Abstract
Background:
Approximately 3.8 million patients in China suffer from familial hypercholesterolemia (FH). Statins and PCSK9 inhibitors are recommended by guidelines as therapeutic agents. Nevertheless, cases in which patients demonstrate statin intolerance and an abnormal response to PCSK9 inhibitors present a significant challenge to the clinical treatment of the condition.
Case presentation:
We report a 56-year-old Chinese female diagnosed with heterozygous familial hypercholesterolemia (HeFH). After taking simvastatin, she had elevated transaminases and creatine kinase levels, leading to a transition to PCSK9 inhibitor therapy. Unfortunately, the patient exhibited an absence of the desired response to three different PCSK9 inhibitors. A novel heterozygous missense variant in the LDLR gene (exon 11, c.1700C > T, p.Thr567Ile) was identified through related gene sequencing and genetic testing also revealed a heterozygous variant in the HTR7 gene. In light of the findings, she was treated with a combination of rosuvastatin and ezetimibe. This treatment resulted in the achievement of target lipid levels. During the follow - up, no adverse events were reported.
Conclusion:
The study highlights that genetic testing should be considered for FH patients who experience failure with PCSK9 inhibitors, as novel LDLR variants may account for resistance and inform personalized treatment.
1 Introduction
FH is an inherited disorder of cholesterol metabolism that results in elevated levels of low-density lipoprotein cholesterol (LDL-C) in the blood. The clinical manifestations of patients are primarily determined by their genotype and exhibit phenotypic heterogeneity (1). Typically, the hereditary condition known as FH is classified into two main types, such as autosomal dominant hypercholesterolemia (ADH) and autosomal recessive hypercholesterolemia (ARH). A patient with ADH has one affected parent and often presents with a positive family history of premature coronary heart disease (CHD). The etiology of ARH is attributed to pathogenic variants in the LDL receptor adaptor protein 1 (LDLRAP1) gene, with the condition manifesting in early childhood (2). In the case of heterozygous individuals or even in homozygous population with altering mutations like the p.(ala431thr) (3), lower levels of LDL-C and milder phenotypes are exhibited in comparison to those with homozygous familial hypercholesterolemia (HoFH). This frequently results in cases being overlooked (1).
The management of FH primarily encompasses dietary and pharmacological interventions (4). Statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are considered the therapeutic options for these patients. However, studies (5–8) have indicated that Asian populations exhibit a comparatively poorer tolerance to statins. On the other hand, it should be noted that not all individuals with FH respond positively to PCSK9 inhibitors (9, 10). Thus, establishing appropriate diagnostic and treatment protocols and elucidating its mechanisms is essential to assure goal attainment for these patients. To the best of our knowledge, there are no case reports of patients with FH for whom all three PCSK9 inhibitors have been ineffective. In this study, we present a case of statin-intolerant HeFH that exhibited an abnormal response to three PCSK9 inhibitors.
2 Case presentation
2.1 Patient information and clinical findings
In April 2021, a 55-year-old female patient was first found to have elevated blood lipids during a health checkup. Notwithstanding this, she did not undergo any lipid-lowering treatment immediately. In March 2022, this patient attended the Outpatient Lipid Management Department due to hyperlipidaemia. Laboratory analysis revealed that the subject's Total Cholesterol (TC) levels were 9.01 mmol/L (normal range: 5.00–5.71 mmol/L), LDL-C levels were 6.09 mmol/L (normal range: <3.4 mmol/L), and lipoprotein(a) [Lp(a)] levels were 794 mg/L (normal range: 1–300 mg/L). The patient exhibited no history of chronic diseases and was not currently taking any medication. The patient's physical examination results are outlined below: heart rate of 65 beats per minute, blood pressure of 123/81mmHg and all markers of myocardial injury, glycosylated hemoglobin, thyroid function and liver and kidney function tests were all within the normal reference range. On the same day, the patient underwent a colour Doppler ultrasound examination of the bilateral carotid arteries. This examination revealed symmetrical diameters of the bilateral carotid arteries, normal intima-media thickness (right IMT: 0.08 cm, left IMT: 0.08 cm), adequate blood flow filling in each segment of the carotid arteries, and normal blood flow velocity and spectral morphology. The patient has two siblings, specifically an older brother and a younger sister. A lipid profile test revealed that the patient's older brother had TC levels exceeding 7.5 mmol/L at the age of 50. To date, the patient's younger sister has exhibited no anomalies in her lipid profile. According to the Simon Broome criteria (11), the patient was diagnosed with possible FH at that time.
2.2 Therapeutic intervention
Firstly, the physician prescribed simvastatin (40 mg, administered once daily) and ezetimibe (10 mg, administered once daily). Following this, a long-term follow-up evaluation of the patient's blood lipid levels was conducted, and a waveform chart was generated based on these levels and the lipid-lowering treatment strategies employed (Figure 1). During subsequent follow-ups, symptoms of intolerance to simvastatin manifested, as indicated by elevated levels of ALT at 64.5 U/L (normal range: 5–40 U/L) and CK at 833 U/L (normal range: 20–174 U/L) concomitant with muscle discomfort. The patient was confirmed to have statin intolerance, and treatment with ezetimibe alone was initiated; however, the patient's lipid levels continued to fall short of the desired target. Two compliance assessments of the patient were conducted using the Morisky Medication Adherence Scale (MMAS) (12) in July and September of 2022, achieving scores of 6 and 7, respectively. These scores suggest a potential for nonadherence to medication. The patient also reported experiencing inadequate exercise and dietary control during this period due to family circumstances.
Figure 1
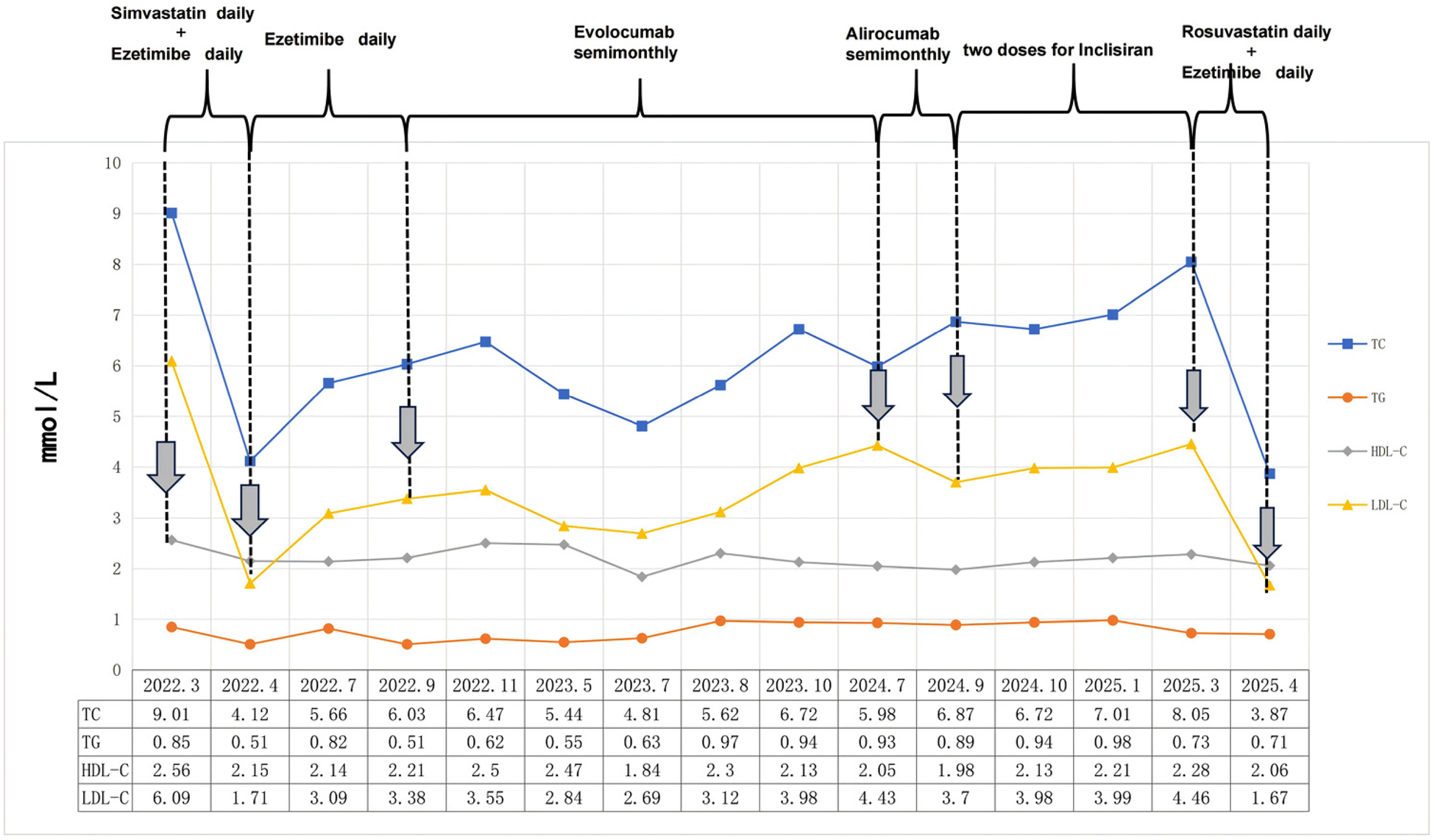
The lipid levels of this patient were monitored over a 3-year follow-up period, and the values are reported in mmol/L.
In September 2022, the therapeutic approach aimed at reducing serum cholesterol levels was transitioned to evolocumab (140 mg twice a month). Nevertheless, over the course of the subsequent ten months, the patient's blood lipid levels initially demonstrated a decline, subsequently followed by an uptick. After that, the treatment regimen was modified to alirocumab, administered at a dosage of 75 mg once every two weeks. After a period of three months during which the aforementioned therapy was administered, the patient's LDL-C level exhibited a decline of only 16.4%. It is acknowledged that the reasons for the abnormal response to PCSK9 inhibitors may include the presence of anti-drug antibodies, increased in vivo PCSK9 concentration, and other factors (13). However, it is regrettable to note that the necessary equipment to detect blood PCSK9 concentration is not currently available at this hospital. Furthermore, the patient declined both genetic testing and other statin medication therapies at that time. Consequently, the decision was taken to administer inclisiran in order to treat the dyslipidaemia. Despite the administration of two standardized doses of inclisiran, the patient's blood lipid levels remained inadequately controlled. Following a comprehensive evaluation of the patient's case, a treatment regimen including rosuvastatin (20 mg daily) and ezetimibe (10 mg daily) was administered. This therapeutic approach resulted in a substantial reduction in blood lipid levels, thereby achieving the desired therapeutic objective. During the subsequent follow-up period, there were no reports of any untoward occurrences.
2.3 Genetic analysis
In March 2025, following the procurement of the patient's consent, genetic testing was conducted to provide further clarification on the diagnosis. A novel heterozygous missense variant in the LDLR gene (exon 11, c.1700C > T, p.Thr567Ile) (Table 1) was identified by gene sequencing. In light of the findings, the patient was diagnosed with HeFH in accordance with the Simon Broome criteria (11) and the diagnostic considerations established by the American Heart Association (14). In this patient, gene sequencing revealed a heterozygous variant in the HTR7 gene (intron1, c.539 + 22547G > A, p.?)(Table 1). Unfortunately, the patient's parents passed away in an accident many years ago, and the siblings of our patient refused to carry out genetic testing.
Table 1
| Gene | Chromosomal location | Transcript Exon | Nucleotide Amino acid | Genotype | Phenotype |
|---|---|---|---|---|---|
| LDL-R | chr19:11226883 | NM_000527.5; exon11 | c.1700C > T (p.Thr567Ile) | Het | HeFH |
| HTR7 | chr10:92594343 | NM_019859.4; intron1 | c.539 + 22547G > A (P.?) | Het | Statin-induced myopathy |
Single nucleotide variants and their associated phenotypes.
3 Discussion
Statins and PCSK9 inhibitors are recommended as lipid-lowering therapies for FH patients (15). However, in this case, elevated levels of transaminases and creatine kinase were observed in the patient during the administration of simvastatin. Over the subsequent 30 months, the patient sequentially utilized three different PCSK9 inhibitors, yet no significant lipid-lowering effect was observed. This presented a challenge in determining the clinical lipid-lowering programmes.
Several studies (16–18) have indicated that the usage rate of statins among the Chinese population is significantly lower than that observed in developed countries. This disparity may be attributed to patients' concerns regarding adverse reactions to statins. The most common adverse reactions to statins are muscle-related adverse reactions and liver dysfunction (18). The genes of ATP-binding cassette transporter B1 (ABCB1) (19), solute carrier organic anion transporter family member 1B1 (SLCO1B1) (20), the cytochrome P450 (CYP) family (21) and 5-hydroxytryptamine (serotonin) receptor 7 (HTR7) (22) have been demonstrated to be associated with statin adverse drug reactions (ADRs).
In this patient, gene sequencing revealed a heterozygous variant in the HTR7 gene (intron1, c.539 + 22547G > A, p.?) (Table 1). Genomic research reveals a statistically significant association between simvastatin-induced myalgia and the rs1935349 variant in the HTR7 gene (22). This statin-specific association explains SAMS occurring in this patient after taking simvastatin. Additionally, it was noted that the incidence of myopathy is much more with simvastation (50%) than with other statins like rosuvastatin (10.8%) (23). In accordance with the results of genetic testing for antihyperlipidaemia medication, the patient was administered rosuvastatin and ezetimibe treatment without any reported adverse reactions.
In consideration of the inadequate compliance with statin therapy demonstrated by patients (24), it can be hypothesised that PCSK9 inhibitors may possess significant therapeutic potential in China. Alirocumab and Evolocumab are fully human monoclonal antibodies that target PCSK9, which were found to be effective and safe in reducing LDL-C (25–27). On 22 August 2023, inclisiran was launched in China. The drug has been demonstrated to target and disrupt the synthesis of liver PCSK9 protein via an intracellular RNA interference mechanism, thereby upregulating the expression of LDL receptors and decreasing LDL-C levels (28). As the world's first siRNA ultra-long-acting lipid-lowering pharmaceutical agent, study (29) in Asian populations has indicated that 71.7% of participants who received 300 mg of inclisiran sodium experienced a reduction in LDL-C of ≥50% by day 330, compared to just 1.5% with placebo. However, it is important to note that a recent study (13) demonstrated that PCSK9 inhibitors exhibited an abnormal response rate of 13.1% in real-world settings, with nearly half of these cases attributable to inadequate adherence. Consequently, the initial step in assessing the unresponsiveness of PCSK9 inhibitors involves monitoring medication adherence. In this case, following treatment with three distinct PCSK9 inhibitors, the patient exhibited an average LDL-C level of 3.67 mmol/L. In view of the fact that all the injections were administered by the nurses, it is imperative to undertake a more thorough analysis in order to ascertain the underlying causes.
Secondly, the availability of PCSK9 assays has the potential to assist clinicians in more effectively diagnosing abnormal responses (30). However, economic factors have been a significant barrier to the implementation of this technology. As indicated by Bruce A. Warden (13), the prevalence of hereditary hemochromatosis (HeFH) was found to be higher among subjects demonstrating unusual responses. Additionally, case reports (30, 31) also support the notion that it is crucial to implement a comprehensive screening process for HeFH and to identify the relevant gene sequences.
A recent study (32) has revealed that LDLR constitutes the primary pathway through which the body eliminates LDL-C, and mutations that affect its conformational changes confer resistance to both PCSK9 and its inhibitors. The LDLR gene (MIM Number: 606945) consists of 18 exons, which are associated with the functional domains of the LDLR protein. In this patient, gene sequencing revealed a novel heterozygous missense variant in the LDLR gene (exon 11, c.1700C > T, p.Thr567Ile). The variant was absent from the control databases of the Exome Sequencing Project, the 1,000 Genomes Project, the Exome Aggregation Consortium and the GnomAD (Genome Aggregation Database). REVEL software predicts that the variant is damage. The results of the functional study on the adjacent site c.1694G > T (p.G565V) provide the basis for the following hypothesis: that the mutation in question represents a missense mutation within the β-propeller domain and may be classified as a class 2 mutation. LDLR binds to apoB100 through two independent interfaces (BS1 and BS2), with BS2 being formed by the β-propeller domain of LDLR binding to the N-terminal domain of apoB100 (33). This facilitates the interaction between LDL-C and LDLR, thereby promoting cellular uptake of LDL-C absorption. Consequently, we further deduce that this patient's elevated LDL-C levels result from inherent LDL-R functional defects, independent of circulating PCSK9 concentrations.
In addition to this, the study (34) suggests that a range of LDLR mutation phenotypes exhibit varied responses to lipid-lowering therapies. It is noteworthy that in patients exhibiting class 2 LDLR variants, statins demonstrated a heightened efficacy in reducing blood lipid levels when compared to those with class 5 LDLR variants (35). During the follow-up period, it was observed that the patient taking simvastatin and rosuvastatin exhibited a reduction in LDL-C levels (71.92% and 62.56%, respectively), demonstrating significant lipid-lowering effects.
It is important to consider several limitations when interpreting the findings of this study. Firstly, as it is a case report from a single center, it may not be representative of the general population. Secondly, the genetic origin of this variant remains to be elucidated due to the absence of parental DNA. Thirdly, further studies are required to elucidate the potential pathogenicity and expression mechanisms of mutated genes, which would help to further understand the structure of LDL and its interaction with LDLR.
4 Conclusion
In this case, genetic testing yielded pivotal insights for an FH patient afflicted with statin intolerance, leading to the identification of the rs1935349 variant in the HTR7 gene. This finding prompted a change to alternative statin regimens, which were shown to successfully avoid adverse drug reactions. On the other hand, genetic testing revealed a novel missense variant in the LDLR gene in this patient, a discovery of significant importance that filled the gaps in the existing gene databases. In summary, genetic testing should be considered for FH patients who experience failure with PCSK9 inhibitors, as novel LDLR variants may account for resistance and inform personalized treatment.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by the ethics committee of Nanjing Drum Tower Hospital Affiliated to Nanjing University Medical School. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Writing – original draft, Validation. HJ: Writing – review & editing, Data curation. YX: Data curation, Writing – review & editing. SY: Writing – review & editing, Project administration. XC: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by funding for the Project of Key Laboratory of Maritime Intelligent Cyberspace Technology (Hohai University), Ministry of Education (EKLMIC202404).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Abifadel M Boileau C . Genetic and molecular architecture of familial hypercholesterolemia. J Intern Med. (2023) 293(2):144–65. 10.1111/joim.13577
2.
Sánchez-Hernández RM Prieto-Matos P Civeira F Lafuente EE Vargas MF Real JT et al Autosomal recessive hypercholesterolemia in Spain. Atherosclerosis. (2018) 269:1–5. 10.1016/j.atherosclerosis.2017.12.006
3.
Das T Mondal S Rawool AK Tarafdar S Ghosh A . Importance of genotype-phenotype correlation in the population screening of familial hypercholesterolemia. Cureus. (2025) 17(2):e79252. 10.7759/cureus.79252
4.
Watts GF Gidding SS Hegele RA Raal FJ Sturm AC Jones LK et al International atherosclerosis society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol. (2023) 20(12):845–69. 10.1038/s41569-023-00892-0
5.
McGowan MP Hosseini Dehkordi SH Moriarty PM Duell PB . Diagnosis and treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc. (2019) 8(24):e013225. 10.1161/JAHA.119.013225
6.
Kim K Johnson JA Derendorf H . Differences in drug pharmacokinetics between East Asians and caucasians and the role of genetic polymorphisms. J Clin Pharmacol. (2004) 44(10):1083–105. 10.1177/0091270004268128
7.
Tomlinson B Chan P Liu ZM . Statin intolerance-an Asian perspective. J Atheroscler Thromb. (2020) 27(5):485–8. 10.5551/jat.50435
8.
Zhang L Zhang S Yu Y Jiang H Ge J . Efficacy and safety of rosuvastatin vs. atorvastatin in lowering LDL cholesterol: a meta-analysis of trials with East Asian populations. Herz. (2020) 45(6):594–602. 10.1007/s00059-018-4767-2
9.
Zhang Z Yang R Zhu J Yang X Luo H Wang H et al Failure of lipid control by PCSK9 inhibitors in compound heterozygous familial hypercholesterolemia complicated with premature myocardial infarction: a case report. Clin Case Rep. (2024) 12(3):e8498. 10.1002/ccr3.8498
10.
Raal F Durst R Bi R Talloczy Z Maheux P Lesogor A et al Efficacy, safety, and tolerability of inclisiran in patients with homozygous familial hypercholesterolemia: results from the ORION-5 randomized clinical trial. Circulation. (2024) 149(5):354–62. 10.1161/CIRCULATIONAHA.122.063460
11.
Betteridge DJ Broome K Durrington PN Mann JI Miller JP Neil HAW et al Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific steering committee on behalf of the simon broome register group. BMJ. (1991) 303(6807):893–6. 10.1136/bmj.303.6807.893
12.
AlGhurair SA Hughes CA Simpson SH Guirguis LM . A systematic review of patient self-reported barriers of adherence to antihypertensive medications using the world health organization multidimensional adherence model. J Clin Hypertens (Greenwich). (2012) 14(12):877–86. 10.1111/j.1751-7176.2012.00699.x
13.
Warden BA Miles JR Oleaga C Ganda OP Duell PB Purnell JQ et al Unusual responses to PCSK9 inhibitors in a clinical cohort utilizing a structured follow-up protocol. Am J Prev Cardiol. (2020) 1:100012. 10.1016/j.ajpc.2020.1000
14.
Gidding SS Champagne MA de Ferranti SD Defesche J Ito MK Knowles JW et al The agenda for familial hypercholesterolemia: a scientific statement from the American heart association. Circulation. (2015) 132(22):2167–92. 10.1161/CIR.0000000000000297
15.
Mach F Baigent C Catapano AL Koskinas KC Casula M Badimon L et al 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41(1):111–88. 10.1093/eurheartj/ehz455
16.
Zhao B He X Wu J Yan S . Adherence to statins and its impact on clinical outcomes: a retrospective population-based study in China. BMC Cardiovasc Disord. (2020) 20(1):282. 10.1186/s12872-020-01566-2
17.
Wang X Li Y Li J Qiu MH Qi ZZ Li XY et al Medication compliance for secondary prevention and long-term outcome among patients with acute coronary syndrome after percutaneous coronary intervention in different regions. Zhonghua Xin Xue Guan Bing Za Zhi. (2021) 49(2):143–9. 10.3760/cma.j.cn112148-20200528-00442
18.
Li JJ Dou KF Zhou ZG Zhao D Ye P Chen H et al Chinese expert consensus on the clinical diagnosis and management of statin intolerance. Clin Pharmacol Ther. (2024) 115(5):954–64. 10.1002/cpt.3213
19.
Hoenig MR Walker PJ Gurnsey C Beadle K Johnson L . The C3435T polymorphism in ABCB1 influences atorvastatin efficacy and muscle symptoms in a high-risk vascular cohort. J Clin Lipidol. (2011) 5(2):91–6. 10.1016/j.jacl.2011.01.001
20.
Brunham LR Lansberg PJ Zhang L Miao F Carter C Hovingh GK et al Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. (2012) 12(3):233–7. 10.1038/tpj.2010.92
21.
Becker ML Visser LE van Schaik RH Hofman A Uitterlinden AG Stricker BH . Influence of genetic variation in CYP3A4 and ABCB1 on dose decrease or switching during simvastatin and atorvastatin therapy. Pharmacoepidemiol Drug Saf. (2010) 19(1):75–81. 10.1002/pds.1866
22.
Ruaño G Thompson PD Windemuth A Seip RL Dande A Sorokin A et al Physiogenomic association of statin-related myalgia to serotonin receptors. Muscle Nerve. (2007) 36(3):329–35. 10.1002/mus.20871
23.
Abed W Abujbara M Batieha A Ajlouni K . Statin induced myopathy among patients attending the national center for diabetes, endocrinology, & genetics. Ann Med Surg (Lond). (2022) 74:103304. 10.1016/j.amsu.2022.103304
24.
Jing R Yao H Yan Q Xue Y Sun W Lu P et al Trends and gaps in statin use for cardiovascular disease prevention in type 2 diabetes: a real-world study in Shanghai, China. Endocr Pract. (2023) 29(10):747–53. 10.1016/j.eprac.2023.07.001
25.
Kastelein JJ Ginsberg HN Langslet G Hovingh GK Ceska R Dufour R et al ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. (2015) 36(43):2996–3003. 10.1093/eurheartj/ehv370
26.
Ginsberg HN Rader DJ Raal FJ Guyton JR Baccara-Dinet MT Lorenzato C et al Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther. (2016) 30(5):473–83. 10.1007/s10557-016-6685-y
27.
Liu YQ Li DD Chai M Cong HL Cong XQ Dai J et al Real world effectiveness of PCSK-9 inhibitors combined with statins versus statins-based therapy among patients with very high risk of atherosclerotic cardiovascular disease in China (RWE-PCSK study). J Geriatr Cardiol. (2021) 18(4):261–70. 10.11909/j.issn.1671-5411.2021.04.005
28.
Wołowiec Ł Osiak J Wołowiec A Wijata A Grześk E Kozakiewicz M et al Inclisiran-safety and effectiveness of small interfering RNA in inhibition of PCSK-9. Pharmaceutics. (2023) 15(2):323. 10.3390/pharmaceutics15020323
29.
Huo Y Lesogor A Lee CW Chiang CE Mena-Madrazo J Poh KK et al Efficacy and safety of inclisiran in Asian patients: results from ORION-18. JACC Asia. (2023) 4(2):123–34. 10.1016/j.jacasi.2023.09.006
30.
Bimal T Ayyalu T Safarova MS Donahoe S Davila C Dayspring T et al Inadequate response to PCSK9 inhibitors. JACC Case Rep. (2025) 30(8):103696. 10.1016/j.jaccas.2025.103696
31.
Dal Pino B Bigazzi F Sbrana F . Inclisiran and lipoprotein apheresis in statin intolerance heterozygous FH patients: a case series. Ther Apher Dial. (2023) 27(5):978–9. 10.1111/1744-9987.14025
32.
Guan Y Liu X Yang Z Zhu X Liu M Du M et al PCSK9 promotes LDLR degradation by preventing SNX17-mediated LDLR recycling. Circulation. (2025) 151(21):1512–26. 10.1161/CIRCULATIONAHA.124.072336
33.
Reimund M Dearborn AD Graziano G Lei H Ciancone AM Kumar A et al Structure of apolipoprotein B100 bound to the low-density lipoprotein receptor. Nature. (2025) 638(8051):829–35. 10.1038/s41586-024-08223-0
34.
Miltiadous G Xenophontos S Bairaktari E Ganotakis M Cariolou M Elisaf M . Genetic and environmental factors affecting the response to statin therapy in patients with molecularly defined familial hypercholesterolaemia. Pharmacogenet Genom. (2005) 15(4):219–25. 10.1097/01213011-200504000-00005
35.
Etxebarria A Benito-Vicente A Palacios L Stef M Cenarro A Civeira F et al Functional characterization and classification of frequent low-density lipoprotein receptor variants. Hum Mutat. (2015) 36(1):129–41. 10.1002/humu.22721
Summary
Keywords
familial hypercholesterolemia, low-density lipoprotein receptor (LDLR), PCSK9 inhibitor, gene mutation, statin intolerance, case report
Citation
Li Y, Jiang H, Xiong Y, Yan S and Chen X (2025) PCSK9 inhibitor failure in a statin-intolerant FH patient with a novel LDLR variant: a case report. Front. Cardiovasc. Med. 12:1644145. doi: 10.3389/fcvm.2025.1644145
Received
17 June 2025
Accepted
12 September 2025
Published
22 September 2025
Volume
12 - 2025
Edited by
Robert Kiss, McGill University, Canada
Reviewed by
Niloufar Javadi, Aurora St. Luke's Medical Center, United States
Tirath Patel, American University of Antigua, Antigua and Barbuda
Anirban Ghosh, AIIMS, India
Terry Gbaa, JB Consulting Limited, United Kingdom
Updates
Copyright
© 2025 Li, Jiang, Xiong, Yan and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xin Chen kaisanju@njglyy.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.