Abstract
Transcatheter edge-to-edge repair is an alternative therapy for patients with severe mitral regurgitation. Here, we report the first case of right ventricular apical thrombus formation following transcatheter edge-to-edge repair in a 54-year-old male with heart failure and reduced ejection fraction. Post-procedural transthoracic echocardiography revealed multiple apical right ventricular thrombi on postoperative day 2. Anticoagulation with warfarin and low-molecular-weight heparin resulted in thrombus resolution, and the patient was discharged uneventfully. This case highlights the importance of vigilant postoperative monitoring and tailored thromboprophylaxis in patients with impaired ventricular function.
Introduction
TEER has emerged as a significant advancement in MR management, offering a safe and effective alternative to surgical intervention, particularly for high-risk patients (1–3). There have been cases of acute thrombus formation during the mitral valve TEER procedure in the left and right atria: at the site of transseptal puncture, as well as on the clip delivery system/guide catheter and on the mitral valve TEER device itself (4, 5). However, RV thrombosis remains undocumented. This report presents a unique case of RV apical thrombus formation following TEER, emphasizing the importance of continuous monitoring and individualized patient management.
Case description
A 54-year-old man presented with progressive exertional dyspnea and chest tightness over one year, significantly worsening in the preceding month to NYHA class III limitations. The patient had a 3/4 holosystolic murmur at the left fifth intercostal space at the midclavicular line. He had coarse breath sounds, abnormal carotid pulsation, but no lower extremity edema. Laboratory evaluation demonstrated elevated D-dimer (11.57 mg/L), international normalized ratio (INR) of 2.0, and markedly elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP: 13,000 pg/mL). The patient maintained sinus rhythm throughout the hospitalization. Coronary computed tomography angiography (CCTA) excluded coronary artery disease, and the patient denied prior hypertension, diabetes, or atherosclerotic history.
Diagnostic assessment and therapeutic intervention
After admission, the patient underwent a comprehensive cardiac ultrasound assessment, including both transthoracic and transesophageal echocardiography. Preoperative transthoracic echocardiography (TTE) demonstrated global cardiomegaly (LV end-diastolic diameter: 72 mm), severe MR (vena contracta: 12 mm), LV ejection fraction (LVEF) 24%, and RV dysfunction (TAPSE: 13 mm) (Figures 1A–F). The echocardiographic findings, including biventricular dilation and systolic dysfunction in the absence of significant coronary artery disease, were consistent with a diagnosis of dilated cardiomyopathy. Further transesophageal echocardiography (TEE) confirmed the regurgitant jet was wide, between the A2 and P2 scallops, with pulse wave Doppler imaging of the pulmonary vein showing systolic flow reversal (Figure 2).
Figure 1
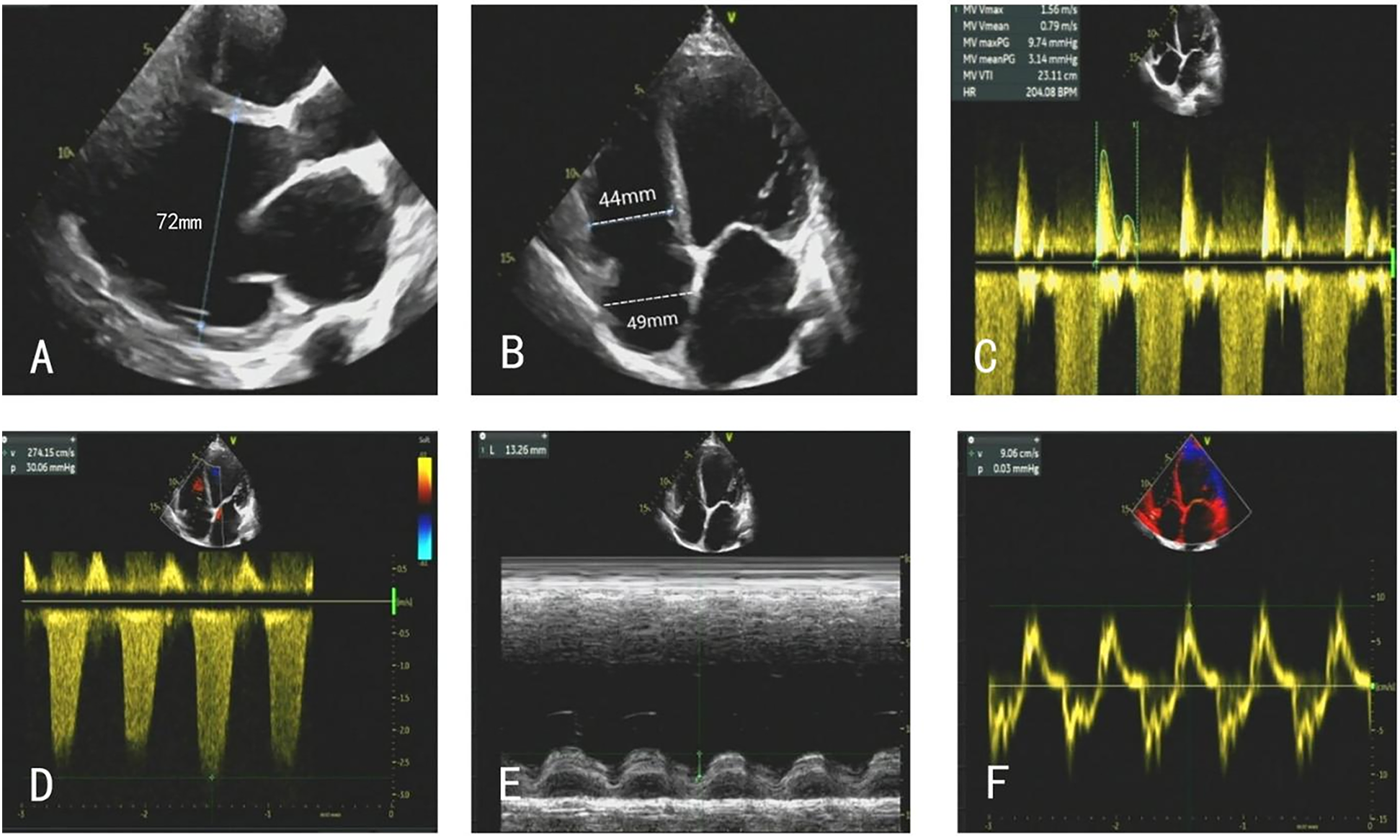
(A–F) TTE on admission showed global cardiomegaly, severe MR, and RV dysfunction.
Figure 2
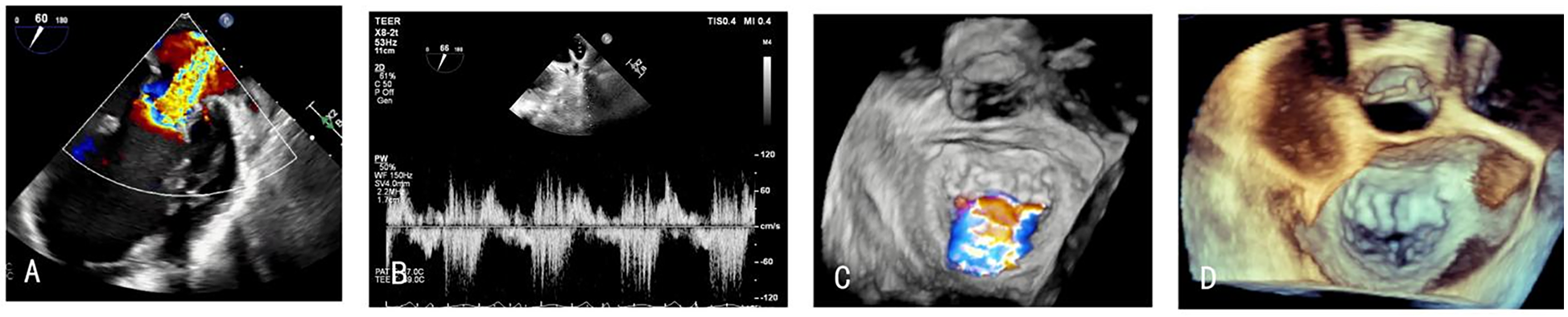
(A,C,D) TEE showing the MR between A2 and P2, (B) pulmonary vein systolic flow reversal before the procedure.
The patient was treated with guideline-directed medical therapy, including furosemide, sacubitril-valsartan, metoprolol, and spironolactone, but the wheezing symptoms were not significantly improved. After reviewing by a multidisciplinary valve team, the patient was deemed a suitable candidate for mitral valve TEER due to elevated surgical risk, poor functional status, suitable valve morphology, and the presence of severe symptomatic mitral regurgitation. Intraoperative right heart catheterization was performed, revealing a mean pulmonary artery pressure of 38 mmHg, confirming the presence of significant pulmonary hypertension. The procedure proceeded smoothly, intraprocedural echocardiographic guidance used two XTR mitral clips in the A2/P2 position. After release, the regurgitant area was reduced to mild, and the lung vein flow normalized. The average pressure gradient of the MV was 2 mm Hg, and there was no apparent stenosis. Meanwhile The thrombus was not detected intraprocedurally.
Postoperatively, antiplatelet therapy was initiated immediately with aspirin 100 mg and clopidogrel 75 mg daily, along with enoxaparin 4,000 AxaIU subcutaneously twice. On the first postoperative day, repeat coagulation tests showed an INR of 1.68, while the NT-proBNP level decreased to 3,490 pg/mL.
On postoperative day 2, repeat TTE demonstrated significant improvement in biventricular systolic function alongside favorable hemodynamic changes. Mitral regurgitation showed substantial reduction in severity, while tricuspid regurgitation exhibited marked improvement from severe to mild. Pulmonary artery systolic pressure decreased to 25 mm Hg from preoperative measurements of 45 mm Hg. Right ventricular remodeling was evidenced by decreased chamber dimensions following TEER. Right ventricular functional parameters demonstrated modest enhancement: TAPSE increased from 13 mm to 15 mm, while peak systolic tissue Doppler velocity (S′) remained stable at 9 cm/s (Figures 3A–D), and no right-to-left shunt was detected. Notably, echocardiographic evaluation revealed multiple apical thrombi within the right ventricular cavity (Figures 3E–G). Subsequent vascular Doppler studies of the lower extremities and pelvic vasculature demonstrated preserved triphasic flow patterns without evidence of deep venous thrombosis.
Figure 3
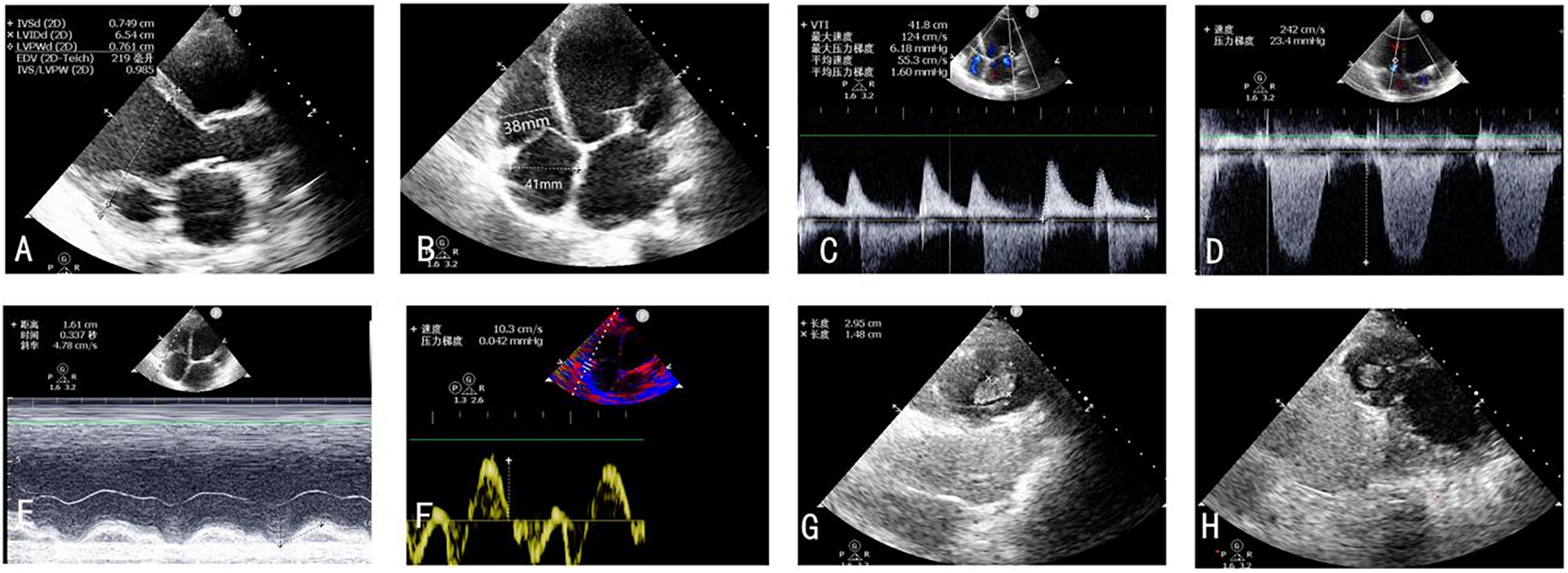
(A–F) TTE postoperative day 2 revealed significant improvement in biventricular systolic function alongside favorable hemodynamic changes. (G,H) Thrombus forming in the right ventricle on the second postoperative day.
The patient was promptly initiated on warfarin 2.5 mg daily for anticoagulation and received subcutaneous dalteparin sodium 5,000 IU every 12 h. Serial monitoring of the INR, clinical status, and imaging findings was conducted throughout the follow-up period. The patient remained demonstrating resolution of heart failure symptoms. Follow-up TTE performed during subsequent evaluations revealed progressive reduction in the size of the right ventricular apical thrombi (Figures 4A–C). The patient was discharged on warfarin 2.5 mg daily for long-term thromboprophylaxis, with a target INR range of 2.0–3.0.
Figure 4
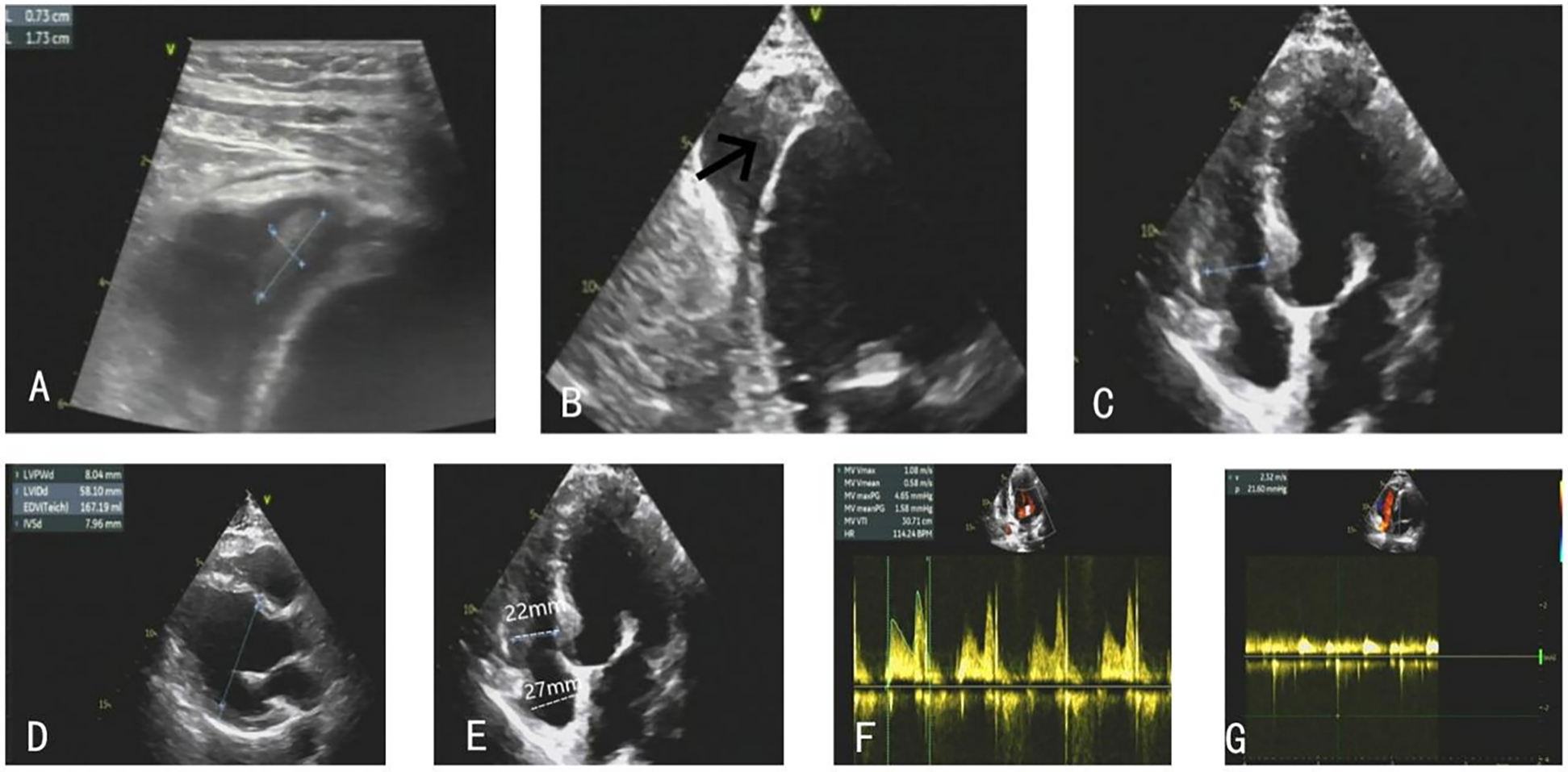
(A–C) The right ventricular thrombus gradually eliminated. (D–G) TTE on the follow-up showed stable clips with mild MR, and the patient's ventricular systolic function significantly improved.
At the 1-month follow-up, repeat TTE demonstrated further diminution of the thrombi. After 7 months of anticoagulation therapy, follow-up echocardiography confirmed complete resolution of the thrombus, stable clips with mild MR, and preserved valvular function. At this time, the INR was therapeutic at 2.31, with a mean mitral valve pressure gradient of 1.6 mmHg and a peak mitral inflow velocity of 108 cm/s (Figures 4D–G). No thromboembolic complications or recurrent thrombus formation were observed during the surveillance period, and noparadoxical embolic events occurred, including stroke, systemic infarction, or hypoxemia.
Discussion
To our knowledge, this is the first case of right ventricular thrombus formation following TEER, an overlooked complication. However, the risk of thrombosis and the best thromboprophylactic therapy remain undetermined at present.
Current evidence suggests a remarkably low incidence of post-TEER ventricular thrombosis, with left ventricular (LV) thrombi reported in 1.1% of cases and no prior RV occurrences (6). To date, thrombogenesis mechanisms remain incompletely understood, proposed mechanisms include synergistic interactions among ventricular dysfunction, hemodynamic alterations, hypercoagulable states, and patient-specific risk factors. Thrombus formation in the LV, particularly in the context of dilated LV, is a known complication in heart failure patients with reduced left ventricular ejection fraction (HFrEF) (7).
The patient had a clearly established predisposition. Pre-operative TTE confirmed severely reduced RV systolic function and dilation, creating a low-flow environment conducive to blood stasis and thrombus formation. This pre-existing RV myopathy is the fundamental underlying risk factor. We propose that the TEER procedure acted as a significant pro-thrombotic stimulus in this vulnerable setting. The acute reduction in left atrial and ventricular filling pressures post-clip deployment alters the loading conditions of the already compromised right heart. This sudden hemodynamic shift could potentially exacerbate relative RV stasis (8). Furthermore, the procedure itself induces a transient inflammatory and hypercoagulable state due to vascular access, device manipulation, and tissue injury, which may have contributed to a systemic pro-thrombotic milieu. Moreover, the significantly elevated D-dimer level raises the possibility of a pre-existing subclinical hypercoagulable state or even a cryptic deep vein thrombosis. Mechanical manipulation during femoral venous access could theoretically have dislodged a small, undetected thrombus, embolizing it to the RV apex where it propagated in the low-flow environment.
Regarding therapeutic alternatives, several theoretical options could be considered for managing right ventricular thrombus, though none are well-established in this specific context. Percutaneous thrombus aspiration or mechanical extraction represents a potential interventional approach, particularly in cases of large, mobile, or high-risk thrombi. However, the procedural risks, including thrombus fragmentation and embolization, must be carefully weighed against the benefits, especially in a patient with recent TEER and impaired ventricular function. Catheter-directed thrombolysis is another option that could be considered in scenarios of hemodynamic compromise or failed anticoagulation, though it carries a significant bleeding risk and lacks evidence in this setting. In our case, given the patient's clinical stability and the absence of embolic events, we opted for a conservative approach with intensified anticoagulation, which ultimately led to complete thrombus resolution. This outcome supports the efficacy of anticoagulation as a first-line strategy in stable patients, while more invasive options may be reserved for those with contraindications to anticoagulation or signs of impending embolism.
Current perioperative antithrombotic strategies for TEER, which typically combine intraprocedural therapeutic anticoagulation with six months of dual antiplatelet therapy post-intervention, remain inadequately supported by clinical evidence (9). LV thrombus formation has been documented despite direct oral anticoagulant (DOAC) therapy, raising questions about their efficacy in this population (6). Emerging data indicate warfarin may offer superior thromboprophylaxis compared to DOACs in TEER patients (6), though recent studies present conflicting conclusions regarding bleeding risks and stroke prevention (10–12). Consequently, optimal thromboprophylaxis strategies remain undefined, necessitating individualized risk-benefit assessments.
Limitations
No preoperative Doppler ultrasound was performed to systematically exclude deep vein thrombosis; however, no obvious DVT was noted during femoral venous access. Preoperative cardiac CT was not performed, but comprehensive TEE before, during, and after the procedure showed no right ventricular thrombus. An SGLT2 inhibitor was not prescribed during initial GDMT optimization, representing a limitation in medical therapy. While the elevated D-dimer was primarily attributed to heart failure, a comprehensive venous duplex ultrasound at admission would have been more complete.
Patient perspective
From a patient's point of view, the described solution was accepted. More than one year after the procedure, the patient has no complaints, and he relieved heart failure symptoms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Suzhou Municipal Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Writing – original draft. JZ: Writing – review & editing. ZG: Writing – review & editing. HZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Gusu Health Personnel Training Project (GSWS2021042 to HF Zhang), General Program of National Natural Science Foundation of China (82270394 to HF Zhang), and Suzhou Key Laboratory of Cardiovascular Disease (SZS202401). Prof. HF Zhang are Associate Fellows at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Nanjing Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1640491/full#supplementary-material
Abbreviations
CCTA, coronary computed tomography angiography; DCM, dilated cardiomyopathy; DOACs, direct oral anticoagulants; GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; INR, international normalized ratio; LA, left atrium; LV, left ventricle; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; MR, mitral regurgitation; MV, mitral valve.
References
1.
Feldman T Kar S Rinaldi M Fail P Hermiller J Smalling R et al Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. (2009) 54(8):686–94. 10.1016/j.jacc.2009.03.077
2.
Whitlow PL Feldman T Pedersen WR Lim DS Kipperman R Smalling R et al Acute and 12-month results with catheter-based mitral valve leaflet repair: the EVEREST II (Endovascular Valve Edge-to-Edge Repair) high risk study. J Am Coll Cardiol. (2012) 59(2):130–9. 10.1016/j.jacc.2011.08.067
3.
Vahanian A Beyersdorf F Praz F Milojevic M Baldus S Bauersachs J et al 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. 10.1093/eurheartj/ehab395. Erratum in: Eur Heart J. (2022) 43(21):2022. doi: 10.1093/eurheartj/ehac051.
4.
Bilge M Saatci Yaşar A Ali S Alemdar R . Left atrial spontaneous echo contrast and thrombus formation at septal puncture during percutaneous mitral valve repair with the MitraClip system of severe mitral regurgitation: a report of two cases. Anadolu Kardiyol Derg. (2014) 14(6):549–50. 10.5152/akd.2014.5355
5.
Huntgeburth M Müller-Ehmsen J Brase C Baldus S Rudolph V . Thrombus formation at the MitraClip system during percutaneous mitral valve repair. JACC Cardiovasc Interv. (2014) 7(9):e111–2. 10.1016/j.jcin.2014.03.010
6.
Tichelbäcker T Körber MI Mauri V Iliadis C Metze C Adler C et al Prevalence of left ventricular thrombus formation after mitral valve edge-to-edge repair. Sci Rep. (2022) 12(1):9096. 10.1038/s41598-022-12944-5
7.
Bakalli A Georgievska-Ismail L Koçinaj D Musliu N Zahiti B Krasniqi A et al Left ventricular and left atrial thrombi in sinus rhythm patients with dilated ischemic cardiomyopathy. Med Arch. (2012) 66(3):155–8. 10.5455/medarh.2012.66.155-158
8.
Umeda H Isotani A Yano M Tomiyama H Shirai S Ando K . Thrombus formation at the left ventricular apex due to split inflow after MitraClip implantation. JACC Cardiovasc Interv. (2019) 12(23):e205–6. 10.1016/j.jcin.2019.07.030
9.
Rosseel L De Backer O Søndergaard L . Clinical valve thrombosis and subclinical leaflet thrombosis in transcatheter aortic heart valves: clinical manifestations, diagnosis, and treatment. Precis Clin Med. (2018) 1(3):111–7. 10.1093/pcmedi/pby016
10.
Fleddermann AM Hayes CH Magalski A Main ML . Efficacy of direct acting oral anticoagulants in treatment of left ventricular thrombus. Am J Cardiol. (2019) 124(3):367–72. 10.1016/j.amjcard.2019.05.009
11.
Iqbal H Straw S Craven TP Stirling K Wheatcroft SB Witte KK . Direct oral anticoagulants compared to vitamin K antagonist for the management of left ventricular thrombus. ESC Heart Fail. (2020) 7(5):2032–41. 10.1002/ehf2.12718
12.
Robinson AA Trankle CR Eubanks G Schumann C Thompson P Wallace RL et al Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol. (2020) 5(6):685–92. 10.1001/jamacardio.2020.0652
Summary
Keywords
transcatheter edge-to-edge repair (TEER), right ventricular apical thrombosis, anticoagulant treatment, severe mitral regurgitation, heart failure
Citation
Cai S, Zhang J, Gou Z and Zhang H (2025) Right ventricular apical thrombus formation after transcatheter edge-to-edge mitral valve repair: a case report. Front. Cardiovasc. Med. 12:1640491. doi: 10.3389/fcvm.2025.1640491
Received
03 June 2025
Revised
07 October 2025
Accepted
24 November 2025
Published
05 December 2025
Volume
12 - 2025
Edited by
Peter Martin Wenaweser, Heart Clinic Zurich, Switzerland
Reviewed by
Alon Shechter, Rabin Medical Center, Israel
Emmanouil Chourdakis, MediClin Heart Center Lahr, Germany
Updates
Copyright
© 2025 Cai, Zhang, Gou and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Haifeng Zhang haifengzhang1395@njmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.