- 1Department of Cardiovascular Medicine, Quzhou KeCheng People’s Hospital, Quzhou, Zhejiang, China
- 2Department of Science and Education, Quzhou KeCheng People’s Hospital, Quzhou, Zhejiang, China
- 3Department of Pharmacy, Quzhou KeCheng People’s Hospital, Quzhou, Zhejiang, China
Diffuse coronary artery spasm (DMV-CAS) is a serious vascular condition characterized by prolonged narrowing of two or more major coronary arteries or their main branches, leading to significant stenosis or blockage (≥70%). This can result in myocardial ischemia, heart attacks, and dangerous arrhythmias. A 68-year-old male with a four-year history of recurrent angina presented with acute-onset chest tightness, palpitations, and syncope. During transport, the patient experienced prehospital cardiopulmonary arrest, with transient return of spontaneous circulation (ROSC) achieved in the emergency department. Electrocardiographic evaluation revealed atrial fibrillation with rapid ventricular rate, pathological Q waves in the inferior, anterior, and anterior septal territories, along with dynamic ST-T abnormalities, including ST elevation in leads II, III, aVF, and V1-6, and ST depression in leads I and aVL. Emergent coronary angiography identified critical multivessel stenoses, with the most significant narrowing observed in the left anterior descending artery. The diagnosis of DMV-CAS was corroborated through angiographic evidence, demonstrating resolution of the spasm following the administration of intracoronary nitroglycerin (200 μg administered bilaterally to the coronary arteries). Despite the implementation of targeted vasodilator therapy, the patient progressed to refractory cardiogenic shock and succumbed in the intensive care unit 1 h after the procedure. This case underscores the rare and severe cardiovascular implications of DMV-CAS, emphasizing the critical need for early and accurate diagnosis of DMV-CAS and the necessity for standardized pharmacological intervention.
Introduction
Diffuse Coronary Artery Spasm (DMV-CAS) is a rare but serious condition characterized by severe, reversible spasm affecting two or more major coronary arteries. This condition is not common in clinical practice and, due to its brief duration, is often difficult to diagnose promptly (1). The occurrence of DMV-CAS may be associated with various factors, including the release of endogenous catecholamines, coronary artery spasm after heart transplantation, and cardiac tamponade, among others (2, 3). Current evidence suggests that DMV-CAS has a lower rate of major cardiovascular events than focal coronary artery spasm and an intermediate prognosis (4). In this report, we describe a rare case of DMV-CAS leading to sudden cardiac death. We hope that this case report will further raise awareness among interventional cardiologists about this rare but critical condition.
Case report
Clinical summary
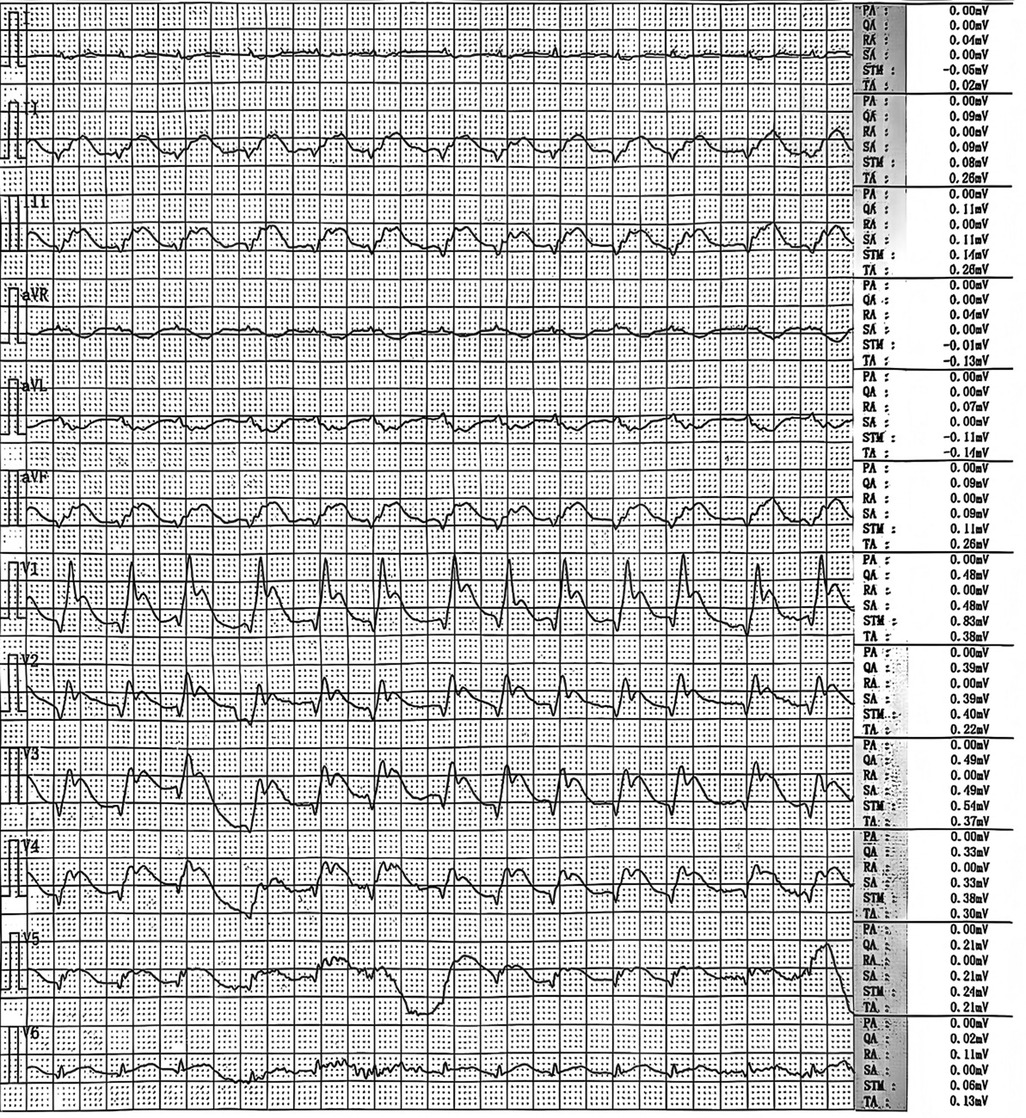
A 68-year-old male patient presented with sudden-onset chest tightness and palpitations at home during the early morning hours, with no identifiable precipitating factors. Upon the arrival of emergency medical services (EMS), the patient was found to be unresponsive and in cardiopulmonary arrest. Continuous chest compressions were administered during transport to the hospital. In the emergency department (ED), advanced resuscitation measures were undertaken, including endotracheal intubation, cardiopulmonary resuscitation (CPR), and defibrillation. After resuscitation, an emergency ECG showed atrial fibrillation with a rapid ventricular rate and changes suggesting acute myocardial infarction (Figure 1). These included pathological Q waves in the inferior, anterior, and anteroseptal walls, ST-segment elevation in leads II, III, aVF, and V1-6, and ST-segment depression with T-wave inversion in leads I and aVL. The patient subsequently experienced recurrent cardiac arrests, necessitating ongoing CPR and repeated defibrillation in the ED.
In light of the acute myocardial infarction diagnosis, the patient received dual antiplatelet therapy (aspirin enteric-coated tablet 300 mg and ticagrelor 180 mg), high-intensity statin therapy (atorvastatin 40 mg), and underwent emergent coronary angiography under endotracheal intubation.
Coronary angiography findings
The initial left coronary angiography revealed no significant stenosis in the left main trunk, an 85% stenosis in the mid-segment of the left anterior descending (LAD) artery, and a 40% stenosis in the mid-segment of the circumflex (LCx) artery, with TIMI 3 flow. Subsequent right coronary angiography encountered technical difficulties due to inadequate catheter engagement. Utilization of a JR4.0 guiding catheter disclosed a slender, elongated proximal right coronary artery (RCA) with approximately 80% stenosis. Administration of intracoronary nitroglycerin (NTG) led to a significant resolution of stenosis, thereby confirming coronary artery spasm as the underlying mechanism.
In light of the discordance between the presumed RCA culprit lesion and the initial ECG findings, a repeat left coronary angiography was conducted. This procedure demonstrated a no-reflow phenomenon in the proximal LAD (TIMI 0 flow) and an 85% stenosis in the proximal LCx. The injection of intracoronary NTG (200 µg) into the left coronary system restored full LAD perfusion (TIMI 3 flow), thereby confirming DMV-CAS as the etiology of the LAD lesion. The proximal LCx stenosis improved to 50% post-spasm relief, verifying the coexistence of underlying atherosclerotic plaque (Figure 2).
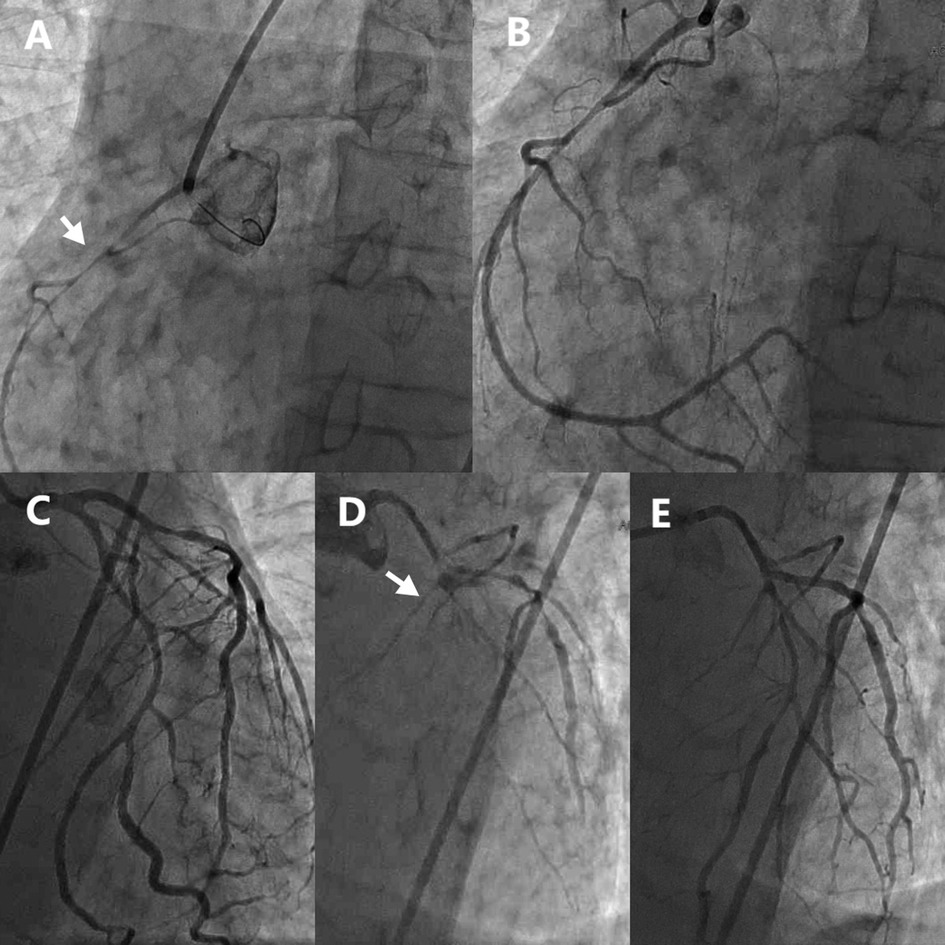
Figure 2. Emergency coronary angiography showed diffuse spasm of multiple coronary arteries. (A) RCA before NTG administration. (B) RCA after NTG administration. (C) Initial LCA imaging findings. (D) LCA second imaging results. (E) LCA after NTG administration. RCA, Right coronary artery; LCA, Left coronary artery; NTG, Nitroglycerin.
Clinical course and past medical history
Following coronary angiography, the patient was transferred to the ICU. Subsequently, he developed progressive bradycardia and hypotension with absent carotid pulses. Immediate CPR was initiated alongside continuous intravenous infusions of epinephrine and norepinephrine. Despite aggressive resuscitation efforts, return of spontaneous circulation (ROSC) was not achieved, and the patient was ultimately declared deceased.
The patient had no documented history of hypertension or diabetes mellitus and denied significant smoking or alcohol use. Neither the patient nor their immediate family members have a history of hypertension, coronary heart disease, or other heart conditions. Three years prior, he underwent coronary angiography for acute myocardial infarction, which revealed: no significant stenosis in the left main trunk, 30% stenosis in the mid-LAD artery, 95% stenosis at the ostium of the first diagonal branch (D1), and 40% stenosis in the mid-LCx artery. Percutaneous transluminal coronary angioplasty (PTCA) was performed on the D1 ostial lesion, resulting in 40% post-PTCA residual stenosis. His postoperative regimen included long-term administration of clopidogrel 75 mg daily, atorvastatin 20 mg daily, and nicorandil 5 mg three times daily. Despite this treatment, he reported persistent anginal symptoms throughout the subsequent three-year period.
Discussion
Coronary artery spasm (CAS) is characterized by the transient and reversible partial or complete occlusion of the coronary arteries resulting from excessive contraction. This phenomenon significantly contributes to myocardial ischemia and can cause a spectrum of coronary artery disease presentations. These manifestations include asymptomatic myocardial ischemia, exertional angina, Prinzmetal's angina, and acute coronary syndromes such as myocardial infarction or sudden death (5). DMV-CAS is a relatively rare yet severe condition marked by intense and reversible spasms occurring in two or more major coronary arteries. Due to its extensive involvement and transient nature, DMV-CAS is diagnostically challenging and often presents as a complication (1). Although the clinical significance of CAS is frequently underestimated, affected patients face an elevated risk of syncope, severe arrhythmias, and sudden death compared to those with classic angina. Consequently, timely diagnosis is crucial for preventing complications associated with CAS (6).
In this case, the patient initially underwent CAG without evidence of left coronary artery (LCA) spasm. However, the operating surgeon reasoned that isolated RCA spasm could not adequately explain the dynamic, widespread ST-segment changes observed on the ECG. Consequently, repeat left coronary angiography was performed, which revealed transient segmental spasm of the LCA. This finding underscores the importance of maintaining a high clinical suspicion for CAS when discrepancies exist between angiographic findings and ECG evidence of ischemia.
CAS can occur with or without obstructive coronary artery disease (CAD), but it is strongly linked to chest pain and ischemia in patients without obstructive CAD (7). Chest pain in CAS typically occurs at rest, often in the early morning, but can also be triggered by exertion. The pain frequently lasts longer than typical angina and may be accompanied by arrhythmias, diaphoresis, nausea/vomiting, or syncope. CAS-associated ECG changes include ST-segment elevation or depression, U-wave inversion, and T-wave abnormalities (8). The primary risk factors for CAS include age, smoking, hypertension, low-density lipoprotein cholesterol (LDL-C), diabetes, and high-sensitivity C-reactive protein (Hs-CRP) levels (9–11). This patient's onset occurred in the early morning, consistent with the typical timing of CAS onset. However, according to the family's description, there were no notable precipitating factors. It is worth noting that this patient lacked traditional cardiovascular comorbidities (hypertension, hyperglycemia, or dyslipidemia) and other typical risk factors. In such patients presenting with recurrent chest pain but lacking traditional risk factors or triggers, clinicians should maintain a high suspicion for CAS. This warrants considering empiric vasodilator therapy even before a definitive diagnosis is established.
The gold standard for diagnosing CAS is invasive coronary angiography combined with pharmacological provocative testing (e.g., acetylcholine) (12, 13). This can be complemented by intravascular imaging techniques such as optical coherence tomography (OCT) for plaque analysis or intravascular ultrasound (IVUS) to assess myocardial bridging (14). Functional assessments like coronary flow reserve (CFR) and index of microcirculatory resistance (IMR) also provide valuable information (14). Non-invasive approaches include ambulatory ECG monitoring for dynamic ST-segment changes, stress echocardiography, and nuclear imaging (8). Current guidelines recommend provocative testing for intermediate-to-high-risk patients, alongside assessing genetic factors and microvascular function (4). This patient had undergone multiple coronary angiography procedures, yet pharmacological provocative testing was not performed, likely due to safety concerns associated with the test and the patient's informed consent preferences, thereby delaying the definitive diagnosis of CAS at an earlier stage.
The management of CAS requires lifestyle modifications and pharmacological strategies. Lifestyle modifications are essential and include: strict smoking cessation, limiting alcohol and caffeine intake, adopting a low-salt, low-fat diet high in antioxidants, engaging in moderate exercise, and practicing stress management. These changes aim to improve endothelial function. Pharmacologically, calcium channel blockers (e.g., diltiazem) are first-line for long-term prevention. The combination of CCB with long-acting nitrates can have a cumulative effect in the early stages of CAS, reducing the incidence of CAS (15). The combination of statins and CCB helps reduce the occurrence of CAS, particularly in patients with elevated low-density lipoprotein cholesterol (LDL-C) levels (16). Additionally, Rho kinase inhibitors (e.g., fasudil) can effectively prevent acetylcholine-induced CAS and myocardial ischemia (17). α1-adrenergic receptor antagonists are an option for refractory vasospastic angina, particularly if symptoms persist despite treatment with dihydropyridine calcium channel blockers (DHP-CCBs) and long-acting nitrates (18). Excessive use of antiplatelet agents may exacerbate CAS, as reported in the literature (19). For this patient, if CCB and long-acting nitrates had been administered during the initial medical contact, the clinical outcome might have been different.
Conclusion
This fatal case of DMV-CAS highlights critical diagnostic and therapeutic challenges. Clinicians should maintain a high index of suspicion for DMV-CAS in patients presenting with:
• Sudden cardiac arrest or profound hemodynamic instability with ECG evidence of widespread, dynamic ST-segment changes (e.g., ST-elevation spanning anterior, inferior, and lateral territories) that are discordant with findings of focal obstructive disease on initial angiography.
• Recurrent angina occurring predominantly at rest or in the early morning hours, especially when refractory to standard antianginal therapies and lacking significant fixed coronary stenosis on prior evaluations.
Early recognition is paramount. Crucially, pharmacologic intervention should not be delayed until definitive angiographic confirmation. At the first point of medical contact (e.g., EMS or ED), prompt administration of sublingual or intravenous nitrates and high-dose CCBs is warranted in suspected cases of acute coronary syndrome where spasm is a potential etiology, particularly with the clinical and ECG features described above. This early vasodilator therapy aims to rapidly relieve spasm, restore coronary flow, and potentially avert the catastrophic cascade of malignant arrhythmias and cardiogenic shock observed here.
The patient's rapid demise despite eventual diagnosis underscores the lethal potential of DMV-CAS and the critical importance of proactive, empiric anti-spasm therapy initiated at the earliest suspicion, even before invasive confirmation. Standardized protocols incorporating these early interventions are essential for improving outcomes in this rare but devastating condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving human participants because the patient's case report does not involve developing research hypotheses and subsequently conducting prospective or systematic studies to publish research results. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JT: Conceptualization, Writing – original draft. YX: Data curation, Writing – original draft. MZ: Data curation, Writing – original draft. CW: Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Quzhou technology projects, China [2022K53, 2023ZD073], Zhejiang Traditional Chinese Medicine Science and Technology Project [2024ZL1218].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CAG, coronary angiography; CAS, coronary artery spasm; CAD, coronary artery disease; CCB, calcium channel blocker; CPR, cardiopulmonary resuscitation; D1, first diagonal branch; DMV-CAS, diffuse coronary artery spasm; ECG, electrocardiogram; ED, emergency department; EMS, emergency medical services; ICU, intensive care unit; LAD, left anterior descending; LCx, left circumflex; LCA, left coronary artery; NTG, nitroglycerin; RCA, right coronary artery; ROSC, return of spontaneous circulation.
References
1. Cai H, Chen S, Wang D. Sudden diffuse spasm of multiple coronary arteries: a case report. Medicine (Baltimore). (2024) 103(2):e36889. doi: 10.1097/MD.0000000000036889
2. Bertic M, Chue CD, Virani S, Davis MK, Ignaszewski A, Sedlak T. Coronary vasospasm following heart transplantation: rapid progression to aggressive cardiac allograft vasculopathy. Can J Cardiol. (2018) 34(12):1687.e9–11. doi: 10.1016/j.cjca.2018.08.022
3. Saxon C, Freeman S, Burke J. Cardiac tamponade-mediated generalized coronary vasospasm presenting as an inferior ST-segment elevation myocardial infarction: a case report. Eur Heart J Case Rep. (2023) 7(5):ytad220. doi: 10.1093/ehjcr/ytad220
4. Hokimoto S, Kaikita K, Yasuda S, Tsujita K, Ishihara M, Matoba T, et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. Circ J. (2023) 87(6):879–936. doi: 10.1253/circj.CJ-22-0779
5. Kondo T, Terada K. Coronary-artery vasospasm. N Engl J Med. (2017) 376(25):e52. doi: 10.1056/NEJMicm1614640
6. Hung M-Y, Kounis NG, Lu M-Y, Hu P. Myocardial ischemic syndromes, heart failure syndromes, electrocardiographic abnormalities, arrhythmic syndromes and angiographic diagnosis of coronary artery spasm: literature review. Int J Med Sci. (2020) 17(8):1071–82. doi: 10.7150/ijms.43472
7. Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC, Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J Am Coll Cardiol. (2012) 59(7):655–62. doi: 10.1016/j.jacc.2011.11.015
8. Yasue H, Mizuno Y, Harada E. Coronary artery spasm—clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci. (2019) 95(2):53–66. doi: 10.2183/pjab.95.005
9. Takaoka K, Yoshimura M, Ogawa H, Kugiyama K, Nakayama M, Shimasaki Y, et al. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol. (2000) 72(2):121–6. doi: 10.1016/s0167-5273(99)00172-2
10. Hung M-J, Hsu K-H, Hu W-S, Chang N-C, Hung M-Y. C-reactive protein for predicting prognosis and its gender-specific associations with diabetes mellitus and hypertension in the development of coronary artery spasm. PLoS One. (2013) 8(10):e77655. doi: 10.1371/journal.pone.0077655
11. Libby P, Bornfeldt KE, Tall AR. Atherosclerosis: successes, surprises, and future challenges. Circ Res. (2016) 118(4):531–4. doi: 10.1161/CIRCRESAHA.116.308334
12. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2013). Circ J. (2014) 78(11):2779–801. doi: 10.1253/circj.cj-66-0098
13. Wong K-T, Tam C-CF, Chung T-S, Lau T-KA, Wong S-F. Coronary vasospasm presenting in a catastrophic way. JACC Case Rep. (2024) 29(18):102554. doi: 10.1016/j.jaccas.2024.102554
14. Spiro J, Ford TJ, Yong A, Zeitz C, Beltrame JF, Watts M, et al. Protocol variation in functional coronary angiography among patients with suspected angina with non-obstructive coronary arteries: a nationwide snapshot of current practice within Australia and New Zealand. Heart Lung Circ. (2024) 33(9):1287–96. doi: 10.1016/j.hlc.2024.04.299
15. Takahashi J, Nihei T, Takagi Y, Miyata S, Odaka Y, Tsunoda R, et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J. (2015) 36(4):228–37. doi: 10.1093/eurheartj/ehu313
16. Oh MS, Yang JH, Lee DH, Park TK, Song YB, Hahn J-Y, et al. Impact of statin therapy on long-term clinical outcomes of vasospastic angina without significant stenosis: a propensity-score matched analysis. Int J Cardiol. (2016) 223:791–6. doi: 10.1016/j.ijcard.2016.08.229
17. Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by the rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. (2002) 105(13):1545–7. doi: 10.1161/hc1002.105938
18. Harris JR, Hale GM, Dasari TW, Schwier NC. Pharmacotherapy of vasospastic angina. J Cardiovasc Pharmacol Ther. (2016) 21(5):439–51. doi: 10.1177/1074248416640161
Keywords: case report, cardiac death, diffuse coronary artery spasm, early diagnosis, standardized drug therapy
Citation: Tang J, Xu Y, Zhang M and Wang C (2025) Case Report: Sudden cardiac death due to spasm of multiple coronary arteries. Front. Cardiovasc. Med. 12:1647748. doi: 10.3389/fcvm.2025.1647748
Received: 16 June 2025; Accepted: 4 August 2025;
Published: 15 August 2025.
Edited by:
Pietro Enea Lazzerini, University of Siena, ItalyReviewed by:
Neda Ćićarić, University Clinical Centre Kragujevac, SerbiaSiu Fung Wong, Hospital Authority, Hong Kong SAR, China
Copyright: © 2025 Tang, Xu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: ChenChen Wang, NDQ2OTU5MTMwQHFxLmNvbQ==
†ORCID:
ChenChen Wang
orcid.org/0000-0001-5763-7174
 JiaQi Tang1
JiaQi Tang1 MingLei Zhang
MingLei Zhang ChenChen Wang
ChenChen Wang