- 1Department of Geriatrics, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Beijing Institute of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 3Department of Ophthalmology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
Introduction: Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of global mortality, particularly among individuals with dyslipidemia. Traditionally, the retina has been considered a key site for examining microvascular changes. Recent evidence, however, indicates that retinal alterations may also reflect macrovascular changes. This study proposes a hypothesis in which Optical Coherence Tomography Angiography (OCTA) is utilized to evaluate retinal vascular changes as a potential biomarker for ASCVD risk assessment in dyslipidemia patients.
Methods: In this cross-sectional study, 261 dyslipidemia patients were recruited and classified into non-ASCVD and ASCVD groups. OCTA was performed on all patients, with the macula and optic disc being the primary areas of assessment. The following parameters were measured: retinal vessel density (VD), retinal nerve fiber layer (RNFL) thickness, retinal thickness, foveal avascular zone (FAZ) area, FAZ perimeter, and VD within a 300 μm width ring surrounding the FAZ (FD). Ultimately, data from 231 eyes were analyzed. Comparisons of OCTA-derived metrics between groups were made, and receiver operating characteristic (ROC) curve analysis was conducted to assess the discriminatory power of these metrics for identifying ASCVD in dyslipidemia patients. The DeLong test was used to compare areas under the ROC curve for these indicators. All statistical tests were two-tailed, with significance set at P < 0.05.
Results: In the ASCVD group, RNFL thickness, superficial capillary plexus (SCP) parafoveal VD, SCP perifoveal VD, macular parafoveal thickness, and FD were significantly lower compared to the non-ASCVD group. ROC curve analysis confirmed the predictive value of these indicators for ASCVD identification in dyslipidemia patients. SCP parafoveal VD, SCP perifoveal VD, and FD correspond to the macular superficial capillary plexus vessel densities. When combined, these indicators formed a new composite measure, macular superficial vessel density (MSVD). The ROC curve further validated MSVD's predictive utility for ASCVD in dyslipidemia patients, with the optimal threshold identified at 143.22% using the Youden index.
Conclusions: OCTA-derived indicators, particularly MSVD, demonstrate significant potential as novel biomarkers for ASCVD risk assessment in dyslipidemia patients.
1 Introduction
Atherosclerotic cardiovascular disease (ASCVD), including coronary atherosclerotic heart disease (CHD), ischemic stroke, and peripheral arterial disease, remains the leading global cause of mortality (1–3). CHD can result in myocardial infarction and sudden cardiac death, ischemic stroke can lead to aphasia, prolonged immobility, and secondary cerebral hemorrhage, and peripheral arterial disease in the lower extremities may cause impaired mobility or necessitate amputation (4–7). These conditions severely impair patients’ quality of life, impose significant economic burdens, and increase mortality rates. Accurate risk stratification and early identification of individuals at high risk are essential to prevent disease initiation, mitigate progression, and reduce associated mortality.
The pathophysiological hallmark of ASCVD is the formation and advancement of atherosclerotic plaques, with dyslipidemia, particularly elevated low-density lipoprotein cholesterol (LDL-C), recognized as a principal risk factor (1–3). Evaluating ASCVD risk in individuals with dyslipidemia, predominantly characterized by elevated LDL-C levels, is crucial for implementing targeted interventions and enhancing clinical outcomes.
Previous ASCVD risk assessment tools have been developed (8–11), but they do not specifically cater to the needs of high-risk individuals with dyslipidemia. The predictive variables used, such as blood pressure, lipid levels, and waist circumference, exhibit considerable fluctuation. This highlights the importance of identifying stable, reliable biomarkers for risk evaluation. The retina, recognized as a window into microvascular health, has gained attention as a potential indicator for coronary artery alterations. Recent studies reveal significant differences in retinal vascular features between CHD patients and controls, with these differences correlating with the extent of coronary artery stenosis (12, 13). This evidence suggests an association between retinal microvascular changes and macrovascular conditions. Thus, the current study investigates the feasibility of assessing ASCVD risk in individuals with dyslipidemia by analyzing retinal vascular characteristics.
Optical coherence tomography angiography (OCTA) represents an advanced, non-invasive imaging technique that enables rapid and quantitative evaluation of retinal vascularity (14). Compared to traditional methods, such as color fundus photography and fundus fluorescein angiography, OCTA offers clear advantages. The former lacks quantitative capabilities, while the latter necessitates the injection of a contrast agent, making it invasive. OCTA provides key metrics, including retinal vascular density (VD), retinal nerve fiber layer (RNFL) thickness, and foveal avascular zone (FAZ) area (15), which together allow for a more thorough examination of retinal microvasculature. Previous investigations utilizing OCTA to assess retinal vascular changes have demonstrated significant alterations in the fundus vessels of patients with hypertension (16), diabetes (17), and dyslipidemia (18) when compared to controls. These conditions are also recognized as risk factors for ASCVD. The aim of this study is to investigate the role of retinal vascular alterations assessed by OCTA as biomarkers for ASCVD risk assessment in dyslipidemia patients.
2 Materials and methods
2.1 Study design and allocation
The study conformed to the ethical guidelines set forth in the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Tongren Hospital, Affiliated to Capital Medical University (approval No. TRECKY2021-227). Written informed consent was obtained from all participants or their legal representatives. The trial was registered under NCT05397158.
A cross-sectional design was employed, enrolling dyslipidemia patients admitted to the Department of Geriatrics, Beijing Tongren Hospital, between December 2022 and March 2024. Inclusion criteria required participants to be aged ≥18 years, have a history or confirmed diagnosis of dyslipidemia during hospitalization, and consent to OCTA examination. Exclusion criteria included glaucoma, inability to comply with OCTA procedures, severe cataract or fundus hemorrhage obstructing OCTA imaging, acute infections, malignancies, active autoimmune diseases, or acute myocardial infarction. Dyslipidemia was defined as LDL-C exceeding target treatment levels according to risk stratification (1–3) or a history of dyslipidemia with prolonged statin and/or proprotein convertase subtilisin/kexin type 9(PCSK9) inhibitor use. Participants were categorized into two groups based on their medical history and hospitalization results: those without ASCVD and those diagnosed with ASCVD. ASCVD was defined as including CHD, ischemic stroke, and peripheral arterial disease (1, 2). CHD included a documented history of acute coronary syndrome (e.g., myocardial infarction or unstable angina), stable angina, or coronary revascularization procedures, such as percutaneous coronary intervention, coronary artery bypass grafting, or other arterial interventions (4, 5). Stroke classification was confined to ischemic stroke (6), while peripheral arterial disease included lower-extremity arterial disease and carotid artery stenosis (7).
2.2 Data collection
Patient data were extracted from the hospital's medical record system. Collected information included demographic variables (sex, age, smoking history, height, and weight), medical conditions [hypertension, diabetes, hyperuricemia, obstructive sleep apnea hypopnea syndrome (OSAHS), CHD, stroke, and peripheral arterial disease], current treatments (antihypertensives, hypoglycemic agents, statins, PCSK9 inhibitors, antiplatelet agents, and anticoagulants), and laboratory results (total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), lipoprotein(a) [Lp(a)], serum creatinine, cystatin C, glycosylated hemoglobin (HbA1c), and estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI (creatinine-cystatin C equation) (19). Furthermore, 24-hour ambulatory blood pressure (ABP) readings, including mean systolic blood pressure (MSBP) and mean diastolic blood pressure (MDBP), coronary angiography or coronary computed tomography angiography (CCTA) results, and peripheral artery ultrasound findings (carotid and lower extremity arteries) were incorporated.
2.3 OCTA
OCTA imaging was conducted on 6 × 6 mm angiograms using the RTVue XR system with Avanti software version 2017.1.0.155 (Optovue, Inc., Fremont, CA, USA). The system's parameters included an image acquisition rate of 70,000 A-scans/second, an axial resolution of 5 μm, and a scan depth range of 2–3 mm, with a transverse range of 2–12 mm. The scanning beam operated at a wavelength of 840 ± 10 nm, spanning from the internal limiting membrane to the retinal pigment epithelium. Images were deemed acceptable if the signal intensity was ≥6. Mydriasis was induced in patients using tropicamide 30 minutes prior to the OCTA examination. The procedures were performed by trained ophthalmic technicians, with data collected from the right eye of each participant (20). For the four participants with functional vision exclusively in the left eye, data from the left eye were included in the analysis.
The retina's key anatomical landmarks include the optic disc and macula (21). The optic disc serves as the point of entry for the central retinal artery, the point of exit for the retinal vein, and the conduit for the optic nerve head, from which the optic nerve emerges. The macula is the region with the highest density of photoreceptor cells. Retinal blood flow is supplied by the central retinal artery and its branching capillaries, which connect to venous branches. The central retinal artery enters through the optic disc, dividing into smaller capillaries. Within the macula, these capillaries narrow, forming a circular-shaped structure devoid of vessels at its center, known as the FAZ.
OCTA facilitates the evaluation of various retinal parameters, including VD in the optic disc region, RNFL thickness, VD and thickness in the macular region, FAZ area and perimeter, and VD within a 300 μm ring surrounding the FAZ (FD) (Figure 1). The retinal vasculature in the optic disc region is organized radially, forming the radial peripapillary capillary (RPC) network, which is divided into two zones: the inside disc and the peripapillary capillary areas. OCTA provides average VD values for both total and small blood vessels within these zones, while RNFL thickness measurements reflect the average in the peripapillary capillary region. In the macular region, the vasculature is categorized into two layers: the superficial capillary plexus (SCP) and the deep capillary plexus (DCP). SCP, extending from the internal limiting membrane to the inner plexiform layer, demonstrates uniform blood flow and regular contours. DCP, located between the inner and outer plexiform layers, consists of finer vasculature with similarly regular morphology. VD measurements are taken within two concentric zones: the parafovea (1–3 mm diameter) and perifovea (3–6 mm diameter). The calculated VD represents the mean value across four quadrants: superior (S), inferior (I), temporal (T), and nasal (N). VD is expressed as a percentage, reflecting the proportion of the area occupied by blood vessels, without distinguishing between arteries and veins. Utilizing the AngioAnalytics license, the OCTA system visualizes retinal VD parameters through color-coded mapping, with distinct colors representing specific VD ranges (e.g., green indicating a VD of 45%–55%). Retinal VD in healthy individuals is solely associated with age (22). Standard RNFL thickness measures approximately 100 μm (23), while macular thickness typically ranges from 200 to 300 μm (14). In contrast, the FAZ area shows considerable variability in healthy individuals with normal vision, ranging from 0.071 mm² to 0.527 mm² (24).
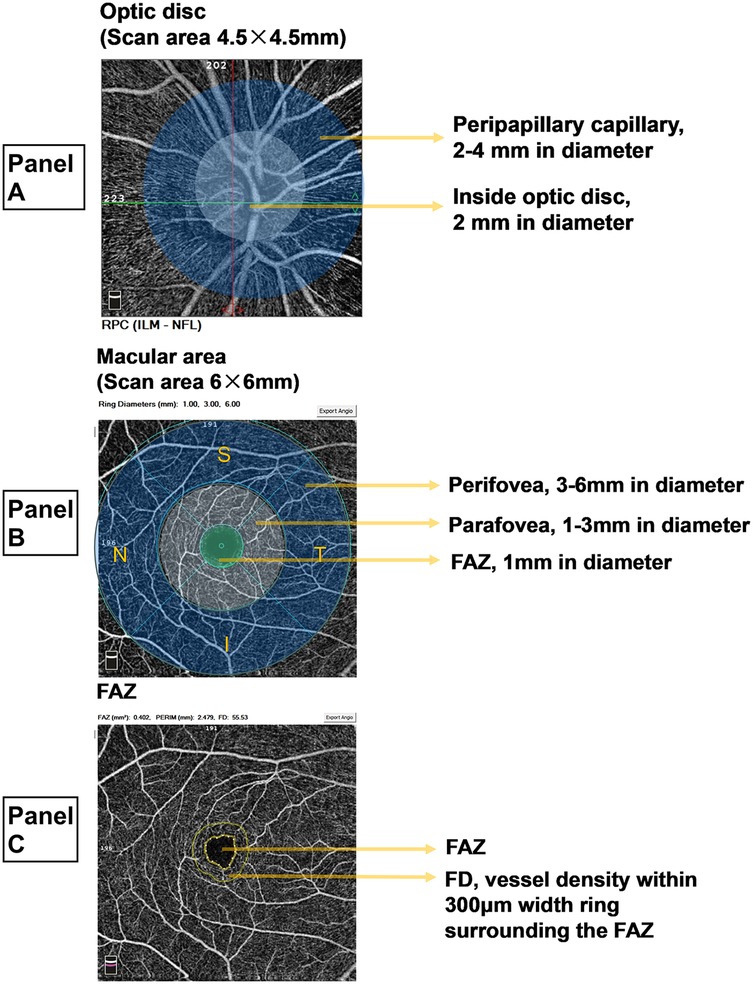
Figure 1. Measurement zones and parameters for OCTA. (A) Optic disc area. The radial peripapillary capillary (RPC) region in the optic disc area encompasses the optic disc (a circular area with a 2-mm diameter) and the peripapillary capillary zone (a ring-shaped area with a diameter of 2-4 mm). The RPC vascular density (VD) is calculated as the mean VD of all vessels across the image and the mean VD of all small vessels. The retinal nerve fiber layer (RNFL) thickness is measured as the average value within the peripapillary capillary region. (B) Macular region. The macular region is analyzed by dividing it into two capillary plexus layers: the superficial capillary plexus (SCP) and the deep capillary plexus (DCP). Both layers share identical measurement divisions, as illustrated using the SCP. The perifoveal region, represented by the outer ring, has a diameter of 3–6 mm, while the parafoveal region, denoted by the inner ring, has a diameter of 1-3 mm. VD measurements within these rings represent the mean values for four quadrants: superior (S), inferior (I), temporal (T), and nasal (N). At the center, the foveal avascular zone (FAZ) is a circular area with a 1-mm diameter. VD is expressed as a percentage, indicating the proportion of vascularized area within the measurement zone. (C) FAZ. The FAZ represents the foveal avascular zone. The FAZ constitutes the central avascular circular zone, while the surrounding ring corresponds to the VD within 300 μm width ring surrounding the FAZ (FD).
Color fundus photography and OCTA examinations were conducted for all patients. An ophthalmologist provided consultation to explain the ophthalmic findings to the patients. As the most commonly employed fundus imaging technique in clinical settings, color fundus photography assists in detecting retinal exudation, hemorrhage, and neovascularization; however, it lacks the ability for quantitative analysis (14). Consequently, the results obtained from color fundus photography in this study served merely as a reference for ophthalmologists during consultations and were not subjected to quantitative evaluation.
2.4 Statistical analysis
Quantitative variables were expressed as mean ± standard deviation for normally distributed data and as median with interquartile range for non-normally distributed data, while qualitative variables were presented as frequencies with percentages. The normality of quantitative data was assessed before analysis. T-tests were applied to compare normally distributed data, whereas non-normally distributed data were evaluated using rank-sum tests. Chi-square tests were utilized for qualitative data analysis. Receiver operating characteristic (ROC) curves were generated to assess the discriminative ability of RNFL thickness, SCP parafovea VD, SCP perifovea VD, macular parafovea thickness, and FD in identifying ASCVD. The areas under the ROC curve (AUC) for these indicators were compared using the DeLong test. The optimal threshold was determined using the Youden index, calculated as sensitivity plus specificity minus one, with the threshold corresponding to the highest Youden index designated as optimal. All statistical tests were two-tailed, with significance set at P < 0.05. Analyses were performed using SPSS 29.0 software (SPSS, Inc., Chicago, IL, USA).
This study examined the relationship between OCTA-derived indices and ASCVD. Previous studies investigating the association between retinal vascular parameters and coronary artery disease typically included sample sizes ranging from 200 to 300 patients. The current study adhered to this sample size, fulfilling the requirements for intergroup comparisons (t-test, rank-sum test) and ROC curve analysis.
3 Results
A total of 261 dyslipidemic patients were initially assessed for eligibility in this study, all of whom were of yellow races. Fourteen patients were excluded due to intolerance to mydriasis, preventing OCTA examination; nine were excluded for having severe cataracts or a history of fundus hemorrhage; four had malignant tumors; and three were in the active phase of autoimmune diseases. Consequently, the final cohort consisted of 231 participants (458 eyes in total), including 170 males (73.6%) with a mean age of 63.68 ± 11.61 years (Figure 2).
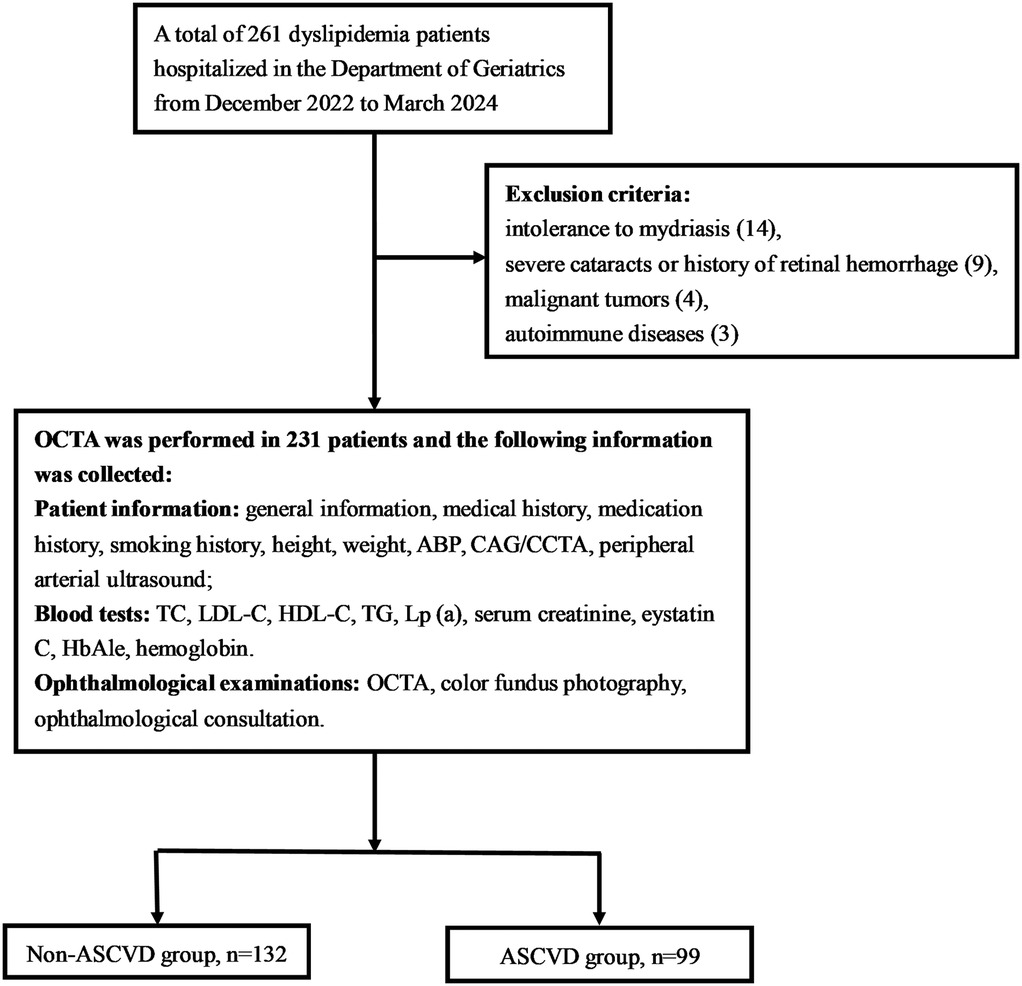
Figure 2. Flow chart. OCTA, optical coherence tomography angiography; ABP, ambulatory blood pressure; CAG, coronary angiography; CCTA, coronary computed tomography angiography; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein Cholesterol; TG, triglyceride; Lp(a), lipoprotein(a).
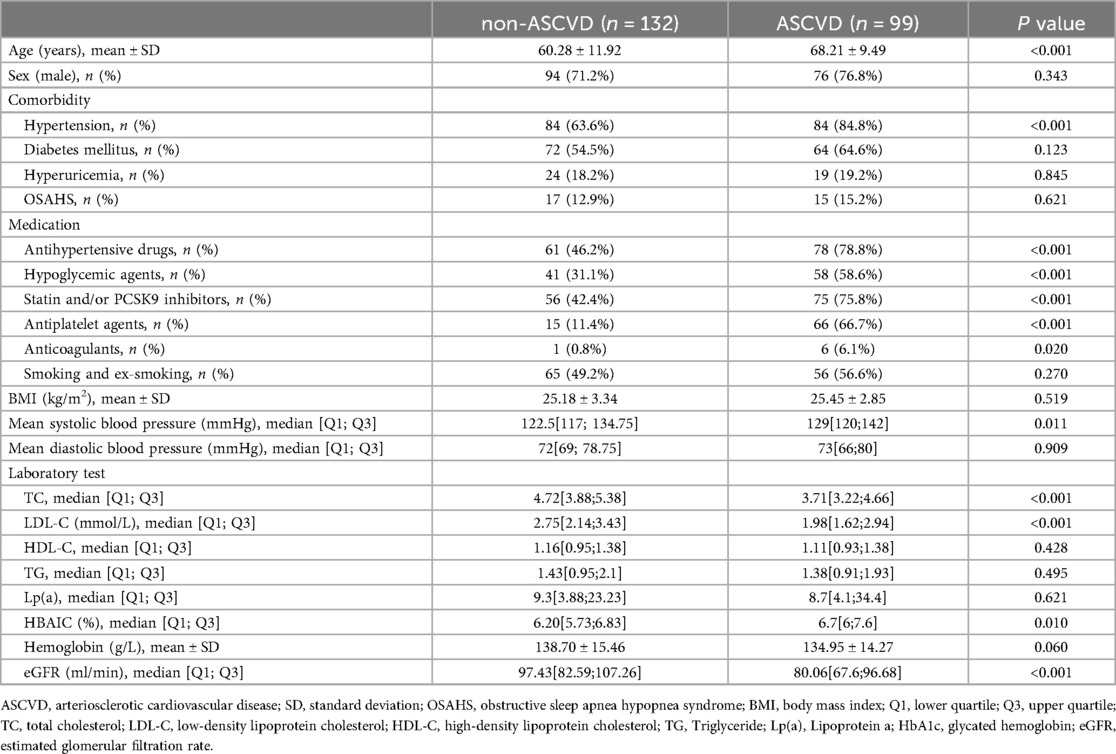
Participants were classified into two groups based on the presence or absence of ASCVD: the non-ASCVD group (n = 132) and the ASCVD group (n = 99). A comparison of baseline characteristics between the groups is provided in Table 1. The ASCVD group was older and had a higher incidence of hypertension, increased use of antihypertensive medications, hypoglycemic agents, statins or PCSK9 inhibitors, antiplatelet agents, and anticoagulants, alongside elevated MSBP and HbA1c levels. Conversely, the non-ASCVD group exhibited higher levels of TC, LDL-C, and eGFR. No significant differences were noted between the groups concerning sex, diabetes, hyperuricemia, OSAHS, smoking status, BMI, MDBP, HDL-C, TG, Lp(a), or hemoglobin.
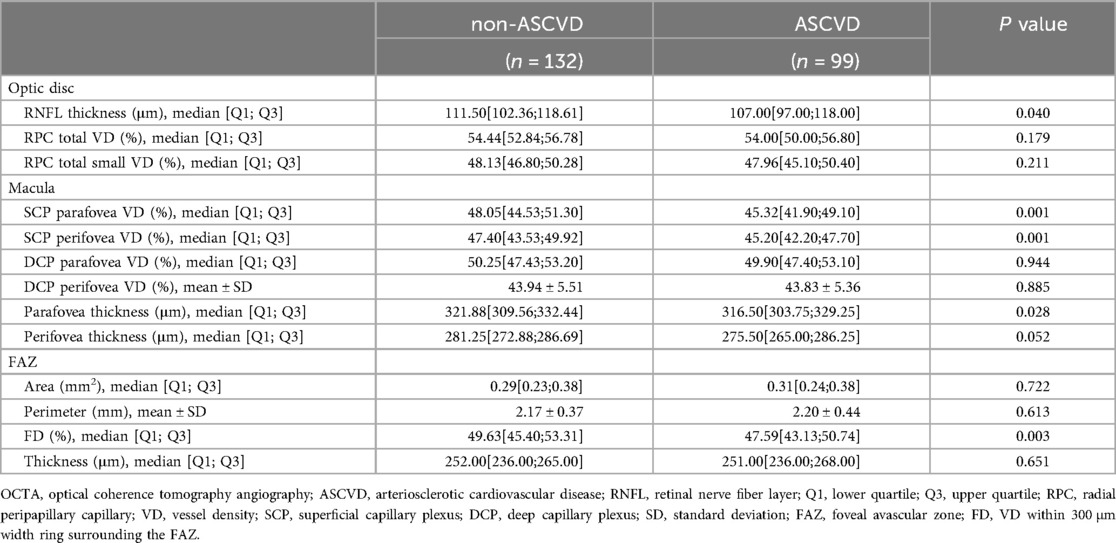
In the optic disc region, the ASCVD group exhibited a reduced RNFL thickness compared to the non-ASCVD group [111.50[102.36;118.61] vs. 107.00[97.00;118.00], P = 0.040]. No significant differences were found in the VD of total or small vessels between the two groups in this region (Table 2).
In the macular region, VD of the parafovea [48.05[44.53;51.30] vs. 45.32[41.90;49.10], P = 0.001] and perifovea [47.40[43.53;49.92] vs. 45.20[42.20;47.70], P = 0.001] in the SCP was significantly lower in the ASCVD group. OCTA imaging revealed a decrease in retinal vasculature density in the ASCVD group, confirmed by processed color images showing more extensive blue areas and fewer green areas (Figure 3). No significant difference was found in the VD of the parafovea [50.25[47.43;53.20] vs. 49.90[47.40;53.10], P = 0.944] or perifovea (43.94 ± 5.51 vs. 43.83 ± 5.36, P = 0.885) within the DCP between the two groups. Retinal thickness in the parafovea (321.88[309.56;332.44] vs. 316.50[303.75;329.25], P = 0.028) and perifovea [281.25[272.88;286.69] vs. 275.50[265.00;286.25], P = 0.052] was reduced in the ASCVD group, with only the parafoveal thickness difference reaching statistical significance (Table 2).
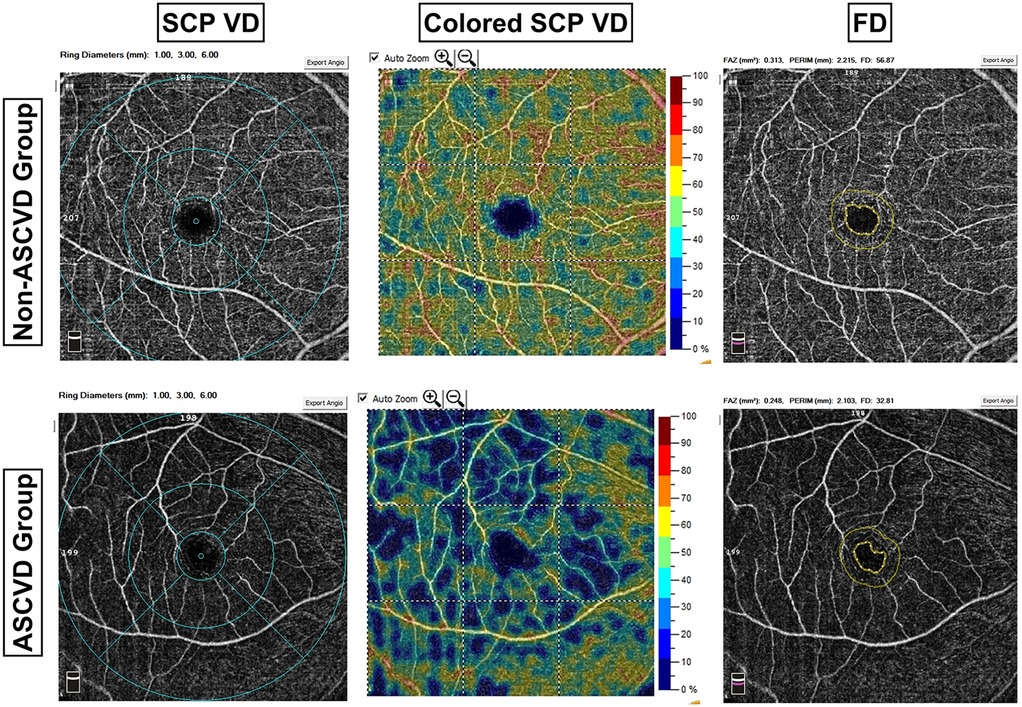
Figure 3. Comparison of OCTA images between groups. ASCVD, arteriosclerotic cardiovascular disease; SCP, superficial capillary plexus; VD, vessel density; FD, VD within 300 μm width ring surrounding the FAZ. The first column illustrates a comparison of SCP VD between the two groups, revealing sparser retinal vessels in the ASCVD group compared to the non-ASCVD group. The OCTA system employed in this study provides a color-coded visualization of retinal VD, as depicted in the second column. Each color corresponds to a specific VD range, with green representing 45%–55% VD. The ASCVD group exhibited a higher prevalence of blue regions and fewer green regions compared to the non-ASCVD group.
In the FAZ, no significant differences were observed between the groups in area, perimeter, or retinal thickness. However, the FD was significantly lower in the ASCVD group compared to the non-ASCVD group [49.63[45.40;53.31] vs. 47.59[43.13;50.74], P = 0.003], which was confirmed by statistical analysis (Table 2). Imaging also showed a decrease in FD in the ASCVD group, further supporting this finding (Figure 3).
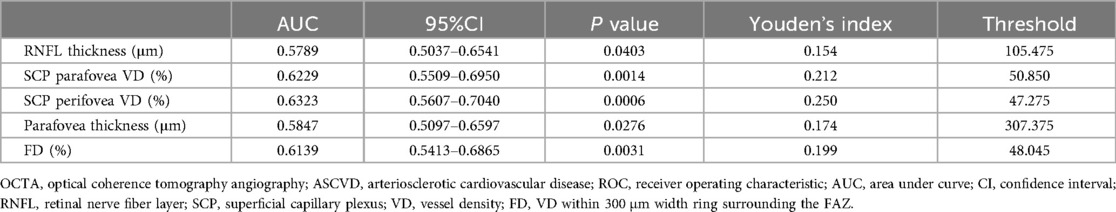
A comparison of OCTA results between the two groups identified RNFL thickness, SCP parafovea VD, SCP perifovea VD, macular parafovea thickness, and FD as key parameters for constructing an ROC curve to evaluate their predictive value for ASCVD in dyslipidemia patients (Table 3 and Figure 4A). The analysis confirmed that all five parameters were indicative of ASCVD risk in this population. The optimal thresholds, determined via the Youden index, were found to be 47.275% for SCP perifovea VD, 50.850% for SCP parafovea VD, and 48.045% for FD.
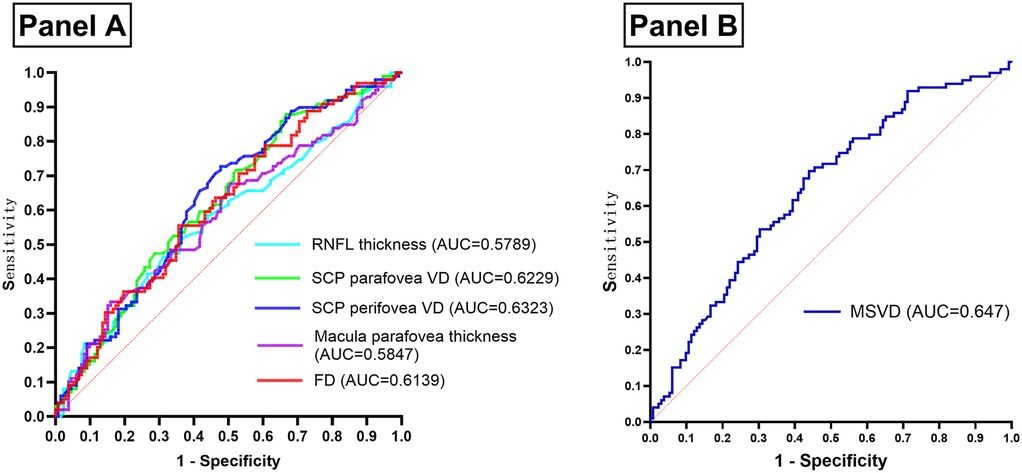
Figure 4. (Panel A) ROC curve of OCTA results assessing the occurrence of ASCVD in patients with dyslipidemia. (Panel B) ROC curve of MSVD assessing the occurrence of ASCVD in patients with dyslipidemia. OCTA, optical coherence tomography angiography; ASCVD, arteriosclerotic cardiovascular disease; ROC, receiver operating characteristic; AUC, area under curve; RNFL, retinal nerve fiber layer; SCP, superficial capillary plexus; VD, vessel density; FD, VD within 300 μm width ring surrounding the FAZ; MSVD, macula superficial vessel density.
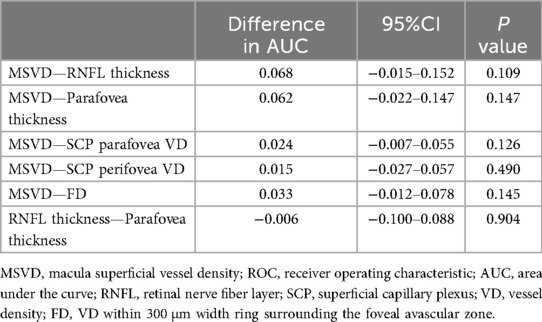
Anatomically, the macular superficial layer, extending from medial to lateral, comprises the FAZ, SCP parafovea, and SCP perifovea, all of which are interconnected vascular regions (Figure 1B). Given the relatively intact terminal vascular ring of the superficial FAZ, FD is defined as the VD within a 300 μm-wide ring surrounding the superficial FAZ. As such, FD, along with SCP parafovea VD and SCP perifovea VD, serves as an indicator of superficial retinal VD. These three parameters were consolidated into a composite measure—macular superficial vessel density (MSVD)—to assess ASCVD risk in dyslipidemia patients. ROC curve analysis revealed an AUC of 0.647 for MSVD (95% CI: 0.576–0.718), which surpassed the AUCs for FD, SCP parafovea VD, and SCP perifovea VD. The maximum Youden index value of 0.258 indicated an optimal threshold of 143.22% (Figure 4B). DeLong test results indicated no statistically significant differences in AUC values when comparing MSVD with FD, SCP parafovea VD, SCP perifovea VD, RNFL thickness, and macular parafovea thickness (Table 4).
4 Discussion
ASCVD includes a range of diseases primarily characterized by atherosclerosis, including CHD, ischemic stroke, and peripheral arterial disease. Recognized as a major global health threat (4–7), various regions have developed tools to assess ASCVD risk. Notably, the ASCVD risk calculator (2013) (9), created by the American College of Cardiology/American Heart Association (ACC/AHA), represents the first predictive model based on comprehensive data from White and Black American populations. The key variables include race (African American, White), gender, age, TC, HDL-C, systolic blood pressure (SBP), antihypertensive medication use, diabetes status, and smoking history. While the model offers satisfactory calibration for populations similar to its development cohort, its accuracy appears limited in other demographic groups (25–28). Furthermore, its predictive endpoints are confined to myocardial infarction and stroke. In 2014, the Joint British Societies (JBS) introduced the JBS3 risk score, a broader cardiovascular risk tool (11). This model extends its endpoints beyond myocardial infarction and stroke to include angina pectoris, coronary revascularization, transient ischemic attack, intermittent claudication, and related conditions. However, external validation of this tool is still pending. In 2016, China developed the Prediction for ASCVD Risk in China (China-PAR) score, which incorporated additional factors such as regional differences (north vs. south) and waist circumference. Its assessment endpoints included CHD-related mortality, myocardial infarction, and stroke (8). In comparison to the ASCVD risk calculator (2013) by the ACC/AHA, China-PAR demonstrates higher predictive accuracy for Chinese populations, but it has yet to be validated for non-Chinese adult cohorts.
Existing risk assessment tools are limited in their applicability across diverse populations and ethnicities. Moreover, these models are tailored to the general population, failing to address the specific needs of individuals with dyslipidemia, a group at heightened risk for ASCVD (1–3). In this study, significant differences in age and SBP were observed between the ASCVD and non-ASCVD groups, consistent with prior research in ASCVD risk models. However, no notable differences were found in HDL-C levels, diastolic blood pressure, or related parameters. Interestingly, TC and LDL-C levels were lower in the ASCVD group compared to the non-ASCVD group, likely reflecting the higher usage of lipid-lowering medications among ASCVD patients. These findings suggest that traditional indicators, such as TC, HDL-C, LDL-C, and DBP, may not be the most reliable markers for assessing ASCVD risk.
The retina, the only tissue with microvessels directly visible in the human body, has long been an important indicator for evaluating the effects of systemic conditions such as hypertension and diabetes on microvascular health (14, 21). Traditionally, color fundus photography is used to assess retinal blood vessels, primarily identifying signs like hemorrhage or neovascularization. The introduction of OCTA has advanced this assessment, offering a faster, non-invasive, and more detailed examination of retinal vascular changes (14, 29–31). Recent studies have established a relationship between retinal vascular parameters and coronary artery diseases. OCTA findings indicate that retinal VD is lower in patients with CHD compared to healthy controls (12, 32). Moreover, in CHD patients, higher Gensini scores correlate with reduced retinal VD (32). Patients with coronary total occlusion (CTO) also show higher Gensini scores and decreased retinal VD compared to those without CTO (33). The Gensini score, a standard clinical tool, assesses the severity of coronary artery stenosis (34). These observations suggest that retinal vasculature offers a distinct view of macrovascular changes. This study supports previous findings, noting that patients with ASCVD exhibit reduced retinal VD, including in the SCP and FD areas, alongside thinner RNFL and macular parafoveal thickness, when compared to individuals without ASCVD.
Among OCTA parameters, a reduction in retinal VD is indicative of vascular stenosis or occlusion. Existing literature confirms that hypertension, diabetes, and dyslipidemia contribute to decreases in retinal VD (16–18, 23, 35), with all three factors being well-established risks for ASCVD. In addition to ischemia caused by retinal vascular changes, RNFL and macular thickness are also influenced by local glucose levels and osmotic pressure (23).
In OCTA imaging, the detection region is divided into the macula and optic disc. Among the evaluated parameters, SCP perifovea VD, SCP parafovea VD, and FD correspond to macular superficial capillary plexus vessel densities and are correlated due to their interconnected capillary network. Combining these parameters results in a new composite measure, MSVD. Although DeLong test results did not demonstrate the superiority of MSVD over other indicators in assessing ASCVD risk in dyslipidemic patients, MSVD streamlines OCTA interpretation and provides practical benefits by enabling risk stratification based on macular scans alone, thus reducing both the scope of examination and associated costs. Considering its economic and clinical potential, MSVD represents a viable method for ASCVD screening in asymptomatic dyslipidemia patients. Its inclusion in future ASCVD risk prediction models merits further exploration.
The study has several limitations: (1) A cross-sectional design is initially selected for feasibility, but its statistical robustness in assessing the value of OCTA findings for evaluating ASCVD in individuals with dyslipidemia is inherently weaker than that of prospective cohort studies. (2) A higher proportion of patients in the ASCVD group are receiving medications (antihypertensive, lipid-lowering drugs, etc.) compared to the non-ASCVD group. Due to the small sample size, the study does not further stratify medication usage. (3) As a single-center study, it lacks the diversity of populations across different regions and ethnic groups, which limits the generalizability of the findings. Future research should involve a multicenter, multiracial prospective cohort study to adjust for confounding factors such as medication use and further assess the predictive value of retinal vascular changes detected by OCTA for ASCVD development in patients with dyslipidemia.
5 Conclusions
This study employs OCTA to assess retinal changes in dyslipidemia patients with ASCVD, identifying significant reductions in SCP perifovea VD, SCP parafovea VD, FD, RNFL, and macular thickness compared to those without ASCVD. These parameters may serve as potential biomarkers for evaluating ASCVD risk in individuals with dyslipidemia. Furthermore, a novel composite indicator, MSVD, is derived by combining the first three vessel density measures, offering an effective tool for ASCVD risk assessment in dyslipidemia patients. The incorporation of MSVD streamlines OCTA interpretation and lowers examination costs. OCTA-derived indicators, particularly MSVD, demonstrate considerable potential for integration into future ASCVD risk assessment models.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Tongren Hospital Affiliated to Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YH: Writing – original draft, Writing – review & editing. G-hW: Conceptualization, Writing – review & editing. M-zQ: Writing – review & editing. KC: Formal analysis, Software, Writing – review & editing. Y-pZ: Writing – review & editing, Investigation. XJ: Investigation, Writing – review & editing. ZZ: Investigation, Writing – review & editing. QiL: Writing – review & editing. QiaL: Writing – review & editing. J-bM: Writing – review & editing, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks to Han-yang Wang for his help in the completion of manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li JJ, Zhao SP, Zhao D, Lu GP, Peng DQ, Liu J, et al. 2023 China guidelines for lipid management. J Geriatr Cardiol. (2023) 20(9):621–63. doi: 10.26599/1671-5411.2023.09.008
2. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41(1):111–88. doi: 10.1093/eurheartj/ehz455
3. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73(24):3168–209. doi: 10.1016/j.jacc.2018.11.002
4. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
5. Chinese Society of Cardiology CMA. Chinese Guidelines for the diagnosis and management of patients with chronic coronary syndrome. Zhonghua Xin Xue Guan Bing Za Zhi. (2024) 52(6):589–614. doi: 10.3760/cma.j.cn112148-20240325-00168
6. Party ISW. National Clinical Guideline for Stroke for the UK and Ireland. (2023). Available online at: www.strokeguideline.org
7. Mazzolai L, Teixido-Tura G, Lanzi S, Boc V, Bossone E, Brodmann M, et al. 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J. (2024) 45(36):3538–700. doi: 10.1093/eurheartj/ehae179
8. Yang X, Li J, Hu D, Chen J, Li Y, Huang J, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: the China-PAR project (prediction for ASCVD risk in China). Circulation. (2016) 134(19):1430–40. doi: 10.1161/CIRCULATIONAHA.116.022367
9. Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. (2014) 63(25 Pt A):2886. doi: 10.1016/j.jacc.2013.11.002
10. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140(11):e596–646. doi: 10.1161/CIR.0000000000000678
11. Board J. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart. (2014) 100(Suppl 2):ii1–ii67. doi: 10.1136/heartjnl-2014-305693
12. Wang J, Jiang J, Zhang Y, Qian YW, Zhang JF, Wang ZL. Retinal and choroidal vascular changes in coronary heart disease: an optical coherence tomography angiography study. Biomed Opt Express. (2019) 10(4):1532. doi: 10.1364/BOE.10.001532
13. Arnould L, Guenancia C, Azemar A, Alan G, Pitois S, Bichat F, et al. The EYE-MI pilot study: a prospective acute coronary syndrome cohort evaluated with retinal optical coherence tomography angiography. Invest Ophthalmol Vis Sci. (2018) 59(10):4299–306. doi: 10.1167/iovs.18-24090
14. Wen-bin W. Atlas of Optical Coherence Tomography Angiography. 1st ed. Beijing: People’s Medical Publishing House (2016).
15. Kalra G, Zarranz-Ventura J, Chahal R, Bernal-Morales C, Lupidi M, Chhablani J. Optical coherence tomography (OCT) angiolytics: a review of OCT angiography quantitative biomarkers. Surv Ophthalmol. (2022) 67(4):1118–34. doi: 10.1016/j.survophthal.2021.11.002
16. Donati S, Maresca AM, Cattaneo J, Grossi A, Mazzola M, Caprani SM, et al. Optical coherence tomography angiography and arterial hypertension: a role in identifying subclinical microvascular damage? Eur J Ophthalmol. (2021) 31(1):158–65. doi: 10.1177/1120672119880390
17. Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. (2017) 124(2):235–44. doi: 10.1016/j.ophtha.2016.10.008
18. He Y, Qin MZ, Cao K, Zhang YP, Jiao X, Zhang Z, et al. Assessment of the impact of low-density lipoprotein cholesterol on retinal vessels using optical coherence tomography angiography. Lipids Health Dis. (2024) 23(1):301. doi: 10.1186/s12944-024-02287-7
19. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385(19):1737–49. doi: 10.1056/NEJMoa2102953
20. Al-Sheikh M, Tepelus TC, Nazikyan T, Sadda SR. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br J Ophthalmol. (2017) 101(4):449–52. doi: 10.1136/bjophthalmol-2016-308764
21. Susan S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 41st ed. London: Elsevier (2015).
22. Takayama K, Kaneko H, Ito Y, Kataoka K, Iwase T, Yasuma T, et al. Novel classification of early-stage systemic hypertensive changes in human retina based on OCTA measurement of choriocapillaris. Sci Rep. (2018) 8(1):15163. doi: 10.1038/s41598-018-33580-y
23. Tombolini B, Borrelli E, Sacconi R, Bandello F, Querques G. Diabetic macular ischemia. Acta Diabetol. (2022) 59(6):751–9. doi: 10.1007/s00592-021-01844-1
24. Sun Z, Yang D, Tang Z, Ng DS, Cheung CY. Optical coherence tomography angiography in diabetic retinopathy: an updated review. Eye (Lond). (2021) 35(1):149–61. doi: 10.1038/s41433-020-01233-y
25. Karmali KN, Goff DJ, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. (2014) 64(10):959–68. doi: 10.1016/j.jacc.2014.06.1186
26. Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, et al. Comparison of application of the ACC/AHA guidelines, adult treatment panel III guidelines, and European society of cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. (2014) 311(14):1416–23. doi: 10.1001/jama.2014.2632
27. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. (2014) 311(14):1406–15. doi: 10.1001/jama.2014.2630
28. Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. (2013) 382(9907):1762–5. doi: 10.1016/S0140-6736(13)62388-0
29. Chua J, Chin CWL, Hong J, Chee ML, Le TT, Ting DSW, et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J Hypertens. (2019) 37(3):572–80. doi: 10.1097/HJH.0000000000001916
30. Shin YI, Nam KY, Lee WH, Ryu CK, Lim HB, Jo YJ, et al. Peripapillary microvascular changes in patients with systemic hypertension: an optical coherence tomography angiography study. Sci Rep. (2020) 10(1):6541. doi: 10.1038/s41598-020-63603-6
31. Borrelli E, Battista M, Sacconi R, Querques G, Bandello F. Optical coherence tomography angiography in diabetes. Asia Pac J Ophthalmol. (2021) 10(1):20–5. doi: 10.1097/APO.0000000000000351
32. Zhong P, Li Z, Lin Y, Peng Q, Huang M, Jiang L, et al. Retinal microvasculature impairments in patients with coronary artery disease: an optical coherence tomography angiography study. Acta Ophthalmol. (2022) 100(2):225–33. doi: 10.1111/aos.14806
33. Zhong P, Hu Y, Jiang L, Peng Q, Huang M, Li C, et al. Retinal microvasculature changes in patients with coronary total occlusion on optical coherence tomography angiography. Front Med (Lausanne). (2021) 8:708491. doi: 10.3389/fmed.2021.708491
34. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51(3):606. doi: 10.1016/s0002-9149(83)80105-2
Keywords: dyslipidemia, ASCVD, risk, biomarkers, retinal vessels, opticalcoherence tomography angiography
Citation: He Y, Wang G-h, Qin M-z, Cao K, Zhang Y-p, Jiao X, Zhang Z, Liu Q, Liu Q and Ma J-b (2025) Retinal vascular alterations as assessment indicators of atherosclerotic cardiovascular disease risk in dyslipidemia patients. Front. Cardiovasc. Med. 12:1634816. doi: 10.3389/fcvm.2025.1634816
Received: 25 May 2025; Accepted: 29 August 2025;
Published: 12 September 2025.
Edited by:
Isabella Russo, University of Turin, ItalyReviewed by:
Alice Verticchio, Icahn School of Medicine at Mount Sinai, United StatesLiu Jian Liu Jian, Northeastern University at Qinhuangdao, China
Copyright: © 2025 He, Wang, Qin, Cao, Zhang, Jiao, Zhang, Liu, Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-hong Wang, Z3VvaG9uZ3dAMTYzLmNvbQ==
 Yu He
Yu He Guo-hong Wang1*
Guo-hong Wang1* Ming-zhao Qin
Ming-zhao Qin Yong-peng Zhang
Yong-peng Zhang Zheng Zhang
Zheng Zhang Jin-bao Ma
Jin-bao Ma