivery of siHmox1 effectively inhibits doxorubicin-induced cardiomyocyte ferroptosis. J Nanobiotechnol. (2024) 22(1):531. doi: 10.1186/s12951-024-02794-w
63. Sun W, Zhao P, Zhou Y, Xing C, Zhao L, Li Z, et al. Ultrasound targeted microbubble destruction assisted exosomal delivery of miR-21 protects the heart from chemotherapy associated cardiotoxicity. Biochem Biophys Res Commun. (2020) 532(1):60–7. doi: 10.1016/j.bbrc.2020.05.044
64. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. (2018) 319(1):62–75. doi: 10.1001/jama.2017.17687
65. Wang S, Chen K, Wang Y, Wang Z, Li Z, Guo J, et al. Cardiac-targeted delivery of nuclear receptor RORα via ultrasound targeted microbubble destruction optimizes the benefits of regular dose of melatonin on sepsis-induced cardiomyopathy. Biomater Res. (2023) 27(1):41. doi: 10.1186/s40824-023-00377-8
66. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. (2018) 15(7):387–407. doi: 10.1038/s41569-018-0007-y
67. Kopechek JA, Mctiernan CF, Chen X, Zhu J, Mburu M, Feroze R, et al. Ultrasound and microbubble-targeted delivery of a microRNA inhibitor to the heart suppresses cardiac hypertrophy and preserves cardiac function. Theranostics. (2019) 9(23):7088–98. doi: 10.7150/thno.34895
68. Crespo-Leiro MG, Costanzo MR, Gustafsson F, Khush KK, Macdonald PS, Potena L, et al. Heart transplantation: focus on donor recovery strategies, left ventricular assist devices, and novel therapies. Eur Heart J. (2022) 43(23):2237–46. doi: 10.1093/eurheartj/ehac204
69. Khush KK, Hsich E, Potena L, Cherikh WS, Chambers DC, Harhay MO, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-eighth adult heart transplantation report - 2021; focus on recipient characteristics. J Heart Lung Transplant. (2021) 40(10):1035–49. doi: 10.1016/j.healun.2021.07.015
70. Bao H, Dai L, Wang H, Jiang T. Ultrasound-targeted sirolimus-loaded microbubbles improves acute rejection of heart transplantation in rats by inhibiting TGF-β1-smad signaling pathway, promoting autophagy and reducing inflammation. Int J Pharm X. (2024) 8:100300. doi: 10.1016/j.ijpx.2024.100300
71. Liu J, Chen Y, Wang G, Jin Q, Sun Z, Lv Q, et al. Improving acute cardiac transplantation rejection therapy using ultrasound-targeted FK506-loaded microbubbles in rats. Biomater Sci. (2019) 7(9):3729–40. doi: 10.1039/C9BM00301K
72. Wang Z, Jiang S, Li S, Yu W, Chen J, Yu D, et al. Targeted galectin-7 inhibition with ultrasound microbubble targeted gene therapy as a sole therapy to prevent acute rejection following heart transplantation in a rodent model. Biomaterials. (2020) 263:120366. doi: 10.1016/j.biomaterials.2020.120366
73. Yi L, Chen Y, Jin Q, Deng C, Wu Y, Li H, et al. Antagomir-155 attenuates acute cardiac rejection using ultrasound targeted microbubbles destruction. Adv Healthcare Mater. (2020) 9(14):e2000189. doi: 10.1002/adhm.202000189
74. Sun J, Singh P, Shami A, Kluza E, Pan M, Djordjevic D, et al. Spatial transcriptional mapping reveals site-specific pathways underlying human atherosclerotic plaque rupture. J Am Coll Cardiol. (2023) 81(23):2213–27. doi: 10.1016/j.jacc.2023.04.008
75. Zhou J, Niu C, Huang B, Chen S, Yu C, Cao S, et al. Platelet membrane biomimetic nanoparticles combined with UTMD to improve the stability of atherosclerotic plaques. Front Chem. (2022) 10:868063. doi: 10.3389/fchem.2022.868063
76. Wu Y, Deng C, Xu J, Wang W, Chen Y, Qin X, et al. Enhanced local delivery of microRNA-145a-5P into mouse aorta via ultrasound-targeted microbubble destruction inhibits atherosclerotic plaque formation. Mol Pharm. (2023) 20(2):1086–95. doi: 10.1021/acs.molpharmaceut.2c00799
77. Yuan H, Hu H, Sun J, Shi M, Yu H, Li C, et al. Ultrasound microbubble delivery targeting intraplaque neovascularization inhibits atherosclerotic plaque in an APOE-deficient mouse model. In Vivo. (2018) 32(5):1025–32. doi: 10.21873/invivo.11342
78. Hu X, Zhao P, Zhang J, Zhu Y, Zhou W, Hong K, et al. Ultrasound-assisted biomimetic nanobubbles for targeted treatment of atherosclerosis. Nanomedicine. (2023) 51:102682. doi: 10.1016/j.nano.2023.102682
79. Yang L, Chen L, Fang Y, Ma S. Downregulation of GSK-3β expression via ultrasound-targeted microbubble destruction enhances atherosclerotic plaque stability in New Zealand rabbits. Ultrasound Med Biol. (2021) 47(3):710–22. doi: 10.1016/j.ultrasmedbio.2020.11.002
80. Yang H, Sun Y, Wei J, Xu L, Tang Y, Yang L, et al. The effects of ultrasound-targeted microbubble destruction (UTMD) carrying IL-8 monoclonal antibody on the inflammatory responses and stability of atherosclerotic plaques. Biomed Pharmacother. (2019) 118:109161. doi: 10.1016/j.biopha.2019.109161
81. Su Y, Xu C, Li K, Wang B, Chen J, Liu L, et al. TGF-β1 and TIMP1 double directional rAAV targeted by UTMD in atherosclerotic vulnerable plaque. Exp Ther Med. (2017) 13(4):1465–9. doi: 10.3892/etm.2017.4101
82. Mackman N. Triggers, targets and treatments for thrombosis. Nature. (2008) 451(7181):914–8. doi: 10.1038/nature06797
83. Doelare SAN, Jean Pierre DM, Nederhoed JH, Smorenburg SPM, Lely RJ, Jongkind V, et al. Microbubbles and ultrasound accelerated thrombolysis for peripheral arterial occlusions: the outcomes of a single arm phase II trial. Eur J Vasc Endovasc Surg. (2021) 62(3):463–8. doi: 10.1016/j.ejvs.2021.05.030
84. Tan ZQ, Ooi EH, Chiew YS, Foo JJ, Ng YK, Ooi ET. Enhancing sonothrombolysis outcomes with dual-frequency ultrasound: insights from an in silico microbubble dynamics study. Comput Biol Med. (2024) 181:109061. doi: 10.1016/j.compbiomed.2024.109061
85. Kandadai MA, Meunier JM, Hart K, Holland CK, Shaw GJ. Plasmin-loaded echogenic liposomes for ultrasound-mediated thrombolysis. Transl Stroke Res. (2015) 6(1):78–87. doi: 10.1007/s12975-014-0376-4
86. Corro R, Urquijo CF, Aguila O, Villa E, Santana J, Rios A, et al. Use of nitric oxide donor-loaded microbubble destruction by ultrasound in thrombus treatment. Molecules. (2022) 27(21):7218. doi: 10.3390/molecules27217218
87. Zhang B, Wu H, Goel L, Kim H, Peng C, Kim J, et al. Magneto-sonothrombolysis with combination of magnetic microbubbles and nanodroplets. Ultrasonics. (2021) 116:106487. doi: 10.1016/j.ultras.2021.106487
88. Wang S, Guo X, Xiu W, Liu Y, Ren L, Xiao H, et al. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci Adv. (2020) 6(31):eaaz8204. doi: 10.1126/sciadv.aaz8204
89. Fournier L, Abioui-Mourgues M, Chabouh G, Aid R, Taille TDL, Couture O, et al. rtPA-loaded fucoidan polymer microbubbles for the targeted treatment of stroke. Biomaterials. (2023) 303:122385. doi: 10.1016/j.biomaterials.2023.122385
90. Zheng X, Pan Y, Zhang Y, Meng K, Zhou J, Wang X, et al. Interventional microbubble enhanced sonothrombolysis on left ventricular assist devices. Adv Sci (Weinh). (2022) 9(21):e2201291. doi: 10.1002/advs.202201291
91. Liang Z, Chen H, Gong X, Shi B, Lin L, Tao F, et al. Ultrasound-induced destruction of nitric oxide-loaded microbubbles in the treatment of thrombus and ischemia-reperfusion injury. Front Pharmacol. (2021) 12:745693. doi: 10.3389/fphar.2021.745693
92. Shi B, Yang Q, Liang Z, Yu R, Li H, Wu Q, et al. Accelerating thrombolysis of arterial thrombus with NO-MBs UTMD therapy. Eur J Pharm Biopharm. (2024) 205:114566. doi: 10.1016/j.ejpb.2024.114566
93. Zhong J, Sun Y, Han Y, Chen X, Li H, Ma Y, et al. Hydrogen sulfide-loaded microbubbles combined with ultrasound mediate thrombolysis and simultaneously mitigate ischemia-reperfusion injury in a rat hindlimb model. J Thromb Haemost. (2021) 19(3):738–52. doi: 10.1111/jth.15110
94. Pan Y, Li Y, Li Y, Zheng X, Zou C, Li J, et al. Nanodroplet-coated microbubbles used in sonothrombolysis with two-step cavitation strategy. Adv Healthcare Mater. (2023) 12(6):e2202281. doi: 10.1002/adhm.202202281
95. Zhang G, Yu C, Zhou M, Wang L, Zhang Y, Luo L. Burden of ischaemic heart disease and attributable risk factors in China from 1990 to 2015: findings from the global burden of disease 2015 study. BMC Cardiovasc Disord. (2018) 18(1):18. doi: 10.1186/s12872-018-0761-0
96. Huang H, Li X, Zheng S, Chen Y, Chen C, Wang J, et al. Downregulation of renal G protein-coupled receptor kinase type 4 expression via ultrasound-targeted microbubble destruction lowers blood pressure in spontaneously hypertensive rats. J Am Heart Assoc. (2016) 5(10):e004028. doi: 10.1161/JAHA.116.004028
97. Fisher DG, Cruz T, Hoch MR, Sharifi KA, Shah IM, Gorick CM, et al. Focused ultrasound-microbubble treatment arrests the growth and formation of cerebral cavernous malformations. Nat Biomed Eng. (2025). doi: 10.1038/s41551-025-01390-z
98. Bai Y, Du Y, Yang Y, Wälchli T, Constanthin PE, Li F. Ultrasound-targeted microbubble destruction increases BBB permeability and promotes stem cell-induced regeneration of stroke by downregulating MMP8. Cell Transplant. (2024) 33:9636897231223293. doi: 10.1177/09636897231223293
99. Zhu Q, He Y, Dong XX, Xu Y, Zhang Y, Liu Z. Microbubble enhanced ultrasound with low mechanical index promotes therapeutic angiogenesis in hind limb ischemia mouse model. Med Phys. (2025) 52(3):1706–16. doi: 10.1002/mp.17539
100. Cao WJ, Rosenblat JD, Roth NC, Kuliszewski MA, Matkar PN, Rudenko D, et al. Therapeutic angiogenesis by ultrasound-mediated MicroRNA-126-3p delivery. Arterioscler, Thromb, Vasc Biol. (2015) 35(11):2401–11. doi: 10.1161/ATVBAHA.115.306506
101. Sun Z, Cai Y, Chen Y, Jin Q, Zhang Z, Zhang L, et al. Ultrasound-targeted microbubble destruction promotes PDGF-primed bone mesenchymal stem cell transplantation for myocardial protection in acute myocardial infarction in rats. J Nanobiotechnol. (2023) 21(1):481. doi: 10.1186/s12951-023-02204-7
102. Yue C, Li R, Li C, Yang T, Huang X, Lei R, et al. Ultrasound-targeted microbubble destruction technology delivering β-klotho to the heart enhances FGF21 sensitivity and attenuates heart remodeling post-myocardial infarction. Int J Mol Med. (2024) 53(6):54. doi: 10.3892/ijmm.2024.5378
103. Wang Z, Chen J, Wang J, Xu M, Yang H, Yang H, et al. MSCs biomimetic ultrasonic phase change nanoparticles promotes cardiac functional recovery after acute myocardial infarction. Biomaterials. (2025) 313:122775. doi: 10.1016/j.biomaterials.2024.122775
104. Cai Q, Li Q, Zhong S, Chen M, Zhong L, Li S, et al. Ultrasound-targeted microbubble destruction rapidly improves left ventricular function in rats with ischemic cardiac dysfunction. Int J Cardiol. (2024) 404:131943. doi: 10.1016/j.ijcard.2024.131943
105. Yang L, Gao T, Huang Y, Wang P-H, Han X-H, Wu J, et al. Ultrasound-targeted β-catenin gene therapy improves the cardiac function in mice after myocardial infarction. Cardiovasc Toxicol. (2025) 25(1):74–84. doi: 10.1007/s12012-024-09946-2
106. Dörner J, Struck R, Zimmer S, Peigney C, Duerr GD, Dewald O, et al. Ultrasound-mediated stimulation of microbubbles after acute myocardial infarction and reperfusion ameliorates left-ventricular remodelling in mice via improvement of borderzone vascularization. PLoS One. (2013) 8(2):e56841. doi: 10.1371/journal.pone.0056841
107. Lammers T, Koczera P, Fokong S, Gremse F, Ehling J, Vogt M, et al. Theranostic USPIO-loaded microbubbles for mediating and monitoring blood-brain barrier permeation. Adv Funct Mater. (2015) 25(1):36–43. doi: 10.1002/adfm.201401199
108. Chen Y, Du M, Yuan Z, Chen Z, Yan F. Spatiotemporal control of engineered bacteria to express interferon-γ by focused ultrasound for tumor immunotherapy. Nat Commun. (2022) 13(1):4468. doi: 10.1038/s41467-022-31932-x
109. Han JP, Kim M, Choi BS, Lee JH, Lee GS, Jeong M, et al. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci Adv. (2022) 8(3):eabj6901. doi: 10.1126/sciadv.abj6901
110. Helfield B, Chen X, Watkins SC, Villanueva FS. Biophysical insight into mechanisms of sonoporation. Proc Natl Acad Sci U S A. (2016) 113(36):9983–8. doi: 10.1073/pnas.1606915113
111. Nan N, Si D, Hu G. Nanoscale cavitation in perforation of cellular membrane by shock-wave induced nanobubble collapse. J Chem Phys. (2018) 149(7):074902. doi: 10.1063/1.5037643
112. Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat Commun. (2021) 12(1):779. doi: 10.1038/s41467-021-21022-9
113. Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. (2019) 23:8. doi: 10.1186/s40824-019-0156-z
114. Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. (2007) 8(8):573–87. doi: 10.1038/nrg2141
115. Bouard D, Alazard-Dany D, Cosset FL. Viral vectors: from virology to transgene expression. Br J Pharmacol. (2009) 157(2):153–65. doi: 10.1038/bjp.2008.349
116. Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. (2016) 45(5):1457–501. doi: 10.1039/C5CS00798D
117. Xie L, Wang J, Song L, Jiang T, Yan F. Cell-cycle dependent nuclear gene delivery enhances the effects of E-cadherin against tumor invasion and metastasis. Signal Transduct Target Ther. (2023) 8(1):182. doi: 10.1038/s41392-023-01398-4
118. Wang J, Li Z, Pan M, Fiaz M, Hao Y, Yan Y, et al. Ultrasound-mediated blood-brain barrier opening: an effective drug delivery system for theranostics of brain diseases. Adv Drug Delivery Rev. (2022) 190:114539. doi: 10.1016/j.addr.2022.114539
119. Mcdannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. (2012) 72(14):3652–63. doi: 10.1158/0008-5472.CAN-12-0128
120. Hynynen K, Mcdannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, et al. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. (2006) 105(3):445–54. doi: 10.3171/jns.2006.105.3.445
121. Mcmahon D, Hynynen K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. (2017) 7(16):3989–4000. doi: 10.7150/thno.21630
122. Wu S-Y, Aurup C, Sanchez CS, Grondin J, Zheng W, Kamimura H, et al. Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping. Sci Rep. (2018) 8(1):7978. doi: 10.1038/s41598-018-25904-9
123. Wang J, Xie L, Shi Y, Ao L, Cai F, Yan F. Early detection and reversal of cell apoptosis induced by focused ultrasound-mediated blood-brain barrier opening. ACS Nano. (2021) 15(9):14509–21. doi: 10.1021/acsnano.1c04029
Keywords: cardiovascular disease, ultrasound, microbubble, ultrasound targeted microbubble destruction (UTMD), blood-brain barrier (BBB), atherosclerosis
Citation: Yang S, Zhang R, Zhang H and Lu Y (2025) Ultrasound-targeted microbubble destruction: a prospective strategy for treating cardiovascular disease. Front. Cardiovasc. Med. 12:1634059. doi: 10.3389/fcvm.2025.1634059
Received: 3 June 2025; Accepted: 6 August 2025;
Published: 1 September 2025.
Edited by:
DeLisa Fairweather, Mayo Clinic Florida, United StatesReviewed by:
Boxuan Ma, Zhejiang University, ChinaAlina Rwei, Delft University of Technology, Netherlands
Copyright: © 2025 Yang, Zhang, Zhang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huali Zhang, Mjk5MDMyODMyN0BxcS5jb20=; Yongping Lu, bHV5b25ncGluZ2FoeW51QDEyNi5jb20=
†These authors share first authorship
 Shuting Yang
Shuting Yang Rong Zhang†
Rong Zhang† Yongping Lu
Yongping Lu