Abstract
A 60-year-old woman was scheduled for elective coronary angiography after a positron emission tomography and computer tomography (PET-CT) cardiac perfusion imaging test showing extensive myocardial ischemia. A few hours before the scheduled angiography, she presented to the emergency room with chest pain and diffuse ST-segment modifications leading to emergent coronary angiography. Recent PET-CT ischemia and viability maps were available at the time of the intervention, favoring a percutaneous coronary intervention for chronic total occlusion (CTO PCI) of the left circumflex artery, apart from the culprit lesion of the left main coronary artery and left anterior descending artery, with good results. A second PET-CT scan 23 days post-PCI showed a reversible perfusion defect of the first diagonal branch territory, which was subsequently treated. A rapid normalization of the left ventricular ejection fraction (LVEF) was noted after revascularization, while the second PET-CT showed no signs of significant myocardial necrosis. This case illustrates the potential role of cardiac imaging perfusion studies in guiding revascularization in complex cases in the context of an acute myocardial infarction (MI).
Learning objectives
• Identify and interpret myocardial perfusion defects by nuclear cardiac imaging, in conjunction with coronary lesions to guide decisions for revascularization.
• Understand the potential role and prognostic significance of myocardial viability in CTO PCI for selected, complex cases.
A 60-year-old woman was referred to our cardiology clinic for chest pain evaluation. The patient reported brief episodes of chest pain on exertion, which began approximately 6 months earlier. The pain is located centrally and radiates to both shoulders. The intensity was variable and appeared principally on exertion, but also after meals. No other symptoms were reported. The physical examination was unremarkable with no signs of heart failure or peripheral vascular disease, while vital signs were in the normal range.
Past medical history
Her medical history was free from prior cardiovascular (CV) events and included substituted hypothyroidism and gastric bypass intervention. The patient presented multiple CV risk factors (type 2 diabetes, arterial hypertension, and dyslipidemia), all well controlled under optimal medical therapy. She led a sedentary life and was overweight (BMI 28.4 kg/m2).
Investigations
A gastroscopy was performed a few days before the cardiologist's visit, showing signs of esophagitis, and a high-dose proton pump inhibitor treatment was initiated. Due to the intermediate-high baseline CV risk and the presence of symptoms, non-invasive testing for myocardial ischemia was scheduled (PET-CT scan). The exam was realized using a Siemens BIOGRAPH VISION® camera, with injection of the 82-Rubidium tracer (600MBq per phase). Stress coronary vasodilation was attained pharmacologically by a selective A2A adenosine receptor agonist (Regadenoson). A CT of the thorax was also obtained concomitantly before the PET image acquisitions, for the application of the attenuation correction algorithm and the estimation of the coronary calcium score. The typical PET-CT scan protocol includes semiquantitative and quantitative left ventricle (LV) perfusion evaluation as well as LV volume measures during the cardiac cycle [LV ejection fraction (EF) estimation]. The exam revealed a massive (70% of the LV) and severe perfusion defect of the anterior, lateral, apical, and septal walls of the LV, both at rest and during stress, with no reversibility, highly suggestive of transmural necrosis (Figure 1). Myocardial blood flow quantification showed a severe decrease in flow and coronary flow reserve (CFR) in the same territories. A transient dilation of the LV was noted, and LVEF was decreased at rest (47%) and even more during stress (40%). The coronary artery calcium score was measured at 2449 Agatston units (AU), denoting extensive coronary artery disease.
Figure 1
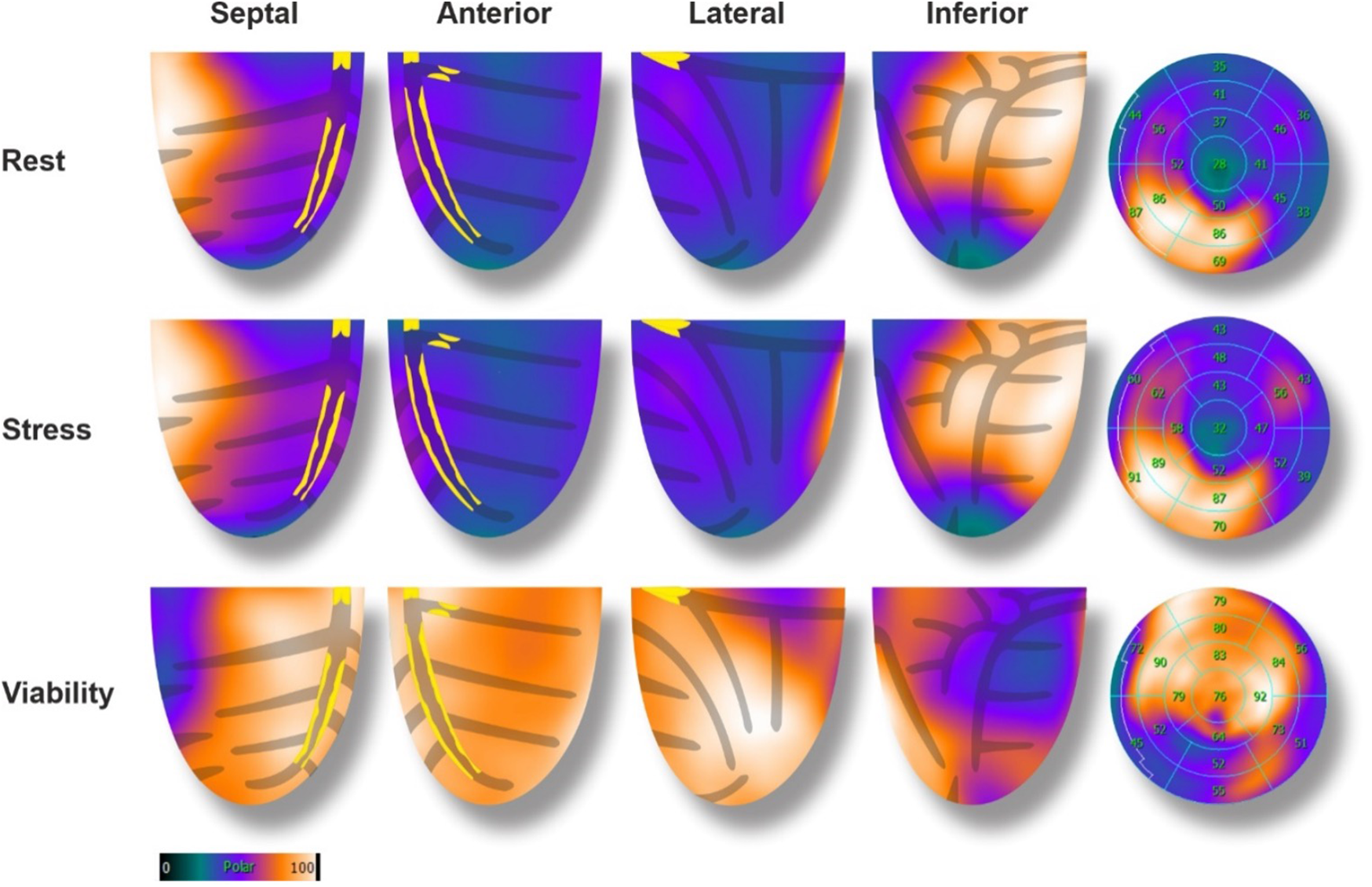
Baseline 82-rubidium PET-CT scan with corresponding glucose metabolism (18F-FDG), and coronary lesions. In perfusion images, a large (70%), severe, anterior, lateral, and apical defect was noted with no reversibility. The 18F-FDG showed enhanced glucose metabolism in the hypo-perfused areas (mirror image), denoting a hibernating state of the myocardial cells.
Two days later, a viability PET-CT exam was performed using the 18F-fluorodeoxyglucose (18F-FDG) tracer (231 MBq) after administration of glucose, 500 mg of Acipimox, and insulin according to standard procedures (1). The exam showed preserved metabolic activity of the LV perfusion defect areas, denoting preserved viability of the anterior, lateral, apical, and septal walls of the LV (hibernation, Figure 1). A coronary angiography was scheduled for the next day.
Management
The same night after dinner, the patient felt the same high-intensity chest pain radiating to both shoulders, which led her to the emergency room. The initial physical examination revealed fine crackles on the lung bases, which rapidly evolved into pulmonary edema and hemodynamic instability. The electrocardiogram (ECG) showed signs of diffuse ischemia with a profound ST-segment depression in DII, DIII, aVF, and V4–V6, with concomitant ST-segment elevation in the aVR derivation. Blood tests revealed a positive high-sensitivity troponin (1,438 ng/L), increased creatinine kinase (CK) (505 U/L), and increased pro-brain natriuretic peptide values (6,775 ng/L).
The patient was sent directly to the catheterization laboratory for coronary angiography in the context of ST depression in more than 6 leads and ST elevation in aVR suggestive of severe multivessel ischemia or left main (LM) coronary disease.
The exam revealed a severe 2-vessel coronary artery disease with a 50%–70% stenosis of the distal LM, sub-occlusive stenosis of the proximal left anterior descending artery (LAD) with diffuse atherosclerosis of the median and distal part, a 70% stenosis of the 1st diagonal artery and total occlusion of the proximal left circumflex artery (LCX) receiving collaterals from the right coronary artery (RCA) (Figure 2).
Figure 2
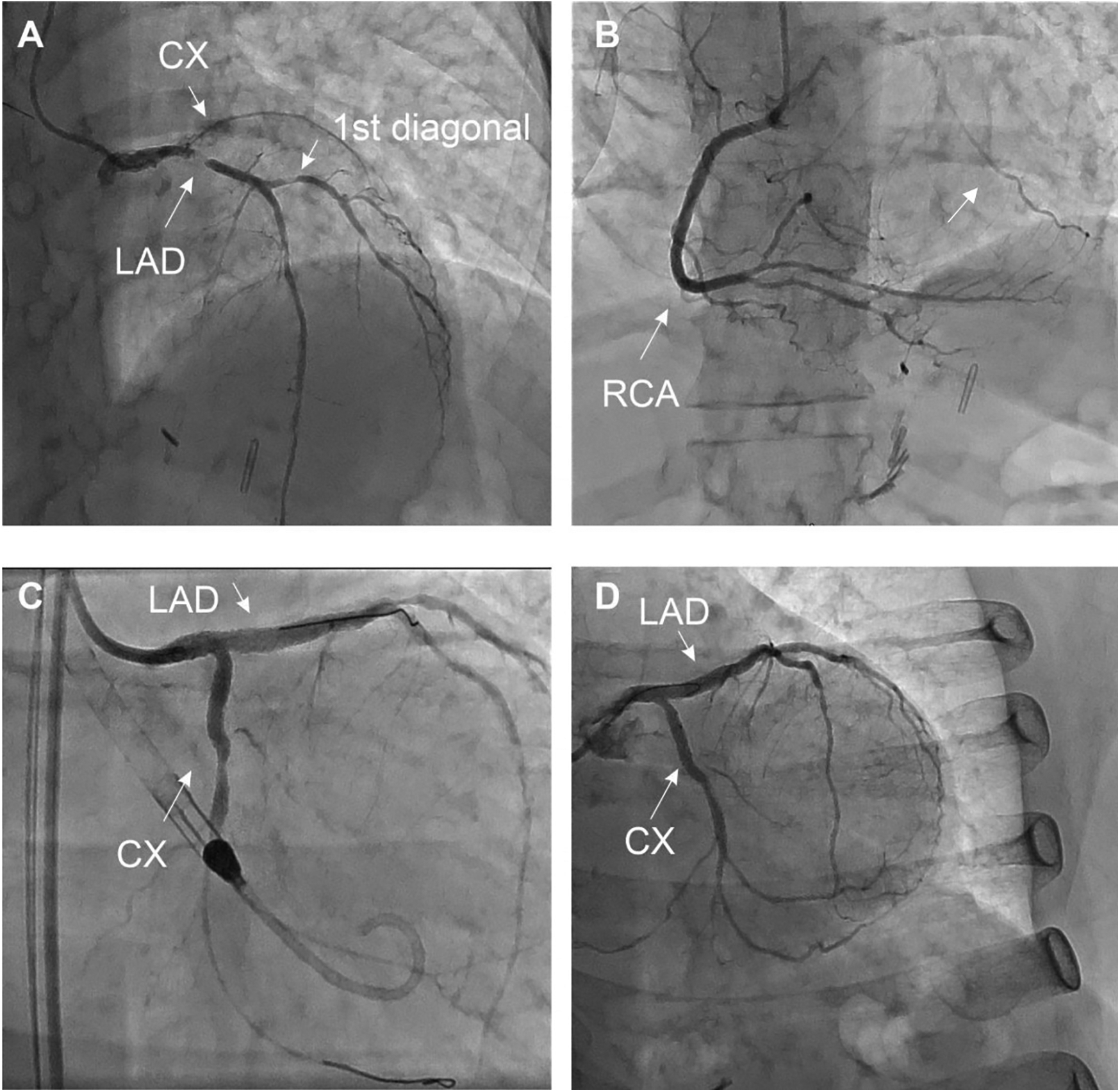
Coronary angiography at the initial presentation (A–C) and final result (D) the patient presented with extended 2-vessel coronary artery disease with a 50%–70% lesion of the distal LM, a sub-occlusive lesion of the proximal LAD with diffuse atherosclerotic infiltration of the median and distal part, à 70% stenosis of the 1st diagonal, and a CTO of the LCX collateralized by the RCA (arrow). MT, LAD, and LCX were initially treated by PCI under mechanical circulatory support (Impella CP system®). The 1st diagonal was treated during the second angiography (D), after documentation of the residual ischemia by PET-CT (Figure 4).
Due to the hemodynamic instability, a mechanical circulatory support was inserted (Impella CP System®, Abiomed) at the beginning of the procedure via femoral access. The procedure included percutaneous coronary intervention (PCI) of the LM and proximal LAD using Shockwave intravascular lithotripsy as well as recanalization of the proximal LCX artery using a Fileder XTA 0.14 wire (Asahi Intecc, JN) to cross the lesion and a T-stenting strategy (Medtronic Onyx stent 2.75 × 22 mm in the LCX with post-dilatation at 3.0 mm, 3.5 × 30 mm Onyx stent in the LAD and LM with proximal optimization à 4.0 mm). No significant complications were noted. The patient was transferred to the intensive care unit (ICU), under double anti-aggregation treatment by Aspirin® and Prasugrel® at standard doses.
The patient rapidly improved with a quick hemodynamic stabilization, leading to the ablation of the Impella CP System® a few hours later. The signs of left heart failure disappeared under diuretics. Transthoracic echocardiography (TTE) at the ICU showed an LVEF of 40% immediately after the ablation of the Impella CP System®. The maximum high sensitivity troponin was measured at 12,408 ng/L, and CK reached 3,706 U/L. No significant arrhythmias were noted, and the patient was discharged from the ICU two days later.
A TTE at day 5 showed complete recovery of the LVEF without any regional abnormalities (Figure 3).
Figure 3
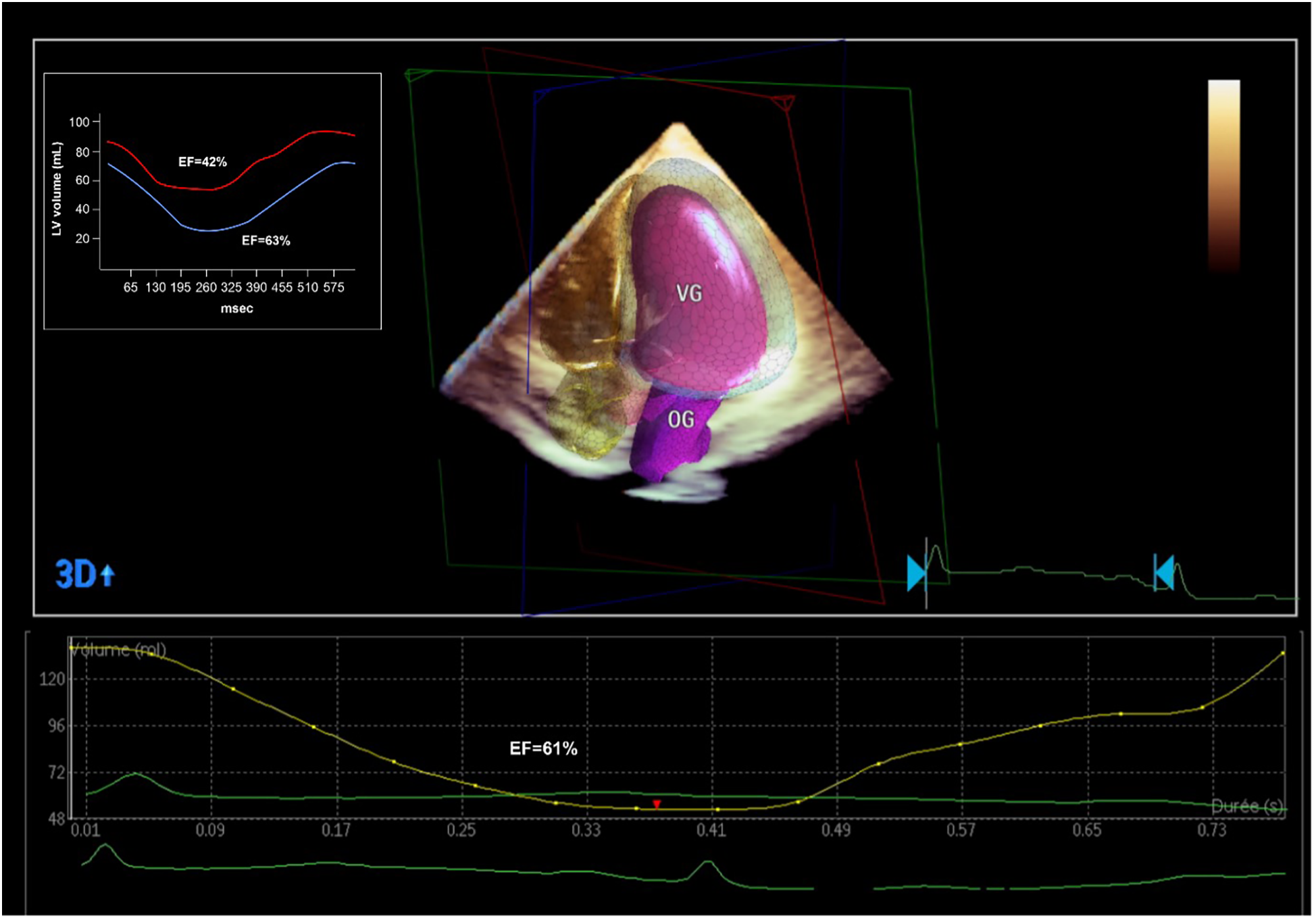
Echocardiographic and PET-CT evaluation of the LVEF after the coronary revascularization. PET-CT showed a significant improvement in LVEF (from 42% to 63%) with a significant decrease globally in LV volumes, denoting an ischemic LV dilatation. 3D echocardiographic evaluation confirmed the normalization of the LVEF.
A second 82-Rubidium PET-CT scan 23 days post-PCI showed significant improvement in myocardial perfusion at rest (4% apical) with a severe but completely reversible perfusion defect of the basal, anterolateral segment of the LV, suggesting a 7%–8% residual ischemia in the area of the 1st diagonal stenosis (Figure 4). The LVEF was significantly increased both at rest (57%) and during pharmacological stress (62%). Subsequently, a PCI of the 1st diagonal branch was performed without complications (2.5 × 12 mm Onyx stent). A control coronary angiography at 18 months showed persistent good results of the LM, LAD, and LCX PCI.
Figure 4
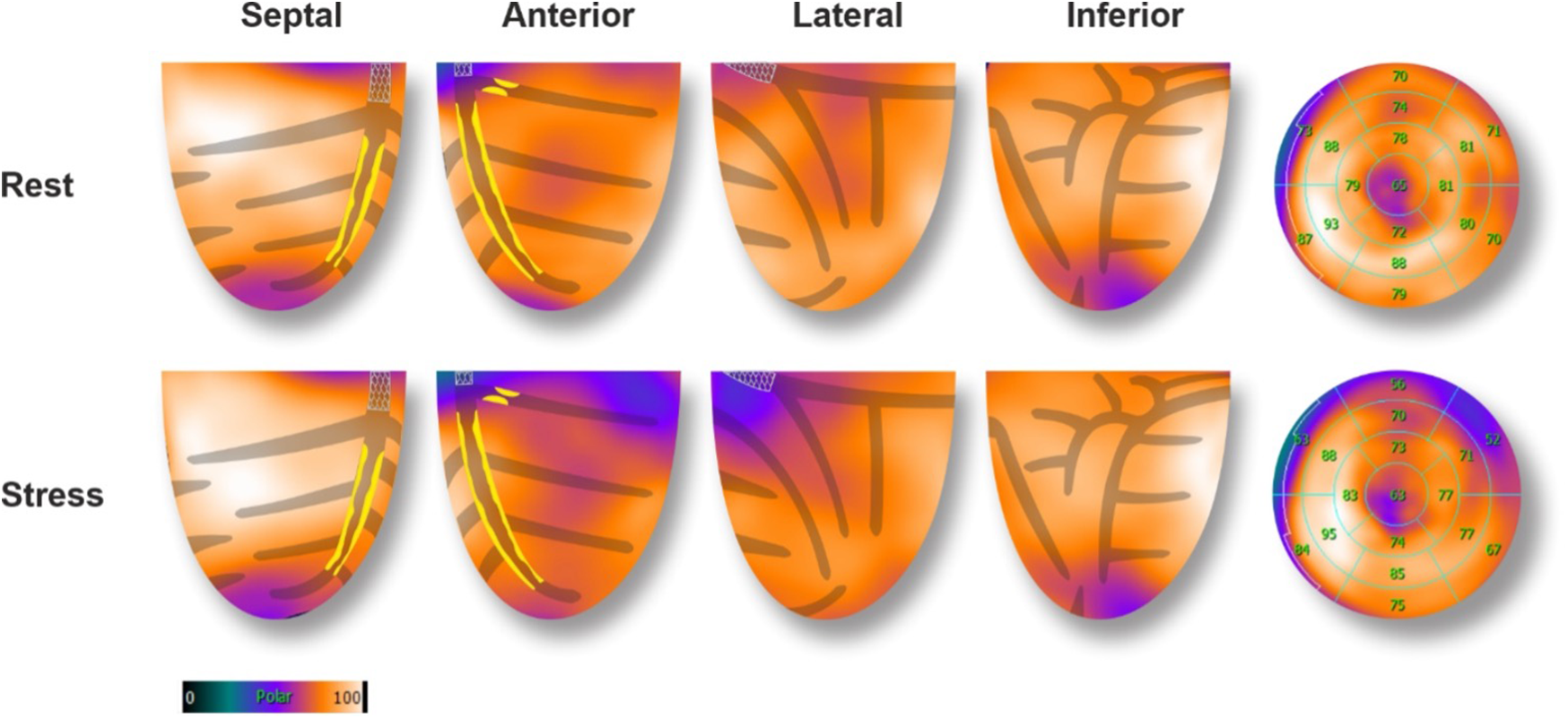
82-Rubidium PET-CT scan 23 days after the PCI of the MT, proximal LAD, and CX arteries. Compared to the baseline exam, images at rest showed a significant improvement in myocardial perfusion after the revascularization. Images during pharmacological stress showed an 8% severe defect in perfusion of the anterolateral basal segment, corresponding to the territory of the 1st diagonal artery (70% focal stenosis of the proximal part). A PCI was subsequently performed (Figure 3).
Discussion
Myocardial ischemia is a key factor for guiding revascularization strategy in patients with stable coronary artery disease (2). The role of myocardial viability, though, is less clear. Historically, many observational studies and meta-analyses have suggested that in patients with dysfunctional myocardium, revascularization is associated with better outcomes and improved ventricular recovery when myocardial viability has been proven by advanced imaging (3). However, more recent controlled trials (STICH and REVIVED-BCI2) showed no benefit in survival with revascularization compared to optimal medical treatment in patients with decreased LVEF despite preserved myocardial viability (4, 5). These findings do not apply in acute, complex cases where viability may help differentiate hibernating myocardium from scar. This is extremely relevant for predicting segmental functional recovery after revascularization in the acute setting, even if this does not translate into improved clinical outcomes in the general population (6). However, this information is rarely available in the context of acute coronary syndromes. In this case, the patient presented to the emergency room with chest pain and ECG signs of diffuse myocardial ischemia. The urgent coronary angiography confirmed the presence of a severe 2-vessel disease involving the distal LM, proximal LAD, and LCX arteries. This was suspected after the initial 82-Rubidium PET-CT scan showing a massive, severe non-reversible perfusion defect covering the anterior, lateral, septal, and apical walls of the left ventricle (Figure 1). Although the LAD lesion was the culprit lesion for the acute coronary syndrome and ECG manifestations of ischemia, the LCX artery was found occluded with morphological aspects pointing to chronic total occlusion (CTO). Typically, in this case without information on the viability of the lateral wall, a recanalization of the LCX artery may not have been attempted, considering the technical challenge. However, the readily available viability test encouraged the operator to attempt the recanalization of the occluded LCX artery in an attempt to prevent further myocardial damage (7). The 18F-FDG viability test performed a few hours before the angiography, confirmed the presence of enhanced glucose metabolism in this area, which denotes a preserved viability of the myocardial cells, thus pointing to the presence of hibernating myocardium and not a scar. This information favored the prompt attempt for the recanalization of the LCX CTO at the time of the treatment of the culprit lesion (LM-LAD). This decision possibly explains the excellent short and mid-term results of the intervention, with the very rapid (in a few hours) restoration of the hemodynamic stability (ablation of the Impella CP system®), the normalization of the LVEF (62% vs. 40%, Figure 3) and the absence of significant myocardial defect at the latest perfusion PET-CT scan (Figure 4).
Although many studies have assessed the role of viability testing in stable coronary artery disease, this case is unique in that myocardial perfusion and viability information were readily available in the context of an acute coronary syndrome. It also reinforces the evidence that, in selected cases, viability assessment may be important for guiding revascularization—especially when performed early after coronary occlusion.
However, it is technically and logistically challenging to implement viability or even perfusion testing during an acute coronary syndrome. These tests often require the administration of vasoactive drugs, which may compromise hemodynamic stability. Additionally, both perfusion and 18F-FDG testing require preparation that can delay angiography, which remains the cornerstone of treatment in this setting.
Conclusion
The present case is an excellent reminder of the importance of early revascularization in achieving favorable results in acute coronary syndromes. It also underscores that, in selected complex cases, viability testing in conjunction with perfusion imaging can play a critical role in guiding revascularization strategy and predicting outcomes.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DA: Resources, Software, Writing – review & editing, Formal analysis, Writing – original draft. LM: Writing – review & editing. FM: Writing – review & editing, Funding acquisition. VG: Conceptualization, Writing – review & editing, Data curation. SN: Writing – review & editing, Validation, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Auerbach MA Schoder H Hoh C Gambhir SS Yaghoubi S Sayre JW et al Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation. (1999) 99:2921–6. 10.1161/01.CIR.99.22.2921
2.
Saraste A Knuuti J . ESC 2019 guidelines for the diagnosis and management of chronic coronary syndromes: recommendations for cardiovascular imaging. Herz. (2020) 45:409–20. 10.1007/s00059-020-04935-x
3.
Allman KC Shaw LJ Hachamovitch R Udelson JE . Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. (2002) 39:1151–8. 10.1016/S0735-1097(02)01726-6
4.
Perera D Ryan M Morgan HP Greenwood JP Petrie MC Dodd M et al Viability and outcomes with revascularization or medical therapy in ischemic ventricular dysfunction: a prespecified secondary analysis of the REVIVED-BCIS2 trial. JAMA Cardiol. (2023) 8:1154–61. 10.1001/jamacardio.2023.3803
5.
Panza JA Ellis AM Al-Khalidi HR Holly TA Berman DS Oh JK et al Myocardial viability and long-term outcomes in ischemic cardiomyopathy. N Engl J Med. (2019) 381:739–48. 10.1056/NEJMoa1807365
6.
Garcia MJ Kwong RY Scherrer-Crosbie M Taub CC Blankstein R Lima J et al State of the art: imaging for myocardial viability: a scientific statement from the American Heart Association. Circ Cardiovasc Imaging. (2020) 13:e000053. 10.1161/HCI.0000000000000053
7.
Neumann FJ Sousa-Uva M Ahlsson A Alfonso F Banning AP Benedetto U et al 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. 10.1093/eurheartj/ehy394
Summary
Keywords
nuclear cardiac imaging, coronary artery disease, myocardial viability, CTO—percutaneous coronary intervention, myocardial ischemia
Citation
Adamopoulos D, Meyer de Stadelhofen L, Mach F, Garibotto V and Noble S (2025) Case Report: PET-CT ischemia and viability maps guiding an emergency revascularization. Front. Cardiovasc. Med. 12:1637403. doi: 10.3389/fcvm.2025.1637403
Received
29 May 2025
Accepted
09 October 2025
Published
29 October 2025
Volume
12 - 2025
Edited by
Heng Ma, Yantai Yuhuangding Hospital, China
Reviewed by
Sun-Joo Jang, Yale University, United States
Qingyu Ji, Second Affiliated Hospital of Baotou Medical College, China
Updates
Copyright
© 2025 Adamopoulos, Meyer de Stadelhofen, Mach, Garibotto and Noble.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Dionysios Adamopoulos dionysios.adamopoulos@hug.ch
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.