Abstract
Background:
Aortic coarctation (CoA) is a congenital cardiovascular condition usually diagnosed in infancy or childhood. Cases in adults are rare and often go undetected because symptoms can be obscured by extensive collateral circulation.
Case description:
A 62-year-old male was admitted to Gansu Provincial Hospital in December 2024 with complaints of recurrent chest tightness, shortness of breath, blurred vision, and tinnitus persisting for over two months. Physical examination revealed significant blood pressure discrepancies between the upper and lower extremities (>50 mmHg). Imaging confirmed severe CoA with nearly complete interruption of the descending aorta, extensive collateral circulation, and complications, including hypertensive crisis with cerebral hemorrhage likely due to extreme hypertension, and bronchiectasis with active pulmonary infection. After multidisciplinary team evaluation, left subclavian artery-to-descending aorta bypass grafting was performed. Postoperatively, blood pressure normalized across all limbs, and the patient remained asymptomatic at the six-month follow-up, with patent graft flow.
Conclusion:
This case of severe CoA in a 62-year-old male highlights the importance of early recognition of atypical presentations in adult patients, the need for individualized surgical strategies, and the benefits of long-term follow-up to ensure successful management and optimal outcomes.
Introduction
Aortic coarctation (CoA) is a congenital cardiovascular anomaly characterized by a localized narrowing of the aortic lumen, most commonly at the isthmus near the ductus arteriosus (1). It accounts for approximately 5%–8% of all congenital heart defects, with an incidence of 3–4 per 10,000 live births (2). CoA is typically diagnosed in infancy or early childhood; however, in rare cases, it may remain undetected until adulthood due to compensatory development of extensive collateral circulation. In adults, the presence of well-established collaterals can obscure classical clinical signs—such as significant discrepancies in blood pressure between the upper and lower extremities—leading to delayed diagnosis (3). When left untreated, longstanding CoA can result in serious complications, including uncontrolled hypertension, intracranial hemorrhage, aortic rupture, and end-organ damage (4). This case report presents a rare instance of severe, undiagnosed CoA in a 62-year-old male, complicated by hypertensive crisis, cerebral hemorrhage, and coexisting bronchiectasis. The case highlights the diagnostic challenges posed by atypical adult presentations and underscores the importance of multidisciplinary collaboration in formulating individualized surgical strategies.
Case description
A 62-year-old male was admitted to Gansu Provincial Hospital in April 2024 with a two-month history of recurrent chest tightness and shortness of breath. These symptoms were accompanied by bilateral blurred vision and high-frequency tinnitus. The patient denied vertigo, syncope, or visual blackout. His family history was unremarkable, and he had no known hereditary conditions. It is noteworthy that, although the patient had longstanding left ventricular hypertrophy, he had not previously experienced classic symptoms of coarctation. This was primarily due to well-developed collateral circulation—including markedly dilated internal thoracic and intercostal arteries—which effectively reduced the pressure gradient and maintained distal perfusion, thereby masking clinical manifestations for many years. Retrospectively, the patient did report a progressive decline in exercise tolerance in the two months prior to admission, but these symptoms became prominent only after the onset of a pulmonary infection (bronchiectasis with active infection). The infection likely increased metabolic demand, exceeded the collateral circulation's compensatory capacity, and triggered overt cardiopulmonary and neurological symptoms, as well as exacerbated the hypertensive crisis.
Two months prior to admission, following a common cold, the patient developed persistent chest discomfort, exertional dyspnea, productive cough with yellow sputum, and progressive reduction in exercise tolerance. Neurological symptoms, including blurred vision and tinnitus, developed concurrently. At the Second Hospital of Lanzhou University, the primary focus was on the neurological symptoms, and no systematic cardiovascular physical examination was performed; only cranial computed tomography (CT) was reported. Cranial CT revealed a cerebral hemorrhage. During the same hospitalization, chest CT incidentally identified features suggestive of CoA, marking the first recognition of this congenital anomaly.
Upon admission to our institution, significant interlimb blood pressure discrepancies were noted: 163/91 mmHg (right upper limb), 159/88 mmHg (left upper limb), 110/82 mmHg (right lower limb), and 102/69 mmHg (left lower limb). On physical examination, cardiac auscultation did not reveal any characteristic murmur suggestive of aortic coarctation (CoA). Cranial CT confirmed a hemorrhagic lesion involving the left basal ganglia, thalamus, and posterior lateral ventricle (approximately 9 ml), alongside multiple chronic infarcts, leukoencephalopathy, and cerebral atrophy. Chest CT revealed multiple bronchiectases with infection in the left lung with concurrent infection, as well as a severe “sandglass-shaped” narrowing at the aortic isthmus, with a minimum lumen diameter of 5 mm and calcification of the descending aortic wall. The aortic sinus was aneurysmally dilated to approximately 46 mm.
Further evaluation using computed tomography angiography (CTA) confirmed severe CoA, with near-complete interruption of the descending aorta at the isthmus and extensive collateral circulation, including prominently dilated and tortuous internal thoracic arteries (Figure 1). The renal arteries were assessed and found to be unremarkable, with preserved renal function throughout hospitalization.
Figure 1
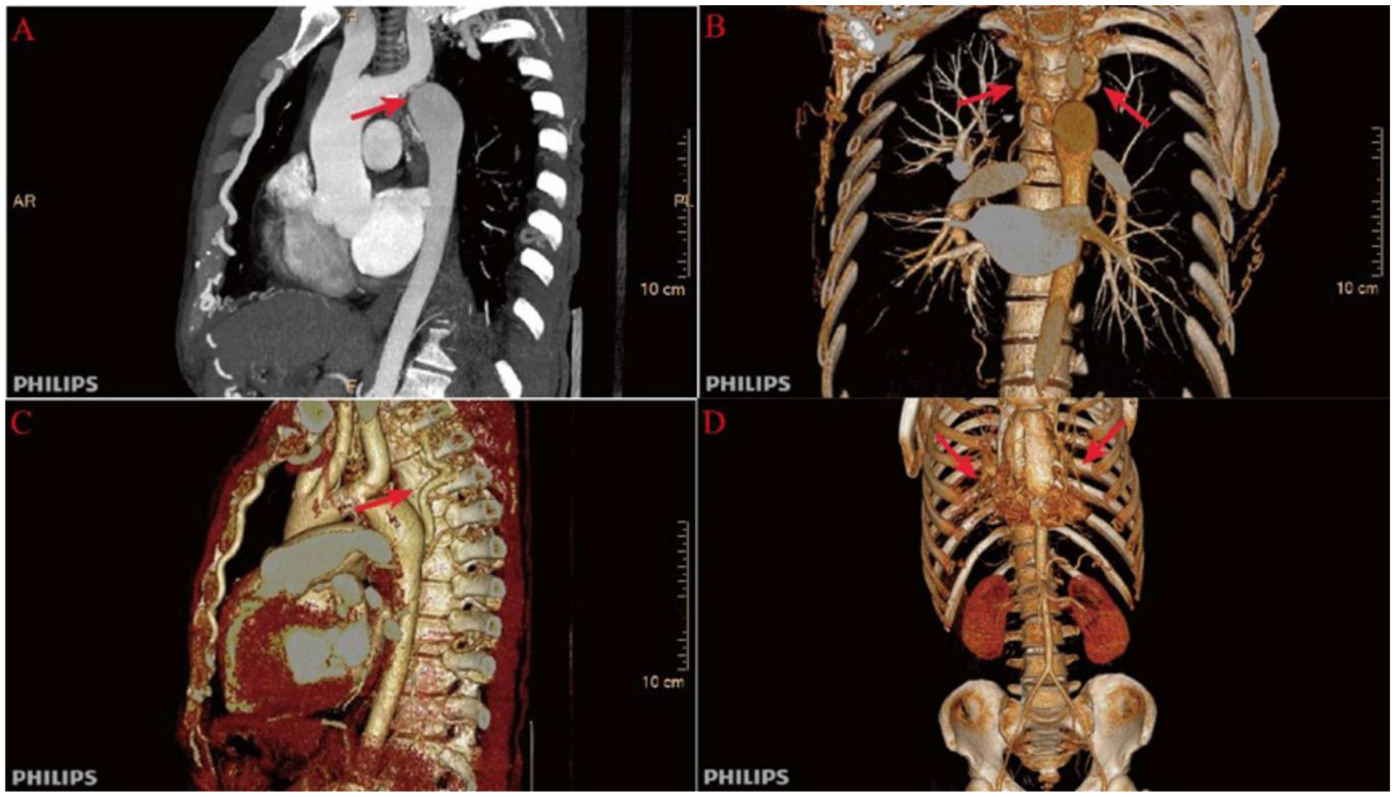
Preoperative computed tomography angiography (CTA) findings. (A) Severe narrowing at the aortic isthmus (“sandglass-shaped” coarctation, arrow); (B, C) Markedly dilated and tortuous paravertebral arteries, demonstrating extensive collateral circulation; (D) Prominent internal thoracic arteries serving as major collateral pathways.
Transthoracic echocardiography revealed a dilated aortic sinus, significant left ventricular hypertrophy, and mild mitral regurgitation, with preserved left ventricular systolic function. The aortic valve was tricuspid. The ascending aorta was within normal limits, while the aortic arch and descending aorta were poorly visualized. A measurable pressure gradient across the coarctation was not obtained by echocardiography due to poor acoustic windows and the complex anatomy. Abdominal and upper limb vascular ultrasound showed a hyperechoic hepatic lesion consistent with hemangioma, along with bilateral carotid intima-media thickening and atherosclerotic plaques. No abnormalities were found in the upper limb arteries or veins.
Cranial magnetic resonance imaging (MRI) showed multiple old lacunar infarcts, gliosis, demyelination (Fazekas grade I), and senile cerebral changes. Magnetic resonance angiography (MRA) revealed no significant abnormalities. Laboratory tests were unremarkable. Based on these findings, the patient was diagnosed with: (1) aortic coarctation complicated by hypertensive crisis, (2) cerebral hemorrhage (recovery phase), and (3) bronchiectasis with active infection.
Initial treatment included triple antihypertensive therapy with nifedipine controlled-release tablets (30 mg once daily), metoprolol sustained-release tablets (47.5 mg once daily), and sacubitril/valsartan (200 mg twice daily), combined with anti-infection therapy. In this case, the use of sacubitril/valsartan was an individualized clinical decision based on the patient's persistent hypertension despite long-term calcium channel blocker therapy and potential cardiac remodeling factors. Although not a first-line antihypertensive option, this regimen partially alleviated the patient's symptoms given the complex clinical context. It must be emphasized that strict evaluation of indications is mandatory when considering its use in patients without left ventricular dysfunction.
Prior to surgery, coronary angiography was performed due to the patient's age and risk factors. The angiogram demonstrated left main trunk dilation, mild plaques in the mid-left anterior descending artery, diffuse dilation of the circumflex artery, and a diminutive right coronary artery, but no significant coronary artery stenosis (Figure 2). These findings effectively excluded significant coronary artery disease preoperatively.
Figure 2
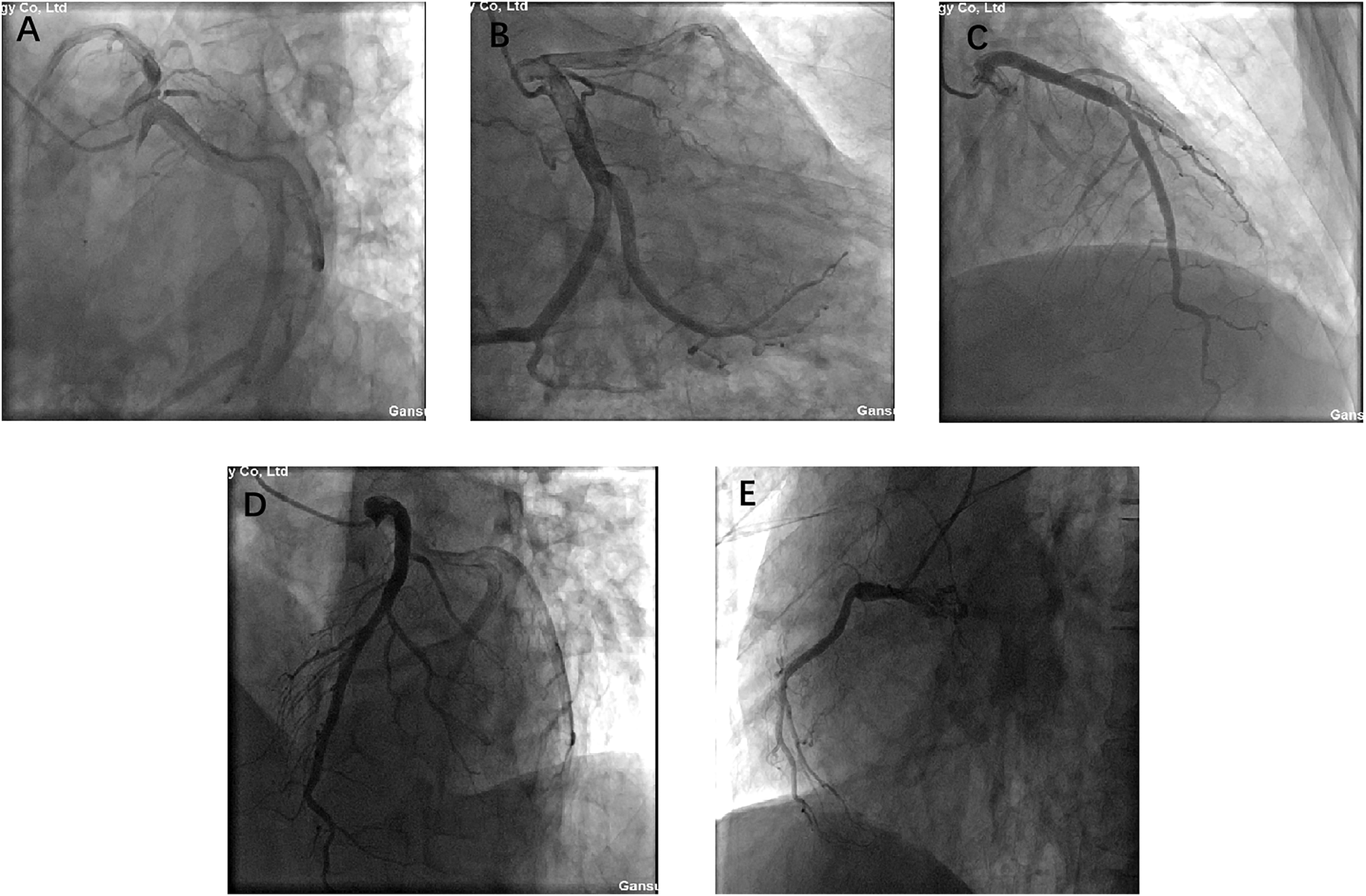
A, B, C, D, and E present coronary angiograms in different projections. The results indicate: dilation of the left main coronary artery, plaque in the mid-to-distal segment of the anterior descending branch, diffuse dilation of the circumflex artery, hypoplasia of the right coronary artery, no significant coronary stenosis, and normal blood flow.
Regarding surgical planning, traditional resection of the coarctation with replacement via left-sided thoracotomy was considered. However, this approach was deemed technically unfeasible and high risk due to (1) the nearly complete aortic interruption and severe calcification evident on imaging, (2) extensive collateral circulation, (3) the risk of complications from aortic arch clamping (including potential spinal ischemia due to interruption of collateral vertebral flow), (4) the patient's comorbidities (recent cerebral hemorrhage and active pulmonary infection), which increased the risks of cardiopulmonary bypass and postoperative complications. Therefore, an extra-anatomic bypass from the left subclavian artery to the descending aorta was selected as the optimal approach, as it avoided direct manipulation of the aortic arch and did not require cardiopulmonary bypass. This strategy is supported by recent literature, which indicates that extra-anatomic bypass provides favorable outcomes in elderly or complex adult CoA cases (7).
After comprehensive evaluation and multidisciplinary team (MDT) discussion, surgical correction was deemed necessary. Given the patient's age, vascular anatomy, and the extent of aortic interruption with heavy calcification, endovascular intervention was considered unfeasible. An extra-anatomic bypass procedure using a left subclavian artery-to-descending aorta synthetic graft was selected as the optimal approach. The left subclavian artery, with an internal diameter of 15 mm, was suitable as the proximal anastomosis site. A post-stenotic aneurysm of the descending aorta was also noted, measuring approximately 54.0 mm × 33.2 mm in maximal cross-sectional diameter.
Surgical procedure details
After induction of general anesthesia and endotracheal intubation, right upper limb blood pressure was 182/96 mmHg, and right lower limb blood pressure was 106/75 mmHg. A standard left thoracotomy was performed through the fourth intercostal space, encountering dense pleural adhesions and abundant collateral vessels (ligated using 7-0 sutures and electrocautery). Single-lung ventilation was initiated to facilitate exposure. The left subclavian artery (proximal segment) and descending aorta (distal to the coarctation) were carefully dissected and isolated. The descending aorta was partially clamped, and a longitudinal arteriotomy (∼4 cm) was made. A 22 mm synthetic vascular graft (Dacron) was anastomosed end-to-side to the descending aorta using 4-0 Prolene, then reinforced with pledgeted sutures. The graft was clamped, and the aortic clamp released to assess for bleeding. The left subclavian artery was similarly clamped, incised, and anastomosed end-to-side to the other end of the graft. The graft was de-aired, and hemostasis confirmed. Intraoperative monitoring showed immediate improvement in lower limb blood pressure (right upper limb: 106/70 mmHg, right lower limb: 108/75 mmHg). No intraoperative complications occurred.
No postoperative anticoagulation or antiplatelet therapy was administered, in accordance with our institutional protocol for this procedure in the absence of other indications.
After surgery, the patient's antihypertensive regimen was gradually reduced in a stepwise fashion. At discharge, only metoprolol was continued, and both nifedipine and sacubitril/valsartan were discontinued. Postoperative blood pressure measurements were balanced across all four limbs: 115/69 mmHg (right upper limb) and 126/75 mmHg (left lower limb). Follow-up CTA confirmed satisfactory graft patency and flow (Figure 3).
Figure 3
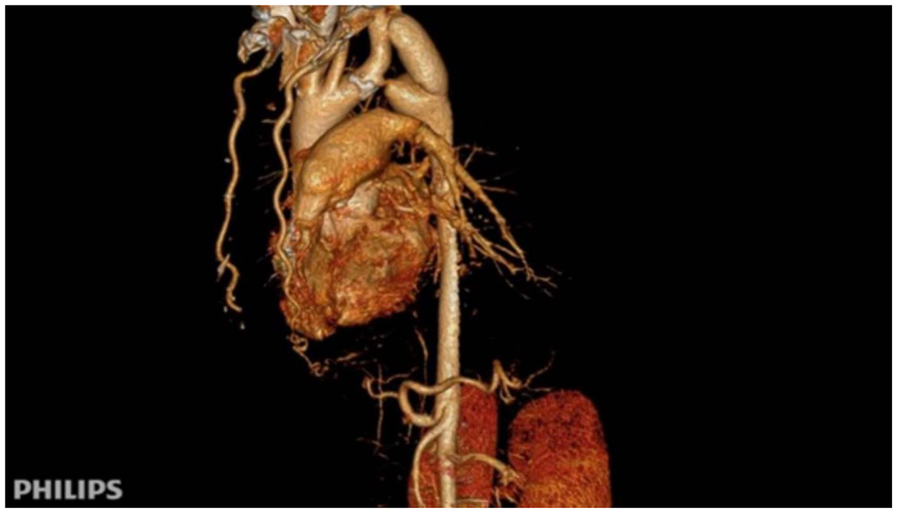
Immediate postoperative CTA following subclavian-to-descending aorta bypass. Patency of the synthetic vascular graft (Dacron) from the left subclavian artery to the descending aorta is demonstrated, with normalization of the aortic lumen distal to the coarctation.
After more than six months of follow-up observation, the patient reported complete resolution of cardiopulmonary and neurological symptoms. Blood pressure remained well controlled, and there was no recurrence of symptoms. Serial imaging demonstrated continued graft patency without evidence of complications such as graft occlusion, pseudoaneurysm, infection, or aneurysmal progression (Figure 4). Additional axial CTA images are provided (Figure 5) to better illustrate the extent of aortic calcification.
Figure 4
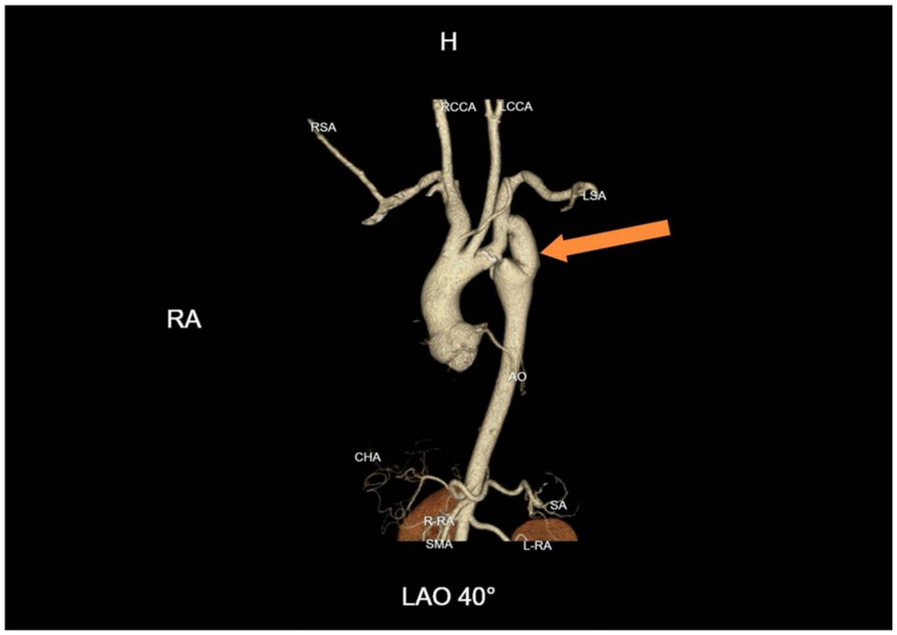
One-year follow-up CTA demonstrating graft patency. Continued patency of the bypass graft with no evidence of pseudoaneurysm, graft occlusion, or aneurysmal progression at the coarctation site.
Figure 5
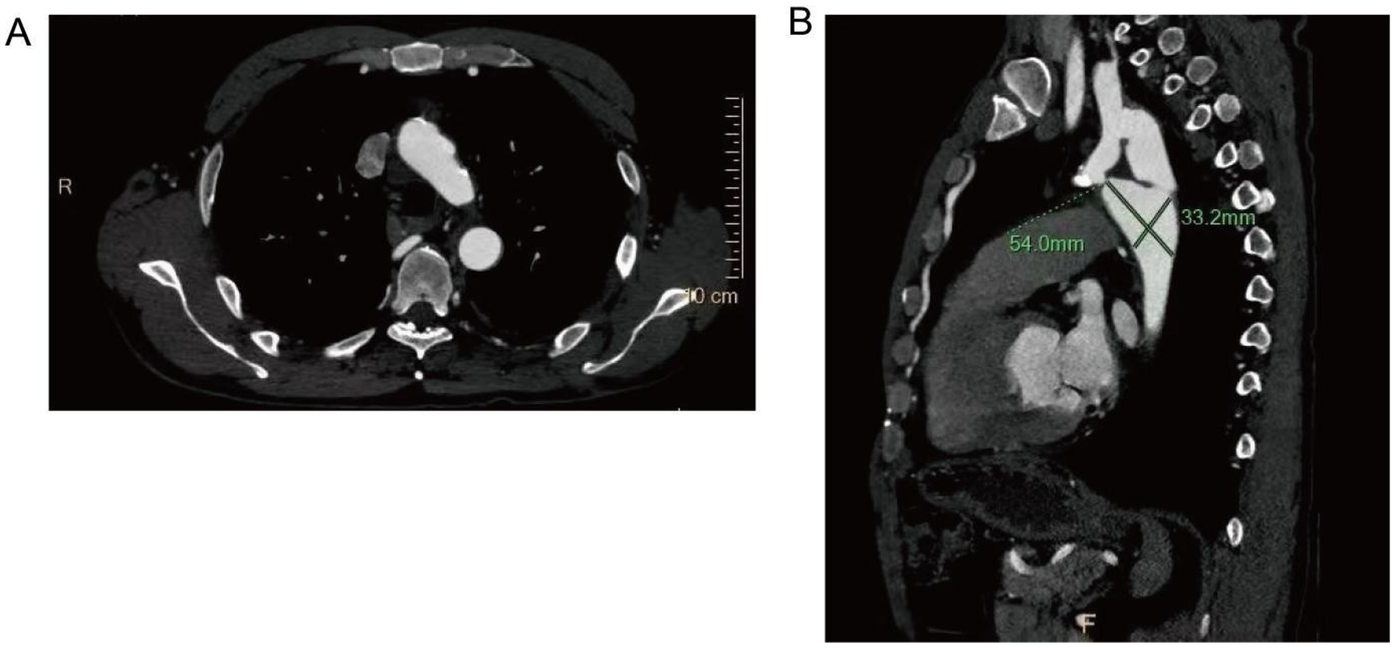
Scattered calcified plaques are observed in the walls of the aortic arch and brachiocephalic trunk, with irregular lumens as shown in (A). The descending aorta shows poststenotic aneurysmal dilation, with dimensional measurements provided in (B).
To contextualize this case, we conducted a literature review of 21 previously reported cases of aortic coarctation in adults and elderly patients (Table 1). Patients ranged in age from 3 months to 65 years, though the majority were adults over 40 years old. Of these, seven underwent prosthetic graft replacement (with three requiring cardiopulmonary bypass), ten were treated with interventional (endovascular) therapy, and one was managed conservatively due to multi-organ involvement. Three cases—including the present patient—were treated with a left subclavian artery-to-descending aorta bypass, all of which had favorable outcomes and symptomatic improvement. The remainder were managed with either resection and prosthetic graft replacement or endovascular intervention (5, 6).
Table 1
| No. | First author (Year) | Country | Age (years) | Sex | Diagnosis | Combined lesions | Treatment plan | Cardiopulmonary bypass | Postoperative complications | Follow-up | Main conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (12) | Niwa K, et al. (2023) | Japan | 56 | M | CoA | – | Descending aorta graft replacement | Not mentioned | None | Not mentioned | Symptom improvement |
| (13) | Nicole Schulick, et al. (2022) | USA | 3 months | F | CoA | – | LSA–DA–descending aortic bypass | None | None | 7 months | Symptom improvement |
| (14) | Arun Sharma, et al. (2024) | India | 24 | M | CoA + BAV | 3) | Ascending–DA–descending aortic bypass | Yes | None | 6 months | Symptom improvement |
| (15) | D B H Verheijen, et al. (2022) | Netherlands | 40 | M | CoA | – | Stent implantation | None | None | 20 months | Symptom improvement |
| (16) | Herrera Mingorance JD, et al. (2022) | Spain | 41 | M | CoA + LV dysfunction | – | Balloon angioplasty + stent | None | None | 8 months | Symptom improvement |
| (17) | Hanazuka T, et al. (2022) | Japan | 22 | F | CoA | – | Balloon angioplasty + stent | Not mentioned | Re-stenosis after 2 years, hematuria | 3 years | Prognosis unclear |
| (18) | Abuji K, et al. (2023) | India | 16 | F | CoA | 3) | LSA–DA–descending aortic bypass | Not mentioned | None | 1 year | Good recovery |
| (19) | Thanigai Arasu, et al. (2024) | India | 6 | M | CoA | – | Ascending–DA–descending aortic bypass | Yes | Recurrent leg weakness | Not mentioned | Symptom improvement |
| (20) | An M Van Berendoncks, et al. (2024) | Belgium | 20 | F | CoA | – | Stent implantation | None | None | 2 years | Symptom improvement |
| (21) | Frank Molina-Ricaurte, et al. (2022) | Chile | 41 | M | CoA + LV dysfunction | – | Aortic graft + ascending–DA–descending bypass | Yes | None | 30 months | Symptom improvement |
| (22) | Mitsuru Sato, et al. (2024) | Japan | 6 | F | CoA | – | Modified graft descending aortic replacement | None | None | 8 years | Symptom improvement |
| (23) | Sawaka Tanabe, et al. (2022) | Japan | 57 | F | CoA + LV dysfunction | 4) | LSA–DA–descending aortic bypass | Not mentioned | None | Not mentioned | Symptom improvement |
| (24) | Sanjay Tyagi, et al. (2022) | India | 43 | F | CoA | – | Balloon angioplasty + stent | Not mentioned | Mild leg weakness | 2 years | Symptom improvement |
| (25) | Davide Reis, et al. (2022) | Portugal | 23 | M | CoA | – | Left cervical–DA bypass + stent | Not mentioned | None | Not mentioned | Symptom improvement |
| (26) | Zhang, et al. (2018) | China | 40 | M | CoA | 3), 4), 5) | Conservative treatment | No | None | 3 months | Good recovery |
| (27) | Onohara, et al. (2014) | Japan | 65 | M | CoA | 3) | Aortic graft replacement | Yes | Not mentioned | 6 months | Good recovery |
| (28) | Natraj Setty, et al. (2019) | India | 9 | M | CoA | 4), 5) | Balloon angioplasty | No | Not mentioned | Not mentioned | Good recovery |
| (29) | Luo, et al. (2020) | China | 44 | M | CoA | 4) | Balloon angioplasty + stent | Not mentioned | None | 2 years | Good recovery |
| (30) | Ghaderian, et al. (2019) | Iran | 3 months | M | CoA | 5) | Balloon angioplasty + stent | Not mentioned | None | Not mentioned | Symptom improvement |
| (31) | Bayar, et al. (2015) | Turkey | 41 | M | CoA | 1), 6) | Drug therapy | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
| (32) | Armistead, et al. (2018) | USA | 26 | M | CoA | 2), 6) | No surgical intervention | Not mentioned | Not mentioned | Not mentioned | Not mentioned |
Overview of published cases of aortic coarctation treatment.
CoA, coarctation of the aorta; BAV, bicuspid aortic valve; LV, left ventricular; LSA, left subclavian artery; DA, descending aorta; –, not applicable/no combined lesion; 1) hypertension; 2) Turner syndrome; 3) bicuspid aortic valve; 4) left ventricular hypertrophy/dysfunction; 5) aortic valve regurgitation; 6) other congenital anomalies.
Discussion
This case report presents a rare instance of CoA in a 62-year-old male, complicated by hypertensive crisis, cerebral hemorrhage, and bronchiectasis. Advanced imaging revealed a near-complete interruption of the descending aorta, accompanied by extensive collateral circulation. An extra-anatomic bypass graft from the left subclavian artery to the descending aorta was successfully performed, effectively eliminating the pressure gradient, normalizing systemic blood pressure, and relieving associated symptoms. At the six-months follow-up, the graft remained patent with no postoperative complications, highlighting the feasibility and durability of this surgical approach in the management of complex adult CoA.
Imaging modalities played a pivotal role in both the diagnosis and the management plan for this patient. In adults suspected of having CoA, contrast-enhanced CTA is generally considered the gold standard for anatomical assessment, allowing for detailed visualization of the coarctation site, extent of collateral formation, and degree of vascular calcification. Echocardiography offers valuable functional information, while magnetic resonance angiography provides a non-radiative alternative for follow-up and comprehensive vessel evaluation. Notably, given the history of cerebral hemorrhage, the patient underwent preoperative cerebrovascular assessment using cranial magnetic resonance angiography (MRA). The MRA results ruled out the presence of intracranial aneurysms, further supporting that the cerebral hemorrhage was more likely attributable to long-term hypertensive cerebrovascular injury rather than an inherent cerebrovascular malformation. This highlights the importance of comprehensive neurovascular imaging in guiding etiological assessment and treatment decisions for such patients with late-stage aortic coarctation presenting with neurological symptoms. In this case, CTA was critical not only in confirming the diagnosis but also in preoperative planning, particularly in assessing the suitability for different intervention options and mapping collateral circulation.
The review included 21 articles reporting 21 cases, with patient ages ranging from 3 months to 65 years. Seven underwent prosthetic graft replacement (three with cardiopulmonary bypass), ten received interventional therapy, and one was managed conservatively due to multi-organ involvement.
In this case, the collateral vessels, which included dilated internal thoracic, intercostal, and vertebral arteries, effectively reduced the pressure gradient across the stenotic segment, delaying end-organ damage and allowing prolonged survival. However, collateral circulation also obscured classical symptoms, leading to incidental detection during an unrelated examination. A similar phenomenon has been reported in previous studies, where the diagnosis of CoA in adults was often incidental and associated with complications such as intracranial hemorrhage, hypertensive crisis, or heart failure (7). This case highlights the need for heightened clinical vigilance in patients with unexplained hypertension, cardiac workload elevation, or neurological symptoms, particularly when associated with upper and lower limb blood pressure discrepancies.
Regarding the pathophysiological relationship between bronchiectasis and CoA in this patient, we propose that long-standing severe CoA resulted in marked dilation of collateral vessels (especially bronchial arteries), which may exert extrinsic compressive effects on adjacent bronchi. Chronic hypoperfusion distal to the coarctation may also contribute to airway ischemia and impaired mucociliary clearance, predisposing to recurrent infections and secondary bronchiectasis.
Given the rarity of adult CoA, especially with near-complete aortic interruption, there are few formal recommendations or consensus guidelines for its management, and most treatment strategies are based on case reports and small case series. The current literature suggests that endovascular stenting has become the first-line treatment for most adults with simple, discrete CoA due to its minimally invasive nature and favorable short-term outcomes (8). Importantly, the presence of aortic wall calcification is not considered an absolute contraindication for catheter-based intervention, and successful stenting has been reported even in calcified segments. However, in cases of severe anatomical abnormality—such as long-segment interruption, extreme tortuosity, or heavy calcification—endovascular approaches may not be feasible or safe, necessitating surgical solutions.
Traditional open repair methods, such as resection with end-to-end anastomosis or patch augmentation, are effective for certain CoA cases but may not be suitable for elderly patients or those with significant collateral circulation and fibrosis at the coarctation site (9). In this context, the left subclavian artery-to-descending aorta bypass grafting performed in this case proved to be a safe and effective alternative. This approach avoided the need for cardiopulmonary bypass, reduced intraoperative risks, and provided physiological hemodynamic improvement through an extra-anatomic bypass. The successful outcome in this patient demonstrates the feasibility and advantages of this technique in elderly patients with complex vascular anatomy.
The diagnostic methods used in this case, particularly CTA, were crucial in confirming the diagnosis and planning the surgical approach. CTA provided detailed visualization of the coarctation site, collateral circulation, and vascular calcification, which guided the choice of bypass grafting (7). Echocardiography and cranial imaging further supported the comprehensive assessment of the patient's condition. This case suggests that in hypertensive patients with persistent symptoms—such as blood pressure discrepancies, neurological manifestations (e.g., blurred vision or tinnitus), or refractory hypertension—vascular anomalies like CoA should be considered. Incorporating imaging techniques (e.g., echocardiography or Doppler ultrasound) at an early stage may improve detection rates of adult CoA. Future advancements in imaging, such as high-resolution magnetic resonance angiography, may further enhance diagnostic accuracy while minimizing patient risk (10).
Although the surgical outcome in this case was successful, alternative treatment strategies may be appropriate for similar cases. Hybrid approaches combining endovascular techniques with open repair could be effective for patients with complex CoA, reducing surgical trauma while addressing extensive vascular abnormalities. Long-term management is crucial for this patient due to the risks of aneurysm formation, graft occlusion, and recurrent hypertension. Given that untreated descending thoracic aortic aneurysm patients have a 5-year survival rate of approximately 54%, with aortic rupture being the leading cause of death, we recommend implementing a standardized postoperative follow-up protocol. This should include regular imaging studies to assess graft patency and aneurysm status, combined with blood pressure monitoring, to enable early detection of complications and timely intervention. Advances in graft materials and techniques, such as bioengineered vascular grafts, may further improve long-term outcomes by reducing thrombosis risk and enhancing biocompatibility (11).
A major limitation of this report is the duration of follow-up. Six months to one year is not sufficient to assess the long-term patency and durability of the graft, nor to fully evaluate risks such as late graft occlusion, pseudoaneurysm, or recurrence of hypertension. Longer-term follow-up and larger case series are needed to better understand the outcomes of surgical and endovascular repair in adult CoA.
This case underscores the importance of individualized treatment strategies for CoA in adult and elderly patients with complex vascular anatomy. It highlights the role of multidisciplinary collaboration in achieving successful outcomes and emphasizes the need for improved diagnostic protocols to enable early detection and management of CoA in adults.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethic Committee of Gansu Provincial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant(s)/patient(s) for the publication of this case report.
Author contributions
BW: Investigation, Methodology, Conceptualization, Writing – review & editing, Writing – original draft, Data curation. ZZ: Methodology, Conceptualization, Investigation, Writing – review & editing, Data curation, Writing – original draft. KC: Writing – review & editing, Formal analysis, Writing – original draft, Conceptualization. XZ: Project administration, Writing – review & editing, Writing – original draft. FG: Investigation, Writing – review & editing, Methodology, Formal analysis, Data curation, Writing – original draft. PX: Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Gansu Provincial Natural Science Foundation (No. 23JRRA1287), Gansu Provincial Hospital Excellent Doctoral Student Training Program (No. 22GSSYD-14), the Gansu Province Traditional Chinese Medicine Research Project (No. GZKZ-2021-7) and Gansu Province Joint Research Fund (No. 24JRRA886).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Law MA Collier SA Sharma S Tivakaran VS . Coarctation of the aorta. In: Statpearls. Treasure Island (FL): StatPearls Publishing (2025).
2.
Raza S Aggarwal S Jenkins P Kharabish A Anwer S Cullington D et al Coarctation of the aorta: diagnosis and management. Diagnostics (Basel). (2023) 13(13):2189. 10.3390/diagnostics13132189
3.
Geggel RL . Coarctation of the aorta: delay in diagnosis and referral basis from infancy to adulthood. J Pediatr. (2022) 242:57–62. 10.1016/j.jpeds.2021.11.066
4.
Geggel RL . Coarctation of the aorta: delay in diagnosis and referral basis from infancy to Adulthood. J Pediatr. (2022) 242:57–62. 10.1016/j.jpeds.2021.11.066
5.
Hirose H Gill I . Left subclavian artery to descending aorta bypass for coarctation physiology after descending aortic repair. Ann Thorac Cardiovasc Surg. (2005) 10:389–90.
6.
Kinoglu B Hokenek F Ugurlucan M Kaplan L . Subclavian to aorta bypass for adult aortic coarctation. Heart Views. (2010) 11(1):24.
7.
Cardoso MRR Crestani AM Souza AS Braga F Brun MM Murakami AN et al Role of computed tomography angiography in the short-term follow-up of aortic coarctation repair. Braz J Cardiovasc Surg. (2024) 39(1):e20230220. 10.21470/1678-9741-2023-0220
8.
Wong HCY Chan AW David E Marta GN Pan NY Koller M et al Should endovascular stenting be used routinely as first-line treatment for malignant superior Vena Cava syndrome?-a critical review in the context of recent advances in oncological treatments. Ann Palliat Med. (2023) 12(4):803–15. 10.21037/apm-22-1293
9.
Vasile CM Laforest G Bulescu C Jalal Z Thambo JB Iriart X . From Crafoord’s end-to-end anastomosis approach to percutaneous interventions: coarctation of the aorta management strategies and reinterventions. J Clin Med. (2023) 12(23):7350. 10.3390/jcm12237350
10.
Kim BK You SH Kim B Shin JH . Deep learning-based high-resolution magnetic resonance angiography (MRA) generation model for 4D time-resolved angiography with interleaved stochastic trajectories (TWIST) MRA in fast stroke imaging. Diagnostics (Basel). (2024) 14(11):1199. 10.3390/diagnostics14111199
11.
Di Francesco D Pigliafreddo A Casarella S Di Nunno L Mantovani D Boccafoschi F . Biological materials for tissue-engineered vascular grafts: overview of recent advancements. Biomolecules. (2023) 13(9):1389. 10.3390/biom13091389
12.
Niwa K Nishikawa H Ueda D Yamashita K . Surgical treatment for coarctation of the aorta. Clin Case Rep. (2023) 11(9):e7953. 10.1002/ccr3.7953
13.
Schulick N Lin Y Fonseca B Campbell DN L Stone M . Surgical management of a collateral arch channel and aortic coarctation. J Card Surg. (2022) 37(2):445–8. 10.1111/jocs.16135
14.
Sharma A Kumar R Bansal V Negi S Kumar H . Surgical management of juxtaductal coarctation of the aorta in adults: a novel technique. Cureus. (2024) 16(8):e66843. 10.7759/cureus.66843
15.
Verheijen DBH Stöger JL van der Kley F Schalij MJ Jongbloed MRM Vliegen HW et al A percutaneous treatment strategy of an adult patient with a bicuspid aortic valve, coarctation of the aorta, and an exceptionally large aneurysm of a collateral artery: case report and literature overview. Front Cardiovasc Med. (2022) 9:1012147. 10.3389/fcvm.2022.1012147
16.
Herrera Mingorance JD Pérez Bailón AM Moreno Escobar JM Salmerón Febres LM . Coarctation of the aorta: an atypical case treated by a double layer stent technique. EJVES Vasc Forum. (2022) 55:23–6. 10.1016/j.ejvsvf.2022.02.005
17.
Hanazuka T Sakata T Ueda H Watanabe M Matsumiya G . Late open conversion after endovascular treatment for the coarctation of aorta in adult due to restenosis with thrombus. J Vasc Surg Cases Innov Tech. (2022) 8(3):338–44. 10.1016/j.jvscit.2022.04.008
18.
Abuji K Vaddavalli VV Kumar D Maheshwari N Gorsi U Kaman L et al Coarctation of the aorta in a young female treated with left subclavian artery to descending thoracic aorta bypass: a case report. J Vasc Bras. (2022) 21:e20220018. 10.1590/1677-5449.202200182
19.
Arasu T Kumar R Khajuria U Komal T . Delayed paraparesis: an unusual complication following coarctation of aorta repair. Ann Card Anaesth. (2024) 27(1):82–4. 10.4103/aca.aca_98_23
20.
Van Berendoncks AM Mannaerts D Berzenji L Jacquemyn Y Hendriks JMH . First diagnosis of severe coarctation of the aorta necessitating percutaneous intervention during pregnancy: a case report. Eur Heart J Case Rep. (2024) 8(10):ytae547. 10.1093/ehjcr/ytae547
21.
Molina-Ricaurte F Sepúlveda E Lucero-Escudero F Sanz-Cucui G Cuevas O . One stage surgical treatment of aortic coarctation associated with bicuspid aortic valve. Report of one case. Rev Med Chil. (2022) 150(3):402–5. 10.4067/s0034-98872022000300402
22.
Sato M Masaki N Sai S . Combined procedure using a double flap as a surgical option for coarctation of the aorta with delayed diagnosis. Gen Thorac Cardiovasc Surg. (2025) 73(1):66–9. 10.1007/s11748-024-02071-5
23.
Tanabe S Morioka K Yamada N Takamori A Kawamura Y Mizuna T . Subclavian-aortic bypass for management of aortic coarctation in adults:report of a case. Kyobu Geka Jpn J Thorac Surg. (2022) 75(8):648–51.
24.
Tyagi S Bansal A Gupta MD Narang P Gupta H Batra V et al Paraparesis in adult aortic coarctation: reversal by stent supported angioplasty. J Cardiol Cases. (2022) 26(3):200–3. 10.1016/j.jccase.2022.04.009
25.
Reis D Gomes T Teixeira A Martins C . Secondary hypertension and aortic coarctation in a young adult: a case report. Radiol Case Rep. (2024) 19(7):2891–4. 10.1016/j.radcr.2024.03.048
26.
Zhang H Feng L . Coarctation of the aorta complicated with intracranial aneurysm: a case report and literature review. World Neurosurg. (2018) 112:25–30. 10.1016/j.wneu.2018.01.011
27.
Onohara D Sato A Tasaki Y Yamada T . Co-existence of severe coarctation of the aorta and aortic valve stenosis in a 65-year-old woman: a case report. Ann Thorac Cardiovasc Surg. (2014) 20 Suppl:750–3. 10.5761/atcs.cr.13-00216
28.
Natraj Setty HSS Shivanand P Narendhiran P Veeresh P Jayashree K Raghu TR et al Coarctation of aorta presenting as spontaneous subarachnoid hemorrhage in a young female: a case report of a rare clinical entity. Cardiol Res. (2019) 10(4):241–4. 10.14740/cr787
29.
Luo W Li J Huang X Cai X . Late diagnosis of coarctation of the aorta in a 44-year-old male: a case report. BMC Cardiovasc Disord. (2020) 20(1):470. 10.1186/s12872-020-01753-1
30.
Ghaderian M Sabri MR . Report of a coarctation of aorta stenting in an infant. Adv Biomed Res. (2019) 8:10. 10.4103/abr.abr_232_18
31.
Bayar N Arslan Ş Üreyen ÇM Küçükseymen S Erol B . A rare combination of vascular anomalies: hypoplastic aortic arch, coarctation of the aorta and poststenotic aneurysm. Turk Kardiyol Dern Ars. (2015) 43(3):272–4. 10.5543/tkda.2015.89084
32.
Armistead S Mullins D Sherick S Ashurst J . Emergent presentation of an adult with undiagnosed coarctation of the aorta. Case Rep Emerg Med. (2018) 2018:5756983. 10.1155/2018/5756983
Summary
Keywords
aortic coarctation, hypertension, vascular surgical procedures, collateral circulation, multidisciplinary care
Citation
Wang B, Zhang Z, Chen K, Zhou X, Gao F and Xie P (2025) Subclavian-to-descending aortic bypass for the treatment of severe late-stage aortic coarctation in a 62-year-old adult: a case report and literature review. Front. Cardiovasc. Med. 12:1635499. doi: 10.3389/fcvm.2025.1635499
Received
26 May 2025
Accepted
22 September 2025
Published
09 October 2025
Volume
12 - 2025
Edited by
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, Italy
Reviewed by
Yeganeh Pasebani, Iran University of Medical Sciences, Iran
Amine Mamoun Boutaleb, Centre Hospitalier Universitaire de Limoges, France
Tran Tien Manh, Hanoi Heart Hospital, Vietnam
Stefan Van Dinter, Radboud University Medical Center, Netherlands
Updates
Copyright
© 2025 Wang, Zhang, Chen, Zhou, Gao and Xie.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Ping Xie 381592934@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.