Abstract
Background:
Endothelial cells (ECs) induce vascular smooth muscle cells (VSMCs) relaxation via nitric oxide (NO), prostacyclin (PGI₂) and hyperpolarizing factors. Recent whole-genomic, single-cell transcriptomic analysis of human vascular cells has revealed angiotypic heterogeneity. However, it remains unknown whether vasorelaxant mediators reiterate this pattern.
Working hypothesis:
The expression of “sentinel” gene transcripts provides a first insight into angiotypic and organotypic heterogeneity of vasorelaxation.
Methods:
The expression of NO- and PGI2-generating enzymes and potassium channels was evaluated by analyzing single-cell RNA-sequencing data derived from the Human Vascular Cell Atlas. The data were transformed into a Seurat object (ShinyCell) and processed using the RStudio software. The results were visualized through uniform manifold approximation (UMAP) and projection representations of single-cell profiles.
Results:
NO synthase (NOS3) expression differed across EC subpopulations, with the highest enrichment in spleen littoral ECs, followed by venous, arterial, and capillary ECs. PGI2 synthase (PTGIS) demonstrated the highest frequency in arterial and venous ECs and VSMCs. At the same time, it was low in capillary, littoral, and lymphatic ECs and pericytes. The PGI2 receptor gene (PTGIR) was expressed in vascular mural cells. A marked angiotypic heterogeneity was noted regarding potassium channels. Overall, the gene transcripts mentioned above were rarely co-expressed. Comparing two cohorts aged 20 to 49 and 50 to 80 revealed NOS3 expression was less frequent in venous and littoral ECs of older individuals. In contrast, arterial and capillary ECs were modestly affected by age. PTGIS frequency was elevated with aging in VSMCs and, to a lesser extent, in venous and arterial ECs. The KCNMA1 gene, which encodes the big potassium channel alpha subunit 1, was almost doubled in the VSMCs from the older group. Finally, organotypic differences were identified in vascular cells derived from the coronary arteries, brain, and uterus.
Conclusion:
This initial report indicates a striking heterogeneity in the expression of genes encoding vasorelaxant pathways in human vascular cells, with age exerting angiotypic influences. Reiteration on a larger number of cells and genes, as well as validation of data using post-transcriptional methods, is warranted to firmly confirm the hypothesis about redundant heterogeneity of vasorelaxant mechanisms.
Graphical Abstract
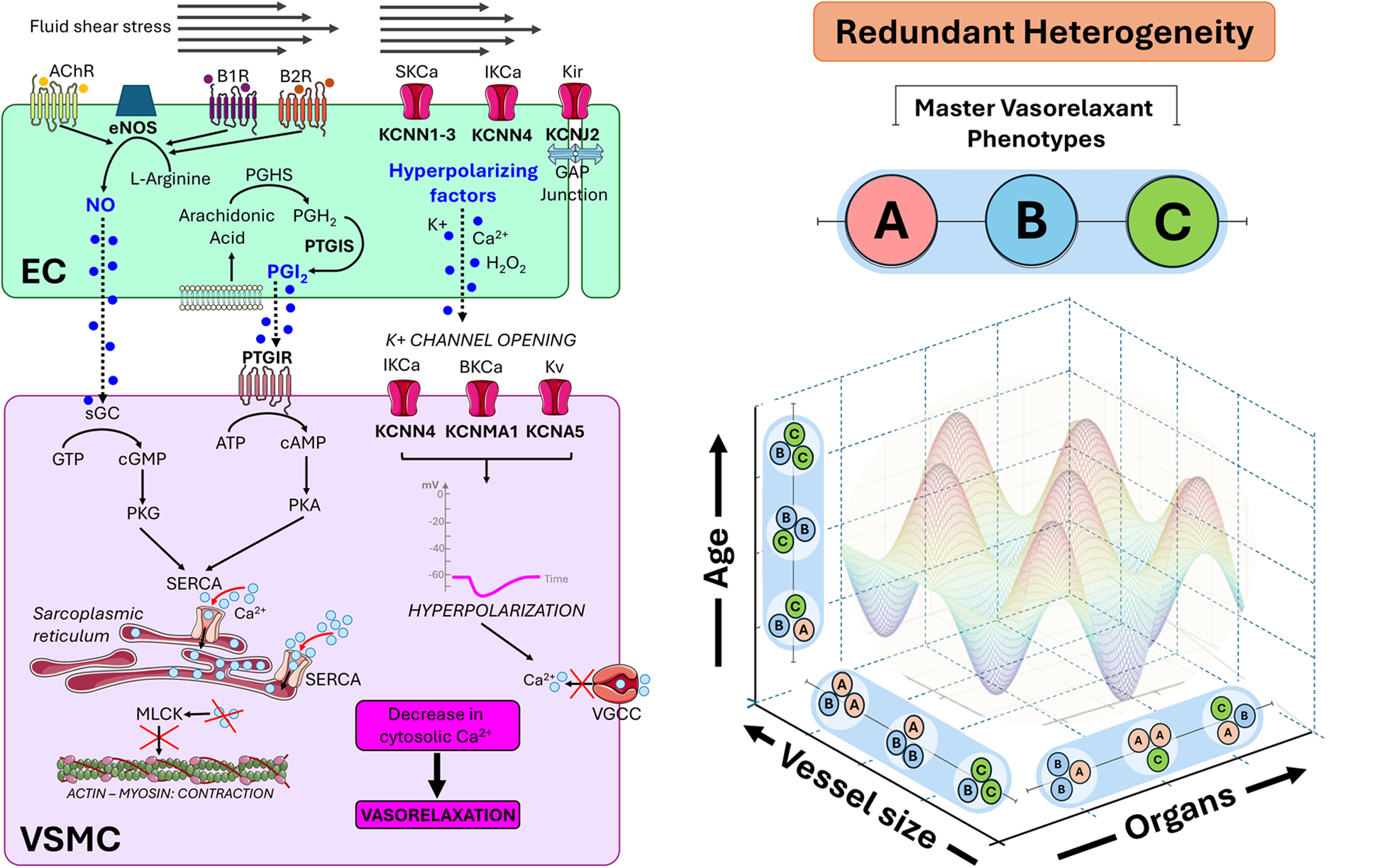
On the left-hand side, the representation of the signalling that originates from endothelial cells (EC) and propagates to vascular smooth muscle cells (VSMC), causing a reduction in cytosolic calcium levels in the latter and subsequent relaxation. Three endothelium-derived relaxing factors—nitric oxide (NO), prostacyclin (PGI2), and hyperpolarizing factors (all indicated in blue)—signal in neighbouring VSMCs through distinct mechanisms. NO and PGI2 signal through the second messengers cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP), respectively, enhancing calcium (Ca2+) uptake into the sarcoplasmic reticulum via sarco/endoplasmic reticulum Ca2+-ATPases (SERCAs). Hyperpolarizing factors promote the opening of potassium (K+) channels, leading to membrane hyperpolarization and the subsequent closure of voltage-gated calcium channels (VGCCs). These mechanisms converge to lower cytosolic Ca2+ levels, diminishing myosin light chain kinase (MLCK) activation, and ultimately promoting vasorelaxation. On the right-hand side, the concept of redundant heterogeneity is introduced. The multiplicity of vasorelaxant mechanisms ensures a first-tier redundancy, which is heterogeneously distributed among vascular cells according to angiotypic (vessel size) and organotypic (different organs) blueprints. Redundant heterogeneity confers the vascular system with peculiar flexibility and resilience to stress, disease, and aging. The coloured harmonic function image exemplifies how the dynamic interaction between vasorelaxant phenotypes and environmental factors results in functional adaptations, with hills and valleys representing increased or reduced vasorelaxation. AchR, acetylcholine receptor; eNOS, endothelial nitric oxide synthase; B1R/B2R, bradykinin receptors; sGC, soluble guanylate cyclase; GTP, guanosine triphosphate; PKG, protein kinase G; PGH2, Prostaglandin H2; PGHS, prostaglandin H synthase; PTGIS, PGI2 synthase; PTGIR, PGI2 receptor; ATP, adenosine triphosphate; PKA, protein kinase A; H2O2, hydrogen peroxide; KCa, potassium calcium-activated channel; Kv, potassium voltage-gated channel; Kir, potassium inward-rectifier channel; SKCa, small conductance calcium-activated potassium channel; IKCa, intermediate-conductance calcium-activated potassium channel; BKCa, big-conductance potassium channel; KCNN1-4, potassium calcium-activated channel subfamily N member 1-4; KCNJ2, inward rectifier K+ channel protein Kir2.1; KCNMA1, calcium-activated potassium channel subunit alpha-1; KCNA5, potassium voltage-gated channel subfamily A member 5. Images from Smart Servier Medical Art: “Receptor green”, “Receptor orange”, “Receptor purple”, Receptor”, “Ion red receptors channel”, “Pink channel”, “Beige receptors channel”, “Tissue junction overview”, “Cell membrane”, “Endoplasmic reticulum” and “Actin”, licensed under CC BY 4.0.
Introduction
The cardiovascular system comprises the heart and a network of large elastic arteries, resistance arteries, arterioles, and terminal capillaries, connected to the venous return. Each arterial segment consists of three concentric layers: (i) the intima, lined with endothelial cells (ECs) that directly interface with the vessel lumen; (ii) the tunica media, which is made up of at least one layer of vascular smooth muscle cells (VSMCs); and (iii) the adventitia, which contains mesenchymal cells, pericytes, immune cells, and nerve terminals. In larger arteries, this outer layer includes a specialized vascular supply system termed “vasa vasorum”. In contrast to arteries and arterioles, capillaries do not possess a VSMC sheet. They are composed of continuous tubes of ECs, covered by a basement membrane and pericytes. These perivascular cells physically and functionally interact with the capillary endothelium through adhesion plaques, gap junctions, and peg-and-socket structures.
Compared to their diameter, arterioles contain a higher proportion of VSMCs and less elastin than large arteries, which accounts for their role as major regulators of vascular resistance and regional blood flow. The tiny capillaries deliver nutrients and oxygen to cells and remove waste products, while also contributing to blood flow modulation through the pericytes' ability to contract and relax. The endothelium acts as a master regulator of vasorelaxation, sensing and transducing chemical stimuli, such as acetylcholine (Ach) and bradykinin (BK), or mechanical forces, such as fluid shear stress from within the artery, into intracellular signalling. The process involves the secretion of diffusible transmitters by ECs, specifically nitric oxide (NO), prostacyclin (PGI2), and the so-called hyperpolarizing factors, which interact to reduce cytosolic Ca2+ levels in VSMCs, allowing relaxation (1). Intercellular gap junctions facilitate bidirectional relaxation currents between small arterioles and upstream arteries, greatly enhancing tissue perfusion (2, 3).
The three endothelium-derived relaxing factors trigger intracellular Ca
2+changes using distinct second messengers.
- (i)
The endothelium-specific NO synthase (eNOS) is responsible for approximately 90% of NO generation from the L-arginine substrate. The signalling from NO involves the activation of soluble guanylate cyclase (sGC), which catalyzes the conversion of guanosine triphosphate (GTP) into inorganic pyrophosphate and the secondary messenger cyclic guanosine monophosphate (cGMP). CGMP activates protein kinase G, which reduces the cytosolic Ca2+ levels by promoting its uptake by sarcoplasmic reticulum calcium-ATPases (SERCA) (4, 5).
- (ii)
PGI2 is a terminal product resulting from the sequential metabolism of arachidonic acid by cyclooxygenase (COX) and prostacyclin synthase (PGIS). The receptor for PGI2, known as IP, is present on various cell types, and signaling through this receptor demonstrates extensive physiological actions, particularly for its potent vasodilatory effects through smooth muscle relaxation and inhibition of platelet aggregation (6). By binding the IP receptor, encoded by the PTGIR gene and highly expressed in the platelets, lung, heart, kidney, and throughout the vasculature, PGI2 triggers physiologic responses mediated by the intracellular accumulation of cyclic adenosine monophosphate (cAMP). CAMP activates protein kinase A (PKA), promoting sarcoplasmic reticulum Ca2+ uptake and inhibiting MLCK via phosphorylation (7).
- (iii)
Hyperpolarizing signalling is a prevalent relaxation mechanism at the level of small arterioles. It functions independently of NO and PGI2, becoming dominant when the two other vasorelaxant pathways are suppressed pharmacologically or downregulated due to endothelial dysfunction (8–10). Hyperpolarization initiates in ECs and propagates to VSMCs via chemical signals, such as potassium (K+), lipid mediators, and hydrogen peroxide, as well as through electrical coupling through myoendothelial gap junctions (10). A crucial step in the hyperpolarization cascade is represented by the opening of K+ channels. The most prominent family of K+ channels are activated by membrane depolarization, with other families consisting of channels that are either activated by a rise in intracellular calcium ions or are constitutively active. A standardized nomenclature for K+ channels has been proposed by the NC-IUPHAR Subcommittees, which has placed cloned channels into groups based on gene family and structure of channels that exhibit 6, 4 or 2 transmembrane domains (11). In the present study, we focused on the following channels: Ca2+-activated K+ (KCa), voltage-gated K+ (Kv), and inward-rectifier K+ (Kir) channels. (i) KCa channels comprise three subtypes: the small-conductance channels (SKCa), intermediate-conductance channels (IKCa), and big-conductance channels (BKCa) (12). (ii) KV channels are activated at more negative membrane voltages than KCa in VSMCs of small arterioles, thus conspicuously contributing to vascular tone control (13). (iii) Kir channels, considered the most prominent channels in ECs but also expressed by capillary pericytes, are activated by laminar flow, G protein-coupled receptor (GPCR) agonists, and K+ efflux from neighbouring cells (14). Kir channels enable a large K+ influx but little outward current outflow under physiological conditions (14). This causes additional K+ efflux and an enhancement of hyperpolarization, which can be eventually transmitted through the endothelial layer to dilate upstream feeding arteries (15).
Over the past twenty years, the concept of vascular cell heterogeneity has revolutionised the field of vascular biology (16). Vascular cells exhibit differences among various organs and tissues, as indicated by variations in overall morphology, intracellular organisation, and gene expression profiles. Furthermore, significant differences in functional characteristics distinguish cells of large vessels from those of the microvasculature, likely due to epigenetic programming, as these differences persist even after isolation and in vitro culture (17, 18). The heterogeneities and the redundancy of vasorelaxant processes are essential for providing the necessary flexibility to perform various physiological duties. Classical research using isolated vascular models and pharmacological inhibitors has helped decipher regional variations in the expression and function of vasorelaxation-related mechanisms. Analyses using single-cell RNA transcriptomics have demonstrated angiotypic and organotypic parallels and diversities that could not be discriminated with classical pharmacological approaches (19, 20). It would be of paramount importance to apply the same approach to decipher vascular vasorelaxant transcriptomic profiles. Filling this knowledge gap will yield substantial clinical and therapeutic consequences. For instance, it may facilitate the development of novel pharmaceuticals that specifically target particular artery beds and organs, thereby reducing off-target effects.
This study conducted a qualitative transcriptomic analysis of key vasodilator pathways in vascular cell subtypes at both the organismal and regional levels, aiming to inform and establish a theoretical basis for future quantitative research. Our working hypothesis was that the expression of “sentinel” gene transcripts would provide a first insight into the angiotypic and organotypic heterogeneity of vasorelaxation. We extracted and analyzed single-cell RNA sequencing data on enzymes, receptors, and channels involved in vasorelaxant pathways from a publicly available Human Vascular Atlas (20). This database provides detailed molecular profiles of endothelial and mural cells, offering a framework for investigating physiological adaptations across various vascular beds in different organs and tissues (20). Results indicate previously unforeseen disparities between vascular cells from arterioles and capillaries or different organs, with further changes being imposed by aging.
Methods
The Human Vascular Atlas is a comprehensive collection of vascular cells from 19 organs and tissues. It integrates arterial, capillary, venous, spleen pulp (littoral), and lymphatic ECs, pericytes, and VSMCs, yielding approximately 67,000 cells from 62 male and female donors. As described in detail previously (20), datasets, other than Tabula Sapiens data, were mapped using CellRanger (version 3.0) and STARsolo (version 2.7.3a) to the human genome (GRCh38 version 3.0.0), with ambient RNA removed using CellBender (version 0.2.0). Pre-processed Tabula Sapiens data were used. Python (version 3), AnnData (versions 0.8.0 and 0.9.1) Pandas (versions 1.5.2 and 1.5.3), NumPy (versions 1.21.6 and 1.23.5), SciPy (versions 1.8.0 and 1.9.3), Matplotlib (version 3.5.2), Seaborn (version 0.12.2) and Scanpy (versions 1.8.2 and 1.9.1) were used for QC and downstream processing. Doublets were identified utilizing Scrublet (version 0.2.3), with cells exhibiting a Scrublet score ≥ 0.4 and an adjusted Benjamini–Hochberg-corrected P ≤ 0.7 classified as doublets and subsequently excluded. Cells of inferior quality were excluded based on the following criteria: minimum read count = 500, minimum gene count = 300, mitochondrial gene percentage ≤ 0.4, ribosomal gene percentage ≤ 0.3. The data were subsequently standardized to 104 and log-transformed [ln(x + 1)] utilizing the SCANPY workflow115. Untransformed reads were preserved in the layer designated as “counts”.
We employed the ShinyCell R package (v2.1.0) to interrogate the associated single-cell RNA-sequencing data to visualize cell metadata and gene expression. The fully interactive Shiny application enables the generation of low-dimensional uniform manifold approximation and projection (UMAP) embeddings, as well as the simultaneous investigation of expression patterns of multiple genes through bubble plots and heatmaps. Briefly, the “Vascular Cell Atlas” dataset (.h5ad, AnnData file) was downloaded in.h5ad format (AnnData file) from https://www.vascularcellatlas.org/ and converted into a Seurat object using the MuDataSeurat package (v 0.0.0.9000). Finally, using the ShinyCell R package, a ShinyCell application was generated from the Seurat object and run via RStudio for data analysis. ShinyCell generation was performed using R (version 4.4.3) and Seurat (version 5.2.1).
Considering the exploratory nature of our study, along with the limited number of cells and donors, we adopted a descriptive approach to reporting the results, without performing quantitative statistical comparisons.
Results
Expression of vascular typical genes
As reported in Supplementary Table S1, ECs exhibited the expected high-level expression of CDH5, VWF, PECAM1, EGFL7, and VWF. These genes were also co-expressed by single cells, as in the case of the PECAM1—EGFL7 duo, showing a double-positive signal in 73% of ECs from capillaries and arterioles. At the same time, mural cells could be classified as VSMCs and pericytes based on the expression of classical markers, including PDGFRB, NOTCH3, ACTA2, MYH11, and RGS5.
Angiotypic differences in the expression of vasorelaxation-related genes
We next analyzed a detailed annotation of organismal vascular cells. Figure 1A illustrates UMAP representations of single-cell profiles. Figures 1B,C visualizes the expression of endothelial and mural typical genes: CDH5, which encodes VE-cadherin, and ACTA2, which encodes alpha-smooth muscle actin. The following panels (Figures 1D–M) represent the UMAP superimposed with expression profiles of genes of interest, with cell type-specific percentage expression reported in Supplementary Table S2.
Figure 1
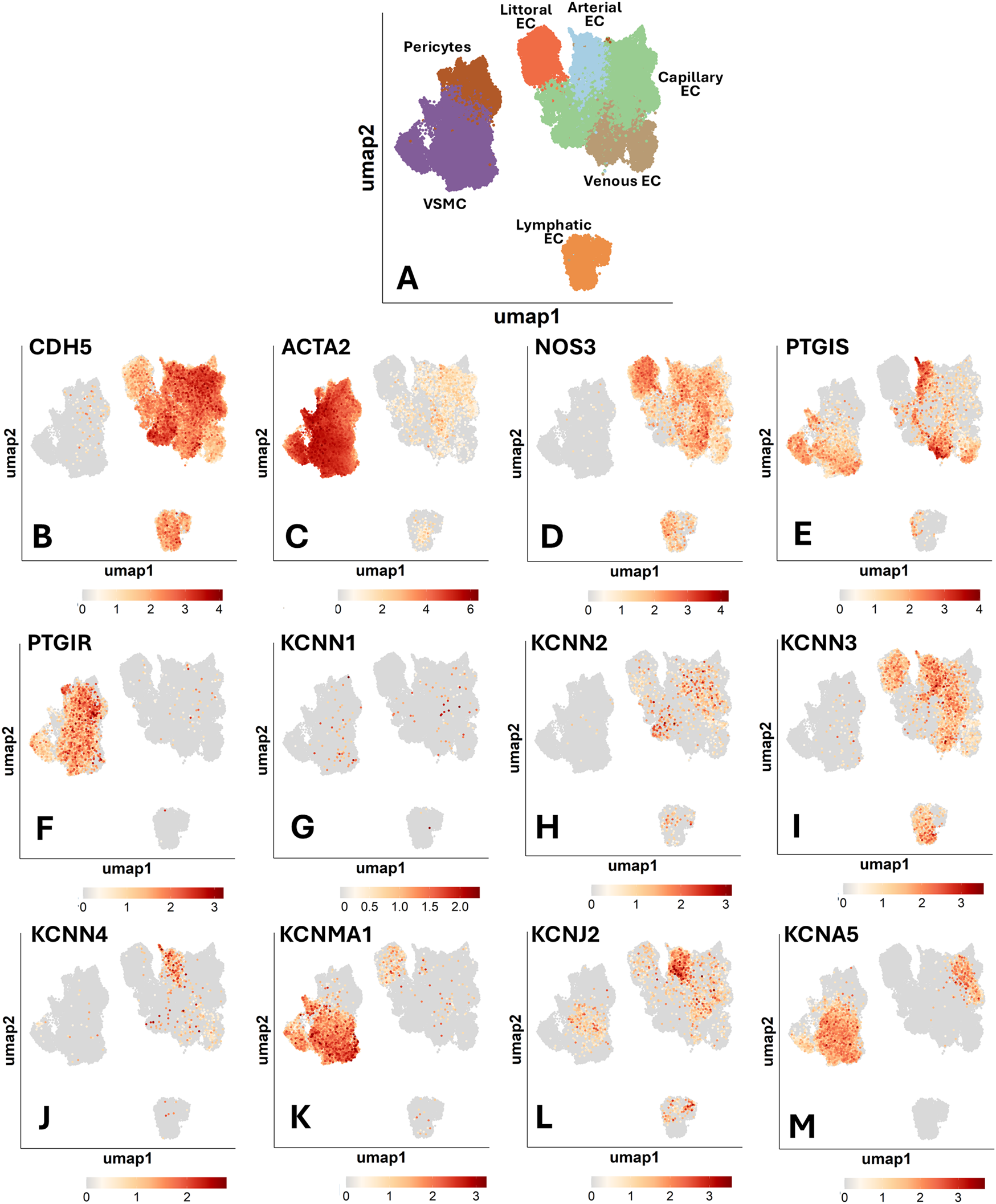
Uniform manifold approximation and projection (UMAP) representations of single-cell profiles and gene expression, side by side. The red scale indicates the level of gene expression. (A) UMAP representations of single-cell profiles. (B) CDH5; (C) ACTA2; (D) NOS3; (E) PTGIS; (F) PTGIR; (G) KCNN1; (H) KCNN2; (I) KCNN3; (J) KCNN4; (K) KCNMA1; (L) KCNJ2; (M) KCNA5.
Nitric oxide synthase
The expression of eNOS was reported to be highly restricted to the endothelium of medium to large arterial blood vessels (21). We observed a marked variation in the abundance of NOS3-positive ECs across the different vascular territories (Figure 1D). When considering the frequency of positive cells, a term used hereon to indicate the proportion of cells with detected expression of a given gene, we found that littoral ECs, spleen sinusoids-lining cells specialized in scavenging senescent blood cells, were the most copious in NOS3 transcripts (62% positive cells). They were followed by venous ECs (33%), arterial ECs (27%), and capillary and lymphatic ECs (both 16%).
Prostacyclin and cognate receptor
PTGIS (Prostacyclin/Prostaglandin I2 synthase) and PTGIR (Prostacyclin/ prostaglandin I2 receptor) are reportedly localized in both ECs and VSMCs (22, 23). As shown in Figure 1E, single-cell analysis revealed a distinct expression pattern for PTGIS, which exhibited the highest frequency in arterial and venous ECs (25% and 27%, respectively). In contrast, PTGIS was low-abundant in capillary (3%), lymphatic (2%) and littoral ECs (<1%). Among mural cells, VSMCs expressed PTGIS (22%), whereas pericytes were negative. On the other hand, PTGIR expression was enriched explicitly in mural cells, with similar frequencies in pericytes and VSMCs (17% and 18%, respectively) (Figure 1F). Again, these data support an angiotypic heterogeneity with a distinct pattern compared with NOS3.
Potassium channels
Within the category of potassium channels (Figures 1G–M), KCNN1 (SKCa1) and KCNN2 (SKCa2) demonstrated low overall expression levels. Regarding the other expressed channels, KCNN3 (SKCa3) was variably expressed among the EC subtypes, with the highest frequency in arterial ECs (23%), followed by littoral ECs (17%), lymphatic ECs (15%), venous ECs (12%), and capillary ECs (5%). In contrast, it was absent in mural cells. Both Kir and Kv channels play a key role in vascular physiology. We found that KCNN4 (IKCa) and KCNJ2 (Kir2.1) were mainly expressed by arterial ECs (8% and 19%, respectively). KCNMA1 (BKCa) was expressed in VSMCs (37%) and was low-expressed in ECs and pericytes. Kv channels are encoded by a large set of KCNx genes. We found that, within this channel family, only KCNA5 (Kv1.5) was detectable in the Human Vascular Atlas database, being expressed in VSMCs (32%) and to a much lesser extent in capillary ECs (2%). Following a specific request from one reviewer, we have examined the expression of KCNQ channels (Kv7), voltage-gated, phosphatidylinositol 4,5-bisphosphate (PIP 2-) modulated K+ channels that play essential roles in regulating the activity of neurons and cardiac myocytes, but also vascular cells. The data showing low expression levels of these channels in the general cell population are reported in Supplementary Table S2. In summary, K+ channels are expressed differently depending on the subtype of vascular cell population.
Gene co-expression
The expression of eNOS is generally considered typical of endothelial cells. In line with this, NOS3-expressing ECs were also positive for canonical endothelial markers, such as PECAM1 and EGFL7. However, as inferred from the percentages mentioned above and illustrated in Figure 2A, a larger proportion of PECAM1- or EGFL7-positive ECs, particularly those from the capillary district, were NOS3-negative. A double negative fraction could also be detected. This data further highlights EC heterogeneity. Figure 2B shows the heatmap of NOS3 and other endothelium-related genes grouped by categorical cell information.
Figure 2
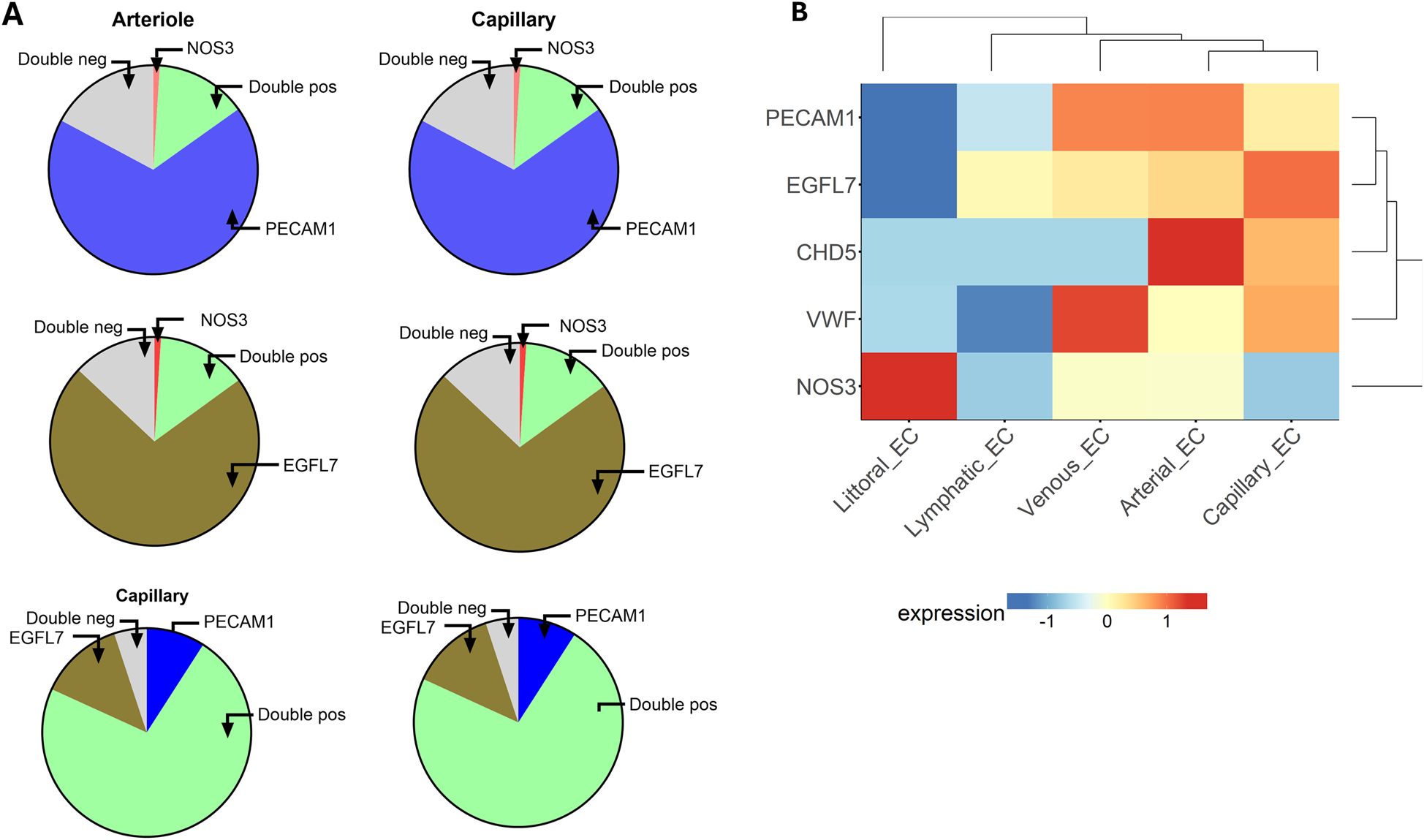
(A) The graphic illustrates the proportions of ECs expressing the indicated genes alone or in combination (double positive) or negative for both genes (double negative). Top, Expression of NOS3 and PECAM1; Middle, NOS3 and EGFL7; Bottom, PECAM1 and EGFL7. (B) Heatmap of endothelium-related genes in cells from different vascular compartments.
We next considered the co-expression levels of the most represented genes, namely NOS3, PTGIS, and KCNN3, in ECs from arterioles and capillaries. As shown in Figure 3, a fraction between 7% and 9% of arterial ECs were double positive, while more than 50% resulted double negative for any possible combinations. The co-expression dropped to 1% or less when considering capillary ECs, with 81 to 92% being double negative. In conclusion, only a modest fraction of arterial ECs, and an even lower proportion of capillary ECs, displayed detectable combinatory expression for the three considered genes.
Figure 3
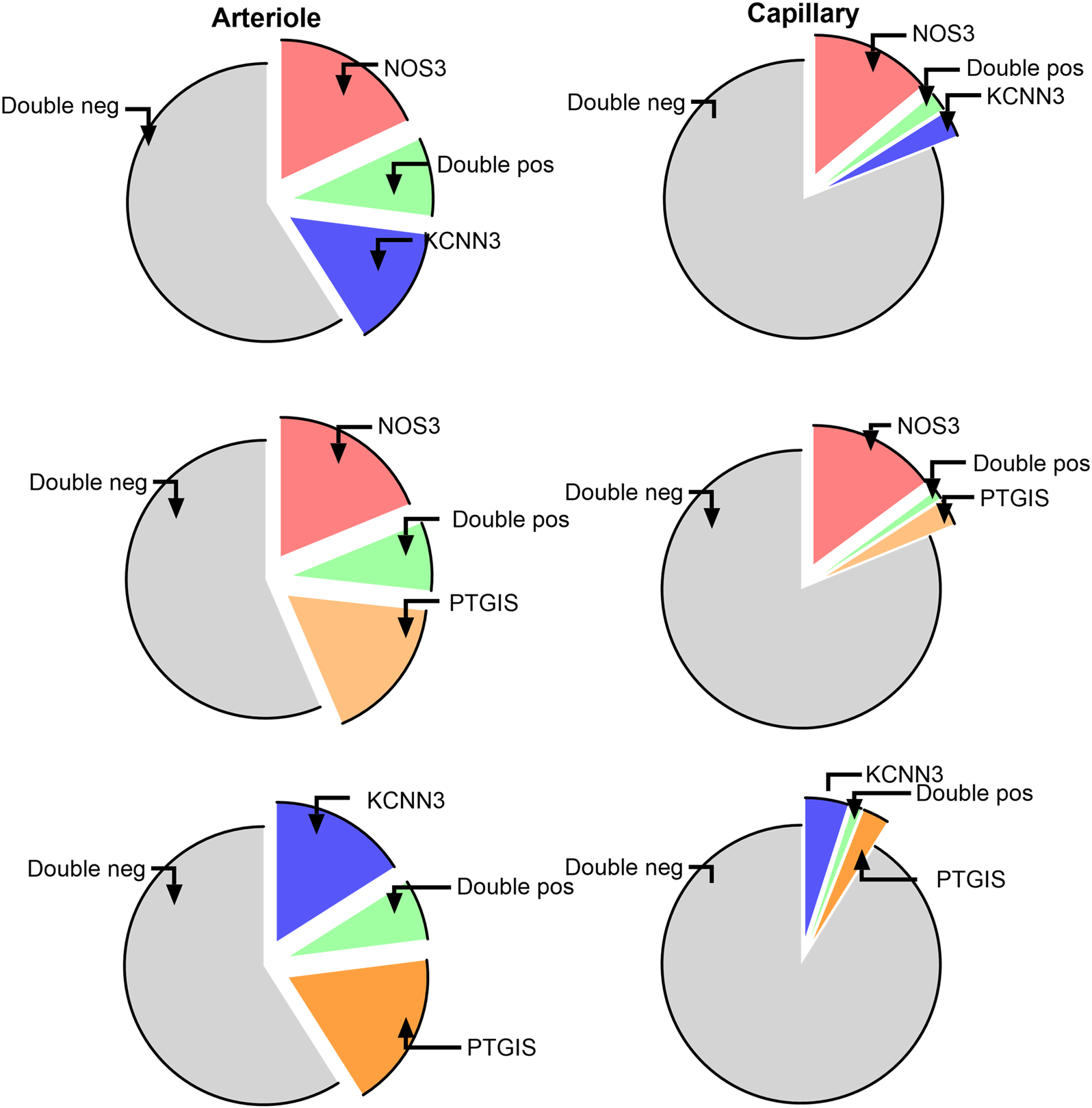
Co-expression of the most represented genes in ECs from arterioles and capillaries. TOP, NOS3 and KCNN3; Middle, NOS3 and PTGIS; Bottom, KCNN3 and PTGIS.
Clustering analysis
Next, we examined the relationship between genes and EC subpopulations, illustrated as a bubble plot (Figure 4A) and a heatmap (Figure 4B), where the colour scale indicates the gene expression level. The dendrogram displays the clustering of samples or genes. Results again highlight the wide heterogeneity of ECs, with the most considerable distance observed between capillary ECs vs. lymphatic ECs.
Figure 4
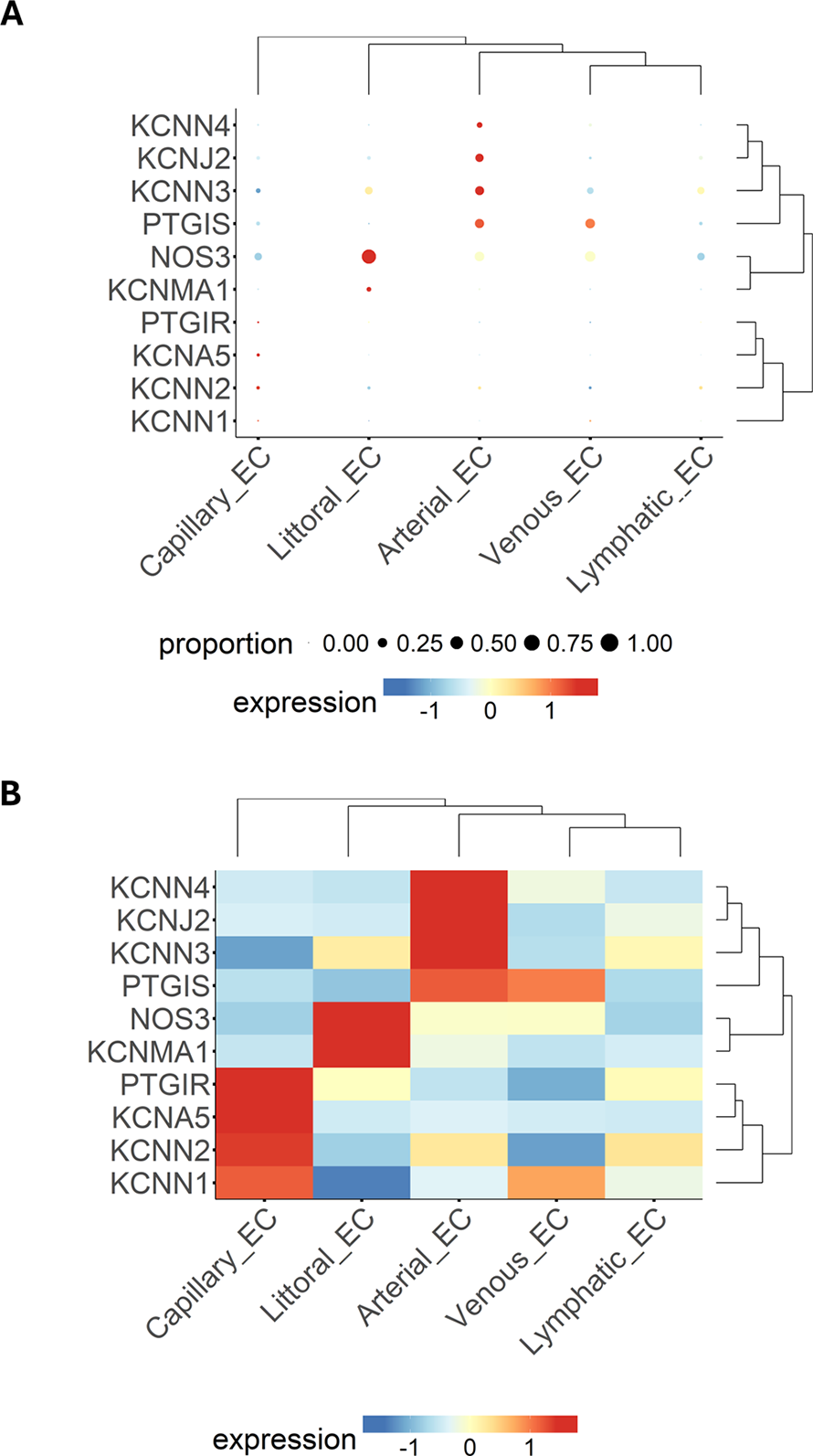
Expression patterns of multiple genes, grouped by categorical cell information, are displayed as either a bubble plot (A) or a heatmap (B). Genes and cell populations are grouped into clusters, and dendrograms illustrate their relative distance. Bubble dimensions are proportional to the number of positive cells, while the heatmap colours identify the expression level as indicated by the scale.
Changes in gene expression with ageing
Advancing age is associated with structural, molecular, and functional alterations affecting all cellular components of the vasculature (24, 25). In this context, hyperpolarization-dependent vasodilation is believed to be a backup system for decreasing NO and PGI2 production. We tested whether older age is associated with transcriptional changes at the single-cell level. To enhance the power of this analysis, we compared two cohorts aged 20 to 49 and 50 to 80 for the most relevantly expressed genes. Figure 5 shows the UMAP representation of gene expression for the two age strata side by side. These data suggest age is a contributor to vascular cell heterogeneity.
Figure 5
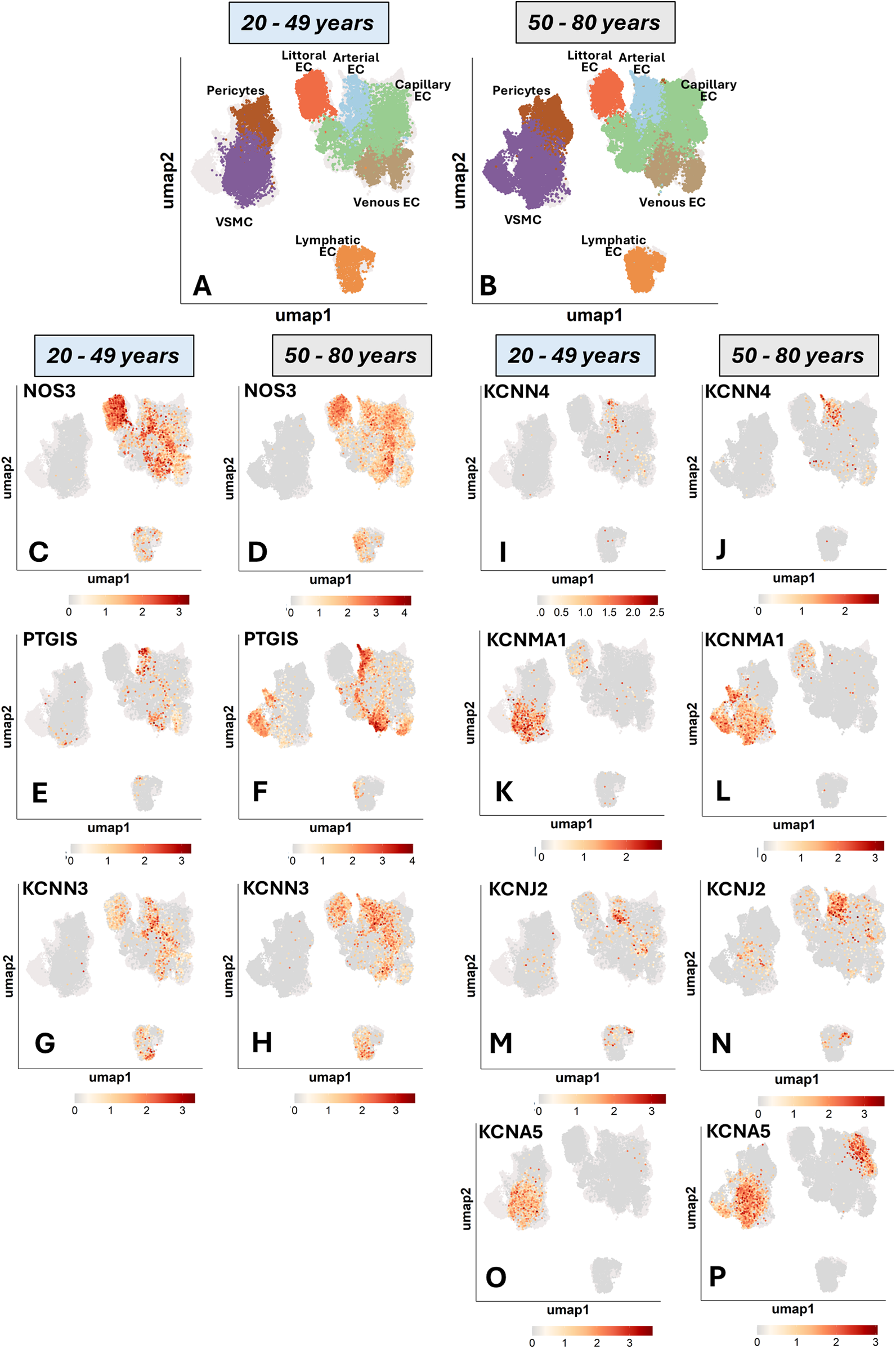
UMAP representations of single-cell profiles and gene expression. The red scale indicates the level of gene expression. (A,B): cell distribution. (C,D): NOS3. (E,F): PTIGS. (G,H): KCNN3. (I,J): KCNN4. (K,L): KCNMA1. (M,N): KCNJ2, and (O,P): KCNA5.
As shown in Figure 6A, NOS positivity was reduced by 11 per cent units in littoral ECs (67% in the younger group and 56% in the older group) and 10 units in venous ECs (40% in the younger group and 30% in the older group). The frequency of NOS3-positive capillary ECs remained similar between the two groups, whereas lymphatic ECs expressing NOS3 were 5 units higher in the old group.
Figure 6
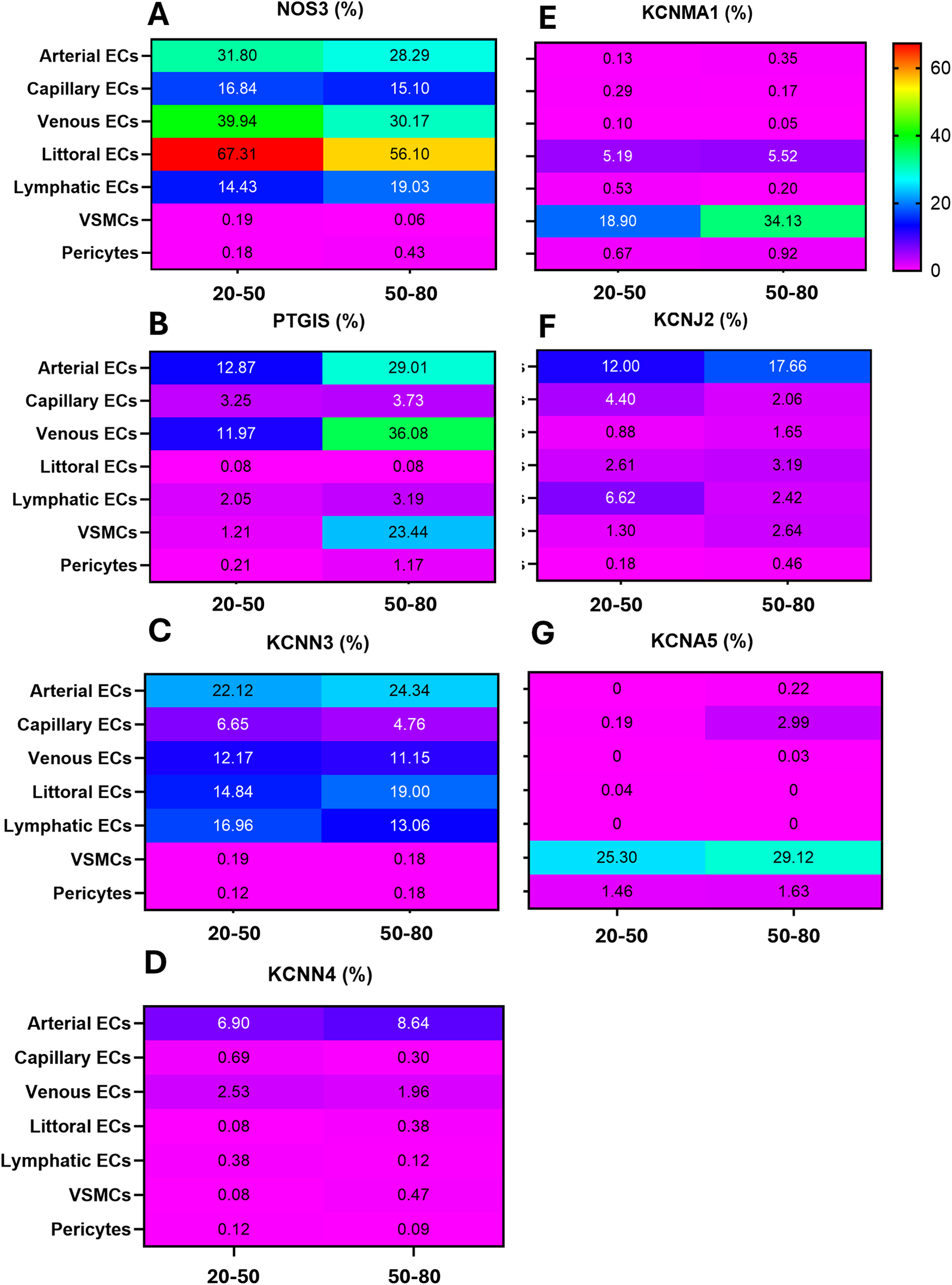
The rainbow heatmap shows the percentage of cells that express a given gene. (A) NOS3; (B) PTGIS; (C) KCNN3; (D) KCNN4; (E) KCNMA1; (F) KCNJ2; (G) KCNA5.
In contrast to the overall eNOS downregulation, PTGIS frequency increased with age in arterial ECs (16 units), venous ECs (14 units), and particularly in VSMCs, which exhibited a 22-unit elevation. In contrast, PTGIS was low-abundant in capillary, littoral, lymphatic ECs, and pericytes from both age groups (Figure 6B).
Next, we examined the relationship between age and K+ channels (Figures 6C–G). Among genes typically expressed by ECs, KCNN3, KCNN4, and KCNJ2 showed modest and variable differences between groups. Regarding mural cells, KCNMA1 and KCNA5 were 15 and 4 units higher in the VSMCs of the older group compared with the young group.
Organotypic differences
Finally, we extracted and analyzed the data concerning ECs and VSMCs obtained from coronary arteries, the brain, and the uterus. Supplementary Table S3 reports the percentage of cells expressing a given gene. Figure 7 shows the UMAP representation of single-cell profiles. Figure 8 provides a violin plot representation of data distribution across different cells and organs.
Figure 7
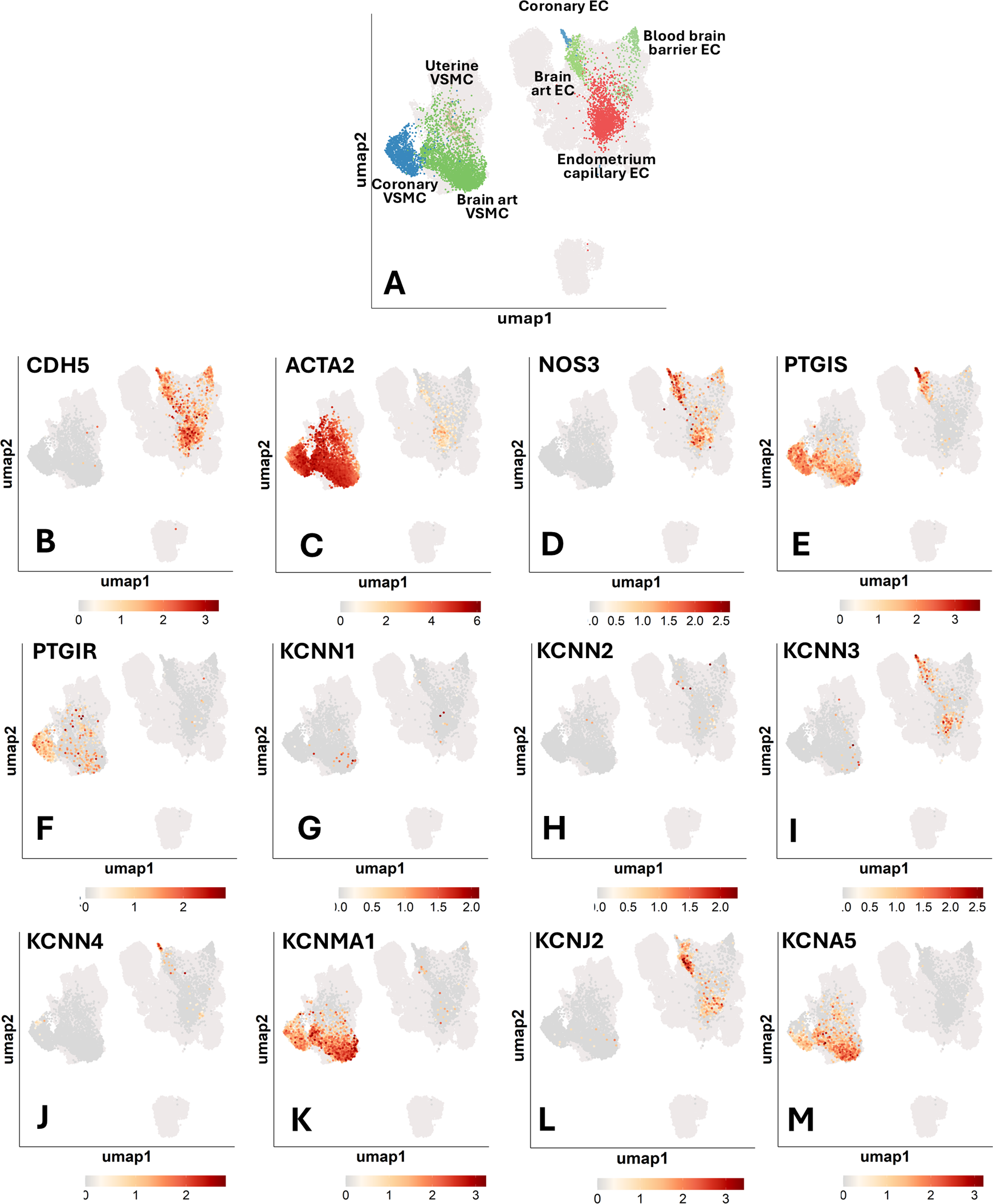
Single-cell transcriptomics of human vascular cells from the coronary arteries, brain, and uterus. UMAP representations of single-cell profiles and gene expression, side by side. The red scale indicates the level of gene expression. (A) UMAP representations of single-cell profiles. (B) CDH5; (C) ACTA2; (D) NOS3; (E) PTGIS; (F) PTGIR; (G) KCNN1; (H) KCNN2; (I) KCNN3; (J) KCNN4; (K) KCNMA1; (L) KCNJ2; (M) KCNA5.
Figure 8
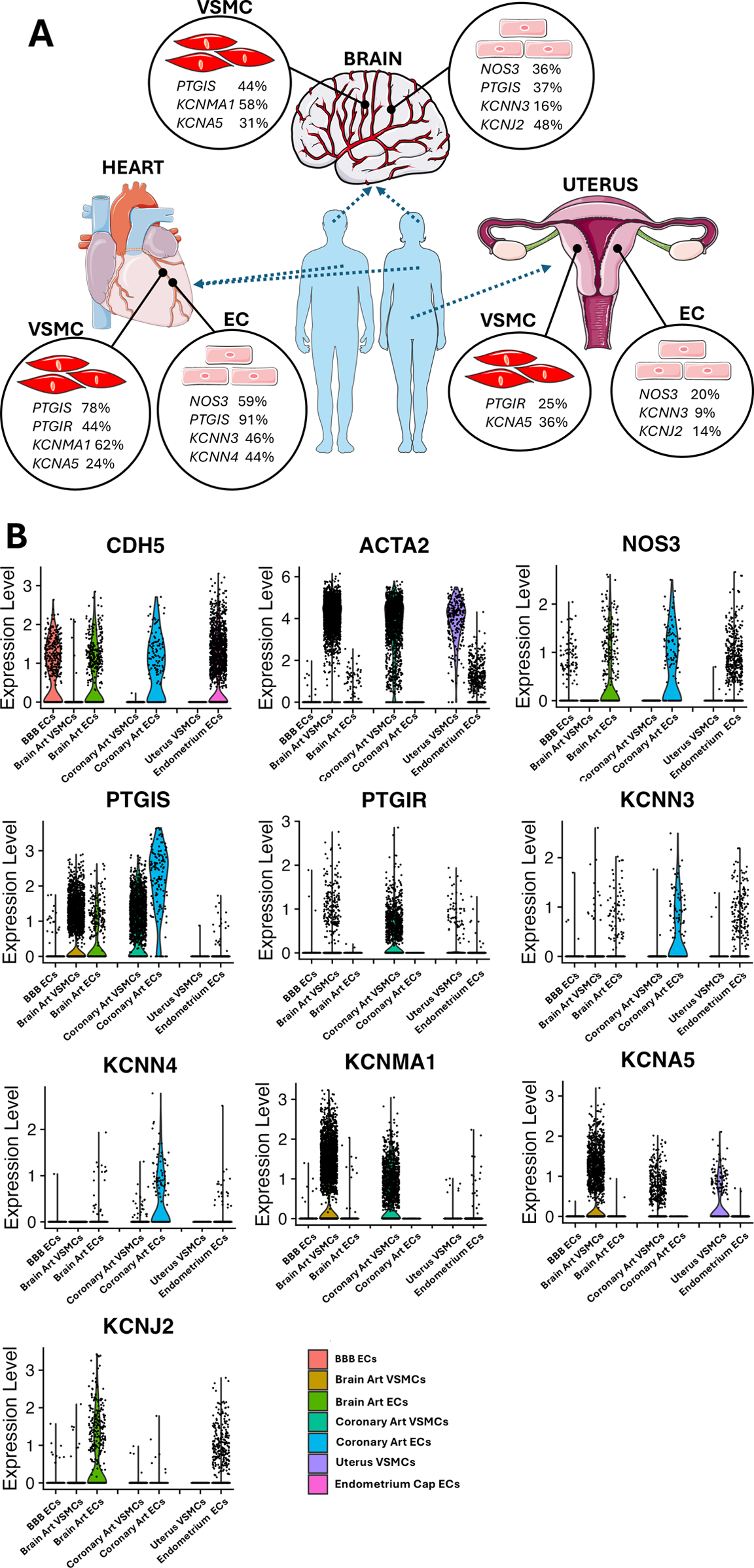
(A) Representative image highlighting the vascular gene transcripts that characterize each organ. Images from “Smart Servier Medical Art: Uterus”, “Heart”, “Brain circulation”, “Endothelium”, “Smooth muscle cell”, “Blue man shape” and “Human shape”, licensed under CC BY 4.0. (B) Coloured violin plot shape area represents the distribution of the expression within each condition tested. Several genes lack a coloured ‘violin’ shape and have instead a vertical line, which indicates that the overall expression of that particular gene is zero in most of the cells. Genes shown are in order: CDH5; ACTA2; NOS3; PTGIS; PTGIR; KCNN3; KCNN4; KCNMA1; KCNA5; KCNJ2.
NOS3 was found to be expressed in ECs, with decreasing frequency in coronary ECs (59%), brain artery ECs (36%), blood-brain barrier (BBB) ECs (24%), and endometrium capillary ECs (20%) (Figure 7D). PTGIS exhibited a distinct expression frequency in arterial ECs and VSMCs from coronary arteries (91% and 78% positive cells, respectively) and brain (37% and 44% positive cells, respectively), whereas it was low expressed in BBB ECs (5% positive cells) and nearly absent in the endometrium capillary ECs and uterine VSMCs (Figure 7E). The PGI2 receptor gene PTGIR was exclusively expressed in VSMCs from coronary arteries (44%), uterus (25%), and brain (6%) and absent in ECs (Figure 6F). KCNN3, KCNN4, KCNMA1, KCNJ2 and KCNA5 emerged as the most predominant K+ channels, whereas KCNN1 and KCNN2 were expressed at very low levels (Figures 7G–M). In particular, KCNN3 was present in all ECs, with the greatest frequency in coronary artery ECs (46%) followed by brain artery ECs (16%) and endometrium capillary ECs (9%). KCNN4 exhibited a similar expression pattern, with higher values in coronary ECs (46%), followed by brain artery ECs (5%), and negligible levels in other tissues. KCNMA1 and KCNA5 were identified in the VSMCs of the coronary arteries (62% and 24%, respectively) and brain (58% and 31%, respectively). Moreover, KCNA5 was detected in VSMCs from the uterus (35%), KCNJ2 was mainly expressed in brain artery ECs (48%), followed by endometrium capillary ECs (14%), coronary ECs (5%) and BBB ECs (3%).
As shown in Figure 8, an organotypic specification is supported by the strikingly higher abundance of NOS3 and PTGIS in coronary artery ECs (59% and 91% positive cells, respectively) as compared to the levels found in the global EC spectrum (27% and 24%, respectively). A similar enrichment was also observed regarding the K+ channels KCNN3 and KCNN4, each being expressed in 46% of coronary artery ECs but only in 23% and 8% of the global ECs. Further evidence for an organotypic identity is provided by the data extracted from coronary artery and brain artery VSMCs. For instance, PTGIS was remarkably more frequent in coronary artery VSMCs (78%) and brain artery VSMCs (43%) as compared with global VSMCs (21%). A similar trend was observed when comparing the abundance of PTGIR transcripts in coronary artery VSMCs (44%) and global VSMCs (18%). A similar organ-specific enrichment was observed concerning KCNMA1 for both coronary artery and brain artery VSMCs (62% and 57%, respectively vs. 37% in global VSMCs) and KCNJ2 limited to coronary artery VSMCs (48% vs. 2% in global VSMCs). Although to a lesser extent, KCNQ1 was expressed with higher frequency (between 10% and 15%) by brain vascular cells compared with the coronary artery and uterine vascular cells (Supplementary Table S3).
Clustering analysis
As shown in Figures 9A,B, coronary artery ECs exhibited a distinct expression pattern compared to brain ECs, BBB ECs, and endometrium ECs. A remarkable difference was that coronary artery ECs were abundant in NOS3 and PTGIS and showed a higher frequency and intensity for KCNN3 and KCNN4. In contrast, brain ECs were characterized by a moderate abundance of NOS3, PTGIS, KCNN3, and KCNJ2, with the latter forming a cluster with a low-abundance, high-intensity gene set formed by KCNN2, KCNA5, and KCNMA1. BBB ECs showed higher PTGIR expression, although at low frequency levels. Endometrium capillary ECs exhibited moderate abundance of NOS3, KCNN3, and KCNJ2, along with the KCNN1 gene expressed with high intensity but low frequency. Moreover, a considerable heterogeneity was identified when comparing VSMCs from the three districts (Figures 9C,D). Coronary artery VSMCs showed a cluster formed by PTGIS and PTGIR, together with KCNN4, which was, however, expressed only at low frequency. Brain artery VSMCs displayed two distinct clusters, namely PTGIS, KCNMA1, and KCNJ2 (the latter being very low abundant but intensely expressed), and KCNN1, KCNN3 and KCNA5 (the first two exhibiting low abundance and intense expression, and the last one being both abundant and intensely expressed). Uterine VSMCs could be distinguished by a low-frequency/intense expression cluster formed by NOS3, KCNN2, and KCNN3. Moreover, they also showed intense and abundant expression levels regarding KCNA5.
Figure 9
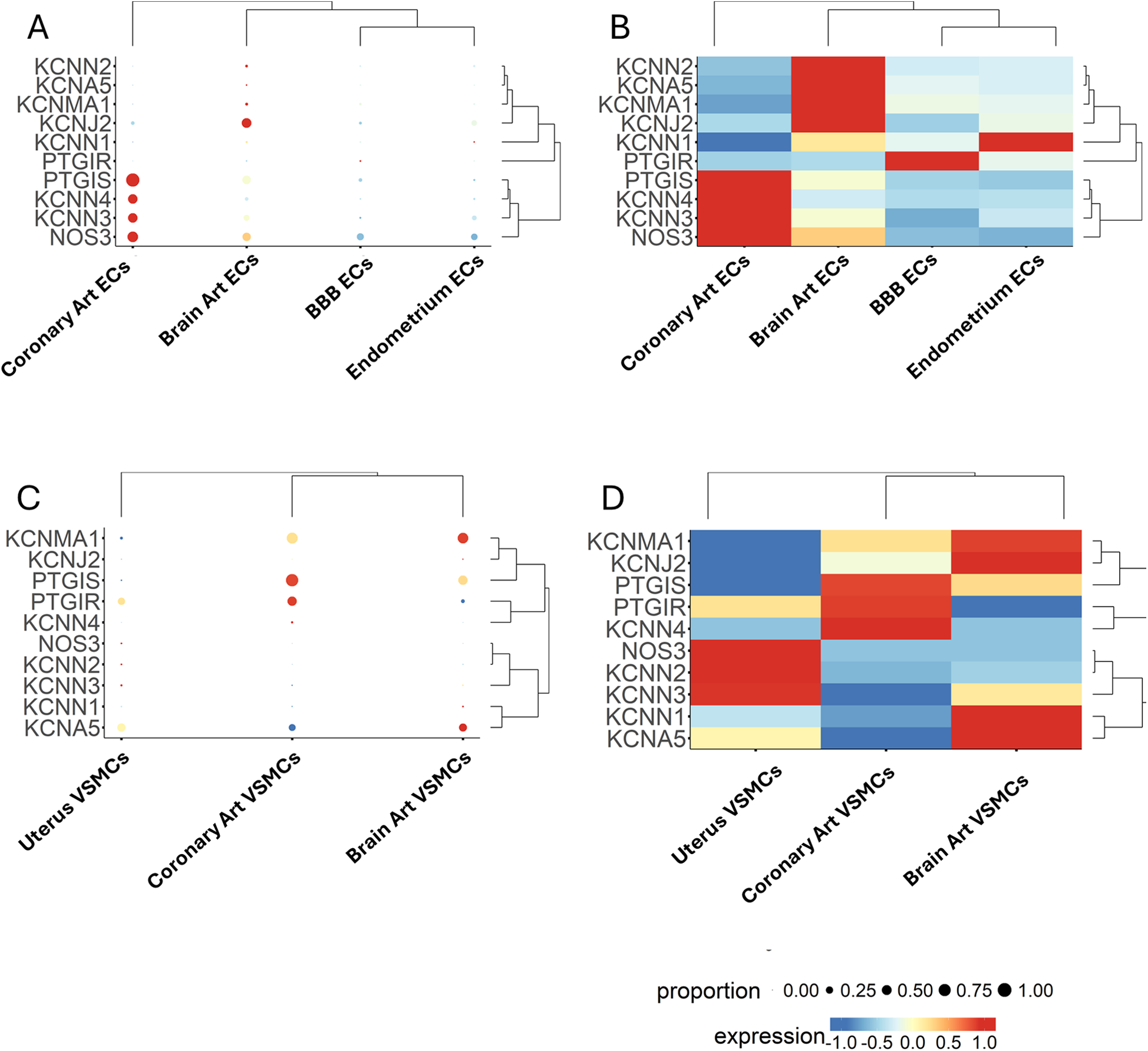
Expression patterns of multiple genes, grouped by categorical cell information, are displayed as either a bubble plot (A, C) or a heatmap (B, D). Genes and cell populations are grouped into clusters, and dendrograms illustrate their relative distance. Bubble dimensions are proportional to the number of positive cells, while the heatmap colours identify the expression level as indicated by the scale.
Discussion
This study examined single-cell transcriptomics of essential genes linked to vasorelaxation. Results indicate a considerable diversity at both the organismal and tissue levels, with age also contributing to variability.
Angiotypic and organotypic diversity: can these concepts be harmonized?
Recent advancements in single-cell genomics and biological network analysis have significantly enhanced our understanding of the variety, flexibility, and modularity of vascular cells (26). These emerging technologies could also help to understand whether the transcriptional profile of vascular cells is tailored to specific organs and tissues or adheres to a more generalized angiotypic program. In a recent groundbreaking study, Barnett and colleagues reported that the global transcriptomic profile of a wide range of human vascular cells primarily corresponds to an angiotypic identity (20). These data contradict previous findings about mouse vascular endothelium, where an organotypic identity reportedly predominated (27). A targeted analysis of specific functions could help resolve the dispute. In this context, we examined the single-cell transcriptomics of key genes related to vasorelaxation in arterial and capillary ECs and arterial VSMCs at both the organismal- and tissue-specific levels. In addition, the expression of the same genes was analyzed in venous, lymphatic, and littoral ECs and pericytes.
Expressional heterogeneity of redundant vasorelaxant pathways
Three separate yet interactive processes regulate vasorelaxation: the NO-cGMP pathway, the PGI2-cAMP pathway, and the hyperpolarization-K+ channels pathway. Cellular heterogeneity and modular redundancy collaborate in enhancing the functional flexibility and adaptability of the vascular system from large arteries to arterioles and capillaries (28). Accordingly, the proportional expression of the three pathways is anticipated to differ along the vascular tree. NO, a crucial signaling molecule, is synthesized by three NOS isoforms, all of which employ L-arginine and molecular oxygen as substrates (29). NOS1 is pivotal in central and peripheral neurons. Its roles encompass the regulation of synaptic plasticity, central control of blood pressure, and vasodilation through peripheral nitrergic neurons. Inducible NOS2 can be expressed in several cell types in response to lipopolysaccharide, cytokines, or other stimuli. It produces substantial amounts of NO, exerting cytostatic effects on parasite target cells. NOS3 is predominantly present in the endothelium. It maintains vasodilation, regulates blood pressure, and possesses several additional vasoprotective effects. We verified the dominant expression of NOS3 in ECs identified by the co-expression of PECAM1 and EGFL7. All other isoforms were not expressed (less than 1% of the cell fraction) except for NOS1 in arterial ECs (7% positive cells) and NOS2 in lymphatic ECS (3% positive cells) (data not shown). Furthermore, the results reveal a remarkable angiotypic variability in NOS3 expression. Littoral ECs exhibited the most abundant expression of NOS3 relative to other EC subtypes. This finding aligns with the fundamental roles of littoral ECs, which filter for antigens, pathogens, and senescent red blood cells, utilizing NO as a permeabilizing and cytotoxic agent (30, 31). The observed abundance of NOS3 transcripts in arterial ECs compared with capillary ECs corresponds with prior findings indicating that this enzyme is predominantly confined to the endothelium of medium to large arterial blood arteries (21). A notable percentage of PECAM1- or EGFL7-positive ECs, especially those from the capillary region, were negative for NOS3. Additional research is required to ascertain the transcriptional repressor limiting NOS3 expression, and to understand whether NOS3-negative cells can be transcriptionally recruited to augment NO synthesis in response to environmental cues, along with gene expression upregulation in constitutively NOS3-positive ECs.
The diverse properties of ECs were corroborated in relation to PTGIS, which had the highest frequency in arterial and venous ECs, while exhibiting low abundance in capillary, lymphatic, and littoral ECs. PTGIS enables to differentiate between VSMCs, positive for this enzyme, and pericytes, which are negative. PTGIR was exclusively expressed in mural cells, exhibiting comparable frequencies in VSMCs and pericytes. By engaging its corresponding receptors, PGI2 exerts cardiovascular actions, including vasodilation and the suppression of VSMC proliferation (32). Moreover, PGI2 is said to influence pericytes to preserve vascular barrier integrity and capillary perfusion (33).
Regarding endothelial K+ channels, we observed a decrease in KCNN3 and KCNJ2 from arterial to capillary ECs. At the same time, the muscular K+ channels KCNMA1 and KCNA5 exhibited a greater abundance in VSMCs than in capillary pericytes. These data are compatible with the major role of muscular arterioles in regulating vascular resistance, with capillaries playing a subsidiary contribution through the contractile activity of pericytes.
Co-expression and clustering analyses further validated the heterogeneity of arterial and capillary ECs. The data indicated that, within each EC subgroup, the most prevalent genes—NOS3, PTGIS, and KCNN3—were infrequently co-expressed, with over 50% of the cells exhibiting negativity for any of the examined genes. In resistance arteries, elevated endothelial Ca2+ levels facilitate vasodilation by activating SKCa/IKCa channels in certain vascular beds and stimulating eNOS in others (34). An interaction between the two pathways can amplify induced vasorelaxation. For instance, endothelial SKCa and IKCa channel activators have been demonstrated to augment agonist-induced membrane hyperpolarization, Ca2+ transients, and the de novo NO generation (34). Based on our findings on limited co-expression, it is plausible to hypothesize that the modularity of vasorelaxant processes is governed by the interaction among various ECs rather than within a distinct group of ECs co-expressing all three genes.
Age-related decline in vasorelaxant gene expression
Increased age correlates with endothelial dysfunction. The possibility that hyperpolarization-dependent vasodilation may compensate for reduced NO generation is intriguing. However, studies in experimental animal models suggest that a diminished expression and activity of K+ channels could also contribute to the dysfunction of older blood vessels (35).
In our study, NOS3 expression was consistently reduced in various EC types from the older cohort, while PTGIS frequency significantly escalated with age in arterial and venous ECs, and VSMCs. Similarly, KCNJ2, which encodes the Inward Rectifier Potassium Channel 2 and is regarded as a facilitator and maintainer of hyperpolarization, exhibited a significant increase in arterial ECs. The large-conductance KCNMA1 was almost twice as frequent in VSMCs of the older group compared to the younger group.
Organotypic specification
The transcriptome analysis revealed similarities and disparities across vascular cells from the brain, heart, and uterus. NOS3 exhibited a threefold greater frequency in coronary artery ECs than endometrium-derived ECs. Similarly, the brain and cardiac endothelium had a higher frequency of PTGIS, starkly contrasting ECs originating from the uterus. Likewise, significant variations in expression were noted with VSMCs. For instance, VSMCs from cerebral and cardiac arteries had high expression levels of PTGIS, whereas uterine VSMCs were devoid of this gene expression. The PTGIR gene was predominantly expressed in coronary and uterine VSMCs compared to cerebral VSMCs.
There is an increasing interest in the organotypic identity of K+ channels, mainly fuelled by the potential for targeted therapeutic applications. KCNN3 was a common component across the three studied organs, whereas KCNJ2 was found in cerebral and endometrium capillary ECs, albeit more extensively in the former. The big conductance BK channels, composed of pore-forming α subunits (BK-α, encoded by the KCNMA1 gene) and regulatory β1 subunits (BK-β1, encoded by the KCNMB1 gene), are prevalent in coronary arteries and modulate myocardial perfusion by linking intracellular Ca2+ homeostasis with excitation-contraction coupling (36–39). The voltage-dependent K+ (KV) subtype 5 channels, encoded by KCNA5, play a crucial role in regulating the precise coupling of coronary blood flow to myocardial metabolism (40). Our findings validated the elevated prevalence of KCNMA1 and KCNA5 gene transcripts in coronary artery VSMCs. This pattern was notably detected in brain VSMCs, but not in uterine VSMCs, which were abundant in KCNA5 but displayed a low frequency of KCNMA1. Evidence suggests that K+ channels play a crucial role in modulating uterine vascular tone and responding to pregnancy and hypoxia (41). Alterations in the expression of these channels have been documented during pregnancy and are regarded as potential therapeutic targets for pregnancy-related disorders (42). Elevated NO production and augmented endothelium-derived hyperpolarizing factor-mediated responses, chiefly via the activation of small and intermediate conductance K+ channels, significantly contribute to endothelium-dependent vasorelaxation of the uterine artery in nonpregnant rats, rising to approximately 70% in pregnant rats (43). Our observations regarding the abundant expression of KCNA5, which encodes the Voltage-Gated Potassium Channel Protein Kv1.5, suggest this channel may have a physiologic role in regulating uterine circulation. Seminal research in non-pregnant sheep showed that the administration of 4-aminopyridine (4-AP), a selective Kv channel blocker, did not affect the baseline uterine blood flow but diminished the estradiol-induced increase in uterine blood flow (44). Dysfunction of Kv channels may contribute to pregnancy complications, as indicated by findings showing hypoxia specifically diminished Kv currents assessed via the patch clamp technique in VSMCs from fetoplacental arteries and triggered vasoconstriction in placental cotyledons (44).
Comparison with global levels confirms organotypic identity
An additional insight favoring organotypic identity is provided by the large enrichment of several genes in ECs and VSMCs from coronary and brain arteries compared with organismal expression levels. For instance, we observed a striking enrichment in NOS3, PTGIS, and PTGIR in coronary artery vascular cells, accompanied by a higher abundance of K+ channels, specifically KCNN3, KCNN4, KCNMA1, and KCNJ2. These data are consistent with the potent vasorelaxant capacity of coronary arteries through multiple, redundant mechanisms. A similar, though lesser enrichment was observed in vascular cells from the brain circulation.
Therapeutic perspectives
There is a great interest in the possibility that single-cell transcriptomics would provide a means to refine personalized treatments. Based on patients' specific requirements, one can opt for a generalized strategy addressing all primary vasorelaxant mechanisms or focus on certain vascular beds informed by identifying angiotypic and organotypic variability. Given its preliminary nature, we are far from claiming that this study can guide a personalized approach. However, in this section, we would like to present how a better understanding of vascular cells' heterogeneity may provide an alternative to complex multidrug treatments. The recent Lacunar Intervention Trial-2 (LACI-2) shows a global methodology (45). This interventional trial aimed to administer increasing dosages of isosorbide mononitrate, a NO donor, and Cilostazol, a phosphodiesterase type 3 inhibitor that enhances the prostacyclin-cAMP pathway in stroke patients. The findings demonstrate that the treatment diminished the risk of significant vascular incidents and improved functional and cognitive results relative to single-agent therapy, while maintaining a favorable safety profile (45). Rinvecalinase alpha (DM199) is a recombinant variant of the endogenous protein tissue kallikrein, presently undergoing trials in patients with strokes and complicated pregnancies (46). It is a bioactive kinin peptide source that concurrently activates the three principal vasorelaxant systems (47). On the other hand, K+ channel modulators offer a novel and appealing approach to target specific arterial beds selectively. Endothelial SKCa and IKCa activators may be appropriate for treating vascular problems in the brain, heart, and uteroplacental unit. Small-molecule activators of KCa2.x and 3.1 channels, including SKA-31, can rapidly suppress myogenic tone in isolated resistance arteries, promote significant vasodilation in intact vascular systems like the coronary circulation, and promptly reduce systemic blood pressure in vivo (48). At the same time, BKCa agonists may be effective for targeting the brain or heart specifically. Several clinical trials using BKCa openers for cardiovascular conditions, including hypertension, ischemic heart disease, stroke, and erectile dysfunction, have been commenced in recent decades. Nevertheless, the inadequate potency and lack of selectivity of these compounds led to the premature cessation of these trials (49). Only one medication candidate, andolast, targeting BKCa channels, remains in clinical development for the treatment of asthma (50). Voltage-sensitive Transcriptional regulators are also appealing. The nuclear factor erythroid 2-related factor 2 (Nrf2) has been shown to upregulate BKCa expression by directly binding to the antioxidant response element (ARE) motif of the KCNMA1 promoter, suggesting that treatment with FDA-approved Nrf2 activators may be an effective strategy for enhancing the treatment of coronary artery disease (38). Finally, activators of Kv channels could provide a means to induce extended vasorelaxation. We have demonstrated that VSMCs from coronary, cerebral, and uterine arteries express KCNA5, which encodes the Kv1.5 potassium channel. Recent studies have proposed Kv7 channels, encoded by KCNQ 1–5 genes, as promising new therapeutic targets to increase fetoplacental blood flow in preeclampsia (51). Nonetheless, this gene family was lowly expressed in VSMCs from our series, except KCNQ1, which was found to be expressed by more than 10% of brain vascular cells.
Conclusions and study limitations
Our data reveal considerable heterogeneity in the expression of vasorelaxant pathway components among vascular cells throughout the arterial tree and different organs. K+ channels and the PGI2-generating enzyme are posited to gain prominence in the ageing vasculature, perhaps offsetting the reduced expression of NOS3. K+ channels are a vital therapeutic target to improve the effectiveness of existing treatments utilizing NO donors and PGI2 analogues.
The study has several limitations intrinsic to its explorative nature. We analyzed a few “sentinels” of relaxation, which may represent a simplification of the complex transcriptomic scenario. This initial attempt justifies additional research to discern the complexity through the inclusion of other vasorelaxant mediators. Similarly, it is premature to infer the physiological implications of these novel data within the enormous body of knowledge on human vascular tissue and bulk cell preparations. A comprehensive discussion on functional repercussions should be deferred until additional data on vascular cells from many human subjects under physiological and pathological settings are available. This will allow for the understanding of how the modification of gene expression through the recruitment or derecruitment of single cell populations for a specific gene correlates with functional changes. Additional restrictions arise from the source database's cell count, which impeded the incorporation of covariates. Rather than examining expression variations across age percentiles, we analyzed two age groups below and above 50. More ageing research will be attainable as the Atlas data is anticipated to be expanded with additional contributions. Another limitation of single-cell data is that the low overall depth of sequencing per cell means that several “drop-out” genes, which are expressed in cells albeit at low levels, are not apparent in the final data. Single-cell sequencing has lower read depth and capture efficiency compared to bulk RNA sequencing or conventional PCR, making it harder to detect lowly expressed genes (52). Deepening the search from general cell populations into cells from specific organs may rule out the possibility of missing relevant gene transcripts. This was the case of the Kv7 channels, a type of voltage-gated K+ channel that plays a crucial role in regulating neuronal excitability and vascular cells' control of blood flow. Their expression in brain vascular smooth muscle cells and endothelium contributes to regulating vessel tone and maintaining the blood-brain barrier (53, 54). Similarly, Kv7 channels have been detected in coronary arteries of animal models and human chorionic plate arteries (51, 55). An analysis of the general cell population indicated that Kv7 gene transcripts were lowly expressed. However, when looking at specific organs, we found an enrichment of the encoding gene transcripts in brain vascular cells at least for the KCNQ1 gene, while remaining low for KCNQ 2–5.
Finally, transcriptome data should be supplemented and validated by evaluating protein levels, potentially integrated with topographic analysis as shown in spatial transcriptomics and proteomics, and epigenetic mechanisms. Despite these limitations, our work lays the groundwork for formulating innovative hypotheses regarding vascular cell heterogeneity, which could potentially benefit patients. The most intriguing inquiries for future investigation involve the requisite number of additional vascular cells necessary to express the examined gene to observe alterations in vasorelaxation, and whether this objective can be therapeutically attained through transcriptional inducers, either independently or in conjunction with traditional activating ligands.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
EA: Data curation, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition. SP: Writing – original draft, Data curation, Writing – review & editing, Methodology, Conceptualization, Software. DW: Writing – original draft, Writing – review & editing. PM: Writing – review & editing, Writing – original draft, Funding acquisition, Investigation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the British Heart Foundation (FS/IBSRF/23/25173, to EA and PM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1634645/full#supplementary-material
References
1.
Augustin HG Koh GY . A systems view of the vascular endothelium in health and disease. Cell. (2024) 187(18):4833–58. 10.1016/j.cell.2024.07.012
2.
Ito M Okamoto R Ito H Zhe Y Dohi K . Regulation of myosin light-chain phosphorylation and its roles in cardiovascular physiology and pathophysiology. Hypertens Res. (2021) 45(1):40–52. 10.1038/s41440-021-00733-y
3.
Welsh DG Tran CHT Hald BO Sancho M . The conducted vasomotor response: function, biophysical basis, and pharmacological control. The Annual Review of Pharmacology and Toxicology. (2017) 58(1):391–410. 10.1146/annurev-pharmtox-010617-052623
4.
Kang Y Liu R Wu J Chen L . Structural insights into the mechanism of human soluble guanylate cyclase. Nature. (2019) 574(7777):206–10. 10.1038/s41586-019-1584-5
5.
Chen K Pittman RN Popel AS . Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxidants and Redox Signaling. (2008) 10(7):1185–98. 10.1089/ars.2007.1959
6.
Wang B Wu L Chen J Dong L Chen C Wen Z et al Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduction and Targeted Therapy. (2021) 6(1):94. 10.1038/s41392-020-00443-w
7.
Majed BH Khalil RA . Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. (2012) 64(3):540–82. 10.1124/pr.111.004770
8.
Wei AD Gutman GA Aldrich R Chandy KG Grissmer S Wulff H . International union of pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. (2005) 57(4):463–72. 10.1124/pr.57.4.9
9.
Dong D Bai Y Cai B . Calcium-Activated potassium channels. Adv Protein Chem Struct Biol. (2015) 104:233–61. 10.1016/bs.apcsb.2015.11.007
10.
Garland CJ Dora KA . Hyperpolarization and the endothelium. Curr Opin Physiol. (2023) 34:100674. 10.1016/j.cophys.2023.100674
11.
Alexander SPH Mathie AA Peters JA Veale EL Striessnig J Kelly E et al The concise guide to PHARMACOLOGY 2023/24: ion channels. Br J Pharmacol. (2023) 180(Suppl 2):S145–S222. 10.1111/bph.16178
12.
Kant S Sellke F Feng J . Metabolic regulation and dysregulation of endothelial small conductance calcium activated potassium channels. Eur J Cell Biol. (2022) 101(2):151208. 10.1016/j.ejcb.2022.151208
13.
Smirnov SV Tammaro P Hutchings SR Smith AL . Role of voltage-gated K+(KV) channels in vascular function. Neurophysiology. (2003) 35(3/4):234–47. 10.1023/b:neph.0000008784.83366.9a
14.
Hibino H Inanobe A Furutani K Murakami S Findlay I Kurachi Y . Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. (2010) 90(1):291–366. 10.1152/physrev.00021.2009
15.
Moshkforoush A Ashenagar B Harraz OF Dabertrand F Longden TA Nelson MT et al The capillary Kir channel as sensor and amplifier of neuronal signals: modeling insights on K+ -mediated neurovascular communication. Proc Natl Acad Sci USA. (2020) 117(28):16626–37. 10.1073/pnas.2000151117
16.
Potente M Mäkinen T . Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol. (2017) 18(8):477–94. 10.1038/nrm.2017.36
17.
Chi J Chang HY Haraldsen G Jahnsen FL Troyanskaya OG Chang DS et al Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. (2003) 100(19):10623–8. 10.1073/pnas.1434429100
18.
Aird WC . Phenotypic heterogeneity of the endothelium. Circ Res. (2007) 100(2):158–73. 10.1161/01.res.0000255691.76142.4a
19.
Chavkin NW Hirschi KK . Single cell analysis in vascular biology. Front Cardiovasc Med. (2020) 7:42. 10.3389/fcvm.2020.00042
20.
Barnett SN Cujba A Yang L Maceiras AR Li S Kedlian V et al An organotypic atlas of human vascular cells. Nat Med. (2024) 30(12):3468–81. 10.1038/s41591-024-03376-x
21.
Fish JE Matouk CC Rachlis A Lin S Tai SC D’Abreo C et al The expression of endothelial nitric-oxide synthase is controlled by a cell-specific histone code. J Biol Chem. (2005) 280(26):24824–38. 10.1074/jbc.m502115200
22.
Helliwell RJ Adams LF Mitchell MD . Prostaglandin synthases: recent developments and a novel hypothesis. Prostaglandins Leukot Essent Fatty Acids. (2003) 70(2):101–13. 10.1016/j.plefa.2003.04.002
23.
Wang JJ Jin S Zhang H Xu Y Hu W Jiang Y et al Molecular recognition and activation of the prostacyclin receptor by anti-pulmonary arterial hypertension drugs. Sci Adv. (2024) 10(6):eadk5184. 10.1126/sciadv.adk5184
24.
Donato AJ Machin DR Lesniewski LA . Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. (2018) 123(7):825–48. 10.1161/circresaha.118.312563
25.
Nicholson WT Vaa B Hesse C Eisenach JH Joyner MJ . Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. (2009) 53(6):973–8. 10.1161/hypertensionaha.108.121483
26.
Trimm E Red-Horse K . Vascular endothelial cell development and diversity. Nat Rev Cardiol. (2022) 20(3):197–210. 10.1038/s41569-022-00770-1
27.
Kalucka J De Rooij LP Goveia J Rohlenova K Dumas SJ Meta E et al Single-Cell transcriptome atlas of murine endothelial cells. Cell. (2020) 180(4):764–779.e20. 10.1016/j.cell.2020.01.015
28.
Hunter P . Understanding redundancy and resilience. EMBO Rep. (2022) 23(3):e54742. 10.15252/embr.202254742
29.
Forstermann U Sessa WC . Nitric oxide synthases: regulation and function. Eur Heart J. (2011) 33(7):829–37. 10.1093/eurheartj/ehr304
30.
Qiu J Wang X Salama ME Hoffman R . Characterization and isolation of splenic littoral cells, a possible cellular niche for extramedullary hematopoiesis in myelofibrosis. Blood. (2015) 126(23):3594. 10.1182/blood.v126.23.3594.3594
31.
Mebius RE Kraal G . Structure and function of the spleen. Nat Rev Immunol. (2005) 5(8):606–16. 10.1038/nri1669
32.
Li Z Luo W Fang S Chen X Lin T Zhou S et al Prostacyclin facilitates vascular smooth muscle cell phenotypic transformation via activating TP receptors when IP receptors are deficient. Acta Physiol. (2020) 231(2):e13555. 10.1111/apha.13555
33.
Muramatsu R Kuroda M Matoba K Lin H Takahashi C Koyama Y et al Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system. J Biol Chem. (2015) 290(18):11515–25. 10.1074/jbc.m114.587253
34.
Ottolini M Daneva Z Chen Y Cope EL Kasetti RB Zode GS et al Mechanisms underlying selective coupling of endothelial Ca2+ signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J Physiol. (2020) 598(17):3577–96. 10.1113/jp279570
35.
Polk FD Hakim MA Silva JF Behringer EJ Pires PW . Endothelial Kir2 channel dysfunction in aged cerebral parenchymal arterioles. AJP Heart Circ Physiol. (2023) 325(6):H1360–72. 10.1152/ajpheart.00279.2023
36.
Carvalho-de-Souza JL Varanda WA Tostes RC Chignalia AZ . BK Channels in cardiovascular diseases and aging. Aging Dis. (2013) 4(1):38–49.
37.
Lu T Jiang B Wang X Lee H . Coronary arterial BK channel dysfunction exacerbates ischemia/reperfusion-induced myocardial injury in diabetic mice. Appl Physiol Nutr Metab. (2016) 41(9):992–1001. 10.1139/apnm-2016-0048
38.
Sun X Qian L Li Y Pfiefer TM Wang X Lee H et al Regulation of KCNMA1 transcription by Nrf2 in coronary arterial smooth muscle cells. J Mol Cell Cardiol. (2020) 140:68–76. 10.1016/j.yjmcc.2020.03.001
39.
Lu T Chai Q Jiao G Wang X Sun X Furuseth JD et al Downregulation of BK channel function and protein expression in coronary arteriolar smooth muscle cells of type 2 diabetic patients. Cardiovasc Res. (2018) 115(1):145–53. 10.1093/cvr/cvy137
40.
Goodwill AG Noblet JN Sassoon D Fu L Kassab GS Schepers L et al Critical contribution of KV1 channels to the regulation of coronary blood flow. Basic Res Cardiol. (2016) 111(5):56. 10.1007/s00395-016-0575-0
41.
Zhu R Xiao D Zhang L . Potassium channels and uterine vascular adaptation to pregnancy and chronic hypoxia. Curr Vasc Pharmacol. (2013) 11(5):737–47. 10.2174/1570161111311050011
42.
Bresnitz W Lorca RA . Potassium channels in the uterine vasculature: role in healthy and complicated pregnancies. Int J Mol Sci. (2022) 23(16):9446. 10.3390/ijms23169446
43.
Hu X Song R Zhang L . Effect of oxidative stress on the Estrogen-NOS-NO-KCA channel pathway in uteroplacental dysfunction: its implication in pregnancy complications. Oxid Med Cell Longevity. (2019) 2019:1–19. 10.1155/2019/9194269
44.
Corcoran J Lacey H Baker PN Wareing M . Altered potassium channel expression in the human placental vasculature of pregnancies complicated by fetal growth restriction. Hypertens Pregnancy. (2008) 27(1):75–86. 10.1080/10641950701826158
45.
Wardlaw JM Woodhouse LJ Mhlanga II Oatey K Heye AK Bamford J et al Isosorbide mononitrate and cilostazol treatment in patients with symptomatic cerebral small vessel disease. JAMA Neurol. (2023) 80(7):682. 10.1001/jamaneurol.2023.1526
46.
Kasner SE Bath PM Hill MD Volpi JJ Giuffre M Masuoka L et al (2025). Recombinant human tissue kallikrein-1 for treating acute ischemic stroke and preventing recurrence. Stroke. Available online at:10.1161/strokeaha.124.048858
47.
Hamid S Rhaleb IA Kassem KM Rhaleb N . Role of kinins in hypertension and heart failure. Pharmaceuticals. (2020) 13(11):347. 10.3390/ph13110347
48.
John CM Mallat RK George G Kim T Mishra RC Braun AP . Pharmacologic targeting of endothelial Ca2+-activated K+ channels: a strategy to improve cardiovascular function. Channels. (2018) 12(1):126–36. 10.1080/19336950.2018.1454814
49.
Guntur D Olschewski H Enyedi P Csáki R Olschewski A Nagaraj C . Revisiting the large-conductance calcium-activated potassium (BKCA) channels in the pulmonary circulation. Biomolecules. (2021) 11(11):1629. 10.3390/biom11111629
50.
Malerba M D'Amato M Radaeli A Giacovelli G Rovati L Arshad S et al Efficacy of andolast in mild to moderate asthma: a randomized, controlled, double-blind multicenter study (the andast trial). Curr Pharm Des. (2015) 21(26):3835–43. 10.2174/1381612821666150407101614
51.
Wei X Zhang Y Yin B Wen J Cheng J Fu X . The expression and function of KCNQ potassium channels in human chorionic plate arteries from women with normal pregnancies and pre-eclampsia. PLoS One. (2018) 13(3):e0192122. 10.1371/journal.pone.0192122
52.
Iqbal F Lupieri A Aikawa M Aikawa E . Harnessing single-cell RNA sequencing to better understand how diseased cells behave the way they do in cardiovascular disease. Arterioscler Thromb Vasc Biol. (2020) 41(2):585–600. 10.1161/atvbaha.120.314776
53.
Zhu Y Sheng Z Yao H Li D . Emerging mechanisms involving brain Kv7 channel in the pathogenesis of hypertension. Biochem Pharmacol. (2022) 206:115318. 10.1016/j.bcp.2022.115318
54.
Celentano C Carotenuto L Miceli F Carleo G Corrado B Baroli G et al Kv7 channel activation reduces brain endothelial cell permeability and prevents kainic acid-induced blood-brain barrier damage. Am J Physiol Cell Physiol. (2024) 326(3):C893–904. 10.1152/ajpcell.00709.2023
55.
Khanamiri S Soltysinska E Jepps TA Bentzen BH Chadha PS Schmitt N et al Contribution of K v 7 channels to basal coronary flow and active response to ischemia. Hypertension. (2013) 62(6):1090–7. 10.1161/hypertensionaha.113.01244
Summary
Keywords
endothelial nitric oxide synthase (eNOS), bradykinin receptors (b1R/b2R), soluble guanylate cyclase (sGC), guanosine triphosphate (GTP), protein kinase g (PKG), prostaglandin H2 (PGH2), prostaglandin H synthase (PGHS)
Citation
Avolio E, Pearce SF, Wambeke D and Madeddu P (2025) Single-cell transcriptomic analysis of the human vascular atlas provides new insights into vasorelaxation redundancy and heterogeneity. Front. Cardiovasc. Med. 12:1634645. doi: 10.3389/fcvm.2025.1634645
Received
24 May 2025
Accepted
15 July 2025
Published
13 August 2025
Volume
12 - 2025
Edited by
Gianfranco Pintus, University of Sharjah, United Arab Emirates
Reviewed by
Rudolf Schubert, Augsburg University, Germany
Joelmir Lucena Veiga da Silva, Universidade Federal da Paraíba, Brazil
Rahme Nese Safakli, University of Surrey, United Kingdom
Updates
Copyright
© 2025 Avolio, Pearce, Wambeke and Madeddu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Paolo Madeddu mdprm@bristol.ac.uk
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.