- 1Department of Cardiology, The First Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
- 2Hebei Key Laboratory of Heart and Metabolism, Shijiazhuang, Hebei, China
Background: Coronary atherosclerotic heart disease (CAD) remains a major global health burden and a leading cause of mortality. Its pathogenesis is closely linked to multiple risk factors, among which inflammation plays a central role. While inflammatory biomarkers such as platelet and monocyte counts have been incorporated into prognostic assessments, their predictive accuracy remains limited. Further investigation of novel inflammatory indices is needed to refine risk stratification and guide clinical management.
Objective: This study aimed to evaluate the prognostic value of the mean platelet volume-to-monocyte count ratio (MMR) for predicting major adverse cardiovascular events (MACE) in patients with newly diagnosed CAD.
Methods: A total of 652 treatment-naïve CAD patients were enrolled. Kaplan–Meier survival analysis and univariate Cox proportional hazards models were applied to assess the association between MMR levels and MACE. Subgroup analyses were performed to test for effect modification. Restricted cubic spline (RCS) models were used to explore the dose–response relationship. The incremental predictive value of MMR beyond conventional risk factors was examined using changes in the concordance index (C-index), net reclassification improvement (NRI), and integrated discrimination improvement (IDI).
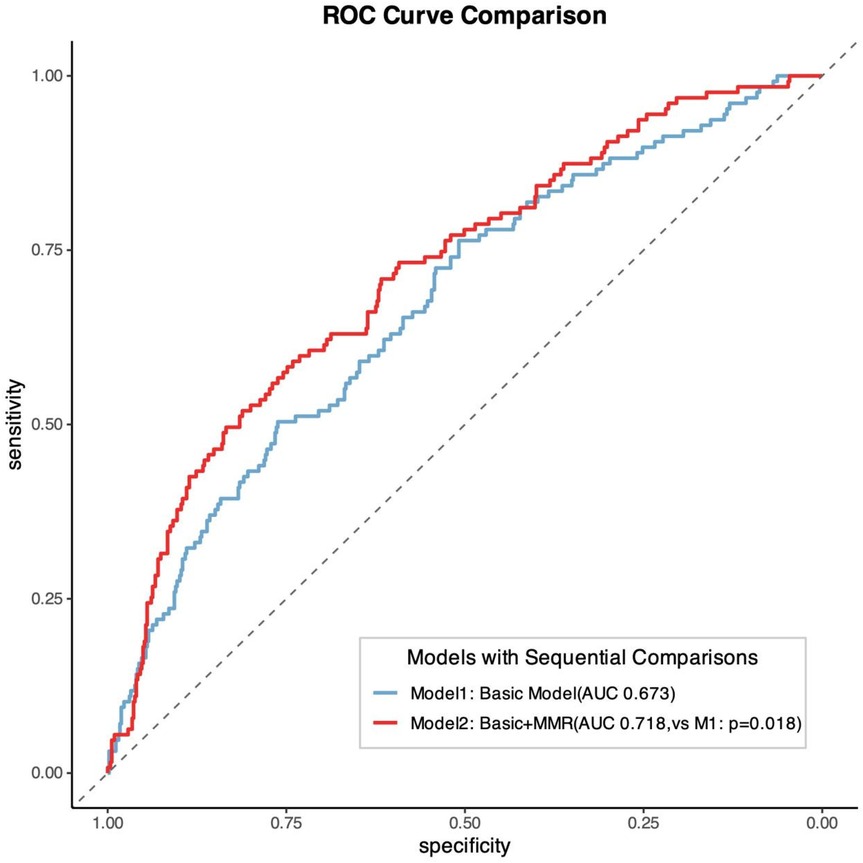
Results: Patients were stratified into quintiles based on MMR values (L1: 7.89–14.43; L2: 14.50–17.96; L3: 18.00–22.16; L4: 22.25–28.53; L5: 28.67–60.67). Kaplan–Meier analysis revealed significantly poorer outcomes in the L3 group compared with other quintiles (log-rank P = 0.0014). RCS analysis demonstrated a significant nonlinear association between MMR levels and MACE risk (P = 0.001), characterized by an inverted U-shaped relationship. Incorporating MMR into conventional risk models significantly improved predictive performance (AUC 0.718 vs. 0.673; P = 0.018).
Conclusion: In newly diagnosed CAD patients, MMR shows a nonlinear, inverted U-shaped association with MACE risk. The addition of MMR to standard risk models enhances prognostic accuracy. Further multicenter prospective studies and mechanistic trials are needed to verify the prognostic value of MMR and to elucidate its mechanism of action.
1 Introduction
The prevalence of coronary artery disease (CAD) has increased significantly. This situation now poses a serious public health threat, endangering population health and having a major global impact (1). CAD pathogenesis involves multifactorial processes (2). Atherosclerosis serves as its primary pathological basis, with complex mechanisms driving progression (3, 4). Inflammatory responses are pivotal in coronary atherosclerosis, where platelet activation and monocyte recruitment/differentiation crucially modulate plaque formation and evolution (5, 6). Upon endothelial injury, platelets adhere to exposed subendothelial matrices and release inflammatory mediators/chemokines, facilitating monocyte adhesion (7, 8). Chemotactic gradients then drive monocytes to infiltrate the intima, polarize into M1 macrophages, phagocytose oxidized lipids, and transform into foam cells—accelerating plaque progression (9). Conversely, when platelet activity is low, macrophages polarize toward the M2 phenotype. M2 macrophages suppress fibrous cap degradation, enhance plaque stability, reduce rupture risk, and prevent thrombosis (10, 11). This evidence indicates a dynamic balance between platelet activity and monocyte function in modulating plaque pathogenesis (12).
Atherosclerosis is recognized as an inflammatory disease, with immune dysregulation playing a central role. Inflammation permeates all stages of atherosclerosis, spurring interest in inflammatory biomarkers (13). Indices like systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) correlate with CAD (14). Although existing biomarkers show prognostic utility (15), their clinical application remains suboptimal due to susceptibility to confounding variables, leading to inconsistent findings (16, 17). Consequently, novel inflammatory indices are needed to enhance prognostic accuracy, alleviate patient burden, and optimize clinical decision-making (18).
The mean platelet volume-to-monocyte count ratio (MMR), an inflammatory index previously linked to chronic obstructive pulmonary disease (COPD) phenotyping (19), has not been investigated in CAD. This study aimed to evaluate MMR's prognostic value in treatment-naïve CAD patients. We hypothesized that integrating MMR would augment traditional models' predictive capacity for CAD outcomes.
2 Methods
2.1 Study design and population
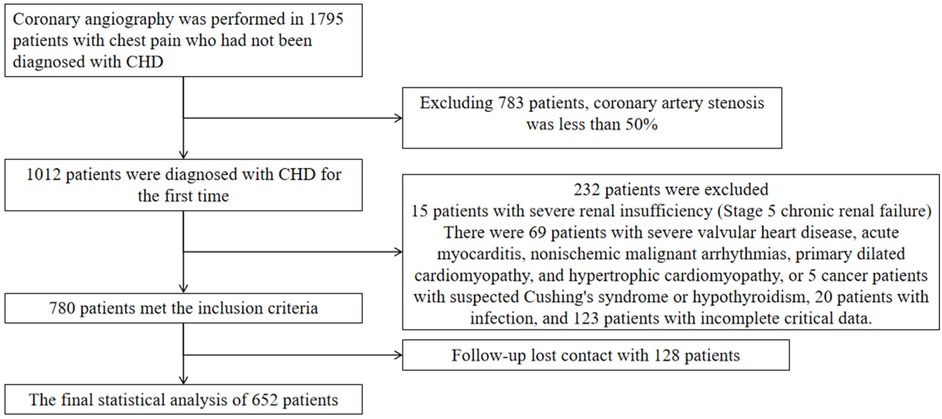
This study enrolled patients who underwent coronary angiography at the First Hospital of Hebei Medical University between August 1, 2018, and March 30, 2020. Eligible participants were newly diagnosed with coronary artery disease (CAD) and had not received prior treatment. All participants provided informed consent for the anonymous use of their clinical data. Exclusion criteria were: angiographic stenosis <50%, confirmed infectious disease, stage 5 chronic kidney disease, heart failure, non-ischemic cardiac conditions (e.g., severe valvular disease, acute myocarditis, malignant arrhythmias of non-ischemic origin, primary dilated cardiomyopathy, hypertrophic cardiomyopathy), suspected malignancy, Conn's syndrome, Cushing's syndrome, hypothyroidism, and incomplete clinical data. These criteria were applied to ensure a homogeneous cohort and to minimize confounding factors affecting inflammatory markers or cardiovascular outcomes. This study was approved by the Ethics Committee of the First Hospital of Hebei Medical University (ethical approval number: 20220362), and conducted in line with the Declaration of Helsinki.
2.2 Follow-up and endpoints
Patients were followed up through outpatient visits or telephone interviews at 1 month, 3 months, 6 months, 1 year, and annually thereafter for up to 5 years. Follow-up data were supplemented and verified using electronic health records. The primary endpoint was major adverse cardiovascular events (MACE), defined as all-cause mortality, nonfatal myocardial infarction, reperfusion therapy, stroke, and readmission for heart failure or severe angina. For the purposes of this study, all-cause mortality, nonfatal myocardial infarction, reperfusion, and stroke were considered MACE(hard endpoints). A total of 652 patients were included in the final analysis (Figure 1).
2.3 Baseline data collection
Baseline demographic and clinical data were collected at admission, including age, sex, body mass index (BMI), smoking status, alcohol consumption, and medical history (hypertension, diabetes, and cardiovascular disease). Laboratory tests were performed on fasting blood samples obtained within 24 h of admission, including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), mean platelet volume, and monocyte count.
2.4 Assessment of anatomical stenosis severity in coronary artery disease
All patients underwent coronary angiography (CAG), and the severity of coronary stenosis was quantified using the Gensini scoring system. This system assigns points based on the degree of luminal narrowing (<25% = 1 point; 25%–49% = 2; 50%–74% = 4; 75%–89% = 8; 90%–98% = 16; total occlusion = 32), which are then multiplied by vessel-specific weighting factors (e.g., left main ×5; proximal left anterior descending ×2.5; mid ×1.5; distal ×1; diagonal branches ×1/0.5; proximal left circumflex ×2.5; distal or posterior descending ×1; posterolateral ×0.5; right coronary segments ×1). The total Gensini score was calculated as the sum of all lesion-specific scores. Assessments were independently reviewed by two board-certified cardiologists, and discrepancies were resolved by consensus.
2.5 Statistical analysis
Statistical analyses were performed using SPSS version 27.0 (IBM Corp., Armonk, NY, USA) and R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria). Baseline characteristics were compared across quintiles of MMR. Categorical variables are presented as n (%), and continuous variables as mean ± standard deviation or median (interquartile range), depending on distribution. Normality was assessed using the Kolmogorov–Smirnov test, and variance homogeneity with Levene's test. Group differences were analyzed using the chi-square test for categorical variables, one-way ANOVA for normally distributed continuous variables, and the Kruskal–Wallis test for non-normally distributed variables.
Kaplan–Meier survival curves with log-rank tests were used to compare event-free survival across MMR groups. Cox proportional hazards models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for MACE, adjusting for clinically relevant confounders and variables significant in univariate analysis. Predictive performance was further assessed using receiver operating characteristic (ROC) curves. Subgroup analyses were conducted by sex, age, hypertension, and diabetes status, with interaction terms tested for effect modification. Restricted cubic spline (RCS) regression was used to evaluate potential nonlinear associations between MMR and MACE risk. A two-sided P value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
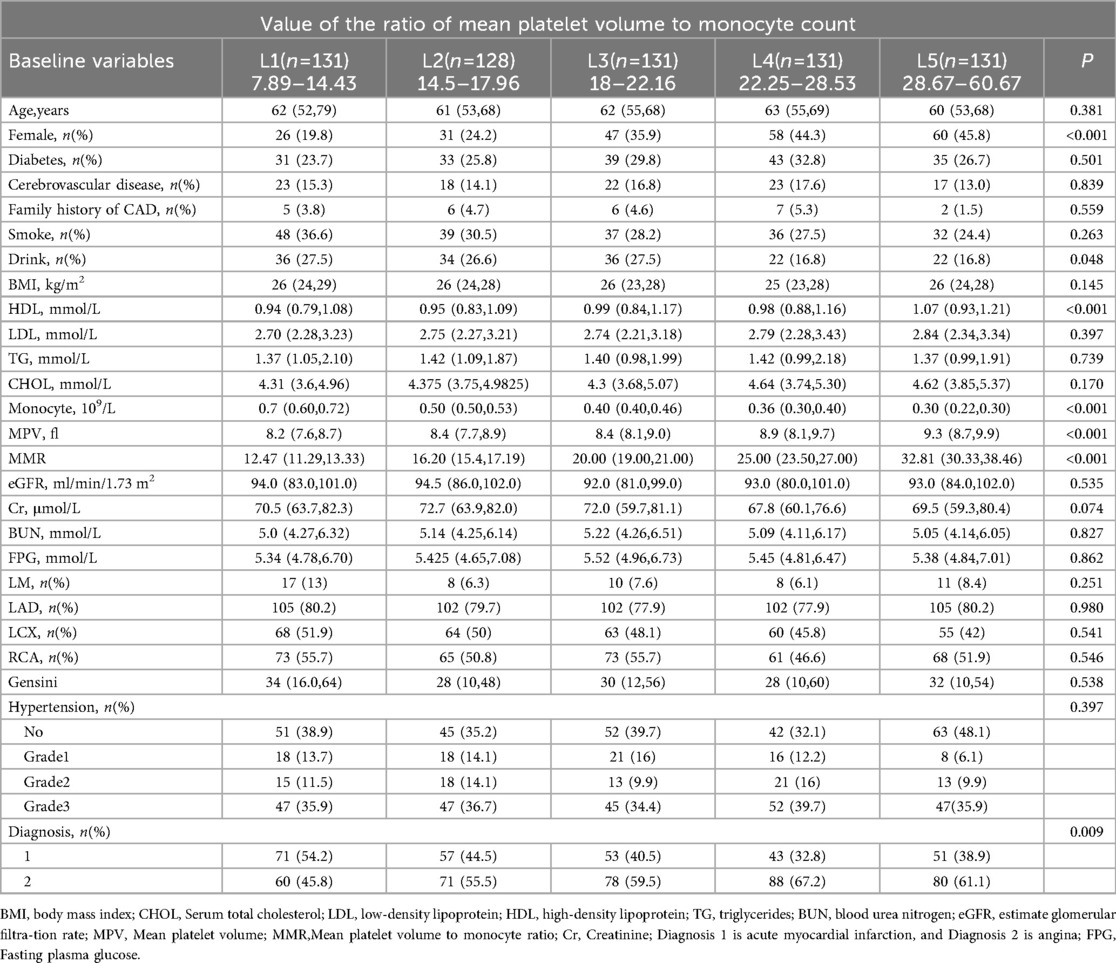
A total of 652 patients were enrolled, including 222 women (34.0%), with a mean age of 60.8 years. Among them, 377 (57.8%) presented with angina pectoris, 181 (27.3%) had diabetes mellitus, and 399 (60.0%) had hypertension. The mean MMR was 21.8. Patients were stratified into quintiles by MMR values: L1 (n = 131; 7.89–14.43), L2 (n = 128; 14.50–17.96), L3 (n = 131; 18.00–22.16), L4 (n = 131; 22.25–28.53), and L5 (n = 131; 28.67–60.67). Significant differences in several clinical variables were observed across quintiles, whereas age, creatinine, blood urea nitrogen, body mass index, estimated glomerular filtration rate, and cholesterol levels showed no significant variation (Table 1).
3.2 MMR levels and risk of experiencing MACE
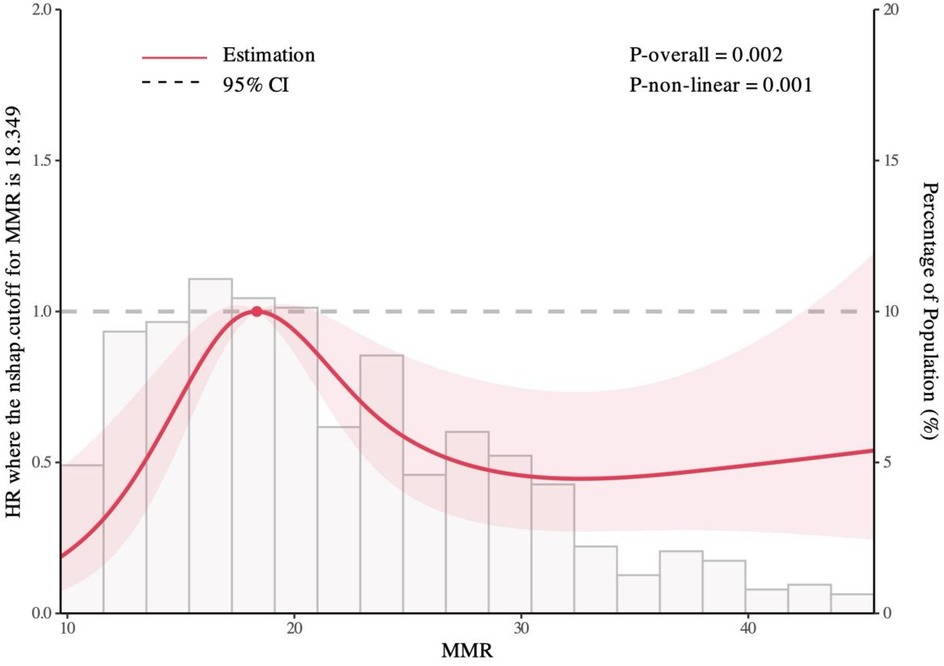
The median follow-up duration was 51 months (IQR: 44 – 46 months). During follow-up, 127 patients (19.5%) experienced MACE, including 10 cardiovascular deaths (1.5%), 3 nonfatal myocardial infarctions (0.4%), 26 revascularizations (3.9%), and 102 rehospitalizations for heart failure or severe angina (15.6%). Kaplan–Meier survival curves by MMR quintiles are shown in Figure 2. Log-rank testing indicated significant differences in survival across groups (P = 0.0014), with patients in the L3 group exhibiting the poorest prognosis. Restricted cubic spline (RCS) analysis further demonstrated a significant nonlinear association between MMR levels and MACE risk (P = 0.001), characterized by an inverted U-shaped curve (Figure 3). The inflection point was identified at MMR = 18.35: below this level, higher MMR was associated with increased risk, whereas above this threshold, higher MMR predicted more favorable outcomes.
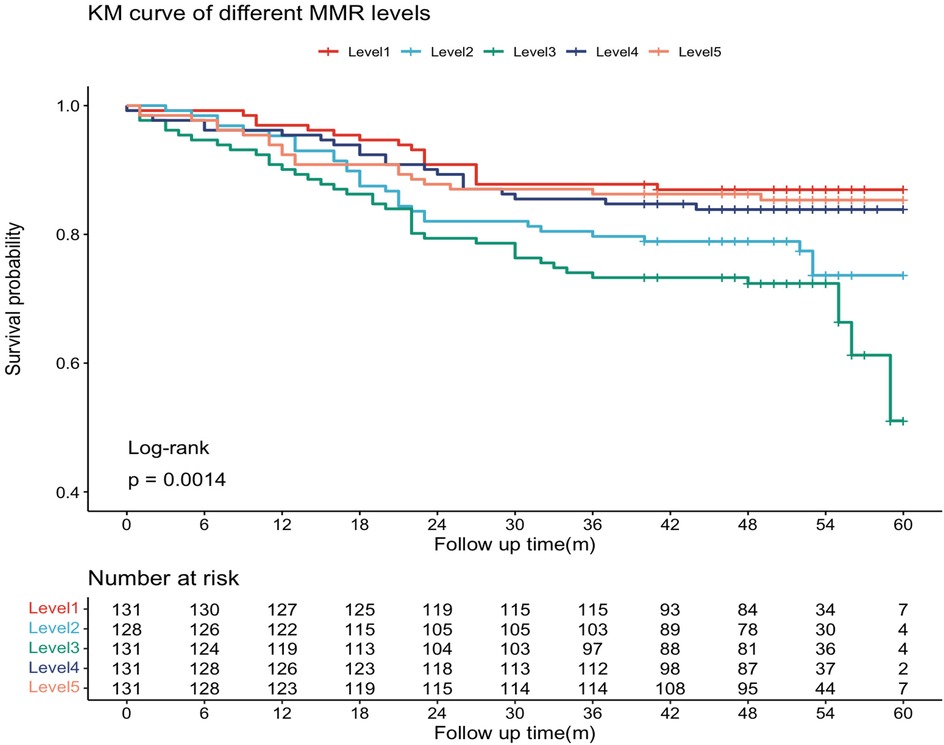
Figure 2. The Kaplan–Meier curves. The MMR was ranked from low to high, and the sample population was divided into five groups by quintile interval.
The MMR values were sorted from low to high. The sample population was divided into five groups at quintile intervals: L1 (n = 131, MMR range 7.89–14.43), L2 (n = 128, MMR range 14.5–17.96), L3 (n = 131, MMR range 18–22.16), L4 (n = 131, MMR range 22.25–28.53), and L5 (n = 131, MMR range 28.67–60.67).
3.3 Independent association between MMR levels and risk of experiencing MACE
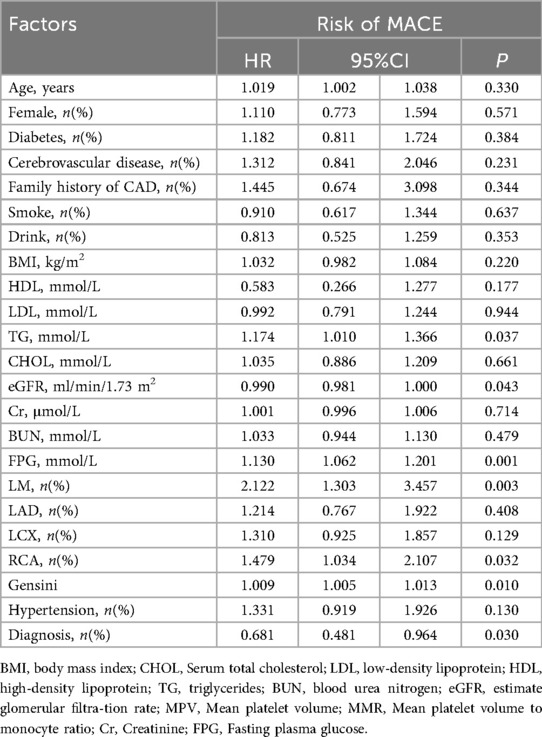
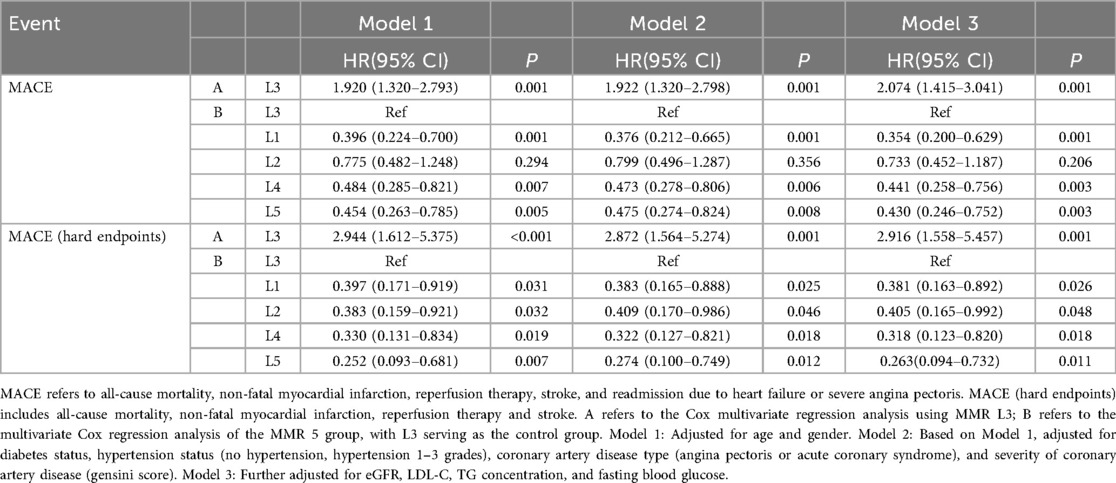
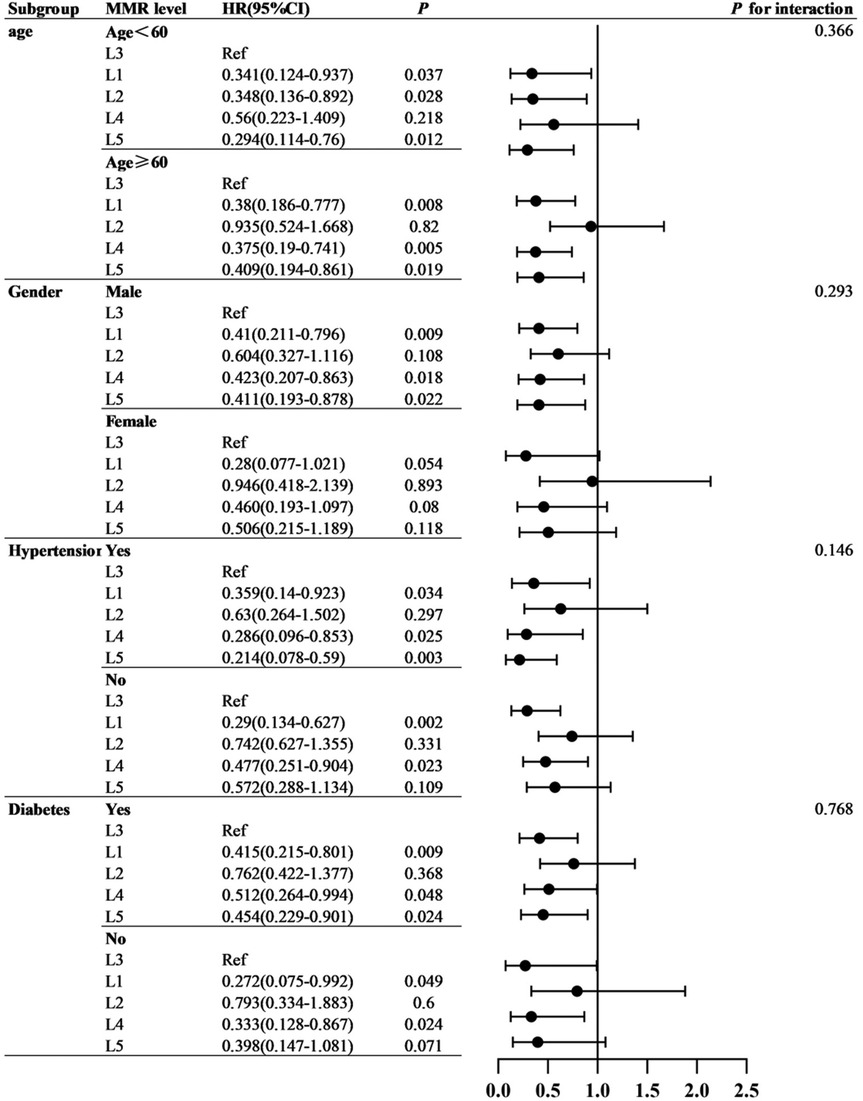
Cox proportional hazards analyses were performed to evaluate the association between MMR and MACE risk. Univariate Cox regression identified several baseline variables associated with MACE (Table 2). Predictive models incorporated covariates from established prognostic frameworks and variables with P < 0.10 in univariate analysis: Model 1 adjusted for age and sex; Model 2 additionally included diabetes, hypertension grade, CAD type, and Gensini score; Model 3 further incorporated eGFR, LDL-C, triglycerides, and fasting glucose. As shown in Table 3, patients in the L3 group had significantly higher risk of both MACE and hard endpoints compared with other quintiles. Specifically, L3 was independently associated with increased MACE risk across all models, with significant differences vs. L1, L4, and L5. Subgroup analyses demonstrated consistent results across sex, age, diabetes, and hypertension strata, with no evidence of significant interaction effects (all interaction P > 0.05). Refer to Figure 4.
3.4 Improvements in cardiovascular risk prediction
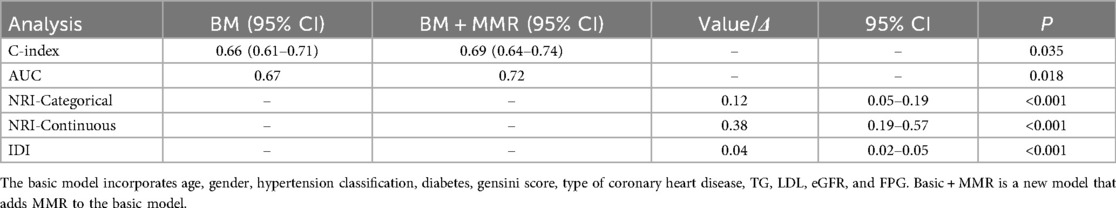
The addition of MMR quintiles to conventional cardiovascular risk models significantly improved prognostic performance. The baseline model, which included age, sex, hypertension grade, diabetes mellitus, CAD type, Gensini score, triglycerides, LDL-C, eGFR, and fasting plasma glucose, yielded a C-index of 0.657 and an AUC of 0.673. After incorporating MMR, the C-index increased to 0.691 (P = 0.035) and the AUC improved to 0.718 (P = 0.018). Moreover, both net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were significantly enhanced (P < 0.001 for both; Figure 5 and Table 4).
The basic model incorporates age, gender, hypertension classification, diabetes, gensini score, type of coronary heart disease, TG, LDL, eGFR, and FPG. Basic + MMR is a new model that adds MMR to the basic model.
4 Discussion
This study evaluated the prognostic significance of MMR in treatment-naïve CAD patients. We found that patients in the intermediate MMR range (18.0–22.16) experienced the highest incidence of MACE, whereas both lower and higher MMR values were associated with more favorable outcomes. Unlike the linear associations typically reported for other inflammatory biomarkers, our restricted cubic spline analysis revealed a distinct nonlinear, inverted U-shaped relationship between MMR and long-term prognosis.
Previous studies have established inflammation as a key driver of atherosclerosis, with indices such as NLR, PLR, and MLR serving as independent prognostic markers in CAD (20, 21). Composite inflammatory indices show utility beyond CAD diagnosis, severity assessment, and prognosis prediction to oncology/hematological disorders (22, 23). However, clinical implementation faces limitations: (1) inconsistent findings regarding their independence as CAD risk predictors (15, 16); (2) substantial threshold variation for MACE prediction; (3) susceptibility to confounders (infections, medications, comorbidities) compromising validity (24); (4) inability to independently predict MACE, necessitating integration into multivariate models (25). Novel indices like systemic inflammation response index (SIRI) exhibit superior MACE predictive value vs. NLR/PLR/MLR. SIRI-incorporated models significantly enhance diagnostic performance (26). In the comparison of the clinical value of composite inflammatory markers and single inflammatory markers, we used MMR, MPV and monocyte count for statistical analysis. Multivariable Cox regression analyses, adjusted for age, gender, hypertension grade, diabetes mellitus, Gensini score, coronary heart disease type (angina pectoris or acute coronary syndrome), triglycerides (TG), LDL cholesterol, eGFR, and FPG, were performed to assess three incremental models: 1. Base model + MMR L3 status 2. Base model + Mean Platelet Volume (MPV) 3. Base model + absolute monocyte count Results demonstrated that MMR L3 was an independent risk factor for MACE (HR = 0.482; 95% CI: 0.329–0.707; P < 0.001). In contrast, neither MPV (HR = 1.066; 95% CI: 0.902–1.260; P = 0.454) nor absolute monocyte count (HR = 0.739; 95% CI: 0.243–2.245; P = 0.594) showed significant associations. This indicates that categorical MMR stratification (L3) demonstrates stronger predictive utility for MACE risk compared to the continuous variables MPV or monocyte count alone. So in some cases, a composite inflammatory measure is more clinically relevant than a single measure. Thus, discovering novel inflammatory biomarkers and refining predictive algorithms remains crucial for optimizing clinical decision-making. After adjusting for factors such as age, gender, history of diabetes and hypertension, coronary heart disease category (angina pectoris or acute myocardial infarction), eGFR, FPG, LDL, TG, and Gensini, we conducted a multivariate Cox regression analysis. Among them, Model a represents the MMR L3 group, Model b represents NLR, Model c represents PLR, and Model d represents MHR. Through the analysis, it was found that the MMR L3 group was an independent risk factor for MACE in newly diagnosed coronary heart disease patients (Model a HR = 2.07, P < 0.001), while the other indicators had no statistical significance (Model b, Model c, and Model d all had P values greater than 0.05). We can observe that the role value of MMR L3 in MACE in newly diagnosed coronary heart disease patients may be due to NLR, PLR, and MHR.
Our study revealed that patients in the MMR L3 quintile (18.0–22.16) exhibited the poorest prognosis compared to other groups. Restricted cubic spline (RCS) analysis confirmed a significant non-linear, inverted U-shaped relationship between MMR and MACE risk—a finding distinct from other composite inflammatory indices (27). The observed inverted U-shaped relationship may reflect a dynamic equilibrium between platelet activity and monocyte function in plaque biology. Platelets modulate monocyte adhesion, differentiation, and polarization. Under pro-inflammatory conditions (28), activated platelets promote M1 macrophage polarization, leading to collagen degradation, reactive oxygen species release, and plaque destabilization (29, 30). Platelet-derived SEMA4D induces M2 polarization (10). M2 macrophages release MMP inhibitors, secrete TGF-β to enhance vascular smooth muscle cell (VSMC) proliferation (strengthening fibrous caps) (31, 32), and produce IL-10 to suppress platelet activation. Platelet-monocyte coculture upregulates M2 markers (CD163) and scavenger receptors (SR-BI, CD36) (11). Notably, CAD patients' platelets exhibit elevated SR-BI/CD36 expression, promoting monocyte differentiation into atheroprotective M2 phenotypes. Additionally, platelet-monocyte aggregates (PMAs) serve as thromboinflammatory hubs through P-selectin/PSGL-1 binding → Mac-1/GPIbα-fibrinogen stabilization (33). This cascade drives plaque destabilization in acute coronary syndromes and restenosis, establishing PMAs as therapeutic targets (e.g., P-selectin inhibitors). Thus, an intermediate MMR range may reflect heightened pro-thrombotic and pro-inflammatory activity, whereas lower or higher MMR levels may favor protective M2-dominated pathways. This mechanistic hypothesis warrants validation through longitudinal and experimental studies.
Our findings indicate that both low and high MMR ranges confer better prognosis compared to the intermediate L3 quintile (18.0–22.16), potentially due to attenuated platelet activity and milder inflammatory responses that stabilize plaques and suppress thrombosis (34, 35). Conversely, MMR values within the 18.0–22.16 range may promote thrombogenesis and plaque vulnerability, worsening clinical outcomes. Analogous to sepsis and severe burns (36), this biphasic pattern suggests bidirectional inflammatory modulation in CAD progression: during early and late disease stages, protective mechanisms (e.g., M2 macrophage polarization, anti-inflammatory cytokine release) may outweigh pathological processes, whereas intermediate phases exhibit dominant pro-thrombotic and pro-inflammatory drivers. This dynamic homeostatic regulation could explain the inverted U-shaped risk curve. However, these hypotheses require validation, as the intricate relationship between platelet indices and monocyte biology remains incompletely characterized. In the future, by repeatedly measuring indicators such as MPV and absolute values of monocytes in patients during the occurrence and development of coronary heart disease, and studying the dynamic changes of MMR and its prognostic relationship with MACE, the possibility of this hypothesis can be tested. Further mechanistic studies are essential to elucidate these interactions, clarify our observations, and optimize translational applications for precision prognostication in CAD management.
Subgroup analyses by age, sex, diabetes, and hypertension status revealed consistent associations, with no significant interaction effects. Although some subgroup estimates did not reach statistical significance, the directionality was consistent with the overall findings. These results suggest the robustness of MMR as a prognostic marker, though larger cohorts are needed to confirm subgroup-specific effects (37).
The addition of MMR to conventional risk models significantly improved prognostic performance, as reflected by higher C-index, AUC, NRI, and IDI values. This supports the role of MMR as an incremental biomarker that enhances existing prognostic frameworks for CAD risk stratification.
5 Limitations
Several limitations should be acknowledged. First, the single-center design with limited demographic diversity may reduce generalizability (38, 39). Second, although patients with overt infection were excluded, residual confounding from subclinical inflammatory states cannot be ruled out. Third, inter-laboratory variability in MPV measurement, and the lack of a universal reference range, may affect MMR reproducibility. Fourth, our analysis focused on baseline values; serial measurements may provide greater insight into dynamic changes in MMR and their prognostic implications. Finally, because hard endpoint events were relatively infrequent, we used a composite MACE definition that included soft endpoints, which may limit interpretability. Larger, multicenter studies with dedicated hard endpoint analyses are warranted (40).
6 Conclusion
In conclusion, MMR is a readily available inflammatory index derived from routine blood tests that demonstrates incremental prognostic value in newly diagnosed CAD patients. The nonlinear, inverted U-shaped association between MMR and MACE highlights the complex role of platelet–monocyte dynamics in atherosclerosis. Further multicenter prospective studies and mechanistic trials are needed to verify the prognostic value of MMR and to elucidate its mechanism of action.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
WF: Writing – review & editing, Writing – original draft. HH: Writing – original draft, Writing – review & editing. JX: Writing – review & editing. PW: Writing – review & editing. QZ: Writing – review & editing. MW: Writing – review & editing. LD: Writing – review & editing. GW: Writing – review & editing. LW: Writing – review & editing. ZC: Writing – review & editing, Writing – original draft. MZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by S&T Program of Hebei [22377771D], National Health Commission Key Research Project (Grant No. YLXX24AIA026), Hebei Province Finance Department Project [ZF2025062], Hebei Provincial Health Commission Project [20190448], The First Hospital of Hebei Medical University ’Spark'. Research Project Fund [XH2018].
Acknowledgments
The authors would like to thank all the participants and technicians involved in the study. Additionally, we are grateful to everyone else who provided assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1643542/full#supplementary-material
Abbreviations
AUC, area under the receiver operating characteristic curve; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, chronic coronary syndrome; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; IDI, integrated discrimination improvement; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; NRI, net reclassification improvement; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; SD, standard deviation; TC, total cholesterol; TG, triglycerides; MPV, mean platelet volume; MMR, the ratio of mean platelet volume to the number of monocytes; Cr, creatinine; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; AMI, acute myocardial infarction; FPG, fasting plasma glucose; RCS, restricted cubic spline.
References
1. Aagaard EN, Kvisvik B, Pervez MO, Lyngbakken MN, Berge T, Enger S, et al. Left ventricular mechanical dispersion in a general population: data from the Akershus Cardiac Examination 1950 study. Eur Heart J Cardiovasc Imaging. (2020) 21(2):183–90. doi: 10.1093/ehjci/jez210
3. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics—2021 update. Circulation. (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
4. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5:56. doi: 10.1038/s41572-019-0106-z
5. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. (2004) 116(6):9–16. doi: 10.1016/j.amjmed.2004.02.006
6. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. (2011) 12(3):204–12. doi: 10.1038/ni.2001
7. Stone GW, Maehara A, Lansky AJ, De Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. (2011) 364(3):226–35. doi: 10.1056/NEJMoa1002358
8. Voronkov NS, Maslov LN, Vyshlov EV, Mukhomedzyanov AV, Ryabov VV, Derkachev IA, et al. Do platelets protect the heart against ischemia/reperfusion injury or exacerbate cardiac ischemia/reperfusion injury? The role of PDGF, VEGF, and PAF. Life Sci. (2024) 5:347. doi: 10.1016/j.lfs.2024.122617
9. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. (2013) 34(10):719–28. doi: 10.1093/eurheartj/ehs411
10. Cui Y, Jiang X, Yang M, Yuan Y, Zhou Z, Gao X, et al. SEMA4D/VEGF surface enhances endothelialization by diminished-glycolysis-mediated M2-like macrophage polarization. Mater Today Bio. (2023) 23:100832. doi: 10.1016/j.mtbio.2023.100832
11. Mehrpouri M, Bashash D, Mohammadi MH, Gheydari ME, Satlsar ES, Hamidpour M. Co-culture of platelets with monocytes induced M2 macrophage polarization and formation of foam cells: shedding light on the crucial role of platelets in monocyte differentiation. Turk J Hematol. (2019) 36(2):97. doi: 10.4274/tjh.galenos.2019.2018.0449
12. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. (2013) 13:709–21. doi: 10.1038/nri3520
13. Mangalesh S, Dudani S. Systemic inflammatory response index over neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio: comparison of prognostic performance in predicting major adverse cardiac events. Ann Med. (2022) 54(1):2151–2. doi: 10.1080/07853890.2022.2104919
14. Li X, Yu C, Liu X, Chen Y, Wang Y, Liang H, et al. A prediction model based on systemic immune-inflammatory index combined with other predictors for major adverse cardiovascular events in acute myocardial infarction patients. J Inflamm Res. (2024) 17:1211–25. doi: 10.2147/JIR.S443153
15. Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Inflammation in ischemic stroke subtypes. Curr Pharm Des. (2012) 18(28):4289–310. doi: 10.2174/138161212802481200
16. Bressi E, Mangiacapra F, Ricottini E, Cavallari I, Colaiori I, Di Gioia G, et al. Impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-year clinical outcomes of patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. J Cardiovasc Transl. (2018) 11(6):517–23. doi: 10.1007/s12265-018-9829-6
17. MozafaryBazargany M, Gholami N, Azimi A, Maadani M, Adimi S, Zamani Kia A, et al. Predictive value of inflammatory indices for one year outcome of primary percutaneous coronary intervention on saphenous vein graft in post-coronary artery bypass grafting patients. Acta Cardiol. (2025) 80(2):109–14. doi: 10.1080/00015385.2024.2413221
18. Odeberg J, Halling A, Ringborn M, Freitag M, Persson ML, Vaara I, et al. Markers of inflammation predict long-term mortality in patients with acute coronary syndrome - a cohort study. BMC Cardiovasc Disord. (2025) 25:190. doi: 10.1186/s12872-025-04608-9
19. Ruder AV, Wetzels SMW, Temmerman L, Biessen EAL, Goossens P. Monocyte heterogeneity in cardiovascular disease. Cardiovasc Res. (2023) 119(11):2033–45. doi: 10.1093/cvr/cvad069
20. Sun T, Chen P, Zheng X. A retrospective analysis from NHANES 2003–2018 on the associations between inflammatory markers and coronary artery disease, all-cause mortality and cardiovascular mortality. PLoS One. (2025) 20(7):e0326953. doi: 10.1371/journal.pone.0326953
21. Xie Y, Cen H, Wang L, Cheng K, Huang L, Lu H, et al. Relationships between inflammatory parameters derived from complete blood count and quantitative flow ratio in patients with stable coronary artery disease. Angiology. (2025) 76(1):51–7. doi: 10.1177/00033197231197804
22. Bao Q, Liu T, Song H, Bao W, Fan W. Prognostic role of inflammatory hematologic indices in predicting acute coronary syndrome in elderly patients with chronic coronary syndrome. J Inflamm Res. (2025) 18:9637–53. doi: 10.2147/JIR.S528161
23. Matsuura K, Osaki A, Nakame A, Fujimoto A, Ichinose Y, Nukui A, et al. MO32–7 high ALC, low NLR and PLR are associated with longer OS in patients with metastatic breast cancer treated with eribulin. Ann Oncol. (2021) 7:32. doi: 10.1016/j.annonc.2021.05.646
24. Steindl A, Nackenhorst M, Puhr H, Marhold M, Sinn K, Hoetzenecker K, et al. Systemic and local inflammation characteristics in patients with cancer after lung transplantation. J Clin Oncol. (2021) 7:39. doi: 10.1200/jco.2021.39.15_suppl.e14527
25. Li Q, Ma X, Shao Q, Yang Z, Wang Y, Gao F, et al. Prognostic impact of multiple lymphocyte-based inflammatory indices in acute coronary syndrome patients. Front Cardiovasc Med. (2022) 9:811790. doi: 10.3389/fcvm.2022.811790
26. Ma M, Wu K, Sun T, Huang X, Zhang B, Chen Z, et al. Impacts of systemic inflammation response index on the prognosis of patients with ischemic heart failure after percutaneous coronary intervention. Front Immunol. (2024) 15:1324890. doi: 10.3389/fimmu.2024.1324890
27. Xie K, Jiang S, Wang Y, Chen H, Wu X, Xu B. Association of immune-inflammatory biomarkers during pregnancy and the postpartum period with postpartum depression symptoms: a cross-sectional and longitudinal retrospective analysis. Brain Behav Immun. (2025) 4:129. doi: 10.1016/j.bbi.2025.05.014
28. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies. Circulation. (2003) 108(15):1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9
29. Pahk K, Kwon H, Eo J, Seo H, Kim S. Coronary plaque instability is associated with inflammatory activity of visceral adipose tissue and carotid artery: a prospective pilot F-18 FDG PET/CT study. Eur Heart J. (2020) 7:41. doi: 10.1093/ehjci/ehaa946.1554
30. Zhang H, Xue C, Reilly M. Abstract 56: coronary artery disease risk alleles at the LIPA locus are associated with higher levels of its mRNA and enzymatic activity in human macrophages. Arterioscl Throm Vas. (2017) 7:37. doi: 10.1161/atvb.37.suppl_1.56
31. Pouyanfard S, Fierro M, Kaufman D. Development of chimeric antigen receptor-expressing iPSC-derived macrophages with improved anti-tumor activity. Blood. (2021) 7:138. doi: 10.1182/blood-2021-148687
32. Yan W, Li T, Yin T, Hou Z, Qu K, Wang N, et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics. (2020) 10(23):10712. doi: 10.7150/thno.46143
33. Canzano P, Brambilla M, Porro B, Cosentino N, Tortorici E, Vicini S, et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. (2021) 2:6. doi: 10.1016/j.jacbts.2020.12.009
34. Wada H, Suzuki M, Matsuda M, Ajiro Y, Shinozaki T, Sakagami S, et al. Impact of smoking status on the relationships of growth differentiation factor 15 with mortality and cardiovascular events in patients with suspected or known coronary artery disease: the ANOX study. Eur Heart J. (2020) 7:41. doi: 10.1093/ehjci/ehaa946.3018
35. Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, et al. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J AM Coll Cardiol. (2008) 7:52. doi: 10.1016/j.jacc.2008.05.031
36. Gibson B, Keller P, Moore-Lotridge S, Summitt J, Schoenecker J. Hyperactivation of plasmin following severe burn is associated with systemic inflammation and coagulopathy. Blood. (2018) 7:132. doi: 10.1182/blood-2018-99-116904
37. Zhao S, Wang Z, Qing P, Li M, Liu Q, Pang X, et al. Comprehensive analysis of the association between triglyceride-glucose index and coronary artery disease severity across different glucose metabolism states: a large-scale cross-sectional study from an Asian cohort. Cardiovasc Diabetol. (2024) 23(1):251. doi: 10.1186/s12933-024-02355-3
38. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. (2015) 162(1):55–63. doi: 10.7326/M14-0697
39. Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S, et al. Prognosis research strategy (PROGRESS) 3: prognostic model research. PLoS Med. (2013) 10(2):e1001381. doi: 10.1371/journal.pmed.1001381
Keywords: mean platelet volume, monocyte count, cardiovascular risk, major adverse cardiovascular events, the mean platelet volume/monocyte ratio
Citation: Fu W, He H, Xu J, Wu P, Zhang Q, Wei M, Duan L, Wang G, Wang L, Cao Z and Zheng M (2025) A study on the correlation between the mean platelet volume to monocyte count ratio and long-term prognosis in patients with newly diagnosed coronary artery disease. Front. Cardiovasc. Med. 12:1643542. doi: 10.3389/fcvm.2025.1643542
Received: 9 June 2025; Accepted: 16 September 2025;
Published: 9 October 2025.
Edited by:
Nikos Stalikas, OLV Aalst, BelgiumReviewed by:
You Chen, First Affiliated Hospital of Xinjiang Medical University, ChinaEmir Becirovic, University Clinical Center Tuzla, Bosnia and Herzegovina
Copyright: © 2025 Fu, He, Xu, Wu, Zhang, Wei, Duan, Wang, Wang, Cao and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zelong Cao, Y2FvemVsb25nQGhlYm11LmVkdS5jbg==; Mingqi Zheng, bXpoZW5nQGhlYm11LmVkdS5jbg==
†These authors have contributed equally to this work
 Wei Fu
Wei Fu Honghou He
Honghou He Jianan Xu1
Jianan Xu1 Zelong Cao
Zelong Cao Mingqi Zheng
Mingqi Zheng