Abstract
We report a rare case involving a 43-year-old male on long-term hemodialysis who developed infective endocarditis (IE) accompanied by a diastolic “tumor plop” sound and a large atrial mass, a presentation more commonly linked to atrial myxomas. The patient initially experienced an upper respiratory tract infection caused by Type I parainfluenza virus, which progressed to severe pneumonia. During hospitalization, physical examination revealed an atypical diastolic “tumor plop” sound, prompting further evaluation. Echocardiography identified a sizable atrial mass measuring 51 mm × 33 mm × 32 mm, which oscillated between the left atrium and ventricle throughout the cardiac cycle. Blood cultures confirmed a bloodstream infection with Rothia dentocariosa. Concurrently, the patient suffered an embolic stroke, likely due to detachment of the cardiac mass. Clinical findings supported a diagnosis of IE with embolic stroke caused by the atrial mass, rather than an atrial myxoma. The patient underwent surgical removal of the mass along with a full course of antibiotic therapy, which led to a significant improvement in clinical status. This case demonstrates that the clinical features of IE in patients receiving hemodialysis may resemble those of an atrial myxoma, including the unusual “tumor plop” sound. It also illustrates the diagnostic and therapeutic challenges encountered in such cases, where rapid identification and treatment are essential for improving outcomes.
1 Introduction
Infective endocarditis (IE) is a life-threatening condition, particularly in individuals with comorbidities such as end-stage renal disease undergoing hemodialysis. These patients face a higher risk of IE due to repeated vascular access, prolonged catheter placement, and frequent exposure to diverse pathogens (1–4). IE commonly presents with fever, the emergence of a new heart murmur, and a risk of embolic events, necessitating a strong clinical suspicion for timely diagnosis (5, 6). A defining feature of IE is the development of vegetations on cardiac valves. However, these vegetations can sometimes mimic other intracardiac masses, such as atrial myxomas, thrombi, and lesions associated with rheumatic heart disease, both clinically and radiographically (see Table 1).
Table 1
| Year | Author | Age/sex | Clinical features | Complications | Pathogen | Main Treatment |
|---|---|---|---|---|---|---|
| 2014 (11) | Anshuan Darbari | 6/male | High fever, erythematous rashes, dyspnea, hepatomegaly, tumor plop | Pulmonary hypertension, vegetation on tricuspid leaflets | Staphylococcus aureus | Surgical excision of vegetation, valve repair, 6-week antibiotic therapy (ceftriaxone/cefixime) |
| 2016 (20) | Talita G. Salani | 19/female | Asymptomatic, right atrium mass | None | None (thrombus) | Anticoagulation therapy, no surgical intervention |
| 2017 (14) | Jun Xu | 63/male | Progressive dyspnea, leg edema, fever, tumor-like mass in the left atrium | Heart failure, bacterial vegetation, severe aortic insufficiency | Corynebacterium striatum, Acinetobacter baumannii (sputum) | Double valve replacement, daptomycin, linezolid |
| 2018 (7) | Gerald paul Fitzgerald. | 23/male | Fever, weight loss, night sweats, fatigue, general malaise, tumor plop | Necrotic and infected atrial myxoma mimicking IE | Streptococcus viridans | Empirical antibiotics; surgical excision of atrial myxoma |
| 2021 (8) | Ovidiu Stiru | 63/male | Chest pain, dyspnea, cardiac mass adjacent to dialysis catheter tip | Right atrial thrombosis, misdiagnosed as myxoma | None | Surgical excision of mass (histopathology confirmed thrombus) |
| 2023 (21) | Mohsen Gholinataj Jelodar | 40/male | Fever, mental confusion | Septic pulmonary embolism, vegetative infected thrombus | Staphylococcus aureus, COVID-19 | Vancomycin and gentamicin, surgical resection of the right atrial mass (histopathology confirmed myxoma) |
| 2024 (13) | Ying-Chi Shen | 55/male | Acute vertigo, unsteady gait, cyanotic fingers, Janeway lesions, Osler nodes, splinter hemorrhages | Posterior circulation embolic stroke, multiple infarctions | None (mimicry of IE by atrial myxoma) | Early surgical excision of left atrial myxoma; dual antiplatelet therapy |
| 2025 (10) | Bhavik Sandip Shah | Teenage/female | Dyspnea, palpitations, a rumbling mid-diastolic murmur | Heart failure | None (RHD, IE, co-existing atrial myxoma) | Mitral valve replacement and complete excision of the atrial myxoma |
Summary of potential differential diagnoses for atrial mass.
IE, infective endocarditis; RHD, rheumatic heart disease.
On rare occasions, IE can result in the formation of a cardiac mass that closely resembles an atrial myxoma (7–10). Atrial myxomas, though benign, are often identified by the distinctive diastolic “tumor plop” sound during auscultation (11, 12). When IE-associated vegetations become sufficiently large and mobile, they may produce a similar murmur, potentially leading to the mistaken diagnosis of a myxoma. This overlap complicates diagnosis, especially when both clinical presentation and auscultation findings align with those typically seen in atrial myxomas.
In the present case, the patient exhibited a large mass in the left atrium, a “tumor plop” sound, and an embolic stroke caused by mass detachment, raising initial concerns for atrial myxoma. However, detailed evaluation through echocardiography and microbiological testing, along with a review of the patient's medical history, revealed the mass to be infective vegetation rather than a myxoma. Although instances of IE mimicking atrial myxoma have been documented (7, 10, 13, 14), such presentations remain uncommon among hemodialysis patients. This case may contribute valuable clinical perspective for diagnosing and managing similar presentations in this high-risk group.
2 Case presentation
2.1 Medical history
A 43-year-old male with a history of more than 3 years of regular hemodialysis was admitted to the nephrology department with a 2-week history of fever on May 13, 2024. Informed consent was obtained, and the study received approval from the hospital's ethics committee. The patient had been diagnosed with stage 5 chronic kidney disease over 3 years prior and had undergone placement of a semi-permanent deep venous catheter for long-term hemodialysis on July 31, 2020. He subsequently received scheduled dialysis. On December 28, 2020, an arteriovenous fistula was surgically created. The hemodialysis catheter was removed on May 17, 2021, and dialysis was continued using the arteriovenous fistula, with treatments performed regularly on Tuesdays, Thursdays, and Saturdays.
The patient began experiencing fever with chills on 2024 May 1, 2 weeks prior to admission, with a maximum temperature of 38°C. He reported a non-productive cough but denied chest tightness, dyspnea, nausea, vomiting, lower limb edema, diarrhea, abdominal pain, rash, or joint pain. He first visited the fever clinic at our hospital, where “epidemic acute upper respiratory viral infection” was suspected. At that time, heart and lung auscultation findings were normal. He was prescribed cephalosporin antibiotics and diclofenac sodium suppositories. However, after a week of ineffective treatment, his symptoms worsened, including increased chest discomfort, dyspnea, and recurrent fever. On May 13, 2024, he visited the emergency department for further care. On the same day, nucleic acid testing returned positive for Type I parainfluenza virus. Complete blood count revealed a white blood cell count of 16.29 × 109/L, with neutrophils at 84.9%, lymphocytes reduced to 5.3%, eosinophils at 0.1%, and an elevated neutrophil count of 13.82 × 109/L. Red blood cell count was low at 3.36 × 1012/L, hemoglobin was reduced to 98 g/L, and platelets measured 114 × 109/L. Despite receiving ertapenem 1 g intravenously once daily and oseltamivir 75 mg orally once daily for 1 week, the patient's fever and chest discomfort persisted. He was therefore admitted for further investigation under the working diagnosis of “fever of unknown origin”.
His medical history included more than 2 years of hypertension, managed with sacubitril/valsartan, and no other notable conditions. On physical examination, the pulse was 136 beats per minute, respiratory rate 22 breaths per minute, blood pressure 106/59 mmHg, and body temperature 38.5°C. Auscultation revealed widespread fine crackles in both lungs, a regular heart rhythm, and a newly detected low-pitched diastolic murmur at the apex. No significant edema was present in the lower limbs. A continuous blowing murmur was heard over the arteriovenous fistula in the left forearm. The initial diagnoses were infectious fever, severe pneumonia (Figure 1A), chronic kidney disease stage 5, secondary hypertension, and hemodialysis status.
Figure 1
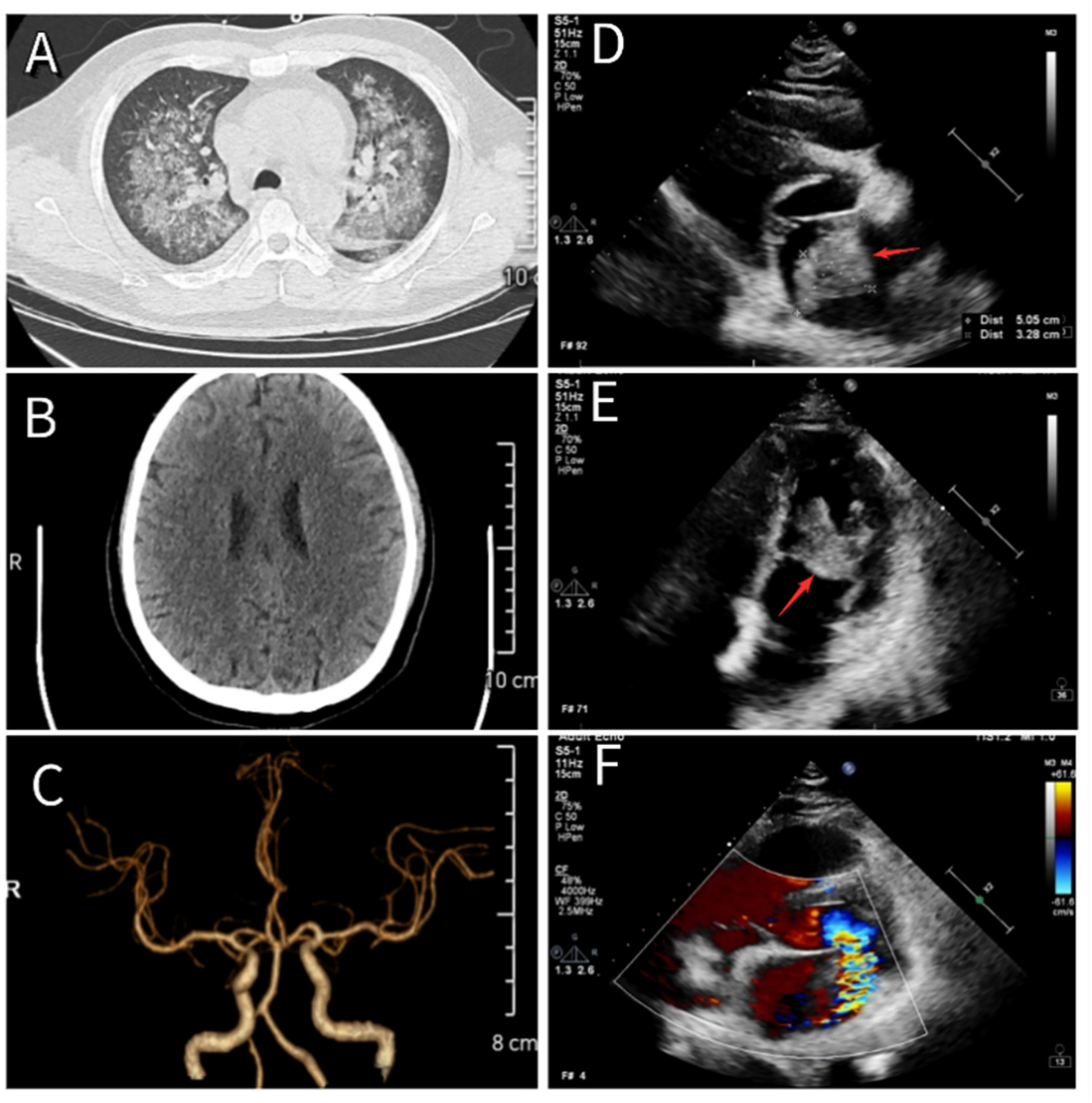
(A) Chest CT showing changes consistent with severe pneumonia. (B) Cranial CT showing no evidence of cerebral infarction. (C) Cranial CTA revealing normal cerebral vasculature. (D,E) Echocardiographic images showing a newly identified atrial mass located at the root of the anterior mitral leaflet, measuring approximately 51 mm × 33 mm × 32 mm (red arrow). (F) The mass moves between the left atrium and ventricle with each cardiac cycle, resulting in severe mitral regurgitation.
2.2 Diagnosis and treatment course
Based on the diagnosis of infectious fever and severe pneumonia, the patient was started on intravenous piperacillin-tazobactam (4.5 g every 12 h) and vancomycin (0.5 g once daily) to provide broad coverage for both Gram-positive and Gram-negative organisms, along with supportive therapy. On the evening of May 24, 2024, he developed acute confusion, delayed responses, and significant chest tightness accompanied by dyspnea. Emergency consultations with neurology and cardiology were initiated. Given the brief onset time, cranial computed tomography angiography (CTA) showed no apparent vascular abnormalities or cerebral embolic lesions (Figures 1B,C). Cardiac auscultation by the cardiologist revealed a characteristic “tumor plop” sound at the apex, leading to immediate bedside transthoracic echocardiography. The scan detected a heterogeneous echogenic mass in the left atrium, approximately 51 mm × 33 mm × 32 mm in size (Figures 1D,E), raising suspicion for either a vegetation associated with IE or an atrial myxoma. Blood cultures collected on May 25 confirmed a bloodstream infection with Rothia dentocariosa. Serial tests showed a rising trend in cardiac troponin, persistent fever, elevated inflammatory markers, and other abnormalities (see Table 2).
Table 2
| Indicators | 5–13 | 5–23 | 5–24 | 5–25 | 5–26 |
|---|---|---|---|---|---|
| Temp (°C) | 38 | 38.5 | 38.2 | 38.1 | 38 |
| BP (mmHg) | 123/70 | 106/59 | 113/67 | 118/71 | 107/62 |
| Alb (g/L) | 34.1 | 24 | 25.1 | 31.9 | 28.5 |
| Hb (g/L) | 98 | 94 | 84 | 86 | 72 |
| hs-CRP (mg/L) | 126 | 106 | 77 | 80 | 119 |
| PCT (ng/ml) | 7.29 | 7.01 | 4.64 | 4.70 | 4.52 |
| TnI (μg/L) | null | 0.145 | 0.085 | 0.085 | 2.250 |
| NT-pro BNP (g/ml) | null | 1,488 | 907 | 879 | 500 |
Summary of key clinical indicators during hospitalization.
5–13 refers to May 13, 2024; subsequent dates follow the same format. Temp, temperature; BP, blood pressure; Alb, albumin; Hb, hemoglobin; hs-CRP, high-sensitivity C-reactive protein; PCT, procalcitonin; TnI, troponin I; NT-pro BNP, N-terminal pro B-type natriuretic peptide.
In light of the patient's worsening condition, a multidisciplinary consultation was held on May 25. Specialists from cardiology, cardiothoracic surgery, infectious diseases, echocardiography, intensive care, and respiratory medicine departments participated. After review, the clinical team reached a consensus diagnosis of IE complicated by embolic stroke due to detachment of the atrial mass. Cardiac valve surgery was advised once the infection became controlled. Following discussions with the patient and his family, transfer arrangements were made. On May 26, he was discharged and referred to the First Affiliated Hospital of Zhejiang University for further treatment.
2.3 Follow-up summaries
After transfer to the First Affiliated Hospital of Zhejiang University, the patient's condition worsened further. He developed increasing chest tightness and dyspnea, accompanied by recurrent confusion and delayed cognitive response. Repeated cranial computed tomography (CT) scans during hospitalization revealed a low-density lesion in the left frontal lobe (Figure 2A), consistent with cerebral infarction. Due to the significant risk of further cerebral embolization from detachment of the large atrial mass and ongoing damage to the mitral valve, cardiac valve surgery was deemed absolutely necessary (6). Taking into account the patient's young age and moderate financial situation, mitral valve mechanical replacement, offering longer durability, was chosen to avoid repeated surgeries. The procedure was performed on June 6, 2024. A postoperative chest CT conducted on June 7 confirmed the prosthetic valve was correctly positioned (Figure 2B). The patient was treated postoperatively with intravenous piperacillin-sulbactam (4.5 g once daily) and daptomycin (0.5 g once daily).
Figure 2
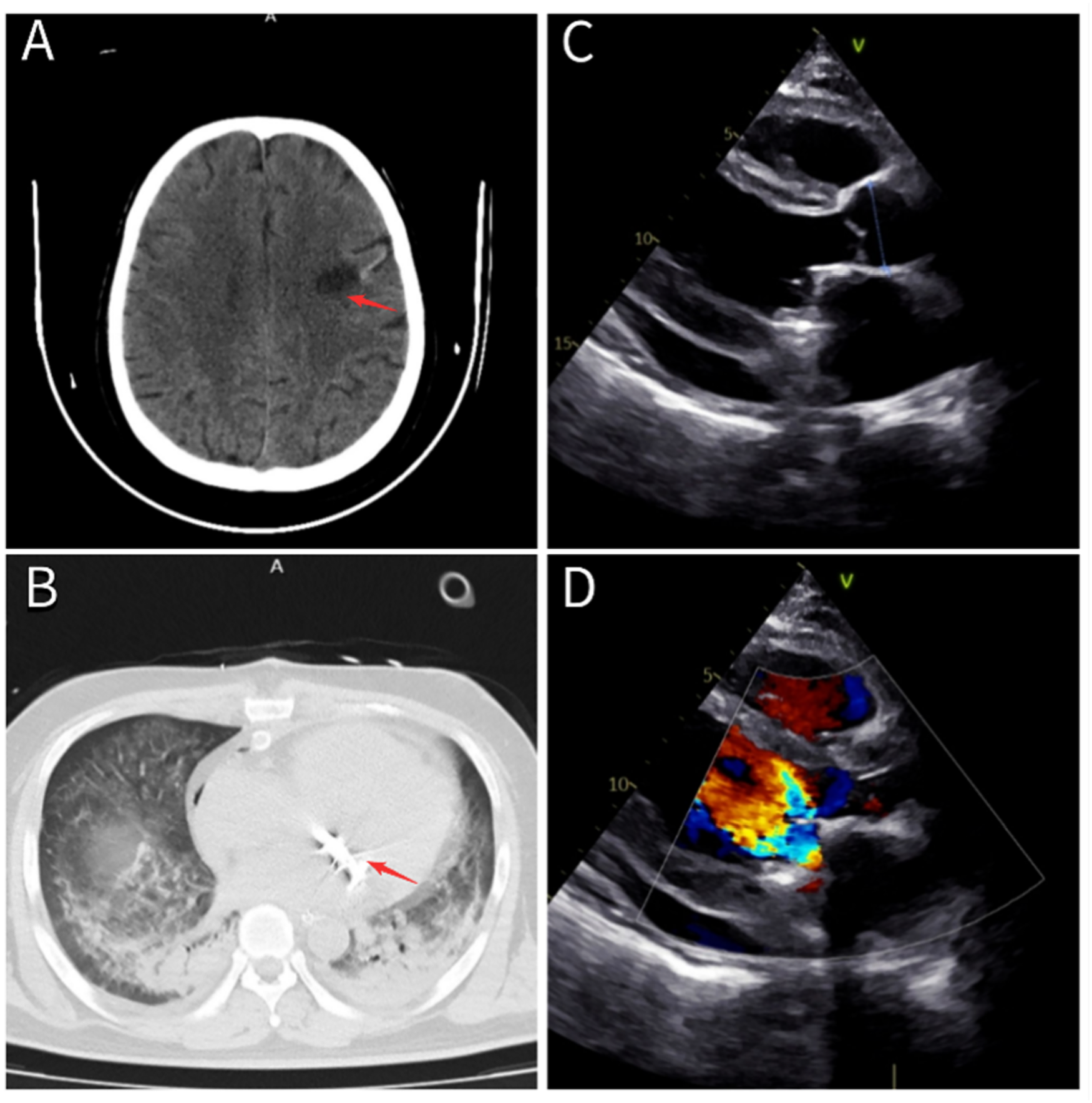
(A) Cranial CT revealing an infarction in the left frontal lobe (red arrow). (B) Chest CT confirming correct placement of the mitral mechanical valve (red arrow). (C,D) Echocardiographic images demonstrating normal function of the mitral mechanical valve with no residual intracardiac masses.
After being discharged on 2024 June 19, the patient resumed maintenance hemodialysis at our hospital's dialysis center. Given the patient's severe pneumonia and sputum culture positive for Klebsiella pneumoniae, the same antimicrobial regimen, piperacillin-sulbactam 4.5 g IV daily and daptomycin 0.5 g IV daily, was continued to complete a 4-week course. On follow-up transthoracic echocardiography performed on July 9, the mitral prosthetic valve demonstrated normal function, with no residual intracardiac masses (Figures 2C,D).
3 Discussion
Infective endocarditis (IE) remains a serious and life-threatening condition in patients with end-stage renal disease undergoing maintenance hemodialysis (1). This group faces increased vulnerability due to factors such as prolonged catheter use, repeated vascular access procedures, and weakened immune responses. The incidence of IE is increased in dialysis patients, yet its timely identification is often hindered by nonspecific symptoms like fever, fatigue, and new-onset heart murmurs (15). The clinical picture is often clouded further by common comorbidities in this population, including anemia, malnutrition, and calcium-phosphorus imbalance (14). In the present case, the intracardiac mass appeared similar to an atrial myxoma on echocardiography and was accompanied by a “tumor plop” sound, blurring the distinction between infective vegetation and a primary cardiac tumor.
The “tumor plop” is a rare auscultatory feature in IE (7, 11), more commonly associated with a large left atrial myxoma. In contrast, cardiac tumors' plops are typically persistent, not acute-onset, and unassociated with fever or elevated inflammatory markers. Additionally, definitive diagnosis of cardiac tumors requires histopathology. In this case, the new-onset “tumor plop” detected between May 1 and 24, combined with the patient's recurrent fever during this period and long-standing hemodialysis history, heightened the consulting cardiologist's suspicion of IE. This clinical constellation prompted a strong recommendation for immediate bedside cardiac ultrasound, with particular attention to the source of the “tumor plop”. The ultrasound revealed a large left atrial mass, providing a critical diagnostic clue for confirming IE. While the excised left atrial mass was not subjected to histological examination, this represents a limitation of this case report. However, based on the patient's clinical background, history, and diagnostic results, IE was considered the more likely cause of the mass.
Several factors likely contributed to the development of the large atrial mass in this patient. Long-term hemodialysis and repeated vascular access procedures, such as arteriovenous fistula punctures, increased the risk of bloodstream infections leading to IE (16). In addition, the previous extended use of a semi-permanent catheter, maintained due to the patient's reluctance to undergo fistula punctures because of pain, further increased susceptibility to infection (17). Compounding this, recurrent febrile episodes following an acute upper respiratory tract infection were not promptly or adequately managed with appropriate antibiotics. The delay in treatment and diagnosis likely played a major role in the progression of IE and the formation of the atrial mass (18).
Our clinical experience with this case provides several key observations. First, regardless of the specific patient population or pathogen, when vegetation in IE becomes large enough and moves between the atrium and ventricle with positional changes, it can produce a “tumor plop” sound similar to that of an atrial myxoma. Second, while the causes of an atrial mass may include IE, thrombus formation, primary cardiac tumors (such as atrial myxoma), or even a combination of these (see Table 1), a differential diagnosis is still possible by assessing infection markers like blood cultures, echocardiographic features, detailed medical history, and physical examination. Third, the presence of a “tumor plop” sound in patients with IE on hemodialysis may suggest a delay in diagnosis and treatment. This is because a mass of sufficient size to produce such a sound typically forms only after prolonged infection.
As such, persistent intermittent fever in hemodialysis patients should not be overlooked or simply attributed to acute upper respiratory tract infection. In many cases, the respiratory infection may act as a trigger or initial clinical indicator rather than the underlying cause. Early and comprehensive clinical evaluation (19), including careful history-taking, physical examination, and echocardiography, might have led to earlier detection of the cardiac mass and potentially prevented the progression to IE and its complications. When severe complications arise in IE among hemodialysis patients, such as heart failure or cerebral embolism as seen in this case, coordinated management involving multiple specialties, timely and targeted antibiotic treatment, and appropriately scheduled surgical intervention are critical for effective care.
4 Conclusion
Regardless of the patient group or pathogen involved, when vegetation in infective endocarditis grows large enough and oscillates between the atrium and ventricle, it can produce a “tumor plop” sound that mimics that of an atrial myxoma. At this stage, infective endocarditis and atrial myxoma can still be distinguished using infection markers such as blood cultures, echocardiographic findings, a thorough medical history, physical examination, and histopathology. In this case, earlier diagnosis and prompt intervention might have prevented the development of the “tumor plop” sound and the resulting complications, including embolic stroke and heart failure. When serious complications do occur in hemodialysis patients with infective endocarditis, multidisciplinary management, timely and effective antibiotic treatment, and well-timed surgical intervention are essential to reducing further embolic events and improving overall prognosis.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jinhua Municipal Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FH: Conceptualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Writing – original draft. XZ: Writing – review & editing. SL: Writing – review & editing. LZ: Writing – review & editing. JY: Writing – review & editing. FZ: Writing – review & editing. YZ: Writing – review & editing. XX: Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The specific funding support is from Jinhua Science and Technology Bureau, with the project number 2023-03-068.
Acknowledgments
We would like to express our gratitude to all contributing authors for their valuable assistance in data collection, analysis, and manuscript preparation. Their support was instrumental in the completion of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ding U Ooi L Wu HHL Chinnadurai R . Infective endocarditis in patients receiving hemodialysis: a current review. Kidney Dis. (2024) 10(6):519–30. 10.1159/000540513
2.
Al-Chalabi S Tay T Chinnadurai R Kalra PA . Risk factors for infective endocarditis in patients receiving hemodialysis: a propensity score matched cohort study. Clin Nephrol. (2023) 100(2):51–9. 10.5414/CN111117
3.
Bentata Y Haloui I Haddiya I Benzirar A El Mahi O Ismailli N et al Infective endocarditis in hemodialysis patients: a 10-year observational single-center study. J Vasc Access. (2022) 23(1):149–53. 10.1177/1129729820970783
4.
Pericàs JM Llopis J Jiménez-Expósito MJ Kourany WM Almirante B Carosi G et al Infective endocarditis in patients on chronic hemodialysis. J Am Coll Cardiol. (2021) 77(13):1629–40. 10.1016/j.jacc.2021.02.014
5.
Baddour LM Wilson WR Bayer AS Fowler VG Jr Tleyjeh IM Rybak MJ et al Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. (2015) 132(15):1435–86. 10.1161/CIR.0000000000000296
6.
Delgado V Ajmone Marsan N de Waha S Bonaros N Brida M Burri H et al 2023 ESC guidelines for the management of endocarditis. Eur Heart J. (2023) 44(39):3948–4042. 10.1093/eurheartj/ehad193
7.
Fitzgerald GP Coughlan JJ Satti Z Arnous S . Atrial myxoma presenting as infective endocarditis. BMJ Case Rep. (2018) 2018:bcr2017223656. 10.1136/bcr-2017-223656
8.
Stiru O Dragulescu R Geana RC Chibulcutean A Raducu L Tulin A et al Catheter-related giant right atrial thrombosis mimicking a myxoma: a case report. Exp Ther Med. (2021) 21(6):603. 10.3892/etm.2021.10035
9.
Arvind B Gupta SK Arava S . All that attaches to atrial septum is not myxoma: deception is everywhere!. Cardiol Young. (2021) 31(10):1680–81. 10.1017/S104795112100250X
10.
Shah BS Lanjewar IC Sawant G Aironi BD . Triple trouble: rheumatic heart disease, infective endocarditis, atrial myxoma. BMJ Case Rep. (2025) 18(2):1–7. 10.1136/bcr-2024-263506
11.
Darbari A Singh D . Tumor plop in a febrile child: unusual presentation of tricuspid valve endocarditis. J Cardiovasc Echogr. (2014) 24(3):86–8. 10.4103/2211-4122.143979
12.
Jagadeesh HV Rangan K Rangashamaiah S Ramegowda KS Nanjappa MC . Clinicopathological correlation of cardiac myxoma—insights from a large volume tertiary cardiac center in south India. J Indian Acad Echocardiogr Cardiovasc Imaging. (2023) 7(1):8–15. 10.4103/jiae.ji-ae_42_22
13.
Shen YC Chang KC Su JJ . Cutaneous manifestations of infective endocarditis as presenting signs of left atrial myxoma in a patient with acute ischemic stroke: a case report. Medicine. (2024) 103(36):e39088. 10.1097/MD.0000000000039088
14.
Xu J Yang Q Li J Zheng X . The left atrial bacterial vegetative mass due to Corynebacterium striatum as a presentation of myxoma: a case report. BMC Infect Dis. (2017) 17(1):368. 10.1186/s12879-017-2468-8
15.
Issa R Chaaban N Salahie A Honnekeri B Parizher G Xu B . Infective endocarditis in patients with end-stage renal disease on dialysis: epidemiology, risk factors, diagnostic challenges, and management approaches. Healthcare. (2024) 12(16):1631. 10.3390/healthcare12161631
16.
Kamde SP Anjankar A . Pathogenesis, diagnosis, antimicrobial therapy, and management of infective endocarditis, and its complications. Cureus. (2022) 14(9):e29182. 10.7759/cureus.29182
17.
Marzouk M Mghaieth F Baffoun N . Infective endocarditis secondary to a hemodialysis catheter revealed by subarachnoid hemorrhage: case report. Tunisie Med. (2024) 102(8):496–99. 10.62438/tunismed.v102i8.5022
18.
Sadeghi M Behdad S Shahsanaei F . Infective endocarditis and its short and long-term prognosis in hemodialysis patients: a systematic review and meta-analysis. Curr Probl Cardiol. (2021) 46(3):100680. 10.1016/j.cpcardiol.2020.100680
19.
Myint N . A case of native mitral valve infective endocarditis in a chronic dialysis patient: the importance of vigilance. Cureus. (2024) 16(9):e69924. 10.7759/cureus.69924
20.
Salani TG Borges CM Urbini CS Schincariol P Quadros KR Ribeiro-Alves MA et al Patient in chronic hemodialysis with right atrial mass: thrombus, fungal endocarditis or atrial myxoma? J Bras Nefrol. (2016) 38(4):462–65. 10.1590/2175-8239-JBN-2015-0151
21.
Gholinataj Jelodar M Mirzaei S Dehghan Chenari H Tabkhi M . Diagnosis of the right atrial myxoma after treatment of COVID-19: a case report. Clin Case Rep. (2023) 11(5):e7216. 10.1002/ccr3.7216
Summary
Keywords
infective endocarditis, hemodialysis, tumor plop, atrial myxoma, embolic stroke
Citation
Huang F, Huang J, Zhang X, Li S, Zhu L, Ying J, Zhou F, Zhang Y and Xu X (2025) Case Report: Reflections on “Tumor Plop” in a febrile hemodialysis patient who had atypical presentation of atrial myxoma as infective endocarditis. Front. Cardiovasc. Med. 12:1645356. doi: 10.3389/fcvm.2025.1645356
Received
11 June 2025
Accepted
12 September 2025
Published
29 September 2025
Volume
12 - 2025
Edited by
Prem Prakash Kushwaha, Case Western Reserve University, United States
Reviewed by
Lovlesh Thakur, Cleveland State University, United States
Yahya Shabi, King Khalid University, Saudi Arabia
Updates
Copyright
© 2025 Huang, Huang, Zhang, Li, Zhu, Ying, Zhou, Zhang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Xuchun Xu xxc619700@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.