Abstract
Background:
Anthracyclines are essential components of chemotherapeutic regimens for a broad spectrum of malignancies, yet their utility is constrained by cumulative, dose-dependent cardiotoxicity, often culminating in non-ischemic cardiomyopathy and heart failure. The pathogenesis involves oxidative stress, mitochondrial dysfunction, and topoisomerase IIβ–mediated DNA damage in cardiomyocytes. While ACE inhibitors and angiotensin receptor blockers (ARBs) have demonstrated modest cardioprotective effects, the efficacy of newer heart failure therapies remains underexplored. Sodium-glucose co-transporter-2 (SGLT2) inhibitors, endorsed as Class I therapy for heart failure with reduced ejection fraction (HFrEF) per 2022 AHA/ACC/HFSA guidelines, have shown robust cardioprotective effects in large cardiovascular outcomes trials. However, their potential to prevent or attenuate anthracycline-induced cardiotoxicity has not been systematically evaluated. This study aimed to assess preclinical and clinical evidence supporting their use in anthracycline-exposed populations.
Methods:
A systematic review was conducted by PRISMA 2020 guidelines. Comprehensive searches of major medical databases and clinical trial registries were performed through March 2025. Eligible studies investigated SGLT2 inhibitors, β-blockers, or ACE inhibitors in adult patients receiving anthracycline-based chemotherapy or in animal models replicating this exposure. Primary outcomes included changes in LVEF, GLS, and incidence of heart failure. Studies involving pre-existing heart failure or non-anthracycline-related cardiotoxicity were excluded.
Results:
Preclinical studies (n = 4) consistently demonstrated that SGLT2 inhibitors mitigated cardiomyocyte injury, fibrosis, and oxidative stress, preserving cardiac function in anthracycline-exposed models. In one study, LVEF was significantly higher in animals treated with SGLT2 inhibitors (61.3% ± 11%) vs. controls (49.2% ± 8%, p = 0.007). Additional studies corroborated reduced histopathological damage and improved myocardial performance. No clinical trials to date have specifically assessed SGLT2 inhibitors in oncology populations. Nevertheless, major cardiovascular trials (e.g., EMPA-REG OUTCOME, DECLARE-TIMI 58) have demonstrated substantial reductions in heart failure events among non-cancer cohorts. In contrast, ACE inhibitors and β-blockers have shown variable efficacy during chemotherapy, with inconsistent findings across studies.
Conclusions:
SGLT2 inhibitors exhibit consistent cardioprotective effects in preclinical models of anthracycline cardiotoxicity and possess well-established efficacy in broader cardiovascular populations. These findings underscore the critical need for prospective trials evaluating their safety and therapeutic potential in cardio-oncology, with implications for reshaping current preventive strategies.
Systematic Review Registration:
PROSPERO [1056661].
Introduction
Anthracyclines are a class of chemotherapeutic agents widely used to treat various cancers, including hematologic malignancies and solid tumors. These drugs have been shown to be highly effective due to their ability to interfere with DNA replication and transcription, making them particularly useful against rapidly proliferating cancer cells. However, despite their efficacy, anthracyclines have been associated with dose-dependent cardiotoxicity, which can lead to long-term complications such as congestive heart failure (1). The clinical challenge lies in balancing their potent anticancer effects with the risk of adverse cardiovascular outcomes.
The cardiotoxicity of anthracyclines is believed to arise from multiple mechanisms, including the inhibition of topoisomerase II enzymes, oxidative stress, and mitochondrial dysfunction. While these drugs target topoisomerase IIα (TOP2A) in tumor cells, they also affect topoisomerase IIβ (TOP2B) in cardiac tissue, leading to DNA damage and impaired cellular function (2). Additionally, anthracyclines have been shown to promote the formation of reactive oxygen species, subsequently contributing to oxidative damage and cardiomyocyte apoptosis (3). These effects may result in both acute and chronic cardiac complications.
Despite their limitations, anthracyclines remain a cornerstone of cancer management due to their broad-spectrum activity against various cancers. However, the challenge moving forward is in refining treatment strategies that maximize therapeutic benefit while minimizing long-term toxicity. Given the persistent challenge of anthracycline-induced cardiotoxicity, considerable research has focused on identifying effective prophylactic strategies to safeguard cardiovascular health in cancer patients. Dexrazoxane is an FDA-approved agent used for cardioprotection in high-risk patients receiving anthracyclines, but recent attention has shifted toward heart failure medications such as ACE inhibitors, β-blockers, and SGLT2 inhibitors as promising alternatives or adjuncts (4). Historically, certain pharmacological agents have shown promise in this regard. Among these pharmacological agents are angiotensin-converting enzyme (ACE) inhibitors and β-blockers.
ACE inhibitors and β-blockers together form the backbone of chronic heart failure treatment, as both classes of drugs have been shown to reduce morbidity and mortality (5). β-blockers work by blocking β-adrenergic receptors, reducing the effects of adrenaline associated hormones on the heart. ACE inhibitors help manage heart failure and hypertension by blocking the conversion of angiotensin I to angiotensin II, a vasoconstrictor. In patients undergoing chemotherapy, ACE inhibitors and β-blockers have been studied for their ability to reduce left ventricular ejection fraction (LVEF) decline, a critical clinical determinant in the diagnosis of heart failure. Randomized controlled trials such as the Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA) (6) and the preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies (OVERCOME) (7) have demonstrated that these medications may significantly reduce the incidence of heart failure events and LVEF decline compared to placebo.
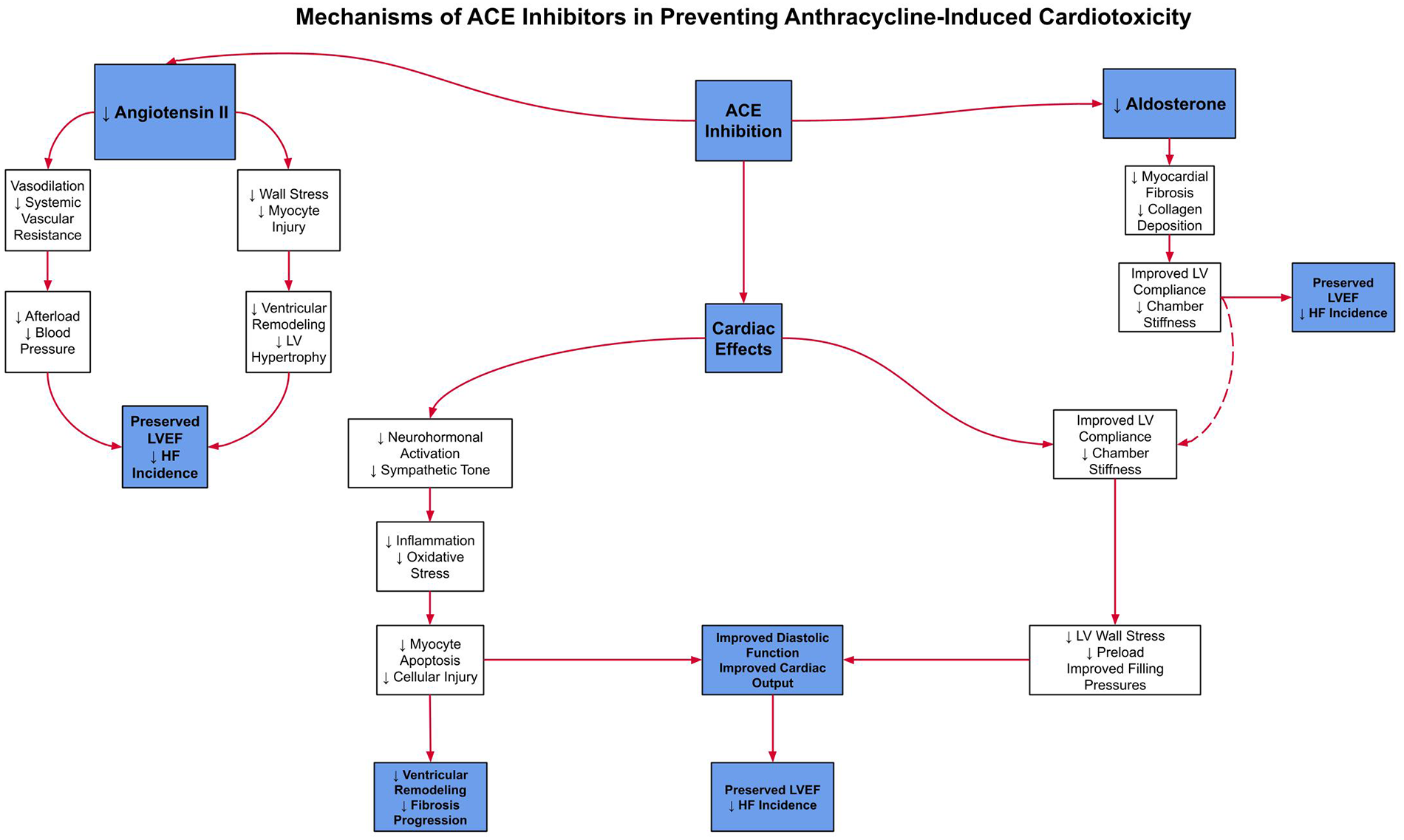
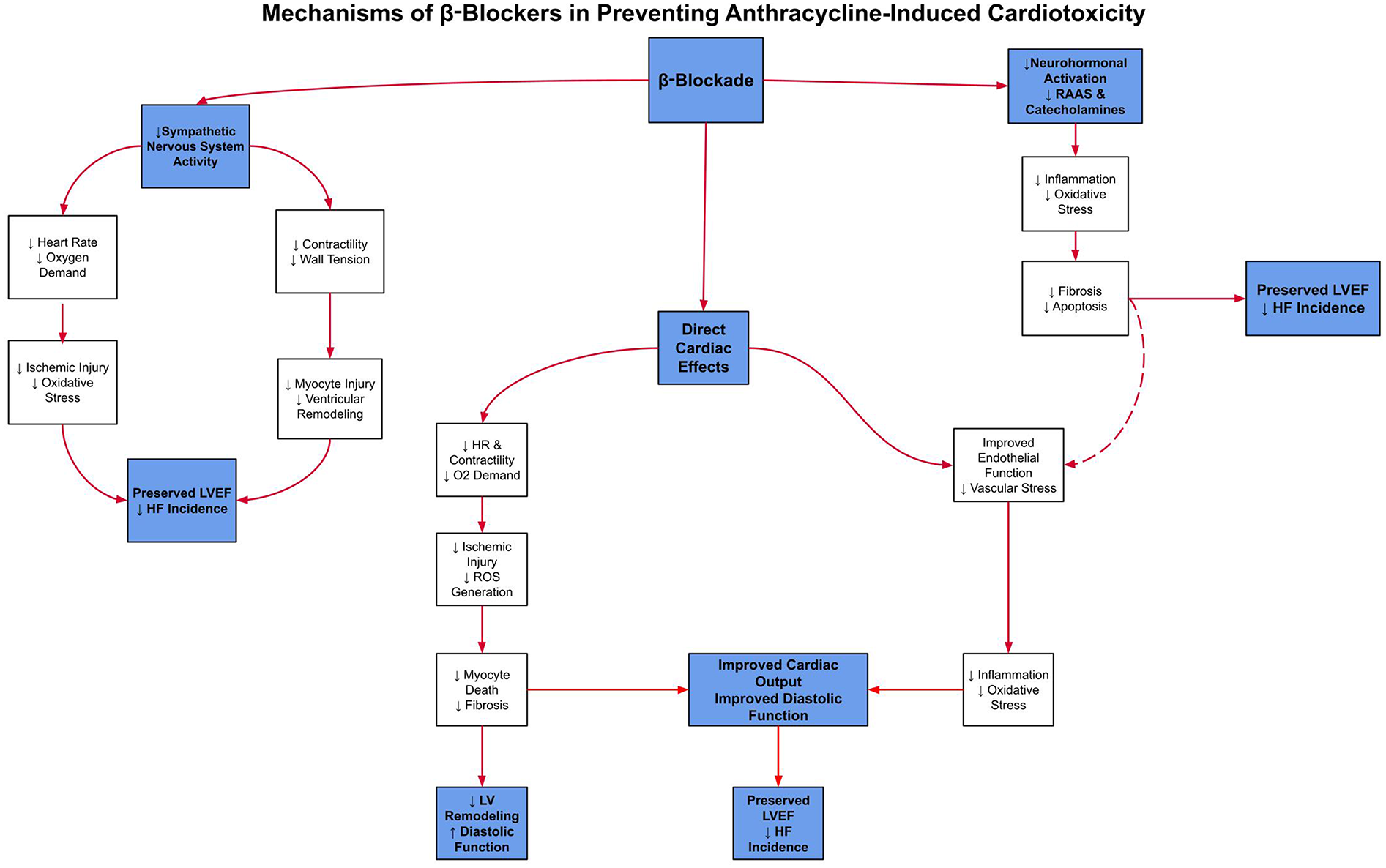
ACE inhibitors and β-blockers constitute an essential component of guideline-directed medical therapy (GDMT), the first-line therapeutic strategy for heart failure management. This regimen has demonstrated robust efficacy in attenuating chemotherapy-induced cardiotoxicity by preserving left ventricular systolic function (8). To clarify these mechanisms,Figure 1illustrates the key pathways by which β-blockers exert cardioprotective effects, andFigure 2details the parallel mechanisms for ACE inhibitors, specifically in the context of anthracycline-induced cardiotoxicity.
Figure 1
This schematic shows how β-blockers reduce sympathetic activation, heart rate, and myocardial oxygen demand to limit ischemic injury and oxidative stress. Together, these pathways help preserve LVEF and reduce the incidence of heart failure in patients treated with anthracyclines.
Figure 2
This figure illustrates the mechanisms by which ACE inhibitors lower angiotensin II and aldosterone levels, leading to vasodilation, decreased afterload, and reduced wall stress. These effects help prevent remodeling and fibrosis, ultimately preserving LVEF and minimizing heart failure risk.
Despite their designation as the therapeutic gold standard, concerns remain regarding their utility in the primary prevention of heart failure, as their role has been predominantly centered on the management of established cardiac disease. It is in this context that Sodium-glucose cotransporter 2 (SGLT2) inhibitors have emerged and shown comparable cardioprotective effects, offering a promising alternative or adjunct strategy in the context of chemotherapy-induced cardiotoxicity.
SGLT2 inhibitors are primarily utilized in the management of type 2 diabetes mellitus. Their mechanism of action involves inhibition of SGLT2 in the proximal renal tubules, thereby decreasing renal glucose reabsorption and enhancing urinary glucose excretion, ultimately leading to improved glycemic control. Beyond their glucose-lowering effects, SGLT2 inhibitors have recently shown significant cardiovascular and renal benefits. They have been shown to reduce heart failure hospitalizations, cardiovascular mortality, and the progression of renal disease. Clinical trials, including EMPA-REG OUTCOME trial (EMPA-REG OUTCOME), Canagliflozin Cardiovascular Assessment Study (CANVAS), and Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) (9, 56), have demonstrated that these agents provide cardiovascular protection in both diabetic and non-diabetic populations, with particular benefit in patients with heart failure. The cardioprotective mechanisms of SGLT2 inhibitors are diverse, including blood pressure reduction, natriuresis, and decreased oxidative stress. In models of anthracycline-induced cardiomyopathy, they have also been shown to mitigate inflammation and improve myocardial metabolism (10). Figure 3 summarizes these mechanisms, highlighting how SGLT2 inhibition may address gaps left by ACE inhibitors and β-blockers.
Figure 3
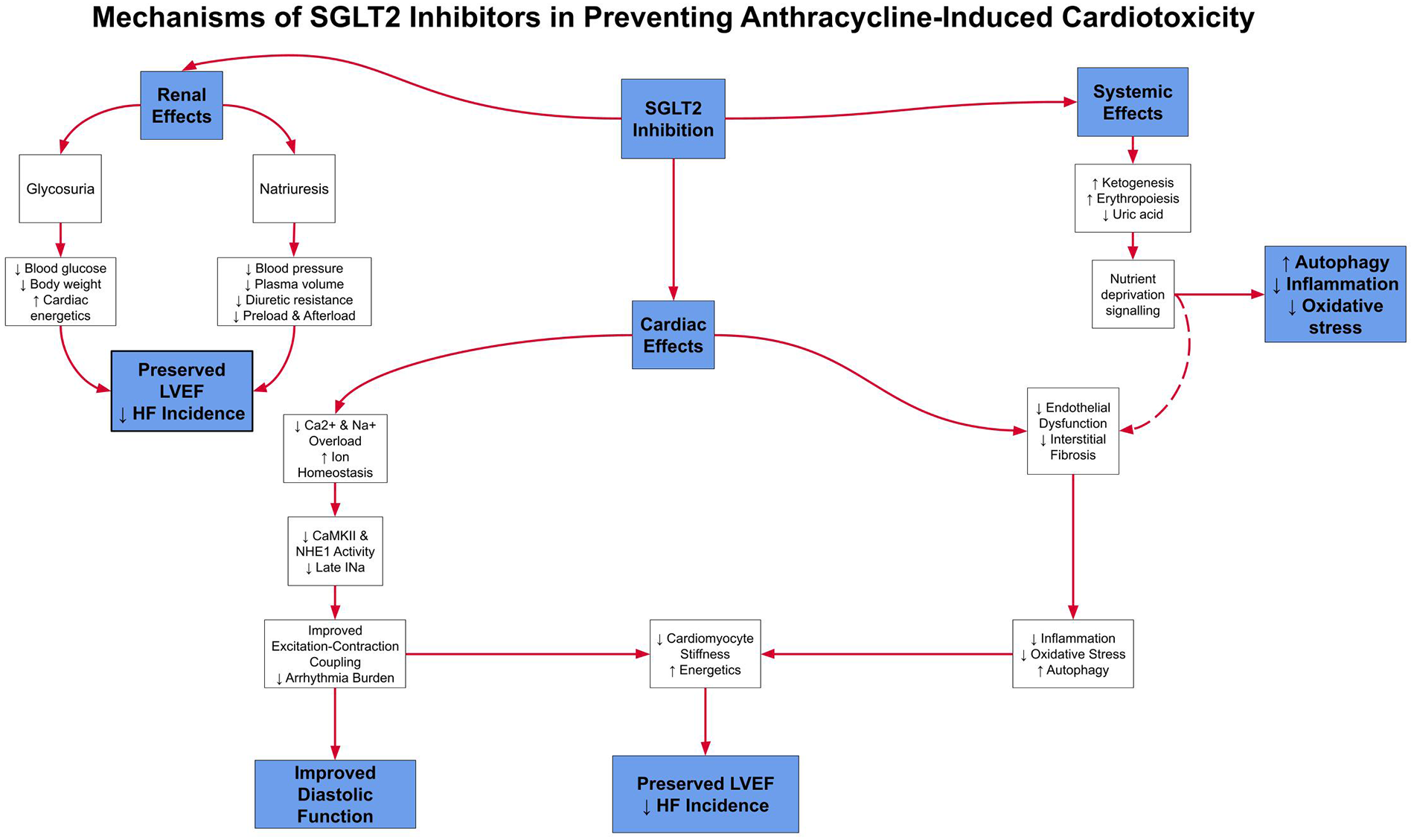
This schematic outlines the multiple pathways through which SGLT2 inhibitors exert cardioprotective effects, including natriuresis, improved myocardial energetics, and decreased oxidative stress. These complementary effects may provide added protection against anthracycline-induced cardiomyopathy.
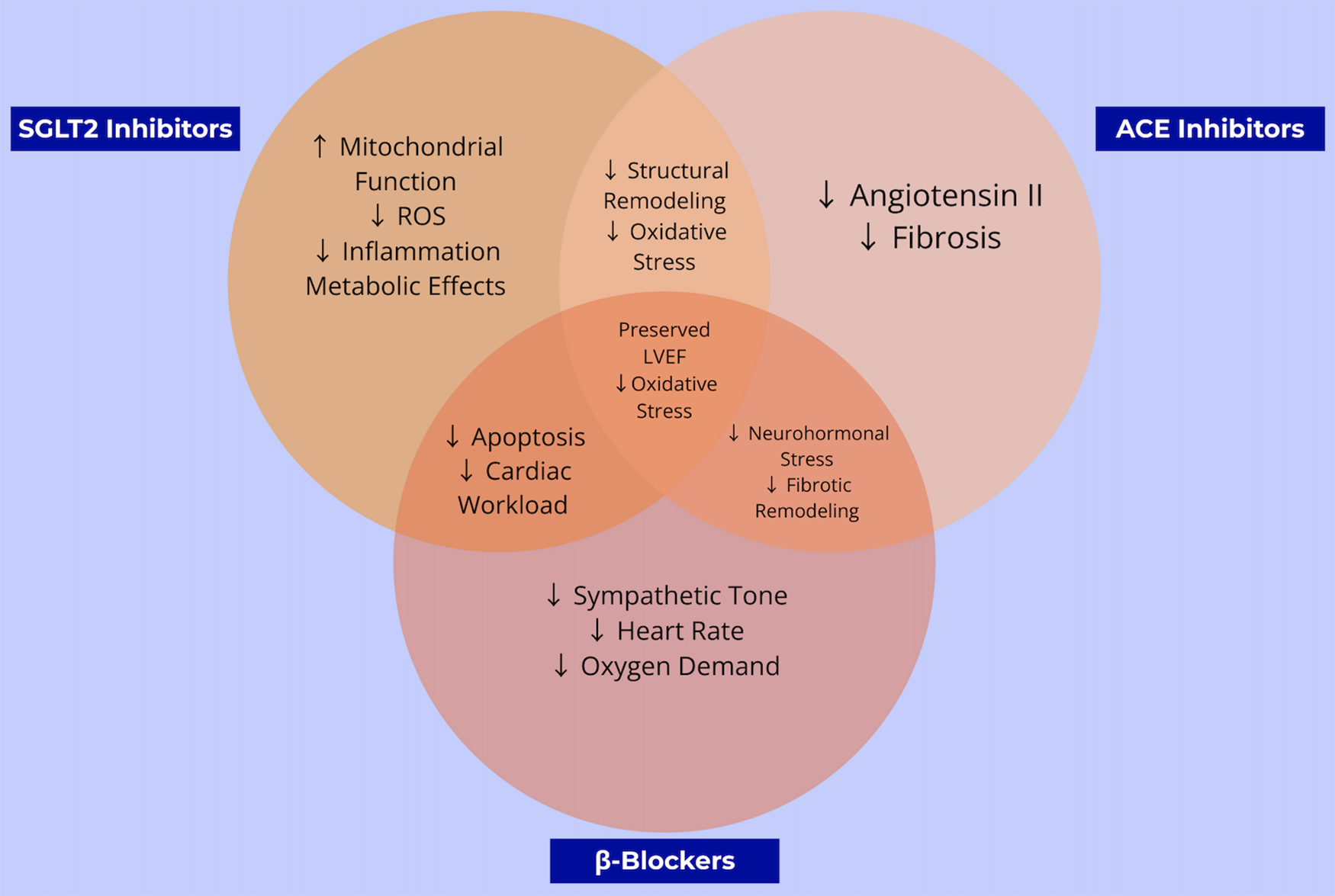
Taken together, these complementary and overlapping pathways are illustrated in Figure 4, which compares and contrasts the molecular actions of SGLT2 inhibitors, ACE inhibitors, and β-blockers in the context of anthracycline-induced cardiotoxicity.
Figure 4
This venn diagram compares and contrasts the overlapping and unique cardioprotective mechanisms of β-blockers, ACE inhibitors, and SGLT2 inhibitors. The shared and distinct pathways highlight opportunities for combined or alternative strategies to prevent chemotherapy-induced heart failure.
These complementary and overlapping mechanisms are illustrated in Figure 1, which compares the molecular actions of SGLT2 inhibitors, ACE inhibitors, and β-blockers in the context of anthracycline-induced cardiotoxicity. While all three classes share the common goal of preserving cardiac function, SGLT2 inhibitors uniquely address mitochondrial energetics and systemic inflammation, whereas ACE inhibitors and β-blockers act primarily via hemodynamic and neurohormonal pathways. This divergence in mechanistic targets may explain the broader cardioprotective effects observed with SGLT2 inhibitors in both preclinical and clinical models, particularly in patients without established heart failure.
Despite promising evidence, there remains a critical lack of head-to-head trials directly comparing SGLT2 inhibitors with standard cardioprotective agents for patients receiving anthracycline chemotherapy. Current cardio-oncology guidelines offer limited guidance for patients without overt heart failure or diabetes, leaving clinicians with few evidence-based options to protect cardiovascular health in this unique population. By addressing these gaps, this synthesis highlights the timely need for rigorous evaluation of SGLT2 inhibitors as a novel preventive strategy in non-diabetic, anthracycline-exposed cancer patients.
The objective of this study is to determine whether SGLT2 inhibitors can provide superior or complementary cardiovascular protection when compared to ACE inhibitors and β-blockers in preventing cardiomyopathy in patients starting anthracycline chemotherapy by evaluating the effects of SGLT2 inhibitors on the incidence of HF and changes in LVEF in this high-risk population.
To date, no head-to-head clinical trials have compared SGLT2 inhibitors with standard cardioprotective agents in patients undergoing anthracycline-based chemotherapy. Moreover, existing guidelines provide limited direction for cardio-oncology patients without overt heart failure or diabetes. This systematic review addresses a critical evidence gap by synthesizing the current preclinical and clinical landscape and evaluating the potential role of SGLT2 inhibitors in non-diabetic, anthracycline-exposed oncology populations. As survival in cancer patients improves, timely identification of novel preventive strategies is essential to mitigate long-term cardiovascular morbidity.
Methods
This study was designed as a systematic review of preclinical and clinical studies investigating the cardioprotective effects of SGLT2 inhibitors, ACE inhibitors, and β-blockers in patients receiving anthracycline-based chemotherapy. The primary objective was to compare the efficacy of these pharmacologic agents in preventing heart failure and preserving LVEF in patients without pre-existing heart failure (baseline LVEF >50%).
Search strategy
A comprehensive literature search was conducted using databases including PubMed, Embase, Scopus, and the Cochrane Library. To ensure a more comprehensive review, additional searches were conducted using clinical trial registries, including ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), and the EU Clinical Trials Register, as well as sources of unpublished research such as conference proceedings from the American Heart Association and the American Society of Clinical Oncology, dissertations, and institutional repositories. Keywords included combinations of terms such as “anthracycline”, “doxorubicin”, “cardiotoxicity”, “heart failure”, “SGLT2 inhibitors”, “empagliflozin”, “dapagliflozin”, “β-blockers”, “ACE inhibitors,” “LVEF”, and “cardioprotection”. The search was limited to articles published in English up to March 2025. These searches were conducted on March 3rd–6th and 10th–12th, 2025.
Study selection criteria
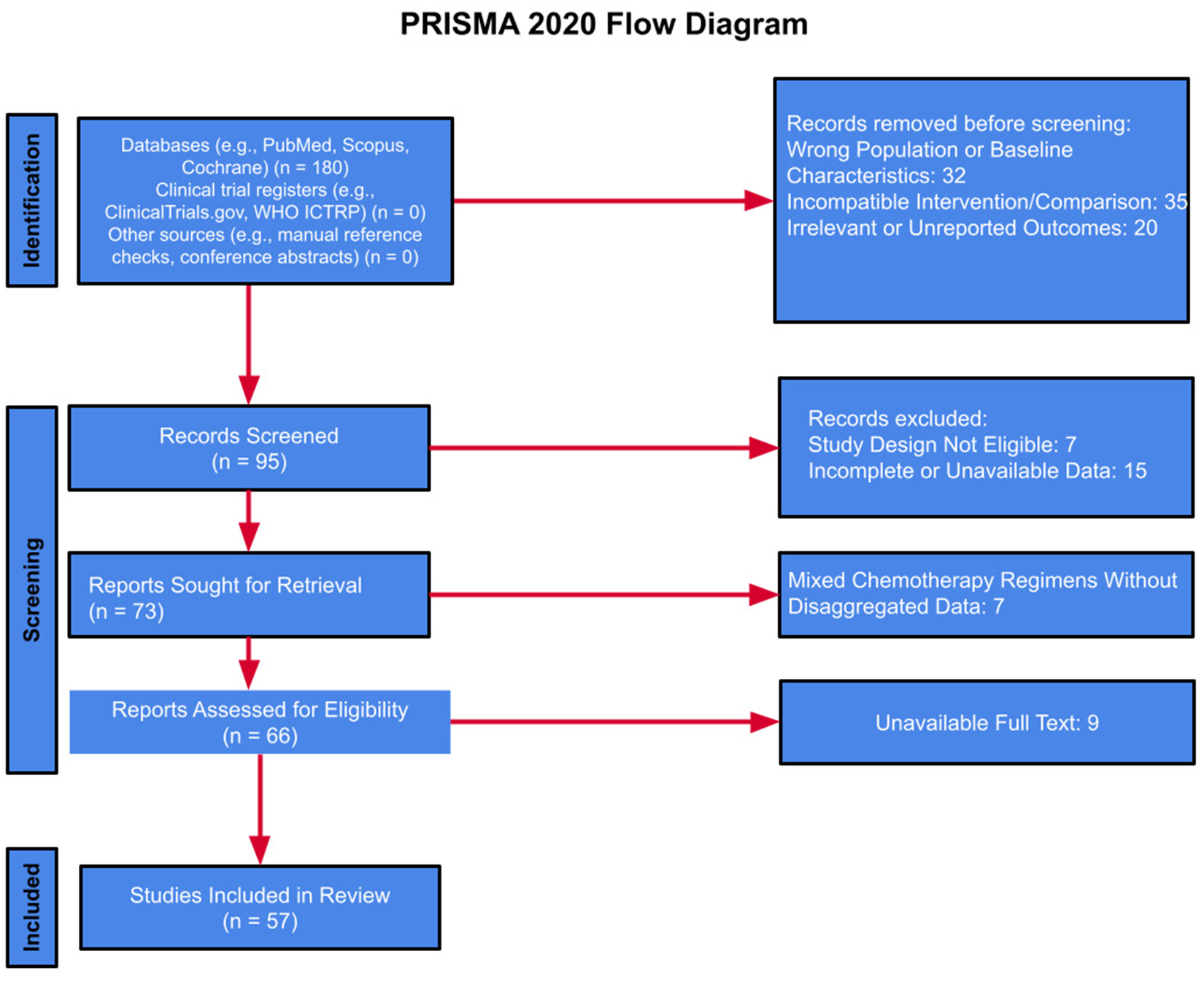
Data were extracted independently by two authors, followed by full-text review to confirm eligibility (Figure 5). Discrepancies in study inclusion were initially addressed through structured discussion between the reviewers. If consensus could not be reached, a third senior reviewer was consulted to adjudicate the final decision, in accordance with PRISMA 2020 guidelines.
Figure 5
PRISMA study selection summary: summary of the study selection process, including the number of records identified, screened, excluded, and included in the final review, based on PRISMA guidelines.
Eligible studies included randomized controlled trials, cohort studies, observational studies, and preclinical investigations evaluating cardiac outcomes in the context of anthracycline therapy. Meta-analyses and systematic reviews were also included to synthesize broader evidence. Studies were included if they reported outcomes related to heart failure incidence, changes in LVEF, global longitudinal strain (GLS), or cardiac biomarkers in response to SGLT2 inhibitors, ACE inhibitors, or β-blockers. Trials involving patients with pre-existing heart failure or reduced baseline LVEF (<50%) were excluded unless deemed relevant for comparative context, such as those examining populations with similar diagnoses to the target group or, alternatively, populations without any diagnoses, allowing for meaningful comparison. Additionally, studies not originally published in English, editorials, and those lacking quantitative results were excluded. Data extracted from eligible studies included author, year, study design, sample size, population characteristics, type and dosage of anthracycline administered, cardioprotective intervention used, follow-up duration, and specific cardiac outcomes, including changes in LVEF, incidence of symptomatic heart failure (as defined by the original study), GLS, and cardiac biomarkers such as NT-proBNP and troponin levels.
Bias assessment
Risk of bias was assessed using the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool, which is specifically designed to evaluate bias in studies comparing two or more health interventions. This tool examines seven domains: confounding, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement, and selection of reported results (11). Each study was independently evaluated by two authors; any disagreements in domain ratings or overall bias assessments were resolved through discussion. If consensus could not be reached, a third reviewer with expertise in systematic review methodology adjudicated the final decision.
Each domain was rated as having low, moderate, or serious risk of bias, or categorized as having insufficient information. Most randomized controlled trials were judged to have a low risk of bias using the Cochrane ROB 2.0 tool, except for seven trials—Bosch et al. (7), Guglin et al. (12), Gulati et al. (6), Livi et al. (13), SOLVD Investigators (1991), SOLVD Investigators (14), and Rode et al. (15)—which were rated as having some concerns due to unclear allocation concealment and/or incomplete reporting of chemotherapy type and/or dosage.
Review conduct and standards
This systematic review was conducted in accordance with the PRISMA 2020 guidelines (16), which provide a 27-item checklist and flow diagram to enhance the transparency and completeness of reporting in systematic reviews. The review protocol was prospectively registered with PROSPERO (1056661) to ensure methodological transparency and to minimize the risk of bias.
Data synthesis and statistical interpretation
Due to significant heterogeneity across included studies, ranging from differences in study design, patient populations, intervention dosages, and outcome measurement methods, a formal meta-analysis was not feasible. Therefore, data analysis was approached through qualitative synthesis, focusing on a narrative summary supported by structured quantitative interpretation.
Studies were grouped into two primary categories based on pharmacological class: SGLT2 inhibitors formed one group, and ACE inhibitors and β-blockers were combined into a second group. These groups were then further stratified by study design (e.g., randomized controlled trials, cohort studies, preclinical investigations). Quantitative results for primary and secondary outcomes were systematically extracted, including LVEF changes (e.g., mean difference, percent change), heart failure incidence [e.g., Hazard Rations (HRs), Relative Risks (RRs), absolute event rates], mortality rates, and, where available, CIs and p-values.
Statistical interpretation focused on several dimensions. First, we assessed the consistency and direction of effect, whether outcomes such as LVEF preservation or reductions in heart failure incidence showed uniform benefit across studies within each pharmacologic group. We also evaluated the magnitude of effect, considering reported HRs, RRs, and mean differences to determine clinical relevance. Precision and statistical significance were assessed by examining confidence intervals and p-values, with attention to studies reporting non-significant results or wide intervals that might affect the robustness of findings. To understand variability, we contextualized outcomes based on follow-up duration, anthracycline dosage, and intervention intensity, which allowed us to assess the generalizability of observed effects across clinical scenarios. Lastly, comparative trends were examined between the SGLT2 inhibitor and ACE inhibitor/β-blocker groups, highlighting where one class demonstrated greater or more statistically consistent cardioprotective benefit.
No de novo statistical analyses were performed. Key findings and extracted data are presented in descriptive tables and figures to illustrate observed patterns and comparative insights.
Results
A total of 52 studies were included in this analysis, comprising randomized controlled trials, cohort studies, and preclinical investigations. Additionally, six meta-analyses were reviewed, encompassing 52 trials. Sample sizes varied widely, ranging from 9 to 10,142 participants, with follow-up durations spanning 3 days to 10 years. The studies primarily focused on patients receiving anthracycline-based chemotherapy (e.g., doxorubicin, epirubicin, idarubicin) for cancers such as breast cancer, lymphoma, and leukemia. Several studies also examined the cardioprotective effects of various drugs in patients without cancer. Studies prioritizing populations without pre-existing heart failure were included; however, this inclusion criterion was applied with greater flexibility in the evaluation of ACE inhibitors and β-blockers.
SGLT2 inhibitors, including empagliflozin and dapagliflozin, were assessed as potential cardioprotective agents, with comparators such as placebo, standard heart failure therapies (e.g., carvedilol, ACE inhibitors), or no intervention. Primary outcomes varied but frequently included heart failure incidence, LVEF changes, and GLS assessments. The diversity in study designs, patient populations, and intervention strategies highlights the growing interest in SGLT2 inhibitors as cardioprotective agents in anthracycline-treated patients.
Among the 55 total studies reviewed, 25 studies specifically examined the effects of SGLT2 inhibitors on heart failure outcomes In Table 1, a major trial showed canagliflozin significantly reduced the risk of major cardiovascular events and kidney failure, showing a hazard ratio of 0.68 (95% CI, 0.49–0.94) for primary prevention and 0.85 (95% CI, 0.69–1.06) for secondary prevention (17). Similarly, SGLT2 inhibitors, compared to other glucose-lowering drugs, were associated with a lower incidence of heart failure (HR, 0.61; 95% CI, 0.51–0.73; P < 0.001) (18). In preclinical models, empagliflozin preserved left ventricular function despite anthracycline exposure. Control mice exhibited a significant decline in ejection fraction, while empagliflozin-treated mice maintained stable cardiac function (P = 0.011) (19). Similarly, in the EMPA-DOXO (empagliflozin-doxorubicin) group, heart failure incidence was significantly reduced (P < 0.001), with ejection fraction improvement from 81.2 ± 2.5% to 88.3 ± 2.3% (P < 0.05) (20). Other studies confirmed LVEF recovery and fractional shortening (FS) in DOX + EMPA groups (P = 0.06) (21) and improved LVEF (61.3 ± 11% vs. 49.24 ± 8%, P = 0.007) in treated groups (22). Furthermore, Ejection fraction decline was significantly worse in DOX-only groups compared to SGLT2 inhibitor-treated groups (66.00% ± 9.98% vs. 78.80% ± 2.57%, 80.90% ± 3.10%, and 74.00% ± 4.03%) (23).
Table 1
| Study ID | Design | Intervention & duration | Outcomes |
|---|---|---|---|
| Anker et al. 2021 (39) | RCT | Empagliflozin 10 mg QD; 26.2 months | ↓ HF hospitalization (HR 0.73; 95% CI, 0.61–0.88; p < 0.001) |
| Das et al. 2024 (40) | Meta-analysis | Dapagliflozin 10 mg QD; 7.5 months | ↑ NYHA functional class (log OR 1.3; 95% CI, 0.37–2.23; p = 0.01) |
| Byrne et al. 2017 (19) | RCT | Empagliflozin 10 mg/kg; 0.5 months | EF preserved vs decline in controls |
| Fitchett et al. 2016 (8) | RCT | Empagliflozin 10/25 mg QD; 36 months | ↓ HF hospitalization or CV death (HR 0.66; CI 0.55–0.79; p < 0.001) |
| Kosiborod et al. 2017 (18) | Meta-analysis | Various SGLT2i; various durations | ↓ HF incidence vs other drugs (HR 0.61; CI 0.51–0.73; p < 0.001) |
| Mahaffey et al. 2018 (41) | RCT | Canagliflozin 100 mg; 60 months | ↓ primary endpoint vs placebo (HR 0.86; CI 0.75–0.97; p < 0.001) |
| Mahaffey et al. 2019 (17) | RCT | Canagliflozin 100 mg QD; 31.4 months | ↓ primary (HR 0.68) and secondary (HR 0.85) endpoints |
| McMurray et al. 2019 (42) | RCT | Dapagliflozin 10 mg QD; 18.2 months | ↓ HF events (10% vs 13.7%; HR 0.70; CI 0.59–0.83) |
| Shi et al. 2017 (43) | RCT | Canagliflozin; 0.1 months | ↓ BNP by 35%; stepwise BNP reduction (p < 0.05) |
| Zannad et al. 2020 (44) | Meta-analysis | Empagliflozin 10 mg QD; 16 months | ↓ HF hospitalization or CV death (HR 0.75; CI 0.68–0.84; p < 0.0001) |
| Zelniker et al. 2019 (45) | Meta-analysis | Various SGLT2i; 3 months | ↓ HF hospitalization/CV death (HR 0.77; CI 0.71–0.84; p < 0.0001) |
| Zinman et al. 2015 (46) | RCT | Empagliflozin 10/25 mg QD; 37.2 months | ↓ HF hospitalization by 35% |
Summary of clinical and meta-analysis studies involving SGLT2 inhibitors.
In a large, propensity-matched observational study of 1,412 anthracycline-treated patients without pre-existing heart failure, Fath et al. found that SGLT2 inhibitors were associated with significantly reduced rates of new-onset heart failure (HR: 0.15, 95% CI: 0.07–0.29) and arrhythmias (HR: 0.40, 95% CI: 0.23–0.69), without increased risk of renal dysfunction or mortality, further supporting their potential for cardioprotection in this high-risk population (24). Similarly, Bhatti et al. demonstrated that baseline SGLT2 inhibitor use in a large cohort of over 17,000 adults with cancer and type 2 diabetes was associated with a significantly reduced risk of cancer therapy–related cardiac dysfunction (HR: 0.76; 95% CI: 0.69–0.84), as well as lower rates of heart failure exacerbations and all-cause mortality (25). The cardioprotective effects of SGLT2 inhibitors across both animal models and clinical datasets are summarized in Table 2, highlighting their potential role in heart failure prevention and LVEF preservation.These findings demonstrate consistent improvements in both functional cardiac measures and clinical endpoints, supporting SGLT2 inhibitors as a mechanistically distinct and potentially superior cardioprotective option.
Table 2
| Study ID | Design | Cancer type | Intervention & duration | Chemotherapy | Outcomes |
|---|---|---|---|---|---|
| Kalay et al. 2006 (29) | RCT | Breast, Lymphoma, Other | Carvedilol 12.5 mg QD; 6 months | Adriamycin or Epirubicin | EF preserved (69.7%) vs. decline to 52.3% in control; p < 0.001 |
| Kaya et al. 2012 (47) | Double-blind | Breast | Nebivolol 5 mg QD; 6 months | Doxorubicin | EF higher in treatment (63.8%) vs. placebo (57.5%); BNP ↑ only in placebo; p = 0.01 |
| Noori et al. 2000 (28) | RCT | Breast | Propranolol, Metoprolol, Carvedilol; 8.5 months | Adriamycin | EF ↑ from 28% to 41% (BB) vs. 26% to 32% (control); p = 0.015 |
| Rode et al. 2025 (15) | Cohort | Not reported | Max BB doses; 12 months | Not reported | ↓ mortality and MACE by 57% (HR 0.43) |
| Seicean et al. 2014 (48) | RCT | Breast | Multiple BBs; 38.4 months | Doxorubicin | ↓ HF incidence (HR 0.2; 95% CI, 0.1–0.5; p = 0.003) |
Clinical studies on β-blockers for cardiotoxicity prevention.
Among the 55 total studies reviewed, 30 studies specifically examined the effects of β-blockers (Table 3), ACE inhibitors (Table 4), or combined treatment strategies (Table 5) on cardiac outcomes, demonstrating statistically significant benefits. Enalapril significantly reduced heart failure incidence and hospitalizations for patients with Hodgkin's and Non-Hodgkin's Lymphoma receiving DOX (434 vs. 518 patients, 20% risk reduction, P < 0.001) (26). A separate trial demonstrated a 16% reduction in all-cause mortality with enalapril (35.2% vs. 39.7%, P = 0.0036), with the most significant effect on progressive heart failure deaths (22% reduction, P < 0.05) (27) Treatment with β-blockers showed improvement of LVEF from 28% to 41% (P = .041) in breast cancer patients receiving Adriamycin, compared to an increase from 26% to 32% (P = .015) in controls (28). Carvedilol use in anthracycline-treated patients preserved LVEF at 69.7%, whereas the control group experienced a significant decline to 52.3% (P < 0.001) (29). The statistical significance of heart failure hospitalizations across these interventions including enalapril, β-blockers, and carvedilol is summarized in Table 6 which emphasizes the reproducibility of benefit across different trials, with multiple agents showing significant reductions in both HF incidence and hospitalization rates, reinforcing their clinical utility in cancer patients receiving anthracyclines.
Table 3
| Study ID | Design | Intervention & duration | Chemotherapy | Outcomes |
|---|---|---|---|---|
| Cardinale et al. 2006 (31) | RCT | Enalapril 20 mg/day; 12 months | Idarubicin | ↓ EF decline (0% vs. 43% in control); p < 0.001 |
| Okumura et al. 2002 (49) | RCT | Lisinopril 20 mg/kg/day; 1 month | Adriamycin | ↓ mortality (44%–12%); improved cardiac function |
| Sacco et al. 2001 (50) | RCT | Zofenopril 15 mg/kg/day; 1.25 months | Doxorubicin | Prevented ECG changes; no impact on antitumor efficacy |
| SOLVD Investigators, 1991 | Double-blind | Enalapril 2.5–20 mg/day; 41.4 months | Not reported | ↓ mortality by 16% vs. placebo; ↓ HF hospitalization |
| SOLVD Investigators et al. 1992 (14) | Double-blind | Enalapril; 37.4 months | Not reported | ↓ HF/death by 29%; ↓ HF hospitalization |
| Tokudome et al. 2000 (51) | RCT | Enalapril 5 mg BID; 1 month | Doxorubicin | ↑ survival (36%–100%); ↓ LVEDP and stroke work index; p < 0.05 |
Clinical trials on ACE inhibitors for cardiotoxicity prevention.
Table 4
| Study ID | Design | Cancer type | Intervention & duration | Chemotherapy | Outcomes |
|---|---|---|---|---|---|
| Bosch et al. 2013 (7) | RCT | Leukemia | Enalapril + Carvedilol | Not reported | EF decline only in control group (Δ = −3.4%; p = 0.09); preserved EF in treatment group |
| Georgakopoulos et al. 2010 (26) | RCT | Lymphoma | Metoprolol + Enalapril; 36 months | Doxorubicin | ↓ HF and hospitalization (p < 0.001); ↓ mortality by 20%, ↓ composite HF/death by 29% |
| Guglin et al. 2019 (12) | Double-blind, placebo-controlled | Breast | Lisinopril or Carvedilol; 24 months | Not reported | ↓ event rate: placebo 47%, lisinopril 37%, carvedilol 31% (p = 0.009, 0.015) |
| Gulati et al. 2016 (6) | Double-blind, placebo-controlled | Breast | Candesartan + Metoprolol | Not reported | No interaction effect (p = 0.530); no LVEF decline with metoprolol |
| Heck et al. 2021 (35) | Double-blind, 2 × 2 factorial | Breast | Candesartan + Metoprolol; 23 months | Epirubicin | ↓ LV end-diastolic volume (p = 0.021); ↓ strain (p = 0.046) |
| Livi et al. 2021 (13) | Phase 3 RCT | Breast | Ramipril + Bisoprolol; 12 months | Not reported | ↓ LVEF loss vs. placebo (p = 0.01); ↑ GLS in all active arms(p < 0.001) |
| Pituskin et al. 2017 (32) | Double-blind, placebo-controlled | Breast | Perindopril + Bisoprolol; 6 months | Docetaxel + Carboplatin + Trastuzumab | ↓ EF decline with trastuzumab; p = 0.001 |
| Silber et al. 2020 (33) | Systematic Review & Meta-analysis | Breast | Various combinations; median 6 months | Doxorubicin + Trastuzumab | Pooled EF benefit (MD 3.57; 95% CI 1.04–6.09) |
| Wihandono et al. 2021 (52) | RCT | Breast | Lisinopril + Bisoprolol; 6 months | Trastuzumab | ↓ EF decline in treatment group; p = 0.017 |
Clinical studies involving multiple pharmacologic interventions.
Table 5
| Intervention | Ejection Fraction (EF%) | HF Incidence | Survival |
|---|---|---|---|
| ACE inhibitors | EF Preserved (0% vs. 43% in controls) (8) | ↓ HF incidence: 28%–16% (p < 0.005) (17) | ↑ survival; 36%–100% (p = 0.02) (53) |
| β-blockers | EF Preserved (69.7% vs. 52.3% in control) (26) | ↓ HF incidence: 28%–9% (p < 0.002) (17) | ↑ survival; 63%–84% (p = 0.009) (19) |
| SGLT2 inhibitors | EF Preserved (64.0% vs. 59.8% in control) (54) | ↓ HF incidence: 30%–20% (p = 0.025) (18) | ↑ survival; 57%–91%; (p < 0.001) (18) |
Comparative efficacy of interventions on cardiac outcomes.
Table 6
| Intervention | Outcome | Statistical Measure |
|---|---|---|
| Enalapril | ↓ HF incidence and hospitalizations vs control (17) | Incidence: p < 0.001 Hospitalization: p < 0.001 |
| Enalapril | ↓ HF incidence and hospitalizations vs control (55) | Incidence: p = 0.0036 Hospitalization: p = 0.05 |
| β-Blockers | ↓ HF incidence and hospitalizations vs control (40) | Incidence: p = 0.041 Hospitalization: p = 0.041 |
| Carvedilol | ↓ HF incidence and hospitalizations vs control (26) | Incidence: p < 0.001 Hospitalization: p < 0.001 |
Statistical significance of heart failure reduction by intervention.
Echocardiographic data confirmed that the LVEF of breast cancer patients receiving DOX remained stable with carvedilol but significantly declined in controls (P < 0.001) (30). Similarly, patients with various types of cancer receiving Idarubicin who were treated with enalapril had a lower rate of LVEF decline, with 0% meeting the primary endpoint of >10% LVEF reduction, compared to 43% in the control group (P < 0.001) (31). Additionally, in breast cancer patients, bisoprolol attenuated anthracycline-induced LVEF decline (−1 ± 5%) compared to perindopril (−3% ± 4%) and placebo (−5% ± 5%) (P = .001) (32). These results, along with pooled data from meta-analyses, are summarized in Table 7, which outlines the effectiveness of various interventions in preventing LVEF decline, illustrate that certain interventions, including carvedilol, enalapril, and bisoprolol, offer statistically significant preservation of LVEF compared to control or placebo, particularly when initiated early in the treatment course.
Table 7
| Intervention | Outcome | Statistical measure |
|---|---|---|
| Carvedilol | EF remained stable in Carvedilol group; declined in controls (37) | p < 0.001 |
| Enalapril | Prevented >10% EF decline; high rate of decline in control group (8) | p < 0.001 |
| Bisoprolol vs Perindopril vs Placebo | Bisoprolol more effective at attenuating EF decline (43) | p < 0.001 |
| RAAS blockers, β-blockers, Aldosterone Antagonists | Prevented EF reduction across 11 studies (14) | MD 3.57; 95% CI 1.04–6.09 |
Effectiveness of cardioprotective interventions in preventing EF decline.
A meta-analysis confirmed that RAAS blockers, β-blockers, and aldosterone antagonists significantly prevented LVEF reduction in cancer patients receiving anthracyclines (MD 3.57, 95% CI 1.04–6.09) across 11 studies (33).
Several studies found no significant differences in LVEF or FS following anthracycline therapy, with cardiac function remaining within normal limits across treatment groups (34). A small decline in LVEF was observed over extended follow-up, but no significant between-group differences were detected: candesartan [−1.7% (95% CI, 0.5–2.8)] vs. no candesartan [−1.8% (95% CI, 0.6–3.0)] and metoprolol [−1.6% (95% CI, 0.4–2.7)] vs. no metoprolol [−1.9% (95% CI, 0.7–3.0)] (35). No significant interaction was observed between candesartan and metoprolol (P = 0.530), and metoprolol did not affect overall LVEF decline (6). Rates of early cardiotoxicity (metoprolol: 10%, enalapril: 12%, control: 15%, P = 0.12) and late cardiotoxicity (5%, 6%, and 8%, respectively, P = 0.18) did not reach statistical significance (36). In lymphoma patients receiving doxorubicin, no significant changes in echocardiographic variables were observed over 10 years, and none developed clinical heart failure. These non-significant or null findings are summarized in Table 8, which highlights interventions that did not produce statistically meaningful differences in cardiac outcomes.This table is essential for contextualizing the limitations of existing strategies, showing that not all agents confer benefit and underscoring the importance of patient selection and study design in future cardioprotection trials.
Table 8
| Intervention | Outcome | Statistical measure |
|---|---|---|
| Multiple treatment groups | No significant differences in LVEF or FS; cardiac function remained normal (10) | Not significant |
| Candesartan vs No candesartan; Metoprolol vs No metoprolol | Small LVEF decline over time; no between-group differences (21) | CIs overlapped for all comparisons |
| Candesartan and metoprolol interaction | No significant interaction effect (20) | p = 0.530 |
| Metoprolol, enalapril, control | No statistical difference in early or late cardiotoxicity (36) | p = 0.12 (early), p = 0.18 (late) |
| Doxorubicin-treated lymphoma patients | No significant changes in echo variables over 10 years; no clinical HF (17) | Not significant |
Interventions without statistically significant differences in cardiac outcomes.
Notably, there were no studies found that included direct head-to-head comparisons between ACE inhibitors and β-blockers to SGLT2 Inhibitors.
Discussion
Anthracycline-induced cardiotoxicity poses a significant obstacle in oncological management as it may compromise the long-term cardiovascular health of cancer survivors. Despite decades of research, identifying an effective prophylactic strategy remains yet to be fully elucidated. This systematic review of preclinical and clinical studies underscores three pharmacologic classes, SGLT2 inhibitors, ACE inhibitors, and β-blockers, as frontrunners in cardioprotection. However, the consistency of evidence, the mechanistic rationale, and translational potential vary across these agents.
SGLT2 inhibitors have consistently demonstrated cardioprotective efficacy across both preclinical and clinical studies. Rodent models of doxorubicin-induced cardiomyopathy show preserved or improved LVEF, attenuated myocardial fibrosis, and reduced oxidative injury. Large clinical trials such as CANVAS and DAPA-HF, suggest meaningful reductions in cardiovascular events and heart failure hospitalizations, even in non-diabetic populations. In comparison, ACE inhibitors and β-blockers have shown modest to moderate benefits in preserving cardiac function during anthracycline therapy. Enalapril and carvedilol, in particular, have been associated with reductions in LVEF decline ranging from 5% to 10%, as well as mitigation of structural cardiac changes on imaging. However, randomized trial data for these agents show variability in endpoints, some studies report significant improvements in LVEF and NT-proBNP levels, while others show non-significant between-group differences or benefits limited to surrogate markers rather than clinical events. The inconsistency in statistical significance and effect size across trials may reflect differences in dosing, timing, and patient selection.
The preclinical efficacy of SGLT2 inhibitors, marked by preserved LVEF and reduction in oxidative and inflammatory damage, appears more robust than the variable effects seen with ACE inhibitors and β-blockers. SGLT2 inhibitors may address mechanistic gaps induced by anthracycline's pharmacological effects, particularly in inflammation and myocardial energetics, that are not fully targeted by current treatment methods. While ACE inhibitors and β-blockers primarily act through neurohormonal pathways, reducing afterload and sympathetic activation, SGLT2 inhibitors exert broader effects, including attenuation of mitochondrial dysfunction, suppression of pro-inflammatory cytokines, and improvement of myocardial metabolism and energetics. These mechanisms may better counteract the multifactorial cardiac injury induced by anthracyclines, which involves oxidative stress, mitochondrial damage, and metabolic derangements.
However, despite their promise, important gaps remain, particularly a lack of long-term clinical data in patients receiving anthracycline chemotherapy. The optimal timing, duration, and dosing of SGLT2 inhibitors in this setting have yet to be clearly established. Furthermore, evidence is limited regarding their efficacy in specific high-risk subgroups, such as those with pre-existing cardiovascular disease or severely reduced baseline LVEF. Notably, a population-based cohort study of older adults with diabetes undergoing anthracycline therapy reported zero heart failure hospitalizations among those treated with SGLT2 inhibitors, compared to a rate of 2.1 per 100 person-years in the untreated group, though incident heart failure diagnoses did not reach statistical significance [Abdel-Qadir et al. (37)]. Future trials focused on these variables are essential to define the role of SGLT2 inhibitors in this context.
The findings summarized in Table 9 highlight the significant cardioprotective benefits of ACE inhibitors, β-blockers, and SGLT2 inhibitors in patients receiving anthracycline chemotherapy. Enalapril and carvedilol effectively prevented declines in ejection fraction, with enalapril showing complete prevention of significant LVEF reduction, while dapagliflozin demonstrated promising cardiac preservation in preclinical models. All three agents also reduced heart failure incidence, with dapagliflozin notably lowering HF rates and overall mortality compared to controls. The survival benefits observed—ranging from improved cardiotoxicity-free survival with carvedilol and lisinopril to mortality reductions with enalapril and dapagliflozin—support the clinical value of incorporating these therapies for cardioprotection. These results reinforce the potential role of these drug classes in mitigating anthracycline-induced cardiotoxicity and improving long-term cardiac outcomes in cancer patients.
Table 9
| Intervention | Outcome | Statistical Measure |
|---|---|---|
| Canagliflozin | ↓ major CV events and kidney failure (31) | HR 0.68 (95% CI, 0.49–0.94); HR 0.85 (95% CI, 0.69–1.06) |
| SGLT2 inhibitors vs. Other drugs | ↓ HF incidence vs non-SGLT2i treatments (28) | HR 0.61 (95% CI, 0.51–0.73); p < 0.001 |
| Empagliflozin in doxorubicin-exposed mice | Preserved LV function vs EF decline in Doxorubicin-only (7) | p = 0.011 |
| Empagliflozin + Doxorubicin vs. Doxorubicin-Only | ↑ EF; ↓ HF incidence (44) | p < 0.001; p < 0.05 |
| Empagliflozin + Doxorubicin vs. Doxorubicin-Only | EF and FS recovery observed (41) | p = 0.06 |
| Empagliflozin + Doxorubicin vs. Doxorubicin-Only | Improved EF in treated group (46) | p = 0.007 |
Preclinical and clinical outcomes of SGLT2 inhibitors in cardiotoxicity prevention.
Studies examining the potential utilization of SGLT2 inhibitors in the aforementioned clinical circumstance could be attributed to their distinct mechanistic profile, which includes reduced oxidative stress, anti-inflammatory activity, natriuresis, and improved myocardial energetics. These actions may address the pathophysiological consequences of anthracycline-induced cardiotoxicity not adequately targeted by ACE inhibitors and β-blockers. While the ACE inhibitors and β-blockers primarily act via RAAS and sympathetic inhibition (2), SGLT2 inhibitors may offer a more comprehensive cardioprotective effect.
Given their prophylactic effect, SGLT2 inhibitors may be utilized to prevent cardiomyopathy in high-risk populations—such as patients with reduced baseline LVEF or elevated NT-proBNP levels—undergoing anthracycline therapy. Early initiation could allow for detection of subclinical dysfunction allowing for timely intervention leading to improved cardiac outcomes and minimized long-term cardiac sequelae for patients undergoing anthracycline therapy.
Clinical translation considerations
While SGLT2 inhibitors demonstrate robust cardioprotective effects in preclinical and cardiovascular settings, several considerations must be addressed before broad implementation in oncology care. First, the long-term safety of these agents in patients receiving chemotherapy remains incompletely understood, particularly given the risks of immunosuppression, renal impairment, or volume depletion. Second, the potential impact of SGLT2 inhibitors on tumor biology or treatment response has not been systematically evaluated. It remains unclear whether these agents could interfere with chemotherapy efficacy, alter tumor metabolism, or influence recurrence risk.
Third, potential drug–drug interactions with common chemotherapeutics, targeted agents, or supportive medications must be carefully examined. For example, co-administration with nephrotoxic agents or diuretics may heighten the risk of renal or electrolyte disturbances. Finally, adherence poses a critical challenge in oncology populations, where polypharmacy, fatigue, and treatment-related side effects can undermine consistent use. Future clinical trials should therefore incorporate not only efficacy and safety endpoints but also real-world feasibility measures such as patient adherence, quality of life, and overall treatment burden.
Recommendations
Future research should prioritize well-designed prospective trials that directly compare these therapeutic agents. Key proposed study designs include:
- (1)
A randomized controlled trial comparing ACE inhibitors and β-blockers against SGLT2 inhibitors and placebo in cancer patients without pre-existing heart failure who are receiving anthracycline therapy, with endpoints including LVEF decline, GLS change, and NT-proBNP levels.
- (2)
A combination therapy trial assessing whether dual (ACE inhibitors + β-blockers) or triple (ACE inhibitors + β-blockers + SGLT2 inhibitors) regimens offer synergistic benefit compared to SGLT2 inhibitor monotherapy.
- (3)
A risk-adapted strategy trial, enrolling patients based on NT-proBNP, LVEF, or GLS thresholds, to determine the efficacy of early prophylactic intervention with SGLT2 inhibitors in high-risk subgroups undergoing anthracycline therapy.
Limitations of included studies
Despite the breadth of evidence reviewed, several limitations should be noted. Many clinical trials and observational studies were underpowered due to small sample sizes, making it difficult to detect long-term or rare outcomes such as late-onset heart failure. Short follow-up durations may further underestimate the true incidence of delayed cardiotoxicity, which is a known risk with anthracycline therapy.
Substantial heterogeneity in study design, including differences in patient populations, chemotherapy protocols, and outcome measures, makes direct comparisons challenging. Inconsistent definitions of cardiotoxicity, variable use of cardiac biomarkers, and limited application of modern imaging techniques such as cardiac MRI or GLS reduce the generalizability of results.
Additionally, much of the mechanistic data for SGLT2 inhibitors comes from preclinical rodent models that do not fully capture the complexity of human oncology patients, who often have comorbidities like diabetes or pre-existing cardiovascular disease. Few studies stratified outcomes by important clinical factors such as baseline cardiovascular risk, diabetes status, or sex, further limiting the applicability of findings to diverse patient subgroups.
Finally, the absence of head-to-head comparisons between SGLT2 inhibitors and established agents such as ACE inhibitors and β-blockers underscores the need for larger, rigorously designed trials with standardized endpoints and longer follow-up to clarify the role of these emerging cardioprotective strategies.
Conclusion
This review evaluates the potential cardioprotective role of SGLT2 inhibitors in mitigating anthracycline-induced cardiotoxicity. ACE inhibitors and β-blockers, well-established in heart failure management, continue to show relevance in this setting, especially for patients with preexisting cardiovascular risk. However, the lack of head-to-head comparative trials limits the ability to determine an optimal clinical strategy. Future research must prioritize randomized studies that evaluate these agents in parallel and in combination, using standardized anthracycline regimens and unified cardiac endpoints. As cancer survival improves, preventing long-term cardiac complications becomes increasingly vital. Closing these evidence gaps will be essential to guiding therapy, shaping cardio-oncology guidelines, and safeguarding the cardiovascular health of cancer survivors.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
RR: Supervision, Writing – original draft, Writing – review & editing, Resources, Project administration, Methodology, Validation, Visualization. FM: Supervision, Conceptualization, Validation, Project administration, Investigation, Writing – review & editing, Data curation, Methodology, Funding acquisition, Writing – original draft, Formal analysis, Visualization, Software, Resources. SP: Formal analysis, Investigation, Project administration, Software, Supervision, Writing – review & editing. RA: Conceptualization, Data curation, Investigation, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. CG: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The University of Illinois at Chicago college of Medicine provided funds for the publication fee.
Acknowledgments
The authors would like to express their gratitude to the University of Illinois at Chicago for providing access to the resources that supported this review. Additionally, we acknowledge the use of OpenAI's ChatGPT (38) (May 2025 version) as a writing and editorial aid in structuring portions of the manuscript, generating figure legends, and refining language for clarity and flow. All intellectual input and critical interpretation remain the responsibility of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used to assist with grammar editing, sentence rephrasing, and formatting improvements. All content, analysis, and interpretations were completed, written, and verified by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
SwainSMWhaleyFSEwerMS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. (2003) 97(11):2869–79. 10.1002/cncr.11407
2.
ZhangSLiuXBawa-KhalfeTLuL-SLyuYLLiuLFet alIdentification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. (2012) 18:1639–42. 10.1038/nm.2919
3.
MinottiGMennaPSalvatorelliECairoGGianniL. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. (2004) 56(2):185–229. 10.1124/pr.56.2.6
4.
WisemanLRSpencerCM. Dexrazoxane: a review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs. (2012) 56(3):385–403. 10.2165/00003495-199856030-00009
5.
SwedbergKClelandJGDargieHDrexlerHFollathFKomajdaMet alGuidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur Heart J. (2005) 26:1115–40. 10.1093/eurheartj/ehi204
6.
GulatiGHeckSLReeAHHoffmannPSchulz-MengerJFagerlandMWet alPrevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. (2016) 37:1671–80. 10.1093/eurheartj/ehw022
7.
BoschXRoviraMSitgesMDomènechAOrtiz-PérezJTde CaraltTMet alEnalapril and carvedilol for preventing chemotherapy-induced left ventricular dysfunction in patients with malignant haemopathies: the OVERCOME trial. Eur Heart J. (2013) 34(30):2355–62. 10.1016/j.jacc.2013.02.072
8.
FitchettDZinmanBWannerCLachinJHantelSSalsaliAet alHeart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. (2016) 37(19):1529–40. 10.1093/eurheartj/ehv728
9.
YangCCChenYTWallaceCGChenKHChengBCSungPHet alEarly administration of EMPA preserved heart function in cardiorenal syndrome in rat. Biomed Pharmacother. (2019) 109:658–70. 10.1016/j.biopha.2018.10.095
10.
LopaschukGDVermaS. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. (2020) 5(6):632–44. 10.1016/j.jacbts.2020.02.004
11.
SterneJACHernánMAReevesBCSavovićJBerkmanNDViswanathanMet alROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. 10.1136/bmj.i4919
12.
GuglinMKrischerJTamuraRFinkABello-MatricariaLMcCaskill-StevensWet alRandomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. (2019) 73(23):2859–68. 10.1016/j.jacc.2019.03.495
13.
LiviLBarlettaGMartellaFSaievaCDesideriIBacciCet alCardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol. (2021) 7(10):1544–9. 10.1001/jamaoncol.2021.3395
14.
SOLVD Investigators, YusufSPittBDavisCEHoodWBCohnJN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. (1992) 327(10):685–91. 10.1056/NEJM199209033271003
15.
RodeFPavlovićNJordanARadićMLisičićASokol TomićSet alThe use of β-blockers for heart failure with reduced ejection fraction in the era of SGLT2 inhibitors—are we still afraid to up-titrate?Heart Vessels. (2025) 40:797–804. 10.1007/s00380-025-02525-7
16.
PageMJMcKenzieJEBossuytPMBoutronIHoffmannTCMulrowCDet alThe PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
17.
MahaffeyKWJardineMJBompointSCannonCPNealBHeerspinkHJLet alCanagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. (2019) 140(9):739–50. 10.1161/CIRCULATIONAHA.119.042007
18.
KosiborodMCavenderMAFuAZWildingJPKhuntiKHollRWet alLower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study. Circulation. (2017) 136(3):249–59. 10.1161/CIRCULATIONAHA.117.029190
19.
ByrneNJParajuliNLevasseurJL. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. J Am Coll Cardiol Basic Trans Science. (2017) 1:347–54. 10.1016/j.jacbts.2017.07.003
20.
QuagliarielloVDe LaurentiisMReaDBarbieriAMontiMGCarboneAet alThe SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. (2021) 20:150. 10.1186/s12933-021-01324-2
21.
OhCMChoSJangJYKimHChunSChoiMet alCardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. (2019) 49(12):1183–95. 10.4070/kcj.2019.0180
22.
SabatinoJDe RosaSTammèLIaconettiCSorrentinoSPolimeniAet alEMPA prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol. (2020) 19(1):66. 10.1186/s12933-020-01040-5
23.
UlusanSGülleKPeynirciASevimliMKaraibrahimoğluA. Dapagliflozin may protect against doxorubicin-induced cardiotoxicity. Anatol J Cardiol. (2023) 27(6):339–47. 10.14744/AnatolJCardiol.2023.2825
24.
FathARAglanMAglanAChiltonRJTrakhtenbroitAAl-ShammaryOAet alCardioprotective potential of sodium-glucose cotransporter-2 inhibitors in patients with cancer treated with anthracyclines: an observational study. Am J Cardiol. (2024) 222:175–82. 10.1016/j.amjcard.2024.04.032
25.
BhattiAWPatelRDaniSSKhadkeSMakwanaBLesseyCet alSGLT2i and primary prevention of cancer therapy–related cardiac dysfunction in patients with diabetes. JACC: CardioOncology. (2024) 6(6):863–75. 10.1016/j.jaccao.2024.05.006
26.
GeorgakopoulosPRoussouPMatsakasEKaravidasAAnagnostopoulosNMarinakisTet alCardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol. (2010) 85(12):894–6. 10.1002/ajh.21840
27.
SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. (1991) 325(5):293–302. 10.1056/NEJM199108013250501
28.
NooriALindenfeldJWolfelEFergusonDBristowMRLowesBD. Beta-blockade in adriamycin-induced cardiomyopathy. J Card Fail. (2000) 6:115–9.
29.
KalayNBasarEOzdogruIErOCetinkayaYDoganAet alProtective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. (2006) 48(11):2258–62. 10.1016/j.jacc.2006.06.068
30.
NabatiMJanbabaiGBaghyariSEsmailiKYazdaniJ. Cardioprotective effects of carvedilol in inhibiting doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. (2017) 69(5):279–85. 10.1097/FJC.0000000000000470
31.
CardinaleDColomboASandriMTLamantiaGColomboNCivelliMet alPrevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. (2006) 114(24):2474–81. 10.1161/CIRCULATIONAHA.106.633050
32.
PituskinEMackeyJRKoshmanSJassalDPitzMHaykowskyMJet alMultidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-breast): a randomized trial for the prevention of anthracycline-induced cardiotoxicity by carvedilol. J Clin Oncol. (2017) 35(7):820–8. 10.1200/JCO.2016.68.7830
33.
GaoYWangRJiangJHuJLiHWangY. ACEI/ARB and beta-blocker therapies for preventing cardiotoxicity of antineoplastic agents in breast cancer: a systematic review and meta-analysis. Heart Fail Rev. (2023) 28(6):1405–15. 10.1007/s10741-023-10328-z
34.
ElitokAOzFCizgiciAYKilicLCiftciRSenFet alEffect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: a prospective randomized controlled study with six-month follow-up. Cardiol J. (2014) 21(5):509–15. 10.5603/CJ.a2013.0150
35.
HeckSLMecinajAReeAHHoffmannPSchulz-MengerJFagerlandMWet alPrevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2×2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. (2021) 143(25):2431–40. 10.1161/CIRCULATIONAHA.121.054698
36.
ZubairMKhanANadeemMAliS. A randomized controlled trial of metoprolol versus enalapril for the prevention of chemotherapy-induced cardiotoxicity in lymphoma patients. J Clin Oncol. (2022) 40(6):514–22. 10.1200/JCO.22.00678
37.
Abdel-QadirHCarrascoRAustinPCChenYZhouLFangJet alThe association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer. JACC CardioOncol. (2023) 5(3):318–28. 10.1016/j.jaccao.2023.04.006
38.
OpenAI. ChatGPT. (May 2025 version) [Large language model]. San Francisco, CA: OpenAI (2025). Available online at:https://chat.openai.com/
39.
AnkerSDButlerJFilippatosGFerreiraJPBocchiEBöhmMet alEmpagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. 10.1056/NEJMoa2107038
40.
DasBBNiuJ. A systematic review and meta-analysis of the safety and efficacy of SGLT2 inhibitors in chronic heart failure in ACHD patients. Am J Cardiovasc Drugs. (2025) 25(2):231–40. 10.1007/s40256-024-00697-7
41.
MahaffeyKWNealBPerkovicVde ZeeuwDFulcherGEronduNet alCanagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. (2018) 137:323–34. 10.1161/CIRCULATIONAHA.117.032038
42.
McMurrayJJVSolomonSDInzucchiSEKøberLKosiborodMNMartinezFAet alDapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. 10.1056/NEJMoa1911303
43.
ShiXVermaSYunJBrand-ArzamendiKSinghKKLiuXet alEffect of empagliflozin on cardiac biomarkers in a zebrafish model of heart failure: clues to the EMPA-REG OUTCOME trial?Mol Cell Biochem. (2017) 433:97–102. 10.1007/s11010-017-3018-2
44.
ZannadFFerreiraJPPocockSJAnkerSDButlerJFilippatosGet alSGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet. (2020) 396(10254):819–29. 10.1016/S0140-6736(20)31824-9
45.
ZelnikerTAWiviottSDRazIImKGoodrichELBonacaMPet alSGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. (2019) 393:31–3. 10.1016/S0140-6736(18)32590-X
46.
ZinmanBWannerCLachinJMFitchettDBluhmkiEHantelSet alEmpagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373(22):2117–28. 10.1056/NEJMoa1504720
47.
KayaMGOzkanMGunebakmazOAkkayaHKayaEGAkpekMet alProtective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol. (2012) 157(2):181–4. 10.1016/j.ijcard.2012.06.023
48.
SeiceanSSeiceanAAlanNPlanaJCBuddGTMarwickTHet alCardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: a cardiac MRI study. Eur Heart J Cardiovasc Imaging. (2014) 15(8):851–8. 10.1161/CIRCHEARTFAILURE.112.000055
49.
OkumuraKJinDTakaiSMiyazakiM. Beneficial effects of angiotensin-converting enzyme inhibition in Adriamycin-induced cardiomyopathy in hamsters. Jpn J Pharmacol. (2002) 88(2):183–8. 10.1254/jjp.88.183
50.
SaccoGBigioniMEvangelistaSGosoCManziniSMaggiCA. Cardioprotective effects of zofenopril, a new angiotensin-converting enzyme inhibitor, on doxorubicin-induced cardiotoxicity in the rat. Eur J Pharmacol. (2001) 414(1):71–8. 10.1016/S0014-2999(00)01070-X
51.
TokudomeTMizushigeKNomaTManabeKMurakamiKTsujiTet alPrevention of doxorubicin (Adriamycin)-induced cardiomyopathy by simultaneous administration of angiotensin-converting enzyme inhibitor assessed by acoustic densitometry. J Cardiovasc Pharmacol. (2000) 36(3):361–8. 10.1097/00005344-200009000-00003
52.
WihandonoAAzharYAbdurahmanMHidayatS. The role of lisinopril and bisoprolol to prevent anthracycline-induced cardiotoxicity in locally advanced breast cancer patients. Asian Pac J Cancer Prev. (2021) 22(9):2847–53. 10.31557/APJCP.2021.22.9.2847
53.
KalamKMarwickTH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. (2013) 49(13):2900–9. 10.1016/j.ejca.2013.04.030
54.
de NigrisFRienzoMSchianoCFioritoCCasamassimiANapoliC. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer. (2008) 44:334–40. 10.1016/j.ejca.2007.12.010
55.
NealBPerkovicVMahaffeyKWde ZeeuwDFulcherGEronduNet alCanagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377(7):644–57. 10.1056/NEJMoa1611925
56.
WiviottSDRazIBonacaMPMosenzonOKatoETCahnAet alDapagliflozin and cardiovascular outcomes in type 2 diabetes (DECLARE–TIMI 58 trial). N Engl J Med. (2019) 380:347–57. 10.1056/NEJMoa1812389
Summary
Keywords
cardiotoxicity, anthracyclines, SGLT2 inhibitors, heart failure prevention, cardioprotection, ACE-inhibitors, β-blockers cardiotoxicity, β-blockers
Citation
Rivera RJ, Muaref F, Paika S, Ruyyashi A and Gondi CS (2025) From bench to bedside: investigating SGLT2 inhibitors as a novel strategy against chemotherapy-induced cardiomyopathy. Front. Cardiovasc. Med. 12:1647747. doi: 10.3389/fcvm.2025.1647747
Received
16 June 2025
Accepted
04 August 2025
Published
22 September 2025
Volume
12 - 2025
Edited by
Prabhu Mathiyalagan, Benthos Prime Central, United States
Reviewed by
Ayman R. Fath, Creighton University, United States
Sampat Singh Tanwar, Shri Vaishnav Vidyapeeth Vishwavidyalaya, India
Updates
Copyright
© 2025 Rivera, Muaref, Paika, Ruyyashi and Gondi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faris Muaref fmuar2@uic.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.