Abstract
Background:
Approximately 25%–30% of patients experience aortic regurgitation (AR) within the first year after left ventricular assist device (LVAD) implantation. However, there is currently no consensus in clinical guidelines regarding the optimal treatment approach for LVAD-associated AR.
Case summary:
We report a case of a female patient who developed AR following LVAD implantation. This patient exhibited an open-like configuration of left ventricular outflow tract (LVOT), with no calcified stenosis in the supravalvular region and a lack of anchoring anatomical structures at the junction of the LVOT and sinus-tubular junction. This anatomical configuration posed a high risk of prosthetic valve displacement during conventional transcatheter aortic valve replacement (TAVR). Therefore, we employed a novel TAVR system (Taurus Trio) equipped with a locator, which effectively prevented downward migration of the prosthetic valve after implantation.
Discussion:
This case indicates the potential advantages and efficacy of the Taurus Trio valve in TAVR for AR Post-LVAD. We plan to conduct long-term follow-up to further explore and optimize the treatment protocol.
Introduction
The Left Ventricular Assist Device (LVAD) functions as an alternative treatment for patients with end-stage heart failure awaiting cardiac transplantation (1). By leveraging a catheter to pump blood from the left ventricle into the aorta, the LVAD effectively reduces the workload of the left ventricle and ensures sustained systemic blood perfusion. As a continuous-flow device, the LVAD generates a persistent transvalvular pressure gradient that exerts a retrograde effect on the aortic valve, keeping it in a persistently closed or partially closed state and leading to leaflet fusion, retraction, and degeneration. In addition, the high-velocity jet from the outflow cannula may impose shear stress and mechanical trauma on the valve leaflets. Together, these factors ultimately contribute to the development and progression of aortic regurgitation (AR) (2). Data from previous studies suggest that the incidence of AR within the first year post-LVAD ranges from 25% to 30% (3). Severe AR can disrupt effective LVAD output by creating a closed-loop circulation, exacerbating heart failure and significantly increasing mortality risk.
Case presentation
Medical history
Our hospital admitted a 53-year-old female patient who presented with a chief complaint of “intermittent chest discomfort for 4 years, with exacerbation over the past week”. In 2020, the patient experienced chest discomfort after physical activity, accompanied by shortness of breath and palpitations. She was subsequently diagnosed with ischemic cardiomyopathy and heart failure at a local hospital. Despite receiving guideline-directed medical therapy (GDMT), her condition persisted with recurrent symptoms and progressive worsening. Consequently, on May 8, 2021, she was initiated on continuous renal replacement therapy, utilizing continuous venous-venous hemofiltration. On May 25, 2022, the patient underwent implantation of a LVAD (HeartCon model) in conjunction with coronary artery bypass grafting (CABG). Postoperatively, her symptoms improved significantly, allowing for discharge. However, in June 2024, the patient presented again with chest discomfort and was admitted to our hospital for further evaluation. At admission, the parameters of LVAD were as follows: pump speed 2,300 rpm, power 5.10 W, and flow rate 7.85 L/min. Laboratory tests revealed an elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) level of 4,864 pg/ml. Echocardiography indicated moderate to severe regurgitation of the aortic valve (Figure 1).
Figure 1
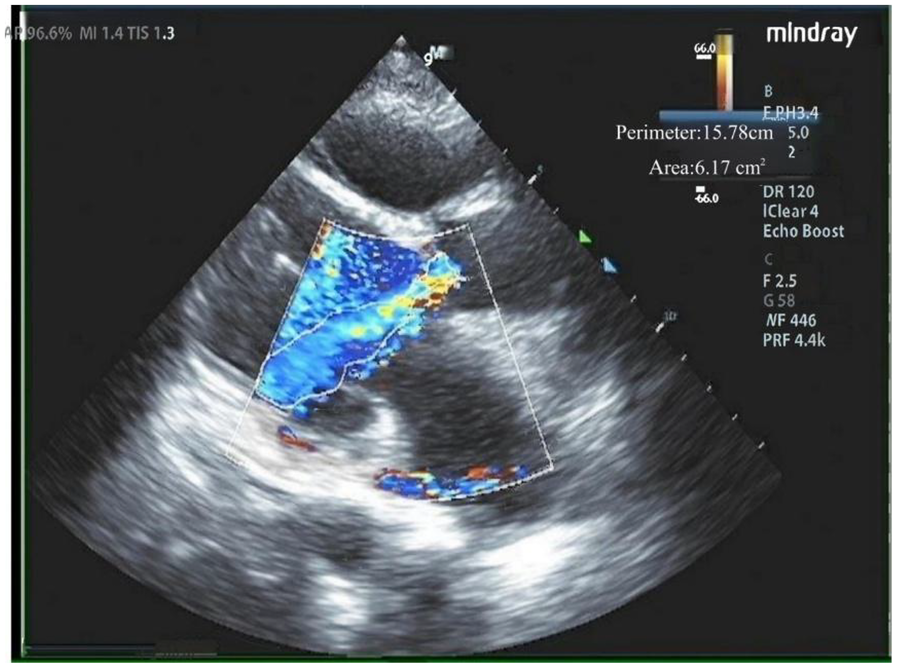
Moderate to severe regurgitation of the aortic valve.
Determination of treatment protocol
Two years after the implantation of LVAD, the patient exhibited moderate to severe aortic valve insufficiency, as indicated by echocardiography. Additionally, NT-proBNP levels were significantly elevated, suggesting an exacerbation of heart failure symptoms. Despite adjustments to the LVAD flow rate, the therapeutic effect remained suboptimal. This was likely attributed to the AR, which caused blood to flow back into the left ventricle from the ascending aorta due to a persistent pressure gradient. This resulted in a closed-loop circulation that compromised LVAD output and adversely affected its overall functionality. Consequently, addressing the AR became imperative. However, given the patient's history of LVAD implantation and CABG, coupled with severe heart failure, the EuroScore II indicated a high surgical mortality risk of 11.2%. Therefore, the risks associated with performing a conventional surgical aortic valve replacement were considered extremely high. Furthermore, although a dedicated transapical device for AR (such as the J-valve) has been developed and is available in China, for patients with an LVAD implanted at the apex, the apical route is anatomically infeasible due to the presence of the LVAD device. This limitation further supported the decision to select the transfemoral approach in this case. Taking into account this comprehensive evaluation, a transcatheter aortic valve replacement (TAVR) via the femoral artery was proposed as a safer and more viable option.
Preoperative CT evaluation
The patient had a regurgitant tri-leaflet aortic valve with a normally shaped, elliptical-like annulus. The valve leaflets showed no thickening, calcification, or commissural fusion. The annular circumference measured 62.3 mm, with a circumference-derived diameter of 19.8 mm. The diameter of the left ventricular outflow tract (LVOT) exceeded that of the annulus during diastole, giving it an overall funnel-like appearance. The supra-annular region was nearly cylindrical, posing significant challenges for anchoring and elevating the risk of valve migration. Specific measurement data can be found in Figure 2. According to current guidelines, in patients with AR, larger self-expanding valves are generally preferred compared with those used for aortic stenosis, in order to achieve stronger radial force and anchoring. The guidelines recommend an oversizing ratio of prosthetic valve diameter relative to the annular diameter of 15%–30%. The valve selection in this case followed these principles. Based on the preoperative CT evaluation and the absence of significant calcification of the native aortic valve, a Taurus Trio 25 valve (size: 28 mm) was ultimately implanted via the right femoral artery approach to achieve optimal radial support and anchoring stability. The Taurus Trio TAVR system is designed on the basis of the JenaValve Trilogy transcatheter heart valve technology (4). Its unique locator elements enable secure anchoring even in the absence of calcification, effectively preventing valve migration toward the LVOT while ensuring precise coaptation with the native leaflets. This design promotes long-term hemodynamic stability and preserves the possibility of future percutaneous coronary interventions (PCI).
Figure 2
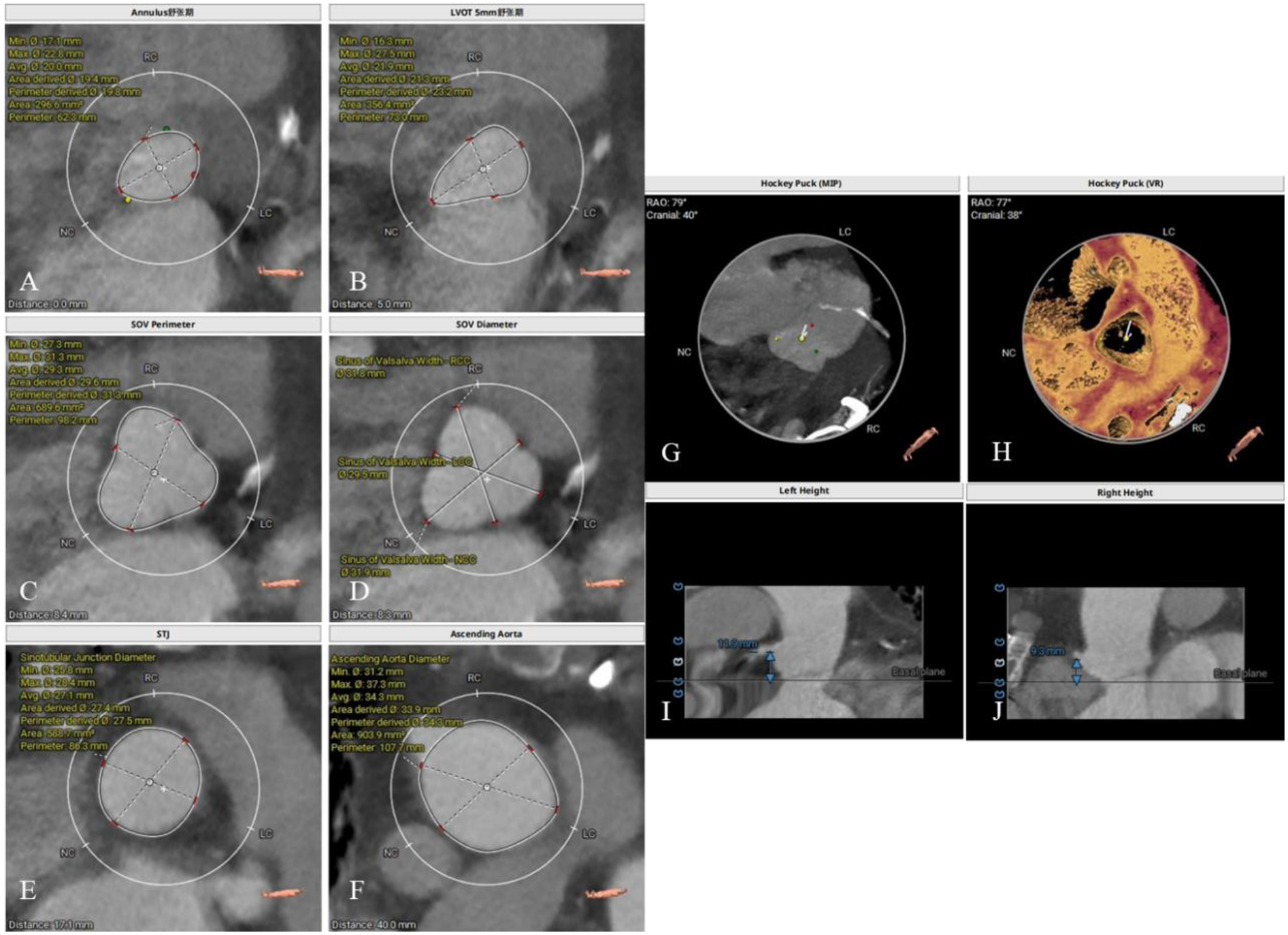
Preoperative CT evaluation. (A) Annular circumference diameter: 19.8 mm; (B) outflow tract circumference diameter: 23.2 mm; (C) sinus of valsalva circumference diameter: 31.3 mm; (D) sinus of valsalva diameter: 31.8 mm, 29.5 mm, 31.9 mm; (E) sinutubular junction circumference diameter: 27.5 mm; (F) aortic circumference diameter 4 cm above the annulus: 34.3 mm; (G,H) no significant calcification observed in the annulus and leaflets; (I) height of the left coronary artery ostium: 11.9 mm; (J) height of the right coronary artery ostium: 9.3 mm.
Surgical procedure
The procedure was successfully performed under general anesthesia in the hybrid operating room via the right femoral artery (Figure 3). LVAD pump speed was maintained at 2,250 rpm, consistent with the preoperative setting. A temporary pacing lead was placed through the left femoral vein, and the left femoral artery was used as secondary access. Under fluoroscopic and echocardiographic guidance, a Taurus Trio-THV 25 valve (28 mm) was implanted via the right femoral artery, with all three locators stably anchored at the sinus base. No rapid pacing was required, and the continuous-flow LVAD did not interfere with valve positioning or deployment. Post-deployment aortography demonstrated only trivial paravalvular leak with patent coronary arteries. Intraoperative transesophageal echocardiography confirmed optimal valve position and morphology, with no paravalvular leak and immediate resolution of aortic regurgitation (Figure 4). Given the patient's long-term warfarin therapy (INR 2–3) due to prior LVAD implantation, the main access site was closed with two ProGlide devices, achieving effective hemostasis, and ultrasound confirmed the absence of bleeding, dissection, or stenosis.
Figure 3
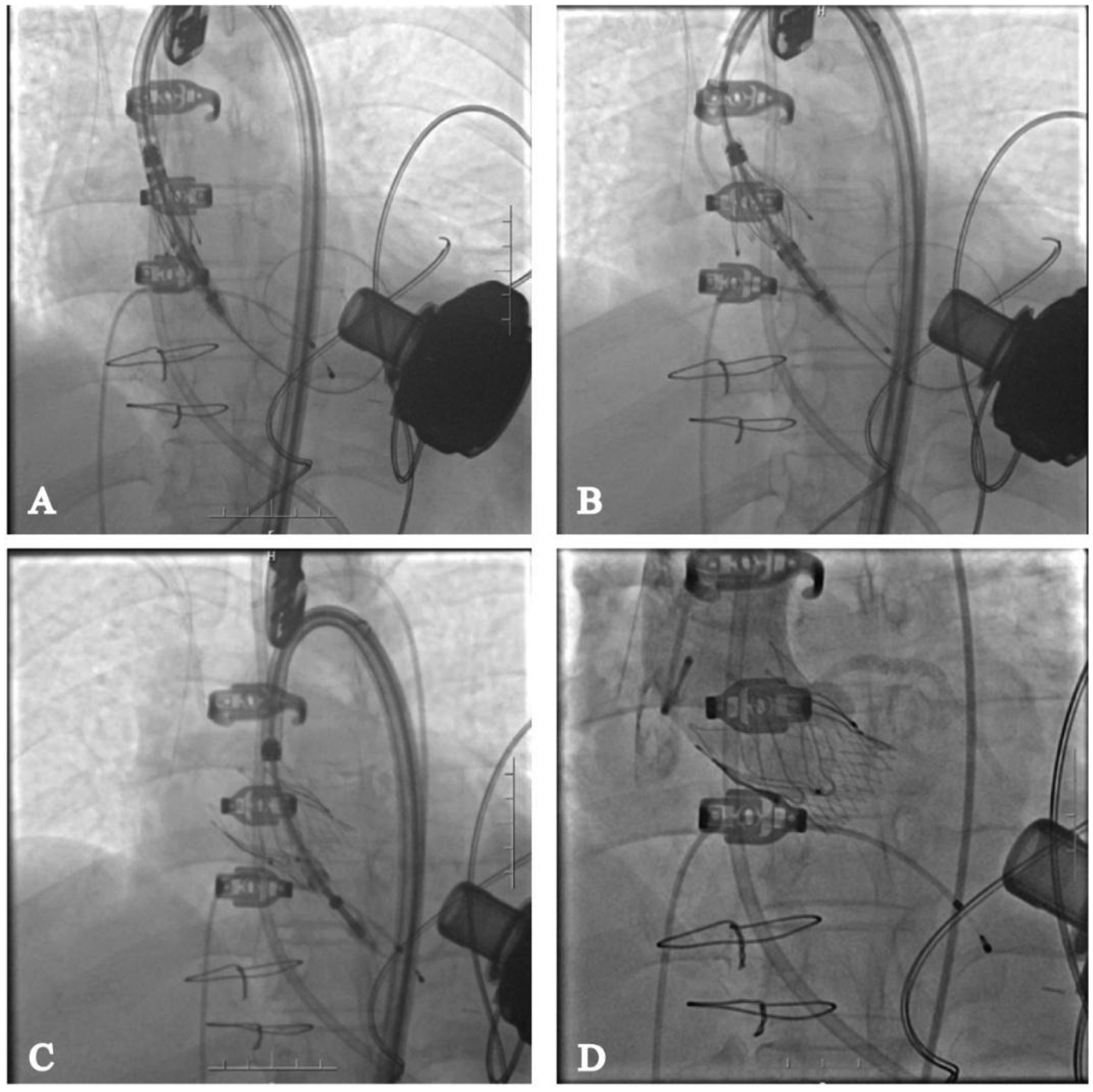
Transcatheter aortic valve replacement via femoral artery. (A) Deliver the valve to the annulus location; (B) deploy the proprietary locator into the sinus of valsalva; (C) release the valve; (D) angiography shows no regurgitation of the aortic valve and no impact on coronary perfusion.
Figure 4
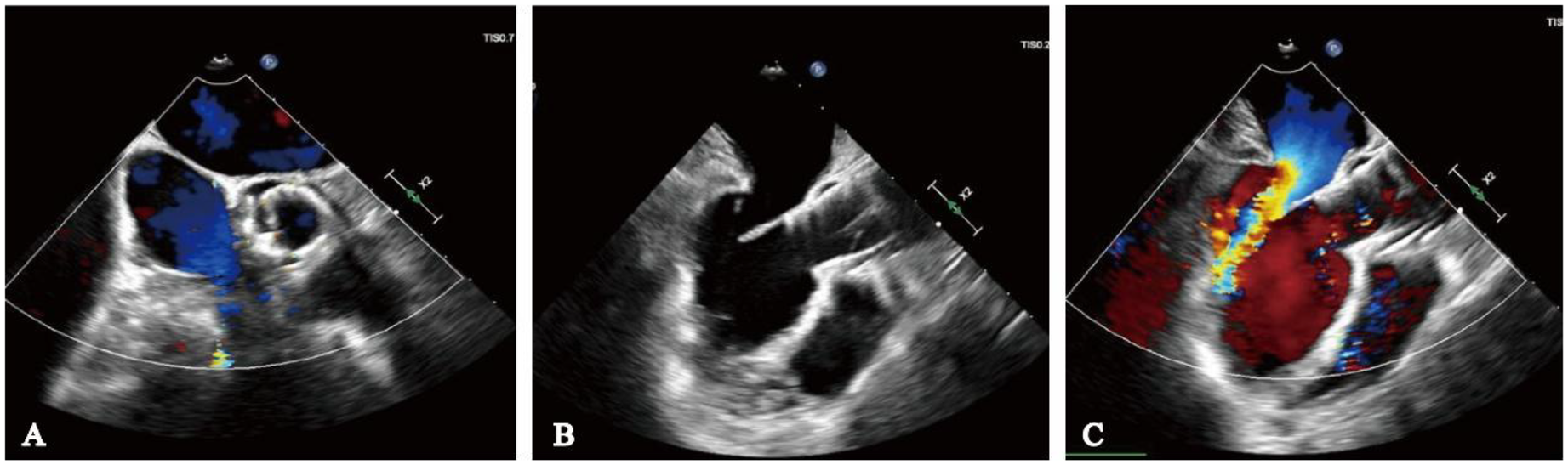
Intraoperative transesophageal echocardiography monitoring. (A) No paravalvular leak on short-axis view; (B) long-axis view indicates adequate valve depth, no impairment of anterior mitral leaflet function; (C) ultrasound color Doppler shows no aortic regurgitation.
Follow-up
At the 1-month follow-up, the patient's symptoms had markedly improved (NYHA functional class II). Transthoracic echocardiography demonstrated normal morphology and function of the prosthetic valve, with no evidence of late prosthesis migration, and spectral Doppler imaging revealed no regurgitation (Figure 5). LVAD parameters remained stable throughout the follow-up period (pump speed 2,200–2,300 rpm, flow approximately 2.5 L/min).
Figure 5
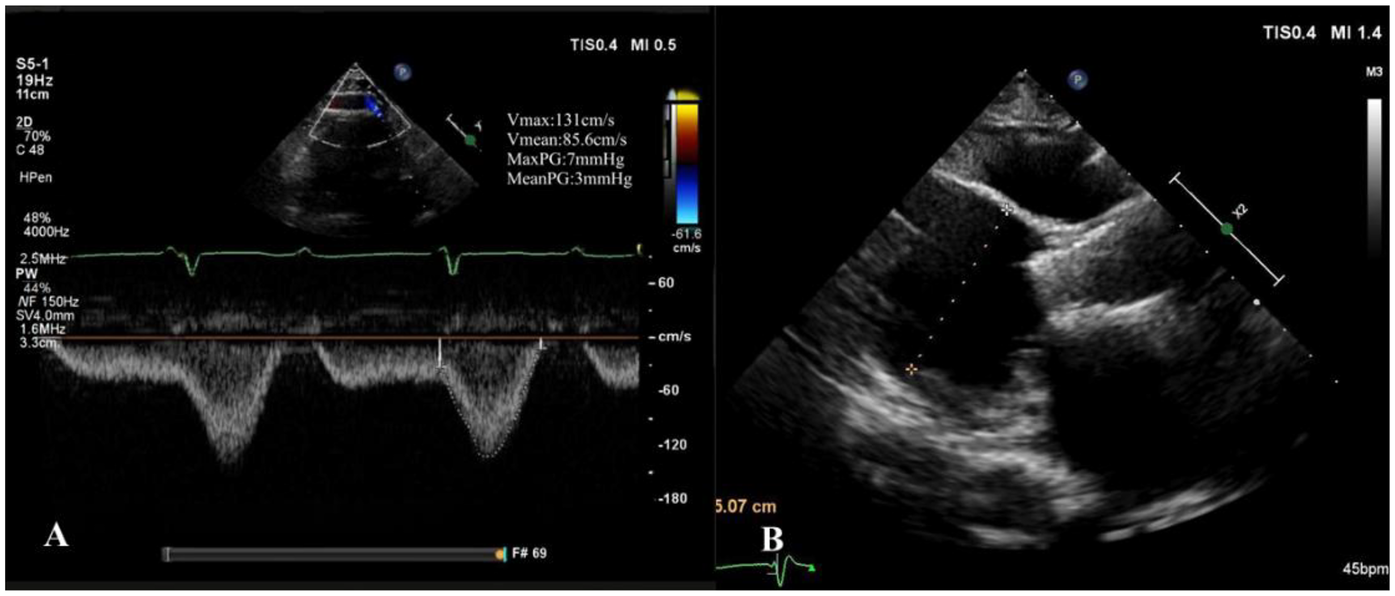
One-month postoperative echocardiographic follow-up. (A) Spectral Doppler showing no evidence of aortic regurgitation; (B) long-axis view demonstrating no late prosthetic valve migration.
Discussion
Heart failure represents the common final pathway of various cardiac diseases and remains a growing global health burden despite advances in primary prevention strategies (5). For patients with end-stage disease refractory to guideline-directed medical therapy (GDMT), cardiac transplantation is the gold standard; however, limited donor availability allows fewer than 10% of eligible patients to undergo transplantation (6). Against this background, left ventricular assist devices (LVADs) have emerged as a crucial alternative, either as a bridge to transplant or as destination therapy (7). With the advent of third-generation continuous-flow LVADs, mid-term survival has approached that of heart transplantation, highlighting their increasing clinical potential (8).
Nevertheless, the hemodynamic characteristics of continuous-flow LVADs predispose to unique complications. Because blood is continuously diverted from the left ventricle into the ascending aorta via the outflow graft, the aortic valve remains closed or minimally opens. This altered physiology impairs valve function and promotes the development of aortic regurgitation (AR) (9). Mechanistically, persistent transvalvular pressure gradients, increased retrograde flow, and LV unloading create a “closed loop” circulation with blood repeatedly regurgitating into the left ventricle (10). This phenomenon attenuates the unloading effect of LVAD support, precipitates recurrent heart failure, and has been associated with increased morbidity and mortality (11).
Accordingly, post-LVAD AR represents a critical challenge to long-term LVAD therapy, yet therapeutic strategies remain limited. The initial management of symptomatic AR typically consists of medical therapy, including diuretics, vasodilators, and LVAD speed reduction (12). However, in cases refractory to medical treatment, surgical aortic valve replacement or heart transplantation remains the primary therapeutic option. In LVAD recipients, repeat sternotomy is often associated with high procedural risk, and not all patients are suitable candidates for transplantation (13, 14). The use of the Amplatzer septal occluder (Abbott) to close the aortic valve has been investigated, but this approach is limited by high rates of AR recurrence (15, 16). Moreover, complete aortic valve closure renders patients fully dependent on LVAD support for systemic perfusion, and any device dysfunction can rapidly become fatal (15, 16).
Transcatheter aortic valve replacement (TAVR), initially developed for severe aortic stenosis, has been increasingly applied to AR, including in LVAD recipients (17). However, several unique challenges arise in the post-LVAD setting. Anatomical considerations include annular dilatation, enlarged left ventricular outflow tract (LVOT), and the absence of calcific anchoring, which compromise secure valve positioning. Registry data of off-label TAVR with conventional devices such as CoreValve and Sapien have demonstrated higher rates of valve embolization, paravalvular leak, and frequent need for a second valve compared with procedures for aortic stenosis (18, 19). Hemodynamic considerations are equally significant: continuous forward flow and increased retrograde flow caused by the LVAD create a complex environment that hinders device stabilization and may necessitate intraoperative adjustments such as pump speed modulation or brief pump pauses to optimize deployment (20, 21).
In recent years, valve systems specifically designed for AR have been developed. The first-generation transapical JenaValve achieved procedural success rates of approximately 97%–100% in non-calcified AR, but valve migration and reintervention remained concerns (22–24). The next-generation transfemoral JenaValve Trilogy, employing a leaflet-locating mechanism for anchoring independent of calcification, demonstrated a 95% technical success rate in the ALIGN-AR trial, with a 30-day composite safety endpoint of 27% and a 1-year all-cause mortality of 7.8%, supporting its favorable safety and efficacy in early follow-up (25).
In addition, China has been actively developing transcatheter valve systems specifically designed for AR. The TaurusTrio (licensed from the Trilogy system by Peijia Medical) has entered pivotal clinical trials at multiple centers and achieved its first successful implantation in 2023, demonstrating favorable early feasibility and potential stability. In the present case, we selected the TaurusTrio system with dedicated locators for TAVR. Its unique design enabled stable positioning and deployment even in the absence of annular calcification and under continuous LVAD flow. Postoperative imaging follow-up confirmed satisfactory valve function without migration or regurgitation, and the patient experienced marked symptomatic improvement, with NYHA functional class improving to II at one month. This case suggests that dedicated AR valves may represent an optimal therapeutic option for patients with LVAD-associated AR and could provide important insights for future clinical practice and guideline development.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Seventh People's Hospital of Zhengzhou. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SC: Project administration, Writing – original draft, Visualization, Conceptualization, Methodology, Investigation. BY: Investigation, Writing – original draft, Visualization, Conceptualization, Project administration, Methodology. XW: Writing – original draft, Investigation, Visualization. PL: Methodology, Writing – original draft. G-YS: Funding acquisition, Validation, Writing – review & editing, Methodology, Visualization, Conceptualization. SZ: Methodology, Conceptualization, Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Henan Province medical science and technology research project (No. LHGJ20220835) and Henan Provincial Key Laboratory of Cardiac Remodeling and Transplantation.
Acknowledgments
We thank the patient and staff who supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Han JJ Acker MA Atluri P . Left ventricular assist devices. Circulation. (2018) 138(24):2841–51. 10.1161/CIRCULATIONAHA.118.035566
2.
Pak SW Uriel N Takayama H Cappleman S Song R Colombo PC et al Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant. (2010) 29(10):1172–6. 10.1016/j.healun.2010.05.018
3.
Bouabdallaoui N El-Hamamsy I Pham M Giraldeau G Parent MC Carrier M et al Aortic regurgitation in patients with a left ventricular assist device: a contemporary review. J Heart Lung Transplant. (2018) 37(11):1289–97. 10.1016/j.healun.2018.07.002
4.
Ranard LS Kaple R Khalique OK Agarwal V Bellumkonda L Bonde P et al First transfemoral implantation of a novel transcatheter valve in an LVAD patient with aortic insufficiency. JACC Case Rep. (2021) 3(17):1806–10. 10.1016/j.jaccas.2021.08.026
5.
Savarese G Becher PM Lund LH Seferovic P Rosano GMC Coats AJS . Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. 10.1093/cvr/cvac013
6.
Colvin M Smith JM Ahn Y Skeans MA Messick E Bradbrook K et al OPTN/SRTR 2020 annual data report: heart. Am J Transplant. (2022) 22(S2):350–437. 10.1111/ajt.16977
7.
Boulet J Wanderley MRB Jr Mehra MR . Contemporary left ventricular assist device therapy as a bridge or alternative to transplantation. Transplantation. (2024) 108(6):1333–41. 10.1097/TP.0000000000004834
8.
Kirschner M Topkara VK Sun J Kurlansky P Kaku Y Naka Y et al Comparing 3-year survival and readmissions between HeartMate 3 and heart transplant as primary treatment for advanced heart failure. J Thorac Cardiovasc Surg. (2025) 169(1):148–59.e143. 10.1016/j.jtcvs.2023.12.019
9.
Wang TS Hernandez AF Felker GM Milano CA Rogers JG Patel CB . Valvular heart disease in patients supported with left ventricular assist devices. Circ Heart Fail. (2014) 7(1):215–22. 10.1161/CIRCHEARTFAILURE.113.000473
10.
Dimarakis I Callan P Khorsandi M Pal JD Bravo CA Mahr C et al Pathophysiology and management of valvular disease in patients with destination left ventricular assist devices. Front Cardiovasc Med. (2022) 9:1029825. 10.3389/fcvm.2022.1029825
11.
Truby LK Garan AR Givens RC Wayda B Takeda K Yuzefpolskaya M et al Aortic insufficiency during contemporary left ventricular assist device support: analysis of the INTERMACS registry. JACC Heart Fail. (2018) 6(11):951–60. 10.1016/j.jchf.2018.07.012
12.
Cowger J Rao V Massey T Sun B May-Newman K Jorde U et al Comprehensive review and suggested strategies for the detection and management of aortic insufficiency in patients with a continuous-flow left ventricular assist device. J Heart Lung Transplant. (2015) 34(2):149–57. 10.1016/j.healun.2014.09.045
13.
Atkins BZ Hashmi ZA Ganapathi AM Harrison JK Hughes GC Rogers JG et al Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J Thorac Cardiovasc Surg. (2013) 146(5):1247–52. 10.1016/j.jtcvs.2013.05.019
14.
Gyoten T Morshuis M Fox H Deutsch MA Hakim-Meibodi K Schramm R et al Secondary aortic valve replacement in continuous flow left ventricular assist device therapy. Artif Organs. (2021) 45(7):736–41. 10.1111/aor.13906
15.
Phan K Haswell JM Xu J Assem Y Mick SL Kapadia SR et al Percutaneous transcatheter interventions for aortic insufficiency in continuous-flow left ventricular assist device patients: a systematic review and meta-analysis. Asaio j. (2017) 63(2):117–22. 10.1097/MAT.0000000000000447
16.
Retzer EM Sayer GT Fedson SE Nathan S Jeevanandam V Friant J et al Predictors of survival following trans-catheter aortic valve closure for left ventricular assist device associated aortic insufficiency. Catheter Cardiovasc Interv. (2016) 87(5):971–9. 10.1002/ccd.26280
17.
Adam M Tamm AR Wienemann H Unbehaun A Klein C Arnold M et al Transcatheter aortic valve replacement for isolated aortic regurgitation using a new self-expanding TAVR system. JACC Cardiovasc Interv. (2023) 16(16):1965–73. 10.1016/j.jcin.2023.07.038
18.
Liu R Fu Z Jiang Z Yan Y Yao J Liu X et al Transcatheter aortic valve replacement for aortic regurgitation: a systematic review and meta-analysis. ESC Heart Fail. (2024) 11(6):3488–500. 10.1002/ehf2.14832
19.
Frerker C Schewel J Schewel D Wohlmuth P Schmidt T Kreidel F et al Expansion of the indication of transcatheter aortic valve implantation–feasibility and outcome in “off-label” patients compared with “on-label” patients. J Invasive Cardiol. (2015) 27(5):229–36. PMID
20.
Bashir H Mendez-Hirata G Schmidt CW Wong A Muuse J Egnaczyk GF et al Transcatheter aortic valve replacement for aortic regurgitation in patients with left ventricular assist devices: an institutional experience. J Soc Cardiovasc Angiogr Interv. (2025) 4(7):103662. 10.1016/j.jscai.2025.103662
21.
Yehya A Rajagopal V Meduri C Kauten J Brown M Dean L et al Short-term results with transcatheter aortic valve replacement for treatment of left ventricular assist device patients with symptomatic aortic insufficiency. J Heart Lung Transplant. (2019) 38(9):920–6. 10.1016/j.healun.2019.03.001
22.
Seiffert M Bader R Kappert U Rastan A Krapf S Bleiziffer S et al Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv. (2014) 7(10):1168–74. 10.1016/j.jcin.2014.05.014
23.
Silaschi M Conradi L Wendler O Schlingloff F Kappert U Rastan AJ et al The JUPITER registry: one-year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv. (2018) 91(7):1345–51. 10.1002/ccd.27370
24.
Seiffert M Diemert P Koschyk D Schirmer J Conradi L Schnabel R et al Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc Interv. (2013) 6(6):590–7. 10.1016/j.jcin.2013.01.138
25.
Vahl TP Thourani VH Makkar RR Hamid N Khalique OK Daniels D et al Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR): a prospective, multicentre, single-arm study. Lancet. (2024) 403(10435):1451–9. 10.1016/S0140-6736(23)02806-4
Summary
Keywords
left ventricular assist device, aortic regurgitation, transcatheter aortic valve replacement, Taurus Trio valve, femoral artery access
Citation
Cheng S, Yang B, Wang X, Lian P, Song G and Zhang S (2025) Transcatheter aortic valve replacement via femoral artery for aortic regurgitation post-LVAD: a case report. Front. Cardiovasc. Med. 12:1643334. doi: 10.3389/fcvm.2025.1643334
Received
08 June 2025
Accepted
23 October 2025
Published
13 November 2025
Volume
12 - 2025
Edited by
Badal Thakkar Sinai Hospital of Baltimore, United States
Reviewed by
Hendrik Ruge, Technical University Munich, Germany
Damian Hudziak, Medical University of Silesia, Poland
Updates
Copyright
© 2025 Cheng, Yang, Wang, Lian, Song and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Guangyuan Song songgy_anzhen@vip.163.com Shenwei Zhang zhangshenwei321@163.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.