Abstract
Background and aim:
There is a lack of evidence comparing anticoagulation and antiplatelet therapy during the midperiod time after left atrial appendage closure (LAAC) in clinical practice (the “early period” is defined as “45 days” after LAAC for a single procedure of LAAC and “3 months” after LAAC for a combined therapy of LAAC and catheter ablation; we defined the time between the “early period” and 6 months following LAAC as the “midperiod”). Our study aims to assess the safety and effectiveness of different anticoagulant therapies in patients undergoing the LAAC procedure with the WATCHMAN device during the midperiod after LAAC implantation in clinical practice.
Methods:
This prospective, single-center cohort study included 374 consecutive patients undergoing percutaneous LAAC with the Watchman device. Patients were divided into two groups: oral anticoagulation (OAC) and antiplatelet therapy (APT). The primary composite endpoint was cardiac mortality, ischemic stroke/ transient ischemic attacks/systemic embolism, and major bleeding events after 6 months following the procedure. The secondary endpoints are cardiovascular death, device-related thrombosis (DRT) events, and each component of the primary endpoint.
Results:
The risk of the primary outcome in the APT group and the OAC group had no statistical difference in multivariable Cox regression (adjusted HR = 0.76; 95% CI: 0.40–1.49; P = 0.447). The secondary endpoints—including cardiac mortality, cardiovascular death, ischemic stroke/systemic embolism, major bleeding events, and DRT events—did not statistically differ between the two groups.
Conclusion:
During the midperiod time after LAAC implantation with the WATCHMAN device, OAC therapy may demonstrate similar safety and efficacy compared with dual antiplatelet therapy.
Background
Atrial fibrillation (AF) is the most common tachyarrhythmia (1, 2). According to the latest AF guidelines, left atrial appendage closure (LAAC) is recommended as an alternative to oral anticoagulants (OACs) for AF patients with high CHA₂DS₂-VASc scores (3).
However, anticoagulant therapy after LAAC has not been well established and remains controversial. The PROTECT-AF and PREVAIL trials, which compared LAAC to warfarin in patients with contraindications to long-term OAC, are among the most influential randomized controlled trials in this field. In these two trials, OAC was continued for at least 45 days after Watchman device implantation and then replaced by APT only after confirming adequate sealing. Based on these findings, expert consensus recommends OAC for 45 days, followed by dual APT until 6 months, and then single APT lifelong maintenance (4, 5).
Recently, studies focusing on anticoagulant therapy in the early period after LAAC (generally, the “early period” is defined as “45 days” after LAAC for a single procedure of LAAC and “3 months” after LAAC for a combined therapy of LAAC and catheter ablation) have demonstrated that OAC is non-inferior to APT in terms of efficacy and safety(6, 7). Furthermore, some cohort studies have already demonstrated that short-term OAC in the early period following LAAC is safe and tends to be associated with a lower rate of device-related thrombosis (DRT) compared to DAPT (8).
However, there is still a lack of strong evidence comparing antithrombotic therapies during the midperiod time after LAAC (we defined the time between the “early period” and 6 months following LAAC as the “midperiod”). Understanding the comparative effectiveness of OAC vs. APT during the midperiod is crucial for optimizing patient outcomes and establishing evidence-based guidelines, particularly in terms of reducing thromboembolic events and major bleeding risks. To the best of our knowledge, only one study—which recruited 555 patients who were divided into either the standard APT group or the lifelong half-dose non-vitamin K oral anticoagulants (NOAC) group—proved that lifelong half-dose NOAC could significantly reduce the risk of the composite endpoint of DRT, thromboembolism, and major bleeding events compared with the standard APT (9). Despite the well-established early-treatment protocols, the optimal antithrombotic strategy during the midperiod following LAAC remains undefined, creating uncertainty in clinical practice and complicating decision-making for clinicians.
In this study, we hypothesized that a proper prolongation of anticoagulant therapy in the midperiod after LAAC may be non-inferior to APT. Our study aimed to assess the safety and effectiveness of OAC vs. APT in patients undergoing LAAC with the WATCHMAN device during the midperiod time following LAAC implantation in clinical practice.
Methods
Study population and clinical follow-up
This study was a prospective, single-centered, hospital-based cohort study that recruited consecutive atrial fibrillation patients undergoing percutaneous LAAC closure with a Watchman device at Beijing ANZHEN Hospital between June 2014 and August 2021.
Inclusion criteria: (1) Age ≥18 years; (2) Non-valvular AF with CHA₂DS₂-VASc score [congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or transient ischemic attacks (TIA) or thromboembolism, vascular disease, age 65–74 years, sex category] ≥2 (men) or ≥3 (women); and (3) Successful WATCHMAN implantation.
Exclusion criteria: (1) Significant peridevice leak (>5 mm) or device-related thrombosis (DRT) at 45-day TEE; and (2) absolute contraindications to APT.
We followed up with most patients who were enrolled into the study via telephone interviews, outpatient interviews, and the WeChat application. Follow-up visits were scheduled 3, 6, and 12 months after treatment and every 6 months thereafter. Data collection was performed by clinical staff who underwent regular training sessions. TEE recordings were evaluated by at least one experienced cardiologist. Interobserver reliability was ensured through periodic calibration of interpretation standards, and all data were subjected to regular auditing for accuracy and completeness. Written informed consent was obtained from each patient, and follow-up information was recorded in the registry database.
Study group
Generally, the “early period” is defined as “45 days” after LAAC for a single procedure of LAAC and “3 months” after LAAC for a combined therapy of LAAC and catheter ablation. We defined the time between the “early period” and 6 months following LAAC as the “midperiod.”
Eligible patients were assigned to either the OAC or APT group at the discretion of the treating physician, based on individual patient characteristics and risk factors. Generally, all patients received OAC for 45 days after LAAC implantation for a single procedure of LAAC or 3 months after a combined therapy of LAAC and catheter ablation. Then, the patients in the APT group were discharged on dual APT for 6 months, followed by lifelong single APT. Those in the OAC group were discharged on OAC for 6 months and switched to lifelong single APT in the absence of absolute contraindications (the allocation of NOACs or vitamin K antagonists depended mainly on the economic circumstances of the patients).
A post-hoc power analysis (α = 0.05, β = 0.20) indicated that 80% power was required to detect a 7.5% absolute risk reduction in the primary endpoint, assuming an event rate of 15% in the APT group; therefore, if 242 patients were included in the OAC group, 128 patients were needed in APT group in this observational study. The observed effect size (7.7% difference) aligned with the exploratory design of the study. We acknowledge this limitation due to the small sample size and note the requirement for larger, multicenter studies to confirm our findings.
LAAC procedure
The Watchman device is a self-expanding, nitinol-framed structure ranging in diameter from 21 to 33 mm with fixation barbs and a permeable polyester fabric covering. Implantation was performed through a 12F sheath following a transseptal approach and was guided by fluoroscopy and intracardiac echocardiography (ICE) to verify proper positioning and stability.
Concomitant catheter ablation procedure
In brief, all ablations used the CARTO3 electroanatomic mapping system. Circumferential pulmonary vein isolation (PVI) was the primary procedure. For paroxysmal AF patients, cavotricuspid isthmus (CTI) ablation was added only if typical atrial flutter (AFL) was diagnosed prior to the procedure; left atrial (LA) roof ablation and mitral isthmus (MI) ablation were rarely performed. For persistent AF, a “2C3L” strategy (PVI plus linear ablation at the LA roof, MI, and CTI) was systematically applied. If AF or organized atrial tachyarrhythmia (OAT) persisted after initial ablation, cardioversion was performed. If unsuccessful or AF immediately recurred, intravenous amiodarone was given before repeat cardioversion. Repeat procedures followed the “2C3L” strategy, targeting conduction gaps and clinical OATs (excluding complex fractionated atrial electrogram ablation). Successful PV isolation and linear block were confirmed by burst pacing from the coronary sinus (200 ms) to induce tachycardia.
Examination
TEE was performed at 45 days following the single procedure of LAAC or at 3 months following the concomitant catheter ablation and LAAC, as well as at the 6- and 12-month follow-ups to examine whether the LAA closure was adequate and the device stable. A residual peridevice flow of >5 mm observed on 45-day TEE was considered a leak, and a visible large thrombus on the device was considered DRT. TEE was performed using a Philips EPIQ CVx system. Two independent cardiologists assessed the leaks and DRT, with discordance resolved by a third reviewer. TEE recordings were acquired through the WeChat application or at the outpatient face-to-face interview. These recordings were assessed by at least one cardiologist at Beijing ANZHEN hospital.
Study outcomes
The primary endpoint of this study was to obtain a composite of cardiac mortality, ischemic stroke/TIA/systemic embolism or major bleeding events after 6 months following the procedure. The secondary endpoints were cardiovascular death, DRT events, and each component of the primary endpoint.
Statistical analysis
Continuous variables were presented as median values with 25th and 75th percentiles, and categorical variables were presented as mean and standard deviations. Baseline characteristics between the OAC group and APT group were assessed via a Fisher’s exact test for dichotomous variables; a two-sample t-test was used for normally distributed continuous variables and a two-sample Wilcoxon rank-sum test was used for variables not normally distributed. Clinical outcomes were compared between the two groups using Cox proportional hazards regression models, adjusted for age, gender, type of AF, body mass index, hypertension, coronary artery disease, diabetes mellitus, chronic heart failure, and previous stroke/TIA/systemic embolism. The Kaplan–Meier estimator was used to calculate the time to event rate of the primary and secondary endpoints, whereas log-rank tests were used to compare incidences of the study endpoints between groups. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated to demonstrate the association between the different utilize of OAC and APT with the clinical outcomes. Missing data were minimal and were addressed using multiple imputation techniques to ensure that all available data were used for analysis. Analyses were performed using the STATA software version 17.
Results
Baseline characteristic
A total of 374 patients who met the inclusion criteria were enrolled in this cohort with a mean follow-up of 26 ± 18 months; out of these, 132 patients (45.5% concomitant catheter ablation) were in the APT group and 242 (21.1% concomitant catheter ablation) were in the OAC group. There were no patients with absolute contraindication to OAC in both groups. The baseline clinical characteristics were similar between the two groups (Table 1). The patients in the APT group had a mean age of 67 years, 22% were women, and 68.2% were diagnosed as having persistent AF; the patients in the OAC group had a mean age of 67 years, 25.6% were women, and 67.0% were diagnosed as having persistent AF. The difference between the two groups was not statistically significant. The difference between the two groups relating to left ventricular ejection fraction (LVEF), CHA2DS2-VASc scores, HAS-BLED score, and comorbidities such as hypertension, diabetes mellitus, chronic heart failure, and coronary artery disease was not statistically significant.
Table 1
| Parameter | OACs group (n = 242) | APT group (n = 132) | P-value |
|---|---|---|---|
| Age, years | 67 (60.74) | 67 (63,73) | 0.291 |
| Female, n (%) | 62 (25.6) | 29 (22.0) | 0.452 |
| Body mass index, kg/m2 | 27.5 (24.0,28.1) | 27.5 (24.0,28.1) | 0.361 |
| Type of AF, n (%) | 0.699 | ||
| Persistent | 162 (67.0) | 90 (68.2) | |
| CHA2DS2-VASc score (continuous) | 4 (3.5) | 4 (3.5) | 0.932 |
| CHA2DS2-VASc score (categorical), n (%) | 0.754 | ||
| 2 | 41 (16.9) | 26 (19.7) | |
| 3 | 50 (20.7) | 31 (23.5) | |
| 4 | 56 (23.1) | 29 (22.0) | |
| ≥5 | 95 (39.3) | 46 (34.9) | |
| HAS-BLED score (continuous) | 1.9 (1.3) | 1.9 (1.2) | 0.497 |
| HAS-BLED score (categorical), n (%) | 0.333 | ||
| 0 | 12 (5.0) | 7 (5.3) | |
| 1 | 81 (31.5) | 46 (34.9) | |
| 2 | 87 (36.0) | 48 (36.4) | |
| 3 | 54 (22.3) | 24 (18.2) | |
| 4 | 8 (3.3) | 4 (3.0) | |
| ≥5 | 0 | 3 (2.3) | |
| EF, % | 57 (58.65) | 57 (56.65) | 0.200 |
| Stroke/TIA, n (%) | 84 (73.0) | 56 (65.1) | 0.278 |
| Diabetes, n (%) | 74 (30.6) | 37 (28.0) | 0.637 |
| Hypertension, n (%) | 176 (72.7) | 97 (73.5) | 0.904 |
| CAD, n (%) | 58 (24.0) | 42 (31.8) | 0.113 |
| Heart failure, n (%) | 24(9.9) | 19(14.4) | 0.235 |
Baseline characteristics of study population.
Values are given as mean ± SD or n (%). AF, atrial fibrillation; TIA, transient ischemic attack.
During the midperiod following LAAC, in the OAC group (n = 242), all patients exclusively received direct oral anticoagulants (DOACs); no patients received warfarin. The specific DOACs used were rivaroxaban (n = 159, 65.7%), edoxaban (n = 53, 21.9%), and dabigatran (n = 30, 12.4%). In the APT group (n = 132), all patients received dual antiplatelet therapy (DAPT). In particular, 81 patients (61.4%) received aspirin in combination with clopidogrel, and the remaining 51 patients (38.6%) received aspirin in combination with ticagrelor. No other antithrombotic regimens were used in the APT group. We appreciate the opportunity to clarify these details.
Primary outcome
There were 20 primary endpoints in the APT group and 18 cases in the OAC group, with rates of 15.15% and 7.44%, respectively, but the difference was not statistically significant. The Kaplan–Meier estimator of the primary outcome is shown in Figure 1. The log-rank test of the composite primary outcome was not significantly different between the APT group and the OAC group. Table 2 shows the primary endpoint in the univariable analysis and multivariable analysis. According to multivariable Cox regression, the risk of the primary outcome did not differ significantly between the APT and OAC groups (adjusted HR = 0.67; 95% CI: 0.33–1.36; P = 0.271).
Figure 1
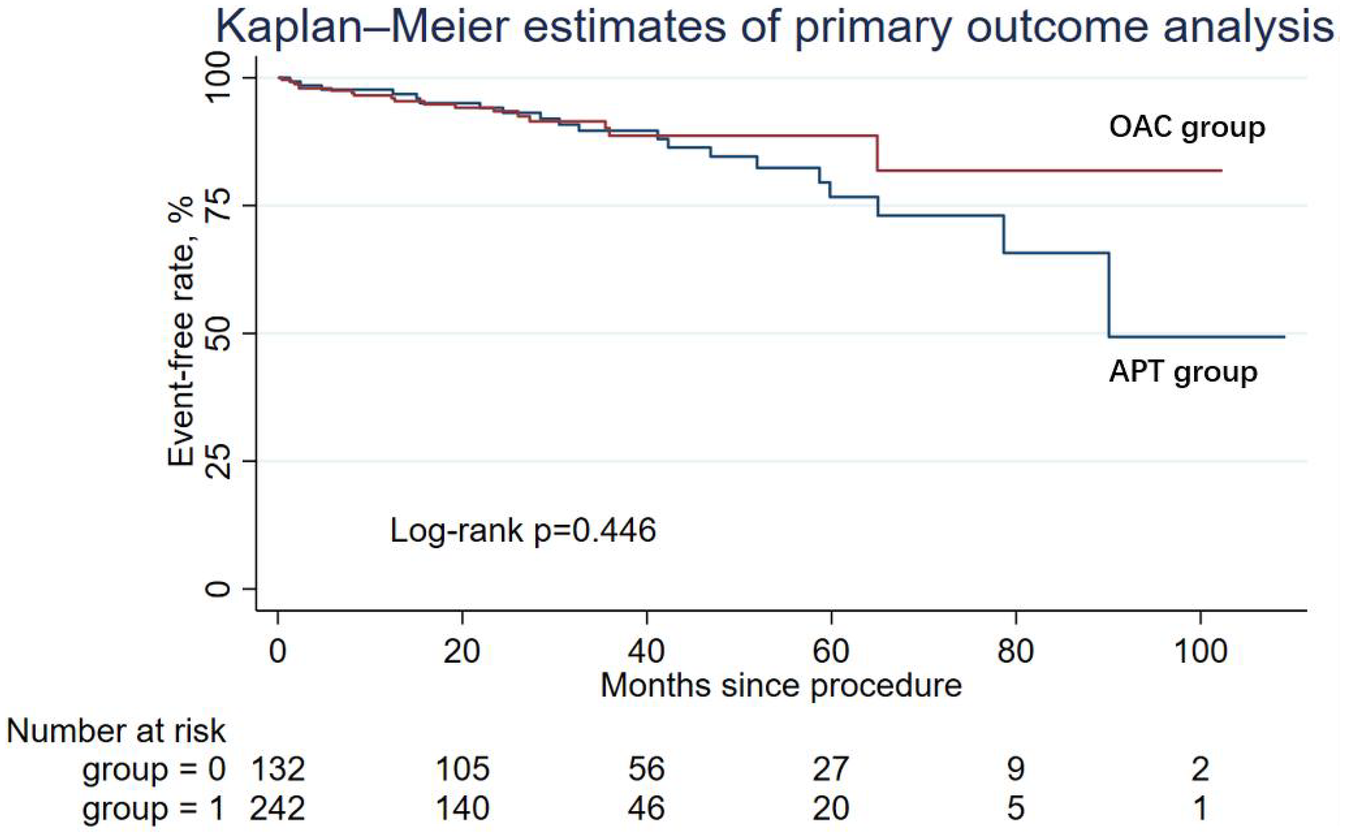
Kaplan-Meier curve showing primary outcome analysis for two groups: OAC (red line) and APT (blue line). The log-rank p-value is 0.446.
Table 2
| Outcome | Incidence rate (%) | Unadjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | |
|---|---|---|---|---|---|---|
| APT group | OAC group | |||||
| Primary endpoint | 15.15 | 7.44 | 0.78 (0.40, 1.49) | 0.447 | 0.67 (0.33, 1.36) | 0.271 |
| Cardiac mortality | 6.82 | 1.65 | 0.59 (0.17, 2.04) | 0.253 | 0.64 (0.14, 2.90) | 0.565 |
| Ischemic stroke/systemic embolism | 3.03 | 1.24 | 0.44 (0.10, 2.00) | 0.289 | 0.37 (0.06, 2.35) | 0.295 |
| Major bleeding events | 1.52 | 0.83 | 0.62 (0.09, 4.43) | 0.637 | 0.50 (0.03, 7.10) | 0.608 |
| DRT events | 2.27 | 0.83 | 0.84 (0.14, 5.23) | 0.854 | 0.79 (0.07, 8.99) | 0.847 |
The incidence rates, the primary endpoints, and the secondary endpoints in the univariable and multivariable Cox regression analysis.
In multivariable Cox analysis, we adjusted for age, gender, type of AF, body mass index, hypertension, coronary artery disease, diabetes mellitus, chronic heart failure, and concomitant AF ablation.
Secondary outcomes
During the follow-up period, nine patients (6.82%) in the APT group and four patients (1.65%) in the OAC group experienced cardiac death, but the difference was not statistically significant in Cox regression (adjusted HR = 0.64; 95% CI: 0.14–2.90; P = 0.565). Similarly, other secondary endpoints, including ischemic stroke/systemic embolism, major bleeding events (adjusted HR = 0.50; 95% CI: 0.03–7.10; P = 0.608), and the DRT events (adjusted HR = 0.79; 95% CI: 0.07–8.99; P = 0.847), were not statistically different between the two groups. The Kaplan–Meier estimator and log-rank test for each secondary endpoint showed an identical result (Figures 2–5); the details of each secondary endpoint are shown in Table 2.
Figure 2
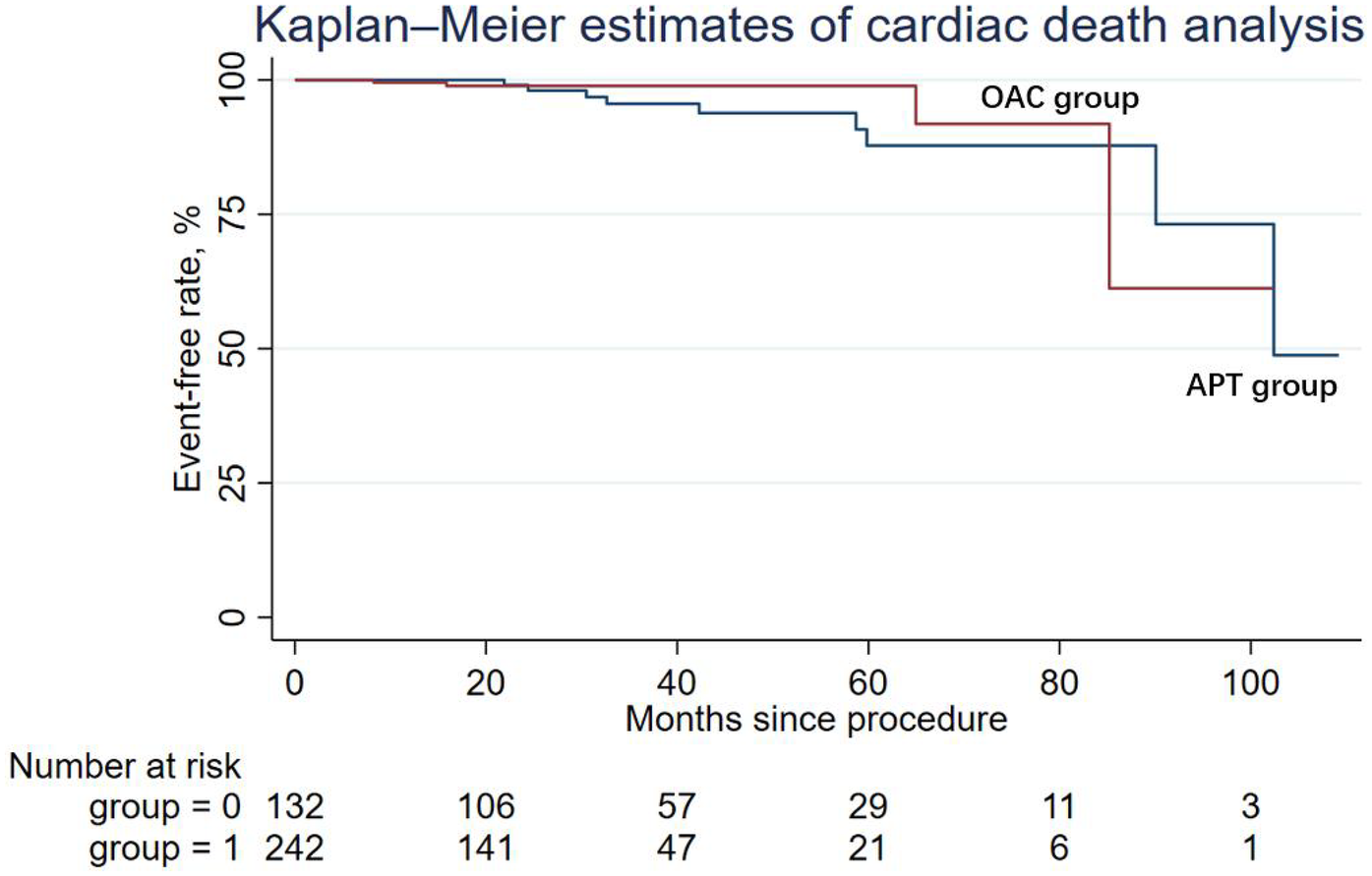
Kaplan-Meier survival curve comparing event-free rates for cardiac death between the OAC and APT groups.
Figure 3
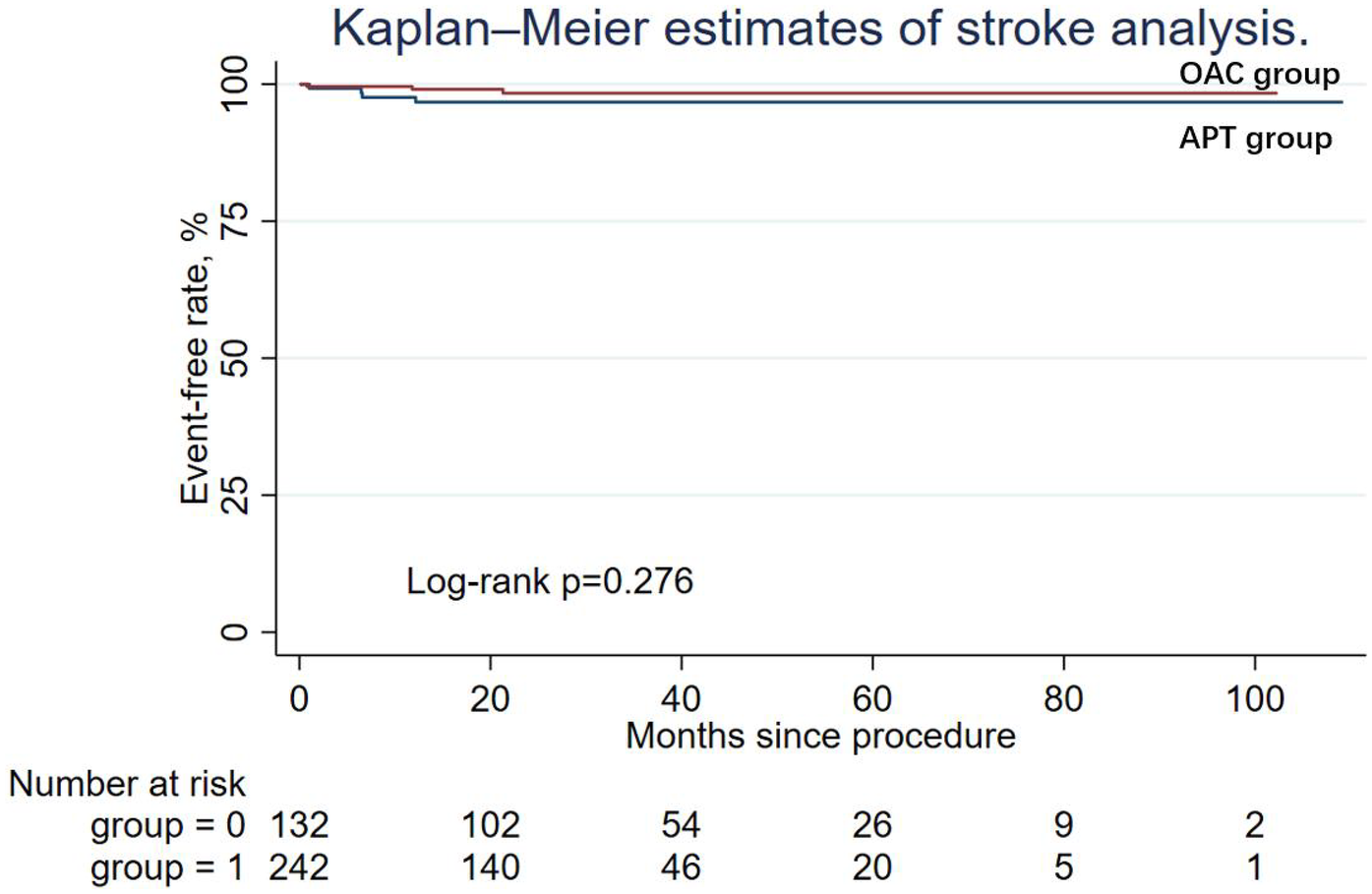
Kaplan-Meier survival curve comparing event-free rates for stroke between the OAC and APT groups.
Figure 4
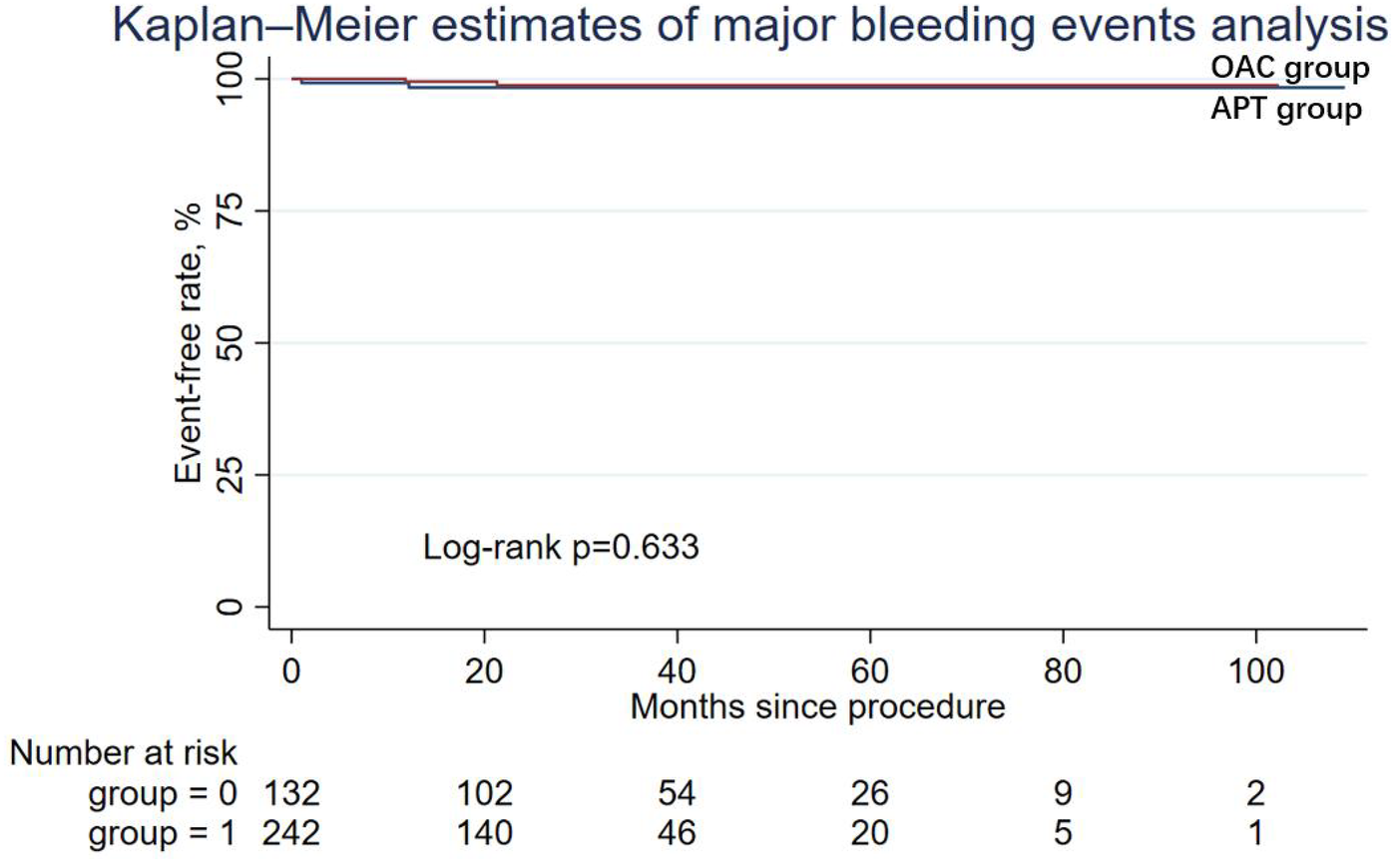
Kaplan-Meier survival curve comparing event-free rates for major bleeding events between the OAC and APT groups.
Figure 5
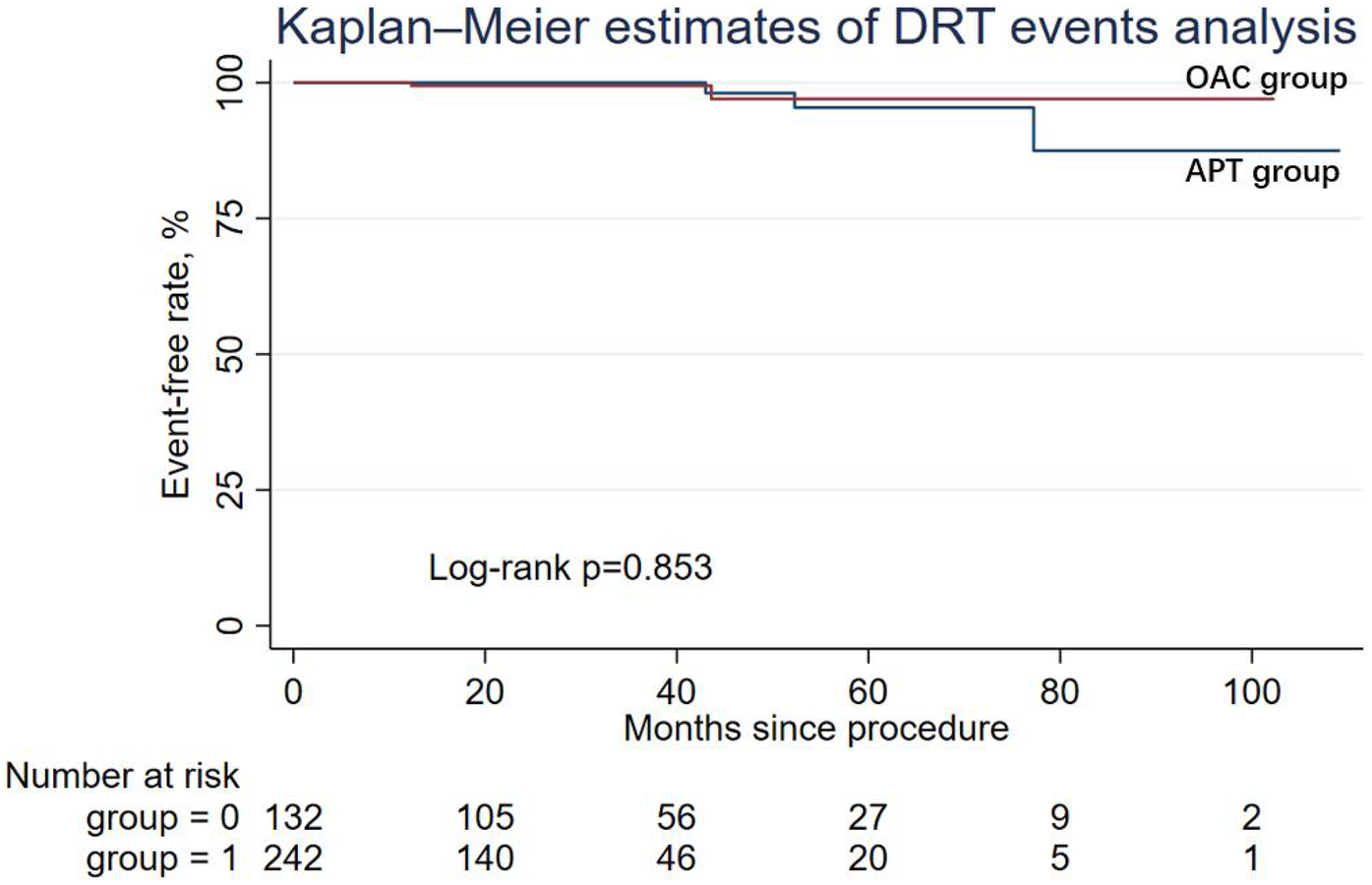
Kaplan-Meier survival curve comparing event-free rates for DRT events between the OAC and APT groups.
Discussion
This was a prospective, single-center cohort study. The major result of this study is that there is no statistical difference between OAC and APT in cardiac death events during the midperiod following LAAC implantation with the WATCHMAN. OAC therapy during the midperiod after LAAC implantation with the WATCHMAN device may demonstrate better safety and efficacy effects compared with dual antiplatelet therapy.
Expert consensus on LAAC and guidelines on atrial fibrillation—citing the results of the PROTECT-AF and PREVAIL trials—recommend the use anticoagulant drugs for 45 days and then continued use of dual antiplatelet drugs for up to 6 months (3, 10). However, the PROTECT-AF and PREVAIL trials were carried out before NOAC was widely used (NOACs were used in only 5% of the patients in the OAC group) (4, 5). Therefore, there was some research focus on comparing the effects of NOAC and APT following implantation of the LAAC device. A meta-analysis of 10 cohort studies involving 2440 patients proved that NOAC was associated with a lower rate of major bleeding events, and all bleeding events were compared with warfarin following WATCHMAN implantation; there were no significant differences between patients receiving NOAC and those receiving warfarin in terms of thromboembolism, cardiac mortality, DRT, and PDL of >5 mm (11).
Recently, several studies have shown the identical benefits of OAC and APT as immediate therapy after LAAC with the WATCHMAN device. A study conducted by Lars Sondergaard et al.—which included 1,018 patients in the OAC group and 509 patients in the APT group (OAC group: 45-day OAC after implantation followed by 6-month single or dual APT; APT group: received APT for variable durations)—found that in the early period after LAAC, OAC therapies did not demonstrate poorer safety and efficacy effects than APT (12). A sub-study of the EWOLUTION study, which included 998 patients with successful WATCHMAN implantation, showed that the risk of a composite ischemic endpoint of stroke, transitory ischemic attack, systemic embolism and device thrombus, or the major bleeding was not significantly different between the patients undergoing dual APT, single APT, OAC, or no therapy in the early period and long period (6 months) following LAAC (13). Another multicenter cohort study included 592 patients who underwent LAAC and received either dual APT or OAC (477 in the dual-APT group and 115 in the OAC group) for a short period following LAAC; the study demonstrated that the composite outcome of death, stroke, and bleeding was not significantly different between the two groups (14). Upon referring to these studies, the instructions for use in European Conformity marked countries were amended, and both antithrombotic regimens were adopted for use in the short period following LAAC with the WATCHMAN device (14).
However, there is still a lack of evidence comparing the effect of anticoagulation and antiplatelet therapy during the midperiod following LAAC. A study by Domenico G. Della Rocca on 555 patients, who were categorized into two groups (standard APT group: guideline recommended therapy which could be briefly described as taking 45 days NOAC and then dual antiplatelet drugs for up to 6 months, then switching to single antiplatelet drug for life; lifelong half-dose NOAC group: half-dose NOAC plus aspirin 81 mg for 45 days after Watchman implantation and lifelong half-dose NOAC monotherapy thereafter), demonstrated that after successful LAAC implantation with the Watchman device, midperiod and long-period half-dose NOAC could significantly reduce the risk of the composite endpoint of DRT, thromboembolism events, and major bleeding compared with a standard, antiplatelet-based, antithrombotic therapy. Although our findings showed numerically lower DRT rates in the OAC group compared with the APT group, the difference was not statistically significant. This result is generally consistent with the observations reported by Della Rocca et al. We believe the main reason for our non-significant result, which diverged from that of Della Rocca, was due to the difference in patient populations (e.g., baseline risk factors, race, and comorbidities of patients) in terms of treatment efficacy (9).
The results of our study showed that the risk of the primary composite endpoint of cardiac mortality, ischemic stroke/TIA/systemic thromboembolism, and major bleeding events was not significantly different; however, the HR of the Cox regression showed a trend of superiority of the OAC group. This finding was in line with the research results of Domenico G. Della Rocca. As the baseline CHA2DS2-VASc and HAS-BLED scores were similar between the APT group and OAC group, the risk of cardiac mortality, ischemic stroke/TIA/systemic thromboembolism, or major bleeding events for the patients who underwent OAC therapy was similar to the risk for patients who underwent APT. And this result reported in our study was also in accordance with previous studies focused on the early period antithrombosis strategies post LAAC the risk of cardiac mortality, ischemic stroke/TIA/systemic thromboembolism or major bleeding events with the use of OAC compared to APT during the midperiod after LAAC implantation. The results of this study indicated that OAC therapy had a superior trend of safety and efficacy compared to APT, including the endpoint of cardiac mortality, cardiovascular death, ischemic stroke/TIA/systemic thromboembolism, and major bleeding events. Although the hazard ratio for the primary composite outcome showed a trend favoring OAC (adjusted HR = 0.67; 95% CI: 0.33–1.36), the difference was not statistically significant. Clinically, this suggests that OAC may provide similar or slightly superior outcomes compared to APT, but larger studies are needed to confirm this trend. We hypothesized that the results may provide new evidence for OAC utilization after the early period following LAAC implantation. Some studies suggest that patients taking one pill show 10%–15% higher adherence rates than those taking two separate pills per day. As a result, taking OAC rather than APT may have a better patient compliance rate. Compared dual APT, the usage of OAC may have a better compliance rate.
In addition, it has been shown that compared with APT, anticoagulant therapy is more effective in preventing DRT. Earlier research suggested that complete endothelialization occurred within 45 days following LAAC; however, a recent meta-analysis showed that 85% of DRT occurred after 45 days following LAAC (15, 16). Therefore, it is of clinical significance to study the antithrombotic regimen during the midperiod following LAAC. Fauchier et al. and a post-hoc analysis of PROTECT AF by Main et al. showed the non-inferior effect of OAC compared to dual APT on DRT risk following LAAC (DRT incidence in the OAC group and the APT group: 8% vs. 79%) (17, 18). These findings were in accordance with the results relating to DRT in our study. Previous research showed that DRT may occur after more than 1 year following LAAC implantation, which may have resulted in the negative DRT event rate comparing the OAC group and APT group of our study and other previous studies (18).
Limitation
There are some limitations to our study: First, this was a non-randomized cohort study and had the limitations and biases inherent to an observational, retrospective study. There may be some unknown confounders which may influence the accuracy of the study results. Second, our study only recruited patients who used a Watchman device. This makes it difficult to generalize the results of our research to other LAAC devices. Third, the small sample size is another limitation of this study, as it may have led to an overinterpretation of the results. We believe that larger multicenter clinical trials are needed to better solve this problem.
Conclusion
In conclusion, OAC therapy during the midperiod following LAAC implantation with the WATCHMAN device may offer comparable safety and efficacy to APT. Further multicenter randomized trials are needed to confirm these findings and identify patient-specific factors that influence treatment response.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This research has undergone review and approval by the Ethics Committee of Beijing Anzhen Hospital affiliated with Capital Medical University. The approval reference number is GZR-2-080. This study strictly adheres to the guidelines and regulations established by the ethics committee and was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants involved in the study.
Author contributions
C-YL: Writing – review & editing, Writing – original draft. LZ: Writing – original draft, Writing – review & editing. J-RZ: Writing – review & editing. L-HH: Writing – review & editing. L-ZG: Writing – review & editing. S-NL: Writing – review & editing. C-HS: Writing – review & editing. D-YL: Writing – review & editing. J-ZD: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Sanna T Diener HC Passman RS Di Lazzaro V Bernstein RA Morillo CA et al Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. (2014) 370(26):2478–86. 10.1056/NEJMoa1313600
2.
Sposato LA Chaturvedi S Hsieh CY Morillo CA Kamel H . Atrial fibrillation detected after stroke and transient ischemic attack: a novel clinical concept challenging current views. Stroke. (2022) 53(3):e94–e103. 10.1161/STROKEAHA.121.034777
3.
Hindricks G Potpara T Dagres N Arbelo E Bax JJ Blomström-Lundqvist C et al 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
4.
Reddy VY Holmes D Doshi SK Neuzil P Kar S . Safety of percutaneous left atrial appendage closure: results from the watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry. Circulation. (2011) 123(4):417–24. 10.1161/CIRCULATIONAHA.110.976449
5.
Holmes DR Jr Kar S Price MJ Whisenant B Sievert H Doshi SK et al Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. (2014) 64(1):1–12. 10.1016/j.jacc.2014.04.029
6.
Maksym J Mazurek T Kochman J Grygier M Kapłon-Cieślicka A Marchel M et al Dual antiplatelet therapy is safe and efficient after left atrial appendage closure. Kardiol Pol. (2018) 76(2):459–63. 10.5603/KP.a2017.0245
7.
Barakat AF Hussein AA Saliba WI Bassiouny M Tarakji K Kanj M et al Initial experience with high-risk patients excluded from clinical trials: safety of short-term anticoagulation after left atrial appendage closure device. Circ Arrhythm Electrophysiol. (2016) 9(6):e004004. 10.1161/CIRCEP.116.004004
8.
Asmarats L Hussein AA Saliba WI Bassiouny M Tarakji K Kanj M et al Short-term oral anticoagulation versus antiplatelet therapy following transcatheter left atrial appendage closure. Circ Cardiovasc Interv. (2020) 13(8):e009039. 10.1161/CIRCINTERVENTIONS.120.009039
9.
Della Rocca DG Magnocavallo M Di Biase L Mohanty S Trivedi C Tarantino N et al Half-dose direct oral anticoagulation versus standard antithrombotic therapy after left atrial appendage occlusion. JACC Cardiovasc Interv. (2021) 14(21):2353–64. 10.1016/j.jcin.2021.07.031
10.
Meier B Blaauw Y Khattab AA Lewalter T Sievert H Tondo C et al EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention. (2015) 10(9):1109–25. 10.4244/EIJY14M09_18
11.
Tan BE Wong PY Lee JZ Tan NY Rao M Cheung JW . Direct oral anticoagulant versus warfarin after left atrial appendage closure with WATCHMAN: updated systematic review and meta-analysis. Curr Probl Cardiol. (2022) 47(11):101335. 10.1016/j.cpcardiol.2022.101335
12.
Søndergaard L Wong YH Reddy VY Boersma LVA Bergmann MW Doshi S et al Propensity-matched comparison of oral anticoagulation versus antiplatelet therapy after left atrial appendage closure with WATCHMAN. JACC Cardiovasc Interv. (2019) 12(11):1055–63. 10.1016/j.jcin.2019.04.004
13.
Boersma LV Schmidt B Betts TR Sievert H Tamburino C Teiger E et al Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J. (2016) 37(31):2465–74. 10.1093/eurheartj/ehv730
14.
Faroux L Cruz-González I Arzamendi D Freixa X Nombela-Franco L Peral V et al Short-term direct oral anticoagulation or dual antiplatelet therapy following left atrial appendage closure in patients with relative contraindications to chronic anticoagulation therapy. Int J Cardiol. (2021) 333:77–82. 10.1016/j.ijcard.2021.02.054
15.
Alkhouli M Busu T Shah K Osman M Alqahtani F Raybuck B . Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol. (2018) 4(12):1629–37. 10.1016/j.jacep.2018.09.007
16.
Schwartz RS Holmes DR Van Tassel RA Hauser R Henry TD Mooney M et al Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv. (2010) 3(8):870–7. 10.1016/j.jcin.2010.04.017
17.
Main ML Fan D Reddy VY Holmes DR Gordon NT Coggins TR et al Assessment of device-related thrombus and associated clinical outcomes with the WATCHMAN left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the PROTECT-AF trial). Am J Cardiol. (2016) 117(7):1127–34. 10.1016/j.amjcard.2016.01.039
18.
Weise FK Bordignon S Perrotta L Konstantinou A Bologna F Nagase T et al Short-term dual antiplatelet therapy after interventional left atrial appendage closure with different devices. EuroIntervention. (2018) 13(18):e2138–46. 10.4244/EIJ-D-17-00901
Summary
Keywords
atrial fibrillation, left atrial appendage closure, anticoagulation, antiplatelet, frail
Citation
Li C-Y, Zhou L, Zhang J-R, Huang L-H, Guo L-Z, Li S-N, Sang C-H, Long D-Y and Dong J-Z (2025) Comparison of oral anticoagulation and antiplatelet therapy during the midperiod after percutaneous left atrial appendage closure. Front. Cardiovasc. Med. 12:1637290. doi: 10.3389/fcvm.2025.1637290
Received
29 May 2025
Accepted
21 October 2025
Published
27 November 2025
Volume
12 - 2025
Edited by
Hugo Ten Cate, Maastricht University Medical Centre, Netherlands
Reviewed by
Jiangtao Yu, Catholic Medical Center Koblenz-Montabaur, Germany
Song Zhang, Second Military Medical University, China
Updates
Copyright
© 2025 Li, Zhou, Zhang, Huang, Guo, Li, Sang, Long and Dong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Song-Nan Li lisongnan@ccmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.