Abstract
Aim:
To assess the incremental value of real-time three-dimensional (3D) transesophageal echocardiography (TEE) in visualizing tricuspid valve (TV) anatomy for procedural planning and guidance of transcatheter edge-to-edge repair (TEER) in cases of severe tricuspid regurgitation (TR).
Materials and methods:
An observational study was conducted on 54 patients with severe TR. The visualization of the TV leaflets during systole was graded semiquantitatively using predefined criteria: 0 points—no visible leaflet border or tissue; 1.25—border only; 2—border and <50% tissue; 3—border and >50% tissue. Each of the three leaflets was evaluated independently in both two-dimensional (2D) and 3D TEE, with a maximum cumulative score of 9. Two thresholds were established: ≥4.5 points as the primary endpoint for adequate visualization for TEER planning and ≥6 points as the secondary endpoint indicating sufficient quality for a detailed morphological assessment.
Results:
In 3D TEE, 77.8% of patients achieved the primary endpoint, and 68.5% reached the secondary threshold. In comparison, 2D TEE enabled 74.1% and 42.6% of patients to meet these respective thresholds. Although the difference in achieving the primary endpoint was not statistically significant (p = 0.82), 3D TEE significantly outperformed 2D TEE in enabling a detailed morphological evaluation (p = 0.012). No significant differences were noted in the visualization quality of the anterior vs. septal leaflets with 3D TEE (67.4% vs. 65.4%, p = 0.800). For the posterior leaflet, 3D TEE provided superior visualization compared with the 2D TEE (p = 0.0008), while still supporting procedural suitability in a comparable proportion of patients (85.4% vs. 89.8%, p = 0.400). Acoustic shadowing from the interatrial septum and aortic root accounted for 92% of inadequate visualizations.
Conclusion:
In this observational study, real-time 3D TEE proved feasible for assessing tricuspid valve anatomy and visualization quality in patients with severe TR who were considered for TEER. Compared with 2D TEE, 3D TEE offered an improved visualization of the posterior leaflet and provided adequate image quality for procedural planning in most patients. Moreover, a statistically significant advantage was observed for 3D TEE over 2D TEE in providing image quality sufficient for a detailed morphological evaluation.
1 Introduction
Severe tricuspid regurgitation (TR) is linked to increased mortality. While traditional surgical methods are the standard treatment, many patients face high surgical risks because of age and comorbidities. The development of less invasive transcatheter techniques offers a promising alternative, allowing high-risk patients to receive effective treatment while reducing the complications associated with open-heart surgery (1–5). Transcatheter tricuspid valve interventions include a clip-based leaflet transcatheter edge-to-edge repair (TEER), transcatheter annuloplasty, and bioprosthetic valve implantation (6–11). Regardless of the interventional method, accurate imaging is crucial for procedural success. Both fluoroscopy and echocardiography are essential for real-time evaluation of cardiac structures and implanted devices during catheter-based procedures. While fluoroscopy provides a precise visualization of catheters and device positioning, echocardiography uniquely enables a real-time assessment of soft tissue anatomy and hemodynamic function. Importantly, echocardiography offers these advantages without exposing operators or patients to ionizing radiation, thereby enhancing procedural safety (12, 13). Assessing the tricuspid valve is particularly challenging due to its unique morphological variability, varying leaflet thickness, and the presence of significant acoustic barriers that impede optimal imaging (14–17). The specificity of tricuspid valve imaging could potentially be improved with three-dimensional (3D) echocardiography, which enables visualization of the region of interest with minimal probe manipulation, although it requires greater expertise from the echocardiographer (18). The American Society of Echocardiography guidelines endorse both the midesophageal and transgastric imaging planes for the two-dimensional (2D) transesophageal echocardiographic (TEE) evaluation of the tricuspid valve (19). However, in the context of intraprocedural TEER guidance, maintaining the probe in a stable midesophageal position is often preferred. This approach not only minimizes the risk of mechanical trauma associated with repeated probe manipulation but also enhances imaging consistency throughout the procedure (20–22). Importantly, one of the key advantages of real-time 3D TEE is its ability to provide a comprehensive, en face visualization of the tricuspid valve from the midesophageal position. This reduces the need for multiple probe adjustments and facilitates both procedural planning and real-time guidance, supporting the feasibility and practical utility of 3D TEE in the TEER setting. While data on the application of 3D TEE in transcatheter tricuspid valve interventions were formerly limited, there has been exponential growth in research publications in recent years, driven by advancements in volumetric imaging and the clinical adoption of implantable devices (23–25). Thus, this study seeks to determine the enhanced utility of real-time 3D TEE in both procedural planning and intraprocedural guidance during TEER for severe TR.
2 Materials and methods
2.1 Study design and population
A total of 54 consecutive patients with severe TR who underwent TEE at a single high-volume center were prospectively included in the study. Patients were prospectively recruited between October 2024 and May 2025. The study protocol received approval from the institutional review board, and informed consent was obtained from all participants.
2.2 Echocardiographic evaluation
Data were collected using a Philips Epiq CVx rev. 4.0 with a transesophageal x8-2t probe and stored on a Philips Xcelera PACS ver. 3.1. A complete preprocedural echocardiographic assessment of the tricuspid valve was assumed to include the following: (i) an en face planar 3D visualization of the tricuspid valve borders and leaflets during systole to provide spatial orientation during the introduction of a delivery system and device implantation (Figure 1); and (ii) a cross-sectional 2D (inflow–outflow right ventricle) view through the device and leaflet at the implantation point (Figure 2).
Figure 1
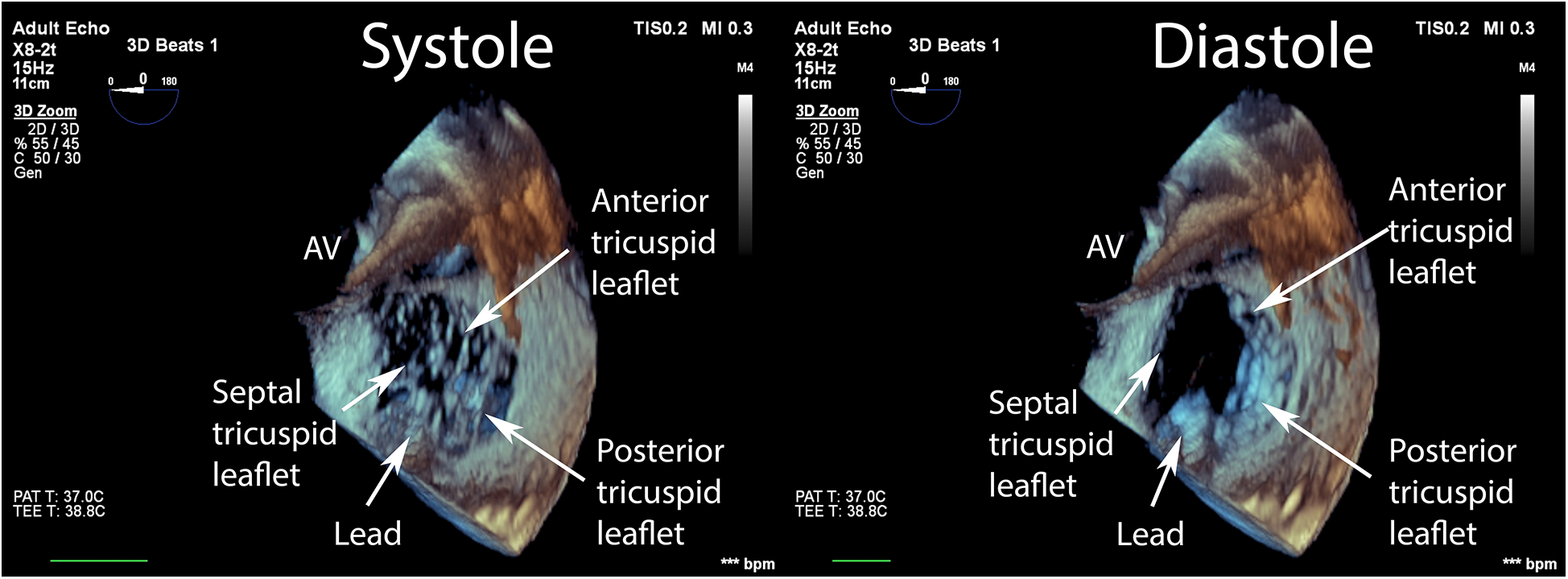
En face view of the tricuspid valve in real-time, three-dimensional transesophageal echocardiography. Visualization of sufficient quality for transcatheter tricuspid valve repair guidance includes the components of the valve: (i) annulus; (ii) septal, anterior, posterior leaflets; (iii) fragments of the subvalvular apparatus and anatomical landmarks: (iv) fragment of the aortic valve annulus, (v) fragment of the intra-atrial septum, and (vi) vena cava superior.
Figure 2
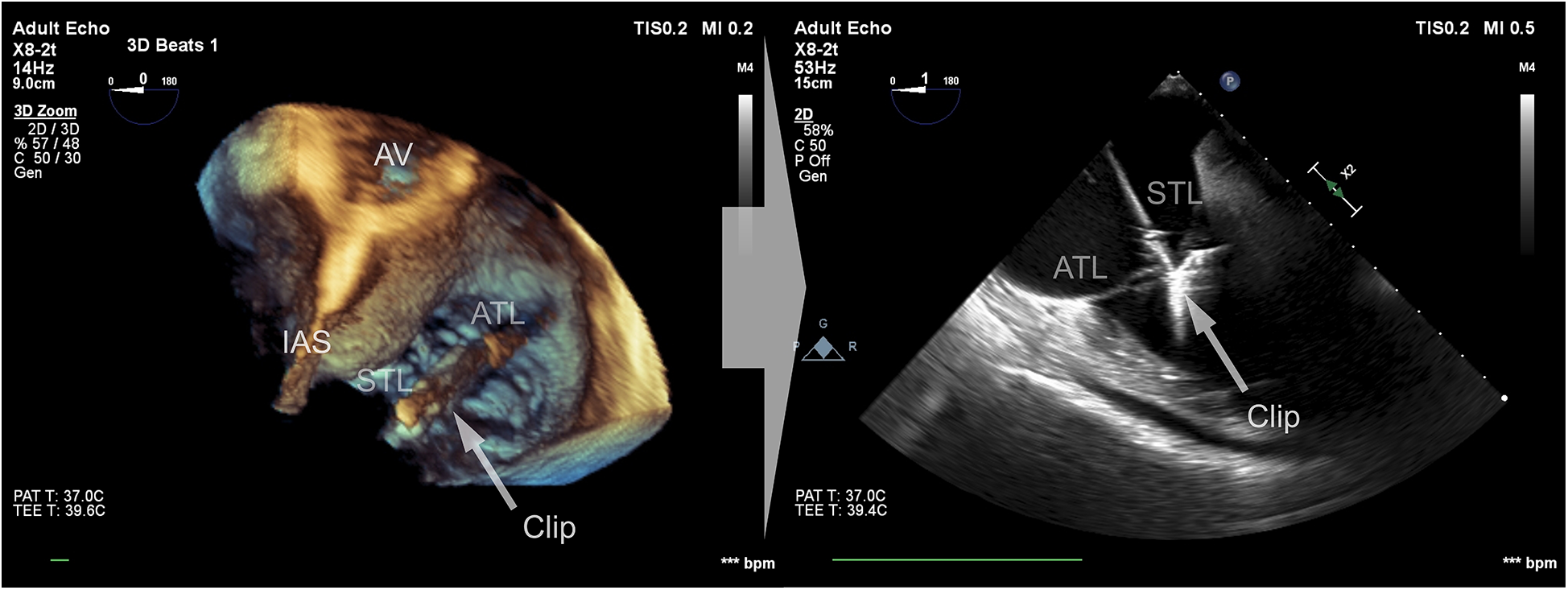
Cross-sectional 2D view of the device and leaflets at the point of implantation based on a 3D view after confirming the perpendicularity between the device and the leaflets.
In our protocol, TEE was performed with the patient under conscious sedation using a split dose of midazolam. Image acquisition began by positioning the probe at the midesophageal level, which is considered optimal for tricuspid valve visualization in a four-chamber view at 0 degrees. By standardizing image acquisition at the midesophageal level, the protocol ensures that both 2D and 3D datasets are directly comparable, thereby enabling a fair assessment of the incremental value provided by 3D TEE. Next, a single-beat, real-time, 3D TEE zoom volume image depicting the components of the tricuspid valve and anatomical landmarks (Figure 1) was acquired. Then, the optimal sector shape, representing a compromise between size and temporal resolution, was selected as the reference for procedural planning and guidance.
The recorded material was reviewed on external workstations with the Philips QLAB 15.5 software with a plug-in by a single experienced echocardiographer. Basic echocardiographic parameters presented in Table 1 were calculated. A real-time 3D TEE zoom volume image was rotated to depict the tricuspid valve complex from the right atrial roof in an en face fashion, and the proper visualization of each leaflet in the systolic phase was semiquantitatively described. The tricuspid valve leaflets were assessed according to the following predetermined criteria: (i) neither leaflet border nor leaflet tissue was identified—0 points; (ii) only a leaflet border, but no leaflet tissue was identified—1.25 points; (iii) a leaflet border and partial leaflet tissue (<50%) were identified—2 points; and (iv) a leaflet border and leaflet tissue (>50%) were identified—3 points. Points scored by each leaflet were summed, and the maximum possible result was 9 points. This subjective scoring system for assessing the visualization quality of the tricuspid valve leaflets was originally adapted from the four-point scale described by Anwar et al. and subsequently modified based on the framework proposed by Kucken et al. (26, 27). In the current study, the scale was further refined by redefining the lower boundary for poor imaging quality to 0 points and introducing an additional increment of 0.25 to the 1-point category. This adjustment was made to underscore the critical importance of precise border visualization, particularly in the context of TEER procedures.
Table 1
| Parameters | Visualization of sufficient quality for TEER | P-value | |
|---|---|---|---|
| Yes (n = 42) | No (n = 12) | ||
| Age (years) | 64.3 (CI 95% 59.9–69.8) | 65.6 (CI 95% 60.8–70.4) | 0.77 |
| Men | 67% | 83% | 0.27 |
| TEE indication | |||
| Arrhythmia | 58.3% | 57.1% | 0.94 |
| Structural | 47.1% | 42.9% | 0.94 |
| Pacemaker leads | 34% | 32% | 0.95 |
| Antero-posterior dimension (mm) | 37.6 (CI 95% 27.7–55.6) | 39.7 (CI 95% 24.3–81.0) | 0.99 |
| Latero-medial dimension (mm) | 43.8 (CI 95% 23.5–56.5) | 38.4 (CI 95% 27.4–50.4) | 0.49 |
| Annulus height (mm) | 5.5 (CI 95% 2.4–11.6) | 8.4 (CI 95% 2.1–12.3) | 0.052 |
| Tricuspid valve 3D perimeter (mm) | 123 (CI 95% 90.9–170.5) | 135.8 (CI 95% 98.4–152.9) | 0.29 |
| Tricuspid valve 2D perimeter (mm) | 116.7 (CI 95% 87.7–163.9) | 121.8 (CI 95% 87.2–147.5) | 0.64 |
| Tricuspid valve 2D area (mm2) | 1,042.2 (CI 95% 567.5–2,560.9) | 995.2 (CI 95% 553.9–1,636.9) | 0.84 |
| Tricuspid valve 3D area (mm2) | 1,104.2 CI 95% (569.4–2,594.4) | 1,054.8 (CI 95% 631.5–1,688.6) | 0.65 |
Baseline characteristics and comparison of tricuspid valve annulus parameters in patients stratified according to the quality of real-time three-dimensional transesophageal echocardiography.
2D, two-dimensional; 3D, three-dimensional; TEE, transesophageal echocardiography; TEER, transcatheter edge-to-edge repair.
As highlighted in the literature, even anatomically suitable patients may have tricuspid leaflets that are exceptionally thin and challenging to visualize. While thin leaflet tissue is less optimal for secure device grasping, the leaflet border or coaptation zone serves as the principal anatomical landmark for effective device anchoring during TEER (28).
The primary endpoint, visualization of sufficient quality for the guidance of TEER, was met when (i) the total score was ≥4.5 points; (ii) at least one leaflet scored 2 points; and (iii) the remaining leaflets scored at least 1.25 points each.
The secondary endpoint, visualization of sufficient quality for a detailed morphologic evaluation of the tricuspid valve, was met when (i) the total score was ≥6 points; and (ii) all leaflets scored at least 2 points each.
The thresholds utilized in the present study were adapted from the methodology described by Kucken et al. (27). In their study, optimal image quality was defined as achieving a score of 15–18 on an 18-point scale, while scores between 10 and 15 indicated sufficient image quality. Given that the maximum achievable score on our proposed scale is 9 points, we have proportionally adjusted the thresholds to more accurately stratify patients into groups of “sufficient quality for TEER guidance” (≥4.5 points) and “detailed morphologic evaluation of the tricuspid valve” (≥6 points).
To enhance the discriminatory power of our scoring system, we introduced an additional criterion for the primary endpoint: at least one leaflet must score a minimum of 2 points, with the remaining leaflets each achieving at least 1.25 points. This modification prevents scenarios in which only two leaflets attain high visualization scores, thereby ensuring a more balanced assessment of the entire tricuspid valve. For the secondary endpoint, the requirement that each leaflet achieve at least 2 points further reduces the likelihood that two leaflets with perfect scores disproportionately influence the overall evaluation.
The potential limitations of this modified, subjective scoring system are discussed in detail in the limitations section.
2.3 Statistical analysis
Continuous variables were expressed as the mean ± standard deviation or median and interquartile range and were compared with Student's t-test or the Mann–Whitney U test as appropriate. Chi-square or Fisher's exact tests were used to compare categorical variables, expressed as counts and percentages. For each individual leaflet, the differences between the 2D and 3D visualization scores were assessed for normality using the Shapiro–Wilk test. Based on the results of the Shapiro–Wilk test, the Wilcoxon signed-rank test was employed to compare the 2D and 3D visualization scores. Finally, the median scores for both 2D and 3D visualizations were calculated and compared to determine which method tended to produce higher-quality scores. Agreement between the measurements was also assessed using the Bland–Altman method. All probability values were two-tailed, and a p-value of < 0.05 was considered statistically significant. The data were processed using MedCalc 14.0 (MedCalc, Ostend, Belgium), Wizard 1.9, and R 4.4.3 with the nortest and effsize packages.
3 Results
In 3D TEE examinations, a visualization of sufficient quality for TEER guidance (the primary endpoint) was achieved in 42 patients (77.8%). The secondary endpoint, defined as quality suitable for a detailed morphologic evaluation of the valve, was achieved in 37 patients (68.5%) (Figure 3). In contrast, 2D TEE enabled 40 patients (74.1%) to reach the primary endpoint and 23 patients (42.6%) to achieve the secondary endpoint (Figure 4). While there was no statistically significant difference between 3D and 2D TEE in the proportion of patients achieving the primary threshold for adequate visualization (p = 0.82), there was a statistically significant difference between 3D and 2D TEE in achieving the higher threshold for the detailed morphological evaluation (p = 0.012). Furthermore, a comparison of echocardiographic parameters and tricuspid valve measurements (Table 1) showed no statistically significant differences between patients with and without sufficient TEE imaging quality.
Figure 3
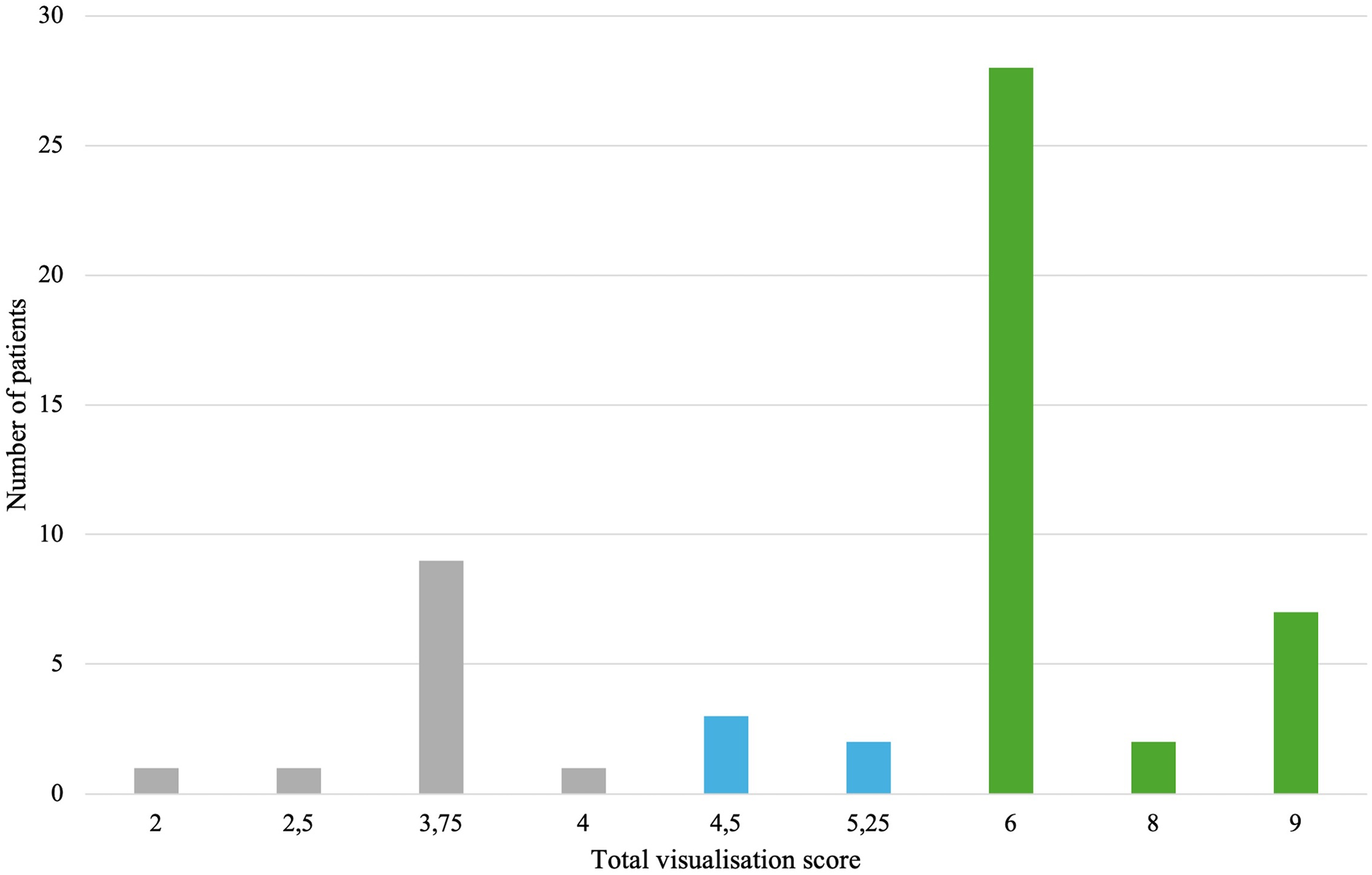
Distribution of total visualization scores for each patient in 3D TEE. Gray color—insufficient visualization quality for procedure guidance, blue color—adequate study quality for guiding TEER (primary endpoint), green color—adequate study quality for a detailed morphological evaluation (secondary endpoint). TEE, transesophageal echocardiography; TEER, transcatheter edge-to-edge repair.
Figure 4
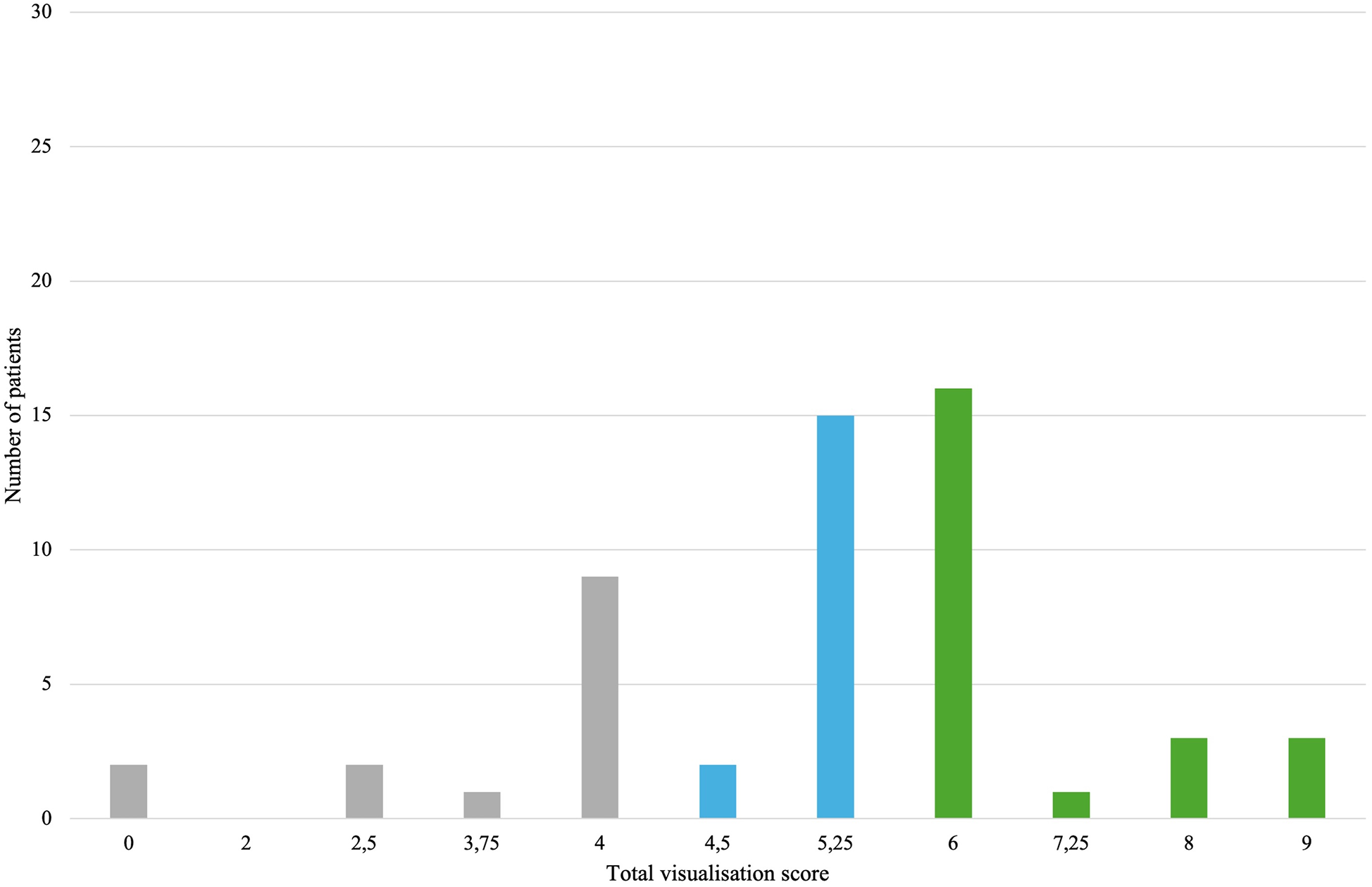
Distribution of total visualization scores for each patient in 2D TEE. Gray color—insufficient visualization quality for procedure guidance, blue color—adequate study quality for guiding TEER (primary endpoint), green color—adequate study quality for a detailed morphological evaluation (secondary endpoint). TEE, transesophageal echocardiography; TEER, transcatheter edge-to-edge repair.
The results of our study indicate that real-time 3D TEE provides sufficient visualization to support transcatheter intervention in 89.8% of patients for both the anterior and the septal leaflets. There was no statistically significant difference between these two leaflets in terms of visualization quality (p = 1.000). A detailed morphological evaluation was achievable in 67.4% of anterior leaflets and 65.4% of septal leaflets, with no significant difference observed between them (p = 0.800). The posterior leaflet was visualized with adequate quality for intervention in 85.4% of patients, which was comparable to the rates for the anterior and the septal leaflets (85.4% vs. 89.8%, p = 0.400). Importantly, a statistically significant difference in visualization scores between 2D and 3D echocardiography was found only for the posterior leaflet. Specifically, 3D TEE yielded significantly higher visualization scores for the posterior leaflet compared with 2D examination (p = 0.0008), as shown in Figure 5.
Figure 5
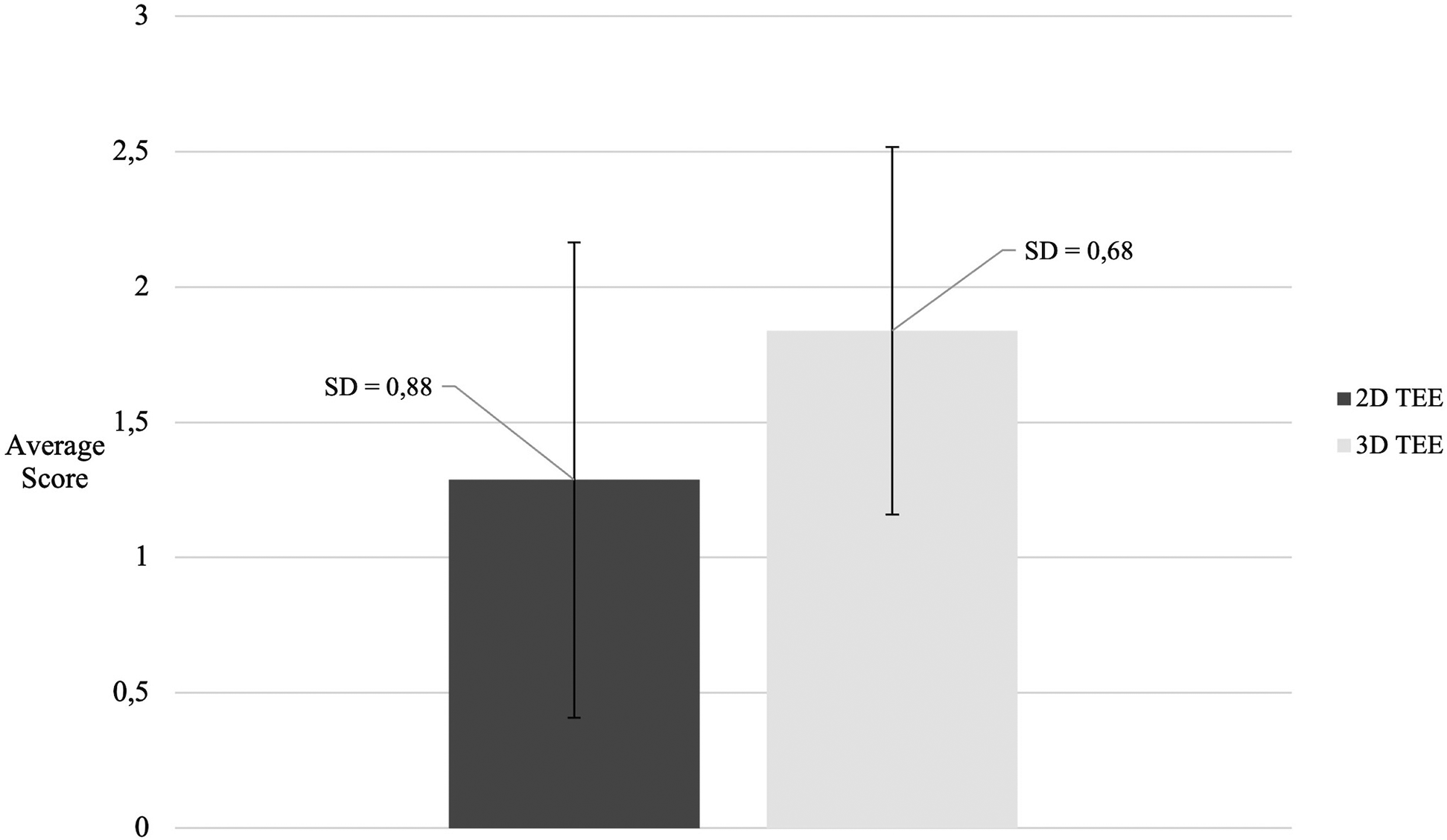
Comparison of posterior leaflet visualization scores between 2D and 3D TEE. TEE, transesophageal echocardiography.
Acoustic shadowing from the intra-atrial septum and the aortic root, causing signal attenuation, was found to be a major factor associated with insufficient tricuspid valve visualization quality for TEER in 11 patients (92%) (Figure 6). In the remaining patient(8%), anatomical mismatch due to tricuspid ring displacement caused by the severe left ventricular dilatation was observed.
Figure 6
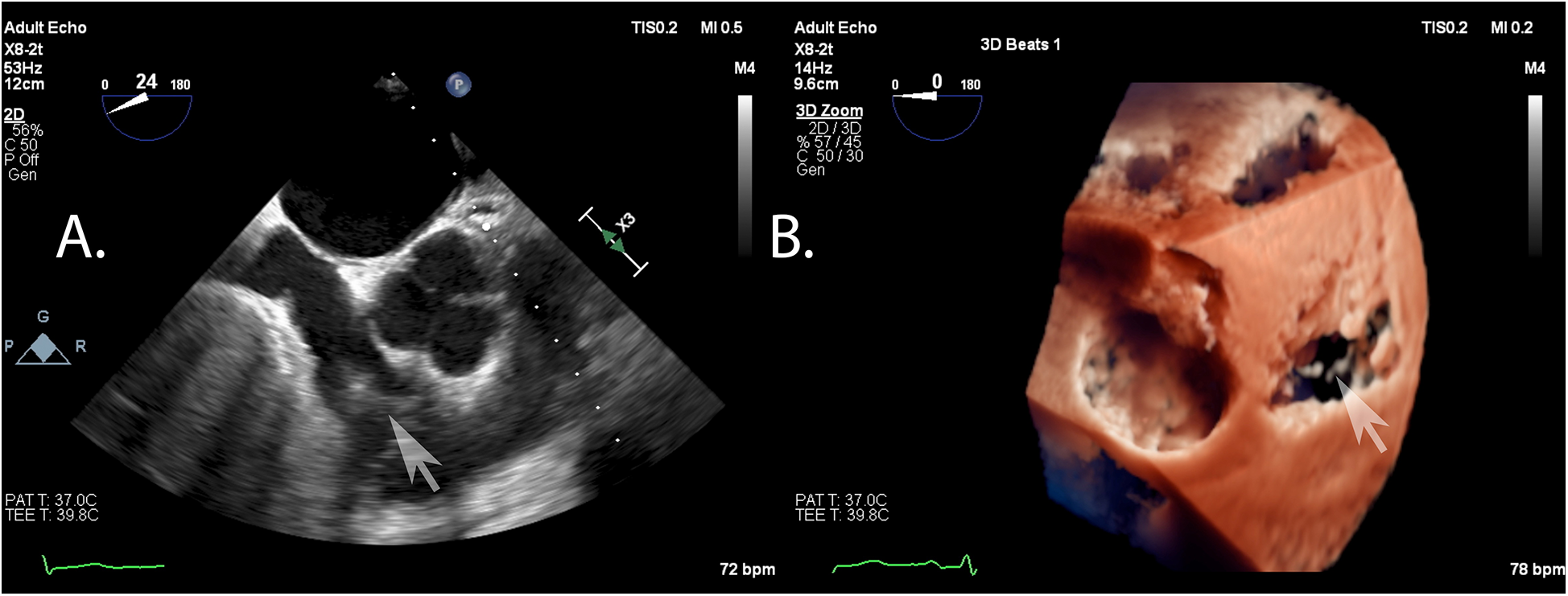
Example of typical artifacts precluding a proper visualization of the tricuspid valve: shadowing from the intra-atrial septum and signal attenuation as seen in the en face view of two- (A) and three-dimensional (B) echocardiography.
4 Discussion
Echocardiography is essential to guide transcatheter mitral and tricuspid interventions, in contrast to the procedures on aortic or pulmonary valves, where fluoroscopy usually provides sufficient visualization (3, 9, 10, 29–32). While initially supported with 2D protocols, the application of 3D echocardiography in both mitral and tricuspid procedures is expected to increase. Although data on real-time 3D TEE in TEER remain limited, we investigated its utility in planning and guiding the intervention in routine clinical practice.
Echocardiographic image quality plays a pivotal role in both the selection of candidates and the procedural success of TEER for TR. As highlighted in the GLIDE score study, image quality was identified as one of the five key anatomical and procedural determinants predicting successful outcomes. Limited echocardiographic visualization can hinder an accurate assessment of leaflet morphology, coaptation gaps, and jet location, which are essential for optimal device positioning and anchoring. The study conducted by Gercek et al. demonstrated that patients with good echocardiographic image quality had significantly higher rates of procedural success, defined as a substantial reduction in tricuspid regurgitation and the achievement of moderate or less residual TR. Conversely, suboptimal image quality was associated with increased procedural complexity and a higher likelihood of residual regurgitation (33). Therefore, a thorough preprocedural echocardiographic evaluation is critical not only for appropriate patient selection but also for maximizing the likelihood of therapeutic success with TEER-TR interventions.
It is well established that the tricuspid valve exhibits considerable morphological variability, with only approximately 54% of individuals having the classic three-leaflet configuration (34). In our study, we did not exclude patients with nonclassical leaflet anatomies. To maintain the clarity and interpretability of the scoring system, we elected not to subdivide the leaflets beyond the three principal anatomical components. In patients in whom a leaflet was anatomically divided or exhibited additional scallops or segments, it was assessed as a single leaflet within the corresponding main category. This approach was similarly applied to other variations in leaflet segmentation.
While this methodological choice simplifies the analysis and facilitates comparison across the cohort, it may not fully capture the spectrum of anatomical diversity present in the population. We acknowledge that this could potentially limit the granularity and real-world applicability of the scoring system, particularly in patients with atypical leaflet morphology.
The main finding of the present study is that real-time 3D TEE provides a good visualization of the tricuspid valve in patients with severe TR. Although the success rate for a visualization of sufficient quality for TEER did not differ between the respective tricuspid valve leaflets in 2D and 3D visualizations, the difference was not statistically significant for the posterior leaflet. Moreover, the individual leaflet analysis revealed a statistically significant difference in 2D and 3D visualization scores solely for the posterior leaflet. These observations may explain the low numbers of septal-posterior implantations, accounting for 20% of interventions, since 2D echocardiography remains the standard for diagnosis and monitoring. Antero-septal clipping remains the primary target in most TEER-TR interventions, as it offers superior visualization (35, 36). Further research is needed to determine whether the improved visualization of the posterior leaflet with 3D echocardiography could lead to a greater adoption of septal-posterior clipping.
4.1 Limitations
Although echocardiographic examinations were standardized, interobserver variability may still be present and pose a major limitation to this study. The consistency of the scoring system among different examiners has yet to be assessed. These findings should be validated in a larger patient population and further analyzed for both interobserver and intraobserver variability. In addition, the clinical relevance of the scoring thresholds has not been validated against procedural outcomes or compared directly with existing scales. External validation in an independent patient cohort is also lacking, which may limit the generalizability of our findings.
A further limitation of this study is the use of a standardized three-leaflet framework for scoring, regardless of the actual anatomical configuration of the tricuspid valve. By not subdividing additional leaflets, scallops, or accessory segments beyond the three principal categories, the analysis may not fully reflect the true morphological diversity encountered in clinical practice. As a result, the granularity and applicability of the scoring system may be reduced in patients with atypical or complex leaflet anatomy. Future studies should consider adapted scoring systems or subgroup analyses to better address the full range of leaflet variations described in the literature.
5 Conclusions
This observational study demonstrates that real-time 3D TEE is a feasible and effective imaging modality for the assessment of TV anatomy and visualization quality in patients with severe TR undergoing evaluation for TEER. The results indicate that 3D TEE provides a superior visualization of the posterior leaflet compared with 2D TEE and achieves adequate image quality for procedural planning and guidance in the majority of investigated cases. Moreover, a statistically significant improvement in image quality for a detailed morphological assessment was demonstrated with 3D TEE compared with 2D TEE. These findings support the incremental value of 3D TEE, particularly for achieving the higher-quality visualization necessary for a detailed morphological evaluation.
However, these findings should be interpreted within the context of the study's design and limitations. While 3D TEE frequently enabled sufficient visualization to support TEER guidance, this study does not establish 3D TEE as the definitive or sole primary imaging tool for all patients. Instead, the results support the utility and added value of 3D TEE as a complementary modality that can enhance intraoperative imaging and procedural feasibility in many, but not all, clinical scenarios.
Further prospective studies, ideally with larger, more diverse patient populations and comparative designs, are warranted to confirm these findings and to clarify the optimal role of 3D TEE in the procedural workflow for transcatheter tricuspid interventions.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Komisja Bioetyczna przy Warszawskim Uniwersytecie Medycznym. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JS: Methodology, Formal analysis, Project administration, Visualization, Writing – review & editing, Data curation, Funding acquisition, Writing – original draft, Investigation, Conceptualization. AR: Validation, Writing – review & editing, Supervision, Project administration, Methodology, Writing – original draft, Investigation, Conceptualization. AK-C: Writing – original draft, Software, Resources, Data curation, Supervision, Investigation, Methodology. ZH: Supervision, Investigation, Methodology, Writing – original draft, Resources. MT: Supervision, Investigation, Formal analysis, Validation, Data curation, Writing – original draft. EO: Data curation, Visualization, Writing – review & editing, Investigation. AP: Data curation, Investigation, Visualization, Writing – review & editing. EP: Methodology, Conceptualization, Investigation, Supervision, Writing – original draft. PS: Resources, Conceptualization, Writing – original draft, Supervision, Software, Project administration, Visualization, Validation, Data curation, Writing – review & editing, Formal analysis, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Topilsky Y Maltais S Medina Inojosa J Oguz D Michelena H Maalouf J et al Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. (2019) 12:433–42. 10.1016/j.jcmg.2018.06.014
2.
Demir OM Regazzoli D Mangieri A Ancona MB Mitomo S Weisz G et al Transcatheter tricuspid valve replacement: principles and design. Front Cardiovasc Med. (2018) 5:1–10. 10.3389/fcvm.2018.00129
3.
Lauten A Figulla HR Unbehaun A Fam N Schofer J Doenst T et al Interventional treatment of severe tricuspid regurgitation. Circ Cardiovasc Interv. (2018) 11:1–9. 10.1161/CIRCINTERVENTIONS.117.006061
4.
Lane CM Eleid MF . Comparative outcomes of transcatheter edge-to-edge repair and tricuspid valve surgery for isolated tricuspid valve regurgitation. Circ Cardiovasc Interv. (2025) 18:12–4. 10.1161/circinterventions.124.014991
5.
Sorajja P Whisenant B Hamid N Naik H Makkar R Tadros P et al Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. (2023) 388:1833–42. 10.1056/nejmoa2300525
6.
Schofer J Bijuklic K Tiburtius C Hansen L Groothuis A Hahn RT . First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J Am Coll Cardiol. (2015) 65:1190–5. 10.1016/j.jacc.2015.01.025
7.
Wengenmayer T Zehender M Bothe W Bode C Grundmann S . First transfemoral percutaneous edge-to-edge repair of the tricuspid valve using the MitraClip system. EuroIntervention. (2016) 11:1541–4. 10.4244/EIJV11I13A296
8.
Lurz P Besler C Noack T Forner AF Bevilacqua C Seeburger J et al Transcatheter treatment of tricuspid regurgitation using edge-to-edge repair: procedural results, clinical implications and predictors of success. EuroIntervention. (2018) 14:e290–7. 10.4244/EIJ-D-17-01091
9.
Taramasso M Hahn RT Alessandrini H Latib A Attinger-Toller A Braun D et al The international multicenter TriValve registry: which patients are undergoing transcatheter tricuspid repair? JACC Cardiovasc Interv. (2017) 10:1982–90. 10.1016/j.jcin.2017.08.011
10.
Taramasso M Alessandrini H Latib A Asami M Attinger-Toller A Biasco L et al Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international TriValve registry. JACC Cardiovasc Interv. (2019) 12:155–65. 10.1016/j.jcin.2018.10.022
11.
Praz F Muraru D Kreidel F Lurz P Hahn RT Delgado V et al Transcatheter treatment for tricuspid valve disease. EuroIntervention. (2021) 17:791–808. 10.4244/eij-d-21-00695
12.
Balzer J , Division of Cardiology, Pneumology and Angiology, Department of Medicine, University Hospital Duesseldorf, Duesseldorf, Germany, Division of Cardiology, Pneumology and Angiology, Department of Medicine, University Hospital Duesseldorf, Duesseldorf, Germany, Division of Cardiology, Pneumology and Angiology, Department of Medicine, University Hospital Duesseldorf, Duesseldorf, Germany, Division of Cardiology, Pneumology and Angiology, Department of Medicine, University Hospital Duesseldorf, Duesseldorf, Germany, ZeusTet alHybrid imaging in the catheter laboratory: real-time fusion of echocardiography and fluoroscopy during percutaneous structural heart disease interventions. Interv Cardiol Rev. (2016) 11:59. 10.15420/icr.2016.11.1.59
13.
Agricola E Meucci F Ancona F Pardo Sanz A Zamorano JL . Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention. (2022) 17:1205–26. 10.4244/eij-d-21-00582
14.
Addetia K Yamat M Mediratta A Medvedofsky D Patel M Ferrara P et al Comprehensive two-dimensional interrogation of the tricuspid valve using knowledge derived from three-dimensional echocardiography. J Am Soc Echocardiogr. (2016) 29:74–82. 10.1016/j.echo.2015.08.017
15.
Dahou A Levin D Reisman M Hahn RT . Anatomy and physiology of the tricuspid valve. JACC Cardiovasc Imaging. (2019) 12:458–68. 10.1016/j.jcmg.2018.07.032
16.
Muraru D Hahn RT Soliman OI Faletra FF Basso C Badano LP . 3-Dimensional echocardiography in imaging the tricuspid valve. JACC Cardiovasc Imaging. (2019) 12:500–15. 10.1016/j.jcmg.2018.10.035
17.
Peters AC Gong FF Rigolin VH . Three-dimensional echocardiography for the assessment of the tricuspid valve. Echocardiography. (2020) 37:758–68. 10.1111/echo.14658
18.
Hahn RT Thomas JD Khalique OK Cavalcante JL Praz F Zoghbi WA . Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc Imaging. (2019) 12:469–90. 10.1016/j.jcmg.2018.07.033
19.
Hahn RT Abraham T Adams MS Bruce CJ Glas KE Lang RM et al Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesth Analg. (2014) 118:21–68. 10.1016/j.echo.2013.07.009
20.
Hilberath JN Oakes DA Shernan SK Bulwer BE D’Ambra MN Eltzschig HK . Safety of transesophageal echocardiography. J Am Soc Echocardiogr. (2010) 23:1115–27. 10.1016/j.echo.2010.08.013
21.
Bavalia N Anis A Benz M Maldjian P Bolanowski PJ Saric M . Esophageal perforation, the most feared complication of TEE: early recognition by multimodality imaging. Echocardiography. (2011) 28:E56–9. 10.1111/j.1540-8175.2010.01291.x
22.
Alkhouli M Eleid MF Michellena H Pislaru SV . Complementary roles of intracardiac and transoesophageal echocardiography in transcatheter tricuspid interventions. EuroIntervention. (2020) 15:1514–5. 10.4244/eij-d-19-01078
23.
Stankovic I Daraban AM Jasaityte R Neskovic AN Claus P Voigt JU . Incremental value of the en face view of the tricuspid valve by two-dimensional and three-dimensional echocardiography for accurate identification of tricuspid valve leaflets. J Am Soc Echocardiogr. (2014) 27:376–84. 10.1016/j.echo.2013.12.017
24.
Mazzola M Giannini C Sticchi A Spontoni P Pugliese NR Gargani L et al Transthoracic and transoesophageal echocardiography for tricuspid transcatheter edge-to-edge repair: a step-by-step protocol. Eur Heart J - Imaging Methods Pract. (2024) 2:1–15. 10.1093/ehjimp/qyae017
25.
Passaniti G Safi LM Granot YN Sarullo FM Caldonazo T Rong LQ et al The use of 3D-echo in edge-to-edge percutaneous tricuspid valve repair. J Clin Med. (2025) 14:684. 10.3390/jcm14030684
26.
Anwar AM Geleijnse ML Soliman OII McGhie JS Frowijn R Nemes A et al Assessment of normal tricuspid valve anatomy in adults by real-time three-dimensional echocardiography. Int J Cardiovasc Imaging. (2007) 23:717–24. 10.1007/s10554-007-9210-3
27.
Kücken T Tamm S Haase-Fielitz A Edlinger CR Neuss M Bannehr M et al Visualisation of the tricuspid valve using a new 3D echocardiographic scoring system. Open Heart. (2020) 7:e001363. 10.1136/openhrt-2020-001363
28.
Cammalleri V Carpenito M Bono MC Mega S Ussia GP Grigioni F . Transcatheter tricuspid valve therapy: from anatomy to intervention. Front Cardiovasc Med. (2021) 8:778445. 10.3389/fcvm.2021.778445
29.
Nickenig G Lurz P Sorajja P Von Bardeleben RS Sitges M Tang GHL et al Percutaneous edge-to-edge repair for tricuspid regurgitation: 3-year outcomes from the TRILUMINATE study. JACC Cardiovasc Interv. (2024) 17(18):2113–22. 10.1016/j.jcin.2024.05.036
30.
Besler C Seeburger J Thiele H Lurz P . Treatment options for severe functional tricuspid regurgitation: indications, techniques and current challenges. E-J Cardiol Pract. (2018) 16:1–13.
31.
Besler C Blazek S Rommel KP Noack T von Roeder M Luecke C et al Combined mitral and tricuspid versus isolated mitral valve transcatheter edge-to-edge repair in patients with symptomatic valve regurgitation at high surgical risk. JACC Cardiovasc Interv. (2018) 11:1142–51. 10.1016/j.jcin.2018.04.010
32.
Yzeiraj E Bijuklic K Tiburtius C Witt J Krause K Steude J et al Tricuspid regurgitation is a predictor of mortality after percutaneous mitral valve edge-to-edge repair. EuroIntervention. (2017) 12:e1817–24. 10.4244/EIJ-D-16-00909
33.
Gerçek M Narang A Körber MI Friedrichs KP Puthumana JJ Ivannikova M et al GLIDE score. JACC Cardiovasc Imaging. (2024) 17:729–42. 10.1016/j.jcmg.2024.04.008
34.
Hahn RT Weckbach LT Noack T Hamid N Kitamura M Bae R et al Proposal for a standard echocardiographic tricuspid valve nomenclature. JACC Cardiovasc Imaging. (2021) 14(7):1299–305. 10.1016/j.jcmg.2021.01.012
35.
Russo G Hahn RT Alessandrini H Andreas M Badano LP Braun D et al Effects of tricuspid transcatheter edge-to-edge repair on tricuspid annulus diameter—data from the TriValve registry. Int J Cardiol. (2024) 405:131934. 10.1016/j.ijcard.2024.131934
36.
Hausleiter J Braun D Orban M Latib A Lurz P Boekstegers P et al Patient selection, echocardiographic screening and treatment strategies for interventional tricuspid repair using the edge-to-edge repair technique. EuroIntervention. (2018) 14:645–53. 10.4244/EIJ-D-17-01136
Summary
Keywords
tricuspid valve, tricuspid valve repair, echocardiography, three-dimensional (3D), transesophageal (TEE), anatomy, transcatheter
Citation
Sobieraj J, Rdzanek A, Kapłon-Cieślicka A, Huczek Z, Tomaniak M, Ostrowska E, Piasecki A, Pędzich E and Scisło P (2025) Heart 3D: echocardiographic and anatomical features of the tricuspid valve in a heterogeneous population with severe regurgitation—implications for edge-to-edge procedure suitability. Front. Cardiovasc. Med. 12:1637158. doi: 10.3389/fcvm.2025.1637158
Received
28 May 2025
Accepted
28 July 2025
Published
22 August 2025
Volume
12 - 2025
Edited by
Muhammed Gerçek, Heart and Diabetes Center North Rhine-Westphalia, Germany
Reviewed by
Felix Rudolph, Heart and Diabetes Center North Rhine-Westphalia, Germany
Max Potratz, Klinik für Allgemeine und Interventionelle Kardiologie Herz- und Diabeteszentrum NRW, Germany
Updates
Copyright
© 2025 Sobieraj, Rdzanek, Kapłon-Cieślicka, Huczek, Tomaniak, Ostrowska, Piasecki, Pędzich and Scisło.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jan Sobieraj jan.sobieraj@wum.edu.pl Adam Rdzanek adam.rdzanek@wum.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.