Abstract
Background:
Early prediction of heart failure (HF) after acute myocardial infarction (AMI) remains a clinical challenge. There is a lack of studies investigating Thymosin α1 expression levels in AMI patients and its relationship with cardiac function post-AMI.
Methods:
This retrospective analysis included patients with AMI from December 2019 to February 2022. The baseline data of two groups were collected. Thymosin α1 expression level of peripheral blood plasma in AMI patients was examined by ELISA. Logistic regression analysis was applied to evaluate risk factors in-hospital cardiac dysfunction after emergency PCI in AMI patients. Receiver operating characteristic (ROC) curve was used to analyze the predictive value of the biomarker.
Results:
A total of 307 hospitalized patients were enrolled in this study, divided into AMI group (n = 274) and non-AMI group (n = 33). The expression level of thymosin α1 in the AMI group was significantly higher than in the non-AMI group. The AMI patients were divided into two subgroups based on the EF values. The sample size was 64 (EF < 50%) and 210 (EF ≥ 50%), respectively. The expression of thymosin α1 in the EF ≥ 50% group was significantly higher than EF < 50% group. Spearman's correlation analysis demonstrated that thymosin α1 was positively correlated with the EF value. Logistic multivariate analysis suggested that thymosin α1, NT-proBNP, and creatine kinase were independent predictors of cardiac function after AMI. The AUC of thymosin α1, NT-proBNP, and creatine kinase was 0.614, 0.714, and 0.724, respectively.
Conclusion:
Thymosin α1 may serve as a potential biomarker to predict cardiac function following AMI. This study may provide novel insights into the potential therapeutic targets for HF following AMI.
1 Introduction
Coronary heart disease remains one of the leading causes of morbidity and mortality worldwide (1). Acute myocardial infarction (AMI) is the most serious manifestation of coronary heart disease, which seriously threatens human health and increases social burden (2). With the advancement of contemporary medicine, especially the implementation of coronary intervention surgeries, many AMI patients can have their culprit vessels opened in a timely and effective manner (3). As a result, the mortality rate has significantly decreased compared to the past (3). However, the complications of AMI cannot be ignored, as they seriously affect the patient's life and health as well as their quality of life. AMI is frequently associated with numerous complications, among which new-onset or chronic heart failure (HF) after discharge is a prevalent one (4). The emergence of HF after an MI can notably enhance the mortality rate and the risk of recurrent hospitalizations for this patient group (5). Therefore, the early and accurate detection of post-MI cardiac dysfunction through clinically effective laboratory indicators, and the subsequent administration of effective treatment, constitute the most efficacious strategies to mitigate the long-term adverse prognosis of this patient group.
Thymosins were initially purified from the calf thymus by Allan L. Goldstein in 1966 as a biologically active substance (6). Subsequent research has disclosed that numerous tissues in the human body can secrete thymosin (7). Due to the isoelectric point, thymosins are divided into isoforms α (pI < 5), β (5 < pI < 7), and γ (pI > 7) (7). T-β4 is the most abundant β-thymosin (constituting approximately 70%–80%) and holds numerous biological effects, including cell proliferation and apoptosis, inflammatory responses, and fibrosis within the body (7–9). A significant number of studies have been conducted on the role of T-β4 in myocardial fibrosis and cardiac function after AMI, suggesting its potential protective mechanisms in the myocardium following AMI (10, 11). Thymosin α1 is related to various biological effects in the body and mainly participates in immune regulation (12). It can promote the maturation and differentiation of T lymphocytes, stimulate the secretion of various lymphokines by mature T cells and NK cells, and augment the body's immune response and resistance to infection (7, 12). Moreover, it has an anti-inflammation protective effect to reduce the damage of central nervous system diseases (13). Given the regulation of thymosin α1 on the immune function of the body, in clinical practice, thymosin α1 is mainly used for the combined treatment of cancers (14), the treatment of hepatitis B virus (15), and the treatment of HIV patients (16). The inflammatory response following AMI plays a crucial role in the progression of myocardial injury. However, the role of Thymosin α1 post-AMI remains poorly understood. The potential of Thymosin α1 as a biomarker for myocardial injury and cardiac dysfunction following AMI is yet to be fully explored.
This study focuses on this issue. By comparing with the control group, the expression level of Thymosin α1 in the peripheral blood of patients with AMI and its relationship with early-onset HF after AMI are clarified. The goal is to identify a novel biomarker for the early clinical detection of HF following AMI, thereby facilitating early intervention and improving long-term outcomes for this patient population.
2 Materials and methods
2.1 Patients
From December 2019 to February 2022, a total of 307 patients were consecutively enrolled as the study subjects, including 274 patients with AMI as the case group, and 33 patients without AMI at enrollment and no prior history of AMI as the control group. All the AMI patients met the diagnostic criteria of the 2023 ESC Guidelines for the management of acute coronary syndromes (17). For AMI patients with confirmed ST-segment elevation myocardial infarction (STEMI), the infarct-related artery (IRA) should be opened within 12 h. For NSTEMI patients, the IRA will be opened within 2, 12, 24, and 72 h according to risk stratification. Moreover, all AMI patients will receive optimal drug therapy according to the guideline (17). The exclusion criteria for this study were as follows: (a) A previous history of AMI; (b) A history of coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI); (c) Severe hepatic/renal insufficiency or chronic obstructive pulmonary disease; (d) Disorders in the hematological, immune, or coagulation systems; (e) The presence of malignant tumors; (f) The existence of psychiatric disorders; (g) Incomplete clinical or laboratory data; (h) Any other circumstances that the researcher deems inappropriate to participate in this study. This study was approved by the Hospital Ethics Committee and the ethical number is 2021-KY-04(K)-(1). All the enrolled patients signed the informed consent form. The study was by the principles of the Declaration of Helsinki and was approved by the Hospital Ethics Committee.
2.2 Clinical or laboratory data
The baseline data of clinical records encompass age, gender, height, weight, systolic blood pressure, heart rate, etc. The past medical history of patients, including hypertension, diabetes, etc., was documented. Blood routine, biochemical markers, and coagulation indicators were all recorded as the fasting blood results in the early morning of patients. Markers of myocardial injury in AMI patients, including troponin, creatine kinase, N-terminal pro-B-type natriuretic peptide (NT-proBNP), etc., were all documented as the highest values during hospitalization. The LVEF assessment was performed within 7 days post-PCI for AMI patients and the Simpson method was used to measure LVEF.
2.3 Thymosin α1 examination by ELISA
Peripheral venous blood samples (5 ml) were collected from all subjects during the initial admission period prior to IRA revascularization. Subsequently, the venous blood was kept at room temperature for 30 min, and the serum was extracted after centrifugation at 1,000 g for 15 min using a centrifuge. The serum was stored at −80°C for subsequent use. The expression of serum thymosin α1 was determined using the enzyme-linked immunosorbent assay (ELISA) kit (Jing Mei Biotechnology, Jiangsu, China. No. JM-03731H1).
2.4 Statistical analysis
Continuous variables with a normal distribution were expressed as mean ± standard deviation (SD), and the independent sample t-test was employed for intergroup comparison; data with a non-normal distribution were expressed as median [interquartile range (IQR)], and the Mann–Whitney U-test was utilized for comparison between groups. Categorical variables were depicted as percentages, and the χ2 test or Fisher's exact test was applied for intergroup comparison.
All the significant variables identified in the intergroup comparison were enrolled in the logistic regression analysis, and the standard error and odd ratio (OR) were documented. The linear relationship between serum thymosin α1 and EF value post-AMI was assessed using the Spearman correlation analysis. The predictive value of Thymosin α1 for cardiac function following AMI was estimated by the receiver operating characteristic (ROC) curve. Statistical significance was considered as P < 0.05. All statistical analyses were performed using SPSS 26.0 statistical software.
3 Results
3.1 Comparison of baseline demographic and clinical data
A total of 307 patients were enrolled in this study, including 274 in the AMI group and 33 in the control group. Baseline data between the two groups, including gender, age, height, weight, BMI, systolic blood pressure, heart rate, smoking rate, drinking rate, prevalence of diabetes, and prevalence of hypertension, showed no statistically significant differences (Table 1).
Table 1
| Variable | AMI (n = 274) | Ccontrol (n = 33) | P-value |
|---|---|---|---|
| Male (n, %) | 219 (79.9) | 23 (69.7) | 0.174 |
| Age (Year) | 65 (55–72) | 66 (59–72) | 0.817 |
| Height (m) | 1.70 (1.64–1.74) | 1.70 (1.59–1.72) | 0.220 |
| Weight (kg) | 70.0 (64.0–78.0) | 67.0 (58.8–82.5) | 0.250 |
| BMI (kg/m2) | 24.68 (22.64–26.57) | 24.22 (22.40–26.94) | 0.596 |
| SBP (mmHg) | 126 (110–141) | 132 (122–140) | 0.290 |
| HR (bpm) | 80 (72–88) | 78 (69–90) | 0.367 |
| Smoking (n, %) | 165 (60.2) | 21 (63.6) | 0.704 |
| Drinking (n, %) | 73 (26.6) | 7 (21.2) | 0.502 |
| Diabetes (n, %) | 58 (21.2) | 6 (18.2) | 0.690 |
| Hypertension (n, %) | 148 (54.0) | 22(66.7) | 0.167 |
Comparison of clinical features between AMI group and control group.
SBP, systolic blood pressure; HR, heart rate.
3.2 Thymosin α1 level was elevated in AMI patients with EF ≥ 50%
Compared with the control group, the expression level of thymosin α1 was significantly elevated in AMI patients (Figure 1). The 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure utilizes the EF value as a crucial basis for chronic HF stratification. HF is classified into two types: HF with reduced ejection fraction (including EF between 41% and 49%, known as HFmrEF, and EF ≤ 40%, known as HFrEF) and HF with preserved ejection fraction (EF ≥ 50%, known as HFpEF) (18). An EF of 50% is an important numerical cut-off for assessing cardiac function reduction. To further clarify the relationship between thymosin α1 and cardiac function in patients with AMI, we categorized AMI patients into two groups based on postoperative echocardiography: one group with an EF < 50% and another group with an EF ≥ 50%. Initially, according to the design, the final number of enrolled patients with an EF < 50% was 64, while the number of patients with an EF ≥ 50% was 210. Baseline data showed no statistically significant differences between the two groups in terms of gender, age, height, weight, systolic blood pressure, heart rate, smoking rate, drinking rate, prevalence of diabetes, and prevalence of hypertension, indicating that the two groups were comparable (Table 2). Furthermore, our study demonstrated that the expression levels of thymosin α1 [2,913.28 (2,648.35–3,247.05) vs. 3,002.50 (2,846.48–3,497.68), P = 0.006] in peripheral blood were significantly higher in patients with an EF ≥ 50% after AMI compared to those with an EF < 50% (Figure 2).
Figure 1
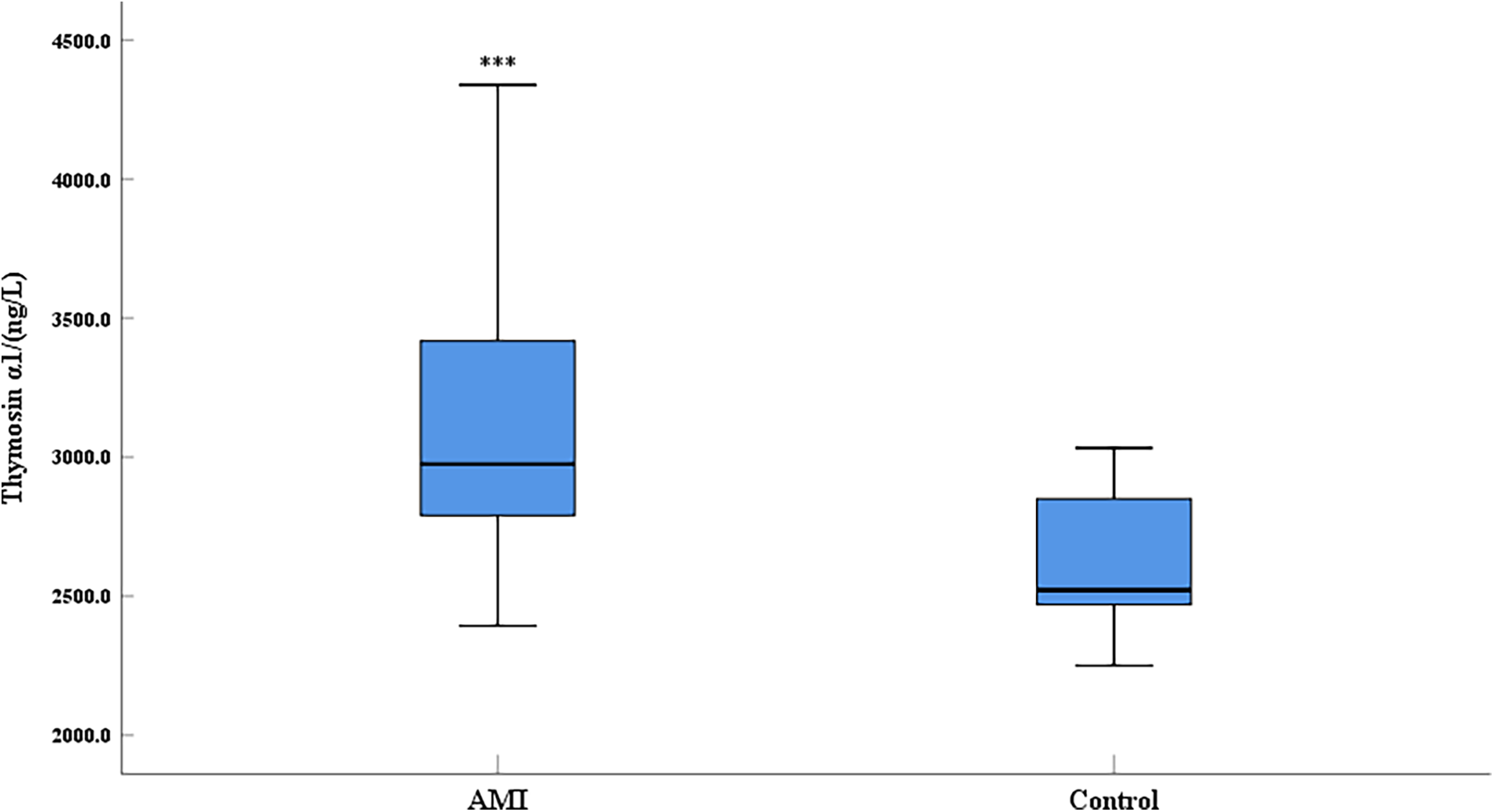
Comparison of serum thymosin α1 level between AMI group and control group (AMI: acute myocardial infarction group, control: control group; ***: P < 0.001).
Table 2
| Variable | LVEF < 50%(n = 64) | LVEF ≥ 50%(n = 210) | P-value |
|---|---|---|---|
| Male (n, %) | 52 (81.3) | 154 (73.3) | 0.199 |
| Age (Year) | 65.00 (60.00–72.75) | 64.00 (54.00–72.00) | 0.123 |
| Height (m) | 1.70 (1.65–1.75) | 1.70 (1.63–1.74) | 0.773 |
| Weight (kg) | 70.00 (60.00–75.00) | 70.50 (65.00–80.00) | 0.090 |
| BMI (kg/m2) | 24.01 (21.97–25.93) | 24.85 (23.14–26.90) | 0.009 |
| SBP (mmHg) | 120 (106–136) | 128 (112–141) | 0.082 |
| HR (bpm) | 80 (71–94) | 80 (73–86) | 0.763 |
| Smoking (n, %) | 42 (65.6) | 123 (58.6) | 0.313 |
| Drinking (n, %) | 19 (29.7) | 54 (25.7) | 0.529 |
| Diabetes (n, %) | 13 (20.3) | 45 (21.4) | 0.848 |
| Hypertension (n, %) | 35 (54.7) | 113(53.8) | 0.902 |
Comparison of clinical features among different ejection fraction groups in AMI patients.
SBP, systolic blood pressure; HR, heart rate.
Bold indicates statistical differences (P < 0.05).
Figure 2
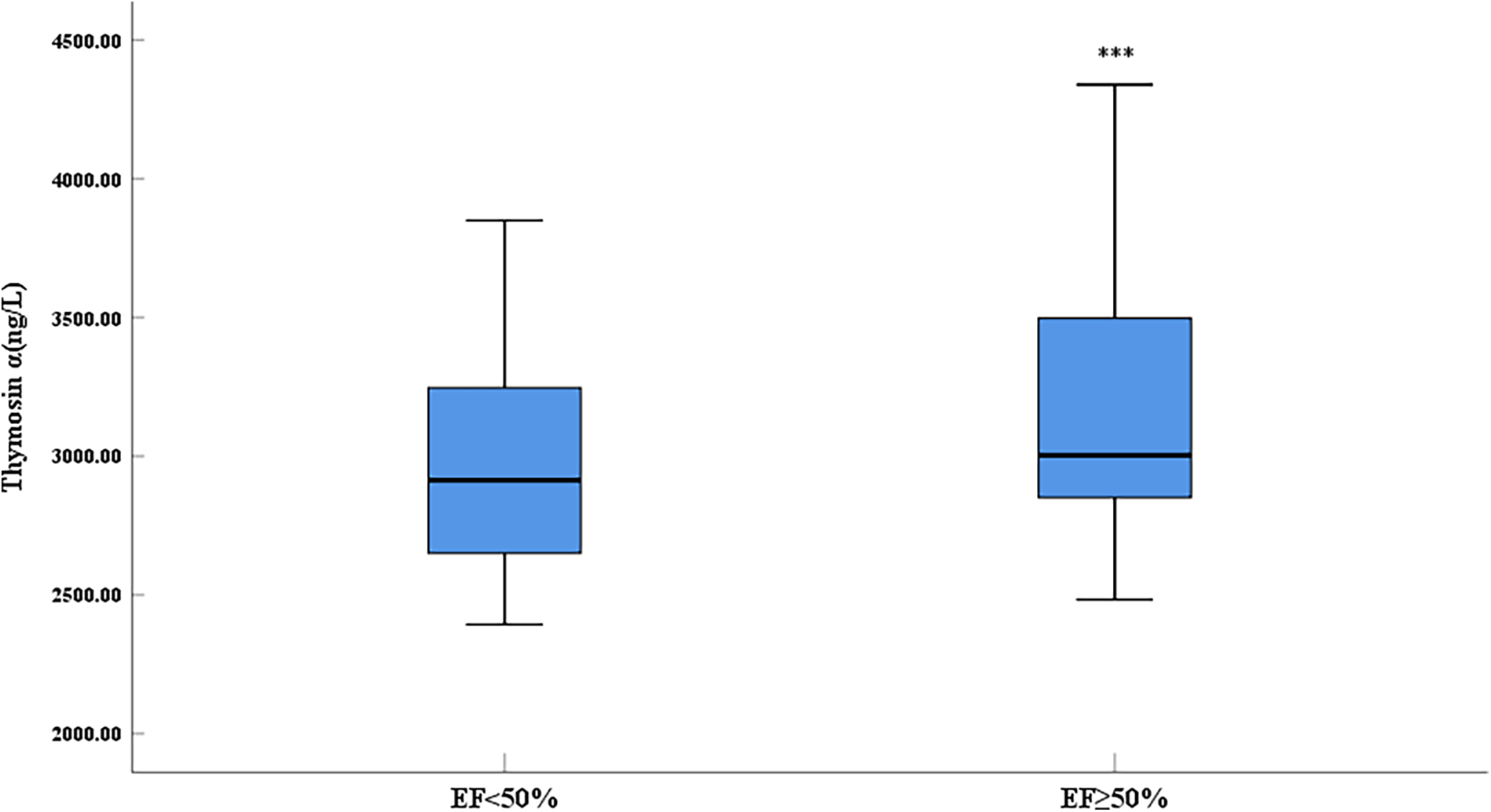
Comparison of serum thymosin α1 level between different LVEF groups in AMI patients (***: P < 0.001).
3.3 Thymosin α1 level was positively correlated to EF value in AMI patients
To further elucidate the relationship between Thymosin α1 level and cardiac function following AMI, a bivariate Spearman correlation analysis was conducted to assess the association between Thymosin α1 level and EF value in all AMI patients. The results revealed that Thymosin α1 level was positively correlated with EF value in patients post-AMI, with higher Thymosin α1 level observed as EF value increased (r = 0.219, P < 0.001) (Figure 3).
Figure 3
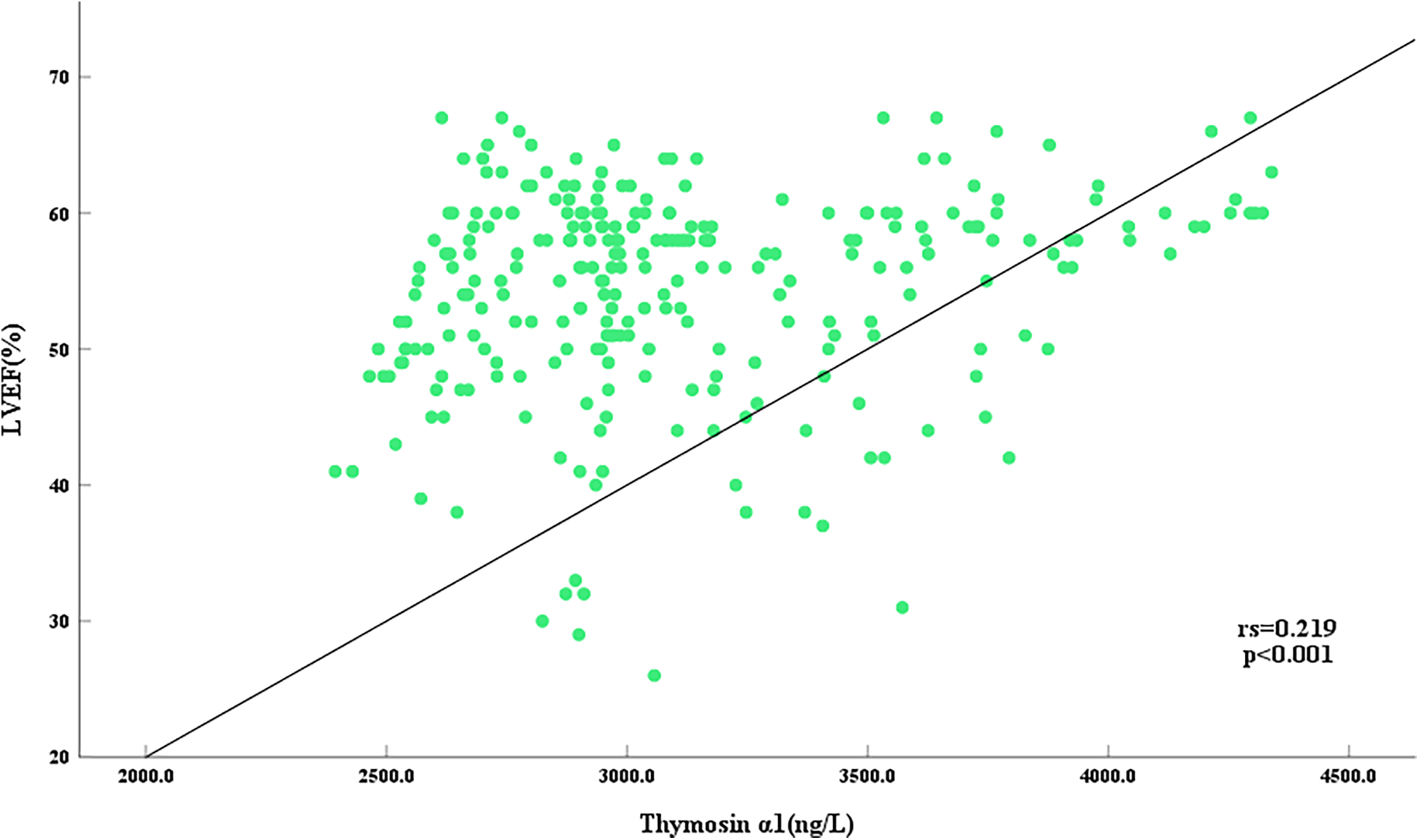
Scatter plot of correlation between serum thymosin α1 level and LVEF in AMI patients.
3.4 Risk factors for EF value in AMI patients
To further investigate the risk factors of cardiac function in AMI patients after opening IRA, we divided AMI patients into two groups: the EF < 50% group and the EF ≥ 50% group, with the specific number of samples in each group remaining the same as before. Firstly, a univariate comparison between the two groups of AMI patients in terms of blood routine, biochemistry, and coagulation was conducted. The results showed that the levels of white blood cell count, neutrophil cell count, and fasting blood glucose level in the EF < 50% group were significantly higher than those in the EF ≥ 50% group. There were no statistically significant differences in other blood routine indicators, biochemical parameters such as liver and kidney function, blood lipid levels, and coagulation markers between the two groups (Table 3). Secondly, we collected myocardial injury markers of the two groups and compared them. The results indicated that the expression levels of troponin I, NT-proBNP, lactate dehydrogenase, creatine kinase, and creatine kinase isoenzyme in the group with poorer cardiac function post-AMI (EF < 50%) were dramatically higher than those in the group with better cardiac function following AMI (EF ≥ 50%) (Table 4).
Table 3
| Variable | LVEF < 50% (n = 64) |
LVEF ≥ 50% (n = 210) |
P-value |
|---|---|---|---|
| Hemoglobin (g/L) | 141.0 (132.3–155.0) | 146.0 (134.5–157.0) | 0.190 |
| Red blood cell (×1012/L) | 4.64 (4.24–5.05) | 4.71 (4.40–5.09) | 0.414 |
| Platelet (×109/L) | 214.5 (182.8–256.0) | 222.5 (187.0–262.3) | 0.492 |
| White blood cell (×109/L) | 10.15 (8.65–12.68) | 9.25 (7.68–11.03) | 0.013 |
| Neutrophil (%) | 75.40 (61.50–83.60) | 73.40 (62.63–81.13) | 0.515 |
| Lymphocyte (%) | 18.40 (10.65–29.40) | 18.15 (12.08–29.33) | 0.530 |
| Monocyte (%) | 5.7 (4.3–7.2) | 5.7 (4.5–7.2) | 0.727 |
| Neutrophil (×109/L) | 7.7 (5.9–9.5) | 6.5 (4.7–8.6) | 0.019 |
| Lymphocyte (×109/L) | 1.6 (1.2–2.7) | 1.8 (1.2–2.4) | 0.870 |
| Monocyte (×109/L) | 0.6 (0.4–0.7) | 0.5 (0.4–0.7) | 0.131 |
| Kalium (mmol/L) | 3.7 (3.5–4.0) | 3.8 (3.4–4.0) | 0.696 |
| Natrium (mmol/L) | 138.0 (136.0–140.8) | 139.0 (136.0–140.0) | 0.818 |
| Chlorinum (mmol/L) | 103 (100–105) | 102 (100–104) | 0.468 |
| Glucose (mmol/L) | 7.06 (6.13–8.58) | 6.45 (5.52–8.30) | 0.001 |
| Glycated hemoglobin (%) | 6.0 (5.7–6.5) | 6.0 (5.7–6.6) | 0.670 |
| Total Protein (g/L) | 72 (68–77) | 72 (69–76) | 0.467 |
| Albumin (g/L) | 42.0 (40.0–43.3) | 42.0 (40.0–44.0) | 0.664 |
| Alanine aminotransferase (U/L) | 35.5 (24.3–54.5) | 36.5 (27.0–46.3) | 0.765 |
| Creatinine (umol/L) | 75.5 (60.0–94.8) | 71.0 (62.0–81.0) | 0.141 |
| Urea nitrogen (mmol/L) | 5.9 (4.9–7.8) | 5.5 (4.8–6.7) | 0.062 |
| Uric acid (μmol/L) | 373.0 (295.3–427.5) | 353.0 (285.8–418.0) | 0.107 |
| Triglyceride (mmol/L) | 1.085 (0.735–1.923) | 1.380 (0.880–1.920) | 0.095 |
| Total cholesterol (mmol/L) | 4.860 (4.093–5.605) | 4.970 (4.280–5.720) | 0.300 |
| High-density lipoprotein (mmol/L) | 1.07 (0.83–1.28) | 1.02 (0.85–1.21) | 0.463 |
| Low-density lipoprotein (mmol/L) | 3.09 (2.55–3.60) | 3.18 (2.49–3.77) | 0.180 |
| Apolipoprotein A1 (g/L) | 1.15 (0.97–1.28) | 1.15 (1.04–1.28) | 0.712 |
| Apolipoprotein B (g/L) | 0.91 (0.72–1.01) | 0.95 (0.80–1.10) | 0.106 |
| Prothrombin time (s) | 11.7 (10.5–11.9) | 11.1 (10.5–11.9) | 0.081 |
| Apolipoprotein E (g/L) | 3.76 (3.24–4.80) | 3.76 (3.04–4.71) | 0.710 |
| Lipoprotein α (g/L) | 23.35 (14.03–36.23) | 16.40 (9.40–29.90) | 0.077 |
| International normalized ratio | 1.02 (0.96–1.05) | 0.96 (0.91–1.04) | 0.081 |
| Activated partial thromboplastin (s) | 26.8 (23.9–29.6) | 26.0 (22.9–28.5) | 0.253 |
| Thrombin time (s) | 16.5 (15.9–17.4) | 16.5 (15.8–17.2) | 0.729 |
| Fibrinogen (g/L) | 2.699 (2.388–3.163) | 2.636 (2.356–2.992) | 0.172 |
| D-dimer (mg/L) | 0.39 (0.22–0.81) | 0.32 (0.20–0.54) | 0.095 |
| C-reactive protein (g/L) | 3.345(0.499–8.628) | 1.110(0.499–6.820) | 0.349 |
Comparison of serum biochemical indexes among different ejection fraction groups in AMI patients.
Bold indicates statistical differences (P < 0.05).
Table 4
| Variable | LVEF < 50% (n = 64) | LVEF ≥ 50% (n = 210) | P-value |
|---|---|---|---|
| Troponin I (μg/L) | 158.86 (44.18–253.73) | 46.67 (12.64–105.35) | <0.001 |
| N-terminal pro-B-type natriuretic peptide (pg/ml) | 2,943 (1,083–5,919) | 972 (556–1,957) | <0.001 |
| Aspartic aminotransferase (U/L) | 129 (40–282) | 92 (46–205) | 0.155 |
| Lactate dehydrogenase (U/L) | 1,083 (679–1,858) | 561 (373–810) | <0.001 |
| Creatine kinase (U/L) | 3,563 (1,668–5,353) | 1,474 (630–2,717) | <0.001 |
| Creatine kinase isoenzyme (U/L) | 286.75 (133.50–413.13) | 140.95 (63.75–239.08) | <0.001 |
| Thymosin α1(ng/L) | 2,913.28 (2,648.35–3,247.05) | 3,002.50 (2,846.48–3,497.68) | 0.006 |
Comparison of myocardial injury markers and serum thymosin α1 levels among different ejection fraction groups in AMI patients.
Bold indicates statistical differences (P < 0.05).
3.5 OR for AMI-related EF value using logistic regression analysis
Logistic regression analysis was conducted on all parameters including thymosin α1 that were notably different by univariate analysis between the EF < 50% group and the EF ≥ 50% group. Risk factors with a remarkable difference in AMI-related EF value included Thymosin α1 (OR = 1.000808, P = 0.048), NT-proBNP (OR = 0.999844, P < 0.001), and creatine kinase (OR = 0.999638, P < 0.001) (Table 5).
Table 5
| Variable | Β value | Wald χ2 | OR(95% CI) | P-value |
|---|---|---|---|---|
| Thymosin α1 (ng/L) | 0.00081 | 3.899 | 1.000808(1.000006–1.001611) | 0.048 |
| N-terminal pro-B-type natriuretic peptide (pg/ml) | −0.00016 | 13.250 | 0.999844(0.999760–0.999928) | <0.001 |
| Creatine kinase (U/L) | −0.00036 | 25.209 | 0.999638(0.999496–0.999779) | <0.001 |
Multivariate logistic regression analysis of LVEF ≥ 50% after AMI.
Bold indicates statistical differences (P < 0.05).
3.6 The ROC analysis of thymosin α1 for predicting AMI-induced cardiac dysfunction
Thymosin α1, NT-proBNP, and creatine kinase were analyzed using the ROC curve to evaluate their predictive value for EF decline caused by AMI. For predicting LVEF < 50% post-AMI, the area under the curve (AUC) of NT-proBNP was 0.714 (0.635–0.792, P < 0.001), with an optimal cutoff of 2,163 pg/ml, and the sensitivity and specificity were 60.9% and 73.4%, respectively (Figure 4). The AUC of creatine kinase was 0.724 (0.651–0.798, P < 0.001), with an optimal cutoff of 1,943 pg/ml, and the sensitivity and specificity were 78.6% and 65.7%, respectively (Figure 4). However, for predicting LVEF ≥ 50% in AMI patients during hospitalization, the AUC of Thymosin α1 level was 0.614 (0.533–0.694, P < 0.001), with an optimal cutoff of 2,961.72 ng/L, and the sensitivity and specificity were 57.6% and 60.9%, respectively (Figure 5).
Figure 4
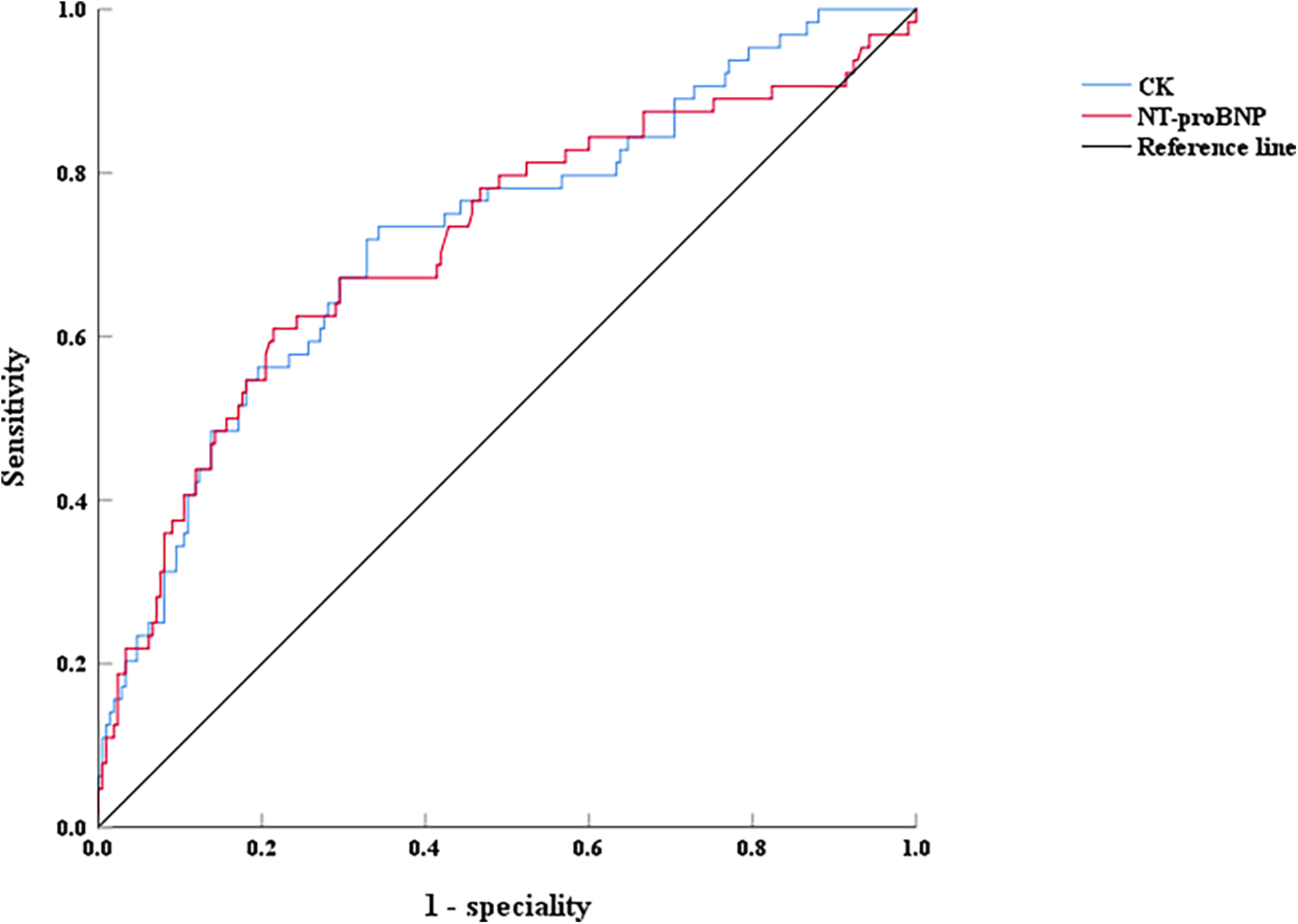
The ROC curves of NT-proBNP and CK on LVEF < 50% after interventional treatment in AMI patients, CK: creatine kinase.
Figure 5
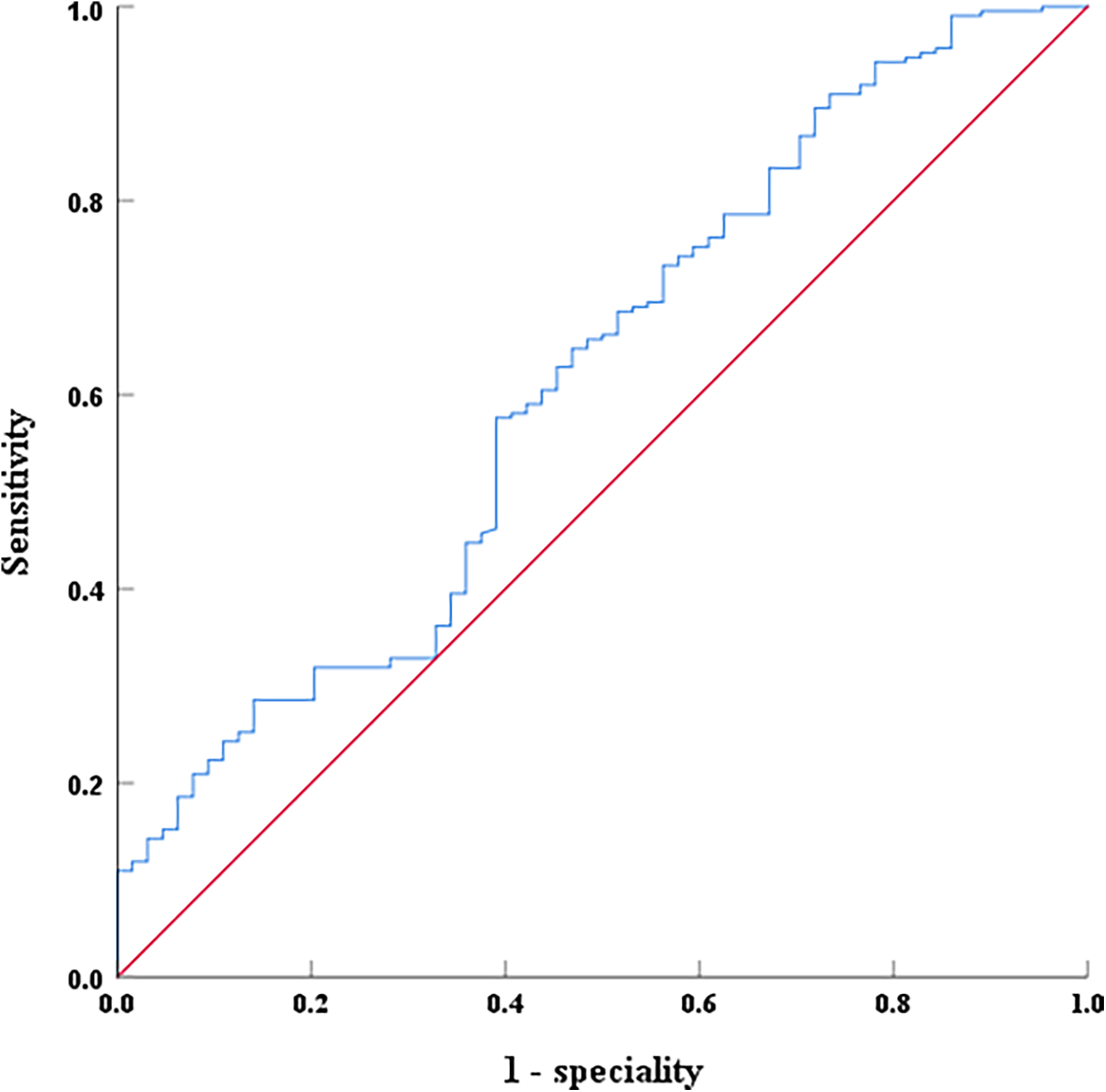
The ROC curves of serum thymosin α1 levels on LVEF ≥ 50% after interventional treatment in AMI patients.
4 Discussion
This study primarily revealed a positive correlation between thymosin α1 level and cardiac function following AMI. Specifically, higher expression levels of thymosin α1 were associated with improved cardiac function after AMI. Additionally, the ROC curve analysis indicated that thymosin α1 was an independent predictor of cardiac function (EF ≥ 50%) following AMI, with its sensitivity and specificity being 57.6% and 60.9%, respectively. Our team formerly reported the relationship between thymosin α1 and cardiac function after acute anterior ST-segment elevation myocardial infarction in the Chinese Journal of Interventional Radiology. Building upon this prior research, an expanded sample size and a broader range of AMI types were examined to draw more reliable conclusions. As far as we are aware, this study was the first to focus on thymosin α1 as a predictive marker for cardiac function following AMI, potentially offering a novel target for the early clinical detection and treatment of HF post-AMI.
A comparison was initially conducted between the control group and the AMI group, followed by a subgroup analysis based on whether the EF value from echocardiography during hospitalization post-AMI exceeded 50%. Myocardial ischemia, hypoxia, and even necrosis occur after AMI, leading to myocardial damage (19). Reperfusion injury of the myocardium after culprit vessel recanalization can further exacerbate myocardial damage (19). Therefore, during the early hospitalization period of AMI, some patients may begin to experience a decline in cardiac function, and the occurrence of HF is also possible (19). Clinically, the EF value from early echocardiography can be used to assess myocardial contractility and indirectly reflect cardiac function post-AMI. A lower EF value indicates worse cardiac function and a higher long-term risk of HF for AMI patients, which in turn suggests a poorer long-term prognosis (20, 21). Therefore, the subgroup analysis in this study based on the EF value during hospitalization post-AMI was justified. Our results indicate that in addition to thymosin α1, the traditional HF marker NT-proBNP and the myocardial injury marker creatine kinase were retained in the logistic multivariate analysis. NT-proBNP is a well-recognized marker of HF, with higher NT-proBNP values correlating with more severe HF. Previous studies have shown that BNP is associated with the larger size of myocardial infarction and the reduction of cardiac function, and is also related to the long-term adverse prognosis of AMI patients (22, 23). Our study concluded that there was a negative correlation between the expression level of NT-proBNP post-AMI and the EF value which was consistent with previous research. Furthermore, our study also identified the predictive value of creatine kinase for the reduction of cardiac function post-AMI. Creatine kinase is a traditional marker of myocardial damage area following AMI and is typically associated with the extent of myocardial damage post-AMI (24, 25), which explains its predictive value for the reduction of cardiac function post-AMI. The retention of these traditional predictive markers of myocardial injury post-AMI for cardiac function in this study further underscores the reliability of the study's conclusions.
Thymosin α1 is a bioactive peptide containing 28 amino acid residues, obtained by cleavage of prothymosin α (composed of 109 amino acid residues) with asparagine endopeptidase (26). It may be involved in cell cycle regulation and indirectly affect transcription and/or DNA replication processes (27). Thymosin α1 can bind to receptors on or near the membrane, triggering biological signal cascade reactions and participating in various physiological processes in the body, especially the regulation of immune responses (28). Currently, the inflammatory response plays a crucial role in the progression of AMI, which can lead to an increased infarct size and reduced cardiac function. Particularly, monocytes and macrophages significantly contribute to the infarct size and remodeling after AMI. Previous studies have demonstrated that anti-inflammatory macrophages (M2-type macrophages) or cardiac repair macrophages in situ post-AMI can alleviate post-infarction myocardial inflammation by secreting anti-inflammatory cytokines, thereby reducing ventricular remodeling and ultimately improving cardiac function (29, 30). A study has shown that thymosin α1 can improve the efficacy of chemotherapy for breast cancer by reversing M2 polarization of efferocytosis-activated macrophages (31). In addition, the latest study indicated that both the exogenously provided and the adenovirus-produced thymosin α1 mediate the tumor-associated macrophages M2 polarization via CD8+ T cells, thereby enhancing the anti-tumor effect of adenovirus (32). Therefore, we could infer that the high expression of thymosin α1 post-AMI may promote the transformation of macrophages infiltrating the myocardium into anti-inflammatory (M2 type) macrophages, thereby exerting a cardioprotective effect and improving cardiac function. This may explain the high expression of thymosin α1 observed in the group with better cardiac function following AMI in our study.
In addition to the association between monocytes/macrophages and cardiac function in AMI, recent studies have demonstrated that regulatory T cells (Tregs), acting as immune response modulators, can also exert cardioprotective effects after AMI and improve cardiac function by modulating the polarization of macrophages (33, 34). Previous studies have shown that in the treatment of cytomegalovirus infection, thymosin α1 could enhance the function of regulatory T cells (Tregs) and reduce Treg cell senescence (35). Furthermore, thymosin α1 could also promote the development and tolerance of Treg cells by regulating DC cells (36). In conclusion, it could be speculated that the up-regulation of thymosin α1 following AMI might reduce the myocardial inflammatory response by enhancing the function of Treg, thereby exerting a cardioprotective effect and improving cardiac function. However, the specific mechanisms underlying these effects require further investigation. To our knowledge, this was the first report demonstrating that thymosin α1 expression was increased following AMI, with higher expression levels correlating with better cardiac function. Through this study, we have uncovered for the first time that thymosin α1 might serve as a protective factor against myocardial injury post-AMI. This finding could provide a novel target for clinically improving cardiac function following AMI and a new therapeutic strategy for reducing the incidence of HF post-AMI.
Although the sample size was increased compared with our previous study, it remained relatively small. The relatively small sample size of the control group and the larger sample size of the case group in our study may influence subsequent research outcomes, such as the moderate sensitivity and specificity of thymosin α1 (AUC = 0.614). Moreover, our study exclusively focuses on Chinese individuals. We plan to increase the total sample size, including additional control group samples and more diverse populations, to a enable more comprehensive analysis. Secondly, the blood samples collected in this study were taken during patients' hospitalization. As myocardial infarction is a dynamic process, we will continue monitoring thymosin α1 levels after coronary artery revascularization to further validate our research conclusions. Thirdly, we identified elevated thymosin α1 as a novel correlate of post-AMI cardiac function recovery. While its predictive power for thymosin α1 remains moderate (AUC = 0.614), this emerging biomarker warrants further investigation. Importantly, our data confirm the expected inverse relationships between LVEF and traditional markers, NT-proBNP (AUC = 0.714) and CK (AUC = 0.724), demonstrating methodological validity while contextualizing thymosin α1's comparatively modest effect size. Subsequent investigations will incorporate serial thymosin α1 measurements at standardized pre-intervention and post-intervention timepoints to delineate its prognostic utility. And the specific protective mechanisms and underlying signaling pathways remain to be elucidated. Lastly, this was a retrospective study which resulted in deficiencies in the data collection process and possible selection bias. Future prospective multicenter studies should investigate thymosin α1 as a potential cardioprotective agent following AMI and elucidate its role in reducing HF incidence.
5 Conclusions
In conclusion, this study discovered that in comparison with the control group, the expression level of thymosin α1 in peripheral blood after AMI was notably elevated, and the better the cardiac function, the higher the expression level of thymosin α1. Thymosin α1 might be a biological indicator for the enhancement of cardiac function following AMI, and it could also be an important bioactive substance for the treatment of cardiac dysfunction after AMI. A prospective, randomized, and multicenter study should be devised to further validate the efficacy of thymosin α1 in improving cardiac function after AMI. Additionally, the two traditional biomarkers for cardiac function post-MI were still maintained in this study, indicating that these traditional predictive markers should not be disregarded.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Investigation, Conceptualization, Funding acquisition, Writing – review & editing, Writing – original draft, Formal analysis, Methodology, Data curation, Resources. Z-FZ: Investigation, Writing – review & editing, Conceptualization, Writing – original draft, Data curation, Methodology, Formal analysis. XJ: Writing – review & editing, Writing – original draft, Visualization, Conceptualization, Validation. YC: Conceptualization, Data curation, Writing – review & editing, Writing – original draft. C-FH: Software, Writing – original draft, Writing – review & editing, Methodology, Formal analysis, Conceptualization. C-XS: Writing – review & editing, Conceptualization, Supervision, Writing – original draft, Validation, Visualization, Methodology, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Shanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (No. ynhg202332).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1736–88. 10.1016/S0140-6736(18)32203-7
2.
Timmis A Vardas P Townsend N Torbica A Katus H De Smedt D et al European Society of cardiology: cardiovascular disease statistics 2021. Eur Heart J. (2022) 43:716–99. 10.1093/eurheartj/ehab892
3.
Damluji AA Bandeen-Roche K Berkower C Boyd CM Al-Damluji MS Cohen MG et al Percutaneous coronary intervention in older patients with ST-segment elevation myocardial infarction and cardiogenic shock. J Am Coll Cardiol. (2019) 73:1890–900. 10.1016/j.jacc.2019.01.055
4.
Harrington J Jones WS Udell JA Hannan K Bhatt DL Anker SD et al Acute decompensated heart failure in the setting of acute coronary syndrome. JACC Heart Fail. (2022) 10:404–14. 10.1016/j.jchf.2022.02.008
5.
Desta L Jernberg T Lofman I Hofman-Bang C Hagerman I Spaak J et al Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART registry (Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. (2015) 3:234–42. 10.1016/j.jchf.2014.10.007
6.
Goldstein AL Slater FD White A . Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. (1966) 56:1010–7. 10.1073/pnas.56.3.1010
7.
Quagliata M Papini AM Rovero P . Therapeutic applications of thymosin peptides: a patent landscape 2018-present. Expert Opin Ther Pat. (2023) 33:865–73. 10.1080/13543776.2023.2298833
8.
Sosne G Christopherson PL Barrett RP Fridman R . Thymosin-beta4 modulates corneal matrix metalloproteinase levels and polymorphonuclear cell infiltration after alkali injury. Invest Ophthalmol Vis Sci. (2005) 46:2388–95. 10.1167/iovs.04-1368
9.
Xing Y Ye Y Zuo H Li Y . Progress on the function and application of thymosin beta4. Front Endocrinol (Lausanne). (2021) 12:767785. 10.3389/fendo.2021.767785
10.
Gladka MM Johansen AKZ van Kampen SJ Peters MMC Molenaar B Versteeg D et al Thymosin beta4 and prothymosin alpha promote cardiac regeneration post-ischaemic injury in mice. Cardiovasc Res. (2023) 119:802–12. 10.1093/cvr/cvac155
11.
Peng H Xu J Yang XP Dai X Peterson EL Carretero OA et al Thymosin-beta4 prevents cardiac rupture and improves cardiac function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. (2014) 307:H741–51. 10.1152/ajpheart.00129.2014
12.
Li J Liu CH Wang FS . Thymosin alpha 1: biological activities, applications and genetic engineering production. Peptides. (2010) 31:2151–8. 10.1016/j.peptides.2010.07.026
13.
Xu Y Jiang Y Wang L Huang J Wen J Lv H et al Thymosin alpha-1 inhibits complete freund’s adjuvant-induced pain and production of microglia-mediated pro inflammatory cytokines in spinal cord. Neurosci Bull. (2019) 35:637–48. 10.1007/s12264-019-00346-z
14.
Garaci E Pica F Rasi G Favalli C . Thymosin alpha 1 in the treatment of cancer: from basic research to clinical application. Int J Immunopharmacol. (2000) 22:1067–76. 10.1016/s0192-0561(00)00075-8
15.
Iino S Toyota J Kumada H Kiyosawa K Kakumu S Sata M et al The efficacy and safety of thymosin alpha-1 in Japanese patients with chronic hepatitis B; results from a randomized clinical trial. J Viral Hepat. (2005) 12:300–6. 10.1111/j.1365-2893.2005.00633.x
16.
Garaci E Rocchi G Perroni L D’Agostini C Soscia F Grelli S et al Combination treatment with zidovudine, thymosin alpha 1 and interferon-alpha in human immunodeficiency virus infection. Int J Clin Lab Res. (1994) 24:23–8. 10.1007/BF02592405
17.
Byrne RA Rossello X Coughlan JJ Barbato E Berry C Chieffo A et al 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44:3720–826. 10.1093/eurheartj/ehad191
18.
McDonagh TA Metra M Adamo M Gardner RS Baumbach A Böhm M et al 2023 Focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 44(37):3627–39. 10.1093/eurheartj/ehad195
19.
Jenca D Melenovsky V Stehlik J Stanek V Kettner J Kautzner J et al Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. (2021) 8:222–37. 10.1002/ehf2.13144
20.
O’Connor CM Hathaway WR Bates ER Leimberger JD Sigmon KN Kereiakes DJ et al Clinical characteristics and long-term outcome of patients in whom congestive heart failure develops after thrombolytic therapy for acute myocardial infarction: development of a predictive model. Am Heart J. (1997) 133:663–73. 10.1016/s0002-8703(97)70168-6
21.
Kelly DJ Gershlick T Witzenbichler B Guagliumi G Fahy M Dangas G et al Incidence and predictors of heart failure following percutaneous coronary intervention in ST-segment elevation myocardial infarction: the HORIZONS-AMI trial. Am Heart J. (2011) 162:663–70. 10.1016/j.ahj.2011.08.002
22.
Niu JM Ma ZL Xie C Zhang ZQ . Association of plasma B-type natriuretic peptide concentration with myocardial infarct size in patients with acute myocardial infarction. Genet Mol Res. (2014) 13:6177–83. 10.4238/2014.February.21.6
23.
Richards AM Nicholls MG Espiner EA Lainchbury JG Troughton RW Elliott J et al B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. (2003) 107:2786–92. 10.1161/01.CIR.0000070953.76250.B9
24.
Mathey D Bleifeld W Buss H Hanrath P . Creatine kinase release in acute myocardial infarction: correlation with clinical, electrocardiographic, and pathological findings. Br Heart J. (1975) 37:1161–8. 10.1136/hrt.37.11.1161
25.
Johnson RN Lubbe WF Mercer CJ Sammel NL Norris RM . Serum myoglobin, creatine kinase and creatine kinase-MB as mutually supportive indices of myocardial infarction and infarct size. Aust N Z J Med. (1982) 12:160–5. 10.1111/j.1445-5994.1982.tb02449.x
26.
Low TL Goldstein AL . The chemistry and biology of thymosin. II. Amino acid sequence analysis of thymosin alpha1 and polypeptide beta1. J Biol Chem. (1979) 254:987–95.
27.
Tao N Xu X Ying Y Hu S Sun Q Lv G et al Thymosin alpha1 and its role in viral infectious diseases. The mechanism and clinical application. Molecules. (2023) 28(8):3539. 10.3390/molecules28083539
28.
Kellici S Burazeri G . Thymosin alpha1: a promising molecule for important clinical applications. Med Arh. (2009) 63:48–50.
29.
Peet C Ivetic A Bromage DI Shah AM . Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. (2020) 116:1101–12. 10.1093/cvr/cvz336
30.
Jia D Chen S Bai P Luo C Liu J Sun A et al Cardiac resident macrophagederived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation. (2022) 145(20):1542–56. 10.1161/CIRCULATIONAHA.121.057549
31.
Wei YT Wang XR Yan C Huang F Zhang Y Liu X et al Thymosin alpha-1 reverses M2 polarization of tumor-associated macrophages during efferocytosis. Cancer Res. (2022) 82:1991–2002. 10.1158/0008-5472.CAN-21-4260
32.
Liu K Kong L Cui H Zhang L Xin Q Zhuang Y et al Thymosin alpha1 reverses oncolytic adenovirus-induced M2 polarization of macrophages to improve antitumor immunity and therapeutic efficacy. Cell Rep Med. (2024) 5:101751. 10.1016/j.xcrm.2024.101751
33.
Wang W Li X Ding X Xiong S Hu Z Lu X et al Lymphatic endothelial transcription factor Tbx1 promotes an immunosuppressive microenvironment to facilitate post-myocardial infarction repair. Immunity. (2023) 56:2342–2357.e10. 10.1016/j.immuni.2023.07.019
34.
Alshoubaki YK Nayer B Lu YZ Salimova E Lau SN Tan JL et al Tregs delivered post-myocardial infarction adopt an injury-specific phenotype promoting cardiac repair via macrophages in mice. Nat Commun. (2024) 15(1):6480. 10.1038/s41467-024-50806-y
35.
Espinar-Buitrago MS Magro-Lopez E Vazquez-Alejo E Munoz-Fernandez MA . Enhanced immunomodulatory effects of thymosin-alpha-1 in combination with polyanionic carbosilane dendrimers against HCMV infection. Int J Mol Sci. (2024) 25(4):1952. 10.3390/ijms25041952
36.
Montagnoli C Perruccio K Bozza S Bonifazi P Zelante T De Luca A et al Provision of antifungal immunity and concomitant alloantigen tolerization by conditioned dendritic cells in experimental hematopoietic transplantation. Blood Cells Mol Dis. (2008) 40:55–62. 10.1016/j.bcmd.2007.06.016
Summary
Keywords
thymosin α1, cardiac function, acute myocardial infarction, biomarker, therapy
Citation
Liu L, Zhou Z-F, Jin X, Chen Y, Hu C-F and Shen C-X (2025) Expression levels of thymosin α1 in acute myocardial infarction patients and its correlation to cardiac function. Front. Cardiovasc. Med. 12:1635557. doi: 10.3389/fcvm.2025.1635557
Received
26 May 2025
Accepted
18 August 2025
Published
12 September 2025
Volume
12 - 2025
Edited by
Andre Rodrigues Duraes, Federal University of Bahia (UFBA), Brazil
Reviewed by
Paolo Puccetti, University of Perugia, Italy
Humberto Villacorta, Fluminense Federal University, Brazil
Antonio Lagoeiro Jorge, Federal Fluminense University, Brazil
Updates
Copyright
© 2025 Liu, Zhou, Jin, Chen, Hu and Shen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Cui-Fen Hu hucuifensuda@126.com Cheng-Xing Shen shenchienx2@hotmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.